Abstract
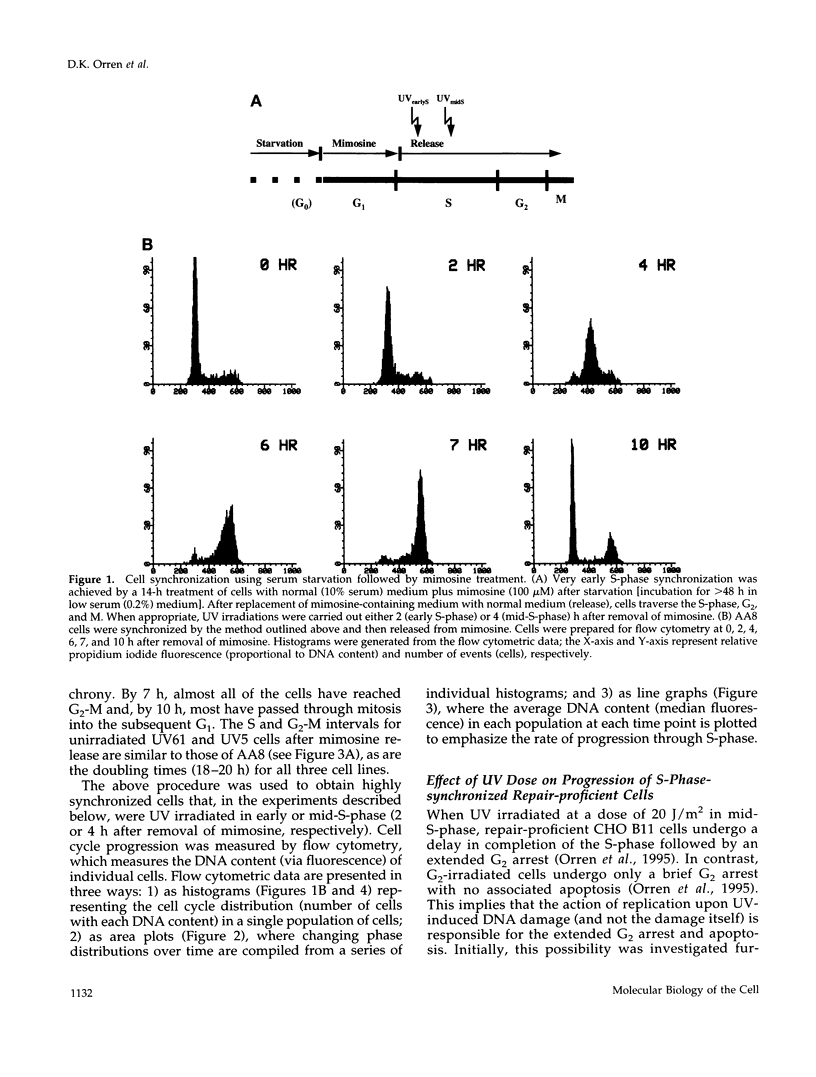
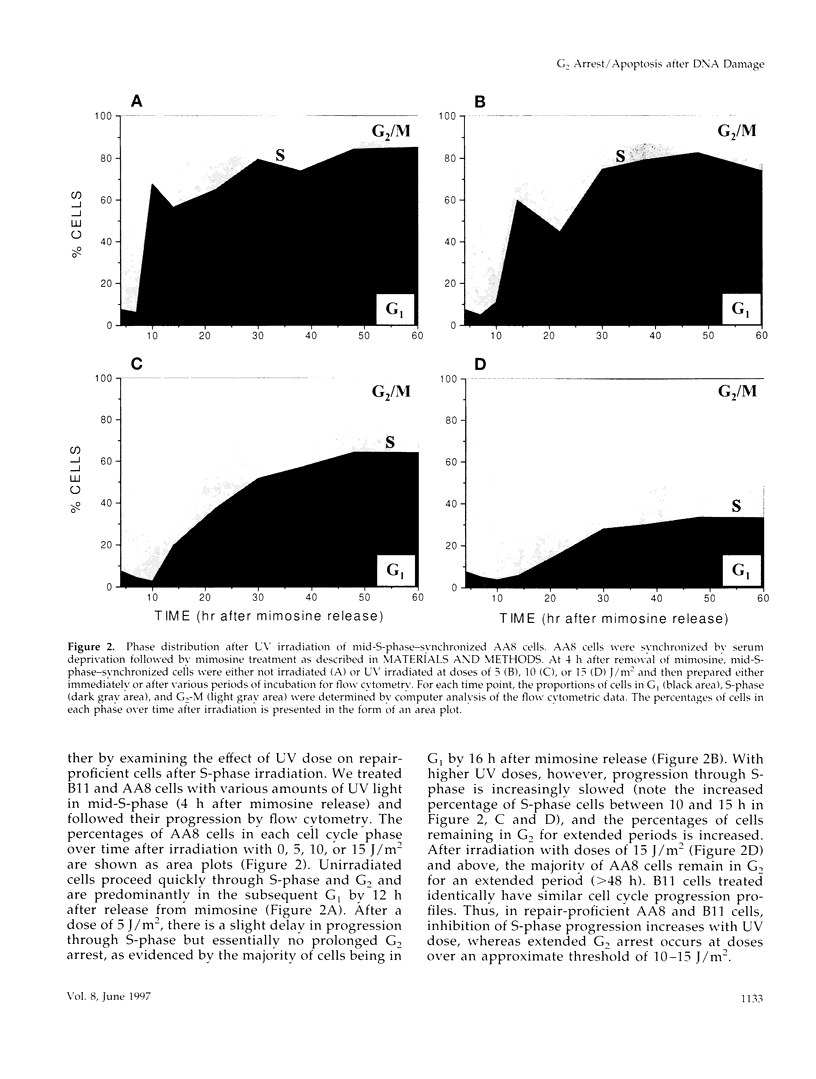
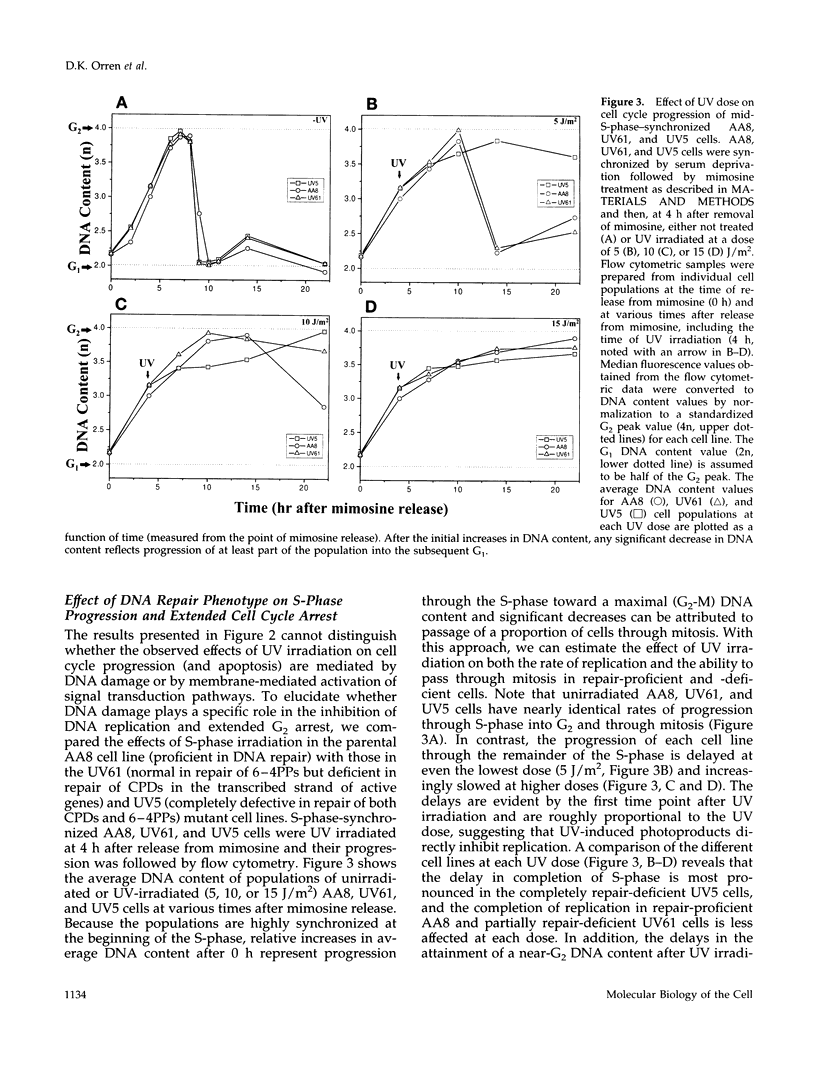
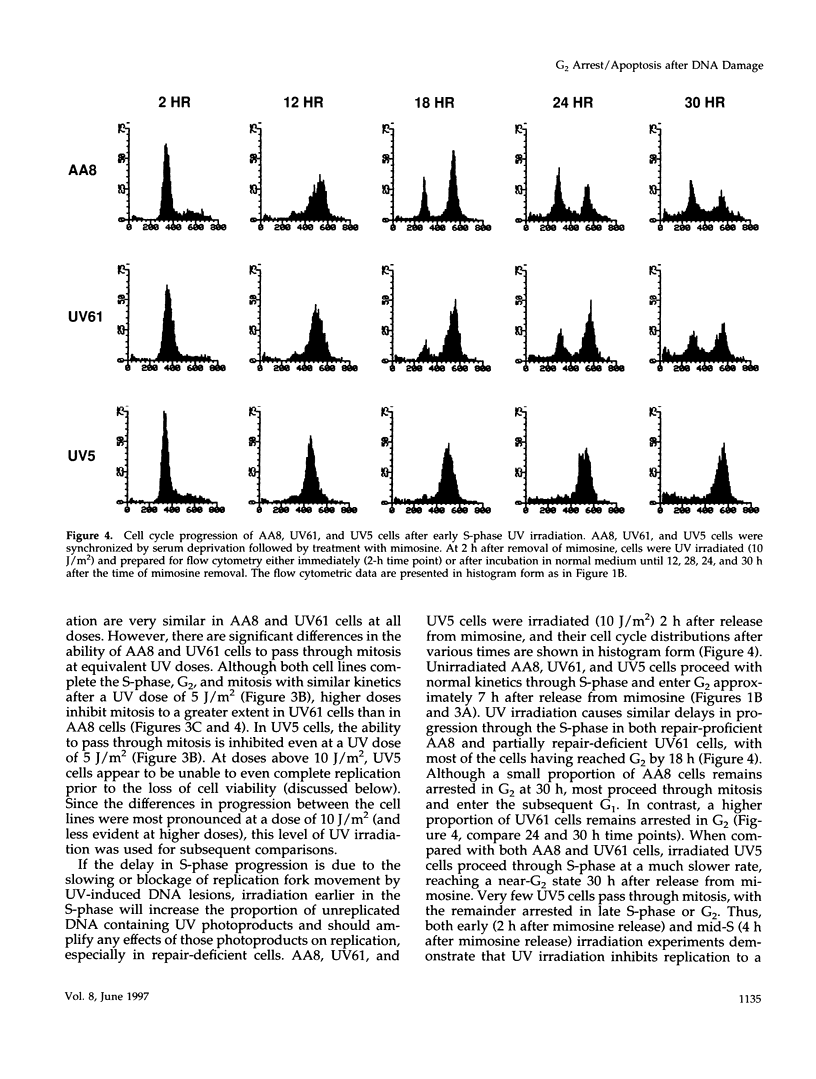
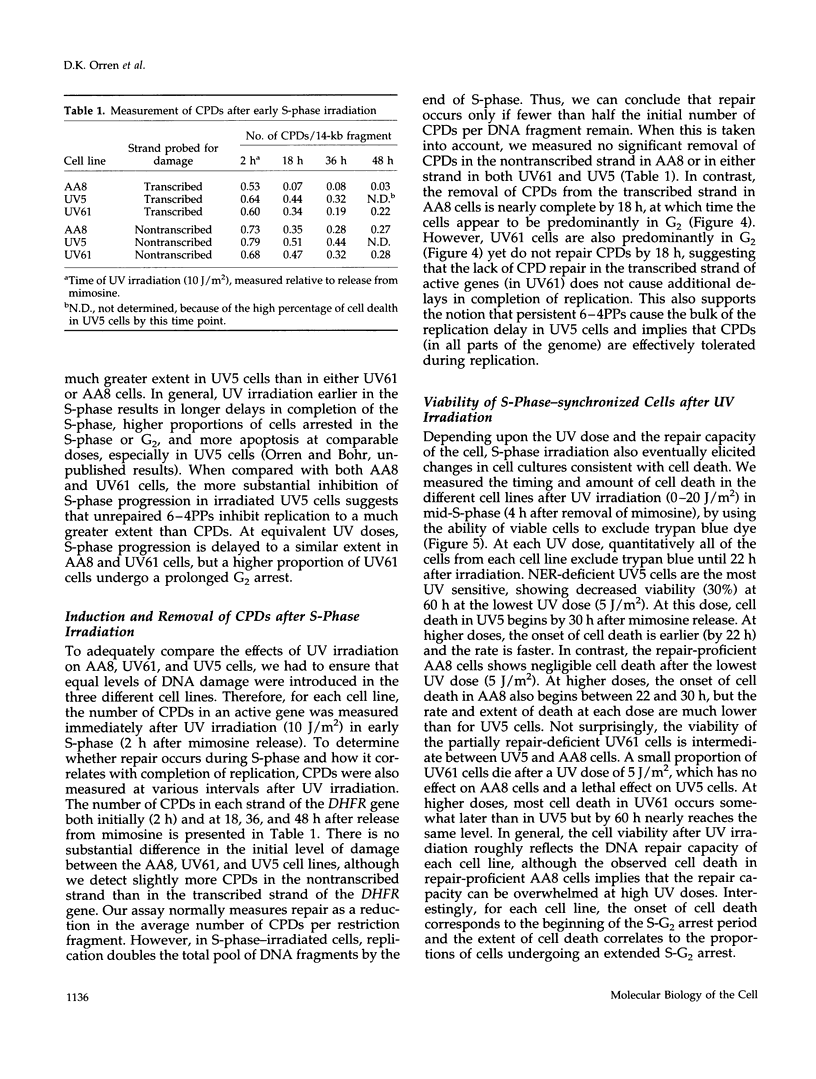
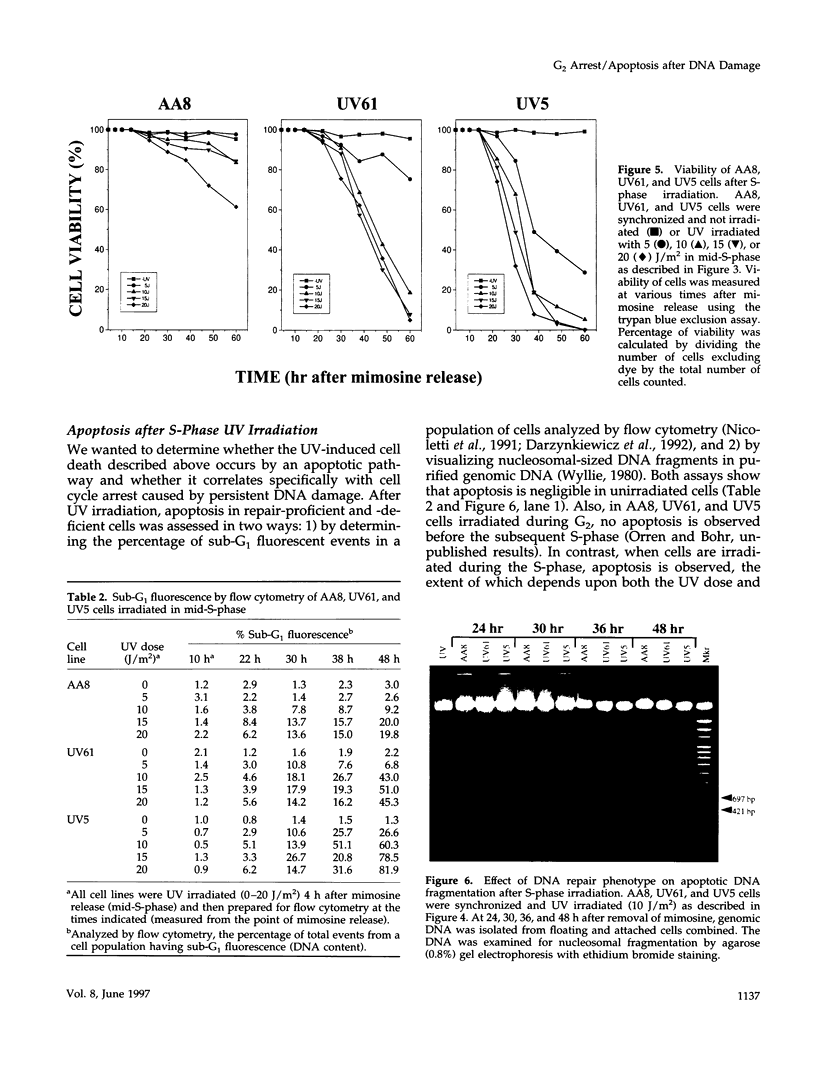
We used genetically related Chinese hamster ovary cell lines proficient or deficient in DNA repair to determine the direct role of UV-induced DNA photoproducts in inhibition of DNA replication and in induction of G2 arrest and apoptosis. UV irradiation of S-phase-synchronized cells causes delays in completion of the S-phase sometimes followed by an extended G2 arrest and apoptosis. The effects of UV irradiation during the S-phase on subsequent cell cycle progression are magnified in repair-deficient cells, indicating that these effects are initiated by persistent DNA damage and not by direct UV activation of signal transduction pathways. Moreover, among the lesions introduced by UV irradiation, persistence of (6-4) photoproducts inhibits DNA synthesis much more than persistence of cyclobutane pyrimidine dimers (which appear to be efficiently bypassed by the DNA replication apparatus). Apoptosis begins approximately 24 h after UV irradiation of S-phase-synchronized cells, occurs to a greater extent in repair-deficient cells, and correlates well with the inability to escape from an extended late S-phase-G2 arrest. We also find that nucleotide excision repair activity (including its coupling to transcription) is similar in the S-phase to what we have previously measured in G1 and G2.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allday M. J., Inman G. J., Crawford D. H., Farrell P. J. DNA damage in human B cells can induce apoptosis, proceeding from G1/S when p53 is transactivation competent and G2/M when it is transactivation defective. EMBO J. 1995 Oct 16;14(20):4994–5005. doi: 10.1002/j.1460-2075.1995.tb00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger C. A., Edenberg H. J. Pyrimidine dimers block simian virus 40 replication forks. Mol Cell Biol. 1986 Oct;6(10):3443–3450. doi: 10.1128/mcb.6.10.