Abstract
AIM: To evaluate the relationship between peptic ulcer disease (PUD) and acute pancreatitis.
METHODS: A cohort of 78 patients with acute pancreatitis were included in this study. The presence of PUD and the Helicobacter pylori (H. pylori) status were assessed by an endoscopic method. The severity of acute pancreatitis was assessed using Ranson’s score, the Acute Physiology and Chronic Health Evaluation (APACHE) II score, computed tomography severity index and the clinical data during hospitalization, all of which were compared between the patients with and without PUD. The risk factors for PUD were also evaluated.
RESULTS: Among 78 patients, 41 patients (52.6%) with acute pancreatitis suffered from PUD, but only 13 (31.7%) patients with PUD were infected by H. pylori. On univariate analysis, male gender, an etiology of alcohol-induced pancreatitis, a history of smoking or alcohol consumption, elevated triglyceride and C-reactive protein levels, and high APACHE II score were significantly associated with PUD. However, on multivariate logistic regression analysis, the APACHE II score (odds ratio: 7.69; 95% confidence interval: 1.78-33.33; P < 0.01) was found to be the only independent risk factor for PUD.
CONCLUSION: Patients with acute pancreatitis are liable to suffer from PUD. PUD is associated with severe acute pancreatitis according to the APACHE II score, and treatment for PUD should be considered for patients with severe acute pancreatitis.
Keywords: Acute pancreatitis, Peptic ulcer disease, Helicobacter pylori, Acute Physiology and Chronic Health Evaluation II score
INTRODUCTION
Acute pancreatitis is a common disease that has shown an increased incidence in the past two decades[1-3]. Acute pancreatitis is caused by an acute inflammatory response resulting from unregulated activation of pancreatic enzymes, which can lead to extrapancreatic complications due to the persistence of hypovolemia, a decreased intravascular volume and multiorgan dysfunction. In fact, patients with acute pancreatitis may complain of various abdominal symptoms such as nausea, vomiting and pain, and these symptoms are sometimes confused with dyspeptic symptoms[4,5]. In a recent study, 65% of patients with acute pancreatitis were found to have acute gastrointestinal mucosal lesions[6]. We hypothesized that the decrease in intravascular volume, and the stress response that diminishes the blood flow could result in upper gastrointestinal ischemia or inflammation, and cause peptic ulcer disease (PUD). The aims of this study were to evaluate the prevalence of PUD among patients with acute pancreatitis, and to compare the clinical characteristics and severity of acute pancreatitis according to the presence of PUD.
MATERIALS AND METHODS
The study was conducted at St. Vincent’s Hospital, a teaching hospital of the the Catholic University of Korea. The medical records, charts and the digitalized picture archived images of consecutive patients who were admitted with acute pancreatitis between February 2008 and August 2009 were collected. The study was approved by the Institutional Review Board of the Catholic University of Korea. The patients included in this study were all older than 17 years of age, had visited our clinic within 2 d of the occurrence of abdominal symptoms, and underwent endoscopic gastroduodenoscopy during hospitalization. The patients with acute pancreatitis due to endoscopic retrograde cholangiopancreatography were excluded. The diagnosis of acute pancreatitis was based on the presence of two of the following three features[7]: (1) acute onset of typical abdominal pain; (2) serum amylase and/or lipase level ≥ 3 times the upper limit of normal; and (3) characteristic findings of acute pancreatitis on an abdominal computed tomography (CT) scan or on ultrasonography. Gallstone pancreatitis was diagnosed by CT or ultrasonography in the absence of another etiology such as excessive alcohol consumption[6-9]. Hyperlipidemia was diagnosed in cases with a serum triglyceride level above 500 mg/mL[6]. The exclusion criteria were previous abdominal surgery, a previous history of acute or chronic pancreatitis, a diagnosis of PUD in the previous 3 mo, a history of taking drugs such as non-steroidal antiinflammatory drugs, aspirin, anticoagulants and/or antiplatelet drugs in the previous month, and incomplete medical records. Our institute routinely recommended endoscopy to confirm and treat acute mucosal lesions in the stomach or duodenum in patients with acute pancreatitis before the patient was permitted any oral intake, and determination of the severity score of acute pancreatitis for evaluating and managing the patients. An ulcer was defined as a lesion with loss of mucosal integrity (a whitish exudate was observed) and the lesion was > 5 mm in size with apparent depth determined by endoscopy[10]. The status of Helicobacter pylori (H. pylori) was evaluated in patients with PUD, who underwent antral or body biopsy for histopathology or a rapid urease test (CLO test). The prevalence of PUD and H. pylori in the patients with acute pancreatitis were evaluated. The severity of acute pancreatitis were evaluated by laboratory data and scores such as Ranson’s score, the Acute Physiology and Chronic Health Evaluation (APACHE) II score and the CT severity index (CTSI) during hospitalization. The clinical characteristics including demographic, laboratory, or radiologic data and the severity of acute pancreatitis in the patients with and without PUD were compared.
