Abstract
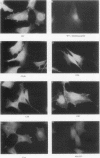
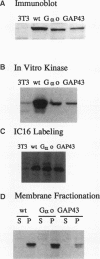
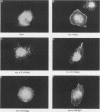
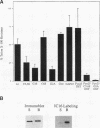
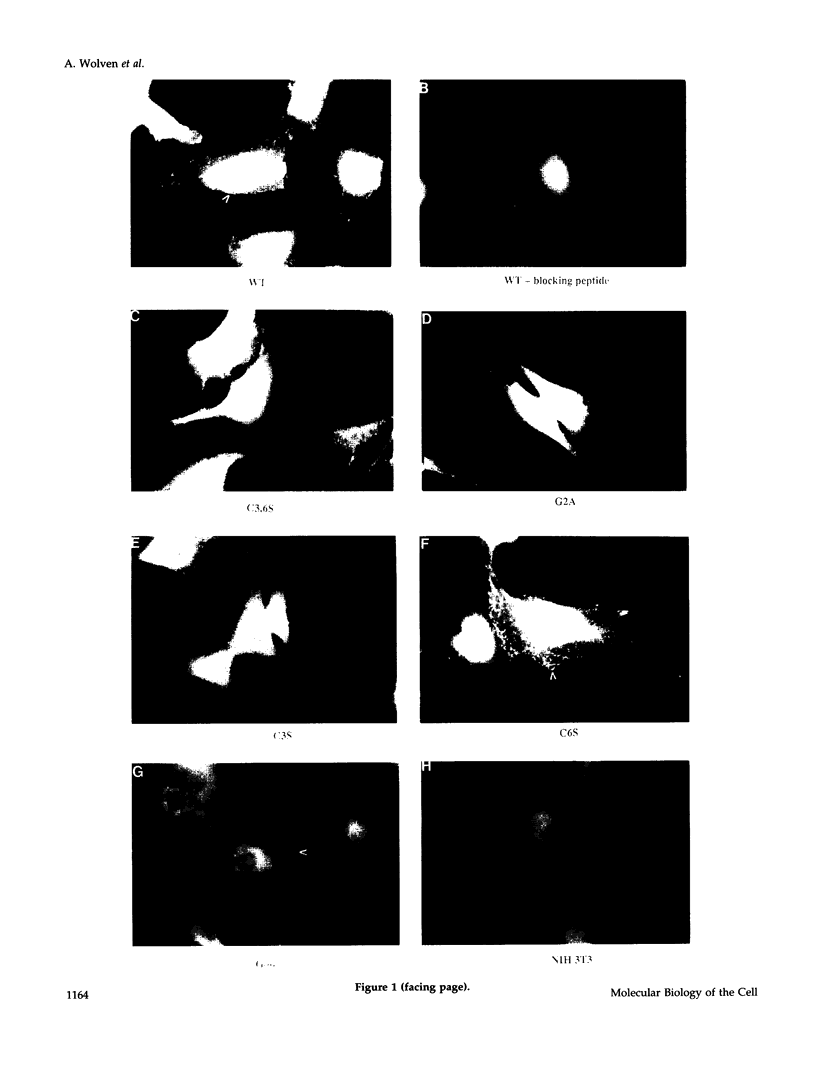
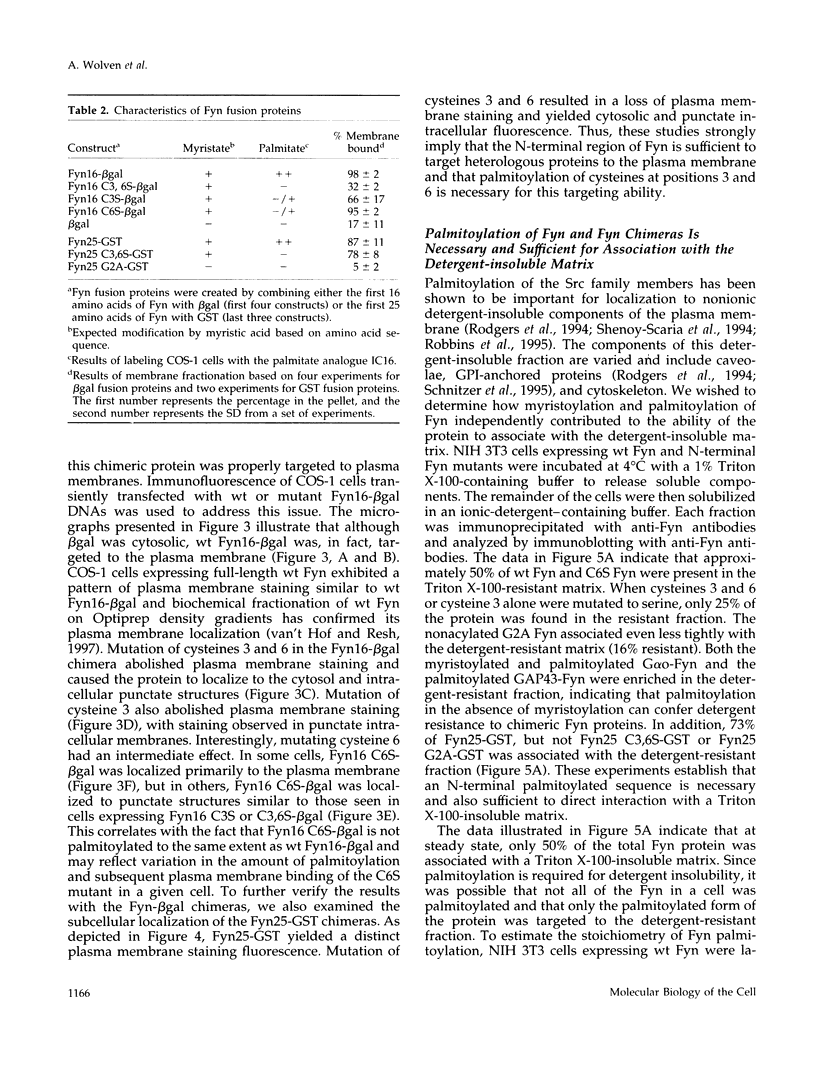
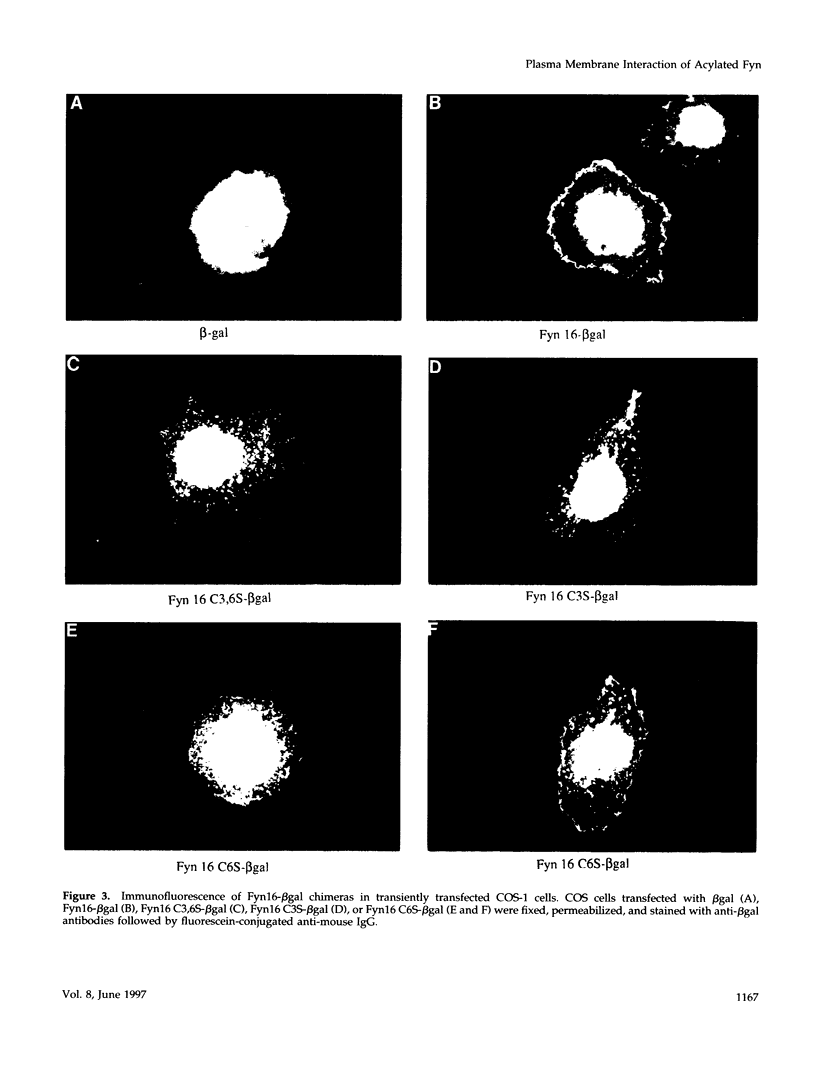
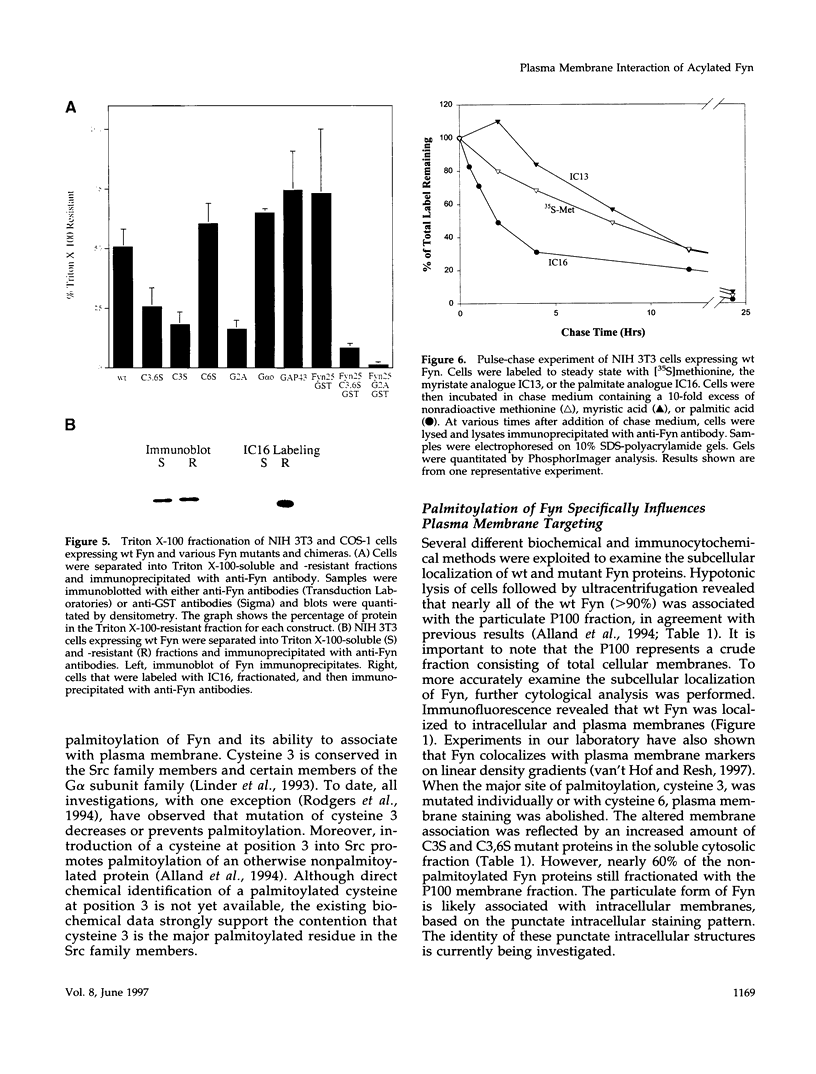
Members of the Src family of protein tyrosine kinases are localized to subspecialized regions of the plasma membrane. Herein we show that the N-terminal SH4 region of the Src family member p59fyn (Fyn) is both necessary and sufficient for targeting of Fyn and heterologous proteins to the plasma membrane and detergent-insoluble subdomains. Attachment of the first 16 amino acids of Fyn to a normally cytosolic protein, beta-galactosidase, resulted in distinct plasma membrane localization of the chimeric protein. Mutation of the palmitoylation site (cysteine-3) within Fyn16-beta-galactosidase or wild-type Fyn abrogated plasma membrane localization, resulting in redistribution of the mutant proteins into intracellular membranes. Substitution of the SH4 motif within Fyn with heterologous sequences from other palmitoylated proteins (G alpha o and GAP43) revealed that the presence of palmitate is sufficient to direct plasma membrane localization independent of surrounding amino acid sequences and myristate. Palmitoylated Fyn chimeras were also enriched in the Triton X-100-resistant matrix, whereas nonpalmitoylated forms of these proteins were detected in the detergent-soluble fraction. The palmitate moiety on Fyn exhibited a half-life of 1.5-2 h. In contrast, the half-life of the polypeptide backbone was 8 h, indicating that palmitoylation is a reversible modification. These studies establish that the palmitoylated SH4 sequence of Fyn can be used to specifically target proteins to the plasma membrane in a reversible manner.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alland L., Peseckis S. M., Atherton R. E., Berthiaume L., Resh M. D. Dual myristylation and palmitylation of Src family member p59fyn affects subcellular localization. J Biol Chem. 1994 Jun 17;269(24):16701–16705. [PubMed] [Google Scholar]
- Ben-Ze'ev A., Duerr A., Solomon F., Penman S. The outer boundary of the cytoskeleton: a lamina derived from plasma membrane proteins. Cell. 1979 Aug;17(4):859–865. doi: 10.1016/0092-8674(79)90326-x. [DOI] [PubMed] [Google Scholar]