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr V. A., Anson R. M. DNA damage, mutation and fine structure DNA repair in aging. Mutat Res. 1995 Oct;338(1-6):25–34. doi: 10.1016/0921-8734(95)00008-t. [DOI] [PubMed] [Google Scholar]
- Bohr V. A. DNA repair fine structure and its relations to genomic instability. Carcinogenesis. 1995 Dec;16(12):2885–2892. doi: 10.1093/carcin/16.12.2885. [DOI] [PubMed] [Google Scholar]
- Bohr V. A., Okumoto D. S., Ho L., Hanawalt P. C. Characterization of a DNA repair domain containing the dihydrofolate reductase gene in Chinese hamster ovary cells. J Biol Chem. 1986 Dec 15;261(35):16666–16672. [PubMed] [Google Scholar]
- Bohr V. A., Smith C. A., Okumoto D. S., Hanawalt P. C. DNA repair in an active gene: removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell. 1985 Feb;40(2):359–369. doi: 10.1016/0092-8674(85)90150-3. [DOI] [PubMed] [Google Scholar]
- Busch D. B., Cleaver J. E., Glaser D. A. Large-scale isolation of UV-sensitive clones of CHO cells. Somatic Cell Genet. 1980 May;6(3):407–418. doi: 10.1007/BF01542792. [DOI] [PubMed] [Google Scholar]
- Calcagnile A., Basic-Zaninovic T., Palombo F., Dogliotti E. Misincorporation rate and type on the leading and lagging strands of UV-damaged DNA. Nucleic Acids Res. 1996 Aug 1;24(15):3005–3009. doi: 10.1093/nar/24.15.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver J. E., Cortés F., Lutze L. H., Morgan W. F., Player A. N., Mitchell D. L. Unique DNA repair properties of a xeroderma pigmentosum revertant. Mol Cell Biol. 1987 Sep;7(9):3353–3357. doi: 10.1128/mcb.7.9.