Abstract
Neurological dysfunction after traumatic brain injury (TBI) is caused by both the primary injury and a secondary cascade of biochemical and metabolic events. Since TBI can be caused by a variety of mechanisms, numerous models have been developed to facilitate its study. The most prevalent models are controlled cortical impact and fluid percussion injury. Both typically use “sham” (craniotomy alone) animals as controls. However, the sham operation is objectively damaging, and we hypothesized that the craniotomy itself may cause a unique brain injury distinct from the impact injury. To test this hypothesis, 38 adult female rats were assigned to one of three groups: control (anesthesia only); craniotomy performed by manual trephine; or craniotomy performed by electric dental drill. The rats were then subjected to behavioral testing, imaging analysis, and quantification of cortical concentrations of cytokines. Both craniotomy methods generate visible MRI lesions that persist for 14 days. The initial lesion generated by the drill technique is significantly larger than that generated by the trephine. Behavioral data mirrored lesion volume. For example, drill rats have significantly impaired sensory and motor responses compared to trephine or naïve rats. Finally, of the seven tested cytokines, KC-GRO and IFN-γ showed significant increases in both craniotomy models compared to naïve rats. We conclude that the traditional sham operation as a control confers profound proinflammatory, morphological, and behavioral damage, which confounds interpretation of conventional experimental brain injury models. Any experimental design incorporating “sham” procedures should distinguish among sham, experimentally injured, and healthy/naïve animals, to help reduce confounding factors.
Key words: craniotomy, cytokines, magnetic resonance imaging, Neurological Severity Scale, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a leading cause of death and disability in the United States, and is responsible for over $6 billion in healthcare costs annually (Finkelstein et al., 2006). Local to global neurological dysfunction associated with TBI is not only caused by the primary immediate insult, but is also caused by the delayed secondary cascade of biochemical and metabolic pathological events. These include impaired aerobic metabolism, altered calcium homeostasis, disrupted amino acid metabolism, and activated inflammatory responses (Alves et al., 2005; Bartnik et al., 2007; Cole et al., 2010; Deshpande et al., 2008; Faria et al., 2007; He et al., 2004; Lloyd et al., 2008; Scafidi et al., 2009; Sun et al., 2008; Wei et al., 2009; Xing et al., 2009). Since the primary injury is immediate, protective measures, such as prevention by helmets and airbags, are most effective in preventing and/or minimizing injury. The onset of this secondary cascade is thought to be delayed for minutes to hours, and lasts for weeks to months. Thus, the secondary cascade provides a potential window of therapeutic opportunity (Adeleye et al., 2010; Arcure and Harrison, 2009; Xiong et al., 2009; Ziebell and Morganti-Kossmann, 2010). These cascade effects are considered to be mainly due to changes within the brain. However, systemic responses may also play a significant role in the pathophysiology of secondary, evolving TBI.
Traumatic brain injury is not a single entity. Rather it is a disorder caused by multiple mechanisms, including low- and high-velocity penetrating injury, blunt trauma, diffuse axonal injury, and blast. Clinically, traumatic brain injuries are generally complicated, often resulting from several mechanisms. To facilitate pre-clinical study of this complex neurological disorder, multiple injury models have been developed to evaluate individual mechanisms. However, while effective for research focused on isolated cellular mechanisms and pathways, in vitro models are incapable of capturing the in vivo complexity of the multi-cellular local, regional, and global brain networks and systemic responses in a whole animal to TBI. Commonly used TBI in vivo models include blast injury (Saljo et al., 2000), lesion administration (Bederson et al., 1986; Persson, 1976), microknife lesion (Jia et al., 2010), weight drop impact (Valable et al., 2010), fluid percussion injury (FPI; Millen et al., 1985; Povlishock and Kontos, 1985), and the most common method, controlled cortical impact (CCI; Dixon et al., 1991; Lighthall, 1988). Briefly, both CCI and FPI involve exposing the animal skull with a midline incision along the scalp, followed by performing a craniotomy above the region of interest, usually centered 1–3 mm lateral to the midline while the animal is anesthetized. The injury device is then stereotaxically positioned and the exposed brain, covered by dura, is then impacted by either a small metal plunger in the case of CCI (severity determined by plunger velocity, depth of penetration, and duration), or a fluid pressure wave in the case of FPI (severity determined by the height from which the pendulum strikes the fluid-filled cylinder and the fluid pulse generating a controllable, measurable pressure wave). After the injury, the craniotomy bone flap is replaced and sealed with dental acrylic or bone wax, and the cranial defect may be filled with surgical dressing, or the craniotomy may be left unsealed. The scalp incision is then closed with either sutures or surgical staples, and the animal is allowed to recover from anesthesia.
For decades, researchers using CCI or FPI models to study brain injury have employed “sham” procedures as a control. To create a “sham” control, the animals are anesthetized, the craniotomy is performed, and the animal is attached to the injury device without triggering the plunger or fluid pulse. A limited survey of the literature since 1989 examined 50 representative articles studying TBI, using either CCI or FPI models in rats. Of these, 12 (24%) articles referred to “sham” control animals, with no explanation or description of the “sham” procedure. Of the remaining 38 studies, 37 (97.4%; 74% of the total number of surveyed papers) used a craniotomy procedure as the control, with only 2 of the 38 studies (5.3%; 4.0% of the total number of surveyed papers) using an additional control that is less than a craniotomy (i.e., naïve rats).
Interestingly, researchers have reported that craniotomy causes significant damage to the brain in several species. Using rat (Olesen, 1987), frog (Crone and Olesen, 1982), cat (Navari et al, 1978), and rabbit (Edvinsson and West, 1972) models, marked changes in brain microvasculature have been demonstrated following craniotomy alone. However, the researchers were unable to definitively determine the cause and/or extent of the damage. Edvinsson and West (1972) attributed the trephine-induced damage to heat generated during the drilling process. Olesen (1987) acknowledged this concept while also speculating that an additional major component of vascular leakage after craniotomy could be due to an inflammatory response, including cyclo-oxygenase metabolites and histamine release, causing alterations of the blood–brain barrier. Navari and associates (1978) conducted a series of experiments in which atmospheric exposure of the surface of the brain caused by the craniotomy resulted in loss of tissue CO2, which resulted in arteriolar vasoconstriction. Despite these early and persisting reports of craniotomy-induced brain injury, including suggested mechanisms, use of the craniotomy procedure has become widespread as a control in TBI pre-clinical research.
Due to the nature of the craniotomy-induced injury, we hypothesized that the sham procedure, specifically the craniotomy, creates a brain injury that may contribute uniquely to the ultimate end-point. Therefore, we compared the effects of sham procedures to baseline values in naïve (i.e., anesthesia only) rats, focusing on evaluating changes in temporal profiles of brain magnetic resonance imaging (MRI), local cytokine concentration, and behavior (Revised Neurological Severity Scale, or NSS-R).
Methods
Animals
All experiments were performed on adult female (10- to 12-week-old, 150–175 g) Wistar rats obtained from Charles River Laboratory (Raleigh, NC), and all procedures were approved by the Uniformed Services University of the Health Sciences Institution for Animal Care and Use Committee (IACUC), in accordance with international guidelines on the ethical use of animals. The animals were maintained in a 12 h reversed light:dark cycle, with feed and water available ad libitum. The 38 rats were randomly assigned to one of three groups: naïve (control; 10 rats), craniotomy performed with an electric drill (drill; 14 rats), or craniotomy performed with a hand-held trephine (trephine; 14 rats); no group received a brain injury. Within each of the three groups, 6 received behavioral testing and MRI analysis, while the remainder were analyzed for cytokine expression.
