Abstract
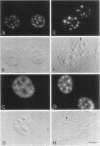
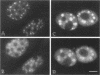
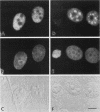
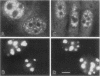
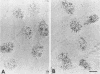
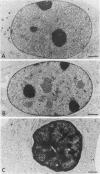
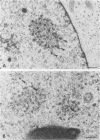
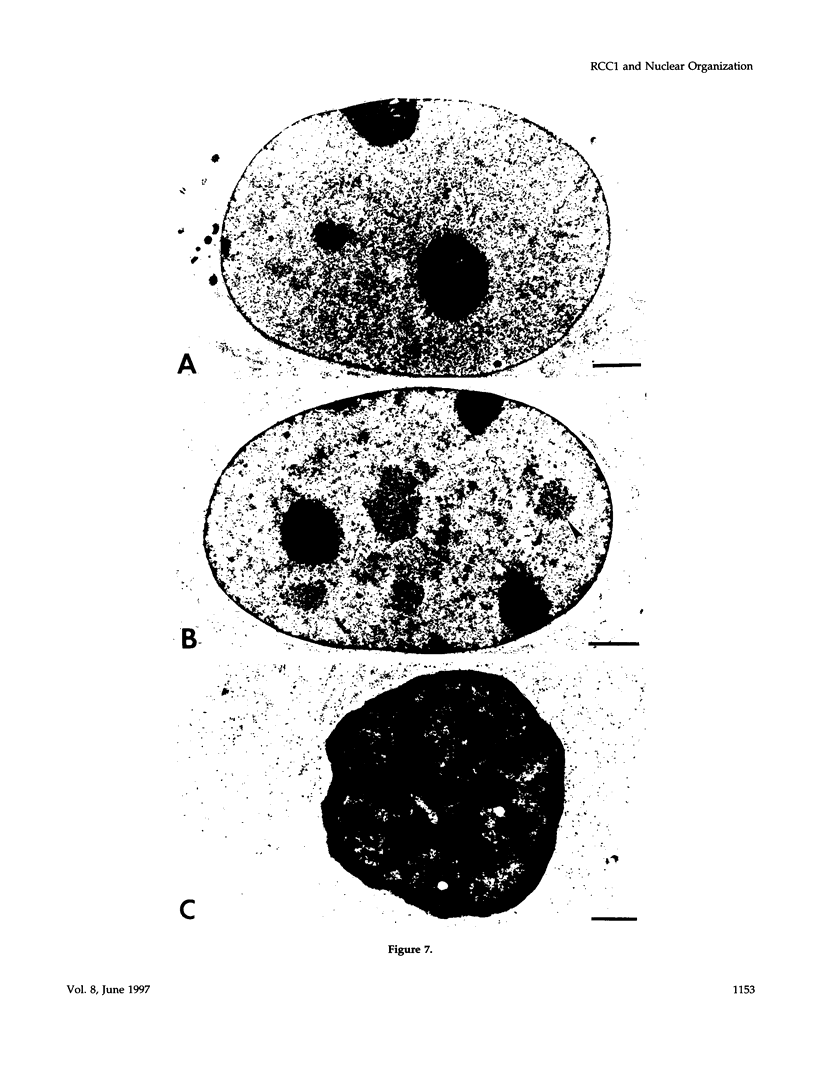
We have examined the effect of RCC1 function on the nuclear organization of pre-mRNA splicing factors and poly(A)+ RNA in the tsBN2 cells, a RCC1 temperature-sensitive mutant cell line. We have found that at 4-6 h after shifting cells from the permissive temperature (32.5 degrees C) to the restrictive temperature (39.5 degrees C), both small nuclear ribonucleoprotein particles and a general splicing factor SC35 reorganized into 4-10 large round clusters in the nucleus, as compared with the typical speckled distribution seen in cells at the permissive temperature. In situ hybridization to poly(A)+ RNA resulted in a similar pattern. Examination by double labeling demonstrated that the redistribution of splicing factors coincides with that of poly(A)+ RNA. Such changes in the nuclear organization of splicing factors and poly(A)+ RNA were not the result of the temperature shift or of chromatin condensation. Cellular transcription was not significantly altered in these cells and extracts made from both the permissive and restrictive temperature were splicing competent. Electron microscopic examination demonstrated that the large clusters containing both splicing factors and poly(A)+ RNA were fused interchromatin granule clusters. In addition, small electron-dense dot-like structures measuring approximately 80 nm in diameter were also observed, most of which are accumulated in enlarged interchromatin granule clusters in the nucleoplasm of RCC1- cells. In spite of the significant changes observed in the nucleoplasm, relatively little alteration was observed in nucleolar structure by both light and electron microscopic examination. The above observations suggest that the RCC1 protein directly or indirectly regulates the organization of splicing components and poly(A)+ RNA in the cell nucleus and that RCC1 may play a role in nuclear organization.
