Abstract
Donor-reactive memory T cells can play an important role in mediating graft rejection following transplantation. Transplant recipients acquire donor-reactive memory T cells not only through prior sensitization with alloantigens, but also through previous exposure to environmental pathogens that are cross-reactive with allogeneic peptide:MHC complexes. Current dogma suggests that most, if not all, memory T cell responses are independent of the requirement for CD28 and/ or CD154/CD40-mediated costimulation in order to mount a recall response. However, heterogeneity among memory T cells is increasingly being appreciated, and one important factor known to impact the function and phenotype of antigen-specific T cell responses is the amount/duration of antigen exposure. Importantly, the impact of antigen exposure on development of costimulation independence is currently unknown. Here, we interrogated the effect of decreased antigen amount/duration during priming on the ability of donor-reactive memory T cells to mediate costimulation blockade-resistant rejection during a recall response following transplantation in a murine model. Recipients possessing donor-reactive memory T cell responses that were generated under conditions of reduced antigen exposure exhibited similar frequencies of antigen-specific T cells at day 30 post infection, but, strikingly, failed to mediate costimulation blockade-resistant rejection following challenge with an OVA-expressing skin graft. Thus, these data demonstrate the amount/ duration of antigen exposure is a critical factor in determining memory T cells' relative requirement for costimulation during the recall response following transplantation.
Keywords: Costimulation, memory T cell, CD8+ T lymphocyte
Introduction
In recent years, it has become increasingly apparent that a large degree of heterogeneity exists among memory T cells in mouse and man (1, 2). Memory T cells possess a wide range of levels of expression of activation markers, trafficking molecules, costimulatory molecules, and inhibitory receptors. At a functional level, memory T cells can vary widely in terms of their proliferative capacity, cytokine production, and cytolytic function upon secondary recall. For example, many groups now have characterized the existence of central (TCM) vs effector (TEM) memory T cells which express different homing receptors that allow them to traffic to the lymphoid organs or peripheral tissues, respectively (3), and initiate the secondary recall response (TCM) or provide surveillance of peripheral tissue sites for invading pathogens (TEM). Additional subsets and functional phenotypes of memory T cells have also recently been described (4). Before this wide range of function and phenotypes of memory T cells was appreciated, it was generally accepted that memory T cells do not require CD28/B7 and/or CD154/CD40 signals for recall responses. This dogma is based on studies showing that memory T cells could become fully activated following in vitro stimulation with B7-deficient APC (5-8), and the finding that CD28-/- mice do not exhibit a gross impairment in their ability to generate memory T cells in response to LCMV infection, or for these memory T cells to respond upon secondary rechallenge (9). Despite these findings, the fact that blockade of CD28 can alleviate established autoimmunity in models of EAE and type 1 diabetes would suggest that memory T cells present in these models could be controlled by blockade of the CD28 pathway (10). Furthermore, psoriasis and rheumatoid arthritis in humans, both of which are thought to be mediated by memory T cells (11), can be successfully treated with CTLA-4 Ig (12, 13). Katsikis et al., working in a model of bacterial infection, found that CD8+ memory T cells arising from adoptively transferred cells required CD28-mediated costimulation for optimal recall responses (14). In sum, these findings suggested that, under certain circumstances, memory T cells may require CD28 and/or CD154 mediated signals in order to mount effective recall responses.
These issues are relevant to the field of transplantation in that it is becoming increasingly well-appreciated that memory T cells elicited via a prior pathogen infection can potentially cross-react with allogeneic peptide:MHC complexes and thereby participate in rejection of the graft (15, 16). A recent study by Amir et al. demonstrated that fully 40% of virus-specific human T cell clones tested exhibited alloreactivity to at least one HLA molecule (17). Furthermore, prior studies have shown that pre-existence of donor-reactive memory T cells (in otherwise unsensitized individuals) correlated with poor graft outcomes (18). Thus, understanding the mechanisms by which pathogen-elicited donor-reactive memory T cells may mount recall responses following transplantation and mediate rejection of an allograft are of critical importance.
Costimulatory requirements of donor-reactive memory T cells during transplantation are particularly relevant in that reagents designed to block costimulatory molecules are currently in late-stage clinical trials for the prevention of graft rejection (19-21). Numerous studies over the last 20 years have demonstrated that blockade of the CD28 and CD154/CD40 costimulatory pathways during transplantation leads to prolonged graft survival in both murine and non-human primate models (22-24). However, it is becoming increasingly well-appreciated that the immune history of a transplant recipient may be a major determinant of the success or failure of tolerance induction strategies (25-32). Importantly, donor-specific memory T cells elicited either by exposure to donor antigens or viral pathogens are refractory to tolerance induction using CD40 and CD28 blockers in protocols that effectively tolerize naïve donor-specific T cells (27, 33). For example, while a costimulation blockade-based tolerance regimen effectively prevented skin graft rejection in naïve mice, mice that had been previously infected with one, two, or three different viruses demonstrated increasing resistance to the induction of tolerance by costimulation blockade (33). Evidence from different murine model systems indicates that both CD4+ and CD8+ donor-specific memory cells can constitute a barrier to tolerance (33, 34). In human transplant recipients, higher levels of donor-specific memory T cells are associated with higher rejection rates (18). However, the functional phenotypes of memory cells are highly heterogeneous, and can be influenced by a variety of different factors. Thus, understanding the individual parameters that give rise to this heterogeneity, especially in terms of relative resistance to costimulation blockade-based therapies for transplantation tolerance induction, remains an important goal.
In this study, we therefore sought to define the priming conditions that influence the relative costimulation requirement of donor-reactive memory T cells. Previous studies have demonstrated that the amount/ duration of antigen exposure can have a profound impact on the function and character of the resulting memory T cell population (35-37). Here we tested the impact of alterations in exposure to antigen during priming on the development of memory T cell responses that are costimulation independent during recall. In order to accomplish this, we made use of a system in which OT-I transgenic T cells were stimulated during the priming phase by OVA-expressing Listeria in order to generate pathogen-elicited donor-reactive memory T cells. Experimental animals received ampicillin post-infection in order to limit the spread of the infection and reduce the amount and duration of antigen exposure of the donor-reactive T cells, and the mice subsequently received an OVA-expressing skin graft to initiate a secondary recall response in the presence or absence of costimulation blockade. Recipients possessing donor-reactive memory T cell responses that had been generated under conditions of reduced antigen exposure exhibited similar frequencies and phenotypes of antigen-specific T cells at day 30 post infection, but, strikingly, failed to mediate costimulation blockade-resistant rejection following challenge with an OVA-expressing skin graft. Thus, these data demonstrate the amount/ duration of antigen exposure is a critical factor in determining memory T cells' relative requirement for costimulation during recall response following transplantation.
Materials and Methods
Mice
Adult male 6- to 8-week old C57BL/6 were purchased from the Jackson Laboratory (Bar Harbor, ME). TCR transgenic OT-I mice were purchased from Taconic, Inc. and were bred onto the Thy1.1+ background. Act-mOVA mice were produced and provided by Dr. Marc Jenkins, Univ. of Minnesota (38). Animals received humane care and treatment in accordance with Emory University Institutional Animal Care and Use Committee guidelines.