- Berthiaume L., Resh M. D. Biochemical characterization of a palmitoyl acyltransferase activity that palmitoylates myristoylated proteins. J Biol Chem. 1995 Sep 22;270(38):22399–22405. doi: 10.1074/jbc.270.38.22399. [DOI] [PubMed] [Google Scholar]
- Bolen J. B., Rowley R. B., Spana C., Tsygankov A. Y. The Src family of tyrosine protein kinases in hemopoietic signal transduction. FASEB J. 1992 Dec;6(15):3403–3409. doi: 10.1096/fasebj.6.15.1281458. [DOI] [PubMed] [Google Scholar]
- Buser C. A., Sigal C. T., Resh M. D., McLaughlin S. Membrane binding of myristylated peptides corresponding to the NH2 terminus of Src. Biochemistry. 1994 Nov 8;33(44):13093–13101. doi: 10.1021/bi00248a019. [DOI] [PubMed] [Google Scholar]
- Cadwallader K. A., Paterson H., Macdonald S. G., Hancock J. F. N-terminally myristoylated Ras proteins require palmitoylation or a polybasic domain for plasma membrane localization. Mol Cell Biol. 1994 Jul;14(7):4722–4730. doi: 10.1128/mcb.14.7.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. A., Sefton B. M. Association between B-lymphocyte membrane immunoglobulin and multiple members of the Src family of protein tyrosine kinases. Mol Cell Biol. 1992 May;12(5):2315–2321. doi: 10.1128/mcb.12.5.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Cervera M., Dreyfuss G., Penman S. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell. 1981 Jan;23(1):113–120. doi: 10.1016/0092-8674(81)90276-2. [DOI] [PubMed] [Google Scholar]
- Clark M. R., Campbell K. S., Kazlauskas A., Johnson S. A., Hertz M., Potter T. A., Pleiman C., Cambier J. C. The B cell antigen receptor complex: association of Ig-alpha and Ig-beta with distinct cytoplasmic effectors. Science. 1992 Oct 2;258(5079):123–126. doi: 10.1126/science.1439759. [DOI] [PubMed] [Google Scholar]
- Clark M. R., Johnson S. A., Cambier J. C. Analysis of Ig-alpha-tyrosine kinase interaction reveals two levels of binding specificity and tyrosine phosphorylated Ig-alpha stimulation of Fyn activity. EMBO J. 1994 Apr 15;13(8):1911–1919. doi: 10.1002/j.1460-2075.1994.tb06460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb B. S., Schaller M. D., Leu T. H., Parsons J. T. Stable association of pp60src and pp59fyn with the focal adhesion-associated protein tyrosine kinase, pp125FAK. Mol Cell Biol. 1994 Jan;14(1):147–155. doi: 10.1128/mcb.14.