Abstract
We examined the hypothesis that vascular and renal dysfunction caused by angiotensin II (Ang II) through increased levels of blood pressure, inflammatory cytokines, and oxidative stress in Sprague–Dawley rats can be prevented by lentiviral-mediated delivery of endothelial heme oxygenase (HO)-1. We targeted the vascular endothelium using a lentiviral construct expressing human HO-1 under the control of the endothelium-specific promoter VE-cadherin (VECAD-HO-1) and examined the effect of long-term human HO-1 expression on blood pressure in Ang II-mediated increases in blood pressure and oxidant stress. A bolus injection of VECAD-HO-1 into the renal artery resulted in expression of human HO-1 for up to 6–9 weeks. Sprague–Dawley rats were implanted with Ang II minipumps and treated with lentivirus carrying either the HO-1 or green fluorescent protein. Renal tissue from VECAD-HO-1-transduced rats expresses human HO-1 mRNA and proteins without an effect on endogenous HO-1. Infusion of Ang II increased blood pressure (p < 0.001) but decreased vascular relaxation in response to acetylcholine, endothelial nitric oxide synthase (eNOS) and phosphorylated eNOS (peNOS) levels, and renal and plasma levels of adiponectin (p < 0.05); in contrast, plasma tumor necrosis factor-α and monocyte chemoattractant protein-1 levels increased. Ang II-treated animals had higher levels of superoxide anion and inducible nitric oxide synthase and increased urinary protein and plasma creatinine levels. Lentiviral transduction with the VECAD-HO-1 construct attenuated the increase in blood pressure (p < 0.05), improved vascular relaxation, increased plasma adiponectin, and prevented the elevation in urinary protein and plasma creatinine in Ang II-treated rats. Endothelial-specific expression of HO-1 also reduced oxidative stress and levels of inflammatory cytokines resulting in increased expression of the anti-apoptotic proteins phosphorylated AKT, phosphorylated AMP-activated protein kinase, peNOS, and eNOS. Collectively, these findings demonstrate that endothelial-specific increases in HO-1 expression attenuate Ang II hypertension and the associated vascular dysfunction that is associated with increases in adiponectin and peNOS and reductions in oxidative stress and levels of inflammatory cytokines.
Cao et al. report that lentiviral vector-mediated delivery of heme oxygenase (HO-1) under control of the endothelium-specific promoter VECAD specifically targets endothelial cells both in vitro and in vivo and endows cardiovascular protection in a rat animal model of hypertension.
Introduction
Hypertension is a multifactorial condition that is associated with increased oxidative stress and vascular dysfunction that predisposes the individual to the development of atherosclerosis and organ damage, especially the kidney. Activation of the renin–angiotensin system is a feature of many forms of hypertension, and infusion of angiotensin II (Ang II) is frequently used in rodents to produce animal models of hypertension. Ang II produces myriad effects, and its actions, via the AT1 receptor, have been implicated in numerous cardiovascular diseases including hypertension. Ang II activates the NAD(P)H oxidase enzyme system and promotes the production of reactive oxygen species (ROS) such as superoxide anion and hydrogen peroxide, which have been implicated in the development of atherosclerosis and several hypertensive diseases (Haugen et al., 2000; Hordijk, 2006). Ang II stimulates the formation of superoxide anion radical (O2•−)/ROS production by human neutrophils that is accompanied by activation of mitogen-activated protein kinase (El et al., 2003), protein tyrosine kinases, and transcriptional factors (Chen et al., 2001). Ang II-mediated augmentation of superoxide production contributes to renal vasoconstriction, cortical hypoxia, and reduced efficiency of O2 usage for sodium transport (Lopez et al., 2003; Kopkan et al., 2006). Increased superoxide production in the thick ascending loop of Henle stimulates sodium reabsorption by decreasing the levels of nitric oxide (NO) (Ortiz and Garvin, 2002a,b) and limits NO availability to the vasa recta, which could lead to decreases in medullary blood flow and blunting of pressure-natriuresis, resulting in hypertension (Mori et al., 2003).
In contrast to the pro-oxidant actions of Ang II, the heme oxygenase (HO) system serves an antioxidant role and is activated in response to oxidative stress. HO catalyzes the rate-limiting step in heme degradation, producing iron, carbon monoxide (CO), and biliverdin, which is rapidly converted to bilirubin, a potent antioxidant (Abraham and Kappas, 2008). HO-1, the inducible isoform, is up-regulated by heme and a variety of non-heme stimuli, including heavy metals, ROS, NO, Ang II, endotoxin, and cytokines (Abraham et al., 1996; Abraham and Kappas, 2005). Recently, induction of HO-1 in vivo was shown to suppress NADPH oxidase-induced oxidative stress (Datla et al., 2007). Thus, the HO system might serve an important protective role in the cardiovascular system against the harmful effects of Ang II-induced oxidative stress (Pflueger et al., 2005). In the rat kidney, HO-1 is expressed mainly in the renal medulla, where it plays an important role in maintaining blood flow to the renal medulla (Olszanecki et al., 2007). Our previous studies indicate that adenovirus- and retrovirus-mediated overexpression of HO-1 blunts Ang II-induced oxidative damage (Quan et al., 2004) and hypertension (Yang et al., 2004) and that pharmacological induction of HO-1 with cobalt protoporphyrin attenuated the increase in blood pressure in a model of renovascular hypertension (Botros et al., 2005).
The current studies were undertaken to address the protective effects of overexpression of HO-1 confined to the endothelium in a rat model of hypertension induced by Ang II infusion. The endothelium is central to cardiovascular disease, and targeting was accomplished by using the endothelium-specific promoter VE-cadherin (VECAD) (Lampugnani et al., 1992), which has been used to selectively express genes in this tissue (Gory et al., 1999). Lentiviral vectors have been used in humans, and there are several clinical trials presently underway (Schambach and Baum, 2008).
We show here that lentiviral vectors carrying the HO-1 construct under the control of the endothelium-specific promoter VECAD specifically target endothelial cells both in vitro and in vivo. Augmentation of superoxide formation by Ang II results in the development of hypertension and endothelial and renal dysfunction; this was prevented by VECAD-HO-1 transduction. HO-1 transduction was associated with elevation of adiponectin levels, activation of the phosphorylated AMP-activated protein kinase (pAMPK)–phosphorylated endothelial NO synthase (peNOS) pathway, and improved endothelial function coincident with decreased levels of inflammatory cytokines and oxidative stress. These results demonstrate a significant vascular and renal protective role for endothelial HO-1 in an Ang II animal model of hypertension.
Materials and Methods
In vitro transduction
Lentiviral vectors expressing either human HO-1 or green fluorescent protein (GFP) cDNA under the control of the endothelial-specific promoter VECAD (Asija et al., 2007) were constructed using the LentiMax system (Lentigen, Baltimore, MD). Human embryonic kidney (HEK) cells and EA.hy926 cells (a human endothelial cell line) were used to evaluate functional transgene expression. Cells were infected with the lentiviral vector (2 μl of 109 transducing units [TU]/ml) carrying either the HO-1 construct under the control of the endothelium-specific promoter VECAD (VECAD-HO-1) or the GFP construct (SCMV-GFP) (Lentigen). GFP and HO-1 mRNA and protein levels were measured by western blotting and real-time (RT) polymerase chain reaction (PCR), and GFP expression was confirmed using confocal laser scanning (Fluoview FV300, Olympus, Center Valley, PA) microscope.