LM-OVA infection and ampcillin treatment
Listeria monocytogenes containing full-length OVA insert with streptomycin-resistance (LM-OVA) was made by H. Shen (39). 5 mls BHI broth (Teknova) supplemented with 50 ug/ml streptomycin was inoculated with LM-OVA and incubated overnight at 37° C. Mice were infected with 104 colony-forming units (CFU) of LM-OVA intraperitoneally in 500 ul of sterile PBS on day 0. Where indicated, mice were treated with 1mg ampicillin (in saline) intraperitoneally and given 2mg/mL ampicillin in drinking water 24 h to d6 post-infection. For CFU quantification, spleens were harvested at the indicated timepoints, processed, and resuspended in 0.5% Triton X-100. Samples were onto BHI plates containing 50 g/ml streptomycin (Teknova). Plates were incubated at 37°C for 24-48 hours and observed for the presence of bacterial colonies.
Quantification of Kb/SIINFEKL complexes
Splenocytes were harvested at the indicated timepoints and stained with CD11b-FITC, mouse IgG1-PE isotype control, B220-PerCP, and CD11c-APC (all BD Pharmingen), and an antibody specific for SIINFEKL/Kb complexes (25-D1.16) (40). The 25-D1.16 was conjugated to R-PE using the Lightning Link R-PE kit (Innova Biosciences) following manufacturer's instructions. The number of peptide:MHC complexes were calculated by normalizing the MFI of 25-D1.16 staining to a standard curve of fluorescent beads bearing a known quantity of R-PE molecules (Bangs Labs) and conducting regression analysis according to the manufacturer's instructions (Bangs Labs).
T Cell Adoptive Transfers
OT-I × Thy1.1+ TCR tg T cells were harvested from spleens, single cell suspensions were prepared and counted, and the frequency of OT-I T cells within the splenocytes preparation was determined prior to adoptive transfer by staining with anti-Vα2, anti-Vβ5, and anti-CD8 (Pharmingen, San Diego, CA). Mice received a single i.v. injection of the indicated number of OT-I T cells along with syngeneic B6 carrier splenocytes.
Flow Cytometric Analyses for Frequency and Absolute Number
At the indicated timepoints, recipients of OT-I T cell transfers were sacrificed, spleens and draining axillary lymph nodes were harvested, and single cell suspensions were prepared. Cells were stained with KLRG-1-PE (Southern Biotech), CD44-PE (BioLegend), CD127-APC (eBiosciences), CD62L-FITC, CD25-APC, Thy1.1-PerCP, and CD8-PacBlue (all BD Biosciences) for flow cytometric analysis on a BD LSRII multi-color flow cytometer. In some cases, the absolute number of antigen-specific T cells was determined by TruCount Bead Analysis (Pharmingen) according to manufacturer's instuctions. Flow cytometric data were analyzed using FlowJo Software (Treestar, San Carlos, CA).
Intracellular Cytokine Staining
For measurement of IFN-γ, TNF, and IL-2 secreting cells, single cell suspensions of splenocytes from adoptive transfer/skin graft recipients (1×106 per well) were incubated in a 96 well plate with 10 nM OVA257-264 (SIINFEKL)(Emory University Microchemical Core Facility) and 10 μg/ml Brefeldin A (Pharmingen, San Diego, CA). After 4 hours in culture, cells were processed using an intracellular staining kit (Pharmingen, San Diego, CA) according to manufacturer's instructions and stained with anti-TNF-PE, anti-IFN-γ-FITC, anti-IL-2 APC, anti-Thy1.1-PerCP, anti-CD8-Pacific Blue, and (all from Pharmingen). Culture media consisted of RPMI 1640 supplemented with 10% FBS (Mediatech, Herndon, VA), 2 mM l-glutamine, 0.01 M Hepes buffer, 100 μg/ml gentamycin (Mediatech), and 5 × 10−5 M 2-mercaptoethanol (Sigma-Aldrich, St. Louis, MO).
Skin Grafting and Costimulation Blockade
Full thickness skin grafts (∼1 cm2) were transplanted onto the dorsal thorax of recipient mice and secured with a plastic adhesive bandage for 5 days. Graft survival was then monitored by daily visual inspection. Rejection was defined as the complete loss of viable epidermal tissue. Where indicated, recipients of skin grafts received treatment with 500 μg each of hamster anti-mouse CD40L mAb (MR-1, BioXcell, West Lebanon, NH) and/or human CTLA-4 Ig (Bristol-Meyers Squibb), administered i.p. on the day of transplantation (day 0) as well as on post-transplant days 2, 4, and 6.
Statistical Analyses
Survival times for skin graft experiments were displayed on Kaplan-Meier curves and compared by log-rank test, and numbers of donor-specific T cells and MFIs of activation markers were compared by Mann-Whitney non-parametric test. Statistical analyses were conducted using GraphPad Prism Software.
Results
Limiting the amount and duration of antigen exposure during priming does not impact magnitude of donor-reactive T cell response
We sought to determine the effects of decreased antigen exposure on the character of donor-reactive memory T cell populations. As such, we began by establishing a model system in which the amount and duration of antigen exposure could be manipulated in vivo. Briefly, we infected naïve B6 mice with OVA-expressing Listeria monocytogenes (LM-OVA). Animals then received ampicillin intraperitoneally at 24 h post-infection, and in their drinking water ad libitum as previously described (41), in order to limit the spread of the infection and reduce the amount and duration of antigen exposure of the donor-reactive T cells. Results indicated that both the magnitude and duration of OVA-expressing Listeria, as measured in ex vivo CFU assays, were reduced in the ampicillin-treated animals (Figure 1A). The area under the curve for the untreated animals was 4251, whereas for the ampicillin-treated animals it was 98.75.
Figure 1. Limiting the duration of infection and peptide presentation in LM-OVA infected mice with ampicillin treatment results in similar peak frequencies and numbers of OT-I T cells.
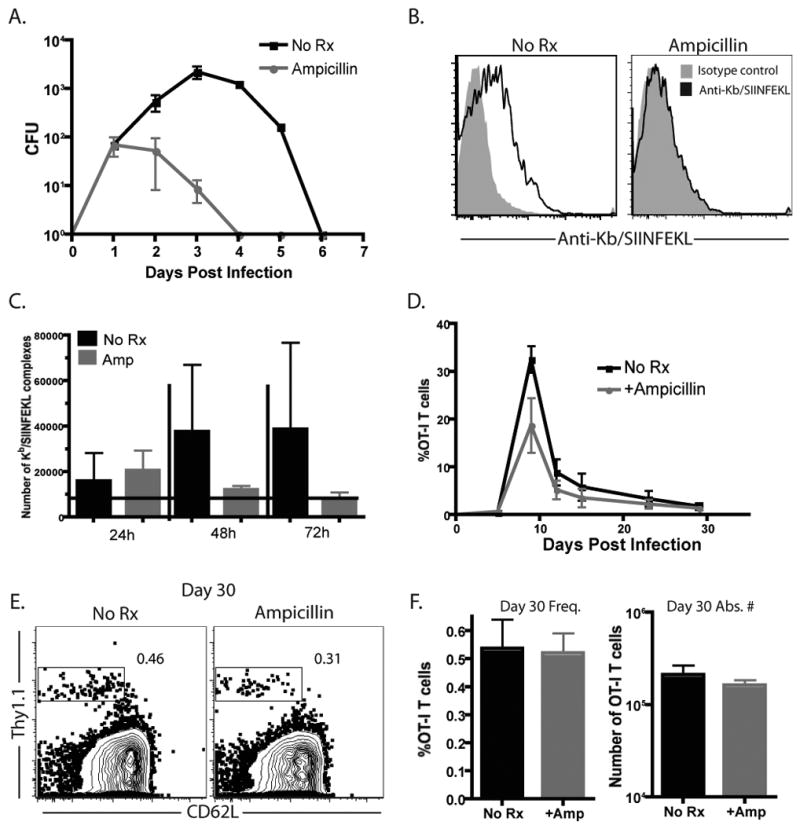
Naïve B6 mice were adoptively transferred with 104 OT-I T cells and infected with 104 CFU of LM-OVA and either left untreated or treated with ampicillin as described in Materials and Methods. A) Mice were sacrificed and spleens were harvested at days 1, 2, 3, 4, and day 6 post-infection and plated as described in Materials and Methods for assessment of bacterial load. Results shown are cumulative from three independent experiments, with 2-3 mice per timepoint. B-C) Spleens harvested at the indicated timepoints were stained for Kb/SIINFEKL expression and enumerated using R-PE MESF beads. B, Representative example of Kb/SIINFEKL staining vs. isotype control at 48 h. C, Cumulative results from 3 mice/group/timepoint demonstrated that Kb/SIINFEKL expression in ampicillin-treated mice at days 2 and 3 post-infection was lower than untreated mice. D-F) Recipients of OT-I T cells were analyzed for the kinetics and magnitude of expansion at days 6, 10, 13, 21, and 30 post-LM-OVA infection. Data shown in C are representative results of the frequency of Thy1.1+CD8+ T cells from four independent experiments of 5 mice per group, per experiment. Data shown in D are a representative example of frequency of Thy1.1+ of CD8+ T cells at day 30 post-infection. Data shown in E are representative results of the frequency (left) and absolute number (right) of Thy1.1+CD8+ T cells detected at day 30 post-infection from three independent experiments of 5 mice per group, per experiment. There is no statistically significant difference in the absolute number of Thy1.1+ CD8+ memory T cells between groups (p=0.8413).