Full text
PDF


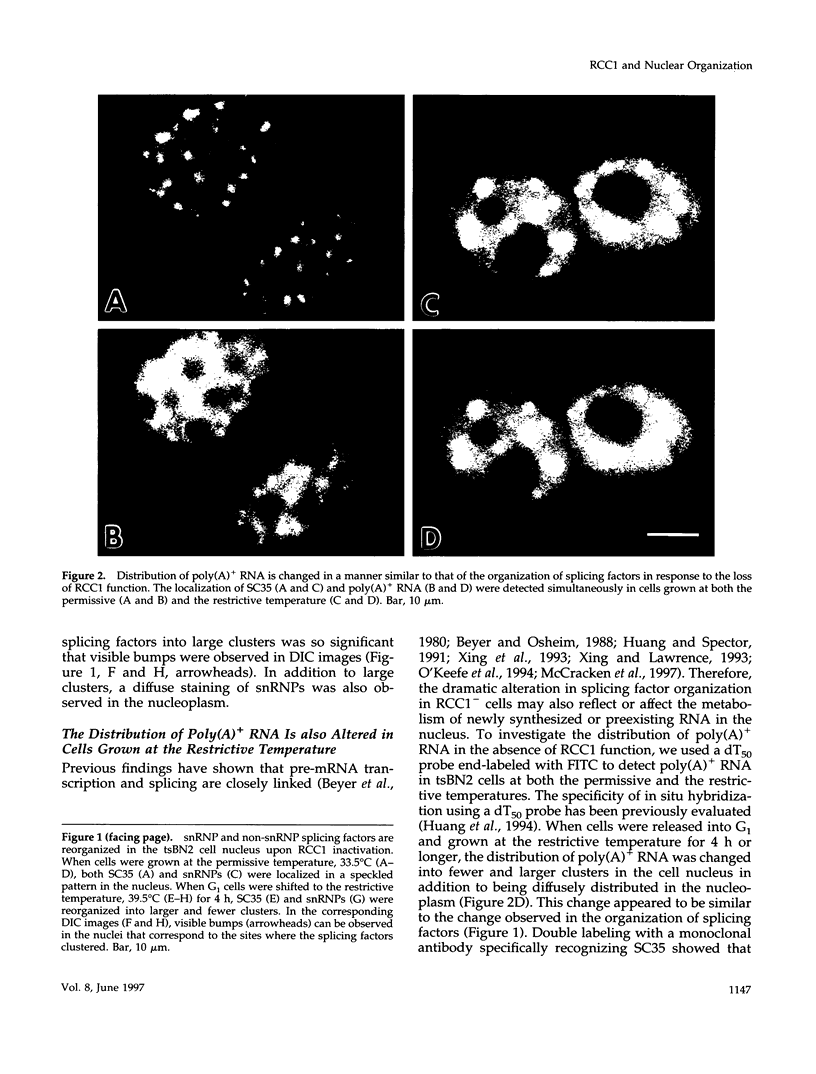

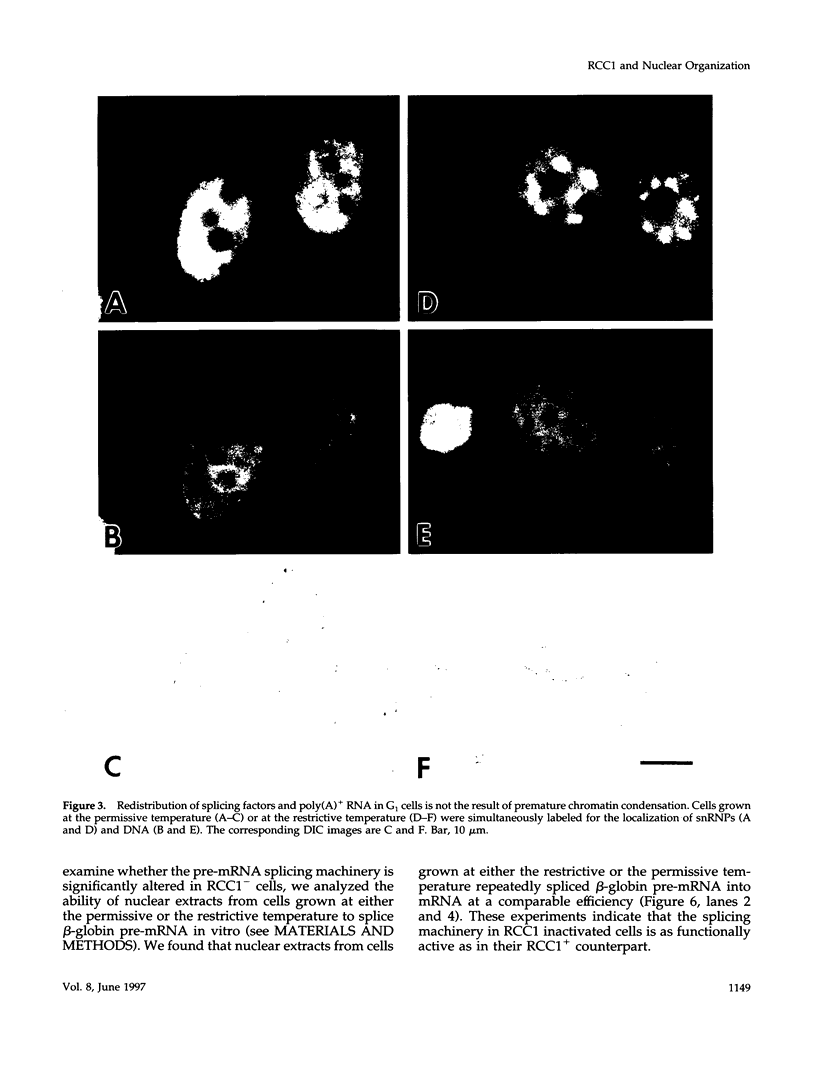
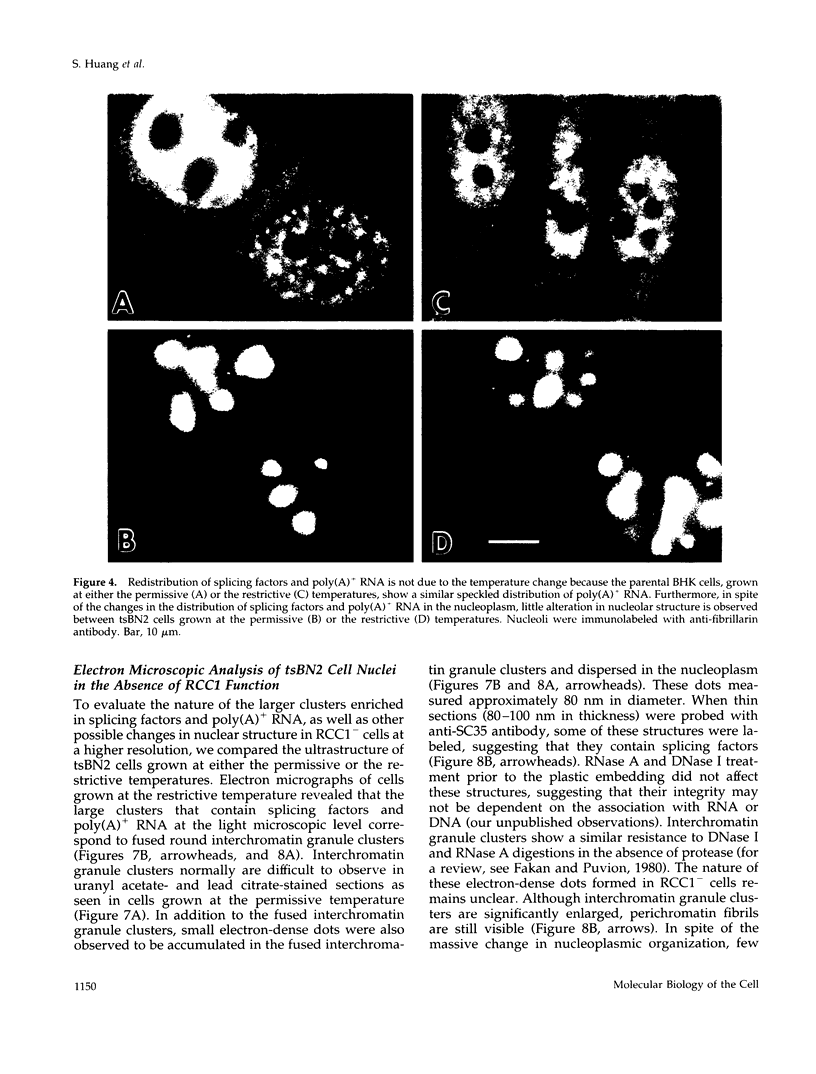
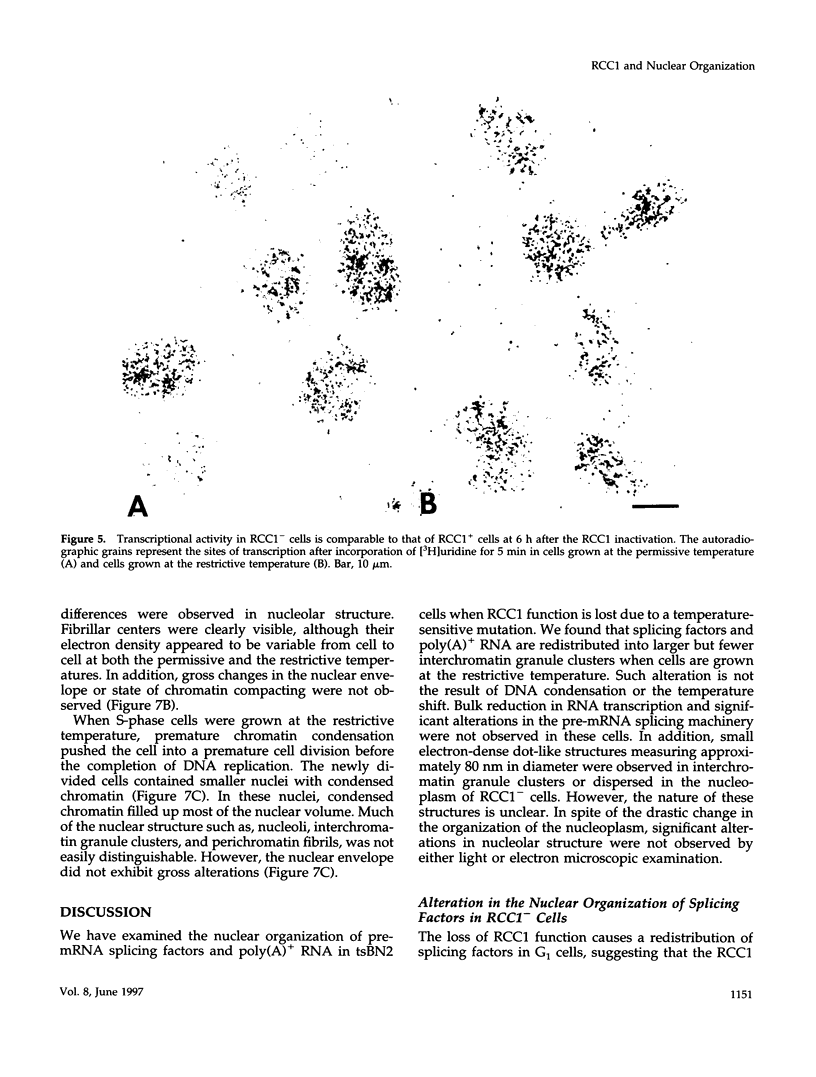





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi M., Clark M. W., Vijayraghavan U., Abelson J. A yeast mutant, PRP20, altered in mRNA metabolism and maintenance of the nuclear structure, is defective in a gene homologous to the human gene RCC1 which is involved in the control of chromosome condensation. Mol Gen Genet. 1990 Oct;224(1):72–80. doi: 10.1007/BF00259453. [DOI] [PubMed] [Google Scholar]
- Amberg D. C., Fleischmann M., Stagljar I., Cole C. N., Aebi M. Nuclear PRP20 protein is required for mRNA export. EMBO J. 1993 Jan;12(1):233–241. doi: 10.1002/j.1460-2075.1993.tb05649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma Y., Seino H., Seki T., Uzawa S., Klebe C., Ohba T., Wittinghofer A., Hayashi N., Nishimoto T. Conserved histidine residues of RCC1 are essential for nucleotide exchange on Ran. J Biochem. 1996 Jul;120(1):82–91. doi: 10.1093/oxfordjournals.jbchem.a021397. [DOI] [PubMed] [Google Scholar]
- Beyer A. L., Miller O. L., Jr, McKnight S. L. Ribonucleoprotein structure in nascent hnRNA is nonrandom and sequence-dependent. Cell. 1980 May;20(1):75–84. doi: 10.1016/0092-8674(80)90236-6. [DOI] [PubMed] [Google Scholar]
- Beyer A. L., Osheim Y. N. Splice site selection, rate of splicing, and alternative splicing on nascent transcripts. Genes Dev. 1988 Jun;2(6):754–765. doi: 10.1101/gad.2.6.754. [DOI] [PubMed] [Google Scholar]
- Bischoff F. R., Ponstingl H. Mitotic regulator protein RCC1 is complexed with a nuclear ras-related polypeptide. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10830–10834. doi: 10.1073/pnas.88.23.10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockdorff N., Ashworth A., Kay G. F., McCabe V. M., Norris D. P., Cooper P. J., Swift S., Rastan S. The product of the mouse Xist gene is a 15 kb inactive X-specific transcript containing no conserved ORF and located in the nucleus. Cell. 1992 Oct 30;71(3):515–526. doi: 10.1016/0092-8674(92)90519-i. [DOI] [PubMed] [Google Scholar]
- Brown C. J., Hendrich B. D., Rupert J. L., Lafrenière R. G., Xing Y., Lawrence J., Willard H. F. The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell. 1992 Oct 30;71(3):527–542. doi: 10.1016/0092-8674(92)90520-m. [DOI] [PubMed] [Google Scholar]