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corda Y., Job C., Anin M. F., Leng M., Job D. Transcription by eucaryotic and procaryotic RNA polymerases of DNA modified at a d(GG) or a d(AG) site by the antitumor drug cis-diamminedichloroplatinum(II). Biochemistry. 1991 Jan 8;30(1):222–230. doi: 10.1021/bi00215a032. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Bruno S., Del Bino G., Gorczyca W., Hotz M. A., Lassota P., Traganos F. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13(8):795–808. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- Devary Y., Gottlieb R. A., Smeal T., Karin M. The mammalian ultraviolet response is triggered by activation of Src tyrosine kinases. Cell. 1992 Dec 24;71(7):1081–1091. doi: 10.1016/s0092-8674(05)80058-3. [DOI] [PubMed] [Google Scholar]
- Donahue B. A., Yin S., Taylor J. S., Reines D., Hanawalt P. C. Transcript cleavage by RNA polymerase II arrested by a cyclobutane pyrimidine dimer in the DNA template. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8502–8506. doi: 10.1073/pnas.91.18.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulić V., Kaufmann W. K., Wilson S. J., Tlsty T. D., Lees E., Harper J. W., Elledge S. J., Reed S. I. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994 Mar 25;76(6):1013–1023. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- Engelberg D., Klein C., Martinetto H., Struhl K., Karin M. The UV response involving the Ras signaling pathway and AP-1 transcription factors is conserved between yeast and mammals. Cell. 1994 May 6;77(3):381–390. doi: 10.1016/0092-8674(94)90153-8. [DOI] [PubMed] [Google Scholar]
- Enoch T., Norbury C. Cellular responses to DNA damage: cell-cycle checkpoints, apoptosis and the roles of p53 and ATM. Trends Biochem Sci. 1995 Oct;20(10):426–430. doi: 10.1016/s0968-0004(00)89093-3. [DOI] [PubMed] [Google Scholar]
