Abstract
Age is a consistent predictor of poor outcome following traumatic brain injury (TBI). Although the elderly population has one of the highest rates of TBI-related hospitalization and death, few preclinical studies have attempted to model and treat TBI in the aged population. Recent studies have indicated that nicotinamide (NAM), a soluble B-group vitamin, improved functional recovery in experimental models of TBI in young animals. The purpose of the present study was to examine the preclinical efficacy of NAM in middle-aged rats. Groups of middle-aged (14-month-old) rats were assigned to NAM (500 mg/kg or 50 mg/kg) or saline alone (1 mL/kg) treatment conditions, and received unilateral cortical contusion injuries (CCI) and injections at 1 h and 24 h following injury. The animals were tested on a variety of tasks to assess vestibulomotor (tapered beam) and cognitive performance (reference and working memory in the Morris water maze), and were evaluated for lesion size, blood–brain barrier compromise, astrocytic activation, and edema formation. In summary, the preclinical efficacy of NAM as a treatment following CCI in middle-aged rats differs from that previously documented in younger rats; while treatment with 50 mg/kg NAM appeared to have no effect, the 500-mg/kg dose worsened performance in middle-aged animals. Histological indicators demonstrated more nuanced group differences, indicating that NAM may positively impact some of the cellular cascades following injury, but were not substantial enough to improve functional recovery. These findings emphasize the need to examine potential treatments for TBI utilizing non-standard populations, and may explain why so many treatments have failed in clinical trials.
Key words: aging, CCI, nicotinamide, recovery of function, therapy, traumatic brain injury
Introduction
The National Institutes of Health (NIH) has stated that traumatic brain injury (TBI) is among the leading causes of acute and chronic disability in the United States. Each year, 1.7 million Americans endure a TBI, and 50,000 die (Centers for Disease Control and Prevention, 2010). Thus brain injury in the U.S. warrants investigation, because it is both a health concern that lacks an effective treatment, and it represents increasingly larger expenditures by the American public. In addition, individuals more than 65 years of age have higher rates of hospitalization and mortality than any other age group (Rutland-Brown et al., 2006). The overall incidence of TBI per 100,000 individuals is 60.6; however, the rate for individuals over 65 years of age is 155.9 (Coronado et al., 2005). One major oversight in the field of TBI research is the paucity of preclinical studies that utilize rodent subjects outside of the normal 3- to 6-month age range.
Research in preclinical studies of TBI indicates that age at the time of injury is relevant to the severity of the injury and the subsequent recovery, with increased age related to heightened injury severity and attenuated functional recovery. Aged and middle-aged animals showed greater tissue damage following injury than their younger counterparts (Hoane et al., 2004b; Shao et al., 2006). Other studies point to an increased vulnerability to and/or decreased endogenous protection against free radical damage following injury (Barnes, 1998; Sandhu and Kaur, 2002; Shao et al., 2006). Age-related changes in glial cells, such as astrocytes and microglia, have likewise been implicated in worsened functioning following TBI (Badan et al., 2003; Onyszchuk et al., 2008, 2009).
Nicotinamide (NAM), a broad-spectrum neuroprotectant, is a soluble B-group vitamin that has been successfully tested in preclinical models of TBI to improve functional recovery following injury. Administration of NAM is a potentially effective treatment because it protects against the neurotoxic effects of poly ADP-ribose polymerase (PARP), it is a precursor of a cellular energy source (NAD), and it is a potent free radical scavenger (Yang et al., 2002). A recent mechanistic review by Maiese and associates noted that the beneficial mechanistic effects of NAM occur through numerous pathways (Maiese et al., 2009). NAM is an antioxidant, it can block proinflammatory cytokines, and it supports cellular energy sources through its promotion of NAD synthesis. Additionally, a review on the relationship between NAM and aging has begun to document this relationship (Xu and Sauve, 2010).
Much of the work in our laboratory has demonstrated that treatment with NAM in young rats (3–6 months of age at the time of injury) significantly reduced injury volume, decreased glial fibrillary acidic protein (GFAP) activation, reduced blood–brain barrier (BBB) breach, reduced acute edema formation, improved motor performance, and generally reduced behavioral impairments and improved outcomes following cortical contusion injury (CCI) and fluid percussion injury (FPI; Hoane et al., 2003, 2006a, 2006b, 2006c, 2008a, 2008b; Holland et al., 2008; Goffus et al., 2010; Quigley et al., 2009). Direct comparisons between a 50-mg/kg and a 500-mg/kg dosing regimen has resulted in some minor dose differences, but in general both dosing regimens have been shown to be effective in facilitating recovery of function following FPI (Hoane et al., 2006c). It has also been shown that NAM treatment (50 and 500 mg/kg), administered at 15 min and 20 h post-FPI, reduced apoptosis at both 24 h and 7 days (Holland et al., 2008). Cortical tissue loss was attenuated with NAM administration, but only beginning at the 7-day time point. In relation to injured controls, we saw a time-dependent modulation of astrocytic activation in the cortex surrounding the injury cavity in NAM-treated rats. Initially NAM reduced GFAP at the 24 h time point following injury, but significantly increased reactivity after 7 days. More importantly, we have also shown that the window of opportunity in young animals for the 50-mg/kg dose is between 6 and 24 h post-CCI, depending on task and treatment regimen (Hoane et al., 2008a, 2008b). Thus there is substantial preclinical evidence in young animals (3–6 months) that NAM significantly improves functional recovery and reduces many pathophysiological variables following TBI. However, it has yet to be determined if NAM will have these same beneficial effects in middle-aged rats.
Given the extensive number of preclinical studies examining treatment efficacy for TBI, it is extremely surprising that few studies have examined a subject sample other than young (3-to 6-month-old) rats. A recent review on experimental TBI and aging has started to draw attention to this issue (Cekic and Stein, 2010). A search of the TBI literature suggests that there are fewer than five studies that have examined novel treatments in middle-aged or aged rats. Thus the primary objective of this study was to examine the therapeutic effects of NAM in a middle-aged sample of Sprague-Dawley rats. The animals were evaluated in a behavioral study to examine recovery of function post-TBI, as well as at acute time points post-TBI to examine several measures of pathophysiology. It was hypothesized that middle-aged animals treated with 50 or 500 mg/kg NAM would perform better than saline-treated injured controls on all measures of performance and injury severity, because this is what has been observed in younger rats.
Methods
Subjects
Male Sprague-Dawley rats (Harlan, Indianapolis, IN) at 14 months of age at the time of injury (mean = 560.86 g, SEM =10.34 g) were used in this study. All animal and surgical procedures were performed as described in the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. The Southern Illinois University Institutional Animal Care and Use Committee reviewed and approved all experimental procedures. Before and after injury, the animals were housed in a university-maintained, Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) accredited vivarium, with a 12-h light/dark schedule, and a controlled environmental temperature of 22°C, in standard housing cages with food and water available ad libitum. A power analysis performed based on the experimental design indicated that groups of 10 animals each result in a power score > 0.85, and was more than adequate to detect significant differences between groups in the behavioral studies. For the anatomical studies groups of 5 animals each result in a power score > 0.85. Specific group sizes for both the behavioral and anatomical experiments are shown in Table 1.
