Abstract
Background
Ischemic postconditioning (PostC) is a recently described cardioprotective modality against reperfusion injury, through series of brief re-flow interruptions applied at the very onset of reperfusion. It is proposed that PostC can activate a complex cellular signaling cascade, in which cell membrane receptors could serve as the upstream triggers of PostC. However, the exact subtypes of such receptors remain controversial or uninvestigated. To this context, the purpose of present study was to determine the definitive role of adenosine A1, bradykinin B1 and B2 receptors in PostC.
Methods and Results
The hearts isolated from adult male C57BL/6J wild-type mice or the mice lacking adenosine A1, or bradykinin B1 or B2 receptors subjected to zero-flow global ischemia and reperfusion in a Langendorff model. PostC, consisting of 6 cycles of 10 sec of reperfusion and 10 sec of ischemia, demonstrated significantly reduced myocardial infarct size (22.8±3.1%, Mean±SEM) as compared with the non-PostC wild-type controls (35.1±2.8%, P<0.05). The infarct-limiting protection of PostC was absent in adenosine A1 receptor knockout mice (34.9±2.7%) or bradykinin B2 receptor knockout mice (33.3±1.7%) and was partially attenuated in bradykinin B1 receptor deficient mice (25.6±2.9%; P>0.05). On the other hand, PostC did not significantly alter post-ischemic cardiac contractile function and coronary flow.
Conclusions
With the use of three distinctive strains of gene knockout mice, the current study has provided the first conclusive evidence showing PostC-induced infarct-limiting cardioprotection could be triggered by activation of multiple types of cell membrane receptors, which include adenosine A1 and bradykinin B2 receptors.
Keywords: Adenosine, Bradykinin, Infarction, Receptors, Reperfusion
Introduction
Ischemic postconditioning (PostC) is a series of brief mechanical interruptions of reperfusion applied at the very onset of reperfusion1. Since the original description of PostC in an in vivo dog model in 20032, this cardioprotective phenomenon has been confirmed by a number of research groups in several mammalian species including rat3–5, mouse3;6;7, rabbit8–12, dog9, and human13. This novel cardioprotective strategy has received considerable interests, mainly due to its potential clinical applicability as a post-ischemic intervention to reduce the cellular damages caused by the pathological factors during the initial minutes of reperfusion. In terms of the timing of intervention, PostC has an advantage over ischemic preconditioning, which has to be applied prior to a sustained ischemic event whose occurrence is usually not easy to predict precisely. In contrast, PostC apparently does not have the same pretreatment timing restrain for preconditioning and therefore it can be used during the routine interventional reperfusion procedures in the patients suffering acute myocardial infarction.
It is now widely accepted that PostC has interrelated passive and active components in its underlying cellular protective mechanisms1;5;14. During the PostC maneuvers, the washout of intra-coronary release of adenosine (possibly bradykinin as well) was significantly delayed3. Furthermore, the PostC-induced intermittent accumulation/release of adenosine and bradykinin could also facilitate the activation of their corresponding receptors on cardiac cell membranes1;14, which in turn triggers the signaling cascade of PostC. Nevertheless, the exact subtypes of adenosine and bradykinin receptors involved in triggering PostC remain controversial or unknown1;3;11;14. To resolve this issue, the present study was undertaken to determine the efficacy of PostC in reducing myocardial infarct size in strains of mice lacking adenosine A1, bradykinin B1 and B2 receptors.
Materials and Methods
Animals
Adult male C57BL/6J wild-type (C57-WT) mice and homozygous (−/−) bradykinin B2 receptor knockout (B2-KO) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Adult male adenosine A1 receptor knockout (A1-KO) mice were bred in the Virginia Commonwealth University (VCU) animal facility using the breeding pairs of homozygous (−/−) A1-KO mice, a generous gift from Dr. Jurgen Schnermann (NIDDK, Bethesda, MD, USA)15. Adult male homozygous (−/−) bradykinin B1 receptor knockout (B1-KO) mice were generated at the Max-Delbrück-Center for Molecular Medicine, Berlin-Buch, Germany according to the published methods16. The B1-KO mice at age of 6 weeks were transferred to VCU animal facility. Because all of the gene-knockout mice used in this study were generated from C57BL/6J background, C57-WT mice were used as the common control group for A1-KO, B1-KO, and B2-KO groups. The animal care and experiments were conducted in conformity with the guidelines on humane use and care of laboratory animals for biomedical research published by NIH (No. 85-23, revised 1996) and the rodent experiment protocol was approved by the Institutional Animal Care and Use Committee of VCU.
