Abstract
Outcome of traumatic brain injury (TBI) is impaired by hyperglycemia, hypotension, and glutamate, and improved by insulin. Insulin reduces glutamate concentration, making it uncertain whether its beneficial effect accrues from euglycemia. Glucagon decreases CNS glutamate, lessens neuronal cell injury, and improves neurological scores in mice after TBI. In vitro, glucagon limits NMDA-mediated excitotoxicity by increasing cAMP and protein kinase A (PKA). NMDA receptor activation couples cerebral blood flow (CBF) to metabolism. Dilation induced by NMDA is impaired after fluid percussion brain injury (FPI) due to upregulation of endogenous tPA, which further disturbs cerebral autoregulation during hypotension after fluid percussion injury (FPI). We hypothesized that glucagon prevents impaired NMDA receptor-mediated dilation after FPI by upregulating cAMP, which decreases release of tPA. NMDA-induced pial artery dilation (PAD) was reversed to vasoconstriction after FPI. Glucagon 30 min before or 30 min after FPI blocked NMDA-mediated vasoconstriction and restored the response to vasodilation. PAD during hypotension was blunted after FPI, but protected by glucagon. Glucagon prevented FPI-induced reductions in CSF cAMP, yielding a net increase in cAMP, and blocked FPI-induced elevation of CSF tPA. Co-administration of the PKA antagonist Rp 8Br cAMPs prevented glucagon-mediated preservation of NMDA-mediated dilation after FPI. The pKA agonist Sp 8Br cAMPs prevented impairment of NMDA-induced dilation. These data indicate that glucagon protects against impaired cerebrovasodilation by upregulating cAMP, which decreases release of tPA, suggesting that it may provide neuroprotection when given after TBI, or prior to certain neurosurgical or cardiac interventions in which the incidence of perioperative ischemia is high.
Key words: autoregulation, cerebral circulation, glucagon, NMDA, signal transduction, tPA, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a leading cause of death and morbidity in the U.S. While damage does occur from the primary insult, secondary injury that results from the release of a myriad of substances, such as excitatory amino acids including glutamate, activated oxygen, neurohormones, signaling molecules, and the like, are thought to play a key role in the development of ultimate outcomes. Several additional risk factors can further exacerbate secondary brain damage, such as hypotension, hypoxia, increased intracranial pressure, and hyperglycemia.
Increased plasma glucose levels within the first 24 h of admission following TBI have been associated with poor outcomes (Salim et al., 2009). Thus early therapeutic intervention to lower blood glucose with insulin may improve the clinical outcome of TBI (Liu-DeRyke et al., 2009). Nonetheless, intensive treatment of hyperglycemia with insulin increased the risk of hypoglycemia, but did not affect follow-up mortality rate and neurological outcome compared to patients receiving conventional insulin therapy (Bilotta et al., 2008), suggesting that both hyper- and hypoglycemia are risk factors for secondary injury. Because insulin has other actions, including decreasing levels of circulating neurotoxic amino acids such as glutamate, our recent studies have focused on using other approaches to decrease glutamate.
Glucagon activates gluconeogenesis by inducing the hepatic uptake of gluconeogenic amino acids, including glutamate (Brockman et al., 1975; Nelson and Cox, 2005). Since the blood–brain barrier is often disrupted after TBI, glucagon-mediated reduction in the systemic circulating levels of glutamate may cause similar effects in the CNS (Gottlieb et al., 2003), where glutamate is elevated post-insult (Zhang et al., 2001). We have recently shown that pre-treatment with glucagon decreases CNS glutamate, lessens neuronal cell injury, and improves neurological scores in mice after TBI, notwithstanding causing an increase in blood glucose, as anticipated (Fanne et al., 2010). However, we could not exclude that glucagon provides neuroprotection through additional mechanisms as well.
N-methyl-d-aspartate (NMDA) receptor activation couples CBF to metabolism (Faraci and Heistad, 1998), which is thought to contribute to the outcome of TBI. Cerebrovasodilation induced by NMDA is impaired after fluid percussion brain injury (FPI) due to upregulation of endogenous tissue plasminogen activator (tPA; Armstead et al., 2005). Impaired NMDA-induced dilation contributes to disturbed cerebral autoregulation during hypotension after FPI (Armstead, 2002). Glucagon activates adenylate cyclase, raising cAMP, while in vitro, glucagon-like peptide 2 limits glutamate and NMDA-mediated excitotoxicity via cAMP and protein kinase A (PKA) (Lovshin et al., 2004). We therefore hypothesized that glucagon, administered as either a pre-or post-treatment, will prevent impaired NMDA receptor-mediated dilation after FPI by upregulating PKA, which will prevent a post-traumatic rise in tPA.