Table 1.
Group Sizes for Each Portion of This Study
| Behavioral study | Acute anatomical study | Edema study | |
|---|---|---|---|
| 500 mg/kg NAM | 8 | 6 | 7 |
| 50 mg/kg NAM | 8 | 6 | 8 |
| Saline-treated | 6 | 6 | 7 |
| Sham-injured | 7 | 5 | 6 |
NAM, nicotinamide.
Surgery
All surgeries were performed under aseptic conditions. The CCI model utilized in the present study was based on previous studies (Hoane et al., 2008b; Quigley et al., 2009). The animals were anesthetized using a mixture of isofluorane (2–4%) and oxygen (0.8 L/min). When the animal became unresponsive (no ocular or pedal reflexes) the head was shaved and scrubbed with 70% alcohol followed by povidone-iodine and placed in a stereotaxic device. A midline incision was made in the skin as well as through the underlying fascia. A circular craniotomy (5.0 mm) was centered 2.4 mm posterior to and 2.4 mm lateral (left) of the bregma. The contusion injury was created with a sterile stainless steel impactor tip (4.0 mm in diameter) that was attached to the Benchmark™ stereotaxic impactor (www.myneurolab.com, Richmond, IL). The injury was induced with an impact speed of 3.0 m/sec and an impact depth of 2.5 mm. The impact tip maintained contact with the brain tissue for 0.5 sec before retraction. To maintain normal body temperature (37°C) during surgery and recovery, the rats were placed on a warm water bed and pump system (EZ Anesthesia, Palmer, PA). Rats receiving sham surgeries underwent identical surgical preparation as injured animals, and received craniotomies, were sutured, and were then transferred to recovery.
Drug administration
Each animal received a two-dose regimen of NAM or saline vehicle administered by injection (at 1 h and 24 h) post-CCI based on previous studies (Hoane et al., 2006c; Holland et al., 2008). The animals were randomly assigned to one of four groups: (a) sham-injured vehicle (0.9% saline 1 mL/kg); (b) CCI-injured vehicle (0.9% saline 1.0 mL/kg); (c) CCI-injured low-dose NAM (50 mg/kg NAM); and (d) CCI-injured high-dose NAM (500 mg/kg NAM). Group assignment was not disclosed until all behavioral testing and histological analyses were completed.
Motor assessment
All animals in the behavioral study were trained on the tapered beam walk task, a measure of vestibulomotor function and motor coordination, 3 days before injury (Hoane et al., 2007; Schallert and Woodlee, 2005). Each animal received two trials on the tapered beam walk task, and three trials were allotted to animals that needed more than two instances of prodding to complete the task in a timely manner. The animals were trained to walk down a 165 cm long, tapering width beam (7.60 cm at the start, and then progressively narrows to a width of 2.54 cm at the end) with a 2 cm ledge. The beam is tapered in width and gets progressively more difficult to more concisely delineate motor ability. The animal's total right hindlimb faults (when the foot stepped off the beam and onto the ledge) and total steps were counted, and eventually evaluated as the percentage of total steps that were faults. The behavior was recorded by video camera and stored for later evaluation. Individuals completing the behavioral task and scoring the tape were blinded to the animal's group assignment. Inter-rater reliability for the scoring of the recorded behavior was also evaluated.
Morris water maze
Animals in the behavioral portion of this study were also tested on the Morris water maze (MWM). The MWM has been widely utilized to assess cognitive performance following TBI (Hoane et al., 2003, 2006c; Lindner et al., 1998). The apparatus consists of a circular, 120 cm diameter blue plastic pool partially filled with water (22°C) to a depth of approximately 41 cm. A clear plastic platform (10 × 10 cm) was submerged approximately 2 cm below the surface in the northeast quadrant of the pool for the entirety of the testing sessions. The animal's progress on the task was evaluated by a video camera affixed above the pool, and the data were processed using the MWM-specific computer software SMART (San Diego Instruments, San Diego, CA).
The animals were assessed on the acquisition of a reference memory task beginning on day 15 post-CCI. On every testing day, each animal received four trials during which it could attempt to locate the submerged platform in the pool, starting at one of four release points (north, south, east, and west quadrants) in random order. In this reference memory task, the platform remained in the same place for each trial on every testing day. The trial was terminated when the rat reached the submerged platform in the northeast quadrant or when 90 sec had elapsed. If the rat did not find the platform within the 90 sec, it was gently guided to the platform and remained on it for at least 10 sec. Each rat remained on the platform for 10 sec, after which it was placed in a warm holding cage for at least 15 min before the next trial.
Additionally, all animals were tested on a working memory task in the MWM. The platform was submerged at the center of a new, randomly chosen quadrant each day for four testing days. Each rat was given four trials per day, starting from one of four randomly selected release points (the inter-trial interval was 15 min). The first trial on each of these four days was considered an information trial and was not included in subsequent analyses. Each trial was ended when the animal located the platform or when 90 sec had elapsed, whichever came soonest. Each animal's performance was evaluated as the distance traveled to the submerged platform or in search of it before 90 sec had elapsed.
Histology
At 28 days post-CCI, the rats in the behavioral portion of the study were euthanized with urethane (3.0 g/kg, 0.5 g/mL IP), and transcardially perfused with 0.9% phosphate-buffered saline (PBS), followed by 10% phosphate-buffered formalin (PBF). Rats in the acute anatomical portion of this study were euthanized 25 h following injury. The brains were post-fixed in PBF following removal from the cranium. A 30% sucrose solution was used to cryopreserve the brains 3 days prior to frozen sectioning. Serial coronal sections (40 μm thick) were sliced using a sliding microtome with an electronic freezing stage and collected in phosphate buffer.
Lesion analysis
For both the behavioral study and the anatomical study, a series of sections were mounted on gelatin-subbed microscope slides, stained with cresyl violet, dehydrated, and cover-slipped. The extent of the lesion was analyzed with an Olympus microscope (BX-51) and an Olympus 13.5-megapixel digital camera (DP-70). Images of sections throughout the extent of the injury coordinates were captured using the digital capturing system, and area measures of the lesioned tissue were determined using the ImageTool software package (http://ddsdx.uthscsa.edu/dig/itdesc.html). The Calvalieri method was used to calculate the volumes of the ipsilateral and contralateral cortices (Coggeshall, 1992). Five stereotaxic coordinates throughout the lesion, at approximately −0.40, −1.40, −2.30, −3.30, and −4.30 mm relative to the bregma, were selected for lesion analysis. The number of sections and the section thickness (40 μm) were multiplied by the mean area of the remaining cortex. The extent of cortical injury was measured by calculating the percent reduction in the injured ipsilateral cortex compared to the contralateral cortex at each level using the formula (1 – (ipsi/contra) × 100), and we have reliably shown that this methodology is sensitive enough to detect treatment-induced reductions in injury size (Hoane et al., 2004a, 2006c, 2008b, 2009).