Model of global ischemia-reperfusion in Langendorff isolated mouse heart
The methodology of Langendorff isolated buffer-perfused mouse heart preparation was previously described in details17;18. In brief, the mouse was anesthetized with pentobarbital sodium (100 mg/kg, 33 IU heparin; i.p.) and the heart was quickly removed from the thorax and placed in icecold buffer. The aortic opening was rapidly cannulated (time delay <3 min) and tied on a 20-gauge blunt needle connected to Langendorff perfusion system. The heart was retrogradely perfused at constant pressure of 55 mm Hg with modified Krebs-Henseleit buffer containing (in mmol/L): 118 NaCl, 24 NaHCO3, 2.5 CaCl2, 4.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, 11 glucose, and 0.5 EDTA. The buffer was continuously gassed with 95% O2 + 5% CO2 (pH ~7.4) and warmed by a heating bath/circulator. The heart temperature was continuously monitored and maintained at 37±0.5°C. Ventricular function was measured by a force-displacement transducer (model FT03, Grass) attached to the apex with surgical thread and metal hook. The resting tension was adjusted to ~0.30 g. The contractile force was continuously recorded with a PowerLab 8SP computerized data acquisition system connected to the force transducer. Coronary flow rate was calculated by timed collection of the outflow perfusate. No electrical pacing was applied to the heart.
Experiment groups and protocol
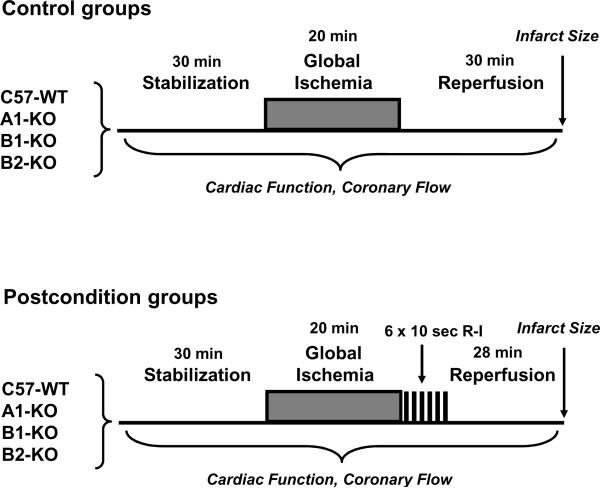
As illustrated inFig. 1, C57-WT control mice and the three strains of receptor deficient mice (i.e. A1-KO, B1-KO, B2-KO) were randomized into the following 8 experiment groups. Group 1: C57-WT (n=8), C57-WT subjected to 20 min of no-flow global ischemia and 30 min of reperfusion; Group 2: C57-WT+PostC (n=8), C57-WT subjected to ischemia and PostC intervention (6 cycles of 10 sec of ischemia and 10 sec of reperfusion) during the initial 2 min of reperfusion; Group 3: A1-KO (n=8), A1-KO subjected to ischemia and reperfusion; Group 4: A1-KO+PostC (n=7), A1-KO subjected to ischemia and PostC; Group 5: B1-KO (n=9), B1-KO subjected to ischemia and reperfusion; Group 6: B1-KO+PostC (n=9), B1-KO subjected to ischemia and PostC; Group 7: B2-KO (n=7), B2-KO subjected to ischemia and reperfusion; and Group 8: B2-KO+PostC (n=7), B2-KO subjected to ischemia and PostC.
Figure 1.
Diagrammatical description of the experiment groups and protocol of global ischemiareperfusion with or without postconditioning in Langendorff isolated perfused heart model. See Methods for abbreviations.
Measurement of infarct size
At the end of experiment, the heart was immediately removed from Langendorff apparatus, weighed, and frozen at −20°C. The frozen heart was manually cut into seven to eight transverse slices (~1 mm thickness), which were incubated in 10% TTC for 30 min at room temperature. TTC was then replaced with 10% formaldehyde for 3–4 hours of fixation before infarct size was measured using a computerized morphometry system (Bioquant 98). The risk area was calculated as total ventricular area minus cavities. Infarct size was calculated as % of risk area.
Data analysis and statistics
The experimental data were presented as the group means and standard errors (SEM). The difference among experimental groups was analyzed with two-way ANOVA followed by Bonferroni post-hoc test for pair-wise comparison. This analysis examines the effects of two variables (i.e. Factor 1: PostC intervention and Factor 2: animal genetic background), both individually and together, on the experimental response. P<0.05 was considered as statistically significant.
Results
Animal exclusion record and morphometric characteristics
Out of 66 mice originally used in the present study, three mice (i.e. 4.5% of the total) were excluded due to persistent arrhythmia or poor pre-ischemic function. The body weight and heart weight were significantly higher in A1-KO and B1-KO mice as compared with the non-PostC C57-WT mice, indicating a genetic background related variation in body/heart mass (Table 1).