- Carmo-Fonseca M., Pepperkok R., Carvalho M. T., Lamond A. I. Transcription-dependent colocalization of the U1, U2, U4/U6, and U5 snRNPs in coiled bodies. J Cell Biol. 1992 Apr;117(1):1–14. doi: 10.1083/jcb.117.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Fonseca M., Pepperkok R., Sproat B. S., Ansorge W., Swanson M. S., Lamond A. I. In vivo detection of snRNP-rich organelles in the nuclei of mammalian cells. EMBO J. 1991 Jul;10(7):1863–1873. doi: 10.1002/j.1460-2075.1991.tb07712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter K. C., Taneja K. L., Lawrence J. B. Discrete nuclear domains of poly(A) RNA and their relationship to the functional organization of the nucleus. J Cell Biol. 1991 Dec;115(5):1191–1202. doi: 10.1083/jcb.115.5.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Dahlberg J. E., Lund E. Diverse effects of the guanine nucleotide exchange factor RCC1 on RNA transport. Science. 1995 Mar 24;267(5205):1807–1810. doi: 10.1126/science.7534442. [DOI] [PubMed] [Google Scholar]
- Coutavas E., Ren M., Oppenheim J. D., D'Eustachio P., Rush M. G. Characterization of proteins that interact with the cell-cycle regulatory protein Ran/TC4. Nature. 1993 Dec 9;366(6455):585–587. doi: 10.1038/366585a0. [DOI] [PubMed] [Google Scholar]
- Dasso M. RCC1 in the cell cycle: the regulator of chromosome condensation takes on new roles. Trends Biochem Sci. 1993 Mar;18(3):96–101. doi: 10.1016/0968-0004(93)90161-f. [DOI] [PubMed] [Google Scholar]
- Dasso M., Seki T., Azuma Y., Ohba T., Nishimoto T. A mutant form of the Ran/TC4 protein disrupts nuclear function in Xenopus laevis egg extracts by inhibiting the RCC1 protein, a regulator of chromosome condensation. EMBO J. 1994 Dec 1;13(23):5732–5744. doi: 10.1002/j.1460-2075.1994.tb06911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeter J., Morphew M., Sazer S. A mutation in the RCC1-related protein pim1 results in nuclear envelope fragmentation in fission yeast. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1436–1440. doi: 10.1073/pnas.92.5.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakan S., Bernhard W. Localisation of rapidly and slowly labelled nuclear RNA as visualized by high resolution autoradiography. Exp Cell Res. 1971 Jul;67(1):129–141. doi: 10.1016/0014-4827(71)90628-8. [DOI] [PubMed] [Google Scholar]