Glial fibrillary acidic protein stain
Three sections evenly spaced throughout the lesion cavity (at −0.40, −2.30, and −4.30 mm, relative to the bregma) were stained for reactive astrocytes. The sections were removed from cryoprotectant, washed with 0.1 M phosphate buffer (PB), and processed for GFAP immunoreactivity using a free floating protocol (Hoane et al., 2003, 2006c; Holland et al., 2008). Tissue sections were incubated with GFAP rabbit anti-cow primary antibody (Z334, diluted 1:3000; Dako North America, Inc., Carpenteria, CA) for 48 h, rinsed in PBS + 0.4 Triton-X (TX) (6 × 5 min), and then incubated with anti-rabbit IgG (BA-1000; Vector Laboratories, Burlingame, CA) for 90 min. The sections were rinsed in PBS + 0.4 TX (6 × 5 min), and then placed in ABC substrate (PK-6100; Vector Laboratories) for 90 min. The sections were rinsed in PBS + 0.4 TX (3 × 5 min), then 0.1 PB (3 × 5 min), and then reacted in a nickel-intensified chromagen solution containing acetate-imidazole buffer, 2.5% nickel ammonium sulfate, 0.05% 3,3′-diaminobenzidine (DAB), and 0.01% H2O2. The tissue sections were mounted on subbed microscope slides, dehydrated, and cover-slipped. The number of GFAP-positive cells surrounding the lesion was determined using cell counts. Two sites from the cortex adjacent to the developing injury cavity, from each of three tissue sections, were viewed with an Olympus video-microscope (BX-51) system at 4× magnification, increased to 40× magnification, and captured. The number of GFAP-positive cells within the field of view (35.29 mm2) was counted based on established methods (Hoane et al., 2003, 2006a). The average number of GFAP-positive cells from the resulting six sites was used for the statistical analysis.
Immunoglobulin G stain
One series of free-floating tissue sections were processed for immunoglobulin G (IgG) immunoreactivity, based on an established protocol (Hoane et al., 2006b). The tissue was rinsed in PBS + 0.2% TX, and incubated with rabbit anti-rat IgG (diluted 1:500; Vector Laboratories) for 1 h. The tissue was then rinsed in PB (3 × 5 min). The tissue sections were incubated with ABC substrate (PK-6100; Vector Laboratories) for 1 h, and were then rinsed in PBS (3 × 5 min). The tissue was visualized with nickel-intensified DAB in a solution containing acetate imidazole buffer, 2.5% nickel sulfate, 0.05% DAB, and 0.01% H2O2. The tissue was rinsed in PBS (2 × 5 min), and then dehydrated in a series of graded alcohols and xylene. Finally, the tissue was mounted onto slides, cover-slipped, and allowed to dry completely. Three sections evenly spaced throughout the lesion cavity (at −0.40, −2.30, and −4.30 mm relative to the bregma) were evaluated for each animal using ImageTool software. The area of IgG infiltration into the tissue was measured. Inter-rater reliability for the scoring of this measure was also evaluated.
Edema analysis
Edema formation was assessed by measuring the water content of the tissue at the site of the injury in separate groups of animals (Hoane et al., 2006a). At 25 h post-injury, the animals were sacrificed using a CO2 overdose, and their brains were rapidly harvested. The 4.0-mm coronal slab containing the injured cortex was placed on a cold plate. Tissue punches (4.0 mm) were made with a steel biopsy punch (Roboz Instruments, Gaithersburg, MD) through the injured cortex or equivalent tissue, ipsilateral cortex, or adjacent caudal cortex, and placed into pre-weighed 1.5 mL tubes. The tubes were then reweighed, and while uncapped, placed in an oven at 65°C for 48 h to dry. The tubes were then removed from the oven and weighed. The percent water content was determined with: [(wet – dry)/wet] * 100.
Data analysis
Behavioral data were analyzed using a mixed model factorial analysis of variance (ANOVA), or one-way between-subjects ANOVA. Treatment was the between factor, and if applicable, day of testing was the within-group factor (SPSS v. 15 for Windows; SPSS Inc., Chicago, IL). The between factor was treatment (500 mg/kg NAM-treated, 50 mg/kg NAM-treated, saline, and sham-injured), and the within-group factor was day of testing. Both the main effects and the interaction effects were considered. Huynh-Feldt corrections and Tukey's honestly significant difference test (Tukey's HSD) were used to control for Type I error in the repeated-measures and post-hoc means comparison, respectively. A significance level of p ≤ 0.05 was used for all statistical analyses. Inter-rater reliability measures were evaluated using the bivariate correlation of scores obtained between the two behavioral raters. Two animals in the saline-treated injured group were excluded from the study due to injury complications and were not replaced.
In the anatomical portion of the study, a one-way ANOVA was completed for which the between-subject factor of treatment (14-month-old 500 mg/kg NAM-treated, 50 mg/kg NAM-treated, saline, and sham-injured) was evaluated. Tukey's HSD was used to control for Type I error, and a significance level of p ≤ 0.05 was used for all statistical analyses. Inter-rater reliability measures were evaluated using the bivariate correlation of scores obtained between the two raters.
Results
Behavioral study: Tapered beam walk task
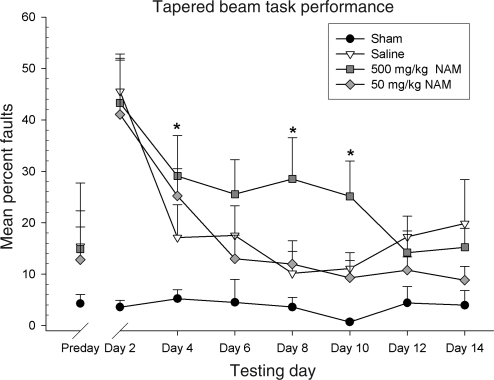
The animal's total right hindlimb faults and total steps were counted and evaluated as a percentage of total steps that were faults on the beam. To examine group differences, performance was evaluated using a repeated-measures ANOVA, with the within-subjects factor of day (2, 4, 6, 8, 10, 12, and 14), and the between-subjects factor of treatment (500 mg/kg NAM, 50 mg/kg NAM, saline, and sham). The interaction of day × treatment was significant [F(11.78,98.32) = 2.21, p = 0.02]. Both the main effect of day [F(3.93,98.32) = 12.40, p < 0.01] and treatment [F(3, 25) = 6.26, p < 0.01] were significant. Further analysis indicated that the 500 mg/kg NAM-treated group performed significantly worse than the saline-treated group on testing day 4 [F(3,25) = 3.20, p = 0.04], day 8 [F(3,25) = 3.90, p = 0.02], and day 10 [F(3,25) = 5.48, p < 0.01]. Inter-rater reliability for the scoring of the recorded behavior was r = 0.98, p < 0.01. Administration of the 50-mg/kg dose of NAM failed to improve recovery on this task compared to the saline-treated group (p < 0.05). Performance on this task is shown in Figure 1.
FIG. 1.
Administration of nicotinamide (NAM) did not improve recovery on the tapered beam walk task in 14-month-old animals. The graph shows plotted mean (±standard error of the mean) percentages of steps that were faults. On days 4, 8, and 10, the 500 mg/kg NAM-treated group faulted significantly more than the saline-treated group. Treatment with 500 mg/kg NAM following injury in 14-month-old animals worsened functional recovery following injury (*p < 0.05), whereas treatment with 50 mg/kg failed to improve recovery.