Craniotomy procedures
The rats were induced with 4% isoflurane and then maintained with 2% in 98% O2 in a vented anesthesia chamber connected to a scrubber. Once anesthetized, the animals were placed in a stereotactic frame in a prone position, with anesthesia delivered via nosecone. The rats were frequently checked for adequate depth of sedation by absent response to toe-pinch. Throughout the procedure, core body temperature was monitored by a rectal thermistor probe and maintained at 36–38°C on a thermopad. The head was restrained by the stereotactic device, and held in a horizontal plane by placement of blunt-tipped interaural and incisor bars. After shaving the top of the animal's head, the surgical site was sterilized with successive applications of 95% ethanol and povidone-iodine. The following steps were all performed using aseptic techniques. A midline incision on the scalp exposed the skull, without requiring muscle retraction. Craniotomy was performed by either an electric drill bit or hand-held trephine. The electric drill utilized a 0.6 mm drill bit (Stoelting, Wood Dale, IL), attached to a variable speed micromotor drill (Stoelting) to penetrate the skull, and carefully outline a 2.5-mm radius bone flap centered at the bregma + 3.0 mm, lateral left 2.7 mm. Forming a bone flap required several circuits of the outline to penetrate the calvaria. After removal of the bone flap, which was done using forceps, an observer experienced in the technique verified that the brain had not been inadvertently penetrated and no visible damage was present. The bone flap was then replaced and secured with bone wax, after less than 4 min of exposure to light and air. For the trephine method, a 5-mm diameter manual trephine (Roboz Surgical Instrument Co., Gaithersburg, MD) was carefully used to penetrate the skull for removal of the bone flap at the same stereotactic coordinates as above. As with the drill sham, an experienced observer verified the integrity of the procedure prior to the replacement of the trephine bone flap. After drill and trephine shams were generated and bone flaps replaced, the incisions were sutured with 4-0 nylon monofilament (Ethicon, Somerville, NJ). Control group (naïve) animals were anesthetized as described for those receiving craniotomy, but were not placed in the stereotaxic frame, and did not undergo scalp incision or craniotomy. After the procedure, the animals were returned to a recovery cage that was placed on a heating pad, and monitored continually until they were bright, alert, and responsive. The time to recovery was typically less than 10 min, at which time the rats were returned to their home cages. To prevent confounding effects on the behavioral assays being performed within 24 h of the surgery, no analgesics were given.
Magnetic resonance imaging
MR images of naïve animals were collected for baseline comparisons. Imaging of drill sham and trephine sham rat brains were performed at 1, 7, and 14 days post-procedure. The rats were induced and maintained with a 4% isoflurane/96% oxygen mixture in an induction chamber throughout the imaging process. While anesthetized, the animals were placed in a MIF 3T (Intera; Philips Medical System, Netherlands, B.V.) that utilizes a solenoid 4-cm radiofrequency receive-only coil (Philips Research Laboratories, Aachen, Germany) for the rat brain. Throughout the procedure, the rats were kept on a heating pad, with a temperature probe to monitor and maintain body temperature between 36 and 38°C.
The MR pulse sequences were as follows: T2-weighted (T2w) turbo spin echo (TSE) sequence, repetition time (TR)/echo time (TE) = 5220/70 msec, flip angle 90°, turbo spin echo factor 11, number of average (NAV) 10, field of view (FOV) 50 mm, slice thickness 0.5 mm, matrix 224 × 256, reconstructed resolution 100 × 100 μm. An automated 2D region-growing algorithm was used to delineate the margin (border) of the brain (MEDx visualization and analysis software; MEDx Software, Germantown, Maryland), allowing calculation of the brain lesion volume. To determine hemispheric volumes, the brains were graphically bisected along the midline from the superior sagittal sinus through the central vein to the base of the brain on each slice. Lesions were measured by manually drawing a region of interest around the visibly altered area. In cases for which the lesion resulted in the loss of parenchyma, the lesion volume was estimated using the natural border of the hemisphere. For contrast-enhanced MRI studies, gadopentetate dimeglumine (GdDTPA, 0.5 M Magnevist; Bayer Health Care Pharmaceuticals, Pine Brook, NJ), was injected through a lateral tail vein at a dose of 0.3 mL/kg. A post-GdDTPA enhanced T1 spin echo weighted (T1wSE) sequence was performed with TR/TE = 550/8.5 msec, flip angle 90°, NAV 4, FOV 50 mm, slice thickness 0.5 mm × 25–28 sections, and matrix 224 × 256, with a reconstructed in plane resolution 100 × 100 μm.
Cytokines
Homogenates of cortical tissue from naïve, drill, and trephine animals were prepared at days 1 and 7 post-surgery. Gross dissection of tissue approximately 3 × 3 × 1.5 mm in size was obtained from the cortex immediately beneath the site of craniotomy. Tissue was homogenized in T-Per Extraction Buffer (Pierce Biotechnology, Rockford, IL), utilizing a Bioruptor UCD-200 ultrasonic disruptor (Diagenode, Sparta, NJ). Protein concentration of samples was determined using a Bradford Protein Assay Kit (Bio-Rad, Hercules, CA). Cytokine levels were determined using the Multi-Array Rat Cytokine Ultra-Sensitive 7-Plex Assay (Meso Scale Discovery, Gaithersburg, MD), which detects interferon-γ (IFN-γ), interleukin-1β (IL-1β), IL-4, IL-5, IL-13, KC-GRO, and TNF-α, and read on a Sector 6000 Imager (Meso Scale Discovery). It should be noted that due to its C-X-C motif, KC-GRO is actually a chemokine and not a cytokine. Cytokine concentration values were determined in reference to a standard curve for each analyte, and normalized to total protein input to calculate analyte amount (pg cytokine/mg protein).
Revised Neurological Severity Scale
From each group, 6 rats were evaluated with a revised neurological severity scale (NSS-R) supplemented by a rotarod motor skill test. The NSS-R used in this study is an adaptation of several pre-existing NSS tests (Hamm, 2001; Mahmood et al., 2001, 2003; Marti et al., 2005; Shohami et al., 1995). The revised NSS-R consists of 10 tests designed to assay motor, sensory, and reflex skills (Table 1). For a completely normal response on each test, zero points were assigned. For an intermediate response demonstrating moderate impairment, one point was assigned. For a complete lack of ability to perform the test, two points were assigned. Therefore, on the 10-task panel the higher the score, the greater the impairment, with 20 points representing the maximum (i.e., worst) outcome.
Table 1.