- Fakan S., Leser G., Martin T. E. Ultrastructural distribution of nuclear ribonucleoproteins as visualized by immunocytochemistry on thin sections. J Cell Biol. 1984 Jan;98(1):358–363. doi: 10.1083/jcb.98.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakan S., Nobis P. Ultrastructural localization of transcription sites and of RNA distribution during the cell cycle of synchronized CHO cells. Exp Cell Res. 1978 May;113(2):327–337. doi: 10.1016/0014-4827(78)90373-7. [DOI] [PubMed] [Google Scholar]
- Fakan S. Perichromatin fibrils are in situ forms of nascent transcripts. Trends Cell Biol. 1994 Mar;4(3):86–90. doi: 10.1016/0962-8924(94)90180-5. [DOI] [PubMed] [Google Scholar]
- Fakan S., Puvion E. The ultrastructural visualization of nucleolar and extranucleolar RNA synthesis and distribution. Int Rev Cytol. 1980;65:255–299. doi: 10.1016/s0074-7696(08)61962-2. [DOI] [PubMed] [Google Scholar]
- Forrester W., Stutz F., Rosbash M., Wickens M. Defects in mRNA 3'-end formation, transcription initiation, and mRNA transport associated with the yeast mutation prp20: possible coupling of mRNA processing and chromatin structure. Genes Dev. 1992 Oct;6(10):1914–1926. doi: 10.1101/gad.6.10.1914. [DOI] [PubMed] [Google Scholar]
- Fu X. D., Maniatis T. Factor required for mammalian spliceosome assembly is localized to discrete regions in the nucleus. Nature. 1990 Feb 1;343(6257):437–441. doi: 10.1038/343437a0. [DOI] [PubMed] [Google Scholar]
- Görlich D., Panté N., Kutay U., Aebi U., Bischoff F. R. Identification of different roles for RanGDP and RanGTP in nuclear protein import. EMBO J. 1996 Oct 15;15(20):5584–5594. [PMC free article] [PubMed] [Google Scholar]
- Hall A. Ras-related GTPases and the cytoskeleton. Mol Biol Cell. 1992 May;3(5):475–479. doi: 10.1091/mbc.3.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan N. C., Traverse K. L., Sullivan D. E., Pardue M. L. The nucleus-limited Hsr-omega-n transcript is a polyadenylated RNA with a regulated intranuclear turnover. J Cell Biol. 1994 Apr;125(1):21–30. doi: 10.1083/jcb.125.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Deerinck T. J., Ellisman M. H., Spector D. L. In vivo analysis of the stability and transport of nuclear poly(A)+ RNA. J Cell Biol. 1994 Aug;126(4):877–899. doi: 10.1083/jcb.126.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Spector D. L. Dynamic organization of pre-mRNA splicing factors. J Cell Biochem. 