Behavioral study: Morris water maze reference memory task
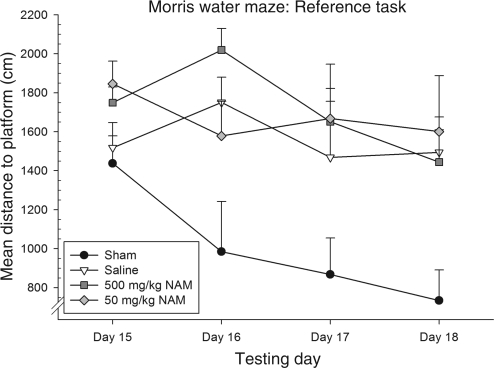
The distance traveled (cm) to the platform was averaged over the four trials for each testing day. To examine group differences, performance was evaluated using a repeated-measures ANOVA, with the within-subjects factor of day (15, 16, 17, and 18), and the between-subjects factor of treatment (500 mg/kg NAM, 50 mg/kg NAM, saline, and sham). The interaction of day × treatment was not significant [F(9,75) = 1.71, p = 0.10]. Both the main effect of day [F(3,98.32) = 4.74, p < .01], and treatment [F(3,25) = 4.76, p < 0.01] were significant. Both the NAM-treated groups were not significantly different from the saline-treated group on any of the testing days.
Further analysis revealed that on day 16, both the saline and the 500 mg/kg NAM-treated groups were significantly more impaired on this task than the sham-injured group (p < 0.04 and p < 0.01, respectively, by Tukey's HSD). NAM administration following TBI not only failed to improve functional recovery, but appears to worsen it on this task at the higher dose. Figure 2 presents performance on this task.
FIG. 2.
Administration of nicotinamide (NAM) did not improve reference memory performance in the Morris water maze (MWM) in 14-month-old animals. The graph shows plotted mean (±standard error of the mean) distances swam to the platform. On day 16, the 500 mg/kg NAM-treated group performed significantly worse than the sham-injured saline-treated group. Trends in performance appear to indicate that performance on this task is sometimes impaired following NAM administration in injured, 14-month-old animals at the higher dose, and ineffective at the lower dose.
Behavioral study: Morris water maze working memory task
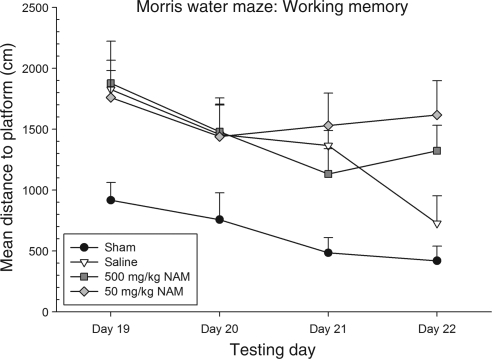
The distance traveled (cm) to the platform was averaged over the last three of the four trials for each testing day. To examine group differences, performance was evaluated using a repeated-measures ANOVA, with the within-subjects factor of day (19, 20, 21, and 22), and the between-subjects factor of treatment (500 mg/kg NAM, 50 mg/kg NAM, saline, and sham). The interaction of day × treatment was not significant [F(9,75) = 1.74, p = 0.09]. Both the main effect of day [F(3,75) = 10.15, p < 0.01], and treatment [F(3,25) = 4.98, p < 0.01] were significant. Neither of the NAM-treated groups performed significantly different from the saline-treated group on any of the testing days.
Significant group differences, however, were found among the treatment groups on this task. It was shown that on day 19, the 500-mg/kg NAM-treated group performed significantly worse than the sham-injured group (p = 0.04 by Tukey's HSD). Both the 50-mg/kg NAM-treated group and the saline-treated group performed significantly more poorly than the sham-injured group on day 21 (p < 0.01 and p = 0.04, respectively, by Tukey's HSD). Lastly, on day 22, both the 500 mg/kg and the 50-mg/kg NAM-treated groups performed significantly worse than the sham-injured group (p = 0.04 and p < 0.01, respectively, by Tukey's HSD). Again, treatment with NAM following TBI appears to have deleterious effects on this cognitive task. Performance on this task is shown in Figure 3.
FIG. 3.
Administration of nicotinamide (NAM) did not improve working memory performance in the Morris water maze (MWM). The graph shows plotted mean (±standard error of the mean) distance swam to the platform. On day 19, the 500 mg/kg NAM-treated group performed significantly worse than the sham-injured group. Both the 500 mg/kg NAM-treated and the saline-treated injured groups performed significantly worse than the sham-injured group on day 21. On day 22, both the NAM-treated injured groups performed significantly worse than the sham-injured group, but the saline-treated group did not.
Behavioral study: Cresyl violet lesion analysis
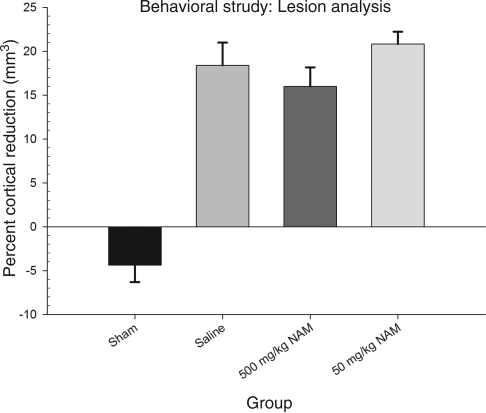
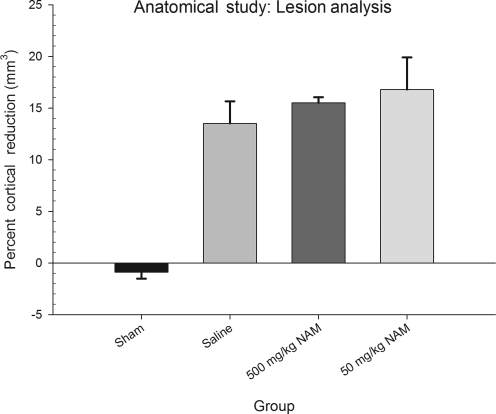
To examine group differences, percent cortical reduction was evaluated using a one-way ANOVA, with treatment (500 mg/kg NAM, 50 mg/kg NAM, saline, and sham) as the between-subjects factor. The overall analysis was statistically significant [F(3,25) = 32.23, p < 0.01]. Neither of the NAM-treated groups differed significantly from the saline-treated injured group (p > 0.05). Inter-rater reliability was r = 0.91. The results of the lesion analysis for all the groups are shown in Figure 4, and histology plates show the extent of the injury at the center of the impact (Fig. 5).
FIG. 4.
Administration of nicotinamide (NAM) did not reduce injury size. Analysis of the percent reduction in injured cortical volume compared to the contralateral cortex is shown. The graph shows plotted mean (±standard error of the mean) percent reductions in cortical volume for each group. Although the injured groups differed significantly from the sham controls, there were no significant differences between the injured groups.