The Revised Neurological Severity Scale
| General balance | Ability to balance and walk on a balance beam |
|---|---|
| Landing test | Display of landing reflex |
| Tail raise test | Observation of limb flexion and extension when lifted by the tail |
| Drag test | Resistance to being gently pulled backward |
| Righting reflex | Ability to right its posture after being placed on its back |
| Nose tap reflex | Withdrawal from a gentle tap on the nose |
| Eye blink response | Normal blinking response to eye stimulation |
| Sound reflex | Display of startle and fear posture |
| Tail reflex | Vocalization following gentle pinch of the tail |
| Paw flexion reflex | Hindlimb withdrawal reflex following gentle foot pinch |
Rotarod test
The accelerated rotarod (Med Associates, Inc., St. Albans, VT) test measures the ability to maintain balance by coordinating movement and making postural adjustments (Rahman et al., 1997; Rustay et al., 2003). A single baseline measurement was obtained, with rats in each group pre-trained on this equipment prior to the start of the study. On each assessment day, the rats were tested three times, for a maximum of 5 min each. The speed of rotation increased from 0 revolutions per minute (rpm) to 35 rpm, with a continual acceleration of 6.3 rpm/min. The mean duration that the rats were able to remain on the device was recorded, and the data are expressed in seconds.
For both the NSS-R and the rotarod tests, a baseline value was determined prior to the administration of the sham/control procedure, and then 1, 7, and 14 days post-procedure. Measurements on individual NSS-R tests were independently assigned by two trained observers with an inter-rater reliability of > 0.80. For each parameter being analyzed, the assigned score was averaged between the two observers.
Statistical analysis
Collected data were analyzed via analysis of variance (ANOVA). When normality tests failed, a Kruskal-Wallis ANOVA on ranks was performed. Significance was based on two-tailed tests, with significance set at p < 0.05. When appropriate, repeated-measures ANOVA (rmANOVA) analyses were performed. For post-hoc comparisons, Holm-Sidak tests were used. The effects of surgery, time, and surgery × time interaction were all quantified. Since lesions in 3 of the trephine group rat brains were below the level of detection at the final time point, three different statistical analyses were performed on mean lesion volume data. The first test was an rmANOVA in which the 3 rats with undetectable lesions at day 14 were not included in the analysis at that time point. Additional statistical analyses included an rmANOVA, in which those same 3 rats were assigned a lesion volume of 0 for the final time point, as well as a more conservative non-parametric Mann-Whitney U test utilizing this same dataset. The results from all three statistical analyses were essentially identical. Data analysis was performed at the conclusion of the project, after all results were collected.
Results
Brain morphology changes after sham procedures
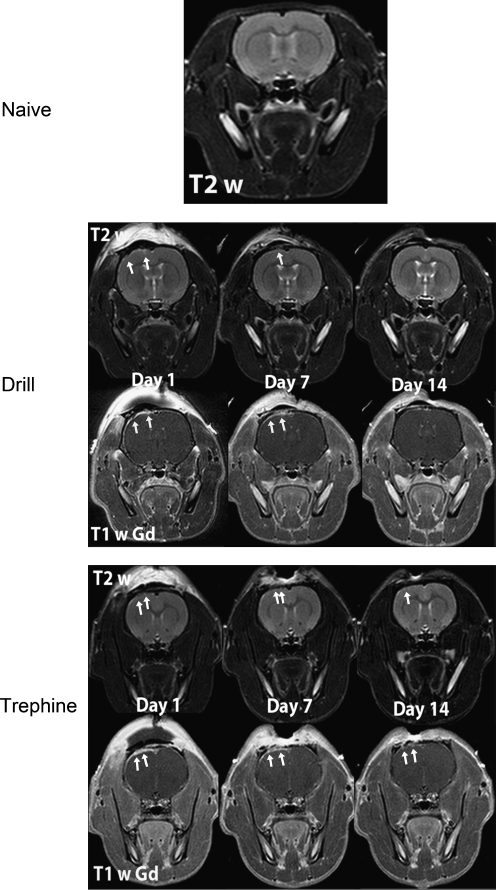
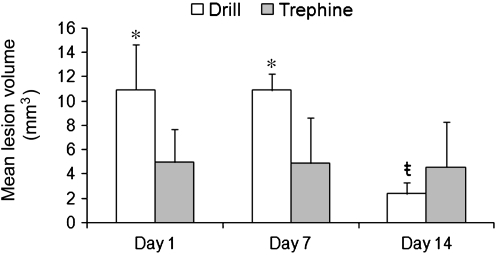
Comparison of both drill and trephine rats to the naïve rats 1 day post-procedure revealed alterations in brain morphology, as determined by MRI images (Fig. 1). Focal dural enhancement underneath the craniotomy was observed in both cohorts of rats on contrast-enhanced MRI. In addition, there were variable degrees of cortical enhancement on post-contrast T1w images in the drill and trephine cohorts over all time points. In T2w images of the drill cohort, 50% of the rats had evidence of subdural and intracerebral bleeding. By comparison, only 33% of the rats in the trephine group had similar findings. These changes persisted across all time points. However, both cohorts of animals exhibited hyperintense regions indicative of edema in the cortex under the craniotomy at each time point. Furthermore, lesion volumes decreased in size by day 14 post-craniotomy (Fig. 2). Importantly, both craniotomy methods generated visible lesions that persisted for 14 days. However, as shown in Figure 2, the initial lesion generated by the drill technique (10.93 ± 3.76 mm3) was significantly larger (p < 0.05) than that generated by the trephine technique (4.97 ± 2.74 mm3), indicating a significantly more severe injury. Interestingly, with both techniques, no difference was observed in lesion volume (p < 0.05) 7 days after craniotomy was performed. Lesions generated by the drill technique showed a significant decrease (p < 0.05) in volume between day 7 (10.91 ±1.35 mm3) and day 14 (2.39 ± 0.99 mm3). By contrast, analysis of mean lesion volume generated using the trephine technique showed no decrease (p < 0.05) between day 7 (4.86 ±3.77 mm3) and day 14 (4.53 ± 3.79 mm3). However, of the trephine rats, 50% (3 of 6) recovered by day 14, such that no lesion was detectable on MRI. By convention, these three rats were removed from the statistical analysis of day 14, rather than assign them a value of zero. The remaining three rats had no change in their lesion volume over time, and therefore the mean value for this group was not changed, despite the reduced number of animals being analyzed.
FIG. 1.
Representative T2w and T1w images post-GdDTPA clearly demonstrate marked damage to the brain following both drill and trephine craniotomies when compared to naïve rat brains. Dural and cortical enhancement was appreciated on T1w post-GdDTPA (arrows), as well as ribbon of enhancement indicative of cerebral edema on T2w images. Images are representative samples from 6 rats from each group (GdDTPA, gadopentetate dimeglumine).
FIG. 2.
Both drill and trephine produced craniotomies causing measurable lesions in the cortex. The lesion produced by the drill was significantly larger than that produced by the trephine. The drill lesion did show significant improvement by day 14 after the craniotomy was performed (all values are mean ± standard error of the mean; n = 6, except for day 14, for which n = 3 for the trephine group; *p < 0.05; ŧ = over time, treatment means differ p < 0.05).
One unexpected result was observed when the volumes of the entire brain, and separate volumes of the ipsilateral and contralateral sides, were determined and compared (Table 2). The ipsilateral side is defined as the side on which the craniotomy was performed, and the contralateral side is the side that did not receive the craniotomy. Neither drill nor trephine craniotomy technique altered the whole brain volume. However, the relative volume of the ipsilateral and contralateral sides, in proportion to total brain volume, was significantly impacted by the technique used to create the craniotomy. On day 1, the ipsilateral side in the drill rats represented 51.12 ± 0.12% of total brain volume, in comparison to 49.98 ± 0.17% seen in the trephine animals. This difference persisted throughout the study (Table 2), as the ipsilateral side remained a significantly (p < 0.05) larger percentage of the total brain volume in the drill condition at every time point. This increase in the ipsilateral hemisphere suggests swelling due to the drill technique, compared to the contralateral hemisphere in the control rats (p < 0.05).