Table 1.
Morphometric Characteristics and Baseline Cardiac Function of the Mice
| C57-WT | C57-WT + PostC | A1-KO | A1-KO + PostC | B1-KO | B1-KO + PostC | B2-KO | B2-KO + PostC | |
|---|---|---|---|---|---|---|---|---|
| (n = 8) | (n = 8) | (n = 8) | (n = 7) | (n = 9) | (n = 9) | (n = 7) | (n = 7) | |
| Body weight, g | 28.1±2.3 | 31.1±0.6 | 35.9±2.5* | 35.4±1.4 | 35.2±1.2* | 36.3±2.2 | 30.1±2.8 | 30.8±2.5 |
| Heart wet weight, mg | 205±9 | 215±6 | 259±13* | 266±7# | 261±18* | 249±13 | 218±20 | 213±18 |
| Coronary flow, ml/min | 1.6±0.2 | 1.8±0.2 | 1.8±0.2 | 2.3±0.2 | 1.6±0.2 | 2.0±0.2 | 2.0±0.3 | 1.8±0.1 |
| Heart rate, bpm | 345±22 | 351±16 | 316±29 | 343±20 | 317±23 | 301±22 | 349±30 | 351±23 |
| Developed force, g | 0.81±0.11 | 0.96±0.18 | 1.03±0.21 | 0.84±0.19 | 0.76±0.15 | 1.00±0.20 | 0.59±0.14 | 0.59±0.12 |
| Rate-force product, g×bpm | 284±46 | 339±67 | 306±55 | 285±63 | 234±46 | 290±63 | 188±30 | 217±56 |
Values are Mean±SEM. Abbreviation: bpm – beats per minute.
indicates P<0.05 versus C57-WT group
indicates P<0.05 versus C57-WT+PostC group (two-way ANOVA with Bonferroni post-hoc test).
Myocardial infarct size
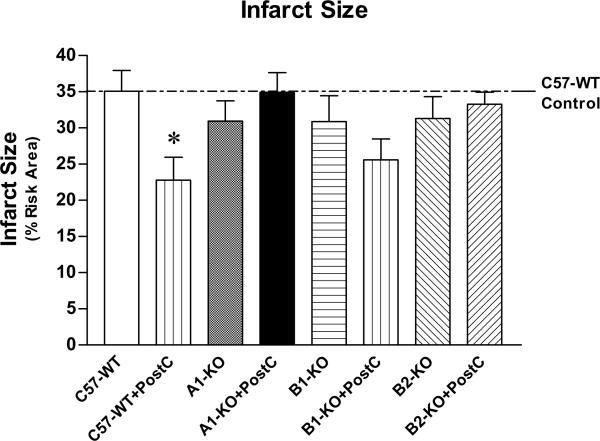
As shown inFigures 2 and 3, PostC resulted in reduction of infarct size following ischemia-reperfusion in C57-WT mice (22.8±3.1%), as compared with the wild-type control mice without PostC (35.1±2.8%; P<0.05). Infarct size in the non-PostC A1-KO and B2-KO was not different from the C57-WT controls (P>0.05). However, the infarct-limiting protection of PostC was completely abolished in mice lacking either adenosine A1 receptors or bradykinin B2 receptors (Figure 3; P>0.05 vs. the corresponding non-PostC A1-KO or B2-KO). Genetic deletion of bradykinin B1 receptors also did not alter myocardial infarct size (i.e. 30.9±3.6% in B1-KO vs. 35.1±2.8% in C57-WT; P<0.05). PostC was able to marginally reduce infarct size to 25.6±2.9% in B1-KO (Figure 3; P>0.05 vs. non-PostC B1-KO group), indicating PostC was partially attenuated in the mice deficient of bradykinin B1 receptors.
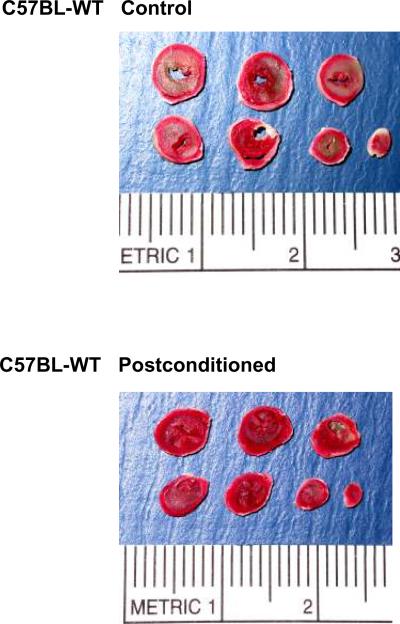
Figure 2.