1996 Aug;62(2):191–197. doi: 10.1002/(sici)1097-4644(199608)62:2<191::aid-jcb7>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Huang S., Spector D. L. Intron-dependent recruitment of pre-mRNA splicing factors to sites of transcription. J Cell Biol. 1996 May;133(4):719–732. doi: 10.1083/jcb.133.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Spector D. L. Nascent pre-mRNA transcripts are associated with nuclear regions enriched in splicing factors. Genes Dev. 1991 Dec;5(12A):2288–2302. doi: 10.1101/gad.5.12a.2288. [DOI] [PubMed] [Google Scholar]
- Huang S., Spector D. L. U1 and U2 small nuclear RNAs are present in nuclear speckles. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):305–308. doi: 10.1073/pnas.89.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-García L. F., Spector D. L. In vivo evidence that transcription and splicing are coordinated by a recruiting mechanism. Cell. 1993 Apr 9;73(1):47–59. doi: 10.1016/0092-8674(93)90159-n. [DOI] [PubMed] [Google Scholar]
- Kadowaki T., Goldfarb D., Spitz L. M., Tartakoff A. M., Ohno M. Regulation of RNA processing and transport by a nuclear guanine nucleotide release protein and members of the Ras superfamily. EMBO J. 1993 Jul;12(7):2929–2937. doi: 10.1002/j.1460-2075.1993.tb05955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornbluth S., Dasso M., Newport J. Evidence for a dual role for TC4 protein in regulating nuclear structure and cell cycle progression. J Cell Biol. 1994 May;125(4):705–719. doi: 10.1083/jcb.125.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krainer A. R., Maniatis T. Multiple factors including the small nuclear ribonucleoproteins U1 and U2 are necessary for pre-mRNA splicing in vitro. Cell. 1985 Oct;42(3):725–736. doi: 10.1016/0092-8674(85)90269-7. [DOI] [PubMed] [Google Scholar]
- Krainer A. R., Maniatis T., Ruskin B., Green M. R. Normal and mutant human beta-globin pre-mRNAs are faithfully and efficiently spliced in vitro. Cell. 1984 Apr;36(4):993–1005. doi: 10.1016/0092-8674(84)90049-7. [DOI] [PubMed] [Google Scholar]
- Krämer A. The structure and function of proteins involved in mammalian pre-mRNA splicing. Annu Rev Biochem. 1996;65:367–409. doi: 10.1146/annurev.bi.65.070196.002055. [DOI] [PubMed] [Google Scholar]