Statistical analysis
The primary end points of the study were the prevalence of PUD associated with acute pancreatitis. The secondary end points were the risk factors for PUD in the patients with acute pancreatitis. The continuous data were expressed as mean ± SE (standard error of the mean) determined using the independent sample Student t-test, while categorical variables were expressed as quantities and were analyzed using the χ2 test. Multiple stepwise logistic regression analysis was used to identify the risk factors for PUD. The analyses were performed with a statistical software package (SPSS, version 15.0; SPSS Inc). A P-value < 0.05 was considered significant for all tests. This research adhered to the principles of the Declaration of Helsinki.
RESULTS
During the study period, a total of 123 consecutive patients with acute pancreatitis and who were not related were enrolled. Patients who did not undergo endoscopic gastroduodenoscopy during hospitalization were excluded (n = 30). Of the remaining 93 patients, 15 were excluded due to a history of acute or chronic pancreatitis (n = 10), a diagnosis of PUD in the previous 3 mo (n = 1), a history of taking drugs such as aspirin in the previous month (n = 3), and incomplete medical records (n = 1).
A total of 78 patients were finally enrolled and included in the analysis. The mean age of the patients was 53.4 ± 1.8 years (range, 18-86 years) and 52 patients (66.7%) were male. The mean time to the endoscopic procedure after admission was 3.3 ± 0.3 d (range, 1-16 days). Forty one patients (52.6%) were found to have PUD. The characteristics of the patients with or without PUD are shown in Table 1. On univariate analysis, male gender (P = 0.03), an etiology of pancreatitis due to alcohol (P = 0.03), a history of smoking (P = 0.05), a history of excessive alcohol consumption (P = 0.01), an elevated triglyceride level (P = 0.05), an elevated C-reactive protein level (P < 0.001) and an elevated APACHE II score (P = 0.001), in particular an APACHE II score ≥ 6 (P < 0.001), were found to be significantly associated with PUD in patients with acute pancreatitis (Table 1). The receiver-operating characteristic (ROC) curve with a cutoff of an APACHE II score of 6 was selected as the highest sensitivity and specificity values for evaluating the factors for PUD [area under the curve (AUC), 0.75; 95% confidence interval (95% CI): 0.67-0.86] (Figure 1). On multivariate logistic regression analysis, only an APACHE II score ≥ 6 was found to be a significant risk factor (Table 2).
Table 1.
Characteristics of the patients with or without peptic ulcer disease (mean ± SE) n (%)
| Ulcer (n = 41) | No ulcer (n = 37) | P value | |
| Age (yrs) | 56.6 ± 2.3 | 49.8 ± 2.9 | 0.07 |
| Gender | |||
| Male | 32 (78.0) | 20 (54.1) | 0.03 |
| Female | 9 (22.0) | 17 (45.9) | |
| BMI (kg/m2) | 23.9 ± 0.5 | 23.3 ± 0.8 | 0.48 |