Table 2.
Brain and Lesion Volume
| |
Day 1 |
Day 7 |
Day 14 |
|
|||
|---|---|---|---|---|---|---|---|
| |
Drill |
Trephine |
Drill |
Trephine |
Drill |
Trephine |
Naive |
| mm3 | mm3 | mm3 | mm3 | ||||
| Brain volume | |||||||
| Whole | 651.00 ± 19.60 | 700.5 ± 10.82 | 681.33 ± 17.15 | 673.4 ± 14.47 | 667.33 ± 17.42 | 669.50 ± 13.37 | 684.83 ± 6.98 |
| Ipsilateral | 332.67 ± 9.13 | 350.17 ± 5.96 | 349.83 ± 7.73 | 333.20 ± 8.14 | 338.67 ± 8.92 | 331.50 ± 7.69 | 340.67 ± 3.72 |
| Contralateral | 318.00 ± 10.70* | 350.50 ± 5.10 | 331.33 ± 9.36 | 340.00 ± 6.88 | 328.67 ± 8.63 | 337.83 ± 5.90 | 344.00 ± 3.34 |
| % of total volume | % of total volume | % of total volume | % of total volume | ||||
|---|---|---|---|---|---|---|---|
| Ipsilateral | 51.12 ± 0.23* | 49.98 ± 0.17 | 51.37 ± 0.28* | 49.47 ± 0.36 | 50.75 ± 0.18* | 49.55 ± 0.34 | 49.74 ± 0.09 |
| Contralateral | 48.82 ± 0.24* | 50.04 ± 0.13 | 48.61 ± 0.25* | 50.50 ± 0.37 | 49.50 ± 0.25* | 50.47 ± 0.28 | 50.23 ± 0.08 |
Magnetic resonance imaging revealed significant morphological alterations in both drill and trephine craniotomy techniques when compared to naïve control rats. The whole brain volume was not affected by the craniotomy; however, there was a significant reduction in the volume of the contralateral side of the brain that received a drill craniotomy, when compared to the naïve or control group. When the volume data of the ipsilateral and contralateral halves of the brain were analyzed as percentages of the entire brain volume, the ipsilateral halves in the trephine rats at each time point were significantly enlarged compared to the drill or naïve animals. Trephine rats also had significantly reduced contralateral halves at each time point compared to drill and naïve animals. All values are mean ± standard error of the mean, n = 6.
Means differ from controls at p < 0.05.
Behavioral analyses correspond to the extent of sham injury
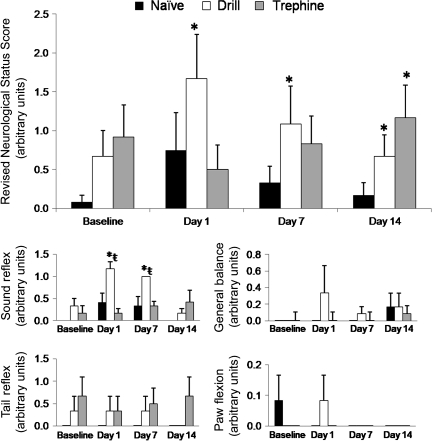
The NSS-R revealed behavior alterations following both craniotomy techniques, despite there being no significant differences in the groups prior to surgery. These alterations did not significantly improve throughout the study, despite a significant improvement in the mean lesion volume between days 7 and 14. The drill technique caused a 250% increase in the NSS-R score between baseline value and the first day post-craniotomy. In addition, the drill rats (1.67 ± 0.57) had a significantly higher NSS-R (p < 0.05), indicative of greater impairment, compared to trephine (0.50 ± 0.32) or naïve animals (0.75 ± 48; Fig. 3). The drill technique resulted in a higher NSS-R (p < 0.05) score that persisted throughout the study when compared to naïve rats. In contrast, the behavioral deficits observed in the trephine group were markedly delayed, not becoming manifest until 2 weeks after craniotomy was performed (Fig. 3).
FIG. 3.
The Revised Neurological Severity Scale (NSS-R) revealed significant sensory and motor behavior impairments in both the drill and trephine rats after the craniotomy. Rats subjected to the drill technique displayed a more immediate response than did the trephine animals. Four components of the NSS-R were analyzed to determine if one particular test was skewing the results; however, with the exception of the test monitoring the sound reflex, none of the individual tasks were affected by the craniotomy (all values are mean ± standard error of the mean, n = 6; *p < 0.05 compared to control animals; ŧ = over time, treatment means differ p < 0.05 compared to baseline values).
To further understand the behavioral changes, the NSS-R was subdivided into its component tests and evaluated (Fig. 3). Although the sound reflex did show significant changes (p < 0.05) in the drill group, both over time and when compared to the naïve and trephine groups, these did not correlate with the amplitude and vector of the overall observed changes in NSS-R scores. Compared to baseline values, the drill rats scored approximately 350% higher on the first day after injury (day 1, 1.17 ± 0.17; baseline, 0.33 ± 0.17). By day 14, this difference had disappeared, although the total NSS-R remained significantly higher in the drill rats at this time. None of the other individual tests revealed significant treatment or time effects (p < 0.05), but instead appeared to be due to incremental shifts on multiple individual tests.
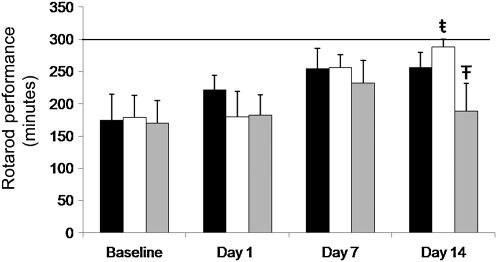
While the naïve rats and trephine rats showed no difference (p > 0.05) between treatments or over time, the drill rats actually showed an improvement in their ability on the rotarod (Fig. 4), and by day 14, remained on the rotarod (287 ± 12.11 sec) significantly longer (p < 0.05) than did the naïve (256.38 ± 23.18 sec) or trephine rats (188.77 ± 43.38 sec). The changes in brain morphology found on MRI and behavioral deficits, in general, demonstrated by the NSS-R indicate that drill craniotomy was more deleterious than trephine and naïve. Therefore, the rotarod results were unique in this regard and unexpected.
FIG. 4.
The ability of the rats to remain on the rotarod was not affected by craniotomy. On day 14 only, the drill rats had a significantly higher rotarod time compared to the trephine rats. Interestingly, by day 14, the drill rats also showed a significant improvement in their ability to remain on the rotarod, compared to their baseline scores. The solid line indicates the maximum possible time on the rotarod (all values are mean ± standard error of the mean, n = 6; ŧ =across time points, means differ p < 0.05; Ŧ = a significant difference, p < 0.05, was observed when comparing trephine to drill rats).
Cytokine and chemokine changes correspond to injury
To better understand the potential mechanisms contributing to anatomical and behavioral changes occurring in both drill and trephine rats, we analyzed the temporal profile of seven inflammatory cytokines in tissue homogenates of cerebral cortex at 1 and 7 days after the sham injury procedure (Table 3).
Table 3.