- Flejter W. L., McDaniel L. D., Johns D., Friedberg E. C., Schultz R. A. Correction of xeroderma pigmentosum complementation group D mutant cell phenotypes by chromosome and gene transfer: involvement of the human ERCC2 DNA repair gene. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):261–265. doi: 10.1073/pnas.89.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D. M., Neilson A., Miyazawa H., DePamphilis M. L., Burhans W. C. Mimosine arrests DNA synthesis at replication forks by inhibiting deoxyribonucleotide metabolism. J Biol Chem. 1995 Apr 21;270(16):9597–9606. doi: 10.1074/jbc.270.16.9597. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Weinert T. A. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989 Nov 3;246(4930):629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Hoffmann J. S., Johnson N. P., Villani G. Conversion of monofunctional DNA adducts of cis-diamminedichloroplatinum (II) to bifunctional lesions. Effect on the in vitro replication of single-stranded DNA by Escherichia coli DNA polymerase I and eukaryotic DNA polymerases alpha. J Biol Chem. 1989 Sep 5;264(25):15130–15135. [PubMed] [Google Scholar]
- Hughes T. A., Cook P. R. Mimosine arrests the cell cycle after cells enter S-phase. Exp Cell Res. 1996 Feb 1;222(2):275–280. doi: 10.1006/excr.1996.0035. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Schimke R. T. Amplification and loss of dihydrofolate reductase genes in a Chinese hamster ovary cell line. Mol Cell Biol. 1981 Dec;1(12):1069–1076. doi: 10.1128/mcb.1.12.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann W. K., Wilson S. J. G1 arrest and cell-cycle-dependent clastogenesis in UV-irradiated human fibroblasts. Mutat Res. 1994 Jan;314(1):67–76. doi: 10.1016/0921-8777(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Ljungman M., Zhang F. Blockage of RNA polymerase as a possible trigger for u.v. light-induced apoptosis. Oncogene. 1996 Aug 15;13(4):823–831. [PubMed] [Google Scholar]