Methods
Closed cranial window and brain injury procedures
Newborn pigs (1–5 days old, 1.0–1.4 kg) of both sexes were studied. All protocols were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Animals were anesthetized with isoflurane (1–2 MAC). Anesthesia was maintained with a-chloralose (30–50 mg/kg, supplemented with 5 mg/kg/h IV). Catheters were inserted into both femoral arteries, one to monitor blood pressure and to sample blood gas tensions and pH, and the other to withdraw blood to determine cerebral blood flow (CBF) using radiolabeled microspheres (see below). The trachea was cannulated, and the animals were ventilated with room air. A heating pad was used to maintain the animals at 37−39°C, monitored rectally.
A cranial window was placed in the parietal skull of the anesthetized animals. The closed cranial window technique for measuring pial artery diameter and collection of cerebrospinal fluid (CSF) for ELISA analysis has been previously described (Armstead, 2002; Armstead et al., 2005).
Protocol
Two types of pial vessels, small arteries (resting diameter 120–160 μm), and arterioles (resting diameter 50–70 μm), were examined to determine whether segmental differences in the effects of FPI could be identified. Typically, 2–3 mL of artificial CSF were flushed through the window over a 30-sec period, and excess CSF was allowed to run off through one of the needle ports. For sample collection, 300 μL of the total cranial window volume of 500 μL was collected by slowly infusing artificial CSF into one side of the window and allowing the CSF to drip freely into a collection tube on the opposite side.
CBF was measured in the cerebral cortex using radioactively-labeled microspheres (Armstead et al., 2009). A catheter was placed in the left ventricle via the right carotid artery to inject the microspheres. In piglets, ligation of one carotid artery has no effect on brain blood flow or its distribution (Laptook et al., 1983; Leffler et al., 1985). In addition to these reports, we prepared piglets by placing an injection catheter in the left atrium via a thoracotomy, and a withdrawal catheter in the femoral artery. The animals were ventilated with 10% CO2 to increase blood flow to the brain. Total brain blood flow and the right cerebral:left cerebral flow ratios were determined before and within 5 min after ligation of the right carotid artery. Right carotid ligation had no effect on brain blood flow or hemispheric distribution, similarly to that observed after placement of the left ventricle injection catheter (Leffler et al., 1989).
In the present study, 15-μm microspheres (300,000–800,000 spheres) containing a known amount of radioactivity were injected into the left ventricle. Reference blood was withdrawn from the femoral artery. After each experiment, the pig was sacrificed and the brain removed and weighed. CBF was determined by counting cerebral cortex brain tissue samples in a gamma counter. Brain tissue sample size varied from 100–500 mg; there were at least 400 spheres/1 g of tissue. There was an adequate number of spheres to accurately determine CBF during hypotension, as shown previously (Armstead, 2002). The energy from each nuclide was separated by differential spectroscopy. Up to five different isotope CBF determinations were made in each piglet. Aliquots of the microsphere solutions injected were used for overlap calculations. The count in each milliliter per minute of blood flow was determined by dividing the counts in the reference withdrawal by the rate of reference withdrawal. Thus blood flow can be calculated as Q = C × R × CR−1, where Q is brain blood flow (in mL/min), C is counts per minute (cpm) in the tissue sample, R is the rate of withdrawal of the reference blood sample (in mL/min), and CR is the total counts in the reference blood sample. CBF thus determined reflects flow to the cerebral cortex both ipsilateral and contralateral to the injury site.
The method used to induce brain FPI has been described previously (Wei et al., 1980). A device designed by the Medical College of Virginia was used. A small opening was made in the parietal skull contralateral to the cranial window. A metal shaft was sealed into the opening on top of the intact dura and fluid was coupled to the brain injury device. The intensity of the injury was 1.9–2.2 atm with a constant duration of 19–23 msec.