| Diabetes | |||
| Yes | 7 (17.7) | 3 (8.1) | 0.31 |
| No | 34 (82.9) | 34 (91.9) | |
| Hypertension | |||
| Yes | 9 (22.0) | 6 (16.2) | 0.52 |
| No | 32 (78.0) | 31 (83.8) | |
| Etiology | |||
| Alcohol | 22 (53.7) | 9 (24.3) | 0.03 |
| Biliary stone | 7 (17.1) | 9 (24.3) | |
| Idiopathic | 6 (14.6) | 14 (37.8) | |
| Others | 6 (14.6) | 5 (13.5 | |
| Smoke | |||
| Yes | 20 (48.8) | 10 (27.0) | 0.05 |
| No | 21 (51.2) | 27 (73.0) | |
| Alcohol | |||
| Yes | 29 (70.7) | 16 (43.2) | 0.01 |
| No | 12 (29.3) | 21 (56.8) | |
| Laboratory | |||
| BUN (mg/dL) | 17.2 ± 1.6 | 17.9 ± 1.7 | 0.78 |
| Cr (mg/dL) | 1.1 ± 0.1 | 1.0 ± 0.1 | 0.34 |
| Amylase (IU/L) | 633.9 ± 123.0 | 564.3 ± 77.9 | 0.64 |
| Lipase (IU/L) | 405.1 ± 75.2 | 518.5 ± 82.6 | 0.31 |
| Triglyceride (mg/dL) | 261.5 ± 71.5 | 111.0 ± 15.6 | 0.05 |
| ESR (mm/h) | 46.6 ± 5.5 | 36.3 ± 4.6 | 0.16 |
| CRP (mg/dL | 15.5 ± 2.3 | 5.2 ± 0.9 | < 0.001 |
| CTSI | 2.1 ± 0.3 | 1.6 ± 0.2 | 0.21 |
| Ranson score | |||
| On admission | 1.3 ± 0.2 | 1.2 ± 0.2 | 0.76 |
| At 48 h | 0.8 ± 0.2 | 0.4 ± 0.1 | 0.07 |
| APACHE II score | 8.0 ± 0.7 | 4.5 ± 0.7 | 0.001 |
| < 6 | 15 (36.6) | 29 (78.4) | < 0.001 |
| ≥ 6 | 26 (63.4) | 8 (21.6) | |
| Time to endoscopy (d) | 3.5 ± 0.5 | 3.0 ± 0.4 | 0.45 |
| Death | 0 (0) | 0 (0) |
BMI: Body mass index; BUN: Blood urea nitrogen; Cr: creatinine; ESR: Erythrocyte sedimentation rate; CRP: C-reactive protein; CTSI: Computed tomography severity index; APACHE: Acute Physiology and Chronic Health Evaluation.
Figure 1.
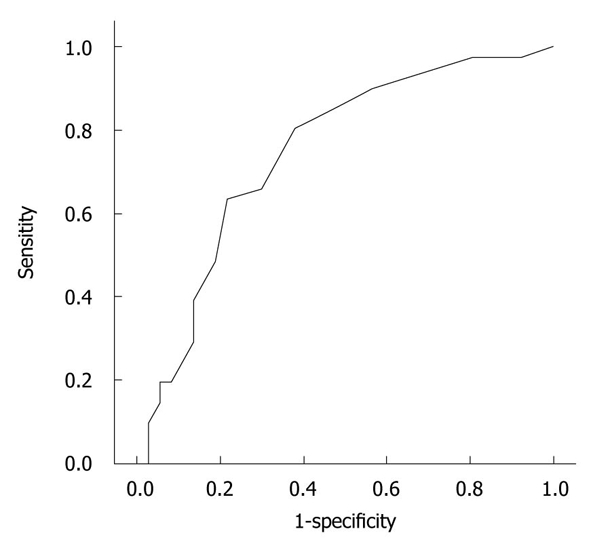
Receiver operating characteristic curve for Acute Physiology and Chronic Health Evaluation II score in predicting the severity of acute pancreatitis (receiver operating characteristic area under the curve, 0.75; 95% confidence interval: 0.64-0.86).
Table 2.
Risk factors for peptic ulcer in patients with acute pancreatitis (multivariate analysis)
| Variables | OR | 95% CI | P value |
| Alcohol-induced | 3.23 | 0.77-14.29 | 0.11 |
| Smoking | 2.86 | 0.65-12.5 | 0.16 |
| APACHE II score ≥ 6 | 7.69 | 1.78-33.33 | < 0.01 |
OR: Odds ratio; CI: Confidence interval; APACHE: Acute Physiology and Chronic Health Evaluation.
Among the 41 patients with PUD and acute pancreatitis, H. pylori was detected in 13 patients (31.7%). Any demographic differences were not found between the H. pylori-positive and -negative groups, except for the location of the ulcer. Among the 13 patients in the H. pylori-positive ulcer group, 10 (76.9%) patients revealed only a gastric ulcer, and a duodenal ulcer was not found. However, for the 28 patients in the H. pylori-negative ulcer group, gastric ulcers were found in 12 patients (42.9%), duodenal ulcers in 12 patients (42.9%), and both gastric and duodenal ulcers in 4 patients (14.3%). The location of the ulcers was different according to the status of H. pylori (Table 3).