- Lock R. B., Ross W. E. Inhibition of p34cdc2 kinase activity by etoposide or irradiation as a mechanism of G2 arrest in Chinese hamster ovary cells. Cancer Res. 1990 Jun 15;50(12):3761–3766. [PubMed] [Google Scholar]
- Lommel L., Carswell-Crumpton C., Hanawalt P. C. Preferential repair of the transcribed DNA strand in the dihydrofolate reductase gene throughout the cell cycle in UV-irradiated human cells. Mutat Res. 1995 Mar;336(2):181–192. doi: 10.1016/0921-8777(94)00055-b. [DOI] [PubMed] [Google Scholar]
- Lommel L., Hanawalt P. C. The genetic defect in the Chinese hamster ovary cell mutant UV61 permits moderate selective repair of cyclobutane pyrimidine dimers in an expressed gene. Mutat Res. 1991 Sep;255(2):183–191. doi: 10.1016/0921-8777(91)90052-q. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Ruley H. E., Jacks T., Housman D. E. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993 Sep 24;74(6):957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- May A., Nairn R. S., Okumoto D. S., Wassermann K., Stevnsner T., Jones J. C., Bohr V. A. Repair of individual DNA strands in the hamster dihydrofolate reductase gene after treatment with ultraviolet light, alkylating agents, and cisplatin. J Biol Chem. 1993 Jan 25;268(3):1650–1657. [PubMed] [Google Scholar]
- Mello J. A., Lippard S. J., Essigmann J. M. DNA adducts of cis-diamminedichloroplatinum(II) and its trans isomer inhibit RNA polymerase II differentially in vivo. Biochemistry. 1995 Nov 14;34(45):14783–14791. doi: 10.1021/bi00045a020. [DOI] [PubMed] [Google Scholar]
- Mellon I., Spivak G., Hanawalt P. C. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell. 1987 Oct 23;51(2):241–249. doi: 10.1016/0092-8674(87)90151-6. [DOI] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. L., Cleaver J. E., Lowery M. P., Hewitt R. R. Induction and repair of (6-4) photoproducts in normal human and xeroderma pigmentosum variant cells during the cell cycle. Mutat Res. 1995 Nov;337(3):161–167. doi: 10.1016/0921-8777(95)00020-k. [DOI] [PubMed] [Google Scholar]