Fourteen experimental groups were studied (all n = 5/cohort): (1 and 2) sham control pre- and post-treated with vehicle (0.9% saline); (3 and 4) FPI, vehicle pre- and post-treated; (5 and 6) FPI pre- and post-treated with tPA (10−7 M); (7 and 8) FPI pre- and post-treated with the plasminogen activator inhibitor (PAI)-1 inhibitor EEIIMD (1 mg/kg IV); (9 and 10) FPI pre- and post-treated with glucagon (25 μg/kg IV); (11 and 12) FPI pre- and post-treated with the PKA antagonist Rp 8Br cAMPs (10−5 M); and (13 and 14) FPI pre- and post-treated with the PKA agonist Sp 8Br cAMPs (10−5 M). In all groups hypotension was induced by the rapid withdrawal of either 5–8 or 10–15 mL blood/kg to induce moderate or severe hypotension (decreases in mean arterial blood pressure of 25% and 45%, respectively; Armstead, 2002). Such decreases in blood pressure were maintained constant for 10 min by withdrawal or reinfusion of additional blood (Armstead, 2002). Moderate and severe hypotension were induced sequentially in each animal (Armstead, 2002). In sham control animals, responses to hypotension (moderate and severe), NMDA, and papaverine (10−8 and 10−6 M, respectively), were obtained initially, and then again 1 h later in the presence of the agent vehicle. In pre-treated animals undergoing FPI, the drugs were administered 30 min before FPI, and the responses to hypotension, NMDA, and papaverine were obtained at 1 h after injury. Drugs were administered 30 min after FPI in post-treated animals. The order of agonist administration was randomized within animal drug treatment groups.
ELISA
Commercially available ELISA kits were used to quantity CSF cAMP and tPA (Assay Designs, Ann Arbor, MI; Innovative Research and Products, Inc., Stamford, CT) concentrations.
Statistical analysis
Pial artery diameter, CBF, and CSF cAMP and tPA values were analyzed using analysis of variance (ANOVA) for repeated measures. If the value was significant, the data were then analyzed by Fisher's protected least significant difference test. An α level of p < 0.05 was considered significant in all statistical tests. Values are represented as mean ± standard error of the mean (SEM) of the absolute value, or as percentage changes from control values.
Results
Glucagon protects against impairment of NMDA receptor-mediated cerebrovasodilation and cerebral autoregulation during hypotension after brain injury
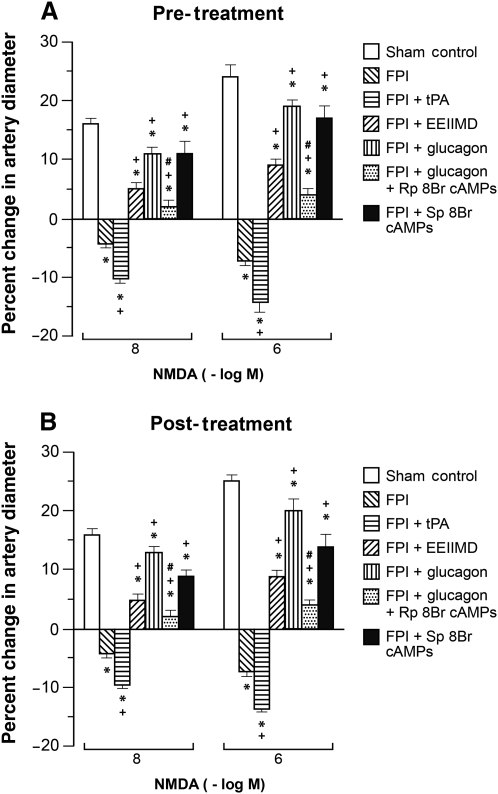
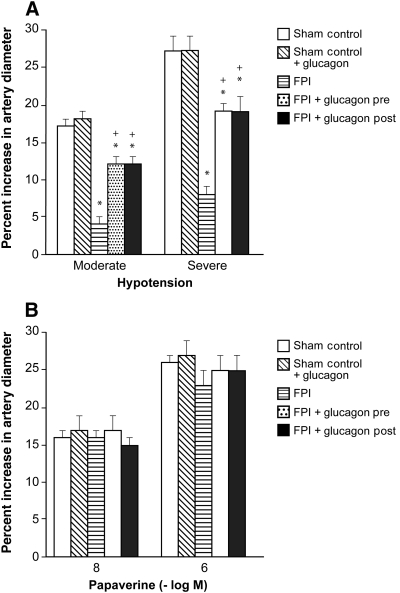
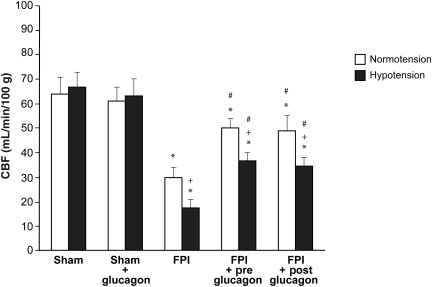
