Abstract
Fabry disease is caused by an X-linked deficiency of the lysosomal enzyme α-galactosidase A (GLA) and has been treated successfully with enzyme replacement therapy (ERT). Gene therapy has been proposed as an alternative to ERT due to the presumed advantages of continuous, endogenous production of the therapeutic enzyme. GLA production in the liver and its therapeutic efficacy in the Fabry mouse have been demonstrated previously with various viral vector systems. In consideration of the potential advantages of using the salivary glands as endogenous GLA biosynthesis sites, we explored the feasibility of this approach in the Fabry mouse. GLA −/0 or −/− mice received an adenoviral vector (2 × 1010 or 1 × 109 viral particles) expressing GLA to the right submandibular gland via oral cannulation of the submandibular duct. Four days later, animals were sacrificed; saliva, plasma, kidney, liver, and brain were collected and assayed using ELISA, Western blot, and a GLA enzymatic activity assay using both traditional fluorescence methods and isotope dilution mass spectrometry by following the U.S. EPA Method 6800. GLA activity was significantly elevated in the serum and liver of both treatment groups, and improvement in the kidney was marginally significant (P < 0.069) in the high-dose group. Notably, we found that liver and salivary gland produce different glycoforms of the GLA transgene. Only small numbers of adenoviral genomes were observed in the livers of treated animals, but in four of 14 in the high-dose groups, liver levels of adenovirus exceeded 20 copies/μg, indicating that the sequestration in the salivary gland was imperfect at high doses. Taken together, these results indicate that the salivary gland-based gene therapy for Fabry disease is promising, and further studies with advanced viral vector gene delivery systems (e.g., adeno-associated virus) for long-term treatment appear to be warranted.
Fabry disease is caused by an X-linked deficiency of the lysosomal enzyme α-galactosidase A (GLA) and has been treated with enzyme replacement therapy (ERT). Here, Passineau and colleagues report results from a gene therapy study in the GLA-knockout mouse intended to provide a proof-of-concept that the salivary glands might be targets for gene transfer to serve as a depot for delivery of enzyme in Fabry disease and possibly other lysosomal storage disorders.
Introduction
Lysosomal storage disorders (LSDs) are a family of related congenital metabolic disorders characterized by the absence of one or more enzymes involved in the degradation and recycling of complex macromolecules within lysosomes (Neufeld, 1991; Gieselmann, 1995). The specific enzyme defect results in accumulation of the substrate normally degraded by the enzyme, which the cell attempts to “store” within engorged lysosomes. This storage burden eventually leads to cellular dysfunction and can result in cell death, with the resultant release of the partially degraded macromolecular substrate into the circulation. Whereas clinical symptoms and target organs vary greatly between LSDs, this fundamental cytopathological mechanism is common to the broad family of LSDs.
Enzyme replacement therapy (ERT) is a biopharmacological strategy wherein a recombinant form of a desired enzyme is synthesized to a clinical grade and administered intravenously to patients lacking that specific enzyme. In the current state of the art, ERT cannot be targeted to specific organs and, therefore, is most effective in enzyme defect disorders wherein the therapeutic enzyme can bind to and enter diseased cells via an endogenous surface receptor such as the cation-independent mannose-6-phosphate (M6P) receptor. For this reason, ERT has been used effectively in a variety of LSDs, as the tissues of patients suffering from these disorders readily take up the biotherapeutic from the systemic circulation in an M6P-dependent manner and direct it to the lysosomal compartments where it is biologically active. In the United States, agalsidase beta (Barngrover, 2002), a recombinant form of α-galactosidase A (GLA), the enzyme deficient in Fabry disease, has been approved by the Food and Drug Administration (FDA) for ERT on a biweekly basis.
Despite the admirable success of ERT in mitigating the progression of Fabry disease in afflicted individuals, this approach has limitations (Beck, 2007). Gene and cell therapies have been proposed and refined as an alternative to the biweekly injection regimen currently in use for the treatment of Fabry disease (Medin et al., 1996; Takenaka et al., 1999, 2000; Ziegler et al., 1999; Jung et al., 2001; Hodges and Cheng, 2006). Numerous gene therapy studies have reported partial or full correction of Fabry signs and symptoms in preclinical models (Hodges and Cheng, 2006). In particular, gene transfer to the liver with adeno-associated virus (AAV) or transposon systems appears quite promising. The translational implications of gene transfer to the liver in humans have been reviewed extensively elsewhere (Seiler et al., 2007; Kamimura and Liu, 2008; Jacobs et al., 2009; LoDuca et al., 2009).
The salivary glands have been proposed as alternative endogenous production sites for systemic gene therapeutics (Voutetakis et al., 2004). This strategy has important safety and practicality advantages over systemic gene transfer, and the salivary glands have been shown previously to direct high levels of functional transgene protein to the plasma via their endocrine secretory pathway (Castle and Castle, 1998; Isenman et al., 1999). Although knowledge of the signals governing sorting between the endocrine and exocrine secretory pathways of the salivary glands remains incomplete, it has been proposed that constitutive proteins preferentially follow the endocrine route (Voutetakis et al., 2005).
We hypothesized that GLA expressed in the salivary glands by means of an adenoviral vector would traffic substantially via the endocrine secretory pathway and enter the plasma. Importantly, previous studies have observed no differences in the trafficking of transgene proteins in the salivary gland secretory pathways when different vector systems are used (e.g., adenovirus or AAV). Herein we report a gene therapy study in the GLA-knockout mouse intended to provide a proof-of-concept that the salivary glands, based on their unique advantages as endogenous biotherapeutic production sites, might be candidates for enzyme replacement gene therapy in Fabry disease and possibly other LSDs for which ERT is being developed. We chose to conduct a very short-term treatment study in order to observe plasma levels of an adenovirus-expressed transgene near their peak (Voutetakis et al., 2005) and correlate with expected accumulation of the transgene in target organs (e.g., kidney).