Representative pictures of tetrazolium chloride-stained heart sections from C57BL/6J wild-type mice subjected to global ischemia-reperfusion alone (top) or with postconditioning (bottom). The infarct areas are in pale color and the viable tissues are in red color.
Figure 3.
Myocardial infarct size (Mean±SEM). *indicates P<0.05 versus C57-WT group (two-way ANOVA with Bonferroni post-hoc test). See Methods for detailed description of the experiment groups and sample numbers.
Baseline and post-ischemic cardiac contractile function
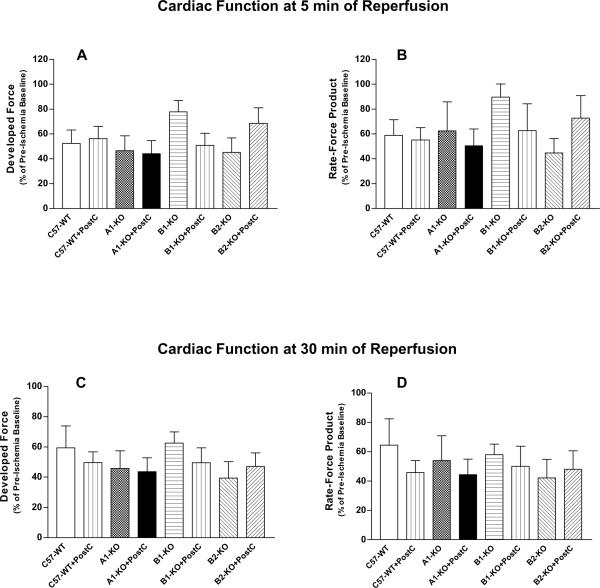
There was no significant difference in the pre-ischemia baseline levels of ventricular developed force and rate-force product among the eight experimental groups (Table 1; P>0.05), despite the baseline contractile function tended to be lower in B2-KO groups. Following the global ischemia-reperfusion, the developed force and rate-force product were remarkably depressed in all of the eight experimental groups, whereas heart rate remained at a constant level similar to the pre-ischemic values at both early (5 min) and late (30 min) time points of reperfusion (Tables 1 and 2;Figure 4). Interestingly, PostC did not improve the post-ischemic cardiac contractile function at either 5 min or 30 min of reperfusion in any strain of the wild-type and gene knockout mice (Table 2 and Figure 4; P>0.05). It is notable that the early post-ischemic functional recovery was remarkably improved in B1-KO group (i.e. 89.8±10.6% of pre-ischemic baseline for rate-force product vs. 58.9±12.6% in C57-WT group; seeFigure 4B), although it failed to reach the statistical significance mainly due to the high intra-group variability for the contractile function parameters (P>0.05 with two-way ANOVA). Furthermore, this trend of functional improvement in B1-KO mice disappeared at the end of 30 min reperfusion (Figure 4D).
Table 2.
Post-Ischemic Cardiac Function
| C57-WT | C57-WT + PostC | A1-KO | A1-KO + PostC | B1-KO | B1-KO + PostC | B2-KO | B2-KO + PostC | |
|---|---|---|---|---|---|---|---|---|
| (n = 8) | (n = 8) | (n = 8) | (n = 7) | (n = 9) | (n = 9) | (n = 7) | (n = 7) | |
| At 5 min of Reperfusion | ||||||||
| Heart rate, bpm | 368±25 | 339±18 | 336±18 | 362±29 | 360±23 | 308±22 | 341±22 | 347±27 |
| Developed force, g | 0.48±0.13 | 0.47±0.14 | 0.53±0.19 | 0.43±0.16 | 0.61±0.15 | 0.58±0.18 | 0.27±0.10 | 0.41±0.14 |
| Rate-force product, g×bpm | 182±46 | 168±53 | 178±62 | 168±66 | 216±53 | 195±69 | 85±25 | 158±61 |
| At 30 min of Reperfusion | ||||||||
| Heart rate, bpm | 345±26 | 309±17 | 324±19 | 321±26 | 288±24 | 301±32 | 354±24 | 334±24 |
| Developed force, g | 0.53±0.15 | 0.48±0.13 | 0.55±0.20 | 0.42±0.16 | 0.55±0.14 | 0.59±0.19 | 0.27±0.14 | 0.28±0.09 |
| Rate-force product, g×bpm | 190±53 | 151±45 | 172±59 | 143±55 | 148±36 | 164±62 | 85±36 | 103±37 |
Values are Mean±SEM. Abbreviation: bpm – beats per minute. No significant difference was found between the groups for any of the functional parameters (two-way ANOVA with Bonferroni post-hoc test).