- Moore P. D., Bose K. K., Rabkin S. D., Strauss B. S. Sites of termination of in vitro DNA synthesis on ultraviolet- and N-acetylaminofluorene-treated phi X174 templates by prokaryotic and eukaryotic DNA polymerases. Proc Natl Acad Sci U S A. 1981 Jan;78(1):110–114. doi: 10.1073/pnas.78.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P., Strauss B. S. Sites of inhibition of in vitro DNA synthesis in carcinogen- and UV-treated phi X174 DNA. Nature. 1979 Apr 12;278(5705):664–666. doi: 10.1038/278664a0. [DOI] [PubMed] [Google Scholar]
- Murray A. W. Creative blocks: cell-cycle checkpoints and feedback controls. Nature. 1992 Oct 15;359(6396):599–604. doi: 10.1038/359599a0. [DOI] [PubMed] [Google Scholar]
- Navas T. A., Zhou Z., Elledge S. J. DNA polymerase epsilon links the DNA replication machinery to the S phase checkpoint. Cell. 1995 Jan 13;80(1):29–39. doi: 10.1016/0092-8674(95)90448-4. [DOI] [PubMed] [Google Scholar]
- Nicoletti I., Migliorati G., Pagliacci M. C., Grignani F., Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991 Jun 3;139(2):271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- O'Connor P. M., Ferris D. K., Pagano M., Draetta G., Pines J., Hunter T., Longo D. L., Kohn K. W. G2 delay induced by nitrogen mustard in human cells affects cyclin A/cdk2 and cyclin B1/cdc2-kinase complexes differently. J Biol Chem. 1993 Apr 15;268(11):8298–8308. [PubMed] [Google Scholar]
- O'Day C. L., Burgers P. M., Taylor J. S. PCNA-induced DNA synthesis past cis-syn and trans-syn-I thymine dimers by calf thymus DNA polymerase delta in vitro. Nucleic Acids Res. 1992 Oct 25;20(20):5403–5406. doi: 10.1093/nar/20.20.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orren D. K., Dianov G. L., Bohr V. A. The human CSB (ERCC6) gene corrects the transcription-coupled repair defect in the CHO cell mutant UV61. Nucleic Acids Res. 1996 Sep 1;24(17):3317–3322. doi: 10.1093/nar/24.17.