Materials and Methods
Animals
Mice of the strain B6;129-Glatm1Kul/J were obtained from Jackson Labs (Bar Harbor, ME) and maintained in the pathogen-free conditions in the Allegheny-Singer Research Institute vivarium, with access to standard chow and water ad libitum. Animal experiments and protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Allegheny-Singer Research Institute. This strain of mice has the GLA gene disrupted by replacement of a portion of exon 3 and intron 4 with a neomycin resistance sequence (Ohshima et al., 1997). Mice were PCR genotyped using primers recognizing the portion of exon 3 containing the insertion/deletion, with the wild-type allele producing an amplicon of 295 base pairs and the disrupted allele producing a 202-base pair amplicon. All animals were individually genotyped by tail biopsy before entering into experimental groups. Approximately equal numbers of males (–/0) and females (–/–) were used in each experimental group.
Construction of Ad-GLA-FLAG
A cDNA sequence corresponding to the human GLA mRNA (GI:125661058) was inserted into the multiple cloning site of the pShuttle-IRES-hrGFP-1 vector (Stratagene, La Jolla, CA) and confirmed with direct sequencing. The resulting shuttle vector was then recombined with the AdEasy backbone by electroporating BJ5183-AD1 Electrocompetent Cells (Stratagene), according to the manufacturer's guidelines, generating the Ad-GLA-FLAG-IRES-hrGFP genome. The adenoviral genome was cut with PacI and transfected into AD-293 cells (Stratagene), and resulting virions were upscaled and purified using double cesium chloride gradient ultracentrifugation. The purified virus was spectrophotometrically measured to be concentrated at 2.04 × 1012 viral particles/ml.
Functional testing of Ad-GLA-FLAG
AD-293 cells were grown to 80% confluence in a 25-cm2 tissue culture flask and infected with 100 viral particles/cell in Dulbecco's modified Eagle medium/F12 50:50 containing 1% penicillin/streptomycin, 1% glutamine, and 2% fetal bovine serum. After 24 hr, cells were scraped and lysed with M-PER cell lysis buffer (Pierce, Rockford, IL). Lysates of mock-infected cells were used as uninfected controls. Lysates were tested for GLA activity in an enzyme bioassay (see below). For immunoprecipitation studies, cellular lysates were processed with an anti-FLAG immunoprecipitation kit (Sigma, St. Louis, MO) per the manufacturer's instructions. In brief, lysates were incubated in the presence of a resin complexed to an anti-FLAG monoclonal antibody (mAb). The protein-bound resin was subjected to multiple wash steps, and the purified product was eluted from the column with 0.1 M glycine buffer at pH 3.5. Eluate from the column was then tested for GLA activity in an enzyme activity assay (see below).
Animal surgery and tissue harvest
Gene transfer to the salivary gland was accomplished as previously described by Voutetakis et al. (2004). In brief, the animal was anesthetized and placed in a stereotactic frame allowing the mouth to be held open. The tongue was retracted, and a thin plastic catheter was placed into the opening of the submandibular duct on one side, advanced ∼1 mm, and held in place with Super Glue. Fifty microliters of vehicle solution carrying the gene transfer vector (2 × 1010 or 1 × 109 viral particles) was infused into the duct on one side via the catheter, and the plunger was left in place for 10 min to allow the vector to contact the salivary epithelial cells. The catheter was then removed and the animal returned to its cage to awaken normally.
Four days after gene delivery, saliva was collected after stimulation with a subcutaneous injection of 0.25 mg/kg pilocarpine. A capillary tube was placed in the animal's mouth, and the saliva was allowed to drain into a microcentrifuge tube. Twenty-five to 50 μl of protease inhibitor (Complete Mini EDTA-free protease inhibitor cocktail tablets; Roche Diagnostics Corp., Indianapolis, IN) was added to the saliva, which was then frozen at −80°C. Blood was collected by orbital bleed into a microcentrifuge tube containing 20 μl of 15% EDTA and placed on ice. The blood was centrifuged at 14,000 rpm for 15 min at 4°C. The serum was removed, placed into a clean microcentrifuge tube, and frozen at −80°C. Animals were then given a lethal dose of ketamine, and liver, kidney, and brain were dissected. Fresh tissue was homogenized in 2 ml of buffer containing 320 mM sucrose, 10 mM Tris-HCl, pH 7.5, 1 mM EGTA, 1 mM EDTA, 0.3% (v/v) 2-mercaptoethanol, and protease inhibitor cocktail tablets (as above) with a Polytron homogenizer (Brinkmann Instruments, Inc., Westbury, NY). The homogenates were centrifuged at 1,000 g for 10 min at 4°C. The supernatant was centrifuged at 100,000 g for 60 min. The concentration of the 100,000 g supernatant (cytosol fraction) was determined using the Bio-Rad Protein Assay (Bio-Rad Life Science Research Products, Hercules, CA).
GLA enzyme bioassay
We utilized a GLA enzyme assay that is adapted from principles used in the clinical diagnosis of Fabry disease in humans (Kint, 1970; Beutler and Kuhl, 1972; Desnick et al., 1973). Fifty microliters of sample (saliva, serum, cell, or tissue lysates) was added to 400 μl of 0.2 M sodium citrate buffer at pH 4.0 and 20 μl of 20 mM 4-methylumbelliferyl α-galactoside (Sigma) and incubated at 37°C for 20 min. After 20 min, 3 ml of high pH stop buffer (0.2 M glycine/NaOH) was added, and sample fluorescence was determined using a VersaFluor spectrofluorometer (Bio-Rad) with 350/50 excitation and 460/10 emission filters. To correct for α-galactosidase B activity, as well as autofluorescence of the sample matrix itself, for each fluid or tissue analyzed, the average emission at 460 nm [EM(460)] for samples from untreated knockout animals (which were individually genotyped to confirm knockout of the GLA gene) was subtracted from emission for samples from the experimental groups.