In order to more precisely quantify the effect of limiting bacterial replication on the degree of antigen exposure, we measured the number of peptide: MHC complexes presented on the surface of CD11c+ APC isolated from LM-OVA infected animals in the presence or absence of ampicillin. Using an antibody specific for SIINFEKL/Kb complexes (25-D1.16) and a flow cytometry bead-based quantification assay (40), the numbers of peptide:MHC complexes were calculated by normalizing the MFI to a standard curve of fluorescent beads bearing a known quantity of fluorescent molecules (Figure 1B). Results obtained using this methodology demonstrated that the numbers of SIINFEKL/Kb complexes per cell were reduced in the spleen at both 48 and 72 hours following infection in the mice that received ampicillin treatment (Figure 1C). By 72h post-infection, staining for SIINFEKL/Kb complexes reached the limit of detection (grey bar) in ampicillin-treated recipients. These data therefore suggested that donor-reactive T cells primed in ampicillin-treated recipients experienced reduced exposure to APC-bound Kb/SIINFEKL complexes relative to untreated control animals.
To assess the impact of this reduced antigen exposure on generation of memory T cells, 104 Thy1.1+ naïve OT-I T cells were adoptively transferred into naïve B6 recipients, which were then infected with LM-OVA in the presence or absence of ampicillin treatment as described above. The magnitude and kinetics of expansion of antigen-specific Thy1.1+ T cells under these conditions were followed over time, and as has been previously reported (41), we did not observe significant differences in either the kinetics of expansion and contraction or the peak burst size in mice treated with ampicillin as compared to untreated controls (Figure 1D). These results corroborate earlier findings demonstrating that CD8+ T cells need only a short exposure to antigen, which results in programmed expansion and differentiation during an antigen-independent phase of the response (42, 43). We then went on to examine OVA-specific Thy1.1+ T cell frequencies and absolute numbers at day 30 post-infection, during the memory phase of the response, and found them to be equivalent in both the ampicillin-treated and untreated recipients (Figure 1E, 1F). Thus, memory T cell frequency was not impacted by decreasing the duration and extent of antigen exposure.
Donor-reactive memory T cells stimulated under conditions of limiting antigen fail to mediate costimulation blockade resistant rejection
The results presented above suggested that limiting antigen exposure during priming did not impact quantitative aspects of memory T cell development. We hypothesized that it may, however, impact qualitative aspects of memory T cell development, specifically with regard to the costimulation independence of memory T cells. To assess the effects of reduced antigen exposure on development of donor-reactive memory T cell requirement for costimulation, 104 Thy1.1+ donor-reactive T cells were adoptively transferred into naïve B6 recipients, which were infected with LM-OVA and treated with ampicillin as described above. At day 30 post-infection, mice were challenged with an OVA-expressing mOVA skin graft, and groups of mice were treated with CTLA-4 Ig and anti-CD154 (MR-1) to block the CD28 and CD40 costimulatory pathways, respectively. Results demonstrated that animals not treated with costimulation blockade, both ampicillin-exposed and unexposed recipients rapidly rejected their skin grafts with second-set kinetics (Figure 2A, MST 13.5 and 11 days, respectively). Consistent with the known costimulation independence of memory T cells, recipients that were treated with costimulation blockade but that had not received ampicillin during priming, also rapidly rejected their skin grafts in a costimulation-independent manner (Figure 2B, MST 13 d). In stark contrast, however, mice that had received ampicillin, and therefore were subjected to attenuated antigen exposure during priming, failed to undergo costimulation blockade-resistant rejection and instead exhibited long-term graft survival (Figure 2B, MST 180 d, p<0.0001). Coupled with our findings that similar frequencies of cells existed in these recipients at the time of graft challenge, these results suggest that limiting antigen exposure during priming inhibits the differentiation of costimulation-independent memory T cells.
Figure 2. Limiting the duration of infection and peptide presentation results in long-term skin graft acceptance following CTLA-4 Ig and anti-CD154 treatment.
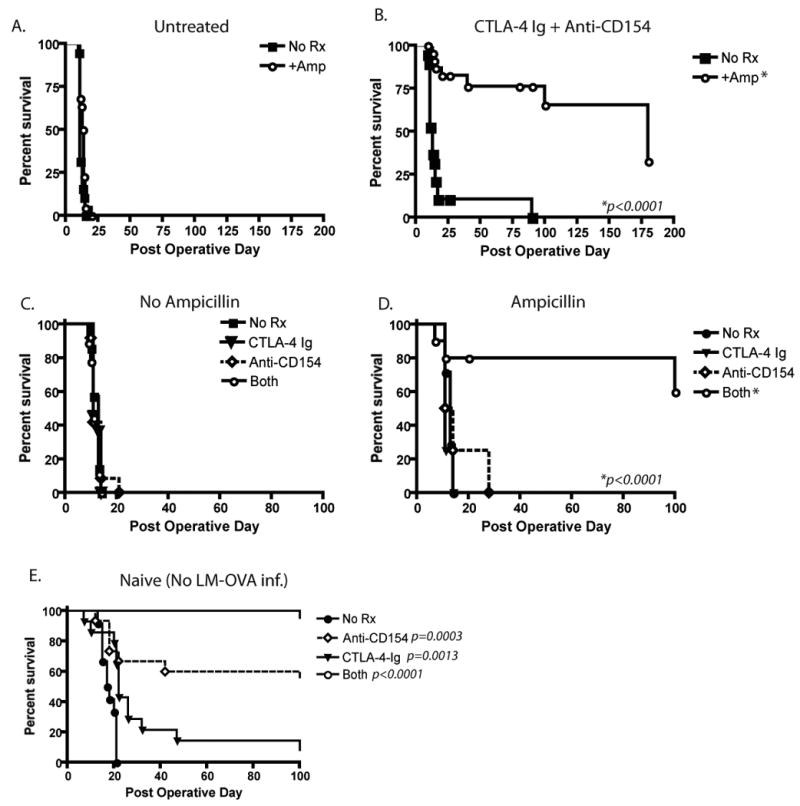
LM-OVA infected, OT-I-transferred recipient mice received mOVA skin grafts and, where indicated, received CTLA-4 Ig alone, anti-CD154 alone, or a combination of the two on days 0, 2, 4, and 6 post transplantation as described in Materials and Methods. A) Untreated controls uniformly experienced graft rejection with accelerated kinetics as compared to naïve mice (MST 11 and 13.5 d for no amp vs amp, respectively), whereas B) costimulation blockade-treated animals receiving ampicillin treatment went on to long-term graft survival (MST 180 d, as compared to 13 d for mice that did not receive ampicillin treatment, p<0.001). Mice that rejected between days 179 and 196 are depicted as having rejected at day 180 post-transplant. C) Recipients experiencing limited antigen exposure demonstrated rejection with CTLA-4 Ig and anti-CD154 alone (MST 11 and 12.5 d, respectively) whereas naïve skin graft recipients under single pathway blockade experienced prolonged graft survival (MST 22 and 189 d, respectively). p=0.0013 for CD28 blockade and p=0.0003 for CD154 blockade as compared to untreated controls. Results shown are from four independent experiments, each experiment with five mice per group.