Animal treatment
The animal studies were conducted in accordance with the National Institutes of Health's Guidelines for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of New York Medical College (Valhalla, NY). Sprague–Dawley rats (9–10 weeks old, 225–250 g in body weight) were divided into four groups—control, Ang II, HO-1, and Ang II + HO-1—and were fed normal chow diet and had free access to water. For the Ang II-treated groups, Alzet osmotic minipumps (Durect Corp., Cupertino, CA) were implanted subcutaneously to deliver Ang II at the rate of 200 ng/min/kg of body weight. The vascular endothelium was targeted by a lentivirus (50 μl of 109 TU/ml) carrying VECAD-HO-1 (Lentigen). VECAD-HO-1 (50 μl, 1–2 × 109 TU/ml in saline) was injected into the left renal artery with a second injection into the tail vein (75 μl of 1 × 109 TU/ml in saline) of Sprague–Dawley rats under anesthesia with sodium pentobarbital (65 mg/kg of body weight, intraperitoneally). Control animals were injected with mock virus (placebo). Rats receiving lentivirus were maintained for 2–3 weeks before treatment with Ang II was initiated. Rats were placed in metabolic cages for urine collection 24 hr before and at 10-day intervals after placement of osmotic minipumps. Blood pressure was measured by the tail cuff method before and at 10-day intervals after lentiviral administration. Prior to the experiment, rats were all acclimated to the tail cuff method. Rats were placed in a heat-controlled box (36–38°C) for approximately 10 min before the tail cuff was applied. The mean of a minimum of five measurements was obtained from each rat. All measurements were determined at the same time of day, between 9:00 and 13:00 h. At day 30, rats were euthanized, and femoral arteries and kidneys were collected for further study. Prior to sacrificing, blood samples were collected into tubes containing potassium EDTA and centrifuged at 2,500 g for 10 min at 4°C to separate the plasma, which was stored at −20°C.
In addition, lentivirus-VECAD-HO-1-antisense (AS) transgenic rats were generated to achieve genetic suppression of HO-1, which was accomplished by delivery of the lentivirus-VECAD-HO-1 gene in the AS orientation using Lenti-VECAD for rat AS (rat HO-1-AS) to newborn Sprague–Dawley rats as described previously (Yang et al., 2004; Olszanecki et al., 2007).
RNA extraction and RT-PCR
Total RNA was extracted from rat kidney or aorta using the RNeasy Protect Mini kit (Qiagen, Gaithersburg, MD) according to the manufacturer's instructions. Total RNA (1 μg) was transcribed into cDNA using the GeneAmp kit (Applied Biosystems, Branchburg, NJ) reverse transcription reagents. Total RNA was analyzed by a quantitative RT-PCR. RT-PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems) on a 7500 HT Fast Real-Time PCR system (Applied Biosystems). Specific primers for human and rat HO-1 were used. The human HO-1 amplification primers were as follows: forward, 5'-CAGGCAGAGAATGCTGAGTTC-3'; and reverse, 5'-GATGTTGAGCAGGAACGCAGT-3' (Abraham, 1998; da Silva et al., 2001). The rat HO-1 amplification primers were as follows: forward, 5'-TGAAGGAGGCCACCAAGGAG-3'; and reverse, 5'-CCCCTGAGAGGTCACCCAGG-3'. Each reaction was performed in triplicate. The comparative threshold cycle method was used to calculate the fold amplification as specified by the manufacturer. All experimental samples were normalized using rat glyceraldehyde 3-phosphate dehydrogenase as an internal control.
Morphology and immunohistochemistry
Kidneys were harvested and cross-sectioned, and a portion was fixed in formalin, paraffin-embedded, and processed for histology using standard techniques. Tissue sections were cut at 3–4 μm thickness. For histopathological evaluation, sections were deparaffined, rehydrated, and stained with hematoxylin–eosin or Sirius Red for collagen. For immunofluorescence, sections were first deparaffined and rehydrated. Sections were then incubated with 1% bovine serum albumin (Sigma-Aldrich, St. Louis, MO) for 1 hr in a humidified chamber at room temperature and treated overnight at 4°C with the primary antibodies, anti-human HO-1 and CD31, diluted 1:50 in Tris buffer (0.1 M, pH 7.4) (TBS). Subsequently, sections were washed in TBS and incubated with fluorescent-conjugated secondary antibodies (Alexa Fluor 488 for HO-1; Alexa Fluor 543 for CD31) diluted 1:200 in TBS for 1 hr at room temperature. Control reactions were performed in the absence of the primary antibody and in the presence of isotype-matched IgGs. Finally, they were stained with 4',6-diamidino-2-phenylindole (Invitrogen, Carlsbad, CA) for 8 min at room temperature, mounted in mounting medium (Dako, Carpinteria, CA), and observed with a confocal microscope (LSM 510 META, Zeiss, Oberkochen, Germany).
Plasma adiponectin, monocyte chemoattractant protein-1, and tumor necrosis factor-α measurements
The high-molecular-weight form of adiponectin, monocyte chemoattractant protein-1 (MCP1), and tumor necrosis factor-α (TNFα) were determined using enzyme-linked immunosorbent assays (Pierce Biotechnology, Inc., Woburn, MA) as previously described (Li et al., 2008).
Assessment of vascular reactivity
The femoral artery was removed, placed in cold Krebs-bicarbonate solution, cleaned of fat and loose connective tissue, and sectioned into rings approximately 3 mm in length. Two rings per artery were obtained, and each ring was mounted on stainless steel hooks and suspended in a 5-ml tissue DMT myograph bath (DMT, Atlanta, GA) filled with Krebs solution (pH 7.4), gassed with 95% O2/5% CO2, and maintained at 37°C. The rings were incubated under a passive tension of 0.2 g for 1 hr. The Krebs buffer solution was replaced every 15 min, and the tension was readjusted each time. Force was recorded from force displacement transducers via the Powerlab system, running Chart 5 software, from ADInstruments, Inc. (Colorado Springs, CO). At the end of the equilibration period, the maximal force generated by addition of a depolarizing solution of KCl (60 mM) was determined. To evaluate acetylcholine-induced vasorelaxation, the rings were preconstricted with phenylephrine (3.5 × 10–7 M) to obtain a stable plateau, and then cumulative dose–response curves to acetylcholine were obtained.
Western blot analysis of renal tissue for HO, endothelial NO synthase, peNOS, inducible NO synthase, AMP-activated protein kinase, pAMPK, AKT, phosphorylated AKT, and adiponectin
Frozen kidneys were pulverized under liquid nitrogen and placed in a homogenization buffer consisting of 10 mM phosphate buffer, 250 mM sucrose, 1 mM EDTA, 0.1 mM phenylmethylsulfonyl fluoride, and 0.1% (v/v) tergitol, pH 7.5. Homogenates were centrifuged at 27,000 g for 10 min at 4°C, the supernatant was decanted, and protein levels were determined followed by immunoblotting with various antibodies.
In brief, proteins in 20 μg of supernatant were separated by 12% SDS-PAGE and transferred to a nitrocellulose membrane. Immunoblotting was performed as previously described (L'Abbate et al., 2007). Chemiluminescence detection was performed with the Amersham (Piscataway, NJ) ECL detection kit, according to the manufacturer's instructions. Antibodies against HO-1 and HO-2 were obtained from Stressgen Biotechnologies Corp. (Victoria, Canada). Antibodies against AKT, AMP-activated protein kinase (AMPK), pAMPK, phosphorylated AKT (pAKT), and adiponectin were obtained from Cell Signaling Technology, Inc. (Beverly, MA), and those against endothelial NO synthase (eNOS), peNOS, and inducible NO synthase (iNOS) were from Santa Cruz Biotechnology (Santa Cruz, CA). HO-1 and HO-2 antibodies were prepared at a dilution of 1:1,000, whereas eNOS, peNOS, iNOS, AMPK, AKT, pAMPK, pAKT, and adiponectin antibodies were diluted 1:5,000.
Measurement of kidney O2− production
Kidney homogenates were placed in plastic scintillation minivials containing 5 μM lucigenin in a final volume of 1 ml of air-equilibrated Krebs' solution (pH 7.4) buffered with 10 mmol HEPES–1 M NaOH, for the detection of O2− (Li et al., 2008). Lucigenin chemiluminescence was measured using a liquid scintillation counter (model LS6000IC, Beckman Instruments, San Diego, CA).