Table 3.
Infection rate of Helicobacter pylori in the patients with peptic ulcer disease (mean ± SE) n (%)
| H. pylori-positive | H. pylori-negative | P value | |
| n (%) | 13 (31.7) | 28 (68.3) | |
| Age (yrs) | 61.2 ± 4.5 | 54.5 ± 2.5 | 0.17 |
| Gender | |||
| Male | 10 (76.9) | 22 (78.8) | 0.91 |
| Female | 3 (23.1) | 6 (21.4) | |
| BMI (kg/m2) | 24.1 ± 1.1 | 23.8 ± 0.6 | 0.76 |
| Smoke | |||
| Yes | 7 (53.8) | 14 (50.0) | 0.82 |
| No | 6 (46.2) | 14 (50.0) | |
| Alcohol | |||
| Yes | 8 (61.5) | 21 (75.0) | 0.38 |
| No | 5 (38.5) | 7 (25.0) | |
| Location of ulcer | |||
| GU only | 10 (76.9) | 12 (42.9) | 0.02 |
| DU only | 0 | 12 (42.9) | |
| GU and DU | 3 (23.1) | 4 (14.3) |
H. pylori: Helicobacter pylori; BMI: Body mass index; GU: Gastric ulcer; DU: Duodenal ulcer.
DISCUSSION
The current study showed that the prevalence of PUD in patients with acute pancreatitis was relatively high (52.6%), and the cause of may be related to the stressful condition of the underlying pancreatitis.
Although the previous literature has reported that the use of histamine 2 (H2) receptor antagonists or proton pump inhibitors (PPI) could prevent stress ulcers in cases of severe pancreatitis[7,11], the published clinical evidence is still scanty and controversial. Only one clinical study reported that acute gastrointestinal lesions occurred in 65% of patients with acute pancreatitis[6], and this rate was higher than that of our study (52.6%). Our strict selection of enrolled patients with only PUD, as demonstrated by endoscopy with definitive criteria, was the reason why a relatively low rate was demonstrated in our study. Because the prevalence of PUD in the general population is known to be about 5% according to recent data[12], the 52.6% prevalence of our data was high, and so PUD seems to be associated with acute pancreatitis.
The pathogenesis of PUD associated with acute pancreatitis is still not understood. However, gastric mucosal ischemia under the stressful condition of acute pancreatitis might be a major factor for peptic ulcer occurring together with acute pancreatitis[6]. Acute pancreatitis may be complicated by the hypovolemic status of the pancreas or extrapancreatic ischemia due to the diminished effective blood volume or hypoperfusion[4,7,11,13], which have been reported to be causative factors for stress ulcers[14]. Another suggestion is that acidic conditions in the intestine develop because of reduced bicarbonate secretion by the pancreas, resulting in patients with pancreatitis becoming susceptible to a duodenal ulcer[7]. Furthermore, intestinal ischemia increases intestinal permeability to bacteria, bacterial products and/or endotoxins, permitting a secondary pancreatic infection, and also stimulates cytokine release, and increases the level of nitric oxide, which serially contributes to ongoing pancreatic injury as well as organ failure[15-17]. In our study, an association between PUD and acute pancreatitis was observed, and the use of antiulcer medication may have an impact on the treatment or prognosis of patients with acute pancreatitis.
PUD disease is a multifactorial disease that has been largely attributed to the presence of H. pylori infection[18-20], and the presence of H. pylori infection in patients with PUD has been reported to range between 61% and 94%[21-23]. However, in our data the prevalence of H. pylori infection was only 31.7%. The distinct difference in location of ulcers between H. pylori-positive and -negative groups was interesting. Why a low prevalence of H. pylori infection was revealed in patients with a duodenal ulcer was unclear. The suggested hypothesis is that inflammation of the pancreas affects sites nearer to the duodenum than the stomach. Because our study excluded patients with a recent drug history that could cause PUD, our study at least demonstrated that the main cause of PUD might be associated with acute pancreatitis.