Figure 4.
Post-ischemic cardiac function, indicated by Developed Force (A and C) and double product of heart rate and developed force (Rate-Force Product; B and D; Mean±SEM) at 5 min (top) or 30 min (bottom) of reperfusion. No statistically significant difference was found among the 8 experiment groups.
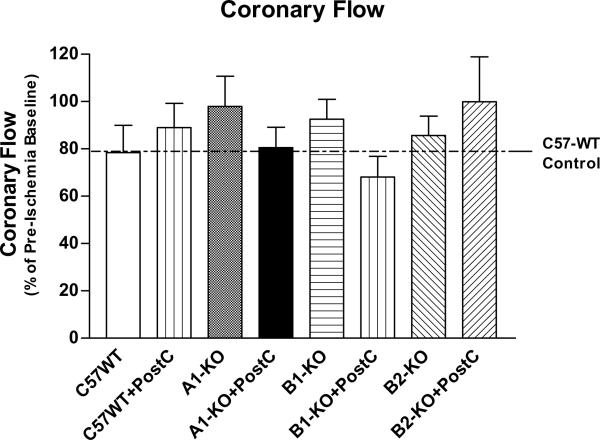
Baseline and post-ischemic coronary flow
Genetic deletion of adenosine A1, or bradykinin B1 or B2 receptors did not alter the pre-ischemic and post-ischemic coronary flow rate (P>0.05;Table 1 andFigure 5). There was only a slight trend towards higher post-ischemic coronary flow in A1-KO mice (Figure 5). PostC also had no effect on the post-ischemic coronary flow as compared with the corresponding non-PostC groups (P>0.05;Figure 5).
Figure 5.
Post-ischemic coronary flow (Mean±SEM). No statistically significant difference in this index of coronary vasodilation was observed among the 8 experiment groups.
Discussion
The present study has demonstrated the existence of PostC against myocardial ischemia-reperfusion injury in C57BL/6J wild-type mice. The six cycles of 10 sec of reperfusion and 10 sec of ischemia PostC maneuvers applied at the very onset of reperfusion significantly reduced infarct size in the globally ischemic-reperfused mouse hearts (Figures 2 and 3). The degree of infarct size reduction of PostC was approximately 35% from the non-PostC controls, which appears to be less potent than the ~42% reduction observed in our previous ischemic preconditioning study in ICR outbred mice17 performed in Langendorff perfused hearts with similar duration of ischemia-reperfusion. These results are comparable to the previous studies demonstrating efficacy of PostC in mice with 31% reduction in infarct size6 and 37% reduction in cardiac troponin I release – a specific marker for cardiac cell necrosis7. These results also support the concept that protective efficacy of PostC is somewhat less robust as compared to ischemic preconditioning based on the findings in conscious rats4 and anesthetized rabbits8. Furthermore, the infarct-limiting effect of PostC was not associated with any improvement in ventricular contractile function at 5 min or 30 min of reperfusion (Table 2 and Figure 4). This dissociation of infarct size and ventricular function was previously observed by our laboratory and others in various settings of preconditioning17–19 and PostC9;19. Some previous studies did report significant improvement in cardiac function following PostC3;7.
More importantly, the present study is the first investigation using adenosine A1, bradykinin B1 or B2 receptors knockout mice to determine the possible participation of these membrane receptors in triggering PostC. This is because the genetic deletion of a particular receptor gene could circumvent many confounding factors involved with the pharmacological antagonists of membrane receptors, such as suboptimal dose, target tissue availability, receptor affinity and selectivity, and other non-specific effects of the drugs. To our knowledge, there were only two previous PostC studies in which the mice with targeted deletion of connexin-436 or adenosine A2A receptors7 were used.
Adenosine A1 receptors and PostC
Despite a general agreement for the involvement of adenosine receptors in cardioprotection at reperfusion such as PostC12, the exact receptor subtype(s) that are responsible for triggering PostC remain controversial. One study addressed this issue by using the blockers of adenosine A1 (DPCPX); A2A (ZM241385) or A3 (MRS1523) receptors at the onset of reperfusion in an in vivo rat model and the authors concluded that A2A and A3, but not A1 receptors are important for PostC-induced cardioprotection3. The role of adenosine A2A receptors was recently confirmed by abrogation of PostC in mice lacking A2A7. However, another in vivo rabbit study reported blockade of PostC by an A2B receptor antagonist, MRS1754, but not by DPCPX and A2A receptor antagonist, 8-(13-chlorostyryl)caffeine11. In contrast, the present study clearly indicated complete loss of PostC-induced cardioprotection in A1-KO mice (Figure 3), which strongly suggest that adenosine A1 receptors are also essential for eliciting PostC. Interestingly, the findings are supported by a recent publication19, which reported that administration of A1 blocker DPCPX (200 nmol/L) abolished PostC-induced infarct size reduction in isolated rabbit hearts subjected to global ischemia-reperfusion. In addition, activation of A1 receptors by endogenous adenosine at reperfusion has been shown to protect the mouse heart20. To explain this apparent discrepancy, we would suspect that the intravenous administration of DPCPX shortly before or at the onset of reperfusion may not effectively block A1 receptors in cardiomyocytes when PostC was employed immediately after reperfusion in the two in vivo studies3;11.