- Martin T. E., Barghusen S. C., Leser G. P., Spear P. G. Redistribution of nuclear ribonucleoprotein antigens during herpes simplex virus infection. J Cell Biol. 1987 Nov;105(5):2069–2082. doi: 10.1083/jcb.105.5.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T., Beach D. Premature initiation of mitosis in yeast lacking RCC1 or an interacting GTPase. Cell. 1991 Jul 26;66(2):347–360. doi: 10.1016/0092-8674(91)90624-8. [DOI] [PubMed] [Google Scholar]
- Mayeda A., Ohshima Y. Short donor site sequences inserted within the intron of beta-globin pre-mRNA serve for splicing in vitro. Mol Cell Biol. 1988 Oct;8(10):4484–4491. doi: 10.1128/mcb.8.10.4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken S., Fong N., Yankulov K., Ballantyne S., Pan G., Greenblatt J., Patterson S. D., Wickens M., Bentley D. L. The C-terminal domain of RNA polymerase II couples mRNA processing to transcription. Nature. 1997 Jan 23;385(6614):357–361. doi: 10.1038/385357a0. [DOI] [PubMed] [Google Scholar]
- Melchior F., Paschal B., Evans J., Gerace L. Inhibition of nuclear protein import by nonhydrolyzable analogues of GTP and identification of the small GTPase Ran/TC4 as an essential transport factor. J Cell Biol. 1993 Dec;123(6 Pt 2):1649–1659. doi: 10.1083/jcb.123.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misteli T., Spector D. L. Serine/threonine phosphatase 1 modulates the subnuclear distribution of pre-mRNA splicing factors. Mol Biol Cell. 1996 Oct;7(10):1559–1572. doi: 10.1091/mbc.7.10.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. S., Blobel G. The GTP-binding protein Ran/TC4 is required for protein import into the nucleus. Nature. 1993 Oct 14;365(6447):661–663. doi: 10.1038/365661a0. [DOI] [PubMed] [Google Scholar]
- Moreno S., Nurse P., Russell P. Regulation of mitosis by cyclic accumulation of p80cdc25 mitotic inducer in fission yeast. Nature. 1990 Apr 5;344(6266):549–552. doi: 10.1038/344549a0. [DOI] [PubMed] [Google Scholar]
- Nishimoto T., Eilen E., Basilico C. Premature of chromosome condensation in a ts DNA- mutant of BHK cells. Cell. 1978 Oct;15(2):475–483. doi: 10.1016/0092-8674(78)90017-x. [DOI] [PubMed] [Google Scholar]