We next evaluated the ability of donor-reactive memory T cells generated under conditions of limited antigen density and duration to mediate graft rejection following single pathway blockade. Specifically, recipients that had been infected with LM-OVA in the presence or absence of ampicillin were allowed to go to memory and then were rechallenged with an mOVA skin graft in the presence of either CTLA-4 Ig or anti-CD154. Results from these experiments demonstrated that in contrast to combined pathway blockade, memory T cells that were generated under conditions of limited antigen exposure were still able to mediate graft rejection following either CD28 or CD154 pathway blockade alone (Figure 2C, D, MST 11 and 12.5 d, respectively). This is in contrast to recipients possessing naïve T cells, which demonstrated significantly prolonged graft survival following CD28 (p=0.0013) or CD154 (p=0.0003) pathway blockade (Figure 2E, MST 22 and 189 days, respectively, compared to 17.5 for untreated controls). These data suggested that in donor-reactive T cell populations that differentiate under conditions of limited antigen exposure, engagement of either the CD28 or CD154/CD40 pathway is sufficient to generate a competent recall response, but that blockade of both pathways results in attenuation of the response and prolongation of graft survival.
Limiting the amount and duration of antigen exposure during priming of donor-reactive T cells resulted in normal cytokine production but reduced expression of KLRG-1 and increased expression of CD127
The above results suggested that cells stimulated under conditions of limited antigen exposure during the priming phase exhibited an increased requirement for costimulation during the recall response. Given these results, we interrogated the stage of differentiation during which differences in these T cell populations could be observed. First, we asked whether effector T cells stimulated under conditions of limited antigen exposure differed significantly from their untreated counterparts during the peak of the response. In order to address this question, we compared the functional and phenotypic characteristics of day 9 OT-I T cells isolated from LM-OVA infected mice that had been left untreated or treated with ampicillin as described above. At day 9 post-infection, splenocytes were harvested and restimulated with OVA peptide to assess cytokine production. Results indicated that T cells from untreated and ampicillin-treated mice produced IFN-γ, TNF, and IL-2 at high frequencies (Figure 3A), and there was no difference in the frequencies of single cytokine-, double-cytokine, or triple cytokine-producing cells (Figure 3B, 3C).
Figure 3. Effector T cells generated under limited antigen stimulation show similar cytokine profiles and decreased KLRG-1 expression.
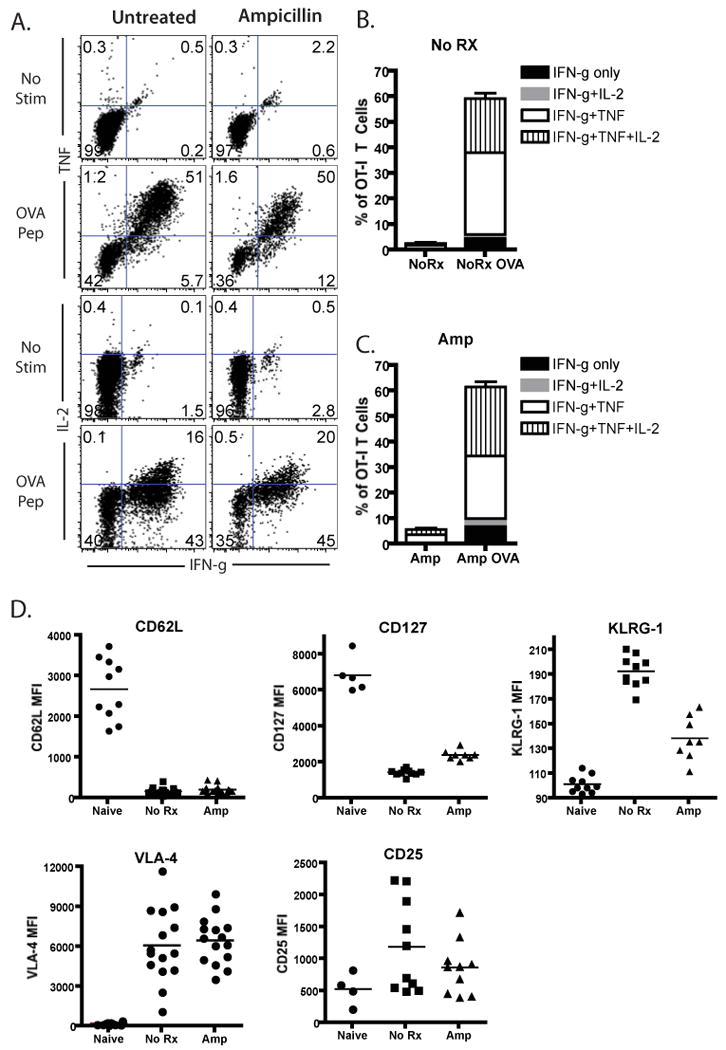
Mice infected with LM-OVA after OT-I transfer were either treated with ampicillin or left untreated and sacrificed 9 days post infection. Splenocytes were harvested for cytokine production assays and assessment of phenotypic expression markers. A-C) Splenocytes from LM-OVA-infected OT-I recipients were restimulated in vitro with SIINFEKL peptide for 4 hours, and analyzed for the presence of intracellular IFN-γ, TNF, and IL-2. Untreated and treated mice showed similar levels of production of IFN-γ, IL-2, and TNF production. Data shown in A are representatives of three independent experiments, where n=5 animals per group per experiment. Data in B and C summarize data for all three independent experiments. D) Thy1.1+ CD8+ T cells from untreated or ampicillin-treated mice were analyzed for surface expression of activation markers. Results indicated decreased KLRG-1 expression on Thy1.1+CD8+ T cells from ampicillin-treated recipients as compared to untreated controls (p<0.001). Results shown in D are cumulative mean fluorescence intensities of Thy1.1+ CD8+ T cells of two-four separate experiments, for a total of five-15 mice per group.
We next compared phenotypic markers of cell differentiation between the two populations, and while no differences were observed in the levels of expression of CD62L, VLA-4, and CD25, we observed a marked reduction in the expression of KLRG-1 on OT-I T cells that had been stimulated under conditions of limited antigen exposure as compared to untreated controls (MFI 193 vs 139, respectively, p<0.001). In addition, a highly statistically significant upregulation of CD127 expression was observed OT-I T cells that had been stimulated under conditions of limited antigen exposure as compared to untreated controls (MFI 2437 vs 1468, respectively, p<0.001).