For organ GLA analysis, we adapted the tandem mass spectrometry (MS/MS) method of Li et al. (2004) as follows: Ten microliters of tissue homogenate was added to 15 μl of a GLA assay cocktail containing 3.33 mmol/L GLA-S (α-galactosidase A synthetic substrate product), 6.67 μmol/L GLA-IS (α-galactosidase A synthetic substrate, 5D enriched product) [GLA-S and GLA-IS per Li et al. (2004); for MS/MS protocol, see Gelb et al. (2006)], 160 mmol/L GLANAc, and 0.142 mol/L sodium acetate, pH 4.6. Samples were incubated at 37°C for 20–24 hr with orbital shaking at 225 ± 25 rpm. After incubation, the reaction was quenched by adding 100 μl of ethyl acetate/methanol 1:1 to each well. We then added 400 μl of ethyl acetate followed by 400 μl of water into each well. The contents of the sample were mixed by aspirating up and down three times and then centrifuged for 5 min at 2,000 g to form the two-phase separation. We transferred 300 μl of the top organic layer of each sample to a 1.7-ml microcentrifuge tube and stored each transferred solution at −80°C until further processing.
For tissue samples (liver, kidney, brain), a solid-phase extraction-based sample cleanup was required prior to optimal MS/MS analysis. The protocol used diol solid-phase extraction (SPE) columns (United Chemical Technologies, Inc., Bristol, PA) with 200 mg of stationary phase with conditioned 1.8 ml of acetonitrile/water 80:20 (Optima Acetonitrile–Fisher, double-distilled H2O–NANOpure ultrapure water system), after which 150 μl from the 300 μl of transferred organic solution was applied to the SPE column. The analytes of interest (natural abundant product of GLA-S and deuterated GLA-IS) were eluted from the column using 1 ml of acetonitrile/water 80:20, and to each eluent was added 2 μl of 90% formic acid. The samples were analyzed by nano-electrospray-quadrupole-time-of-flight tandem mass spectrometry (nano-ESI-QTOF-MS-MS) using the Agilent (Santa Clara, CA) chip cube with infusion chip (Agilent no. G4240-61002) and QTOF (model 6530) instrumentation.
The following conditions were used for the analysis: flow rate from syringe pump, 70 μl/hr; capillary voltage, 1,500 V; fragmentor voltage, 150 V; nitrogen gas temperature, 325°C; nitrogen gas flow rate, 4 L/min; run time, 1 min; collision cell exit potential, 20 V. In MS mode, the product of the GLA-S was detected at 484.2806 m/z; the deuterated GLA-IS was detected at 489.3119 m/z in MS/MS mode. Multiple reaction monitoring transitions monitored for these ions were as follows: 484.2806 m/z fragments to 384.2282 m/z (GLA-S) and 489.3119 m/z fragments to 389.2595 m/z (GLA-IS) (in this document, these MS/MS peaks will henceforth be referred to as the 384 m/z peak and the 389 m/z peak). The mass accuracy was ± 5 ppm. The 1-min analyses were done in triplicate, and the 384/389 peak height isotope dilution mass spectrometric (IDMS) ratio was used to evaluate the GLA activity. The IDMS isotopic ratio method and its mathematical foundation are given in the U.S. EPA Method 6800 published in 2008. IDMS MS/MS replaced the traditional fluorescence method when it became clear that analysis of organ homogenates was limited by autofluorescence and detection limits of the fluorescence protocol.
For all GLA assays, protein concentration of each sample was determined and fluorescent or MS/MS readings were corrected for protein concentration. Relative GLA activity is expressed as percentage of wild-type.
Western blot analysis of FLAG-GLA in tissue lysates
Tissue lysate samples (25 μg of total protein) were treated for 1.5 hr with either 50 U of endoglycosidase H (Endo H) or 15 U of N-glycosidase F (PNGase F) at 37°C, or no glycosidases as a control, and resolved on a 10% SDS-PAGE gel. After transfer to nitrocellulose, membranes were probed with an anti-FLAG polyclonal antibody (pAb; in Tris-buffered saline with Tween-20) overnight at 4°C, followed by incubation with an anti-rabbit horseradish peroxidase secondary antibody. Bands were detected with the ECL detection reagents (GE Healthcare, Waukesha, WI).
RT-PCR for adenoviral genomes
DNA from mouse liver was isolated using a DNeasy Kit (Qiagen, Valencia, CA). Ad5 E4 gene copy number in the samples was determined in quantitative real-time PCR. Primers for amplifying the E4 gene were as follows: forward, 5'-GGAGTGCGCCGAGACAAC-3'; reverse, 5'-TGGATGCCACAGGATTCCAT and the probe 5'-TGGCATGACACTACGACCAACACGATCT-3'. As endogenous control, mouse β-actin gene was amplified with the following primers: forward, 5'-CGAGCGGTTCCGATGC-3'; reverse, 5'-TGGATGCCACAGGATTCCAT-3' and the probe 5'-AGGCTCTTTTCCAGCCTTCCTTCTTGG. To determine the viral copy number, fluorescent readings of the samples were compared to the standard curve created using DNA with a known number of viral copies.
Statistical analyses
Data are reported as means ± SD. Differences in relative GLA activity among the untreated, low-dose, and high-dose treatment groups were compared using either one-way analysis of variance (ANOVA) or Kruskal–Wallis one-way ANOVA on ranks if Levene's test for the equality of variances was violated. A Games–Howell post-hoc procedure test was performed to evaluate differences between the means of the three groups. For comparison of plasma between untreated and high-dose groups, an independent samples t test was used. A P value of <0.05 was considered to indicate statistical significance. Statistical analysis was performed using SPSS version 14.0 (SPSS Inc., Chicago, IL).