Temporal Profile of Seven Inflammatory Cytokines in Tissue Homogenates of Cerebral Cortices from Animals in the Study Groups
| |
Day 1 |
Day 7 |
|
||
|---|---|---|---|---|---|
| |
Drill |
Trephine |
Drill |
Trephine |
Naive |
| Cytokine | pg cytokine/mg protein | pg cytokine/mg protein | pg cytokine/mg protein | ||
| KC-GRO | 5.21 ± 2.58* | 19.26 ± 9.30*,ŧ,+ | 1.61 ± 0.47 | 0.85 ± 0.34 | 0.91 ± 0.07 |
| IL-1β | 8.30 ± 3.36 | 18.52 ± 4.15*,ŧ,+ | 6.42 ± 5.75 | 8.98 ± 2.35 | 5.29 ± 1.43 |
| IFN-γ | 0.19 ± 0.06* | 0.34 ± 0.07*,+ | 0.14 ± 0.03 | 0.34 ± 0.08*,+ | 0.17 ± 0.02 |
| TNF-α | 0.78 ± 0.20 | 2.08 ± 0.46 | 0.86 ± 0.27 | 1.30 ± 0.33 | 1.77 ± 0.59 |
| IL-13 | 0.05 ± 0.01 | 0.09 ± 0.01 | 0.03 ± 0.02 | 0.10 ± 0.02*,+ | 0.05 ± 0.01 |
Both techniques caused significant increases in cytokine concentrations in the cortical region directly beneath the craniotomy. Immediately after the craniotomy, both drill and trephine rats had significantly (p < 0.05) higher concentrations of KC-GRO, while the trephine group had significantly elevated IL-1β and IFN-γ compared to naïve animals. In the trephine group, expression of IFN-γ remained elevated 7 days after injury, compared to both naïve and drill rats. Further, in the trephine rats, the concentrations of KC-GRO, IL-1β, and IFN-γ were increased (p < 0.05) at day 1, compared to drill animals, although only IFN-γ remained elevated at day 7 (p < 0.05). All values are mean ± standard error of the mean (n = 4).
Means differ from naïve controls, p < 0.05.
For each individual craniotomy method, means differ from day 1 to day 7, p < 0.05.
Means differ between drill and trephine animals, p < 0.05.
IL-1β, interleukin1β; IFN-γ, interferon-γ; TNF-α, tumor necrosis factor-α; IL-13, interleukin-13.
As shown in Table 3, significantly elevated (p < 0.05) levels of KC-GRO were detected at day 1 in both drill (5.21 ± 2.58 pg cytokine/mg protein) and trephine rats (19.26 ± 9.30 pg cytokine/mg protein) compared to control animals (0.91 ± 0.07 pg cytokine/mg protein). Further, the KC-GRO concentrations in the trephine rats were significantly higher (p < 0.05) than the drill animals at this same time point. KC-GRO levels in the contralateral cortex of both drill and trephine animals were significantly lower (p < 0.05) than cortex on the ipsilateral side, and near levels measured in naïve control samples (data not shown). In both drill and trephine rats, KC-GRO levels did not remain elevated and were no different (p < 0.05) from naïve control values by day 7. A significantly increased (p < 0.01) level of IL-1β was also measured in trephine animals (18.52 ± 4.15 pg cytokine/mg protein) at 1 day after sham procedure compared to control animals (5.29 ± 1.43 pg cytokine/mg protein) and drill animals (8.30 ± 3.36 pg cytokine/mg protein). Similarly to that of KC-GRO, the elevated level of IL-1β in trephine animals (8.98 ± 2.35 pg cytokine/mg protein) returned to levels found in the naïve control group by day 7. IL-1β cytokine levels did not change in the drill group in comparison to naïve controls, at either day 1 (8.30 ± 3.36 pg cytokine/mg protein) or day 7 (6.42 ± 5.75 pg cytokine/mg protein). IFN-γ levels were increased (p < 0.05) in the trephine group at both days 1 and 7 in comparison to the control and drill animals. IL-13 concentrations in the trephine group 7 days (0.10 ± 0.02 pg cytokine/mg protein) after the craniotomy were significantly higher than levels in either naïve (0.05 ± 0.01 pg cytokine/mg protein) or drill (0.03 ± 0.02 pg cytokine/mg protein) rats. In all samples analyzed, IL-4 and IL-5 were below detectable levels (LLOD = 0.958 and 24.1 pg/mL, respectively, data not shown).
Discussion
The key result of this study is that despite the care taken in performing rat craniotomies, the use of both electric drill and manual trephine resulted in quantifiable structural and functional damage to the underlying brain. This occurred despite the fact that the surgeon performing craniotomies has 15 years of experience, and an experienced independent researcher verified that there was no visible dural damage post-procedure. Despite these precautions, both drill and trephine caused morphological, behavioral, and biochemical changes consistent with traumatic brain injury, although the use of the trephine appeared to be the less damaging than the drill. Both the trephine and drill procedures took approximately the same length of time. Therefore, the difference in the severity of the post-surgical effects can be attributed to the physio-mechanical effects of the technique, and is not due to a neuroprotective effect exerted by a longer period under anesthesia in the trephine group.
The most plausible explanation for trauma caused by the craniotomy is the disruption of the extensive network of nerve fibers and blood vessels connecting the lissencephalic rat brain to the skull by removal of the bone flap. Kosaras and associates (2009) recently published an exhaustive description of the innervation and vascularization of the rat calvaria (Kosaras et al., 2009). After years of dedicated work, they described a vast fine mesh, or network, of blood vessels and efferent nerves from the brain innervating the skull bone marrow and emissary canals, but also concentrated in the suture regions allowing nerve access to the scalp. Nerve fibers embedded within and passing through the calvaria appear to be sensory and probably autonomic (sympathetic or parasympathetic nerves). Those transiting the sutures are primarily sensory pain fibers. Disruption of these blood vessels and nerves may well initiate an immediate primary neurovascular injury (by electric drill heat and vibration and/or removing and replacing the bone flap alone), followed by a more severe delayed secondary cascade injury of activated biochemical pathways leading to local cytotoxic edema.
The effects of craniotomy procedures extend beyond the vasculature within the calvaria. Navari and colleagues (1978) compared the effects of a craniotomy alone to a technique in which the bone flap was temporarily replaced with a cranial window, allowing visualization of the cortical surface of the brain without concurrent exposure to the atmosphere (Navari et al., 1978). The researchers were then able to demonstrate that exposure of the cortical surface of the brain to the atmosphere had a significant effect on pial arterial diameter. Interestingly, this effect was observed within minutes of atmospheric exposure. Since the craniotomies used in preparation for injury methods are typically left exposed for several minutes while the device is put in place, the injury administered, and the bone flap replaced, this could have a confounding effect. Even without the injury being administered, there is still sufficient atmospheric exposure to result in altered vascular physiology. Similar results were observed by Olesen (1987), who observed significant leakiness of brain vasculature after craniotomy. These changes in blood flow could have profound effects by initiating cascades of inflammatory mediators, altering local metabolism by altering the supply of oxygen and biochemical substrates, and disrupting the blood–brain barrier.