Effect of reduced antigen exposure on memory T cell generation and function
Because we had observed differences in KLRG-1 and CD127, both markers of T cell differentiation status, at day 9 post-infection, we next sought to characterize the phenotypes and functionality of cells stimulated under conditions of limiting antigen exposure at day 30 post-infection (memory timepoint). Again, adoptive transfer recipients of OT-I T cells were infected with LM-OVA in the presence or absence of ampicillin. At day 30 post-infection, splenocytes were harvested and assayed for ability to produce cytokines following a short period of in vitro restimulation. No differences were observed in the abilities of cells primed under conditions of limiting antigen exposure to produce IFN-γ, TNF, and/or IL-2 following ex vivo restimulation (Figures 4A-4C). Furthermore, at this memory timepoint, no differences were observed in the expression of KLRG-1, CD127, CD62L, CD28 or CD25.
Figure 4. Memory CD8+ T cells generated under conditions of limited antigen results in similar levels of cytokine production and similar phenotypic profiles.
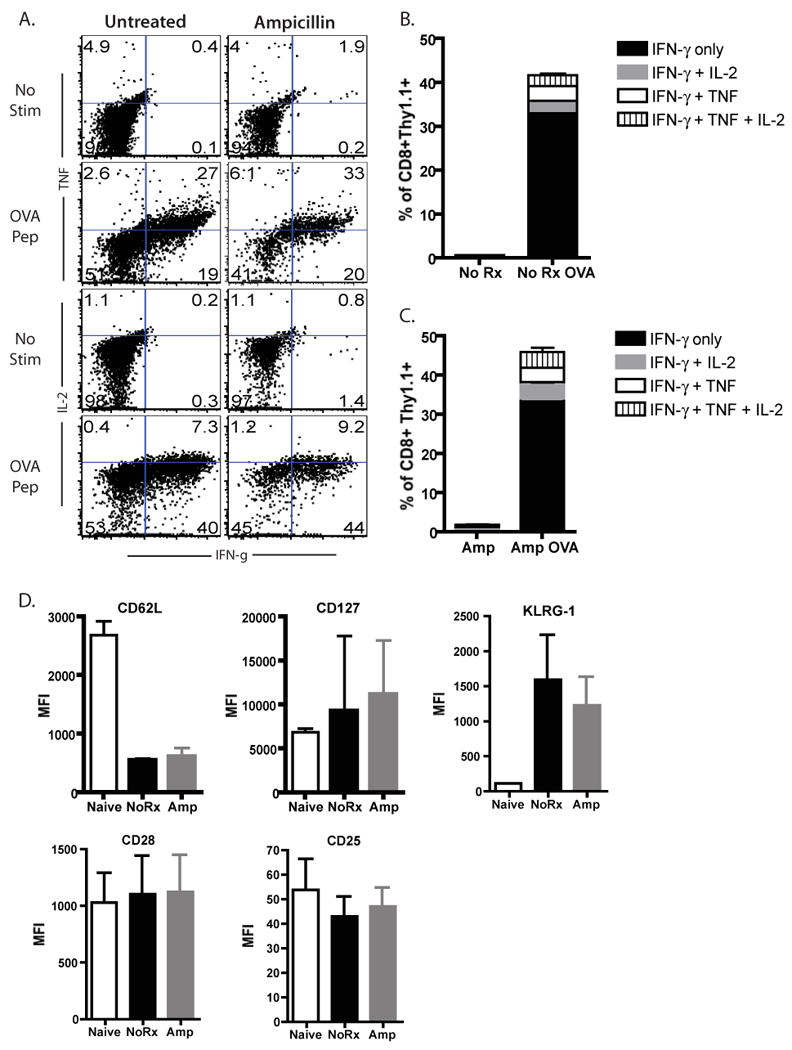
Mice infected with LM-OVA after OT-I transfer were either treated with ampicillin or left untreated, sacrificed 30 days post infection and splenocytes were harvested for cytokine production assays and assessment of phenotypic expression markers. A-C) Splenocytes from LM-OVA-infected OT-I recipients were restimulated in vitro with SIINFEKL peptide for 4 hours, and analyzed for the presence of intracellular IFN-γ, TNF, and IL-2. Untreated and ampicillin-treated mice showed similar levels of production of IFN-γ, IL-2, and TNF. Data shown in A are representatives of two independent experiments, where n=5 animals per group per experiment. Data in B show summary data for both independent experiments. D) CD8+Thy1.1+ T cells from untreated or ampicillin-treated recipients were analyzed at day 30 post-infected for expression of CD62L, CD127, KLRG-1. CD28, and CD25. Mean fluorescence intensities of Thy1.1+ CD8+ T cells of two separate experiments are shown, for a total of five- 10 mice per group.
Memory T cells primed under conditions of limited antigen exposure exhibit diminished recall responses in the presence of costimulation blockade
As memory T cells primed under conditions of limiting antigen exposure were phenotypically indistinguishable from their untreated counterparts at day 30 post-infection, we sought to assess their ability to respond upon rechallenge with antigen. Briefly, recipients of OT-I T cells that had been infected with LM-OVA in then left untreated or treated with ampicillin were challenged with an mOVA skin graft on day 30 post infection (Figure 5A). Groups of mice were either left untreated following graft placement or were treated with CTLA-4 Ig and anti-CD154. Ten days post transplantation, mice were sacrificed and spleens and draining LN were analyzed for the donor-reactive T cell response. While a slight decrease in both the frequency (Figure 5B) and absolute number (Figure 5C) of donor-reactive CD8+ T cells was observed in the spleens of costimulation blockade-treated animals that had not received ampicillin as compared to controls, donor-reactive T cell responses were significantly diminished in terms of both frequency (Figure 5B and 5C, left panel, p<0.05) and absolute number (Figure 5C, right panel, p<0.05) in the spleens of mice that received ampicillin during the priming phase of the OVA-specific response, and costimulation blockade during the recall response to an OVA-expressing skin graft as compared to untreated controls. Statistically significant differences between all other groups were not observed. Similar observations were also made in the draining LN (data not shown).
Figure 5. Memory T cells primed under conditions of limited antigen exposure exhibit diminished recall responses in the presence of costimulation blockade.