Results
Ad-GLA-FLAG expresses an enzymatically functional GLA transgene
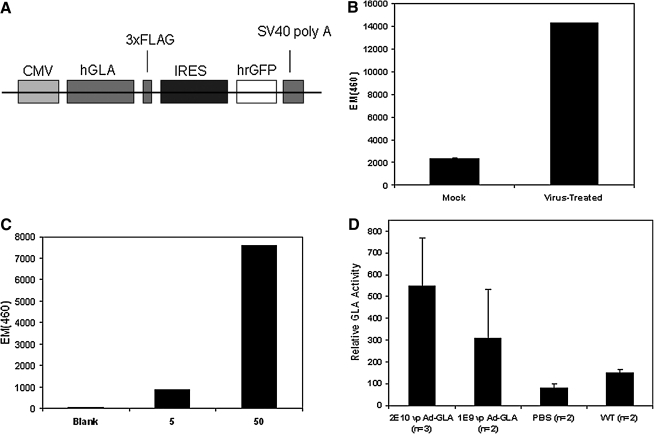
A schematic of the expression cassette for our gene transfer vector is shown in Fig. 1A. We first sought to demonstrate the ability of this adenoviral vector to express a functional GLA transgene using the classical assay principle of specific cleavage of 4-methylumbelliferyl α-galactoside to 4-methylumbelliferone (4-MU) by GLA at pH 4.0. 4-MU emits light at 460 nm when excited by 365-nm light, and thus relative GLA activity can be assayed using a spectrofluorometer. Figure 1B shows relative GLA activity in 293 cells infected with our Ad-GLA-FLAG virus relative to mock-infected controls, demonstrating substantial enhancement of GLA activity. To demonstrate that this enhancement of GLA activity was due to our transgene, we performed a FLAG immunoprecipitation (to isolate our FLAG-tagged GLA transgene from endogenous sources of GLA-like activity) on the cellular lysates assayed in Fig. 1B and performed a cell-free GLA activity assay on the eluate from the FLAG column, with the results presented in Fig. 1C. FLAG-tagged enzyme retained by the column cleaved 4-methylumbelliferyl α-galactoside in a dose-dependent manner. Finally, we delivered either 2 × 1010 or 1 × 109 viral particles (“high dose” and “low dose,” respectively, with PBS infusions serving as negative controls) of Ad-GLA-FLAG to the right submandibular gland of Fabry mice. Four days later, mice were sacrificed and GLA enzyme assays performed on lysates of the salivary glands. Figure 1D shows GLA activity in mice treated with Ad-GLA-FLAG compared with PBS-treated negative controls and untreated wild-type positive controls. In the aggregate, these experiments demonstrate that our virus expresses an enzymatically functional, FLAG-tagged form of human GLA in the mouse salivary gland.
FIG. 1.
(A) Schematic of the expression cassette packaged in our Ad-GLA-FLAG gene transfer vector. The CMV promoter drives expression of GLA fused to a C-terminal FLAG sequence followed by a stop codon. Downstream from the stop codon, an internal ribosomal entry site (IRES) initiates expression of green fluorescent protein (GFP). (B) GLA assay of cellular lysates from AD-293 cells 24 hr after infection with 100 viral particles of Ad-GLA-FLAG (mock-infected cells are negative controls). (C) GLA assay of anti-FLAG column eluate at two different sample concentrations (5 or 50 ml of eluate in 400 ml of citrate buffer with 20 ml of substrate; note: protein concentration of eluate was not determined). (D) GLA assay of salivary gland tissue homogenates 48 hr after delivery of Ad-GLA-FLAG to the right submandibular gland. Glands from animals receiving a saline infusion served as negative controls. Error bars are ± SEM.
GLA transgene produced in the salivary gland enters the endocrine secretory pathway as a different glycoform from the identical transgene produced in the liver
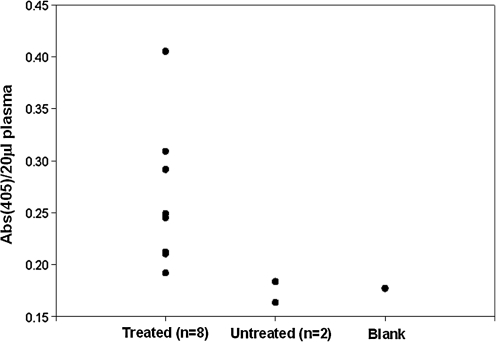
To first test our hypothesis that expression of our GLA transgene in the salivary gland would result in its endocrine secretion, we screened serum from eight treated (2 × 1010 viral particles) animals 3 days post treatment against two untreated knockouts as negative controls using an ELISA assay where an anti-FLAG mAb was used as the capture antibody and an anti-GLA pAb served as the detection antibody. Results of this experiment are shown in Fig. 2. All treated animals showed higher absorption at 405 nm [Abs(405)] relative to controls, with notable variability.
FIG. 2.
ELISA performed on serum samples from high-dose treated (n = 8) and untreated (n = 2) animals 4 days post treatment using an anti-FLAG mAb as a capture antibody and an anti-GLA pAb as detection antibody. Data are presented as Abs(405) of a 20-μl serum sample on a per-animal basis.
We next sought to characterize the organ trafficking of GLA entering the endocrine secretory pathway, and performed a series of experiments wherein we delivered either 2 × 1010 or 1 × 109 viral particles (“high dose” and “low dose,” respectively) of Ad-GLA-FLAG to the right submandibular gland of Fabry mice. Four days later, animals were sacrificed. We performed Western blot analysis of a relevant affected organ in Fabry disease (kidney), as well as a bystander organ (liver) and an organ that should not allow entry of the transgene (brain) in animals wherein the FLAG-tagged transgene GLA had been demonstrated to be expressed in the salivary gland. As a positive control, we used animals with a tail-vein injection of Ad-GLA-FLAG, relying on precedent from Ziegler et al. (1999), which showed that this method results in systemic circulation of the GLA transgene after its production in the liver.