- Nishitani H., Ohtsubo M., Yamashita K., Iida H., Pines J., Yasudo H., Shibata Y., Hunter T., Nishimoto T. Loss of RCC1, a nuclear DNA-binding protein, uncouples the completion of DNA replication from the activation of cdc2 protein kinase and mitosis. EMBO J. 1991 Jun;10(6):1555–1564. doi: 10.1002/j.1460-2075.1991.tb07675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe R. T., Mayeda A., Sadowski C. L., Krainer A. R., Spector D. L. Disruption of pre-mRNA splicing in vivo results in reorganization of splicing factors. J Cell Biol. 1994 Feb;124(3):249–260. doi: 10.1083/jcb.124.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs R. L., Lischwe M. A., Spohn W. H., Busch H. Fibrillarin: a new protein of the nucleolus identified by autoimmune sera. Biol Cell. 1985;54(2):123–133. doi: 10.1111/j.1768-322x.1985.tb00387.x. [DOI] [PubMed] [Google Scholar]
- Phelan A., Carmo-Fonseca M., McLaughlan J., Lamond A. I., Clements J. B. A herpes simplex virus type 1 immediate-early gene product, IE63, regulates small nuclear ribonucleoprotein distribution. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9056–9060. doi: 10.1073/pnas.90.19.9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J., Hunter T. Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell. 1989 Sep 8;58(5):833–846. doi: 10.1016/0092-8674(89)90936-7. [DOI] [PubMed] [Google Scholar]
- Saitoh H., Cooke C. A., Burgess W. H., Earnshaw W. C., Dasso M. Direct and indirect association of the small GTPase ran with nuclear pore proteins and soluble transport factors: studies in Xenopus laevis egg extracts. Mol Biol Cell. 1996 Sep;7(9):1319–1334. doi: 10.1091/mbc.7.9.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh H., Dasso M. The RCC1 protein interacts with Ran, RanBP1, hsc70, and a 340-kDa protein in Xenopus extracts. J Biol Chem. 1995 May 5;270(18):10658–10663. doi: 10.1074/jbc.270.18.10658. [DOI] [PubMed] [Google Scholar]
- Sazer S., Nurse P. A fission yeast RCC1-related protein is required for the mitosis to interphase transition. EMBO J. 1994 Feb 1;13(3):606–615. doi: 10.1002/j.1460-2075.1994.tb06298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenstedt G., Saavedra C., Loeb J. D., Cole C. N., Silver P. A. The GTP-bound form of the yeast Ran/TC4 homologue blocks nuclear protein import and appearance of poly(A)+ RNA in the cytoplasm. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):225–229. doi: 10.1073/pnas.92.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. L., Fu X. D., Maniatis T. Associations between distinct pre-mRNA splicing components and the cell nucleus. EMBO J. 1991 Nov;10(11):3467–3481. doi: 10.1002/j.1460-2075.1991.tb04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. L. Higher order nuclear organization: three-dimensional distribution of small nuclear ribonucleoprotein particles. Proc Natl Acad Sci U S A. 1990 Jan;87(1):147–151. doi: 10.1073/pnas.87.