Measurements of serum and urinary creatinine and urinary protein
Urinary protein excretion was measured using the BCA assay (Pierce, Rockford, IL). Serum and urinary creatinine levels were measured by an enzyme-linked immunoassay (Cayman Chemical Co., Ann Arbor, MI) according to the manufacturer's instructions.
Statistical analysis
The data are presented as mean ± SE values. Statistical significance (p < 0.05) of differences between the experimental groups was determined by Fisher's method of analysis for multiple comparisons. For comparison between treatment groups, the Null hypothesis was tested by either a single-factor analysis of variance for multiple groups or an unpaired t test for two groups.
Results
Endothelial-specific transduction
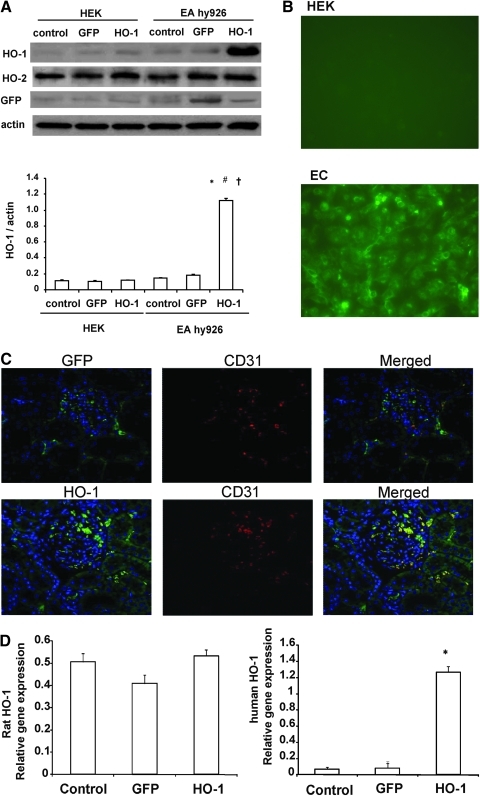
Transduction specificity was determined using cultured kidney-derived HEK cells and human umbilical vein endothelial cell-derived EA.hy926 cells. After transduction with either VECAD-HO-1 or VECAD-GFP, HO-1 and GFP expression was found only in EA.hy926 cells and not in HEK cells (Fig. 1A). The presence of GFP-positive cells was confirmed in the endothelial cells using confocal microscopy (Fig. 1B). The levels of HO-1 in EA.hy296 cells were significantly (p < 0.01) higher than in controls, indicating that VECAD-HO-1 and VECAD-GFP specifically target endothelial cells. The expression of HO-1 persisted for the duration of the experimental protocols (6–9 weeks). Immunohistochemistry of kidneys isolated from lentivirus-GFP- and lentivirus-human HO-1-treated rats showed co-localization of the endothelial-specific marker CD31 (platelet endothelial cell adhesion molecule) and HO-1 or GFP (Fig. 1C), demonstrating the cell-specific targeting of the viral vectors. Renal tissues from VECAD-HO-1-transduced rats express human HO-1 mRNA and protein without affecting levels of endogenous rat HO-1 (Fig. 1D).
FIG. 1.
(A and B) In vitro transduction with lentivirus-VECAD-HO-1 or lentivirus-VECAD-GFP in HEK and EA.hy926 (EC) cells. Blots are representative of three separate experiments. Data are shown as mean band density normalized relative to β-actin and are mean ± SE values (n = 3). *p < 0.01 versus control, #p < 0.01 versus GFP, †p < 0.05 versus HEK. (C) Immunofluorescence analysis of GFP, CD31, and human HO-1 in renal cortical sections from rats transduced with lentivirus-VECAD-GFP (upper panels) and with lentivirus-VECAD-HO-1 (lower panels). (D) Expression of human and rat HO-1 mRNA in renal tissues following bolus injection of lentivirus-VECAD-HO-1. Quantitative RT-PCR revealed a marked expression of human HO-1 without affecting rat HO-1 6 weeks after lentivirus administration. Data are mean ± SEM values of three independent experiments. *p < 0.05 versus lentivirus-GFP.
Blood pressure and inflammation in Ang II-treated animals
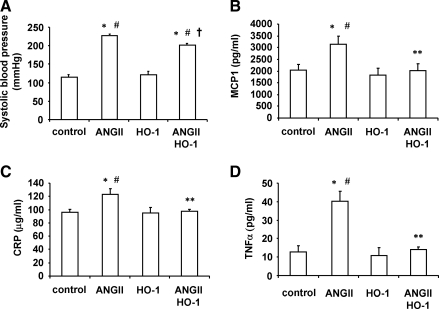
Systolic blood pressure was significantly (p < 0.01) increased in Ang II-treated Sprague–Dawley rats (222.6 ± 2.8 mm Hg) compared with control rats (112.2 ± 2.2 mm Hg) and remained elevated (215.2 ± 2.9 mm Hg) throughout the experiment. The elevation of the systolic blood pressure was attenuated by VECAD-HO-1 transduction in Ang II-treated Sprague–Dawley rats (194.9 ± 2.4 mm Hg). In the VECAD-HO-1-transduced control rats, blood pressure (118 ± 2.0 mm Hg) was unchanged (Fig. 2A). Similar patterns were noted for plasma MCP1 (Fig. 2B), C-reactive protein (Fig. 2C), and TNFα (Fig. 2D). Plasma MCP1 was increased (p < 0.0.5) in Ang II-treated rats compared with control and VECAD-HO-1-transduced rats (p < 0.05). Plasma C-reactive protein was also significantly (p < 0.05) increased in Ang II-treated rats and decreased after lentivirus-VECAD-HO-1 transduction. Likewise, plasma TNFα was higher (p < 0.05) in Ang II-treated rats compared with both control and VECAD-HO-1-transduced rats. VECAD-HO-1 transduction reduced (p < 0.05) plasma TNFα in Ang II-treated rats.
FIG. 2.
Effect of Ang II and VECAD-HO-1 transduction on (A) blood pressure, (B) MCP1, (C) C-reactive protein (CRP), and (D) TNFα in Sprague–Dawley rats. Blood pressure was measured by the tail cuff method. Data are mean ± SE values (n = 6 for each group). *p < 0.05 versus control, #p < 0.05 versus HO-1, **p < 0.05, †p < 0.01 versus Ang II.
HO-1 targeting to vascular system increases adiponectin release
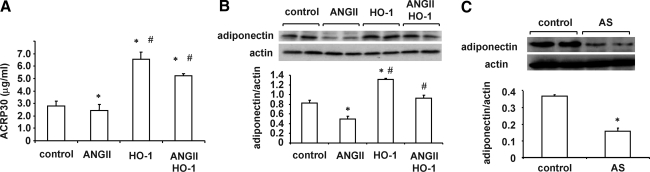
As seen in Fig. 3A, infusion of Ang II resulted in the suppression of adipocyte release and/or synthesis of adiponectin. In contrast, rats treated with lentivirus-VECAD-HO-1 exhibited a significant increase in circulating adiponectin levels compared with lentivirus-VECAD-mock (p < 0.05). Furthermore, infusion of Ang II in rats transduced with lentivirus-VECAD-HO-1 resulted in the continued expression of high levels of circulating adiponectin compared with rats infused with Ang II alone (p < 0.05). Because circulating adiponectin affects renal function, we examined whether adiponectin is present in renal tissue and the effect of Ang II on renal content of adiponectin. As seen in Fig. 3B, Ang II infusion decreased (p < 0.05) adiponectin levels in renal tissue. Rats transduced with lentivirus-VECAD-HO-1 exhibited an increase (p < 0.05) of renal content of adiponectin. To ascertain if targeting the vascular system is involved in vascular protection, we administered lentivirus-VECAD expressing the rat HO-1 gene in the AS orientation. As seen in Fig. 3C, adiponectin protein levels in renal tissue were decreased in rats transduced with lentivirus-VECAD-HO-1-AS compared with rats that received lentivirus-VECAD-mock (p < 0.01). These results showed that targeting vascular endothelial cells with the HO-1 gene positively affects adipocyte-mediated adiponectin synthesis.