An ulcer was clearly defined and selected to exclude other mucosal lesions such as erythema, erosion and edema that were seen on endoscopy. The reason is that by excluding subjective ambiguous mucosal lesions and including the clinically significant meaningful lesion, the prevalence or characteristics of the ulcer could be evaluated. Another reason is that in our country, patients who have PUD revealed on endoscopy can receive the medical benefits of health insurance for treatment with drugs such as PPI. The patients were not placed on ulcer prophylaxis at admission with acute pancreatitis because prescription of anti-ulcer medications such as PPI or H2 blockers under health insurance need documentation of an ulcer by endoscopy.
The APACHE II score was the only independent risk factor associated with PUD in our study. Many prognostic factors for acute pancreatitis have been previously suggested, such as laboratory markers, radiologic views and scoring systems. A previous study reported that the occurrence of an acute gastric mucosal lesion was not related to the severity of acute pancreatitis[6]. However, that study lacked a proven method, such as the APACHE scoring system, for evaluating the severity of acute pancreatitis. The APACHE II score reflects the systemic or physiologic response to inflammation-driven stress during the course of acute pancreatitis[24], and it was reported to be superior to the Balthazar CTSI for predicting organ failure[25-27]. On the basis of the highest sensitivity and specificity values that were generated from the ROC curves, the cutoff of an APACHE II score of 6 was selected for evaluating the factors for PUD (AUC, 0.75; 95% CI: 0.64-0.86). The score of 6 was somewhat low to reflect the severity in patients with acute pancreatitis[10,15], and may be due to the exclusion of critically ill patients.
The potential limitations of our study were the small number of patients ultimately enrolled, and the retrospective design. However, the subjects of this study were limited to patients with acute pancreatitis and who underwent endoscopy under strict criteria that considered the patient’s history of medication or surgery. Our clinic has routinely recommended that hospitalized patients are evaluated, and the severity of acute pancreatitis predicted, by using scoring systems for acute pancreatitis such as Ranson’s score, the APACHE II score and the CTSI. Another limitation of this study was the exclusion of critically ill patients who did not undergo endoscopy, which affected the exact prevalence of PUD, but this would cause underestimation of the prevalence of PUD because some of these patients had a possibility of having PUD combined with severe acute pancreatitis.
In conclusion, patients with acute pancreatitis have a strong possibility of suffering from PUD. In particular, if patients are diagnosed with severe acute pancreatitis, based on the APACHE II scoring system, then treatment for PUD is strongly recommended.
COMMENTS
Background
Acute pancreatitis is caused by an acute inflammatory response due to unregulated activation of pancreatic enzymes, which can lead to extrapancreatic complications because of the persistence of hypovolemia, a decreased intravascular volume and multiorgan dysfunction. The possibility of inflammation due to ischemia in the upper gastrointestinal tract may exist. In fact, patients with acute pancreatitis may complain of various abdominal symptoms such as nausea, vomiting and pain, and these symptoms can sometimes be confused with dyspeptic symptoms; these patients may have complications of acute gastrointestinal mucosal lesions.
Research frontiers
The authors hypothesized that the reduction in intravascular volume and the stress response that diminishes the blood flow will affect the upper gastrointestinal lesion and cause peptic ulcer diseases (PUD). In analysis of PUD, we also tried to identify the status of Helicobacter pylori (H. pylori) infection through endoscopy.
Innovations and breakthroughs
Patients with acute pancreatitis are liable to suffer from PUD, and PUD is related to severe acute pancreatitis according to the Acute Physiology and Chronic Health Evaluation (APACHE) II score with a cutoff value of 6. Among patients with acute pancreatitis, a low prevalence of H. pylori infection was revealed in patients with PUD, especially in patients with a duodenal ulcer.
Applications
In the authors’ study, an association between PUD and acute pancreatitis was observed and the use of antiulcer medication may be recommended for patients with acute pancreatitis to relieve symptoms of the suspected ulcer, especially in severe acute pancreatitis. By identifying a definite relationship between acute pancreatitis and PUD, this study provides a challenge to clarify the basic mechanisms of these two diseases. In addition, a large prospective study to confirm these observations is required.
Peer review
This study retrospectively reviewed the clinical records of the patients of acute pancreatitis (AP) focusing on the relationship between AP and gastroduodenal PUD and found a positive relationship between APACHE II score and PUD.
Acknowledgments
The authors thank Mrs Yu-Jung Kim for help with the literature and statistical research.