NMDA, glutamate, papaverine (10−8 and 10−6 M), and hypotension (moderate and severe) elicited reproducible pial small artery and arteriole dilation under sham control conditions (data not shown). Vasodilation in response to NMDA and glutamate was reversed to vasoconstriction after FPI in pial small arteries (Fig. 1). Glucagon (25 μg/kg IV) given 30 min before (pre-injury) or 30 min after injury (post-injury) blocked NMDA receptor-mediated vasoconstriction and restored the response to vasodilation (Fig. 1). Pial artery dilation during hypotension was un- affected by glucagon (25 μg/kg IV) in the absence of FPI (Fig. 2). However, glucagon given before or after FPI attenuated loss of pial artery dilation during hypotension (Fig. 2). Papaverine-induced dilation was also unchanged by glucagon in the absence of FPI (Fig. 2). Importantly, papaverine-induced pial artery dilation was unchanged by glucagon pre- and post-FPI (Fig. 2). Similar observations were made for pial arterioles. CBF was similarly unchanged by glucagon in the absence of FPI (Fig. 3). CBF was reduced by FPI and reduced further during hypotension, indicating disturbed cerebral autoregulation, but was significantly preserved/restored by glucagon (Fig. 3).
FIG. 1.
Influence of NMDA (10−8 and 10−6 M) on pial small artery diameter before (sham control) and 1 h after FPI in animals (A) pre- and (B) post-treated (30 min before or 30 min after) with tPA (10−7 M), EEIIMD (1 mg/kg IV), glucagon (25 μg/kg IV), glucagon + Rp 8Br cAMPs (10−5 M), and Sp 8Br cAMPs (10−5 M; n = 5; *p < 0.05 compared to controls; +p < 0.05 compared to FPI non-treated; #p < 0.05 compared to the corresponding glucagon value; NMDA, N-methyl-d-aspartate; FPI, fluid percussion injury; tPA, tissue plasminogen activator).
FIG. 2.
(A) Influence of hypotension and (B) papaverine (10−8 and 10−6 M) on pial small artery diameter before (sham control), and after FPI, in the absence and presence of pre- and post-treatment with glucagon (25 μg/kg IV; n = 5; *p < 0.05 compared to controls; +p < 0.05 compared to the corresponding FPI alone value; FPI, fluid percussion injury).
FIG. 3.
Influence of fluid percussion injury (FPI) on cerebral blood flow (CBF) during normotension and hypotension in the absence and presence of pre- and post-treatment with glucagon (25 μg/kg IV; n = 5; *p < 0.05 compared to the corresponding sham control value; +p < 0.05 compared to the corresponding normotension value; #p < 0.05 compared to the corresponding FPI alone value).
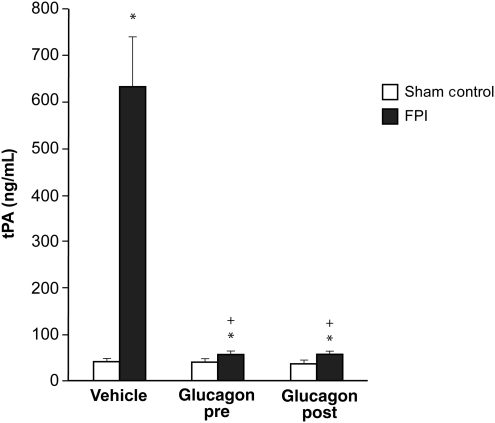
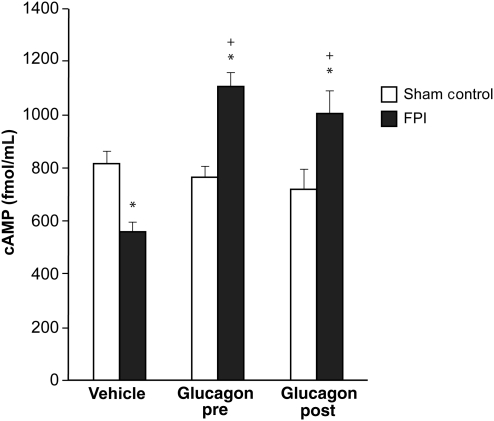
Glucagon blocks FPI-induced elevation of CSF tPA, and prevents FPI-induced reductions in CSF cAMP
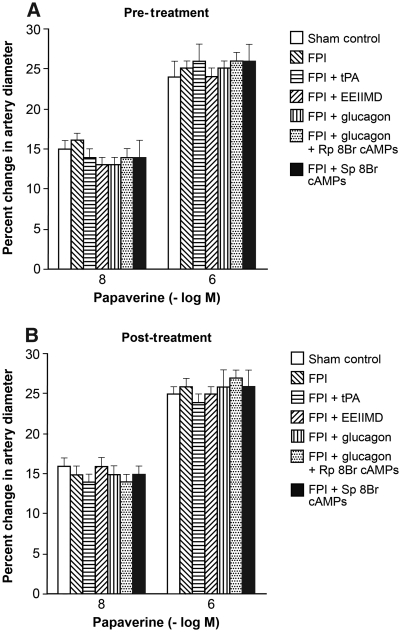