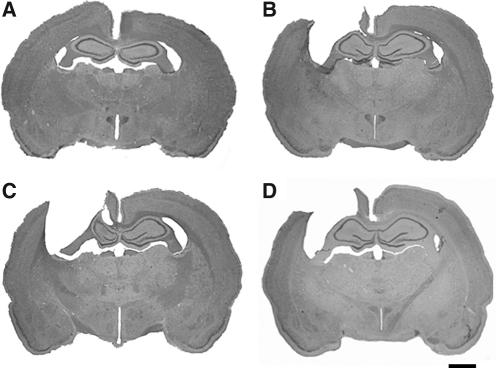
FIG. 5.
Shown are coronal brain sections (40 μm, cresyl violet stain) at the center of the injury cavity (−2.30 mm relative to the bregma) for the different experimental groups. Images shown are representative images from each group: (A) sham, (B) saline, (C) 50 mg/kg NAM, and (D) 500 mg/kg NAM (scale bar = 2.0 mm; NAM, nicotinamide).
Anatomical study: Cresyl violet lesion analysis
Groups in the acute anatomical study were examined for the reduction of cortical volume, with group (500 mg/kg NAM, 50 mg/kg NAM, saline, and sham) as the between-subjects factor. The analysis revealed that there was a statistically significant effect of group [F(3,19) = 15.13, p < 0.01]. Each of the injured groups demonstrated significantly larger lesion cavities than the sham-injured group, but none of the groups differed significantly from one another (p > 0.05). Inter-rater reliability was r = 0.95. The results of the lesion analysis for all the groups are shown in Figure 6.
FIG. 6.
Administration of nicotinamide (NAM) did not reduce injury size at acute time points. Analysis of the percent reduction in injured cortical volume compared to the contralateral cortex is shown. The graph shows plotted mean (±standard error of the mean) percent reductions in cortical volume for each group. The analysis revealed no statistically significant differences between any of the injured groups in lesion cavity formation.
Anatomical study: Glial fibrillary acidic protein stain analysis
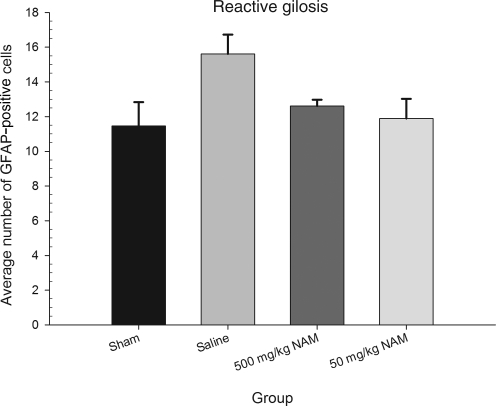
The average number of GFAP-positive cells was evaluated using a one-way ANOVA, with group (500 mg/kg NAM, 50 mg/kg NAM, saline, and sham-injured) as the between-subjects factor. The analysis revealed that there was a statistically significant effect of group [F(3,10) = 3.81, p < 0.05]. The results of the lesion analysis for all the groups are shown in Figure 7. Additionally, GFAP-positive activation of the sham-injured group was significantly lower than in the saline-treated group. Interestingly, neither of the NAM-treated groups differed significantly from the 14-month-old sham-injured group.
FIG. 7.
Administration of nicotinamide (NAM) did not significantly reduce reactive gliosis following injury. The graph shows the mean (±standard error of the mean) number of activated astrocytes in cortical areas around the injury cavity. Although administration of NAM reduced glial fibrillary acidic protein (GFAP) expression to some degree compared to saline-treated animals, this effect was not statistically significant.
Anatomical study: Immunoglobulin G stain analysis
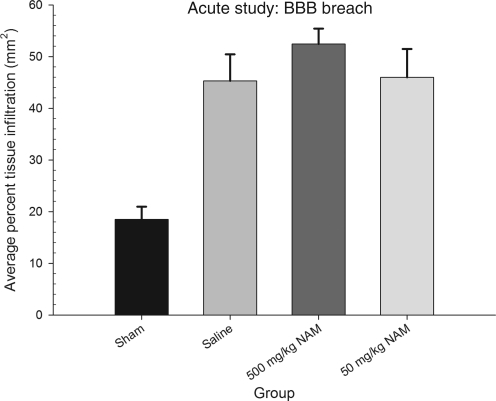
The percent surface area darkened by IgG infiltration was measured for each section, and then averaged over the three sections to indicate the average percent BBB compromise for each animal. The one-way ANOVA revealed that there was a statistically significant effect of treatment [F(3,11) = 14.12, p < 0.01]. Again, the percent BBB breach in each of the injured groups differed from that of the sham-injured group, but none significantly differed from one another (p > 0.05). Inter-rater reliability was r = .95. Figure 8 displays the results of this analysis.
FIG. 8.
Administration of nicotinamide (NAM) did not reduce BBB breech. The graph shows mean (±standard error of the mean) percent IgG-positive tissue in the whole section. All injured groups showed significantly more staining than the sham-injured group. However, there were no statistically significant differences between the injured groups.
Edema analysis
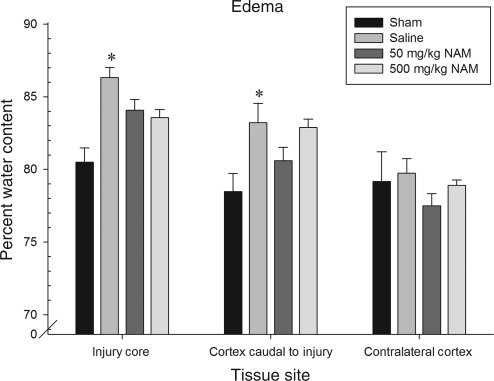
The groups were examined for percent water content to assess post-injury swelling. Three tissue sites were examined: the injury site, contralateral to the injury site, and caudal adjacent cortex. To examine group differences, each tissue site was evaluated using a one-way ANOVA, with the between-subjects factor of treatment (500 mg/kg NAM, 50 mg/kg NAM, saline, and sham). The analysis revealed that there was a statistically significant effect of treatment in edema formation both at the injury site [F(3,24) = 6.16, p < 0.01], and in the ipsilateral caudal cortex adjacent to the injury site [F(3,24) = 4.11, p = 0.02]. Independent-samples t-tests revealed that both the 500 mg/kg and 50 mg/kg NAM-treated groups had significantly less swelling than the saline-treated group (p = 0.05 and p = 0.02, respectively). Additionally, in the tissue sample adjacent to the injury site, independent samples t-tests revealed that only the saline- and 500-mg/kg NAM-treated groups had significantly more edema than the sham-injured group. The 500 mg/kg NAM-treated group also had significantly more edema formation than the 50-mg/kg NAM-treated group (p > 0.05). The results of the edema analysis for all groups are shown in Figure 9.
FIG. 9.