FIG. 3.
(A) Plasma adiponectin (ACRP30) levels of Sprague–Dawley rats. Data are mean ± SE values (n = 6) for each group. *p < 0.05 versus control, #p < 0.05 versus Ang II. (B) Renal adiponectin in Sprague–Dawley animals. Immunoblots were performed with antibodies against rat adiponectin. Data are shown as mean band density normalized relative to β-actin (n = 6 for each group). *p < 0.05 versus control, #p < 0.05 versus Ang II. (C) Renal adiponectin of VECAD-HO-1-AS rats. *p < 0.05 versus control.
Vascular reactivity, superoxide production, and iNOS levels
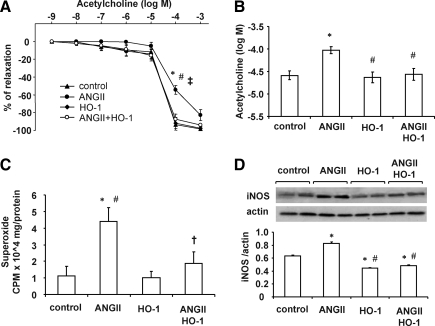
Figure 4A shows the dose–response curves for acetylcholine-induced vasorelaxation of femoral artery rings precontracted with phenylephrine. In rats infused with Ang II, the acetylcholine dose–response curve was shifted to the right, and the 50% effective concentration (Fig. 4B) increased from 10−6 M to 10−4 M in rings isolated from control rats. Treatment of rats with lentivirus carrying the HO-1 construct prevented the Ang II-induced impairment of endothelial function as the dose–response curves and the 50% effective concentration for acetylcholine were not different from those of control rats. The impairment of endothelial-dependent vasorelaxation was proportional to renal superoxide generation (Fig. 4C). Thus, Ang II infusion resulted in a fourfold increase in renal superoxide levels compared with the control (p < 0.01), and this increase was greatly attenuated in Ang II-infused rats that received the HO-1 construct. Arteries from Ang II-treated Sprague–Dawley rats demonstrated a marked (p < 0.01) increase in the levels of superoxide, suggesting an induction of the oxidative stress response. VECAD-HO-1 transduction reduced (p < 0.05) superoxide production in Ang II-treated animals (Fig. 4C). Figure 4D displays the effect of lentivirus-VECAD-HO-1 on artery iNOS levels. Ang II infusion caused a significant increase (p < 0.05) in iNOS protein expression. Administration of lentivirus-VECAD-HO-1 prevented the Ang II-mediated increase in iNOS levels (p < 0.05).
FIG. 4.
(A) Femoral arteries were precontracted with phenylephrine for dose-dependent (10–9–10–3 M) vasorelaxant responses to acetylcholine. Vessels from Ang II-treated Sprague–Dawley treated rats demonstrated a decreased response compared with control rats that was prevented by VECAD-HO-1 transduction. *p < 0.05 versus control, #p < 0.05 versus HO-1, †p < 0.05 versus Ang II+HO-1. (B) 50% effective concentration for acetylcholine. Results are mean ± SE values (n = 6). *p < 0.01 versus control, #p < 0.05 versus Ang II. (C) Artery renal superoxide levels. *p < 0.01 versus control, #p < 0.01 versus HO-1, †p < 0.05 versus Ang II. (D) Artery iNOS expression. Data are shown as mean band density normalized relative to β-actin. *p < 0.05 versus control, #p < 0.05 versus Ang II.
Effect of Ang II on lentivirus-VECAD-HO-1 transduction
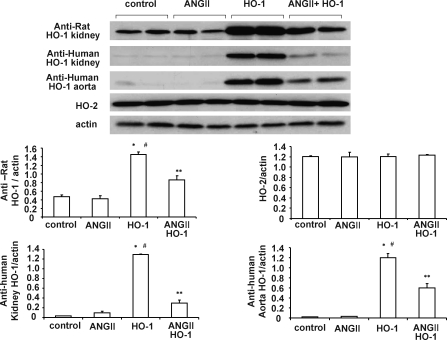
As seen in Fig. 5, human HO-1 expression in renal tissue was not affected by Ang II infusion. Similarly, western blot analysis showed that anti-rat HO-1 detected both human and rat HO-1; however, both anti-rat HO-1 and anti-human HO-1 showed that lentivirus-VECAD HO-1 was expressed at high levels in both renal and aortic tissue. Lentivirus-VECAD HO-1 delivery did not affect endogenous HO-2 expression. In rats treated with VECAD HO-1 and infused with Ang II, HO-1 expression was reduced compared with rats that were treated with the lentiviral vector but did not receive Ang II.
FIG. 5.
Western blot and densitometry analysis of HO-1 and HO-2 in kidney and aorta of rats treated with either Ang II or vehicle solution and transduced with VECAD-HO-1. Immunoblots were performed with antibodies against rat HO-1 and HO-2. Data are shown as mean band density normalized relative to β-actin (n = 6 for each group). *p < 0.01 versus control, #p < 0.01 versus Ang II, **p < 0.05 versus HO-1.
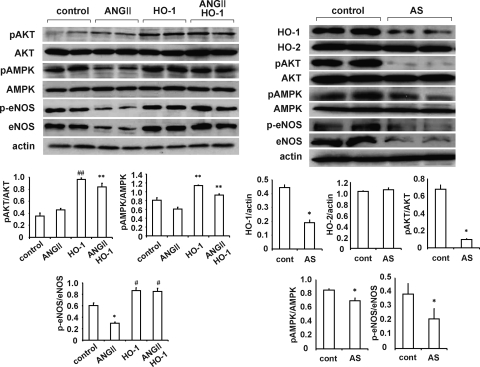
Effect of lentivirus-VECAD human HO-1 and lentivirus-VECAD rat HO-1-AS on signaling molecules and peNOS
Administration of lentivirus-VECAD-HO-1 increased renal pAKT, pAMPK and peNOS levels compared with control rats or rats administered Ang II (Fig. 6, left panel; p < 0.05). This effect is considered to be due to selective expression of human HO-1 in the vascular system. Moreover, administration of lentivirus-VECAD HO-1 prevented the decline in peNOS that resulted from Ang II infusion. As seen in Fig. 6, right panel, administration of lentivirus-VECAD rat HO-1-AS resulted in decreased HO-1 levels (p < 0.05) compared with control renal tissue. The decrease in endogenous HO-1 seen after lentivirus-VECAD rat HO-1-AS administration was associated with a significant decrease (p < 0.05) in levels of the signaling molecules pAKT and pAMPK and levels of peNOS.
FIG. 6.
Western blot and densitometry analysis of AMPK, pAMPK, AKT, pAKT, eNOS, and peNOS in kidney from Sprague–Dawley rats. Rats were treated with Ang II and transduced with (left panel) sense and (right panel) AS VECAD-HO-1. Data are shown as mean band density normalized to β-actin or as pAMPK/AMPK, pAKT/AKT, or peNOS/eNOS ratio (n = 6 for each group). *p < 0.05 versus control (cont), #p < 0.05 versus Ang II, **p < 0.05 versus HO-1, ##p < 0.05 versus Ang II.