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orren D. K., Petersen L. N., Bohr V. A. A UV-responsive G2 checkpoint in rodent cells. Mol Cell Biol. 1995 Jul;15(7):3722–3730. doi: 10.1128/mcb.15.7.3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulovich A. G., Hartwell L. H. A checkpoint regulates the rate of progression through S phase in S. cerevisiae in response to DNA damage. Cell. 1995 Sep 8;82(5):841–847. doi: 10.1016/0092-8674(95)90481-6. [DOI] [PubMed] [Google Scholar]
- Petersen L. N., Orren D. K., Bohr V. A. Gene-specific and strand-specific DNA repair in the G1 and G2 phases of the cell cycle. Mol Cell Biol. 1995 Jul;15(7):3731–3737. doi: 10.1128/mcb.15.7.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachsenmaier C., Radler-Pohl A., Zinck R., Nordheim A., Herrlich P., Rahmsdorf H. J. Involvement of growth factor receptors in the mammalian UVC response. Cell. 1994 Sep 23;78(6):963–972. doi: 10.1016/0092-8674(94)90272-0. [DOI] [PubMed] [Google Scholar]
- Selby C. P., Sancar A. Molecular mechanism of transcription-repair coupling. Science. 1993 Apr 2;260(5104):53–58. doi: 10.1126/science.8465200. [DOI] [PubMed] [Google Scholar]
- Spivak G., Hanawalt P. C. Translesion DNA synthesis in the dihydrofolate reductase domain of UV-irradiated CHO cells. Biochemistry. 1992 Jul 28;31(29):6794–6800. doi: 10.1021/bi00144a021. [DOI] [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Jacks T., Cory S. DNA damage can induce apoptosis in proliferating lymphoid cells via p53-independent mechanisms inhibitable by Bcl-2. Cell. 1994 Oct 21;79(2):329–339. doi: 10.1016/0092-8674(94)90201-1. [DOI] [PubMed] [Google Scholar]
