Abstract
OBJECTIVES
The purposes of this study were to quantify the time and effort involved in obtaining prenatal consent for the Neonatal Research Network Surfactant Positive Airway Pressure and Pulse Oximetry Randomized Trial (SUPPORT) and to determine whether the enrolled infants were representative of the eligible population.
METHODS
Eligible subjects were likely to deliver in the SUPPORT gestational age window (24–27 6/7; weeks). Data included who approached the subjects for consent, how often they approached, the duration of each contact, whether consent was obtained, and whether subjects were enrolled in the trial. Eligible, nonenrolled infants entered into the Neonatal Research Network Generic Database throughout the period of SUPPORT enrollment were compared with enrolled infants.
RESULTS
A total of 2826 women were identified at 18 sites, 2228 were approached for consent, and 1219 (54.7%) agreed. For 76.9% of those approached, <3 visits (mean: 2.0 ± 1.2 visits) were required to complete the consent process. Of the 659 infants with consent who were delivered within the study window, 611 were enrolled. Mothers who received a neonatal consultation were more likely to give consent (P < .001). The proportion of infants not exposed to steroids was significantly greater in the nonapproached group than in the approached group (20.0% vs 3.4%; P < .0001).
CONCLUSION
In a trial that involved preterm infants and required prenatal consent, >5 women were identified as being likely to deliver in the SUPPORT gestational age window for each 1 who delivered an enrolled infant.
Keywords: informed consent, prenatal, neonatal
Informed consent ensures that subjects enrolled in research trials are appropriately informed of the risks and benefits and come to the trial voluntarily. Traditionally, consent is obtained directly from the subjects when they are in a condition to understand and to participate in the process. In trials involving infants who will receive an intervention at or near the time of birth, the process requires approaching families before delivery.
Funding for clinical trials often is based on a model of capitation in which a site is paid on the basis of the number of subjects enrolled. Funds may be awarded for screening for eligible subjects, but usually centers are not paid for the time required to seek or to obtain consent for subjects who are not enrolled. Because prospective data comparing the number of families that are approached in the prenatal period with the number of infants who are enrolled have not been available, the current model may not account adequately for time spent by study staff members to screen, to approach, and to enroll all eligible subjects.
The Surfactant Positive Airway Pressure and Pulse Oximetry Trial in Extremely Low Birth Weight Infants (SUPPORT) was a randomized, factorial, 2 × 2 design, multicenter trial conducted by the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN). The trial compared prospectively continuous positive airway pressure therapy and a protocol-driven, limited, ventilatory strategy begun in the delivery room and continued in the NICU with early (<1-hour) intratracheal administration of surfactant, followed by conventional mechanical ventilation. Infants also were assigned randomly to a prospective comparison of a lower pulse oxygen saturation target range (85%–89%) and a higher, more-conventional, pulse oxygen saturation target range (91%–95%) until the infant no longer required ventilatory support or oxygen, by using purpose-altered oximeters. Early screening and enrollment in the SUPPORT suggested that prenatal screening and consent were labor-intensive, and the number of patients enrolled seemed to be much smaller than the number screened. Therefore, this prospective, secondary study was designed to quantify the prenatal screening and consent process, to determine the time, effort, and other factors that contribute to successful enrollment of patients in a complex trial conducted by an experienced, multicenter, trial network. The second objective of the study was to determine whether the subjects enrolled in the trial were representative of the overall eligible population.
METHODS
This was a prospective cohort study of the prenatal consent practices of research personnel in the SUPPORT. Data for this secondary study were collected during the second half of the SUPPORT. Eligible infants were infants born at NRN centers at gestational ages (GAs) of 24 0/7 to 27 6/7 weeks, without known malformations, for whom full postnatal treatment was planned. SUPPORT enrollment began in February 2005 and ended in February 2009.
Eligible subjects for the prenatal consent secondary study were women the obstetric/perinatal staff members thought were likely to deliver in the SUPPORT GA window. Clinical data were collected by trained research coordinators, and all analyses were performed at a central data coordinating center (RTI International, Research Tri-angle Park, NC). If the parents were not approached for consent, then the reason was recorded. If the parents were approached, then the staff members recorded the total number of times the parents were approached, the duration of the attempts made, and whether permission was obtained from the obstetrician to approach the parents for prenatal consent. Because a neonatal consultation often is performed as part of clinical management in this population, staff members recorded whether a consultation was performed, whether consent was discussed during the consultation, and whether consent was obtained at the time of the consultation. The institutional review boards for some centers required that a consultation be completed before consent, and this was also recorded. Women who were approached were asked whether they were asked to participate in any other studies involving themselves or their infants. Finally, the study staff members were asked to estimate the time required to obtain a decision regarding consent.
Data were collected at each of the 18 NICHD NRN centers until 50 mothers had delivered within the GA window at the center. Data forms were keyed by the centers and transmitted electronically to the data coordinating center. As part of the ongoing NRN Generic Database(GDB)observational study, data were collected routinely for inborn infants at NRN centers, including most of those who met the GA eligibility criteria for the SUPPORT. These data were used to identify eligible, nonenrolled infants and to evaluate whether infants enrolled in the SUPPORT represented the eligible NRN population.