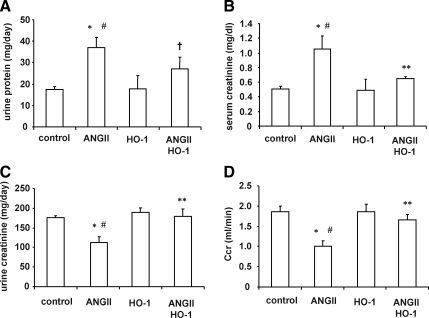
Effect of Ang II and lentivirus-VECAD-HO-1 transduction on renal injury markers
Urinary protein excretion and serum creatinine levels were significantly increased (p < 0.01 and p < 0.05, respectively) by the Ang II treatment (Fig. 7A and B). Lentivirus-VECAD-HO-1 administration reduced the increase in urinary protein excretion (p < 0.05) and serum creatinine levels (p < 0.05) in Ang II-treated animals, indicating that targeting vascular expression of HO-1 has a beneficial effect on renal function. Delivery of human HO-1 alone had no effect on either urinary protein or serum creatinine levels compared with control animals (Fig. 7A and 7B), although urinary creatinine excretion was decreased by Ang II infusion. This was prevented by lentivirus-VECAD-HO-1 treatment (Fig. 7C). Figure 7D shows that Ang II administration decreased creatinine clearance, which was prevented by lentivirus-VECAD-HO-1 transduction in Sprague–Dawley rats.
FIG. 7.
Effect of Ang II and up-regulation of HO-1 by gene transduction on renal function in Sprague–Dawley animals. Results are mean ± SE values (n = 6 for each group). (A) Urinary protein excretion. *p < 0.01 versus control, #p < 0.01 versus HO-1, †p < 0.05 versus Ang II. (B) Serum creatinine. *p < 0.05 versus control, #p < 0.05 versus HO-1, **p < 0.05 versus Ang II. (C) Urinary creatinine. *p < 0.05 versus control, #p < 0.05 versus HO-1, **p < 0.05 versus Ang II. (D) Creatinine clearance (Ccr). *p < 0.05 versus control, #p < 0.05 versus HO-1, **p < 0.05 versus Ang II.
Discussion
We report for the first time that a lentiviral vector carrying the HO-1 construct under the control of the endothelium-specific promoter VECAD specifically targets endothelial cells both in vitro and in vivo and endows cardiovascular protection to rats infused with Ang II. Lentiviral vectors have been shown to stably transduce renal and cardiac cells in vivo (Gusella et al., 2002a,b; Der et al., 2008), although the use of lentiviral vectors for the treatment of kidney diseases has been limited. Recently, lentiviral vectors carrying the HO-1 construct together with the antioxidant response element were successfully used to transduce human endothelial cells. Antioxidant response element–driven HO-1 overexpression inhibited nuclear factor κB activation and the subsequent vascular cell adhesion molecule-1 expression induced by TNFα (Hurttila et al., 2008). Similarly, overexpression of HO-1 using a VECAD promoter in endothelial cells prevented cell death that resulted from hyperglycemia (Asija et al., 2007). The VECAD promoter is widely used to direct endothelial-specific expression of numerous genes (Gory et al., 1999). We used lentiviral vectors (Huentelman et al., 2005) to achieve a longer duration of transgene expression. Hence, a bolus intravenous injection of lentiviral constructs expressing either HO-1 or GFP under the control of the VECAD promoter directed the expression of these genes to the vascular endothelium as assessed by their co-localization with CD31, an endothelium-specific marker. The vascular expression of both HO-1 and GFP was evident 6–9 weeks after transduction.
In the present report we demonstrate that overexpression of HO-1 ameliorated and prevented the negative cardiovascular and renal effects of Ang II infusion, including the increase in blood pressure and the accompanying impairment of endothelium-dependent vasorelaxation and creatinine clearance associated with increased oxidative stress and inflammation. Moreover, this is the first study to suggest the existence of an HO-1–adiponectin axis that may be central to renoprotection in this animal model of hypertension. Thus, improvements of endothelial and renal function were accompanied by increases in adiponectin levels and the pAMPK–peNOS pathway. Our previous studies showed that adenoviral- and retroviral-based vectors that overexpress HO-1 in the rat prevented the development of hypertension in both the spontaneously hypertensive rat and an Ang II-dependent model (Sabaawy et al., 2001; Yang et al., 2004). In contrast, we found that genetic suppression of HO-1, using HO-1-AS, elevated blood pressure and exacerbated renal damage in the two-kidney, one-clip model of renovascular hypertension (Olszanecki et al., 2007).
Overexpression of HO-1 has been shown to lower blood pressure in animal models of hypertension (Cao et al., 2009), and the induction of HO-1 has a cytoprotective role against renal injury secondary to rhabdomyolysis (Nath et al., 1992), ischemia–reperfusion injury (Maines et al., 1993), glomerulonephritis (Datta et al., 1999), renal transplant rejection (Avihingsanon et al., 2002), and nephrotoxin (e.g., cisplatin) administration (Agarwal et al., 1995). Under these conditions, the protective effects of increased levels of HO-1 were related to the increased degradation of the toxic free heme moiety and stimulation of the efflux of pro-oxidant iron from the cells as well as to the biological actions of bilirubin, a potent antioxidant (Stocker, 2004), and CO, a vasodilatory and anti-inflammatory molecule (Ryter and Otterbein, 2004). Recently, inhibition of apoptosis was proposed as a new mechanism for HO-mediated cytoprotection. Several lines of evidence point to an anti-apoptotic role of HO. The absence of the HO-1 gene significantly increases the susceptibility of both fibroblasts and vascular smooth muscle cells to stressful and toxic insults (Poss and Tonegawa, 1997; Li Volti et al., 2002), whereas, on the other hand, fibroblasts (Ferris et al., 1999) and neurons (Chen et al., 2000) overexpressing HO-1 are resistant to stress-mediated cell death. Furthermore, both pharmacological HO-1 induction and HO-1 gene overexpression render human renal epithelial cells resistant to cisplatin-induced apoptosis (Shiraishi et al., 2000). In contrast, HO-1–/– mice exhibit increased susceptibility to renal apoptosis and necrosis after treatment with cisplatin (Shiraishi et al., 2000). Our previous studies showed that genetic suppression of HO-1 exacerbates renal damage, which can be reversed by an increase in the anti-apoptotic signaling pathway (Olszanecki et al., 2007). Thus, it appears that the induction of renal HO-1 results in an anti-apoptotic phenotype.
Ang II-mediated increases in O2− and ROS contribute to endothelial cell apoptosis and dysfunction (Li and Shah, 2004). These perturbations may be reversed by the overexpression of antioxidant enzymes and the administration of antioxidants (Paravicini and Touyz, 2006). In the present study, lentivirus-mediated overexpression of HO-1 resulted in decreased O2− generation, which may be secondary to a decrease in the levels of heme-dependent protein NADPH oxidase (Kwak et al., 1991) and/or an increase in the levels of endothelial cell superoxide dismutase (Turkseven et al., 2005). In addition, the heme degradation products, CO and bilirubin, have potent anti-inflammatory and anti-apoptotic activities and antioxidant properties (Abraham and Kappas, 2008). Up-regulation of HO-1 also resulted in decreases of TNFα and MCP1 levels in the Ang II animal model. TNFα contributes to an increase in renal inflammation, and TNFα inhibition reduces renal injury in DOCA-salt hypertensive rats (Elmarakby et al., 2008). MCP1 can induce oxidative stress and activate the mitogen-activated protein kinase system and nuclear factor κB. In a variety of chronic kidney diseases, the renal expression of MCP1 and/or the levels of MCP1 predict the severity of tubulointerstitial disease and the risk for loss of kidney function (Yoshimoto et al., 2004). MCP1 was significantly up-regulated in the kidney of mutant mice unable to express HO-1 (Nath et al., 2001). The expression of HO-1 in the proximal tubule in proteinuric human kidney disease correlates with the severity of proteinuria, hematuria, and tubulointerstitial disease (Pedraza-Chaverri et al., 2006). Interestingly, constitutive overexpression of HO-1 by proximal tubular epithelial cells reduced the production of MCP1 by these cells in response to albumin. Thus, the induction of HO-1 appears to provide a favorable cellular environment for survival by the endowment of antioxidative and anti-inflammatory properties. Indeed, HO-1 overexpression results in a phenotype that is antioxidative, anti-inflammatory, and anti-apoptotic. Our results show that the generation of renal O2− was prevented by increased HO-1 expression, and this effect was accompanied by concomitant decreases in TNFα and MCP1 levels and increases in pAKT and pAMPK expression.