- Taft S. A., Ling S. Y., Griffiths T. D. Effect of UV light on sister-chromatid exchanges, activation of alternative sites of replicon initiation and thymidine incorporation in CHO AA8, UV61 and UV5 cells. Mutat Res. 1991 Nov;255(3):257–264. doi: 10.1016/0921-8777(91)90029-o. [DOI] [PubMed] [Google Scholar]
- Thomas D. C., Kunkel T. A. Replication of UV-irradiated DNA in human cell extracts: evidence for mutagenic bypass of pyrimidine dimers. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7744–7748. doi: 10.1073/pnas.90.16.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H., Mitchell D. L., Regan J. D., Bouffler S. D., Stewart S. A., Carrier W. L., Nairn R. S., Johnson R. T. CHO mutant UV61 removes (6-4) photoproducts but not cyclobutane dimers. Mutagenesis. 1989 Mar;4(2):140–146. doi: 10.1093/mutage/4.2.140. [DOI] [PubMed] [Google Scholar]
- Troelstra C., Odijk H., de Wit J., Westerveld A., Thompson L. H., Bootsma D., Hoeijmakers J. H. Molecular cloning of the human DNA excision repair gene ERCC-6. Mol Cell Biol. 1990 Nov;10(11):5806–5813. doi: 10.1128/mcb.10.11.5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troelstra C., van Gool A., de Wit J., Vermeulen W., Bootsma D., Hoeijmakers J. H. ERCC6, a member of a subfamily of putative helicases, is involved in Cockayne's syndrome and preferential repair of active genes. Cell. 1992 Dec 11;71(6):939–953. doi: 10.1016/0092-8674(92)90390-x. [DOI] [PubMed] [Google Scholar]
- Weber C. A., Salazar E. P., Stewart S. A., Thompson L. H. Molecular cloning and biological characterization of a human gene, ERCC2, that corrects the nucleotide excision repair defect in CHO UV5 cells. Mol Cell Biol. 1988 Mar;8(3):1137–1146. doi: 10.1128/mcb.8.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Yamaizumi M., Sugano T. U.v.-induced nuclear accumulation of p53 is evoked through DNA damage of actively transcribed genes independent of the cell cycle. Oncogene. 1994 Oct;9(10):2775–2784. [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]