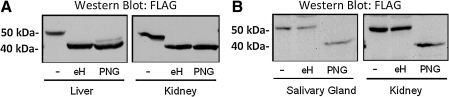
Surprisingly, we found that GLA-FLAG produced in the salivary gland, whether detected in the gland or in remote organs, consistently migrated on Western blot with an apparent molecular weight of ∼8–10 kDa higher than GLA-FLAG produced in the liver. We rationalized that this difference in apparent molecular weight was due to differential glycosylation of the GLA-FLAG transgene when produced in salivary gland versus the liver. To test this possibility, tissue lysates from tail vein-injected and salivary gland-treated animals were incubated with either Endo H (which removes unprocessed high-mannose N-glycans) or PNGase F (which removes both high-mannose and complex-type N-glycans), resolved by SDS-PAGE, and analyzed by Western blot. As shown in Fig. 3, FLAG-GLA from both the kidney and the liver of the tail vein-injected mice was completely sensitive to Endo H treatment, indicating that the enzyme bears exclusively high mannose-type N-glycans. In contrast, however, the electrophoretic mobility of FLAG-GLA from the kidney and salivary gland of the salivary gland-transduced mice was insensitive to Endo H and could only be shifted upon PNGase F treatment, demonstrating that it bears complex-type N-glycans. Together, these results suggest that unique glycoforms of GLA are produced by the two methods of GLA transgene expression.
FIG. 3.
Different glycoforms of GLA are produced by the liver (A; tail-vein injection) and salivary gland (B; salivary-gland cannulation). Representative Western blots of FLAG-GLA from various tissues following treatment with Endo H (eH) or PNGase F (PNG) or with no treatment (–) are shown.
The GLA-FLAG adenovirus is not entirely contained within the salivary gland at high doses
Due to the presence of tight junctions between the cellular components of the salivary gland (Maria et al., 2008), it has been assumed in prior studies that viral vectors delivered to the salivary gland via salivary duct catheterization remain mainly sequestered within the gland and do not spread systemically throughout the animal (Voutetakis et al., 2004; Adriaansen et al., 2008). Because this assumption has important implications for safety in translating this strategy to humans, we tested this assumption by performing quantitative PCR analysis of liver samples for the presence of adenoviral genomes. The liver was chosen based on previous reports that show >99% of an adenoviral vector delivered systemically to a mouse is retained by the liver within 1 hr (Alemany et al., 2000).
Figure 4 shows results of quantitative PCR experiments detecting the adenoviral E4 genomic region performed in duplicate on liver homogenates from high- and low-dose groups, with two untreated knockout animals serving as negative controls. Although the average number of adenoviral genomes per microgram of liver was negligible (0–20 copies/μg), four of the 14 high-dose animals had >20 copies/μg (157, 398, 206, and 88 copies/μg). Further, when we examined the GLA transgene in the liver and kidney of these four animals, we found the GLA transgene to be Endo H-sensitive, indicating that it had been produced locally within the liver. Endo H-sensitive GLA could not be detected in the remaining high-dose animals or in any of the low-dose animals (data not shown). Based on these results, the four high-dose animals with >20 adenoviral copies/μg of liver tissue were excluded from subsequent biochemical analysis. Adenoviral genomes were not detected in livers from nontreated knockout animals.
FIG. 4.
RT-PCR for adenoviral genomes in liver homogenates. Data are presented as adenoviral E4 region copy number/μg of tissue protein. Error bars are ± SEM.
The GLA transgene is functional after trafficking to remote organs
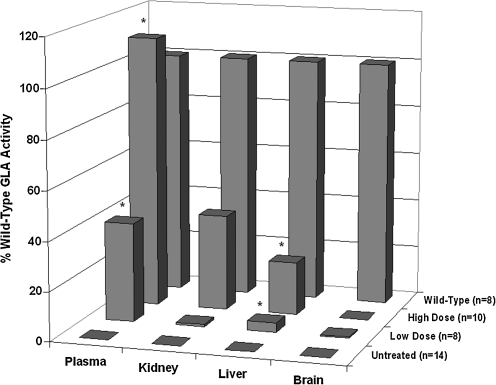
The therapeutic regimen used in this study was very short (4 days) in the context of a lifelong disease, but the expression levels of adenovirus in the salivary gland at that time point are near peak (Voutetakis et al., 2005). We hypothesized that with the relatively high expression levels driven by adenovirus, we might be able to gain some insight into whether the relative affinities of various organs for the therapeutic transgene might affect the kinetics of biochemical correction over longer treatment regimens. Accordingly, we chose to first sample the plasma to establish semiquantitative levels of GLA transgene, and then we studied GLA activity in (1) an organ that is severely afflicted in Fabry's disease (kidney), (2) an organ that is not critically involved in Fabry disease (liver), and (3) the brain, which by virtue of the blood–brain barrier is known to exclude therapeutic GLA. Due to the high and variable background tissue homogenates contribute to the fluorescent GLA activity assay, biochemical analysis of kidney, liver, and brain tissues was performed with an MS/MS-based assay modified from that of Li et al. (2004).
Relative GLA activities in plasma, kidney, liver, and brain in the various experimental groups are presented in Fig. 5. Plasma GLA activity was significantly (p < 0.05) elevated relative to untreated animals in both the low-dose (40% of wild-type) and high-dose (111% of wild-type) groups. In the kidney, there was a trend toward higher GLA activity relative to the control in the high-dose group (39% of wild-type), which was not significant (p < 0.069) due to high intragroup variability in the high-dose group. In the liver, both low- and high-dose groups had significantly (p < 0.05) elevated GLA activity relative to the untreated group (3.8% and 21.5% of wild-type, respectively). Finally, GLA activity in the brain in both treatment groups was <1% of wild-type. However, two of the 10 high-dose animals showed exceptionally high levels of brain GLA activity and were excluded from analysis as outliers as being >5 standard deviations above the mean.
FIG. 5.
GLA activity measured in serum and organ homogenates in the various treatment groups (z-axis). Serum (50 μl) was assayed, and average EM(460) for the untreated knockout animals determined the value of background (0% activity). The activity of experimental groups is expressed as the percentage of average activity in wild-type animals. For organ homogenates, a ratio of GLA-product to GLA-IS was determined and corrected for protein concentration. Product/IS ratio for untreated knockout animals determined the value of background (0% activity). The activity of experimental groups is expressed as the percentage of average activity in wild-type animals. *p < 0.5, significantly different.