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone R. D., Mulligan R. C. High-efficiency gene transfer into mammalian cells: generation of helper-free recombinant retrovirus with broad mammalian host range. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6349–6353. doi: 10.1073/pnas.81.20.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degtyarev M. Y., Spiegel A. M., Jones T. L. Increased palmitoylation of the Gs protein alpha subunit after activation by the beta-adrenergic receptor or cholera toxin. J Biol Chem. 1993 Nov 15;268(32):23769–23772. [PubMed] [Google Scholar]
- Degtyarev M. Y., Spiegel A. M., Jones T. L. Palmitoylation of a G protein alpha i subunit requires membrane localization not myristoylation. J Biol Chem. 1994 Dec 9;269(49):30898–30903. [PubMed] [Google Scholar]
- Dunphy J. T., Greentree W. K., Manahan C. L., Linder M. E. G-protein palmitoyltransferase activity is enriched in plasma membranes. J Biol Chem. 1996 Mar 22;271(12):7154–7159. doi: 10.1074/jbc.271.12.7154. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Zokas L. Novel tyrosine kinase substrates from Rous sarcoma virus-transformed cells are present in the membrane skeleton. J Cell Biol. 1989 Jun;108(6):2401–2408. doi: 10.1083/jcb.108.6.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock J. F., Paterson H., Marshall C. J. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell. 1990 Oct 5;63(1):133–139. doi: 10.1016/0092-8674(90)90294-o. [DOI] [PubMed] [Google Scholar]
- Huang M. M., Bolen J. B., Barnwell J. W., Shattil S. J., Brugge J. S. Membrane glycoprotein IV (CD36) is physically associated with the Fyn, Lyn, and Yes protein-tyrosine kinases in human platelets. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7844–7848. doi: 10.1073/pnas.88.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong J., Lublin D. M. Amino-terminal palmitate or polybasic domain can provide required second signal to myristate for membrane binding of p56lck. Biochem Biophys Res Commun. 1995 Feb 15;207(2):868–876. doi: 10.1006/bbrc.1995.1266. [DOI] [PubMed] [Google Scholar]
- Kypta R. M., Goldberg Y., Ulug E. T., Courtneidge S. A. Association between the PDGF receptor and members of the src family of tyrosine kinases. Cell. 1990 Aug 10;62(3):481–492. doi: 10.1016/0092-8674(90)90013-5. [DOI] [PubMed] [Google Scholar]
- Lenk R., Ransom L., Kaufmann Y., Penman S. A cytoskeletal structure with associated polyribosomes obtained from HeLa cells. Cell. 1977 Jan;10(1):67–78. doi: 10.1016/0092-8674(77)90141-6. [DOI] [PubMed] [Google Scholar]