Footnotes
Peer reviewer: Shoichiro Sumi, MD, PhD, Associate Professor, Department of Organ Reconstruction, Institute for Frontier Medical Sciences, Kyoto University, Sakyo-ku, Kyoto, 606-8507, Japan
S- Editor Sun H L- Editor Cant MR E- Editor Lin YP
References
- 1.Imrie CW. Acute pancreatitis: overview. Eur J Gastroenterol Hepatol. 1997;9:103–105. [PubMed] [Google Scholar]
- 2.Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med. 2006;354:2142–2150. doi: 10.1056/NEJMcp054958. [DOI] [PubMed] [Google Scholar]
- 3.Jaakkola M, Nordback I. Pancreatitis in Finland between 1970 and 1989. Gut. 1993;34:1255–1260. doi: 10.1136/gut.34.9.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008;371:143–152. doi: 10.1016/S0140-6736(08)60107-5. [DOI] [PubMed] [Google Scholar]
- 5.Tenner S. Steinberg WM. Acute pancreatitis. In: Feldman M, Friedman LS, Brandt LJ, eds , editors. Sleisenger and Fordtran’s gastrointestinal and liver disease. 8th edition: Pathophysiology/diagnosis/management. Philadelphia: Saunders; 2006. pp. 1241–1269. [Google Scholar]
- 6.Chen TA, Lo GH, Lin CK, Lai KH, Wong HY, Yu HC, Hsu PI, Chen HH, Tsai WL, Chen WC. Acute pancreatitis-associated acute gastrointestinal mucosal lesions: incidence, characteristics, and clinical significance. J Clin Gastroenterol. 2007;41:630–634. doi: 10.1097/01.mcg.0000225638.37533.8c. [DOI] [PubMed] [Google Scholar]
- 7.Banks PA, Freeman ML. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379–2400. doi: 10.1111/j.1572-0241.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- 8.Bank S, Indaram A. Causes of acute and recurrent pancreatitis. Clinical considerations and clues to diagnosis. Gastroenterol Clin North Am. 1999;28:571–589, viii. doi: 10.1016/s0889-8553(05)70074-1. [DOI] [PubMed] [Google Scholar]
- 9.Law NM, Freeman ML. Emergency complications of acute and chronic pancreatitis. Gastroenterol Clin North Am. 2003;32:1169–1194, ix. doi: 10.1016/s0889-8553(03)00089-x. [DOI] [PubMed] [Google Scholar]
- 10.Yeomans ND, Naesdal J. Systematic review: ulcer definition in NSAID ulcer prevention trials. Aliment Pharmacol Ther. 2008;27:465–472. doi: 10.1111/j.1365-2036.2008.03610.x. [DOI] [PubMed] [Google Scholar]
- 11.Toouli J, Brooke-Smith M, Bassi C, Carr-Locke D, Telford J, Freeny P, Imrie C, Tandon R. Guidelines for the management of acute pancreatitis. J Gastroenterol Hepatol. 2002;17 Suppl:S15–S39. doi: 10.1046/j.1440-1746.17.s1.2.x. [DOI] [PubMed] [Google Scholar]
- 12.Groenen MJ, Kuipers EJ, Hansen BE, Ouwendijk RJ. Incidence of duodenal ulcers and gastric ulcers in a Western population: back to where it started. Can J Gastroenterol. 2009;23:604–608. doi: 10.1155/2009/181059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muddana V, Whitcomb DC, Khalid A, Slivka A, Papachristou GI. Elevated serum creatinine as a marker of pancreatic necrosis in acute pancreatitis. Am J Gastroenterol. 2009;104:164–170. doi: 10.1038/ajg.2008.66. [DOI] [PubMed] [Google Scholar]
- 14.Fennerty MB. Pathophysiology of the upper gastrointestinal tract in the critically ill patient: rationale for the therapeutic benefits of acid suppression. Crit Care Med. 2002;30:S351–S355. doi: 10.1097/00003246-200206001-00002. [DOI] [PubMed] [Google Scholar]
- 15.Ammori BJ, Becker KL, Kite P, Snider RH, Nylén ES, White JC, Barclay GR, Larvin M, McMahon MJ. Calcitonin precursors: early markers of gut barrier dysfunction in patients with acute pancreatitis. Pancreas. 2003;27:239–243. doi: 10.1097/00006676-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Rahman SH, Ammori BJ, Holmfield J, Larvin M, McMahon MJ. Intestinal hypoperfusion contributes to gut barrier failure in severe acute pancreatitis. J Gastrointest Surg. 2003;7:26–35; discussion 35-36. doi: 10.1016/S1091-255X(02)00090-2. [DOI] [PubMed] [Google Scholar]