Another explanation for the damage caused by the drill technique is the mechanical stress involved. The use of an electric dental drill bit to create the bone flap may have induced a variety of physical stressors causing damage to the underlying brain. The friction of a high-speed drill bit generates local heat that could be transmitted to the surface of the brain. The rapid vibration of the drill bit, while not directly reaching the brain, certainly permeates the calvaria, and may be capable of disrupting local nerve fibers and/or vasculature, resulting in similar problems as those described above. This raises the question of whether a similar phenomenon would be observed in mice, which have a significantly thinner skull in comparison to rats, altering the transmission of heat and vibration. The process of creating the bone flap may result in the generation of a very fine bone powder. It is conceivable that this residue causes dural irritation, initiating an inflammatory cascade resulting in neurovasculature changes, such as swelling, manifest as the brain lesions observed in this study. However, with the exception of differences in volume, the lesions generated by both the drill and trephine were morphologically similar, with enhancement of the dura and underlying cerebral cortex seen on post-contrast T1w MRI, and a ribbon of hyperintensity in the brain. Therefore it seems that the effect of the craniotomy is the result of specific biological characteristics.
Damage to the network of blood vessels connecting the brain to the calvaria explains many of the morphological alterations observed in both techniques. The trephine technique generates a craniotomy by slow, steady, grinding pressure to penetrate the calvaria. In contrast, the drill technique uses high-frequency rotational forces to penetrate the calvaria, much like the drill in a dentist's office. Despite the stark mechanical differences in generating the bone flap, the post-surgical lesions, while differing in volume, appeared identical morphologically. Further, the changes in the volumes of the two hemispheres of the brain seen in the drill rats, measured as a percentage of total brain volume, are likely due to swelling of the ipsilateral hemisphere associated with the vascular disruption and edema, which causes compression of the contralateral hemisphere.
The physical primary injury caused by removal and replacement of the bone flap initiates a secondary cascade of events. The increase in proinflammatory cytokine signaling, specifically the elevated KC-GRO and IFN-γ expression seen in both drill and trephine animals compared to naïve controls, confirms that damage and inflammatory processes result from these craniotomies performed during the sham procedure itself. Interestingly, not only was there a greater increase in the expression of specific cytokines after the craniotomy, but there was also a wider range of cytokines that responded to the trephine procedure. This may be associated with the extent of the damage generated by the craniotomy technique. Homogenates of cortical tissue were generated from directly beneath the craniotomy site (see methods section). The trephine results in a smaller lesion volume, which may indicate more intact neurovasculature, less swelling, and less debris. In contrast, the drill technique may have had a greater proportion of dead cells at the center of the lesion from which the cortical punch was collected. However, it should be noted that these observations are based on the imaging results and need to be correlated with histological analysis in future studies. These effects may facilitate faster and more thorough penetration by cytokines into the sampled brain tissue in trephine compared to the drill rats, potentially accounting for the increases in KC-GRO and IL-1β noted above. KC-GRO levels are increased at early time points post-injury in closed-head injury models (Semple et al., 2010a, 2010b; Valles et al., 2006). The increase in the expression of this protein in both craniotomy groups suggests that the sham procedure itself can induce a confounding factor if this research is repeated using an open-head injury model. This study did not investigate the cellular source of the KC-GRO found after craniotomy, but previous studies have implicated activated microglia and astrocytes in the production of KC-GRO in vitro (Janabi et al., 1999; Zhai et al., 2004). The cytokines altered in this study promote neuronal survival, activate microglia for early inflammatory processes, and mediate infiltration of neutrophils and macrophages of blood-borne cell types into brain tissue (Clausen et al., 2008, 2009). The lack of observable changes in TNF-α in animals of either type of craniotomy procedure is notable. TNF-α is suggested to be a contributor to decreased cognitive function shortly after injury, but it is also a mediator of long-term restoration of function (Scherbel et al., 1999). In TBI animal studies, TNF-α levels are maximal as early as 1 h post-injury (Harrington et al., 2005; Stover et al., 2000), and return to baseline levels rapidly.
One important fact to note is that behavioral effects in the present study were observed based on the overall NSS-R and not on individual task performance. This result underscores the value of functional assessments that include a variety of tasks, such as the newly developed NSS-R, which includes 10 tasks and a scoring system that differentiates function on a three-point scale for each behavior. As experimental technology improves, the differences between naïve, craniotomy, and true TBI models will become increasingly obvious. This is particularly true when craniotomy techniques are used as controls in mild TBI models, for which the distinction between damage due to the craniotomy may be difficult to distinguish from the primary impact used to cause the mild TBI. In fact, experiments today that show no difference in the results when comparing craniotomy to true naïve or mild TBI may ultimately reveal significant changes due to technical improvements in the assays (Abdel Baki et al., 2009; Bramlett et al., 1999). Clearly, craniotomy in the rat results in distinct brain injury.
The assays performed in this study generated a unique “footprint” of injury following the craniotomy. However, the authors would like to point out that additional factors beyond the craniotomy could play a small role in the results seen here. For example, the surgical incision alone could induce an inflammatory response that could contribute to the neurological effects observed in this study. However, the localized effect of the cytokine expression, with significant elevations seen in the ipsilateral cortex but not the contralateral cortex, suggests that the inflammatory mediators were generated locally by activated microglia, and not by influx from a systemic effect precipitated by the surgery.
Ultimately, these results raise the question of the appropriate control for traumatic brain injury research models, especially CCI and FPI, that utilize craniotomy. Craniotomy alone results in traumatic brain injury with morphological, biochemical, and behavioral correlates. Although the manual trephine causes less injury than the electric drill, there is demonstrable, significant injury nonetheless. Yet over the last 20 years, researchers using these two models have frequently adopted the practice of using craniotomy as the sole control, and have often used the phrase “uninjured controls” when referring to these sham animals, with no other animals used for comparison. Craniotomy data are frequently omitted because it is considered control. Not only are these animals not “uninjured,” we contend that their injury is distinct, significant, and unrelated to the deliberate injury model. Additionally, the consistency of the results indicates that the effects observed are not inadvertent damage, but are secondary to the technique itself. However, the importance of using experienced surgeons that meticulously monitor for inadvertent damage cannot be overstated.
Finally, the changes observed in this study, particularly the significantly increased expression in cytokines that can initiate numerous secondary cascades, may confound studies examining any of a number of post-traumatic changes in brain physiology. The goal of TBI research is ultimately to understand the pathophysiological mechanisms of injury, and more importantly, to develop effective treatments that restore the victims to their pre-injury state. Therefore, great care should be taken in selecting the appropriate models and controls for comparing post-traumatic changes in the brain, and for evaluating the efficacy of potential treatments. Given the distinct injury caused by craniotomy procedures, it may be beneficial to forego this procedure as a control group and use naïve or anesthesia-only animals as controls.
Disclaimer
The opinions expressed herein belong solely to the authors. They do not nor should they be interpreted as representative of or endorsed by the Uniformed Services University of the Health Sciences, U.S. Army, U.S. Navy, Department of Defense, or any other agency of the federal government.
Acknowledgments
This work was supported by a grant from the Department of Defense (60885-CNRM), and was performed at the Center for Neurosciences and Regenerative Medicine (CNRM) and at the National Institutes of Health (NIH). Additional support for this work was provided by the Comprehensive National Neuroscience Program (CNNP, award number W81XWH-07-0679), and the Intramural Research Program in the Clinical Center at the NIH.
Author Disclosure Statement
No competing financial interests exist.