Administration of nicotinamide (NAM) did reduce edema following injury in some situations. The graph shows mean (±standard error of the mean) percent water content for the tissue, with higher water content indicating more swelling. At the injury site, both NAM-treated groups had significantly less swelling than the saline-treated group. In the cortex adjacent to the injury, only the saline- and 500-mg/kg NAM-treated groups demonstrated significantly more swelling than the sham-injured group and the 50-mg/kg group. Additionally, the 500 mg/kg NAM-treated group had significantly more swelling than the 50 mg/kg NAM-treated group (*p < 0.05).
Discussion
The primary goal of this study was to examine the preclinical efficacy of NAM therapy in a middle-aged sample of rats. In the present study, unlike previous work with NAM in young animals (Goffus et al., 2010; Hoane et al., 2003, 2006c, 2008a, 2008b; Quigley et al., 2009), middle-aged (14-month-old) animals did not demonstrate enhanced functional recovery on behavioral indices of recovery. Performance of the 50-mg/kg NAM-treated group on both the tapered beam walk task and the MWM tasks did not demonstrate enhanced functional improvement over time, performing much like the saline-treated animals. Likewise, the 500 mg/kg NAM-treated group did not demonstrate improvement in functional recovery, and at times performed worse than the saline-treated animals on the same behavioral measures. Although these differences were not uniformly evident, the trend appeared repeatedly over the differing behavioral evaluations. On several of the behavioral testing days, the NAM-treated groups performed significantly worse than sham-injured animals, even when the saline-treated group did not. These findings indicate that NAM-administration following TBI provides little preclinical efficacy, and may actually make recovery slightly worse in middle-aged animals. These behavioral effects are in stark contrast to all of the studies we have performed testing various dosing regimens of NAM in young samples (3–5 months of age) of rats, with either the CCI or FPI models (Goffus et al., 2010; Hoane et al., 2003, 2006a, 2006b, 2006c, 2008a, 2008b; Holland et al., 2008; Quigley et al., 2009). It is assumed that these effects will only get worse as the age of the subjects increase at the time of injury. Although the focus of this study was not on examining the effect of the aging process on NAM treatment, that does not preclude the fact that this is an interesting and understudied question. Currently we are performing a longitudinal study to examine the effect of the aging process on recovery of function and NAM therapy. Animals were injured and treated with a regimen of NAM starting at 4 months of age, and their recovery of function is being assessed throughout their lifespan. Thus the implications of this study suggest that much more work needs to be done in the field of aging and TBI.
Histological and pathophysiological measures of injury severity examined in the present study impart important findings relevant to the preclinical efficacy of NAM in a middle-aged sample of rats. Lesion cavity size was not significantly different among the injured groups in either the behavioral and anatomical studies; thus treatment with NAM failed to reduce the extent of injury in the middle-aged rats, unlike what we have repeatedly seen in young rats (Goffus et al., 2010; Hoane et al., 2003, 2006c, 2008a, 2008b; Holland et al., 2008). Likewise, BBB breach following CCI was also not significantly reduced by NAM treatment in middle-aged rats, which has been shown to occur in younger animals (Hoane et al., 2006b). Cortical levels of reactive astrocytes were reduced with NAM treatment following CCI; however, this reduction was not statistically significant. All three of these pathophysiological markers have been shown to be improved with NAM treatment in young rats following CCI or FPI (Goffus et al., 2010; Hoane et al., 2003, 2006b, 2006c, 2008a; Holland et al., 2008). Thus it appears that the worsened behavioral outcome evidenced in the behavioral study with NAM treatment may be associated with the inability of NAM to offset standard pathophysiological markers post-injury in middle-aged rats.
The edema evaluation revealed more positive findings regarding NAM therapy. At the injury core, both groups treated with NAM had significantly less swelling than the saline-treated group. This finding supports previous work in our laboratory in young animals that demonstrated the ability of NAM to reduce edema post-CCI (Hoane et al., 2006a). However, further away from the injury core, this effect was diminished; only the 50 mg/kg NAM-treated group demonstrated a significant reduction in water content compared to the saline-treated group and the 500 mg/kg group. In this same region, the 500 mg/kg NAM-treated group had significantly more swelling than the 50 mg/kg group. A beneficial effect in edema reduction was observed with the 50 mg/kg NAM-treated group, suggesting that some beneficial efficacy may remain in aged animals. In one regard, the beneficial effects of NAM on edema in the present study are the only effects seen in these 14-month-old rats that replicate our findings in younger samples. However, our previous finding was with the 500 mg/kg dose in young rats (Hoane et al., 2006a), and this particular dose in 14-month-old rats was significantly worse than the lower 50 mg/kg dose, when assessed outside of the injury core. Thus the mechanisms by which NAM may reduce post-injury edema still appear to be partially intact in the aged animals; however, it appears to be dose-dependent and did not result in behavioral sparing or improvement.
As highlighted in a recent review by Maiese and colleagues, the multitude of cellular effects that NAM exerts on the body makes it difficult to elucidate exactly which are responsible for the behavioral effects of the vitamin (Maiese et al., 2009). As an antioxidant, NAM may exert both protective and destructive effects on cellular death in an injury state. Likewise, NAM's effects on the PARP system, which utilizes the energy source NAD+ to repair breaks in DNA fragments, appear to be twofold: while NAM supports PARP activity by providing it with a precursor of its energy source, research also indicates that heightened PARP activity may endanger cell survival because of the depletion of NAD+. Consistent with some findings here, NAM can block a multitude of proinflammatory cytokines that become upregulated following injury, reducing tissue swelling. As noted, NAM has incredible potential to prevent cell injury and promote cell recovery in numerous conditions and diseases, but these effects are sensitive to each of the pathways through which the vitamin exerts its effects (Maiese and Chong, 2003; Maiese et al., 2009). Notably, NAM is also a sirtuin inhibitor, and its effects with the mammalian sirtuin receptor (SIRT1) are linked to cellular metabolism as well as cell longevity and survival (Chong and Maiese, 2008; Maiese et al., 2009). Age at the time of administration is another variable that must be considered relevant to NAM's effectiveness.
Although the point of this study was not to determine the mechanisms behind the paradoxical effects found in the current study, some explanation may exist in the literature. In a review on the actions of NAM during cell life and longevity, Li and associates (2006) summarized that “one must approach therapy with nicotinamide with caution, since nicotinamide may have detrimental effects with cellular aging that appear to be concentration dependent,” which was first stated in an earlier publication (Chong et al., 2005). The review by Li and colleagues (2006) does a nice job describing the potential mechanisms by which NAM in the aged brain may lead to problems, such as increased apoptosis and sirtuin inhibition, and that NAM appears unable to prevent cellular injury in states of intracellular acidification. Thus the dose-dependent behavioral effects seen in the present study appear to support the cellular/molecular effects of NAM described above. In addition, it is not known if the bioavailability of NAM changes in middle-aged rats. It is also likely that the half-life of NAM is different in middle-aged rats than in younger rats, although there are no current data on NAM's half-life in the literature. There are also possibly numerous differences in gene expression related to NAM therapy in middle-aged versus young rats that may account for the observed discrepancies in treatment efficacy. More research is needed to understand and counteract this effect. It is possible that low-dose NAM as one arm of polytherapy may be the best approach to treatment of TBI in the aging brain.