Cortical periarachnoid CSF tPA concentrations were increased within 1 h after FPI. Glucagon (25 μg/kg IV) given 30 min before or 30 min after FPI blocked tPA upregulation post-insult (Fig. 4). Conversely, the reduction in CSF cAMP concentration after FPI was blocked entirely and an increase in CSF cAMP was seen in animals given glucagon pre- and post-FPI (Fig. 5). tPA (10−7M, approximately the concentration observed in CSF after FPI; Armstead et al., 2005; Fig. 1) aggravated NMDA- and glutamate-induced pial artery vasoconstriction after FPI (Fig. 1). EEIIMD, a PAI-1-based antagonist of tPA signaling (1 mg/kg IV), given before or after injury, blocked NMDA- and glutamate-induced vasoconstriction after FPI, and partially restored/preserved dilation in response to these excitatory amino acids (Fig. 1). Co-administration of the PKA antagonist Rp 8Br cAMPs (10−7 M) with glucagon impaired dilation in response to NMDA after FPI compared to glucagon alone, whereas the PKA agonist Sp 8Br cAMPs protected against impairment of NMDA dilation, similarly to the effect of glucagon (Fig. 1). In contrast, responses to papaverine were unchanged after FPI, tPA, EEIIMD, Rp 8Br cAMPs, Sp 8Br cAMPs, and glucagon (Fig. 6).
FIG. 4.
Influence of fluid percussion injury (FPI) on cerebrospinal fluid (CSF) tissue plasminogen activator (tPA) (ng/mL) in vehicle (0.9% saline), and glucagon (0.25 μg/kg IV) pre- and post-treated pigs (n = 5; *p < 0.05 compared to sham controls; +p < 0.05 compared to the corresponding vehicle value).
FIG. 5.
Influence of fluid percussion injury (FPI) on cerebrospinal fluid (CSF) cAMP (fmol/mL) in vehicle (0.9% saline), and glucagon (0.25 μg/kg IV) pre- and post-treated pigs (n = 5; *p < 0.05 compared to sham controls; +p < 0.05 compared to corresponding vehicle animals; cAMP, cyclic adenosine monophosphate).
FIG. 6.
Influence of papaverine (10−8 and 10−6 M) on pial artery diameter before (sham control) and 1 h after FPI in animals (A) pre- and (B) post-treated (30 min before or 30 min after) with tPA (10−7 M), EEIIMD (1 mg/kg IV), glucagon (25 μg/kg IV), glucagon + Rp 8Br cAMPs (10−5 M), and Sp 8Br cAMPs (10−5 M; n = 5; FPI, fluid percussion injury; tPA, tissue plasminogen activator).
Blood chemistry
There were no statistically significant differences in blood chemistry between sham control, FPI, and FPI-drug treated animals during CBF and pial artery diameter measurements of hypotension (Table 1). The amplitude of the pressure pulse, used as an index of injury intensity, was equivalent in FPI-vehicle and FPI-drug treated animals (1.9 ± 0.1 atm).
Table 1.
Blood Chemistry and Arterial Blood Pressure During Normotension and Hypotension
| Normotension | Moderate hypotension | Severe hypotension | |
|---|---|---|---|
| pH | 7.45 ± 0.03 | 7.44 ± 0.04 | 7.43 ± 0.04 |
| Pco2 (mm Hg) | 36 ± 5 | 37 ± 6 | 36 ± 7 |
| Po2 (mm Hg) | 92 ± 10 | 90 ± 10 | 88 ± 11 |
| MAP (mm Hg) | 72 ± 8 | 54 ± 6 | 40 ± 5 |
MAP, mean arterial pressure.