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. L. Macromolecular domains within the cell nucleus. Annu Rev Cell Biol. 1993;9:265–315. doi: 10.1146/annurev.cb.09.110193.001405. [DOI] [PubMed] [Google Scholar]
- Spector D. L., O'Keefe R. T., Jiménez-García L. F. Dynamics of transcription and pre-mRNA splicing within the mammalian cell nucleus. Cold Spring Harb Symp Quant Biol. 1993;58:799–805. doi: 10.1101/sqb.1993.058.01.087. [DOI] [PubMed] [Google Scholar]
- Tachibana T., Imamoto N., Seino H., Nishimoto T., Yoneda Y. Loss of RCC1 leads to suppression of nuclear protein import in living cells. J Biol Chem. 1994 Oct 7;269(40):24542–24545. [PubMed] [Google Scholar]
- Turner B. M., Franchi L. Identification of protein antigens associated with the nuclear matrix and with clusters of interchromatin granules in both interphase and mitotic cells. J Cell Sci. 1987 Mar;87(Pt 2):269–282. doi: 10.1242/jcs.87.2.269. [DOI] [PubMed] [Google Scholar]
- Tyc K., Steitz J. A. U3, U8 and U13 comprise a new class of mammalian snRNPs localized in the cell nucleolus. EMBO J. 1989 Oct;8(10):3113–3119. doi: 10.1002/j.1460-2075.1989.tb08463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S., Sekiguchi T., Nishitani H., Miyauchi K., Ohtsubo M., Nishimoto T. Premature chromosome condensation is induced by a point mutation in the hamster RCC1 gene. Mol Cell Biol. 1990 Feb;10(2):577–584. doi: 10.1128/mcb.10.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y., Johnson C. V., Dobner P. R., Lawrence J. B. Higher level organization of individual gene transcription and RNA splicing. Science. 1993 Feb 26;259(5099):1326–1330. doi: 10.1126/science.8446901. [DOI] [PubMed] [Google Scholar]
- Xing Y., Lawrence J. B. Nuclear RNA tracks: structural basis for transcription and splicing? Trends Cell Biol. 1993 Oct;3(10):346–353. doi: 10.1016/0962-8924(93)90105-a. [DOI] [PubMed] [Google Scholar]
- Yokoyama N., Hayashi N., Seki T., Panté N., Ohba T., Nishii K., Kuma K., Hayashida T., Miyata T., Aebi U. A giant nucleopore protein that binds Ran/TC4. Nature. 1995 Jul 13;376(6536):184–188. doi: 10.1038/376184a0. [DOI] [PubMed] [Google Scholar]