References
- Abdel Baki S.G. Kao H.Y. Kelemen E. Fenton A.A. Bergold P.J. A hierarchy of neurobehavioral tasks discriminates between mild and moderate brain injury in rats. Brain Res. 2009;1280:98–106. doi: 10.1016/j.brainres.2009.05.034. [DOI] [PubMed] [Google Scholar]
- Adeleye A. Shohami E. Nachman D. Alexandrovich A. Trembovler V. Yaka R. Shoshan Y. Dhawan J. Biegon A. D-cycloserine improves functional outcome after traumatic brain injury with wide therapeutic window. Eur. J. Pharmacol. 2010;629:25–30. doi: 10.1016/j.ejphar.2009.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves O.L. Bullock R. Clausen T. Reinert M. Reeves T.M. Concurrent monitoring of cerebral electrophysiology and metabolism after traumatic brain injury: an experimental and clinical study. J. Neurotrauma. 2005;22:733–749. doi: 10.1089/neu.2005.22.733. [DOI] [PubMed] [Google Scholar]
- Arcure J. Harrison E.E. A review of the use of early hypothermia in the treatment of traumatic brain injuries. J. Spec. Oper. Med. 2009;9:22–25. doi: 10.55460/6EAQ-Z4AP. [DOI] [PubMed] [Google Scholar]
- Bartnik B.L. Lee S.M. Hovda D.A. Sutton R.L. The fate of glucose during the period of decreased metabolism after fluid percussion injury: a 13C NMR study. J. Neurotrauma. 2007;24:1079–1092. doi: 10.1089/neu.2006.0210. [DOI] [PubMed] [Google Scholar]
- Bederson J.B. Bartkowski H.M. Moon K. Halks-Miller M. Nishimura M.C. Brant-Zawadski M. Pitts L.H. Nuclear magnetic resonance imaging and spectroscopy in experimental brain edema in a rat model. J. Neurosurg. 1986;64:795–802. doi: 10.3171/jns.1986.64.5.0795. [DOI] [PubMed] [Google Scholar]
- Bramlett H.M. Dietrich W.D. Green E.J. Secondary hypoxia following moderate fluid percussion brain injury in rats exacerbates sensorimotor and cognitive deficits. J. Neurotrauma. 1999;16:1035–1047. doi: 10.1089/neu.1999.16.1035. [DOI] [PubMed] [Google Scholar]
- Clausen B.H. Lambertsen K.L. Babcock A.A. Holm T.H. Dagnaes-Hansen F. Finsen B. Interleukin-1beta and tumor necrosis factor-alpha are expressed by different subsets of microglia and macrophages after ischemic stroke in mice. J. Neuroinflammation. 2008;5:46. doi: 10.1186/1742-2094-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen F. Hanell A. Bjork M. Hillered L. Mir A.K. Gram H. Marklund N. Neutralization of interleukin-1beta modifies the inflammatory response and improves histological and cognitive outcome following traumatic brain injury in mice. Eur. J. Neurosci. 2009;30:385–396. doi: 10.1111/j.1460-9568.2009.06820.x. [DOI] [PubMed] [Google Scholar]
- Cole J.T. Mitala C.M. Kundu S. Verma A. Elkind J.A. Nissim I. Cohen A.S. Dietary branched chain amino acids ameliorate injury-induced cognitive impairment. Proc. Natl. Acad. Sci. USA. 2010;107:366–371. doi: 10.1073/pnas.0910280107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C. Olesen S.P. Electrical resistance of brain microvascular endothelium. Brain Res. 1982;241:49–55. doi: 10.1016/0006-8993(82)91227-6. [DOI] [PubMed] [Google Scholar]
- Deshpande L.S. Sun D.A. Sombati S. Baranova A. Wilson M.S. Attkisson E. Hamm R.J. DeLorenzo R.J. Alterations in neuronal calcium levels are associated with cognitive deficits after traumatic brain injury. Neurosci. Lett. 2008;441:115–119. doi: 10.1016/j.neulet.2008.05.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon C.E. Clifton G.L. Lighthall J.W. Yaghmai A.A. Hayes R.L. A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Edvinsson L. West K.A. Experimental cerebral heat lesions produced by trephine craniotomy in rabbits. Acta Pathol. Microbiol. Scand. A. 1972;80:134–138. doi: 10.1111/j.1699-0463.1972.tb00278.x. [DOI] [PubMed] [Google Scholar]
- Faria M.H.G. Muniz L.R.F. De Vasconcelos P.R.L. Ketone bodies metabolism during ischemic and reperfusion brain injuries following bilateral occlusion of common carotid arteries in rats. Acta Cirurgica Brasileira. 2007;22:125–129. doi: 10.1590/s0102-86502007000200009. [DOI] [PubMed] [Google Scholar]
- Finkelstein E.C.P. Miller T. Oxford University Press; New York: 2006. The Incidence and Economic Burden of Injuries in the United States. associates. [Google Scholar]
- Hamm R.J. Neurobehavioral assessment of outcome following traumatic brain injury in rats: an evaluation of selected measures. J. Neurotrauma. 2001;18:1207–1216. doi: 10.1089/089771501317095241. [DOI] [PubMed] [Google Scholar]
- Harrington J.F. Messier A.A. Levine A. Szmydynger-Chodobska J. Chodobski A. Shedding of tumor necrosis factor type 1 receptor after experimental spinal cord injury. J. Neurotrauma. 2005;22:919–928. doi: 10.1089/neu.2005.22.919. [DOI] [PubMed] [Google Scholar]
- He X.S. Xiang Z. Zhou F. Fu L.A. Shuang W. Calcium overloading in traumatic axonal injury by lateral head rotation: a morphological evidence in rat model. J. Clin. Neurosci. 2004;11:402–407. doi: 10.1016/j.jocn.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Janabi N. Hau I. Tardieu M. Negative feedback between prostaglandin and alpha- and beta-chemokine synthesis in human microglial cells and astrocytes. J. Immunol. 1999;162:1701–1706. [PubMed] [Google Scholar]
- Jia J. Yan M. Lu Z. Sun M. He J. Xia C. Regulated expression of pancreatic triglyceride lipase after rat traumatic brain injury. Mol. Cell Biochem. 2010;335:127–136. doi: 10.1007/s11010-009-0249-4. [DOI] [PubMed] [Google Scholar]
- Kosaras B. Jakubowski M. Kainz V. Burstein R. Sensory innervation of the calvarial bones of the mouse. J. Comp. Neurol. 2009;515:331–348. doi: 10.1002/cne.22049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighthall J.W. Controlled cortical impact: a new experimental brain injury model. J. Neurotrauma. 1988;5:1–15. doi: 10.1089/neu.1988.5.1. [DOI] [PubMed] [Google Scholar]
- Lloyd E. Somera-Molina K. Van Eldik L.J. Watterson D.M. Wainwright M.S. Suppression of acute proinflammatory cytokine and chemokine upregulation by post-injury administration of a novel small molecule improves long-term neurologic outcome in a mouse model of traumatic brain injury. J. Neuroinflammation. 2008;5:28. doi: 10.1186/1742-2094-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood A. Lu D. Wang L. Li Y. Lu M. Chopp M. Treatment of traumatic brain injury in female rats with intravenous administration of bone marrow stromal cells. Neurosurgery. 2001;49:1196–1203. discussion 1203–1194. [PubMed] [Google Scholar]
- Mahmood A. Lu D. Lu M. Chopp M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery. 2003;53:697–702. doi: 10.1227/01.neu.0000079333.61863.aa. discussion 702–693. [DOI] [PubMed] [Google Scholar]