GLA is undetectable in the saliva
As previous work in the field has shown that transgenes expressed in the salivary gland do not distribute exclusively to the endocrine or exocrine pathways, but rather a mixture of both, we collected saliva from all animals in this study to compare GLA activity in serum against an equivalent volume of saliva. However, GLA activity in neither the wild-type nor the treatment groups was significantly different from that of untreated knockout animals, with the exception of one animal where GLA activity above baseline was detectable in the saliva. This led us to speculate that either (1) the GLA transgene does not enter the exocrine secretory pathway or (2) GLA loses its enzymatic activity in the saliva.
To test the former theory, we performed Western blot analysis of saliva samples from these same animals, and probed these blots for the FLAG tag on the transgene, with a size-matched (49.3 kDa) synthetic fusion protein comprised of FLAG-tagged bacterial alkaline phosphatase serving as a positive control. We were unable to visualize a FLAG-positive band of a size corresponding to GLA's apparent molecular weight (data not shown). We speculate that both the relatively low concentration of protein in the saliva matrix may complicate this analysis, because GLA secreted into the blood will accumulate in organs, as opposed to GLA secreted into the saliva, which will be routinely washed out in the physiological salivary flow. Nevertheless, significant secretion of GLA into the saliva should be detectable with Western blot or enzymatic assay, and we therefore conclude that GLA is not majorly secreted into the saliva.
Gender does not obviously affect endocrine secretion of GLA from the salivary glands
Post-hoc analysis was carried out to probe correlation between organ/plasma GLA activity and gender in all experimental groups, and no significant correlation could be established. We acknowledge that this study is lightly powered to address this question, and therefore we cannot entirely exclude the possibility that gender might affect this mechanism and/or host response to the enzyme (as in Vedder et al., 2008).
Discussion
This study is the first to explore the intriguing concept of salivary gland-based gene therapeutics as a potential treatment for an LSD. We have demonstrated that a GLA transgene expressed in the salivary gland enters the plasma, disseminates to remote organs, and partially rescues the GLA-knockout phenotype. Furthermore, we observed both dose dependence of this phenomenon at this very early (4 days post treatment) time point and a specific salivary gland-produced GLA glycoform, strengthening our theory that a salivary gland endocrine secretory mechanism governs this effect. These data with adenovirus now provide a rationale for further studies to explore the cumulative effect of this gene therapy approach over longer treatment regimens using, for example, AAV to attempt treatment over months to years.
The issue of saliva/serum distribution of transgenes expressed in the salivary glands has been a major research topic in prior studies using this approach, mainly because the signals governing these distinct secretory pathways in the salivary gland are poorly understood. Whereas these prior studies have reported various saliva/serum distribution ratios, notably between erythropoietin and human growth hormone when directly compared (Voutetakis et al., 2005; Samuni et al., 2008a,b), this is to our knowledge the first study that has examined the ratio of functional transgenes between the two compartments. Based on our findings, we conclude that GLA secreted into the plasma is functional, whereas GLA activity is absent in the saliva (although this is likely due to absence of the enzyme rather than its inactivation). This observation is not entirely unexpected, given the constitutive nature of GLA secretion under native conditions. It should be noted that, from the standpoint of translating this gene therapy strategy to humans, paucity of the transgene in the saliva could be a positive, in that undesired gastrointestinal side effects would presumably be minimized.
The relative organ biodistribution of GLA in the Fabry mice treated in this study is interesting, given the considerable work that has already been published concerning GLA enzyme replacement in this mouse model. Specifically, systemically injected adenovirus has been used previously to endogenously express GLA in a Fabry mouse (Ziegler et al., 1999). Later, this group used both AAV2 (Ziegler et al., 2004) and AAV8 (Ziegler et al., 2007) to deliver GLA primarily to the liver, with impressive results. Exogenously produced GLA has been used for enzyme replacement in a Fabry mouse in preclinical studies (Ioannou et al., 2001), leading to the approval by the FDA of agalsidase beta for the treatment of Fabry disease in humans.
One question we considered in designing and interpreting these experiments is whether detection of the GLA transgene in the blood indicates native endocrine secretion, or whether endocrine secretion occurs only due to overflow of the exocrine pathway or even destruction of infected cells with the consequent dumping of the gene product into the blood. This consideration is particularly important in light of a recent report by Adriaansen et al. (2008), wherein they reported that in “high dose” (1 × 1010 viral particles) mice a human parathyroid hormone (hPTH) transgene was detected in the serum at levels above baseline after administration of an hPTH-expressing adenovirus, whereas hPTH levels in a “low dose” (1 × 109 viral particles) group of mice were indistinguishable from baseline.
Our finding that GLA activity is enhanced in serum of both high- and low-dose groups in our study suggests that a genuine, unsaturated endocrine secretory mechanism is involved in at least the low-dose group. Two caveats should be made however: (1) in our experiments, 1 × 109 viral particles were delivered unilaterally to the submandibular gland, whereas in the Adriaansen study this dose is split equilaterally (thus, our low dose is effectively twice that of the low dose in the Adriaansen study); (2) two different detection methods, with likely differing sensitivities, were used in the two studies. Taken together, and in light of the clinical observation that only 1–5% of normal enzyme activity is needed to prevent some LSDs (Muenzer and Fisher, 2004), these observations call for more sensitive transgene detection methods (perhaps based on mass spectrometry) in future studies involving this gene therapy strategy for Fabry disease.