An increase in the endothelium HO-1 expression was associated with an increase in adiponectin levels that was accompanied by concomitant increases in the levels of renal eNOS, peNOS, pAKT, and pAMPK. These changes were associated with improved resistance to oxidants and apoptosis, thereby protecting the kidney. Our results support the idea that adiponectin is critical for endothelial cell survival and function (Ouchi et al., 2004) via the activation of eNOS, pAKT, and pAMPK. AKT is critical to cell survival (Franke et al., 1997; Kim et al., 2001; Tsang et al., 2005; Varma et al., 2005). Phosphorylation of AKT augments ATP synthesis and promotes the association of hexokinase with the mitochondrial voltage-dependent anion channel to promote voltage-dependent anion channel closure and block the release of cytochrome c (Gottlob et al., 2001). Activation of AMPK is important in cellular energy homeostasis (Marsin et al., 2000) and also reduces inflammation and improves insulin sensitivity and glucose tolerance (Bandyopadhyay et al., 2006; Yang et al., 2008). Both pAMPK and pAKT utilize eNOS as a substrate and increase the levels of peNOS (Chen et al., 1999; Dimmeler et al., 1999; Abraham and Kappas, 2008). Our findings are especially novel inasmuch as they implicate HO-1 expression as a key regulator of peNOS levels, possibly via an increase in adiponectin–pAMPK and, thereby, of renal and vascular function. These results support the importance of the temporal relationship of HO-1 and adiponectin in cytoprotection via increases in pAMPK and pAKT and subsequently peNOS and NO availability. Further support for this pathway is derived from observations in Sprague–Dawley rats treated with HO-1-AS in which much lower levels of adiponectin, pAMPK, pAKT, and peNOS were observed compared with control animals, implicating a link with HO-1.
Our previous studies indicate that up-regulation of HO-1 expression increased adiponectin levels in both diabetic and obese animal models (L'Abbate et al., 2007; Kim et al., 2008; Li et al., 2008; Peterson et al., 2008). Adiponectin has many beneficial effects in a number of cardiovascular diseases (Han et al., 2007; Hopkins et al., 2007), most notably a negative relationship between adiponectin and hypertension (Cao et al., 2009). Obese African Americans have reduced adiponectin levels associated with albuminuria (Sharma et al., 2008). Adiponectin deficiency in mice resulted in oxidative stress, fusion of podocyte foot processes in the kidney glomerulus, and urinary albumin excretion (Sharma et al., 2008), all of which were reversed by adiponectin treatment, likely through activation of AMPK. Furthermore, total serum adiponectin levels are an independent determinant of arteriosclerosis in IgA nephropathy patients, suggesting that adiponectin may prevent renal arteriosclerosis and protect renal function (Iwasa et al., 2008). Moreover, the presence of proteinuria, an important predictor of endothelial dysfunction in early diabetic nephropathy, is associated with lower levels of adiponectin (Yilmaz et al., 2008). Our present results show that lentivirus-mediated HO-1 gene transduction not only increased plasma adiponectin, but also up-regulated the levels of adiponectin in renal tissue, contributing to the renoprotective effects. It is of particular interest that adiponectin synthesis by adipocytes is affected by vascular HO-1 levels, leading to increases in circulating adiponectin that reaches renal tissue as determined by western blot analysis of kidneys isolated from lentivirus-VECAD-HO-1 rats. Inhibition of vascular HO-1 using lentivirus-VECAD-rat HO-1-AS decreased renal levels of adiponectin and the signaling molecules pAKT, pAMPK, and peNOS.
Collectively, the results of the present study demonstrate that lentiviral vectors carrying the HO-1 construct under the control of the endothelium-specific promoter VECAD specifically target endothelial cells. The resultant up-regulation of HO-1 protects against Ang II-induced vascular and renal dysfunction and is associated with increased adiponectin, pAMPK, and pAKT. The present study highlights the existence of an HO-1–adiponectin regulatory axis that, along with the pAMPK–peNOS pathway, can be manipulated to ameliorate the deleterious cardiovascular and renal effects of Ang II in an animal model of hypertension.
Acknowledgments
This work was supported by grants DK068134, HL55601, and HL34300 (to N.G.A.) and HL088421 and HL088421-S1 (to D.E.S.) from the National Institutes of Health. All authors had full access to the data and take responsibility for their integrity. All authors have read and agree with the manuscript as written. We thank Jennifer Brown for her outstanding editorial assistance in the preparation of the manuscript.
Author Disclosure Statement
The authors declare no conflicting financial interests.
References
- Abraham N.G. Quantitation of heme oxygenase (HO-1) copies in human tissues by competitive RT/PCR. Methods Mol. Biol. 1998;108:199–209. doi: 10.1385/0-89603-472-0:199. [DOI] [PubMed] [Google Scholar]
- Abraham N.G. Drummond G.S. Lutton J.D. Kappas A. The biological significance and physiological role of heme oxygenase. Cell. Physiol. Biochem. 1996;6:129–168. [Google Scholar]
- Abraham N.G. Kappas A. Heme oxygenase and the cardiovascular-renal system. Free Radic. Biol. Med. 2005;39:1–25. doi: 10.1016/j.freeradbiomed.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Abraham N.G. Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- Agarwal A. Balla J. Alam J., et al. Induction of heme oxygenase in toxic renal injury: A protective role in cisplatin nephrotoxicity in the rat. Kidney Int. 1995;48:1298–1307. doi: 10.1038/ki.1995.414. [DOI] [PubMed] [Google Scholar]
- Asija A. Peterson S. Stec D.E. Abraham N.G. Targeting endothelial cells with heme oxygenase-1 gene using VE-cadherin promoter attenuates hyperglycemia-mediated cell injury and apoptosis. Antioxid. Redox Signal. 2007;12:2065–2074. doi: 10.1089/ars.2007.1804. [DOI] [PubMed] [Google Scholar]
- Avihingsanon Y. Ma N. Csizmadia E., et al. Expression of protective genes in human renal allografts: A regulatory response to injury associated with graft rejection. Transplantation. 2002;73:1079–1085. doi: 10.1097/00007890-200204150-00011. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay G.K. Yu J.G. Ofrecio J. Olefsky J.M. Increased malonyl-CoA levels in muscle from obese and type 2 diabetic subjects lead to decreased fatty acid oxidation and increased lipogenesis; thiazolidinedione treatment reverses these defects. Diabetes. 2006;55:2277–2285. doi: 10.2337/db06-0062. [DOI] [PubMed] [Google Scholar]
- Botros F.T. Schwartzman M.L. Stier C.T., Jr., et al. Increase in heme oxygenase-1 levels ameliorates renovascular hypertension. Kidney Int. 2005;68:2745–2755. doi: 10.1111/j.1523-1755.2005.00745.x. [DOI] [PubMed] [Google Scholar]