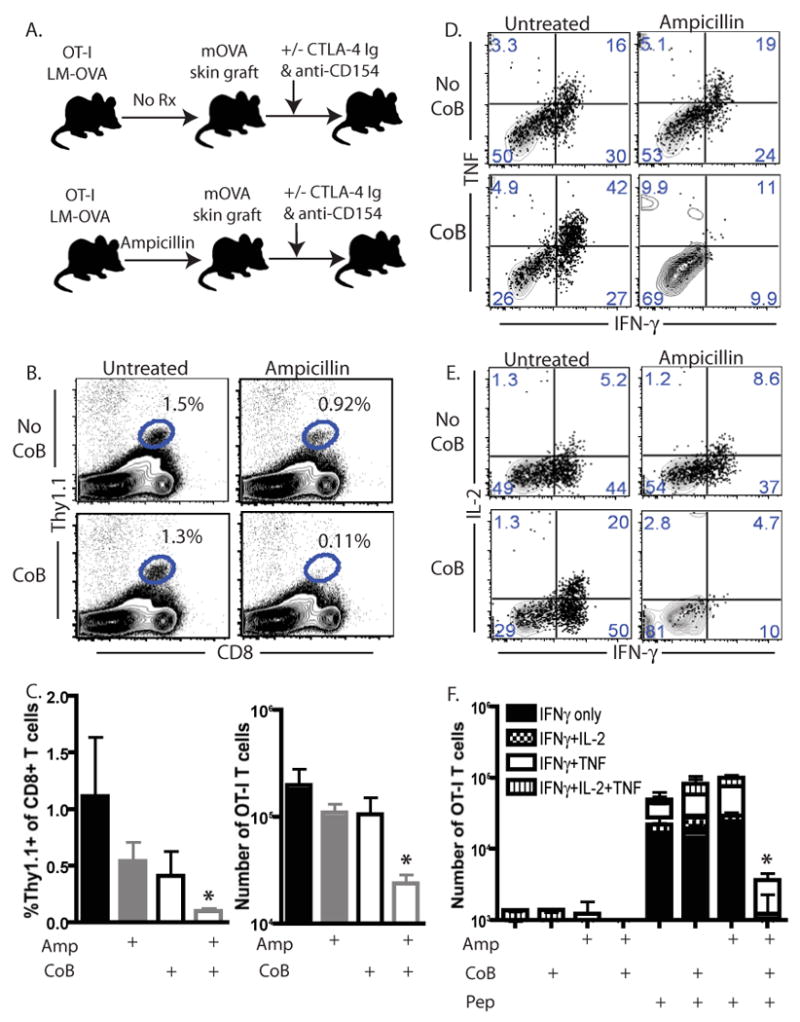
A) Experimental design schematic showing that untreated or ampicillin-treated LM-OVA infected animals were grafted with an OVA-expressing skin graft at day 30 post-infected. Mice were then left untreated or treated with CTLA-4 Ig and anti-CD154 as described in Materials and Methods. Six days after transplantation, splenocytes and draining lymph nodes were harvested for cytokine production assays and assessment of phenotypic expression markers. B, C) Frequencies of Thy1.1+ CD8+ T cells were analyzed in spleen. Frequencies shown are %Thy1.1+ of total CD8+ T cells. *p<0.05 as compared to untreated controls receiving neither ampicillin nor costimulation blockade. C, right panel) Absolute numbers were calculated using TruCount analysis. *p<0.05 as compared to untreated controls receiving neither ampicillin nor costimulation blockade. Results shown are representative (B) and summarized data (C) from three independent experiments with a total of 8-10 mice per group. D-F) Splenocytes from indicated groups were harvested on day six post-transplant and restimulated in vitro to assess cytokine production. Representative flow plots for IFN-γ/ TNF production (D) and IFN-γ/ IL-2 production (E) are shown. F, Summary data from two independent experiments with a total of 8-9 mice per group are shown. Results indicated that whereas costimulation blockade treatment of untreated mice did not diminish cytokine responses upon recall (p=NS), costimulation blockade treatment of ampicillin-treated animals resulted in a significant decrease in cytokine producing cells (*p<0.01).
In order to examine the functionality of the remaining cells, intracellular cytokine staining was performed on splenocytes isolated from the different treatment groups following a four-hour in vitro restimulation (Figures 5D and 5E). Interestingly, we observed that following a secondary rechallenge with an OVA-expressing skin graft, cytokine responses in mice that were treated with ampicillin during the priming phase of the response were potently inhibited by in vivo administration of costimulation blockade (Figure 5F, p<0.01). In contrast, costimulation blockade had no effect on recall cytokine responses of cells isolated from mice that had not received ampicillin during the priming phase (Figure 5F, p=NS).
Discussion
In this study we addressed the role of antigen amount/duration in the differentiation of costimulation-independent memory T cells. While memory T cells are widely considered to be costimulation-independent, there is a great deal of heterogeneity within memory T cell populations, stemming from factors such as strength of stimulus, cytokine microenvironment, clonal competition, and duration of antigen exposure (1, 36). Here, we found that memory T cells that were primed under conditions of limited antigen exposure exhibited a reduced frequency of KLRG-1hi cells and increased CD127 expression during the peak of the response, and an increased requirement for CD28 and CD154/CD40-mediated costimulation during the recall response to a transplant. From these data we conclude that the amount and/or duration of antigen exposure during the priming phase is a critical parameter in determining the relative costimulation requirement for memory T cells upon recall.
The amount/duration of exposure to antigen has been shown to impact memory T cell differentiation in other studies. For example, using the same model of LM-OVA infection followed by ampicillin treatment, Williams et al. showed that limiting the amount of antigen resulted in decreased frequencies of antigen-specific TEM cells and increased frequencies of TCM cells (41). As a result, a striking decrease was observed in the frequencies of tissue-homing memory T cells such as those found in the liver. Likewise, Sarkar et al. showed that antigen-specific CD8+ that were exposed to LCMV infection for a shorter time period more rapidly differentiated from effector memory T cells (TEM) into central memory T cells (TCM) as compared to those which were exposed to antigen for a longer period of time (44). While we did not observe an increase in antigen-specific CD62Lhi cells in T cell populations stimulated under conditions of reduced antigen exposure in our model, we did observe an increase in CD127 and decrease in KLRG-1 expression at day 9 post-infection, both markers that have been shown to be expressed by correlated with long-lived memory precursors (45, 46). Interestingly, other studies have showed that the generation of TCM was dependent upon the initial precursor frequency of antigen-specific T cells, in that antigen-specific populations stimulated at an initial high frequency underwent rapid conversion from TEM to TCM following antigen exposure as compared to those stimulated at an initial low frequency (44, 47). It has been demonstrated that the likely mechanism underlying this effect is the competition of cells within the responding population for antigen/APCs (37). Taken together, our data along with these studies therefore demonstrate that T cells stimulated under conditions of limiting antigen amount/duration are induced to more rapidly differentiate into CD127hi KLRG-1lo long-lived memory precursors and/or TCM-like cells as compared to those receiving prolonged antigen exposure.
While the studies discussed above examined the impact of reduced antigen exposure on memory T cell phenotype and differentiation status, our study is the first to explore the impact of reduced antigen exposure on the costimulatory requirements of memory T cells. After rechallenging mice with an OVA-expressing skin graft 30 days post infection in the presence of CD28 and/or CD154 blockade, we observed that donor-reactive memory T cells stimulated under conditions of limited antigen exposure exhibited costimulatory requirements that were intermediate relative to naïve and fully differentiated memory T cells. Specifically, our results showed that mice possessing donor-reactive memory T cells stimulated under reduced antigen exposure failed to mount a vigorous immune response and reject their grafts when CD28 and CD154 costimulatory pathways were both blocked. In this way, memory T cells stimulated under conditions of reduced antigen exposure behaved more like naïve T cells. However, when either CD28 or CD154 single pathway blockade was administered, the mice rejected their grafts more rapidly than did mice possessing naïve donor-reactive T cells. Thus, taken together, these results suggest that a spectrum of costimulatory requirements may exist within the heterogeneous memory T cell pool.
The data presented here are consistent with previous reports examining the costimulatory requirements of memory T cells. Specifically, Katsikis et al., working in a model of bacterial infection, found that memory T cells arising from high-frequency adoptively transferred cells required CD28-mediated costimulation for optimal recall responses (14). As discussed above, conditions of high T cell precursor frequency likely lead to competition for antigen and hence reduced antigen stimulation of a given T cell clone (37, 48). Thus, in this model where individual T cell stimulation was likely low, costimulatory requirements of memory T cells were relatively increased. Also consistent with our findings that memory T cells stimulated under conditions of reduced antigen exposure possessed a more TCM-like phenotype (increased CD127, decreased KLRG-1) and exhibited increased requirement for costimulation upon recall are the report of Oberbarnscheidt et al., which demonstrated that TCM were more dependent on CD28/CD154-mediated costimulatory signals than TEM, which could mediate rejection of a graft despite treatment with reagents to block these pathways (49). Taken together, these findings suggest that, under certain circumstances, memory T cells may require CD28 and/or CD154 mediated signals in order to mount effective recall responses. The results reported here demonstrate that the amount/duration of antigen during primary exposure is one such factor, and is therefore critical for the development of costimulation independent memory T cell responses.