- Marti M. Mela F. Fantin M. Zucchini S. Brown J.M. Witta J. Di Benedetto M. Buzas B. Reinscheid R.K. Salvadori S. Guerrini R. Romualdi P. Candeletti S. Simonato M. Cox B.M. Morari M. Blockade of nociceptin/orphanin FQ transmission attenuates symptoms and neurodegeneration associated with Parkinson's disease. J. Neurosci. 2005;25:9591–9601. doi: 10.1523/JNEUROSCI.2546-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millen J.E. Glauser F.L. Fairman R.P. A comparison of physiological responses to percussive brain trauma in dogs and sheep. J. Neurosurg. 1985;62:587–591. doi: 10.3171/jns.1985.62.4.0587. [DOI] [PubMed] [Google Scholar]
- Navari R.M. Wei E.P. Kontos H.A. Patterson J.L., Jr. Comparison of the open skull and cranial window preparations in the study of the cerebral microcirculation. Microvasc. Res. 1978;16:304–315. doi: 10.1016/0026-2862(78)90064-x. [DOI] [PubMed] [Google Scholar]
- Olesen S.P. Leakiness of rat brain microvessels to fluorescent probes following craniotomy. Acta Physiol. Scand. 1987;130:63–68. doi: 10.1111/j.1748-1716.1987.tb08112.x. [DOI] [PubMed] [Google Scholar]
- Persson L. Cellular reactions to small cerebral stab wounds in the rat frontal lobe. An ultrastructural study. Virchows Arch. B Cell Pathol. 1976;22:21–37. doi: 10.1007/BF02889204. [DOI] [PubMed] [Google Scholar]
- Povlishock J.T. Kontos H.A. Continuing axonal and vascular change following experimental brain trauma. Cent. Nerv. Syst. Trauma. 1985;2:285–298. doi: 10.1089/cns.1985.2.285. [DOI] [PubMed] [Google Scholar]
- Rahman M.A. Grunberg N.E. Mueller G.P. Disulfiram causes sustained behavioral and biochemical effects in rats. Pharmacol. Biochem. Behav. 1997;56:409–415. doi: 10.1016/s0091-3057(96)00222-5. [DOI] [PubMed] [Google Scholar]
- Rustay N.R. Wahlsten D. Crabbe J.C. Influence of task parameters on rotarod performance and sensitivity to ethanol in mice. Behav. Brain Res. 2003;141:237–249. doi: 10.1016/s0166-4328(02)00376-5. [DOI] [PubMed] [Google Scholar]
- Saljo A. Bao F. Haglid K.G. Hansson H.A. Blast exposure causes redistribution of phosphorylated neurofilament subunits in neurons of the adult rat brain. J. Neurotrauma. 2000;17:719–726. doi: 10.1089/089771500415454. [DOI] [PubMed] [Google Scholar]
- Scafidi S. O'Brien J. Hopkins I. Robertson C. Fiskum G. McKenna M. Delayed cerebral oxidative glucose metabolism after traumatic brain injury in young rats. J. Neurochem. 2009;109(Suppl. 1):189–197. doi: 10.1111/j.1471-4159.2009.05896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherbel U. Raghupathi R. Nakamura M. Saatman K.E. Trojanowski J.Q. Neugebauer E. Marino M.W. McIntosh T.K. Differential acute and chronic responses of tumor necrosis factor-deficient mice to experimental brain injury. Proc. Natl. Acad. Sci. USA. 1999;96:8721–8726. doi: 10.1073/pnas.96.15.8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple B.D. Bye N. Rancan M. Ziebell J.M. Morganti-Kossmann M.C. Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2-/- mice. J. Cereb. Blood Flow Metab. 2010a;30:769–782. doi: 10.1038/jcbfm.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple B.D. Kossmann T. Morganti-Kossmann M.C. Role of chemokines in CNS health and pathology: a focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. J. Cereb. Blood Flow Metab. 2010b;30:459–473. doi: 10.1038/jcbfm.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohami E. Novikov M. Bass R. Long-term effect of HU-211, a novel non-competitive NMDA antagonist, on motor and memory functions after closed head injury in the rat. Brain Res. 1995;674:55–62. doi: 10.1016/0006-8993(94)01433-i. [DOI] [PubMed] [Google Scholar]
- Stover J.F. Schoning B. Beyer T.F. Woiciechowsky C. Unterberg A.W. Temporal profile of cerebrospinal fluid glutamate, interleukin-6, and tumor necrosis factor-alpha in relation to brain edema and contusion following controlled cortical impact injury in rats. Neurosci. Lett. 2000;288:25–28. doi: 10.1016/s0304-3940(00)01187-3. [DOI] [PubMed] [Google Scholar]
- Sun D.A. Deshpande L.S. Sombati S. Baranova A. Wilson M.S. Hamm R.J. DeLorenzo R.J. Traumatic brain injury causes a long-lasting calcium (Ca2+)-plateau of elevated intracellular Ca levels and altered Ca2+ homeostatic mechanisms in hippocampal neurons surviving brain injury. Eur. J. Neurosci. 2008;27:1659–1672. doi: 10.1111/j.1460-9568.2008.06156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valable S. Francony G. Bouzat P. Fevre M.C. Mahious N. Bouet V. Farion R. Barbier E. Lahrech H. Remy C. Petit E. Segebarth C. Bernaudin M. Payen J.F. The impact of erythropoietin on short-term changes in phosphorylation of brain protein kinases in a rat model of traumatic brain injury. J. Cereb. Blood Flow Metab. 2010;30:361–369. doi: 10.1038/jcbfm.2009.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valles A. Grijpink-Ongering L. de Bree F.M. Tuinstra T. Ronken E. Differential regulation of the CXCR2 chemokine network in rat brain trauma: implications for neuroimmune interactions and neuronal survival. Neurobiol. Dis. 2006;22:312–322. doi: 10.1016/j.nbd.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Wei H.H. Lu X.C. Shear D.A. Waghray A. Yao C. Tortella F.C. Dave J.R. NNZ-2566 treatment inhibits neuroinflammation and pro-inflammatory cytokine expression induced by experimental penetrating ballistic-like brain injury in rats. J. Neuroinflammation. 2009;6:19. doi: 10.1186/1742-2094-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing G. Ren M. Watson W.A. O'Neil J.T. Verma A. Traumatic brain injury-induced expression and phosphorylation of pyruvate dehydrogenase: a mechanism of dysregulated glucose metabolism. Neurosci. Lett. 2009;454:38–42. doi: 10.1016/j.neulet.2009.01.047. [DOI] [PubMed] [Google Scholar]
- Xiong Y. Chopp M. Lee C.P. Erythropoietin improves brain mitochondrial function in rats after traumatic brain injury. Neurol. Res. 2009;31:496–502. doi: 10.1179/174313208X353703. [DOI] [PubMed] [Google Scholar]
- Zhai Q. Luo Y. Zhang Y. Berman M.A. Dorf M.E. Low nuclear levels of nuclear factor-kappa B are essential for KC self-induction in astrocytes: requirements for shuttling and phosphorylation. Glia. 2004;48:327–336. doi: 10.1002/glia.20087. [DOI] [PubMed] [Google Scholar]
- Ziebell J.M. Morganti-Kossmann M.C. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics. 2010;7:22–30. doi: 10.1016/j.nurt.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]