Our finding that advenoviral genomes were present in the livers of animals in the high-dose group compares with a similar finding when AAV was used in the salivary gland (Katano et al., 2006) and complicates interpretation of the organ biochemistry results. It has been assumed that tight junctions between ductal and acinar cells in the salivary gland (Maria et al., 2008) mainly sequester the virus, but our data illustrate that this sequestration is imperfect. As cannulation of the glands involves filling the intraductal labyrinth with carrier solution, we hypothesize that distention of the gland could result in vector leaking into the blood and delivery to the liver. This is a cautionary finding for other gene therapy strategies involving the salivary gland, and argues that RT-PCR detection of viral vectors in the liver is an important quality-control step in such studies. Indeed, Voutetakis et al. (2004) and Katano et al. (2006) did include this important step, whereas more recent studies have not always done so. In our case, we are reasonably confident that GLA produced in the salivary gland underlies the biochemical improvement because we have been able to differentiate liver-produced versus salivary gland-produced GLA based on the nature of its glycosylation.
Our surprising finding that GLA produced in the salivary gland is exclusively of the complex-type glycoform, in contrast to the liver, where the transgene is produced exclusively as the high-mannose glycoform, warrants further investigation. Ablation of carbohydrate recognition on lysosomal enzymes has been shown to alter biodistribution, even resulting in transit of the blood–brain barrier (Grubb et al., 2008). This might lead to differences in biodistribution between our system and liver-directed gene transfer such as that described by Ziegler et al. (1999). More investigation will be needed to fully characterize the potential therapeutic implications of this differential glycosylation.
One of the most positive aspects of the gene therapy strategy used in this study is its potential to achieve a constant “trickle” of GLA as opposed to the bolus injections of GLA now used to treat the disease in humans. The blood half-life of agalsidase beta is dose-dependant and varies from 45 to 100 min, and thus tissues must absorb the therapeutic during a relatively short window following infusion and retain it for the 2-week period between doses. The potential advantages of constant endogenous production of the therapeutic have been shown in prior studies. Now that it has been demonstrated that a GLA transgene expressed in the salivary gland can traffic via an endocrine pathway to multiple tissues and exert therapeutic activity, future studies should address long-term expression vectors (e.g., AAV) to further explore the translational potential of this gene therapy strategy for Fabry disease and possibly other LSDs.
Acknowledgments
This work was supported by NIH grants 1K99DE018188-01 and 5R00DE018188-03 to M.J.P. and 5R01GM086524-03 to R.S. The work was partially supported by NSF grants 0421152 and 0821401 to H.M.K. The authors would like to thank Dr. Changyu Zhang for his editorial comments, Diane Vido for help with statistical analysis, and the Centers for Disease Control and Prevention Newborn Screening and Molecular Biology Branch for providing the GLA-S and GLA-IS reagents.
Author Disclosure Statement
The authors certify that no competing financial interests exist relevant to this manuscript.
References
- Adriaansen J. Perez P. Goldsmith C.M., et al. Differential sorting of human parathyroid hormone after transduction of mouse and rat salivary glands. Hum. Gene Ther. 2008;19:1021–1028. doi: 10.1089/hum.2008.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemany R. Suzuki K. Curiel D.T. Blood clearance rates of adenovirus type 5 in mice. J. Gen. Virol. 2000;81:2605–2609. doi: 10.1099/0022-1317-81-11-2605. [DOI] [PubMed] [Google Scholar]
- Barngrover D. Fabrazyme—recombinant protein treatment for Fabry's disease. J. Biotechnol. 2002;95:280–282. [PubMed] [Google Scholar]
- Beck M. New therapeutic options for lysosomal storage disorders: enzyme replacement, small molecules and gene therapy. Hum. Genet. 2007;121:1–22. doi: 10.1007/s00439-006-0280-4. [DOI] [PubMed] [Google Scholar]
- Beutler E. Kuhl W. Purification and properties of human alpha-galactosidases. J. Biol. Chem. 1972;247:7195–7200. [PubMed] [Google Scholar]
- Castle D. Castle A. Intracellular transport and secretion of salivary proteins. Crit. Rev. Oral Biol. Med. 1998;9:4–22. doi: 10.1177/10454411980090010301. [DOI] [PubMed] [Google Scholar]
- Desnick R.J. Allen K.Y. Desnick S.J., et al. Fabry's disease: enzymatic diagnosis of hemizygotes and heterozygotes. Alpha-galactosidase activities in plasma, serum, urine, and leukocytes. J. Lab. Clin. Med. 1973;81:157–171. [PubMed] [Google Scholar]
- Gelb M.H. Turecek F. Scott C.R. Chamoles N.A. Direct multiplex assay of enzymes in dried blood spots by tandem mass spectrometry for the newborn screening of lysosomal storage disorders. J. Inherit. Metab. Dis. 2006;29:397–404. doi: 10.1007/s10545-006-0265-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieselmann V. Lysosomal storage diseases. Biochim. Biophys. Acta. 1995;1270:103–136. doi: 10.1016/0925-4439(94)00075-2. [DOI] [PubMed] [Google Scholar]
- Grubb J.H. Vogler C. Levy B., et al. Chemically modified beta-glucuronidase crosses blood–brain barrier and clears neuronal storage in murine mucopolysaccharidosis VII. Proc. Natl. Acad. Sci. U.S.A. 2008;105:2616–2621. doi: 10.1073/pnas.0712147105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges B.L. Cheng S.H. Cell and gene-based therapies for the lysosomal storage diseases. Curr. Gene Ther. 2006;6:227–241. doi: 10.2174/156652306776359522. [DOI] [PubMed] [Google Scholar]