The behavioral performance of the aged animals on the tasks evaluated here is consistent with previous findings from our laboratory. It has been shown that although the 14-month-old injured animals showed improvement on the MWM task, their improvement was less robust than that noted in younger animals (Hoane et al., 2004b). Several studies using middle-aged rats (12–16 months of age) have demonstrated impairments on sensorimotor and cognitive evaluations, including several employed in this study (Barnes, 1998; Frick et al., 1995; Hamm et al., 1991, 1992; Hoane et al., 2004b; Lindner et al., 1999; Unterberg et al., 1994). However, none of these studies evaluated preclinical efficacy.
Given that there is an extensive literature examining preclinical efficacy in rodent models of TBI, it is surprising that only a handful of studies have used anything but young animals. The epidemiology of human TBI would suggest that this is problematic. Work by Stein and colleagues examining the preclinical efficacy of progesterone indicate that its effectiveness is maintained in both male and female aged subjects (Cutler et al., 2007; Katsuri and Stein, 2009). Much like the effects noted in young adult rats, treatment with progesterone following injury attenuated edema, reduced apoptosis, and improved locomotor performance in aged animals (Cutler et al., 2007; Katsuri and Stein, 2009). Interestingly, in a recent study, it has been shown that experimentally-induced vitamin D3 deficiency reduces the effectiveness of progesterone in aged rats (20 months old; Cekic and Stein, 2010). The combination of vitamin D3 and progesterone has been shown to enhance cell survival following excitotoxic insult (Atif et al., 2009). As such, it appears that the preclinical efficacy of each potential drug therapy must be investigated in both young and aged samples. It is likely that a drug's mechanism of action may dictate its ability to be efficacious in a multitude of age groups.
In summary, the effects of age following TBI need to be more thoroughly investigated in order to both effectively reduce injury severity, as well as to design therapeutic interventions that will address the specific mechanisms of interest in an effective fashion. In order to do so, it is imperative that preclinical evaluations of TBI need to include more aged samples of animals, in order to understand the way in which the phenomenon of aging impacts the preclinical efficacy of drugs aimed at treating TBI. It is not enough to simply study the effects of age on TBI; instead novel therapies must be screened in specific age categories. Given the incidence of TBI in the aged population, much more research is needed in this area of study.
Acknowledgments
Funding provided by NIH grant NS045647.
Author Disclosure Statement
No competing financial interests exist.
References
- Atif F. Sayeed I. Ishrat T. Stein D.G. Progesterone with vitamin D affords better neuroprotection against excitotoxicity in cultured neurons than progesterone alone. Mol. Med. 2009;15:328–336. doi: 10.2119/molmed.2009.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badan I. Buchhold B. Hamm A. Gratz M. Walker L. Platt D. Kessler C. Popa-Wagner K. Accelerated glial reactivity to stroke in aged rats correlates with reduced functional recovery. J. Cereb. Blood Flow Metab. 2003;23:845–854. doi: 10.1097/01.WCB.0000071883.63724.A7. [DOI] [PubMed] [Google Scholar]
- Barnes C.A. Aging and the physiology of spatial memory. Neurobiol. Aging. 1998:563–568. doi: 10.1016/s0197-4580(88)80114-3. [DOI] [PubMed] [Google Scholar]
- Cekic M. Stein D.G. Traumatic brain injury and aging: Is a combination of progesterone and vitamin D hormone a simple solution to a complex problem? Neurotherapeutics. 2010;7:81–90. doi: 10.1016/j.nurt.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Injury Prevention and Control: Traumatic Brain Injury. 2010. Sep 22, 2010. http://www.cdc.gov/traumaticbraininjury/statistics.html http://www.cdc.gov/traumaticbraininjury/statistics.html Retrieved.
- Chong Z.Z. Maiese K. Enhanced tolerance against early and late apoptotic oxidative stress in mammalian neurons through nicotinamidase and sirtuin mediated pathways. Curr. Neurovasc. Res. 2008;5:159–170. doi: 10.2174/156720208785425666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong Z.Z. Lin S.H. Li F. Maiese K. The sirtuin inhibitor nicotinamide enhances neuronal cell survival during acute anoxic injury through AKT, BAD, PARP, and mitochondrial associated “anti-apoptotic” pathways. Curr. Neurovasc. Res. 2005;2:271–285. doi: 10.2174/156720205774322584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall R.E. A consideration of neural counting methods. Trends Neurosci. 1992;15:9–13. doi: 10.1016/0166-2236(92)90339-a. [DOI] [PubMed] [Google Scholar]
- Coronado V.G. Thomas K.E. Sattin R.W. Johnson R.L. The CDC traumatic brain injury surveillance system: Characteristics of persons aged 65 years and older hospitalized with a TBI. J. Head Trauma Rehabil. 2005;20:215–228. doi: 10.1097/00001199-200505000-00005. [DOI] [PubMed] [Google Scholar]
- Cutler S.M. Cekic M. Miller D.M. Wali B. Vanlandingham J.W. Stein D.G. Progesterone improves acute recovery after traumatic brain injury in the aged rat. J. Neurotrauma. 2007;24:1475–1486. doi: 10.1089/neu.2007.0294. [DOI] [PubMed] [Google Scholar]
- Frick K.M. Baxter M.G. Markowska A.L. Olton D.S. Price D.L. Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiol. Aging. 1995;16:149–160. doi: 10.1016/0197-4580(94)00155-3. [DOI] [PubMed] [Google Scholar]
- Goffus A.M. Anderson G.D. Hoane M.R. Sustained delivery of nicotinamide limits cortical injury and improves functional recovery following traumatic brain injury. Oxid. Med. Cell Longev. 2010;3:145–152. doi: 10.4161/oxim.3.2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm R.J. Jenkins L.W. Lyeth B.G. White-Gbadebo D. Hayes R.L. The effect of age on outcome following traumatic brain injury in rats. J. Neurosurg. 1991;75:916–921. doi: 10.3171/jns.1991.75.6.0916. [DOI] [PubMed] [Google Scholar]
- Hamm R.J. White-Gbadebo D. Lyeth B.G. Jenkins L.W. Hayes R.L. The effect of age on motor and cognitive deficits after traumatic brain injury in rats. Neurosurgery. 1992;31:1072–1077. doi: 10.1227/00006123-199212000-00013. [DOI] [PubMed] [Google Scholar]
- Hoane M.R. Akstulewicz S.L. Toppen J. Treatment with vitamin B3 improves functional recovery and reduces GFAP expression following traumatic brain injury in the rat. J. Neurotrauma. 2003;20:1189–1198. doi: 10.1089/089771503770802871. [DOI] [PubMed] [Google Scholar]