Bradykinin B1 receptors and PostC
The pathophysiological function of bradykinin B1 receptors in ischemia-reperfusion injury remains ambiguous. From a few previous studies on B1-KO mice, both of beneficial21 and detrimental22;23 effects of B1 receptors on myocardial infarction and/or remodeling have been proposed. Our current study also yielded a blurry picture on the role of B1 receptors in PostC. We found that PostC produced a marginally smaller infarct size in B1-KO mice (25.6±2.9%) than the non-PostC B1-KO mice (30.9±3.6%; P>0.05;Figure 3). These results suggest that PostC was only partially attenuated in the absence of B1 receptors. Therefore, we believe that bradykinin B1 receptors do not play role in triggering PostC.
Bradykinin B2 receptors and PostC
Contrary to the above-mentioned controversies concerning the role of adenosine A1 and bradykinin B1 receptors in myocardial reperfusion injury and cardioprotection, there is a unanimous agreement on the crucial importance of bradykinin B2 receptors in myocardial protection, such as ischemic and pharmacologic preconditioning24;25. The present study further supports the notion that bradykinin B2 receptors are also indispensable in PostC, because the infarct-limiting effect of PostC observed in C57-WT mice was completely lost in B2-KO mice (Figure 3). Our results also showed that genetic deficiency of bradykinin B2 receptors did not significantly modify myocardial tolerance to ischemia-reperfusion injury, indicated by the similar infarct size (i.e. 35.1±2.8% in C57-WT vs. 31.3±3.0% in B2-KO; P>0.05;Figure 3). Similar results were observed after in vivo ischemia-reperfusion in B2-KO mice23;25. Furthermore, a recent study showed that pharmacological inhibition of B2 receptors by administration of HOE140 or WIN64338 blocked the infarct size reduction afforded by PostC or intermittent bradykinin infusion at the onset of reperfusion26. These results further suggest that the intact presence of bradykinin B2 receptors at the early onset of reperfusion is critical for transmitting the cell survival signals of PostC.
Role of other G protein-coupled receptors in PostC
It is notable that other types of G protein-coupled receptors could also involve with the signaling cascades of PostC. As we previously discussed, in addition to the involvement of adenosine A1 demonstrated by our present study and others19, other subtypes of adenosine receptors were also shown to be indispensable for PostC by several research groups. These G protein-coupled receptors include adenosine A2A3;7, A2B11, and A33. Furthermore, a few recent studies suggested that opioid receptors are the likely participants in PostC signaling27–29, which essentially confirmed the concept originally proposed by Gross and colleagues that pharmacological activation of opioid receptors at the early phase of reperfusion is as protective as preconditioning with the opioid receptor agonists in the rat heart30. However, it remains unclear how these G protein-coupled receptors cross-talk with each other, which leads to loss of Post-induced cardioprotection in the absence of one of these receptors.
Conclusion
The present study using three distinctive strains of gene knockout mice has provided conclusive evidence for essential role of both adenosine A1 and bradykinin B2 receptors on infarct-limiting protection of PostC in globally ischemic-reperfused mouse hearts. Future studies are necessary to elucidate the exact signaling cascades following the PostC-induced activation of each of these membrane receptor subtypes that ultimately lead to the cytoprotective phenotype against cardiac reperfusion injury.
Acknowledgments
Funding Sources This study was supported by the grants from American Heart Association National Center (0530157N to Dr Xi) and National Institutes of Health (HL51045, HL59469, HL79424 to Dr Kukreja).
Footnotes
This work was presented at the American Heart Association Scientific Sessions, Orlando, FL, November 4–7, 2007.