- Cao J. Inoue K. Li X., et al. Physiological significance of heme oxygenase in hypertension. Int. J. Biochem. Cell Biol. 2009;41:1025–1033. doi: 10.1016/j.biocel.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. Gunter K. Maines M.D. Neurons overexpressing heme oxygenase-1 resist oxidative stress-mediated cell death. J. Neurochem. 2000;75:304–313. doi: 10.1046/j.1471-4159.2000.0750304.x. [DOI] [PubMed] [Google Scholar]
- Chen K. Vita J.A. Berk B.C. Keaney J.F., Jr. c-Jun N-terminal kinase activation by hydrogen peroxide in endothelial cells involves SRC-dependent epidermal growth factor receptor transactivation. J. Biol. Chem. 2001;276:16045–16050. doi: 10.1074/jbc.M011766200. [DOI] [PubMed] [Google Scholar]
- Chen Z.P. Mitchelhill K.I. Michell B.J., et al. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443:285–289. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- da Silva J.L. Zand B.A. Yang L.M., et al. Heme oxygenase isoform-specific expression and distribution in the rat kidney. Kidney Int. 2001;59:1448–1457. doi: 10.1046/j.1523-1755.2001.0590041448.x. [DOI] [PubMed] [Google Scholar]
- Datla S.R. Dusting G.J. Mori T.A., et al. Induction of heme oxygenase-1 in vivo suppresses NADPH oxidase derived oxidative stress. Hypertension. 2007;50:636–642. doi: 10.1161/HYPERTENSIONAHA.107.092296. [DOI] [PubMed] [Google Scholar]
- Datta P.K. Koukouritaki S.B. Hopp K.A. Lianos E.A. Heme oxygenase-1 induction attenuates inducible nitric oxide synthase expression and proteinuria in glomerulonephritis. J. Am. Soc. Nephrol. 1999;12:2540–2550. doi: 10.1681/ASN.V10122540. [DOI] [PubMed] [Google Scholar]
- Der S.S. Grobe J.L. Yuan L., et al. Cardiac overexpression of angiotensin converting enzyme 2 protects the heart from ischemia-induced pathophysiology. Hypertension. 2008;51:712–718. doi: 10.1161/HYPERTENSIONAHA.107.100693. [DOI] [PubMed] [Google Scholar]
- Dimmeler S. Fleming I. Fisslthaler B., et al. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- El B.R. Alvarez M. Monteseirin J., et al. Oxidative stress is a critical mediator of the angiotensin II signal in human neutrophils: Involvement of mitogen-activated protein kinase, calcineurin, and the transcription factor NF-kappaB. Blood. 2003;102:662–671. doi: 10.1182/blood-2002-09-2785. [DOI] [PubMed] [Google Scholar]
- Elmarakby A.A. Quigley J.E. Imig J.D., et al. TNF-alpha inhibition reduces renal injury in DOCA-salt hypertensive rats. Am. J. Physiol Regul. Integr. Comp. Physiol. 2008;294:R76–R83. doi: 10.1152/ajpregu.00466.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris C.D. Jaffrey S.R. Sawa A., et al. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat. Cell Biol. 1999;1:152–157. doi: 10.1038/11072. [DOI] [PubMed] [Google Scholar]
- Franke T.F. Kaplan D.R. Cantley L.C. PI3K: Downstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- Gory S. Vernet M. Laurent M., et al. The vascular endothelial-cadherin promoter directs endothelial-specific expression in transgenic mice. Blood. 1999;93:184–192. [PubMed] [Google Scholar]
- Gottlob K. Majewski N. Kennedy S., et al. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 2001;15:1406–1418. doi: 10.1101/gad.889901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusella G.L. Fedorova E. Hanss B., et al. Lentiviral gene transduction of kidney. Hum. Gene Ther. 2002a;13:407–414. doi: 10.1089/10430340252792530. [DOI] [PubMed] [Google Scholar]
- Gusella G.L. Fedorova E. Marras D., et al. In vivo gene transfer to kidney by lentiviral vector. Kidney Int. 2002b;61(1 Suppl):S32–S36. doi: 10.1046/j.1523-1755.2002.0610s1032.x. [DOI] [PubMed] [Google Scholar]
- Han S.H. Quon M.J. Kim J.A. Koh K.K. Adiponectin and cardiovascular disease: Response to therapeutic interventions. J. Am. Coll. Cardiol. 2007;49:531–538. doi: 10.1016/j.jacc.2006.08.061. [DOI] [PubMed] [Google Scholar]
- Haugen E.N. Croatt A.J. Nath K.A. Angiotensin II induces renal oxidant stress in vivo and heme oxygenase-1 in vivo and in vitro. Kidney Int. 2000;58:144–152. doi: 10.1046/j.1523-1755.2000.00150.x. [DOI] [PubMed] [Google Scholar]
- Hopkins T.A. Ouchi N. Shibata R. Walsh K. Adiponectin actions in the cardiovascular system. Cardiovasc. Res. 2007;74:11–18. doi: 10.1016/j.cardiores.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordijk P.L. Regulation of NADPH oxidases: The role of Rac proteins. Circ. Res. 2006;98:453–462. doi: 10.1161/01.RES.0000204727.46710.5e. [DOI] [PubMed] [Google Scholar]
- Huentelman M.J. Grobe J.L. Vazquez J., et al. Protection from angiotensin II-induced cardiac hypertrophy and fibrosis by systemic lentiviral delivery of ACE2 in rats. Exp. Physiol. 2005;90:783–790. doi: 10.1113/expphysiol.2005.031096. [DOI] [PubMed] [Google Scholar]
- Hurttila H. Koponen J.K. Kansanen E., et al. Oxidative stress-inducible lentiviral vectors for gene therapy. Gene Ther. 2008;15:1271–1279. doi: 10.1038/gt.2008.75. [DOI] [PubMed] [Google Scholar]
- Iwasa Y. Otsubo S. Ishizuka T., et al. Influence of serum high-molecular-weight and total adiponectin on arteriosclerosis in IgA nephropathy patients. Nephron Clin. Pract. 2008;108:c226–c232. doi: 10.1159/000119717. [DOI] [PubMed] [Google Scholar]
- Kim A.H. Khursigara G. Sun X., et al. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol. Cell Biol. 2001;21:893–901. doi: 10.1128/MCB.21.3.893-901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.H. Burgess A.P. Li M., et al. Heme oxygenase-mediated increases in adiponectin decrease fat content and inflammatory cytokines, tumor necrosis factor-alpha and interleukin-6 in Zucker rats and reduce adipogenesis in human mesenchymal stem cells. J. Pharmacol. Exp. Ther. 2008;325:833–840. doi: 10.1124/jpet.107.135285. [DOI] [PubMed] [Google Scholar]
- Kopkan L. Castillo A. Navar L.G. Majid D.S. Enhanced superoxide generation modulates renal function in ANG II-induced hypertensive rats. Am. J. Physiol. Renal Physiol. 2006;290:F80–F86. doi: 10.1152/ajprenal.00090.2005. [DOI] [PubMed] [Google Scholar]
- Kwak J.Y. Takeshige K. Cheung B.S. Minakami S. Bilirubin inhibits the activation of superoxide-producing NADPH oxidase in a neutrophil cell-free system. Biochim. Biophys. Acta. 1991;1076:369–373. doi: 10.1016/0167-4838(91)90478-i. [DOI] [PubMed] [Google Scholar]
- L'Abbate A. Neglia D. Vecoli C., et al. Beneficial effect of heme oxygenase-1 expression on myocardial ischemia-reperfusion involves an increase in adiponectin in mildly diabetic rats. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H3532–H3541. doi: 10.1152/ajpheart.00826.2007. [DOI] [PubMed] [Google Scholar]
- Lampugnani M.G. Resnati M. Raiteri M., et al. A novel endothelial-specific membrane protein is a marker of cell-cell contacts. J. Cell Biol. 1992;118:1511–1522. doi: 10.1083/jcb.118.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.M. Shah A.M. Endothelial cell superoxide generation: Regulation and relevance for cardiovascular pathophysiology. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287:R1014–R1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- Li M. Kim D.H. Tsenovoy P.L., et al. Treatment of obese diabetic mice with a heme oxygenase inducer reduces visceral and subcutaneous adiposity, increases adiponectin levels, and improves insulin sensitivity and glucose tolerance. Diabetes. 2008;57:1526–1535. doi: 10.2337/db07-1764. [DOI] [PubMed] [Google Scholar]