In this study we used a model in which antigen expression was attenuated via ampicillin treatment to limit the bacterial infection in order to assess the impact of antigen exposure. While this model has been widely used in the literature as a means of limiting antigen exposure (41, 43), it has two caveats in that: 1) it does not distinguish between a change in the magnitude vs. duration of antigen expression, and 2) attenuation of the infection also limits the exposure of the responding T cells to other components of the inflammatory response to infection and the innate immune system, which has been shown to impact the function and phenotype of the responding memory T cell population (50-52). These factors may also secondarily impact the context of antigen presentation, the quantity and/or quality of CD4+ T cell help, the quantity and duration of co-stimulation, the cytokine milieu, and the generation of tissue-homing donor-reactive memory T cells. Thus, future studies will aim to address the role of antigen dose vs duration and the non-specific inflammatory response on the development of costimulation-independent memory T cell responses, and to define the cellular and molecular mechanisms underlying this effect.
In summary, this study establishes the amount/ duration of exposure to antigen as a critical element in determining the relative costimulatory requirements for memory T cells during the recall response to a transplant. Given the increasingly-appreciated role of pathogen-specific, allo-crossreactive memory T cells in mediating graft rejection (17, 33), these results suggest that there may be significant heterogeneity within these allo-crossreactive memory T cell populations with regard to their costimulatory requirements. Thus, more extensive characterization of the function and phenotypes of allo-crossreactive memory T cells may shed light on their abilities to mediate costimulation blockade-resistant rejection following transplantation. Furthermore, our results firmly establish the fact that some memory T cell populations are susceptible to the effects of costimulatory blockade in vivo. Further work designed to elucidate the specific molecules and pathways that are differentially expressed and/ or regulated in these memory T cells may result in the identification of novel targets for the inhibition of donor-reactive memory T cell responses following transplantation.
References
- 1.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodland DL, Kohlmeier JE. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat Rev Immunol. 2009;9:153–161. doi: 10.1038/nri2496. [DOI] [PubMed] [Google Scholar]
- 3.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 4.Hikono H, Kohlmeier JE, Takamura S, Wittmer ST, Roberts AD, Woodland DL. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. The Journal of experimental medicine. 2007;204:1625–1636. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.London CA, Lodge MP, Abbas AK. Functional responses and costimulator dependence of memory CD4+ T cells. J Immunol. 2000;164:265–272. doi: 10.4049/jimmunol.164.1.265. [DOI] [PubMed] [Google Scholar]
- 6.Kim SK, Schluns KS, Lefrancois L. Induction and visualization of mucosal memory CD8 T cells following systemic virus infection. J Immunol. 1999;163:4125–4132. [PubMed] [Google Scholar]
- 7.Bachmann MF, Gallimore A, Linkert S, Cerundolo V, Lanzavecchia A, Kopf M, Viola A. Developmental regulation of Lck targeting to the CD8 coreceptor controls signaling in naive and memory T cells. The Journal of experimental medicine. 1999;189:1521–1530. doi: 10.1084/jem.189.10.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Croft M, Bradley LM, Swain SL. Naive versus memory CD4 T cell response to antigen. Memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types including resting B cells. J Immunol. 1994;152:2675–2685. [PubMed] [Google Scholar]
- 9.Suresh M, Whitmire JK, Harrington LE, Larsen CP, Pearson TC, Altman JD, Ahmed R. Role of CD28-B7 interactions in generation and maintenance of CD8 T cell memory. J Immunol. 2001;167:5565–5573. doi: 10.4049/jimmunol.167.10.5565. [DOI] [PubMed] [Google Scholar]
- 10.Khoury SJ, Akalin E, Chandraker A, Turka L, Linsley PS, Sayegh MH, Hancock WW. CD28-B7 costimulatory blockade by CTLA4-Ig prevents actively induced experiemental autoimmune encephalomyelitis and inhibits Th1 but spares Th2 cytokines in the central nervous system. J Immunol. 1995;155:4521–4524. [PubMed] [Google Scholar]
- 11.Bluestone JA, St Clair EW, Turka LA. CTLA4Ig: bridging the basic immunology with clinical application. Immunity. 2006;24:233–238. doi: 10.1016/j.immuni.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Abrams JR, Lebwohl MG, Guzzo CA, Jegasothy BV, Goldfarb MT, Goffe BS, Menter A, Lowe NJ, Krueger G, Brown MJ, Weiner RS, Birkhofer MJ, Warner GL, Berry KK, Linsley PS, Krueger JG, Ochs HD, Kelley SL, Kang S. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Invest. 1999;103:1243–1252. doi: 10.1172/JCI5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kremer JM, Westhovens R, Leon M, Di Giorgio E, Alten R, Steinfeld S, Russell A, Dougados M, Emery P, Nuamah IF, Williams GR, Becker JC, Hagerty DT, Moreland LW. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349:1907–1915. doi: 10.1056/NEJMoa035075. [DOI] [PubMed] [Google Scholar]
- 14.Borowski AB, Boesteanu AC, Mueller YM, Carafides C, Topham DJ, Altman JD, Jennings SR, Katsikis PD. Memory CD8+ T cells require CD28 costimulation. J Immunol. 2007;179:6494–6503. doi: 10.4049/jimmunol.179.10.6494. [DOI] [PubMed] [Google Scholar]
- 15.Ford ML, Larsen CP. Overcoming the memory barrier in tolerance induction: molecular mimicry and functional heterogeneity among pathogen-specific T-cell populations. Curr Opin Organ Transplant. 2010;15:405–410. doi: 10.1097/MOT.0b013e32833b7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bingaman AW, Farber DL. Memory T cells in transplantation: generation, function, and potential role in rejection. Am J Transplant. 2004;4:846–852. doi: 10.1111/j.1600-6143.2004.00453.x. [DOI] [PubMed] [Google Scholar]
- 17.Amir AL, D'Orsogna LJ, Roelen DL, van Loenen MM, Hagedoorn RS, de Boer R, van der Hoorn MA, Kester MG, Doxiadis II, Falkenburg JH, Claas FH, Heemskerk MH. Allo-HLA reactivity of virus-specific memory T cells is common. Blood. 2010;115:3146–3157. doi: 10.1182/blood-2009-07-234906. [DOI] [PubMed] [Google Scholar]
- 18.Heeger PS, Greenspan NS, Kuhlenschmidt S, Dejelo C, Hricik DE, Schulak JA, Tary-Lehmann M. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol. 1999;163:2267–2275. [PubMed] [Google Scholar]
- 19.Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, Massari P, Mondragon-Ramirez GA, Agarwal M, Russo GD, Lin CS, Garg P, Larsen CP. A Phase III Study of Belatacept-based Immunosuppression Regimens versus Cyclosporine in Renal Transplant Recipients (BENEFIT Study) Am J Transplant. 2010;10:535–546. doi: 10.1111/j.1600-6143.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 20.Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, Lang P, Grinyo J, Halloran PF, Solez K, Hagerty D, Levy E, Zhou W, Natarajan K, Charpentier B. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353:770–781. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- 21.Durrbach A, Pestana JM, Pearson T, Vincenti F, Garcia VD, Campistol J, Rial MdC, Florman S, Block A, Russo GD, Xing J, Garg P, Grinyó J. A Phase III Study of Belatacept Versus Cyclosporine in Kidney Transplants from Extended Criteria Donors (BENEFIT-EXT Study) Am J Transplant. 2010;10:547–557. doi: 10.1111/j.1600-6143.2010.03016.x. [DOI] [PubMed] [Google Scholar]