- 17.Ammori BJ, Barclay GR, Larvin M, McMahon MJ. Hypocalcemia in patients with acute pancreatitis: a putative role for systemic endotoxin exposure. Pancreas. 2003;26:213–217. doi: 10.1097/00006676-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Hentschel E, Brandstätter G, Dragosics B, Hirschl AM, Nemec H, Schütze K, Taufer M, Wurzer H. Effect of ranitidine and amoxicillin plus metronidazole on the eradication of Helicobacter pylori and the recurrence of duodenal ulcer. N Engl J Med. 1993;328:308–312. doi: 10.1056/NEJM199302043280503. [DOI] [PubMed] [Google Scholar]
- 19.Patchett S, Beattie S, Leen E, Keane C, O'Morain C. Helicobacter pylori and duodenal ulcer recurrence. Am J Gastroenterol. 1992;87:24–27. [PubMed] [Google Scholar]
- 20.Kalaghchi B, Mekasha G, Jack MA, Smoot DT. Ideology of Helicobacter pylori prevalence in peptic ulcer disease in an inner-city minority population. J Clin Gastroenterol. 2004;38:248–251. doi: 10.1097/00004836-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Sugiyama T, Nishikawa K, Komatsu Y, Ishizuka J, Mizushima T, Kumagai A, Kato M, Saito N, Takeda H, Asaka M, et al. Attributable risk of H. pylori in peptic ulcer disease: does declining prevalence of infection in general population explain increasing frequency of non-H. pylori ulcers? Dig Dis Sci. 2001;46:307–310. doi: 10.1023/a:1005600831851. [DOI] [PubMed] [Google Scholar]
- 22.Jyotheeswaran S, Shah AN, Jin HO, Potter GD, Ona FV, Chey WY. Prevalence of Helicobacter pylori in peptic ulcer patients in greater Rochester, NY: is empirical triple therapy justified? Am J Gastroenterol. 1998;93:574–578. doi: 10.1111/j.1572-0241.1998.167_b.x. [DOI] [PubMed] [Google Scholar]
- 23.Vorobjova T, Maaroos HI, Uibo R, Wadström T, Wood WG, Sipponen P. Helicobacter pylori: histological and serological study on gastric and duodenal ulcer patients in Estonia. Scand J Gastroenterol Suppl. 1991;186:84–89. doi: 10.3109/00365529109103992. [DOI] [PubMed] [Google Scholar]
- 24.Triantopoulou C, Lytras D, Maniatis P, Chrysovergis D, Manes K, Siafas I, Papailiou J, Dervenis C. Computed tomography versus Acute Physiology and Chronic Health Evaluation II score in predicting severity of acute pancreatitis: a prospective, comparative study with statistical evaluation. Pancreas. 2007;35:238–242. doi: 10.1097/MPA.0b013e3180619662. [DOI] [PubMed] [Google Scholar]
- 25.Chatzicostas C, Roussomoustakaki M, Vardas E, Romanos J, Kouroumalis EA. Balthazar computed tomography severity index is superior to Ranson criteria and APACHE II and III scoring systems in predicting acute pancreatitis outcome. J Clin Gastroenterol. 2003;36:253–260. doi: 10.1097/00004836-200303000-00013. [DOI] [PubMed] [Google Scholar]
- 26.De Sanctis JT, Lee MJ, Gazelle GS, Boland GW, Halpern EF, Saini S, Mueller PR. Prognostic indicators in acute pancreatitis: CT vs APACHE II. Clin Radiol. 1997;52:842–848. doi: 10.1016/s0009-9260(97)80079-7. [DOI] [PubMed] [Google Scholar]
- 27.Papachristou GI, Muddana V, Yadav D, O'Connell M, Sanders MK, Slivka A, Whitcomb DC. Comparison of BISAP, Ranson's, APACHE-II, and CTSI scores in predicting organ failure, complications, and mortality in acute pancreatitis. Am J Gastroenterol. 2010;105:435–441; quiz 442. doi: 10.1038/ajg.2009.622. [DOI] [PubMed] [Google Scholar]