- Li Volti G. Wang J. Traganos F., et al. Differential effect of heme oxygenase-1 in endothelial and smooth muscle cell cycle progression. Biochem. Biophys. Res. Commun. 2002;296:1077–1082. doi: 10.1016/s0006-291x(02)02054-5. [DOI] [PubMed] [Google Scholar]
- Lopez B. Salom M.G. Arregui B., et al. Role of superoxide in modulating the renal effects of angiotensin II. Hypertension. 2003;42:1150–1156. doi: 10.1161/01.HYP.0000101968.09376.79. [DOI] [PubMed] [Google Scholar]
- Maines M.D. Mayer R.D. Ewing J.F. Induction of kidney heme oxygenase-1 (HSP32) mRNA and protein by ischemia reperfusion: Possible role of heme as both promoter of tissue damage and regulator of HSP32. J. Pharmacol. Exp. Ther. 1993;264:457–462. [PubMed] [Google Scholar]
- Marsin A.S. Bertrand L. Rider M.H., et al. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr. Biol. 2000;10:1247–1255. doi: 10.1016/s0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- Mori T. Cowley A.W., Jr. Angiotensin II-NAD(P)H oxidase-stimulated superoxide modifies tubulovascular nitric oxide cross-talk in renal outer medulla. Hypertension. 2003;42:588–593. doi: 10.1161/01.HYP.0000091821.39824.09. [DOI] [PubMed] [Google Scholar]
- Nath K.A. Balla J. Jacob H.S., et al. Induction of heme oxygenase is a rapid protective response in rhabdomyolysis in the rat. J. Clin. Invest. 1992;90:267–270. doi: 10.1172/JCI115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath K.A. Vercellotti G.M. Grande J.P., et al. Heme protein-induced chronic renal inflammation: suppressive effect of induced heme oxygenase-1. Kidney Int. 2001;59:106–117. doi: 10.1046/j.1523-1755.2001.00471.x. [DOI] [PubMed] [Google Scholar]
- Olszanecki R. Rezzani R. Omura S., et al. Genetic suppression of HO-1 exacerbates renal damage: Reversed by an increase in the antiapoptotic signaling pathway. Am. J. Physiol. Renal Physiol. 2007;292:F148–F157. doi: 10.1152/ajprenal.00261.2006. [DOI] [PubMed] [Google Scholar]
- Ortiz P.A. Garvin J.L. Interaction of O2− and NO in the thick ascending limb. Hypertension. 2002a;39:591–596. doi: 10.1161/hy0202.103287. [DOI] [PubMed] [Google Scholar]
- Ortiz P.A. Garvin J.L. Superoxide stimulates NaCl absorption by the thick ascending limb. Am. J. Physiol. Renal Physiol. 2002b;283:F957–F962. doi: 10.1152/ajprenal.00102.2002. [DOI] [PubMed] [Google Scholar]
- Ouchi N. Kobayashi H. Kihara S., et al. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J. Biol. Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paravicini T.M. Touyz R.M. Redox signaling in hypertension. Cardiovasc. Res. 2006;71:247–258. doi: 10.1016/j.cardiores.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Pedraza-Chaverri J. Murali N.S. Croatt A.J., et al. Proteinuria as a determinant of renal expression of heme oxygenase-1: Studies in models of glomerular and tubular proteinuria in the rat. Am. J. Physiol. Renal Physiol. 2006;290:F196–F204. doi: 10.1152/ajprenal.00230.2005. [DOI] [PubMed] [Google Scholar]
- Peterson S.J. Drummond G. Kim D.H., et al. L-4F treatment reduces adiposity, increases adiponectin levels and improves insulin sensitivity in obese mice. J. Lipid Res. 2008;49:1658–1669. doi: 10.1194/jlr.M800046-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflueger A. Croatt A.J. Peterson T.E., et al. The hyperbilirubinemic Gunn rat is resistant to the pressor effects of angiotensin II. Am. J. Physiol. Renal Physiol. 2005;288:F552–F558. doi: 10.1152/ajprenal.00278.2004. [DOI] [PubMed] [Google Scholar]
- Poss K.D. Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc. Natl. Acad. Sci. U.S.A. 1997;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan S. Yang L. Shenouda S., et al. Expression of human heme oxygenase-1 in the thick ascending limb attenuates angiotensin II-mediated increase in oxidative injury. Kidney Int. 2004;65:1628–1639. doi: 10.1111/j.1523-1755.2004.00562.x. [DOI] [PubMed] [Google Scholar]
- Ryter S.W. Otterbein L.E. Carbon monoxide in biology and medicine. Bioessays. 2004;26:270–280. doi: 10.1002/bies.20005. [DOI] [PubMed] [Google Scholar]
- Sabaawy H.E. Zhang F. Nguyen X., et al. Human heme oxygenase-1 gene transfer lowers blood pressure and promotes growth in spontaneously hypertensive rats. Hypertension. 2001;38:210–215. doi: 10.1161/01.hyp.38.2.210. [DOI] [PubMed] [Google Scholar]
- Schambach A. Baum C. Clinical application of lentiviral vectors—concepts and practice. Curr. Gene Ther. 2008;8:474–482. doi: 10.2174/156652308786848049. [DOI] [PubMed] [Google Scholar]
- Sharma K. Ramachandrarao S. Qiu G., et al. Adiponectin regulates albuminuria and podocyte function in mice. J. Clin. Invest. 2008;118:1645–1656. doi: 10.1172/JCI32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi F. Curtis L.M. Truong L., et al. Heme oxygenase-1 gene ablation or expression modulates cisplatin- induced renal tubular apoptosis. Am. J. Physiol. Renal Physiol. 2000;278:F726–F736. doi: 10.1152/ajprenal.2000.278.5.F726. [DOI] [PubMed] [Google Scholar]
- Stocker R. Antioxidant activities of bile pigments. Antioxid. Redox. Signal. 2004;6:841–849. doi: 10.1089/ars.2004.6.841. [DOI] [PubMed] [Google Scholar]
- Tsang A. Hausenloy D.J. Mocanu M.M., et al. Preconditioning the diabetic heart: The importance of akt phosphorylation. Diabetes. 2005;54:2360–2364. doi: 10.2337/diabetes.54.8.2360. [DOI] [PubMed] [Google Scholar]
- Turkseven S. Kruger A. Mingone C.J., et al. Antioxidant mechanism of heme oxygenase-1 involves an increase in superoxide dismutase and catalase in experimental diabetes. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H701–H707. doi: 10.1152/ajpheart.00024.2005. [DOI] [PubMed] [Google Scholar]
- Varma S. Lal B.K. Zheng R., et al. Hyperglycemia alters PI3k and Akt signaling and leads to endothelial cell proliferative dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H1744–H1751. doi: 10.1152/ajpheart.01088.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L. Quan S. Nasjletti A., et al. Heme oxygenase-1 gene expression modulates angiotensin II-induced increase in blood pressure. Hypertension. 2004;43:1221–1226. doi: 10.1161/01.HYP.0000126287.62060.e6. [DOI] [PubMed] [Google Scholar]
- Yang X. Ongusaha P.P. Miles P.D., et al. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature. 2008;451:964–969. doi: 10.1038/nature06668. [DOI] [PubMed] [Google Scholar]
- Yilmaz M.I. Saglam M. Qureshi A.R., et al. Endothelial dysfunction in type-2 diabetics with early diabetic nephropathy is associated with low circulating adiponectin. Nephrol. Dial. Transplant. 2008;23:1621–1627. doi: 10.1093/ndt/gfm828. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K. Wada T. Furuichi K., et al. CD68 and MCP-1/CCR2 expression of initial biopsies reflect the outcomes of membranous nephropathy. Nephron Clin. Pract. 2004;98:c25–c34. doi: 10.1159/000079924. [DOI] [PubMed] [Google Scholar]