Discussion
Several key new findings emerged from this study. First, it was observed that glucagon given before or after FPI prevented impairment of NMDA receptor- and glutamate-mediated pial artery vasodilation. Cerebrovasodilation induced by NMDA that had been reversed to vasoconstriction by FPI was restored to vasodilation after FPI by glucagon. Second, hypotension-induced pial artery dilation was blunted after FPI, but the impairment was significantly reduced by glucagon. Additionally, CBF was decreased by FPI and decreased further during hypotension, but both brain injury-induced reductions in CBF were also significantly blunted by glucagon. Since pial artery dilation induced by papaverine was unchanged by FPI and glucagon, injury associated impairment of vasodilation was not an epiphenomenon nor nonspecifically altered by glucagon. Administration of glucagon in the absence of injury also had no influence on pial artery dilation induced by hypotension. Additionally, impairment of NMDA-mediated cerebrovasodilation contributes to disturbed cerebral autoregulation during hypotension (Armstead, 2002), thereby indicating the functional pathophysiological significance of impaired excitatory amino acid-mediated vasodilation and its prevention by glucagon after FPI. Because in some cohorts glucagon was administered 30 min after brain injury, results of the present study support our recent observations showing protection with post-injury treatment in the mouse (Fanne et al., 2010). Thus glucagon may provide a novel critical care pathway for the treatment of victims of TBI, but future studies will be needed to determine its therapeutic window in the treatment setting.
Another important result of the present study relates to the mechanism whereby glucagon improved cerebral hemodynamics after FPI. We previously reported an increase in tPA associated with FPI that contributed to reversing NMDA-mediated pial artery dilation to vasoconstriction (Armstead et al., 2005). Results of the present study show that glucagon prevents upregulation of tPA after FPI, indicating that this may serve as one mechanism by which it prevents impairment of NMDA-induced pial artery dilation after injury. EEIIMD, a hexapeptide derivative of the endogenous plasminogen activator inhibitor-1, prevented impairment of NMDA-mediated vasodilation after FPI, consistent with prior studies (Armstead et al., 2005), which provides additional support for the role of tPA as an obligatory intermediary in the impairment of cerebrovascular activity. Since EEIIMD also prevents cerebral hypoperfusion after FPI (Armstead et al., 2009), reversing NMDA-mediated vasodilation to vasoconstriction by tPA is likely to contribute directly to reduced CBF.
A decrease in cAMP concentration in CSF after FPI has been noted previously (Armstead 1997; Atkins et al., 2007). New results in the present study show that glucagon not only blocks the injury-associated decrease in CSF cAMP, but it also increases levels above those observed prior to FPI. Administration of a PKA agonist mimicked the protection of dilation seen in response to NMDA receptor activation observed with glucagon, while a PKA antagonist impaired dilation to NMDA compared to glucagon. Taken together, these data suggest that glucagon protects NMDA dilation after FPI through a cAMP-PKA-dependent mechanism, in addition to blocking upregulation of tPA. We speculate that PKA acts upstream of tPA to prevent its release and/or action, but acknowledge that we cannot exclude a potential downstream role as well. Additional studies will be needed to clarify these sequential relationships.
A link between tPA and glutamatergic neurotransmission has been made, in that kainic acid injection into the hippocampus is associated with cell death in wild-type, but not tPA-null, mice (Tsirka et al., 1995). Other work has indicated that tPA cleaves the NR-1 subunit of NMDA to increase the influx of calcium (Nicole et al., 2001; Wang et al., 1998), though subsequent studies suggest an interaction with the NR2B subunit of NMDA instead (Pawlak et al., 2005). Regardless of the debate as to which subunit or mechanism is involved, it is widely accepted that tPA interacts with glutamate receptors, which are important mediators of excitotoxicity in ischemic CNS disorders.