- Linder M. E., Middleton P., Hepler J. R., Taussig R., Gilman A. G., Mumby S. M. Lipid modifications of G proteins: alpha subunits are palmitoylated. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3675–3679. doi: 10.1073/pnas.90.8.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Fisher D. A., Storm D. R. Analysis of the palmitoylation and membrane targeting domain of neuromodulin (GAP-43) by site-specific mutagenesis. Biochemistry. 1993 Oct 12;32(40):10714–10719. doi: 10.1021/bi00091a023. [DOI] [PubMed] [Google Scholar]
- Mori S., Rönnstrand L., Yokote K., Engström A., Courtneidge S. A., Claesson-Welsh L., Heldin C. H. Identification of two juxtamembrane autophosphorylation sites in the PDGF beta-receptor; involvement in the interaction with Src family tyrosine kinases. EMBO J. 1993 Jun;12(6):2257–2264. doi: 10.1002/j.1460-2075.1993.tb05879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura H., Resh M. D. Differential binding of pp60c-src and pp60v-src to cytoskeleton is mediated by SH2 and catalytic domains. Oncogene. 1994 Aug;9(8):2293–2303. [PubMed] [Google Scholar]
- Okamura H., Resh M. D. p80/85 cortactin associates with the Src SH2 domain and colocalizes with v-Src in transformed cells. J Biol Chem. 1995 Nov 3;270(44):26613–26618. doi: 10.1074/jbc.270.44.26613. [DOI] [PubMed] [Google Scholar]
- Parenti M., Viganó M. A., Newman C. M., Milligan G., Magee A. I. A novel N-terminal motif for palmitoylation of G-protein alpha subunits. Biochem J. 1993 Apr 15;291(Pt 2):349–353. doi: 10.1042/bj2910349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peitzsch R. M., McLaughlin S. Binding of acylated peptides and fatty acids to phospholipid vesicles: pertinence to myristoylated proteins. Biochemistry. 1993 Oct 5;32(39):10436–10443. doi: 10.1021/bi00090a020. [DOI] [PubMed] [Google Scholar]
- Peseckis S. M., Deichaite I., Resh M. D. Iodinated fatty acids as probes for myristate processing and function. Incorporation into pp60v-src. J Biol Chem. 1993 Mar 5;268(7):5107–5114. [PubMed] [Google Scholar]
- Peseckis S. M., Resh M. D. Fatty acyl transfer by human N-myristyl transferase is dependent upon conserved cysteine and histidine residues. J Biol Chem. 1994 Dec 9;269(49):30888–30892. [PubMed] [Google Scholar]