- Hoane M.R. Becerra G.D. Shank J.E. Tatko L. Pak E.S. Smith M. Murashov A.K. Transplantation of neuronal and glial precursors dramatically improves sensorimotor function but not cognitive function in the traumatically injured brain. J. Neurotrauma. 2004a;21:163–174. doi: 10.1089/089771504322778622. [DOI] [PubMed] [Google Scholar]
- Hoane M.R. Gilbert D.R. Holland M.A. Pierce J.L. Nicotinamide reduces acute cortical neuronal death and edema in the traumatically injured brain. Neurosci. Lett. 2006a;408:35–39. doi: 10.1016/j.neulet.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Hoane M.R. Kaplan S.A. Ellis A.L. The effects of nicotinamide on apoptosis and blood-brain barrier breakdown following traumatic brain injury. Brain Res. 2006b;1125:185–193. doi: 10.1016/j.brainres.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Hoane M.R. Kaufman N.A. Vitek M.P. Mckenna S.E. COG1410 improves cognitive performance and reduces cortical neuronal loss in the traumatically injured brain. J. Neurotrauma. 2009;26:1–10. doi: 10.1089/neu.2008.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoane M.R. Lasley L.A. Akstulewicz S.L. Middle age increases tissue vulnerability and impairs sensorimotor and cognitive recovery following traumatic brain injury in the rat. Behav. Brain Res. 2004b;153:189–197. doi: 10.1016/j.bbr.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Hoane M.R. Pierce J.L. Holland M.A. Anderson G.D. Nicotinamide treatment induces behavioral recovery when administered up to 4 hours following cortical contusion injury in the rat. Neuroscience. 2008a;154:861–868. doi: 10.1016/j.neuroscience.2008.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoane M.R. Pierce J.L. Holland M.A. Birky N.D. Dang T. Vitek M.P. Mckenna S.E. The novel apolipoprotein E-based peptide COG1410 improves sensorimotor performance and reduces injury magnitude following cortical contusion injury. J. Neurotrauma. 2007;24:1108–1118. doi: 10.1089/neu.2006.0254. [DOI] [PubMed] [Google Scholar]
- Hoane M.R. Pierce J.L. Kaufman N.A. Beare J.E. Variation in chronic nicotinamide treatment after traumatic brain injury can alter components of functional recovery independent of histological damage. Oxid. Med. Cell Longev. 2008b;1:46–53. doi: 10.4161/oxim.1.1.6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoane M.R. Tan A.A. Pierce J.L. Anderson G.D. Smith D.C. Nicotinamide treatment reduces behavioral impairments and provides cortical protection after fluid percussion injury in the rat. J. Neurotrauma. 2006c;23:1535–1548. doi: 10.1089/neu.2006.23.1535. [DOI] [PubMed] [Google Scholar]
- Holland M.A. Tan A.A. Smith D.C. Hoane M.R. Nicotinamide treatment provides acute neuroprotection and GFAP regulation following fluid percussion injury. J. Neurotrauma. 2008;25:140–152. doi: 10.1089/neu.2007.0312. [DOI] [PubMed] [Google Scholar]
- Katsuri S.B. Stein D.G. Progesterone decreases cortical and sub-cortical edema in young and aged ovariectomized rats with brain injury. Restor. Neurol. Neurosci. 2009;27:265–275. doi: 10.3233/RNN-2009-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. Chong Z.Z. Maiese K. Cell life versus cell longevity: the mysteries surrounding the NAD+ precursor nicotinamide. Curr. Med. Chem. 2006;13:883–895. doi: 10.2174/092986706776361058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner M.D. Cain C.K. Plone M.A. Frydel B.R. Blaney T.J. Emerich D.F. Hoane M.R. Incomplete nigrostriatal dopaminergic cell loss and partial reductions in striatal dopamine produce akinesia, rigidity, tremor, and cognitive deficits in middle-aged rats. Behav. Brain Res. 1999;102:1–16. doi: 10.1016/s0166-4328(98)00160-0. [DOI] [PubMed] [Google Scholar]
- Lindner M.D. Plone M.A. Cain C.K. Frydel B.R. Francis J.M. Emerich D.F. Sutton R.L. Dissociable long-term cognitive deficits after frontal versus sensorimotor cortical contusions. J. Neurotrauma. 1998;15:199–216. doi: 10.1089/neu.1998.15.199. [DOI] [PubMed] [Google Scholar]
- Maiese K. Chong Z.Z. Nicotinamide: necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol. Sci. 2003;24:228–232. doi: 10.1016/S0165-6147(03)00078-6. [DOI] [PubMed] [Google Scholar]
- Maiese K. Chong Z.Z. Hou J. Shang Y.C. The vitamin nicotinamide: translating nutrition into clinical care. Molecules. 2009;14:3446–3485. doi: 10.3390/molecules14093446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyszchuk G. He Y. Berman N.E.J. Brooks W.M. Detrimental effects of aging on outcome from traumatic brain injury: A behavioral, magnetic resonance imaging and histological study in mice. J. Neurotrauma. 2008;26:153–171. doi: 10.1089/neu.2007.0430. [DOI] [PubMed] [Google Scholar]
- Onyszchuk G. Levine S.M. Brooks W.M. Berman N.E.J. Post-acute pathological changes in the thalamus and internal capsule in aged mice following controlled cortical impact injury: A magnetic resonance imaging, iron histochemical, and glial immunohistochemical study. Neurosci. Lett. 2009;452:204–208. doi: 10.1016/j.neulet.2009.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley A. Tan A.A. Hoane M.R. The effects of hypertonic saline and nicotinamide on sensorimotor and cognitive function following cortical contusion injury in the rat. Brain Res. 2009;1304:138–148. doi: 10.1016/j.brainres.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutland-Brown W. Langlois J.A. Thomas K.E. Xi Y.L. Incidence of traumatic brain injury in the United States, 2003. J. Head Trauma Rehabil. 2006:456–465. doi: 10.1097/00001199-200611000-00009. [DOI] [PubMed] [Google Scholar]
- Sandhu S.K. Kaur G. Alterations in oxidative stress scavenger system in aging rat brain and lymphocytes. Biogerontology. 2002;3:161–173. doi: 10.1023/a:1015643107449. [DOI] [PubMed] [Google Scholar]
- Schallert T. Woodlee M.T. In: Orienting and Placing. The Behavior of the Laboratory Rat. I.Q. Whishaw., editor; B. Kolb., editor. Oxford University Press; New York: 2005. pp. 129–140. [Google Scholar]
- Shao C. Roberts K.N. Markesbery W.R. Scheff S.W. Lovel M.A. Oxidative stress in head trauma and aging. Free Radic. Biol. Med. 2006:77–85. doi: 10.1016/j.freeradbiomed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Unterberg A. Schneider G.H. Gottschalk J. Lanksch W.R. Development of traumatic brain edema in old versus young rats. Acta Neurochir. (Wien.) 1994;60:431–433. doi: 10.1007/978-3-7091-9334-1_117. [DOI] [PubMed] [Google Scholar]
- Xu P. Sauve A.A. Vitamin B3, the nicotinamide adenine dinucleotides and aging. Mech. Ageing Dev. 2010;131:287–298. doi: 10.1016/j.mad.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Yang J. Klaidman L.K. Nalbandian A. Oliver J. Chang M.L. Chan P.H. Adams J.D., Jr The effects of nicotinamide on energy metabolism following transient focal cerebral ischemia in Wistar rats. Neurosci. Lett. 2002;333:91–94. doi: 10.1016/s0304-3940(02)01005-4. [DOI] [PubMed] [Google Scholar]