Activation of NMDA receptors elicits cerebrovasodilation and may represent one of the mechanisms for the coupling of local metabolism to blood flow (Faraci and Heistad, 1998). More recently it was observed that tPA is critical for the full expression of the flow increase evoked by activation of the mouse whisker barrel cortex (Park et al., 2008). Specifically, tPA promoted NO synthesis during NMDA receptor activation by modulating phosphorylation of nNOS (Park et al., 2008). These findings suggest that tPA is a key factor in linking NMDA receptor activation to NO synthesis and functional hyperemia. Our previous studies showed that the PAI-1 derived hexapeptide, EEIIMD, blocked the reversal of NMDA-induced dilation to vasoconstriction, as well as reductions in baseline pial artery diameter after FPI (Armstead, 2002), indicating that the interaction between tPA and NMDA is altered in the setting of TBI. One way in which this relationship may change after brain injury comes from our independent observation that tPA promotes upregulation of the JNK and ERK isoforms of mitogen-activated protein kinase in the presence of brain injury, both of which impair cerebral hemodynamics (Armstead et al., 2010).
In conclusion, our data indicate that glucagon protects against impaired cerebrovasodilation induced by NMDA after FPI by blocking upregulation of endogenous tPA through activation of cAMP protein kinase A-dependent pathways. Since glucagon also helped to maintain autoregulation during hypotension after FPI, these data provide insight into the underlying pathways involved in neuroprotection. These data suggest that administration of glucagon may help provide neuroprotection when given immediately after TBI, or prior to certain neurosurgical or cardiac interventions in which the incidence of perioperative ischemia is high.
Acknowledgments
This research was supported by grants from the National Institutes of Health (NS53410 and HD57355 to W.M.A.; HL76406, CA83121, HL76206, HL07971, and HL81864 to D.B.C.; and HL77760 and HL82545 to A.A.R.H.), the University of Pennsylvania Institute for Translational Medicine and Therapeutics (to D.B.C.), and the Israeli Science Foundation (to A.A.R.H.).
Author Disclosure Statement
No competing financial interests exist.
References
- Armstead W.M. Age dependent NMDA contribution to impaired hypotensive cerebral hemodynamics following brain injury. Develop. Brain Res. 2002;139:19–28. doi: 10.1016/s0165-3806(02)00511-4. [DOI] [PubMed] [Google Scholar]
- Armstead W.M. Cines D.B. Bdeir K. Bdeir Y. Stein S.C. Higazi A.A.R. uPA modulates the age dependent effect of brain injury on cerebral hemodynamics through LRP and ERK MAPK. J. Cereb. Blood Flow Metab. 2009;29:524–533. doi: 10.1038/jcbfm.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstead W.M. Cines D.B. Higazi A.A.R. Plasminogen activators contribute to age dependent impairment of NMDA cerebrovasodilation after brain injury. Develop. Brain Res. 2005;156:139–146. doi: 10.1016/j.devbrainres.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Armstead W.M. Kiessling J.W. Riley J. Cines D.B. Higazi A.A.R. tPA contributes to impaired NMDA cerebrovasodilation after traumatic brain injury through activation of JNK MAPK. Neurological Res. 2010 doi: 10.1179/016164110X12807570509853. DOI 10.1179/016164110X12807570509853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstead W.M. Role of impaired cAMP and calcium-sensitive K channel function in altered cerebral hemodynamics following brain injury. Brain Res. 1997;768:177–184. doi: 10.1016/s0006-8993(97)00641-0. [DOI] [PubMed] [Google Scholar]
- Atkins C.M. Oliva A.A. Alonso O.F. Pearse D.D. Bramlett H.M. Dietrich W.D. Modulation of the cAMP pathway after traumatic brain injury. Exp. Neurology. 2007;208:145–158. doi: 10.1016/j.expneurol.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilotta F. Caramia R. Cernak I. Paolini F.P. Doronzio A. Cuzzone V. Intensive insulin therapy after severe traumatic brain injury: a randomized clinical trial. NeuroCrit. Care. 2008;9:159–166. doi: 10.1007/s12028-008-9084-9. [DOI] [PubMed] [Google Scholar]