- 22.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, Cho HR, Aruffo A, Hollenbaugh D, Linsley PS, Winn KJ, Pearson TC. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 23.Linsley PS, Wallace PM, Johnson J, Gibson M, Greene JL, Ledbetter JA, Singh C, Tepper MA. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992;257:792–795. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 24.Kirk AD, Harlan DM, Armstrong NN, Davis TA, Dong Y, Gray GS, Hong X, Thomas D, Fechner JH, Knechtle SJ. CTLA4Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci USA. 1997;94:8789–8794. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neujahr DC, Chen C, Huang X, Markmann JF, Cobbold S, Waldmann H, Sayegh MH, Hancock WW, Turka LA. Accelerated memory cell homeostasis during T cell depletion and approaches to overcome it. J Immunol. 2006;176:4632–4639. doi: 10.4049/jimmunol.176.8.4632. [DOI] [PubMed] [Google Scholar]
- 26.Pantenburg B, Heinzel F, Das L, Heeger PS, Valujskikh A. T cells primed by Leishmania major infection cross-react with alloantigens and alter the course of allograft rejection. J Immunol. 2002;169:3686–3693. doi: 10.4049/jimmunol.169.7.3686. [DOI] [PubMed] [Google Scholar]
- 27.Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2:501–509. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- 28.Welsh RM, Markees TG, Woda BA, Daniels KA, Brehm MA, Mordes JP, Greiner DL, Rossini AA. Virus-induced abrogation of transplantation tolerance induced by donor- specific transfusion and anti-CD154 antibody. J Virol. 2000;74:2210–2218. doi: 10.1128/jvi.74.5.2210-2218.2000. In Process Citation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams AB, Shirasugi N, Jones TR, Williams MA, Durham MM, Ha J, Dong Y, Guo Z, Newell KA, Pearson TC, Larsen CP. Conventional immunosuppression is compatible with costimulation blockade-based, mixed chimerism tolerance induction. Am J Transplant. 2003;3:895–901. doi: 10.1034/j.1600-6143.2003.00155.x. [DOI] [PubMed] [Google Scholar]
- 30.Williams MA, Onami TM, Adams AB, Durham MM, Pearson TC, Ahmed R, Larsen CP. Cutting edge: persistent viral infection prevents tolerance induction and escapes immune control following CD28/CD40 blockade-based regimen. J Immunol. 2002;169:5387–5391. doi: 10.4049/jimmunol.169.10.5387. [DOI] [PubMed] [Google Scholar]
- 31.Valujskikh A, Lakkis FG. In remembrance of things past: memory T cells and transplant rejection. Immunol Rev. 2003;196:65–74. doi: 10.1046/j.1600-065x.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 32.Lakkis FG, Sayegh MH. Memory T cells: a hurdle to immunologic tolerance. J Am Soc Nephrol. 2003;14:2402–2410. doi: 10.1097/01.asn.0000085020.78117.70. [DOI] [PubMed] [Google Scholar]
- 33.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, Wherry EJ, Onami T, Lanier JG, Kokko KE, Pearson TC, Ahmed R, Larsen CP. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111:1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y, Heeger PS, Valujskikh A. In Vivo Helper Functions of Alloreactive Memory CD4+ T Cells Remain Intact Despite Donor-Specific Transfusion and Anti-CD40 Ligand Therapy. Journal of Immunology. 2004;172:5456–5466. doi: 10.4049/jimmunol.172.9.5456. [DOI] [PubMed] [Google Scholar]
- 35.Sarkar JK, Mitra AC, Mukherjee MK. The minimum protective level of antibodies in smallpox. Bulletin of the World Health Organization. 1975;52(3):307–311. [PMC free article] [PubMed] [Google Scholar]
- 36.Kalia V, Sarkar S, Ahmed R. Fine-tuning CD4+ central memory T cell heterogeneity by strength of stimulation. Eur J Immunol. 2008;38:15–19. doi: 10.1002/eji.200738044. [DOI] [PubMed] [Google Scholar]
- 37.Blair DA, Lefrancois L. Increased competition for antigen during priming negatively impacts the generation of memory CD4 T cells. Proc Natl Acad Sci U S A. 2007;104:15045–15050. doi: 10.1073/pnas.0703767104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am J Transplant. 2003;3:1355–1362. doi: 10.1046/j.1600-6135.2003.00246.x. [DOI] [PubMed] [Google Scholar]
- 39.Shen H, Miller JF, Fan X, Kolwyck D, Ahmed R, Harty JT. Compartmentalization of bacterial antigens: differential effects on priming of CD8 T cells and protective immunity. Cell. 1998;92:535–545. doi: 10.1016/s0092-8674(00)80946-0. [DOI] [PubMed] [Google Scholar]
- 40.Porgador A, Yewdell JW, Deng Y, Bennink JR, Germain RN. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity. 1997;6:715–726. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- 41.Williams MA, Bevan MJ. Shortening the infectious period does not alter expansion of CD8 T cells but diminishes their capacity to differentiate into memory cells. J Immunol. 2004;173:6694–6702. doi: 10.4049/jimmunol.173.11.6694. [DOI] [PubMed] [Google Scholar]
- 42.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mercado R, Vijh S, Allen SE, Kerksiek K, Pilip IM, Pamer EG. Early programming of T cell populations responding to bacterial infection. J Immunol. 2000;165:6833–6839. doi: 10.4049/jimmunol.165.12.6833. [DOI] [PubMed] [Google Scholar]
- 44.Sarkar S, Teichgraber V, Kalia V, Polley A, Masopust D, Harrington LE, Ahmed R, Wherry EJ. Strength of stimulus and clonal competition impact the rate of memory CD8 T cell differentiation. J Immunol. 2007;179:6704–6714. doi: 10.4049/jimmunol.179.10.6704. [DOI] [PubMed] [Google Scholar]
- 45.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 46.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancois L. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ford ML, Koehn BH, Wagener ME, Jiang W, Gangappa S, Pearson TC, Larsen CP. Antigen-specific precursor frequency impacts T cell proliferation, differentiation, and requirement for costimulation. J Exp Med. 2007;204:299–309. doi: 10.1084/jem.20062319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oberbarnscheidt MH, Ng YH, Chalasani G. The roles of CD8 central and effector memory T-cell subsets in allograft rejection. Am J Transplant. 2008;8:1809–1818. doi: 10.1111/j.1600-6143.2008.02335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rahman AH, Cui W, Larosa DF, Taylor DK, Zhang J, Goldstein DR, Wherry EJ, Kaech SM, Turka LA. MyD88 plays a critical T cell-intrinsic role in supporting CD8 T cell expansion during acute lymphocytic choriomeningitis virus infection. J Immunol. 2008;181:3804–3810. doi: 10.4049/jimmunol.181.6.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang T, Chen L, Ahmed E, Ma L, Yin D, Zhou P, Shen J, Xu H, Wang CR, Alegre ML, Chong AS. Prevention of allograft tolerance by bacterial infection with Listeria monocytogenes. J Immunol. 2008;180:5991–5999. doi: 10.4049/jimmunol.180.9.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]