- Picard D., Yamamoto K. R. Two signals mediate hormone-dependent nuclear localization of the glucocorticoid receptor. EMBO J. 1987 Nov;6(11):3333–3340. doi: 10.1002/j.1460-2075.1987.tb02654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleiman C. M., Abrams C., Gauen L. T., Bedzyk W., Jongstra J., Shaw A. S., Cambier J. C. Distinct p53/56lyn and p59fyn domains associate with nonphosphorylated and phosphorylated Ig-alpha. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4268–4272. doi: 10.1073/pnas.91.10.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh M. D. Myristylation and palmitylation of Src family members: the fats of the matter. Cell. 1994 Feb 11;76(3):411–413. doi: 10.1016/0092-8674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Robbins S. M., Quintrell N. A., Bishop J. M. Myristoylation and differential palmitoylation of the HCK protein-tyrosine kinases govern their attachment to membranes and association with caveolae. Mol Cell Biol. 1995 Jul;15(7):3507–3515. doi: 10.1128/mcb.15.7.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson L. J., Busconi L., Michel T. Agonist-modulated palmitoylation of endothelial nitric oxide synthase. J Biol Chem. 1995 Jan 20;270(3):995–998. doi: 10.1074/jbc.270.3.995. [DOI] [PubMed] [Google Scholar]
- Rodgers W., Crise B., Rose J. K. Signals determining protein tyrosine kinase and glycosyl-phosphatidylinositol-anchored protein targeting to a glycolipid-enriched membrane fraction. Mol Cell Biol. 1994 Aug;14(8):5384–5391. doi: 10.1128/mcb.14.8.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg K. G., Heuser J. E., Donzell W. C., Ying Y. S., Glenney J. R., Anderson R. G. Caveolin, a protein component of caveolae membrane coats. Cell. 1992 Feb 21;68(4):673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- Samelson L. E., Phillips A. F., Luong E. T., Klausner R. D. Association of the fyn protein-tyrosine kinase with the T-cell antigen receptor. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4358–4362. doi: 10.1073/pnas.87.11.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor O., Robbins K. C. Substrate specificity for normal but not mutationally activated variants of src family kinases. J Biol Chem. 1993 Oct 5;268(28):21014–21020. [PubMed] [Google Scholar]