- Brockman R.P. Bermann E.N. Joo P.K. Manns J.G. Effects of glucagon and insulin on net hepatic metabolism of glucose precursors in sheep. Am. J. Physiol. 1975;229:1344–1349. doi: 10.1152/ajplegacy.1975.229.5.1344. [DOI] [PubMed] [Google Scholar]
- Fanne R.A. Nassar T. Mazuz A. Waked O. Heyman S.N. Hijazi N. Goelman G. Higazi A.A.R. Neuroprotection by glucagons: role of gluconeogenesis. J. Neurosurg. 2010 doi: 10.3171/2010.4.JNS10263. DOI: 10.3171/2010.4.JNS10263. [DOI] [PubMed] [Google Scholar]
- Faraci F.M. Heistad D.D. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol. Rev. 1998;78:53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- Gottlieb M. Wang Y. Teichberg V.I. Blood-mediated scavenging of cerebrospinal fluid glutamate. J. Neurochem. 2003;87:119–126. doi: 10.1046/j.1471-4159.2003.01972.x. [DOI] [PubMed] [Google Scholar]
- Laptook A.K. Stonestreet B.S. Oh W. The effect of carotid artery ligation on brain blood flow in newborn piglets. Brain Res. 1983;276:51–59. doi: 10.1016/0006-8993(83)90547-4. [DOI] [PubMed] [Google Scholar]
- Leffler C.W. Busija D.W. Fletcher A.M. Beasley D.G. Hessler J.R. Green R.S. Effects of indomethacin upon cerebral hemodynamics of newborn pigs. Pediatr. Res. 1985;19:1160–1164. doi: 10.1203/00006450-198511000-00009. [DOI] [PubMed] [Google Scholar]
- Leffler C.W. Busija D.W. Mirro R. Armstead W.M. Beasely D.G. Effects of ischemia on brain blood flow and oxygen consumption of newborn pigs. Am. J. Physiol. 1989;257:H1917–H1926. doi: 10.1152/ajpheart.1989.257.6.H1917. [DOI] [PubMed] [Google Scholar]
- Liu-DeRyke X. Collingridge D.S. Orme J. Roller D. Zurasky J. Rhoney D.H. Clinical impact of early hyperglycemia during acute phase of traumatic brain injury. NeuroCrit. Care. 2009;11:151–157. doi: 10.1007/s12028-009-9228-6. [DOI] [PubMed] [Google Scholar]
- Lovshin J.A. Huang Q. Seaberg R. Brubaker P.L. Drucker D.J. Extrahypothalamic expression of the glucagon-like peptide-2 receptor is coupled to reduction of glutamate-induced cell death in cultured hippocampal cells. Endocrinology. 2004;145:3495–3506. doi: 10.1210/en.2004-0100. [DOI] [PubMed] [Google Scholar]
- Nelson D. Cox M. Gluconeogenesis. In: A.L. Leninger AL., editor. Principles of Biochemistry. 4th. WH Freeman; New York: 2005. pp. 543–549. [Google Scholar]
- Nicole O. Docagne F. Ali C. Margaill I. Carmeliet P.L. MacKenzie E.T. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor mediated signaling. Nat. Med. 2001;7:59–64. doi: 10.1038/83358. [DOI] [PubMed] [Google Scholar]
- Park L. Gallo E.F. Anrather J. Wang G. Norris E.H. Paul J. Key role of tissue plasminogen activator in neurovascular coupling. Proc. Natl. Acad. Sci. USA. 2008;105:1073–1078. doi: 10.1073/pnas.0708823105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak R. Melchor J.P. Matys T. Skrzpiec A.E. Strickland S. Ethanol-withdrawal seizures are controlled by tissue plasminogen activator via modulation of NR2B-containing NMDA receptors. Proc. Natl. Acad. Sci. 2005;102:443–338. doi: 10.1073/pnas.0406454102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim M. Hadjizacharia P. Dubose J. Brown C. Inaba K. Chan L.S. Persistent hyperglycemia in severe traumatic brain injury: an independent predictor of outcome. Am. Surg. 2009;75:25–29. [PubMed] [Google Scholar]
- Tsirka S.E. Gualandris A. Amaral D.G. Strickland S. Excitotoxin-induced neuronal degeneration and seizure are mediated by tissue plasminogen activator. Nature. 1995;377:340–344. doi: 10.1038/377340a0. [DOI] [PubMed] [Google Scholar]
- Wang F. Tsirka S.E. Strickland S. Stiege P.E. Lipton S.A. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat. Med. 1998;4:228–231. doi: 10.1038/nm0298-228. [DOI] [PubMed] [Google Scholar]
- Wei E.P. Dietrich W.D. Povlischock J.T. Navari R.M. Kontos H.A. Functional, morphological, and metabolic abnormalities of the cerebral microcirculation after concussive brain injury in cats. Circ. Res. 1980;46:37–47. doi: 10.1161/01.res.46.1.37. [DOI] [PubMed] [Google Scholar]
- Zhang H. Zhang X. Zhang T. Chen L. Excitatory amino acids in cerebrospinal fluid of patients with severe head injury. Clin. Chem. 2001;47:1458–1462. [PubMed] [Google Scholar]