Disclosures None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhao ZQ, Vinten-Johansen J. Postconditioning: reduction of reperfusion-induced injury. Cardiovasc Res. 2006;70:200–211. doi: 10.1016/j.cardiores.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 2.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 3.Kin H, Zatta AJ, Lofye MT, Amerson BS, Halkos ME, Kerendi F, Zhao ZQ, Guyton RA, Headrick JP, Vinten-Johansen J. Postconditioning reduces infarct size via adenosine receptor activation by endogenous adenosine. Cardiovasc Res. 2005;67:124–133. doi: 10.1016/j.cardiores.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Tang XL, Sato H, Tiwari S, Dawn B, Bi Q, Li Q, Shirk G, Bolli R. Cardioprotection by postconditioning in conscious rats is limited to coronary occlusions <45 min. Am J Physiol Heart Circ Physiol. 2006;291:H2308–H2317. doi: 10.1152/ajpheart.00479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsang A, Hausenloy DJ, Mocanu MM, Yellon DM. Postconditioning: a form of “modified reperfusion” protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ Res. 2004;95:230–232. doi: 10.1161/01.RES.0000138303.76488.fe. [DOI] [PubMed] [Google Scholar]
- 6.Heusch G, Buchert A, Feldhaus S, Schulz R. No loss of cardioprotection by postconditioning in connexin 43-deficient mice. Basic Res Cardiol. 2006;101:354–356. doi: 10.1007/s00395-006-0589-0. [DOI] [PubMed] [Google Scholar]
- 7.Morrison RR, Tan XL, Ledent C, Mustafa SJ, Hofmann PA. Targeted deletion of A2A adenosine receptors attenuates the protective effects of myocardial postconditioning. Am J Physiol Heart Circ Physiol. 2007;293:H2523–H2529. doi: 10.1152/ajpheart.00612.2007. [DOI] [PubMed] [Google Scholar]
- 8.Argaud L, Gateau-Roesch O, Raisky O, Loufouat J, Robert D, Ovize M. Postconditioning inhibits mitochondrial permeability transition. Circulation. 2005;111:194–197. doi: 10.1161/01.CIR.0000151290.04952.3B. [DOI] [PubMed] [Google Scholar]
- 9.Couvreur N, Lucats L, Tissier R, Bize A, Berdeaux A, Ghaleh B. Differential effects of postconditioning on myocardial stunning and infarction: a study in conscious dogs and anesthetized rabbits. Am J Physiol Heart Circ Physiol. 2006;291:H1345–H1350. doi: 10.1152/ajpheart.00124.2006. [DOI] [PubMed] [Google Scholar]
- 10.Darling CE, Jiang R, Maynard M, Whittaker P, Vinten-Johansen J, Przyklenk K. Postconditioning via stuttering reperfusion limits myocardial infarct size in rabbit hearts: role of ERK1/2. Am J Physiol Heart Circ Physiol. 2005;289:H1618–H1626. doi: 10.1152/ajpheart.00055.2005. [DOI] [PubMed] [Google Scholar]
- 11.Philipp S, Yang XM, Cui L, Davis AM, Downey JM, Cohen MV. Postconditioning protects rabbit hearts through a protein kinase C-adenosine A2b receptor cascade. Cardiovasc Res. 2006;70:308–314. doi: 10.1016/j.cardiores.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Yang XM, Philipp S, Downey JM, Cohen MV. Postconditioning's protection is not dependent on circulating blood factors or cells but involves adenosine receptors and requires PI3-kinase and guanylyl cyclase activation. Basic Res Cardiol. 2005;100:57–63. doi: 10.1007/s00395-004-0498-4. [DOI] [PubMed] [Google Scholar]
- 13.Staat P, Rioufol G, Piot C, Cottin Y, Cung TT, L'Huillier I, Aupetit JF, Bonnefoy E, Finet G, Andre-Fouet X, Ovize M. Postconditioning the human heart. Circulation. 2005;112:2143–2148. doi: 10.1161/CIRCULATIONAHA.105.558122. [DOI] [PubMed] [Google Scholar]
- 14.Gross ER, Gross GJ. Ligand triggers of classical preconditioning and postconditioning. Cardiovasc Res. 2006;70:212–221. doi: 10.1016/j.cardiores.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, Schnermann J. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci U S A. 2001;98:9983–9988. doi: 10.1073/pnas.171317998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pesquero JB, Araujo RC, Heppenstall PA, Stucky CL, Silva JA, Jr., Walther T, Oliveira SM, Pesquero JL, Paiva AC, Calixto JB, Lewin GR, Bader M. Hypoalgesia and altered inflammatory responses in mice lacking kinin B1 receptors. Proc Natl Acad Sci U S A. 2000;97:8140–8145. doi: 10.1073/pnas.120035997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xi L, Hess ML, Kukreja RC. Ischemic preconditioning in isolated perfused mouse heart: reduction in infarct size without improvement of post-ischemic ventricular function. Mol Cell Biochem. 1998;186:69–77. [PubMed] [Google Scholar]