- Schaller M. D., Borgman C. A., Cobb B. S., Vines R. R., Reynolds A. B., Parsons J. T. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer J. E., McIntosh D. P., Dvorak A. M., Liu J., Oh P. Separation of caveolae from associated microdomains of GPI-anchored proteins. Science. 1995 Sep 8;269(5229):1435–1439. doi: 10.1126/science.7660128. [DOI] [PubMed] [Google Scholar]
- Schroeder H., Leventis R., Shahinian S., Walton P. A., Silvius J. R. Lipid-modified, cysteinyl-containing peptides of diverse structures are efficiently S-acylated at the plasma membrane of mammalian cells. J Cell Biol. 1996 Aug;134(3):647–660. doi: 10.1083/jcb.134.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahinian S., Silvius J. R. Doubly-lipid-modified protein sequence motifs exhibit long-lived anchorage to lipid bilayer membranes. Biochemistry. 1995 Mar 21;34(11):3813–3822. doi: 10.1021/bi00011a039. [DOI] [PubMed] [Google Scholar]
- Shenoy-Scaria A. M., Dietzen D. J., Kwong J., Link D. C., Lublin D. M. Cysteine3 of Src family protein tyrosine kinase determines palmitoylation and localization in caveolae. J Cell Biol. 1994 Jul;126(2):353–363. doi: 10.1083/jcb.126.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy-Scaria A. M., Gauen L. K., Kwong J., Shaw A. S., Lublin D. M. Palmitylation of an amino-terminal cysteine motif of protein tyrosine kinases p56lck and p59fyn mediates interaction with glycosyl-phosphatidylinositol-anchored proteins. Mol Cell Biol. 1993 Oct;13(10):6385–6392. doi: 10.1128/mcb.13.10.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal C. T., Zhou W., Buser C. A., McLaughlin S., Resh M. D. Amino-terminal basic residues of Src mediate membrane binding through electrostatic interaction with acidic phospholipids. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):12253–12257. doi: 10.1073/pnas.91.25.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman L., Resh M. D. Lysine residues form an integral component of a novel NH2-terminal membrane targeting motif for myristylated pp60v-src. J Cell Biol. 1992 Oct;119(2):415–425. doi: 10.1083/jcb.119.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugie K., Kawakami T., Maeda Y., Kawabe T., Uchida A., Yodoi J. Fyn tyrosine kinase associated with Fc epsilon RII/CD23: possible multiple roles in lymphocyte activation. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9132–9135. doi: 10.1073/pnas.88.20.9132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sármay G., Pecht I., Gergely J. Protein-tyrosine kinase activity tightly associated with human type II Fc gamma receptors. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4140–4144. doi: 10.1073/pnas.91.10.4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timson Gauen L. K., Kong A. N., Samelson L. E., Shaw A. S. p59fyn tyrosine kinase associates with multiple T-cell receptor subunits through its unique amino-terminal domain. Mol Cell Biol. 1992 Dec;12(12):5438–5446. doi: 10.1128/mcb.12.12.5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timson Gauen L. K., Linder M. E., Shaw A. S. Multiple features of the p59fyn src homology 4 domain define a motif for immune-receptor tyrosine-based activation motif (ITAM) binding and for plasma membrane localization. J Cell Biol. 1996 Jun;133(5):1007–1015. doi: 10.1083/jcb.133.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twamley-Stein G. M., Pepperkok R., Ansorge W., Courtneidge S. A. The Src family tyrosine kinases are required for platelet-derived growth factor-mediated signal transduction in NIH 3T3 cells. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7696–7700. doi: 10.1073/pnas.90.16.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twamley G. M., Kypta R. M., Hall B., Courtneidge S. A. Association of Fyn with the activated platelet-derived growth factor receptor: requirements for binding and phosphorylation. Oncogene. 1992 Oct;7(10):1893–1901. [PubMed] [Google Scholar]
- Wedegaertner P. B., Bourne H. R. Activation and depalmitoylation of Gs alpha. Cell. 1994 Jul 1;77(7):1063–1070. doi: 10.1016/0092-8674(94)90445-6. [DOI] [PubMed] [Google Scholar]
- Zhou W., Parent L. J., Wills J. W., Resh M. D. Identification of a membrane-binding domain within the amino-terminal region of human immunodeficiency virus type 1 Gag protein which interacts with acidic phospholipids. J Virol. 1994 Apr;68(4):2556–2569. doi: 10.1128/jvi.68.4.2556-2569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber M. X., Strittmatter S. M., Fishman M. C. A membrane-targeting signal in the amino terminus of the neuronal protein GAP-43. Nature. 1989 Sep 28;341(6240):345–348. doi: 10.1038/341345a0. [DOI] [PubMed] [Google Scholar]
- van't Hof W., Resh M. D. Rapid plasma membrane anchoring of newly synthesized p59fyn: selective requirement for NH2-terminal myristoylation and palmitoylation at cysteine-3. J Cell Biol. 1997 Mar 10;136(5):1023–1035. doi: 10.1083/jcb.136.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]