- Ioannou Y.A. Zeidner K.M. Gordon R.E. Desnick R.J. Fabry disease: preclinical studies demonstrate the effectiveness of alpha-galactosidase A replacement in enzyme-deficient mice. Am. J. Hum. Genet. 2001;68:14–25. doi: 10.1086/316953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenman L. Liebow C. Rothman S. The endocrine secretion of mammalian digestive enzymes by exocrine glands. Am. J. Physiol. 1999;276:E223–E232. doi: 10.1152/ajpendo.1999.276.2.E223. [DOI] [PubMed] [Google Scholar]
- Jacobs F. Feng Y. Van Craeyveld E., et al. Species differences in hepatocyte-directed gene transfer: implications for clinical translation. Curr. Gene Ther. 2009;9:83–90. doi: 10.2174/156652309787909562. [DOI] [PubMed] [Google Scholar]
- Jung S.C. Han I.P. Limaye A., et al. Adeno-associated viral vector-mediated gene transfer results in long-term enzymatic and functional correction in multiple organs of Fabry mice. Proc. Natl. Acad. Sci. U.S.A. 2001;98:2676–2681. doi: 10.1073/pnas.051634498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura K. Liu D. Physical approaches for nucleic acid delivery to liver. AAPS J. 2008;10:589–595. doi: 10.1208/s12248-008-9067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katano H. Kok M.R. Cotrim A.P., et al. Enhanced transduction of mouse salivary glands with AAV5-based vectors. Gene Ther. 2006;13:594–601. doi: 10.1038/sj.gt.3302691. [DOI] [PubMed] [Google Scholar]
- Kint J.A. Fabry's disease: alpha-galactosidase deficiency. Science. 1970;167:1268–1269. doi: 10.1126/science.167.3922.1268. [DOI] [PubMed] [Google Scholar]
- Li Y. Scott C.R. Chamoles N.A., et al. Direct multiplex assay of lysosomal enzymes in dried blood spots for newborn screening. Clin. Chem. 2004;50:1785–1796. doi: 10.1373/clinchem.2004.035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoDuca P.A. Hoffman B.E. Herzog R.W. Hepatic gene transfer as a means of tolerance induction to transgene products. Curr. Gene Ther. 2009;9:104–114. doi: 10.2174/156652309787909490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maria O.M. Kim J.W. Gerstenhaber J.A., et al. Distribution of tight junction proteins in adult human salivary glands. J. Histochem. Cytochem. 2008;56:1093–1098. doi: 10.1369/jhc.2008.951780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medin J.A. Tudor M. Simovitch R., et al. Correction in trans for Fabry disease: expression, secretion and uptake of alpha-galactosidase A in patient-derived cells driven by a high-titer recombinant retroviral vector. Proc. Natl. Acad. Sci. U.S.A. 1996;93:7917–7922. doi: 10.1073/pnas.93.15.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muenzer J. Fisher A. Advances in the treatment of mucopolysaccharidosis type I. N. Engl. J. Med. 2004;350:1932–1934. doi: 10.1056/NEJMp048084. [DOI] [PubMed] [Google Scholar]
- Neufeld E.F. Lysosomal storage diseases. Annu. Rev. Biochem. 1991;60:257–280. doi: 10.1146/annurev.bi.60.070191.001353. [DOI] [PubMed] [Google Scholar]
- Ohshima T. Murray G.J. Swaim W.D., et al. alpha-Galactosidase A deficient mice: a model of Fabry disease. Proc. Natl. Acad. Sci. U.S.A. 1997;94:2540–2544. doi: 10.1073/pnas.94.6.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuni Y. Cawley N.X. Zheng C., et al. Sorting behavior of a transgenic erythropoietin-growth hormone fusion protein in murine salivary glands. Hum. Gene Ther. 2008a;19:279–286. doi: 10.1089/hum.2007.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuni Y. Zheng C. Cawley N.X., et al. Sorting of growth hormone-erythropoietin fusion proteins in rat salivary glands. Biochem. Biophys. Res. Commun. 2008b;373:136–139. doi: 10.1016/j.bbrc.2008.05.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler M.P. Cerullo V. Lee B. Immune response to helper dependent adenoviral mediated liver gene therapy: challenges and prospects. Curr. Gene Ther. 2007;7:297–305. doi: 10.2174/156652307782151452. [DOI] [PubMed] [Google Scholar]
- Takenaka T. Qin G. Brady R.O. Medin J.A. Circulating alpha-galactosidase A derived from transduced bone marrow cells: relevance for corrective gene transfer for Fabry disease. Hum. Gene Ther. 1999;10:1931–1939. doi: 10.1089/10430349950017293. [DOI] [PubMed] [Google Scholar]
- Takenaka T. Murray G.J. Qin G., et al. Long-term enzyme correction and lipid reduction in multiple organs of primary and secondary transplanted Fabry mice receiving transduced bone marrow cells. Proc. Natl. Acad. Sci. U.S.A. 2000;97:7515–7520. doi: 10.1073/pnas.120177997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedder A.C. Breunig F. Donker-Koopman W.E., et al. Treatment of Fabry disease with different dosing regimens of agalsidase: effects on antibody formation and GL-3. Mol. Genet. Metab. 2008;94:319–325. doi: 10.1016/j.ymgme.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Voutetakis A. Kok M.R. Zheng C., et al. Reengineered salivary glands are stable endogenous bioreactors for systemic gene therapeutics. Proc. Natl. Acad. Sci. U.S.A. 2004;101:3053–3058. doi: 10.1073/pnas.0400136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voutetakis A. Bossis I. Kok M.R., et al. Salivary glands as a potential gene transfer target for gene therapeutics of some monogenetic endocrine disorders. J. Endocrinol. 2005;185:363–372. doi: 10.1677/joe.1.06171. [DOI] [PubMed] [Google Scholar]
- Ziegler R.J. Yew N.S. Li C., et al. Correction of enzymatic and lysosomal storage defects in Fabry mice by adenovirus-mediated gene transfer. Hum. Gene Ther. 1999;10:1667–1682. doi: 10.1089/10430349950017671. [DOI] [PubMed] [Google Scholar]
- Ziegler R.J. Lonning S.M. Armentano D., et al. AAV2 vector harboring a liver-restricted promoter facilitates sustained expression of therapeutic levels of alpha-galactosidase A and the induction of immune tolerance in Fabry mice. Mol. Ther. 2004;9:231–240. doi: 10.1016/j.ymthe.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Ziegler R.J. Cherry M. Barbon C.M., et al. Correction of the biochemical and functional deficits in Fabry mice following AAV8-mediated hepatic expression of alpha-galactosidase A. Mol. Ther. 2007;15:492–500. doi: 10.1038/sj.mt.6300066. [DOI] [PubMed] [Google Scholar]