- 18.Xi L, Jarrett NC, Hess ML, Kukreja RC. Essential role of inducible nitric oxide synthase in monophosphoryl lipid A-induced late cardioprotection: evidence from pharmacological inhibition and gene knockout mice. Circulation. 1999;99:2157–2163. doi: 10.1161/01.cir.99.16.2157. [DOI] [PubMed] [Google Scholar]
- 19.Donato M, D'Annunzio V, Berg G, Gonzalez G, Schreier L, Morales C, Wikinski RL, Gelpi RJ. Ischemic postconditioning reduces infarct size by activation of A1 receptors and KATP channels in both normal and hypercholesterolemic rabbits. J Cardiovasc Pharmacol. 2007;49:287–292. doi: 10.1097/FJC.0b013e31803c55fe. [DOI] [PubMed] [Google Scholar]
- 20.Peart J, Headrick JP. Intrinsic A1 adenosine receptor activation during ischemia or reperfusion improves recovery in mouse hearts. Am J Physiol Heart Circ Physiol. 2000;279:H2166–H2175. doi: 10.1152/ajpheart.2000.279.5.H2166. [DOI] [PubMed] [Google Scholar]
- 21.Xu J, Carretero OA, Sun Y, Shesely EG, Rhaleb NE, Liu YH, Liao TD, Yang JJ, Bader M, Yang XP. Role of the B1 kinin receptor in the regulation of cardiac function and remodeling after myocardial infarction. Hypertension. 2005;45:747–753. doi: 10.1161/01.HYP.0000153322.04859.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lagneux C, Bader M, Pesquero JB, Demenge P, Ribuot C. Detrimental implication of B1 receptors in myocardial ischemia: evidence from pharmacological blockade and gene knockout mice. Int Immunopharmacol. 2002;2:815–822. doi: 10.1016/s1567-5769(02)00022-x. [DOI] [PubMed] [Google Scholar]
- 23.Yin H, Chao J, Bader M, Chao L. Differential role of kinin B1 and B2 receptors in ischemia-induced apoptosis and ventricular remodeling. Peptides. 2007;28:1383–1389. doi: 10.1016/j.peptides.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kositprapa C, Ockaili RA, Kukreja RC. Bradykinin B2 receptor is involved in the late phase of preconditioning in rabbit heart. J Mol Cell Cardiol. 2001;33:1355–1362. doi: 10.1006/jmcc.2000.1396. [DOI] [PubMed] [Google Scholar]
- 25.Yang XP, Liu YH, Scicli GM, Webb CR, Carretero OA. Role of kinins in the cardioprotective effect of preconditioning: study of myocardial ischemia/reperfusion injury in B2 kinin receptor knockout mice and kininogen-deficient rats. Hypertension. 1997;30:735–740. doi: 10.1161/01.hyp.30.3.735. [DOI] [PubMed] [Google Scholar]
- 26.Penna C, Mancardi D, Rastaldo R, Losano G, Pagliaro P. Intermittent activation of bradykinin B2 receptors and mitochondrial KATP channels trigger cardiac postconditioning through redox signaling. Cardiovasc Res. 2007;75:168–177. doi: 10.1016/j.cardiores.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Chen Z, Li T, Zhang B. Morphine Postconditioning Protects Against Reperfusion Injury in the Isolated Rat Hearts. J Surg Res. 2008;145:287–294. doi: 10.1016/j.jss.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 28.Jang Y, Xi J, Wang H, Mueller RA, Norfleet EA, Xu Z. Postconditioning prevents reperfusion injury by activating delta-opioid receptors. Anesthesiology. 2008;108:243–250. doi: 10.1097/01.anes.0000299437.93898.4a. [DOI] [PubMed] [Google Scholar]
- 29.Zatta AJ, Kin H, Yoshishige D, Jiang R, Wang N, Reeves JG, Mykytenko J, Guyton RA, Zhao ZQ, Caffrey JL, Vinten-Johansen J. Evidence that cardioprotection by postconditioning involves preservation of myocardial opioid content and selective opioid receptor activation. Am J Physiol Heart Circ Physiol. 2008;294:H1444–H1451. doi: 10.1152/ajpheart.01279.2006. [DOI] [PubMed] [Google Scholar]
- 30.Gross ER, Hsu AK, Gross GJ. Opioid-induced cardioprotection occurs via glycogen synthase kinase beta inhibition during reperfusion in intact rat hearts. Circ Res. 2004;94:960–966. doi: 10.1161/01.RES.0000122392.33172.09. [DOI] [PubMed] [Google Scholar]