To determine the representativeness of the population enrolled in the SUPPORT, data from the GDB were obtained for infants born at NRN centers during the period of SUPPORT enrollment who met the enrollment criteria for SUPPORT participation. For eligible but nonenrolled infants, information was collected on prenatal care, prenatal steroid treatment, and demographic characteristics. These data were compared for infants who were or were not enrolled in the SUPPORT, to assess whether the trial had enrolled a representative sample of the available population. In addition, comparisons were made between eligible infants whose mothers were or were not approached for the SUPPORT.
We performed bivariate statistical analyses by using χ2 tests and Student’s t tests. All statistical analyses were performed by using SAS 9.1.3 (SAS Institute, Cary, NC). P values of <.05 were considered statistically significant. This trial was approved by the institutional review boards of all participating centers and RTI International.
RESULTS
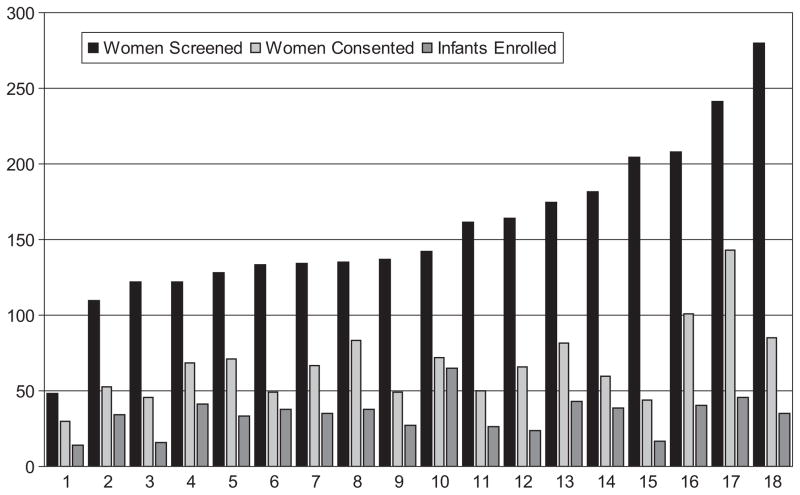
Between October 2005 and February 2009, a total of 2826 women at 18 NRN centers were identified as being at risk of delivering a premature infant between 24 and 27 6/7 weeks of gestation and were otherwise eligible for participation in the SUPPORT. At 13 centers, >50 women (range: 51–90 women) delivered in the GA window before the centers stopped collecting data. All data collected by participating centers were included in this analysis. The results of analyses using only the first 50 women from the centers that overenrolled were not significantly different. Screening, enrollment, and consent numbers according to center are presented in Fig 1.
FIGURE 1.
Numbers of women who were screened, provided consent, and were enrolled, according to center.
A total of 2228 women were approached for informed consent. The 2 most-frequent reasons for not attempting to obtain consent were inability to obtain consent from the mother and lack of time before delivery (Table 1). Reasons mothers were considered unable to give consent included being in active labor, being too young, or having a mental or physical health issue that precluded approach. Common reasons within the “other” category were fetal abnormalities and language barriers that made informed consent impossible. Of note relative to the issue of language is the fact that, despite available, telephone-based, translation services, some institutional review boards do not allow research consent without a written consent form in the primary language of the person involved.
TABLE 1.
Reasons for Not Approaching Mothers for Consent
| Reason | n (%) |
|---|---|
| Mother not able to provide consent | 141 (23.6) |
| Insufficient time | 140 (23.4) |
| Early discharge | 73 (12.2) |
| Study staff members not available | 38 (6.4) |
| Consultation not performed when required before consent | 18 (3.0) |
| Not aware of admission | 35 (5.9) |
| Other | 153 (25.5) |
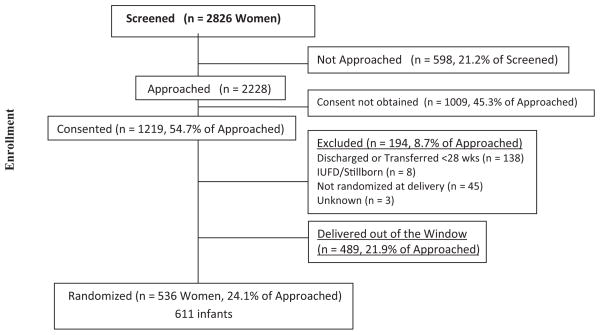
Study coordinators or research nurses made 74.7% of all approaches. For 76.9% of the women approached, consent was attempted <3 times (overall mean: 2.0 ± 1.2 times). The frequency of approaches ranged from 1 time (n = 900) to 11 times (n = 2). The median time spent to obtain a decision regarding consent was <1 hour, regardless of the decision. The estimated GAs at the time of screening were not significantly different for those who consented and those who did not. Of the 2228 mothers who were approached, 1219 (54.7%) gave consent, and 581 (26.1%) subsequently delivered within the SUPPORT GA window. Of those, 536 (92.3%) delivered infants who were enrolled (Fig 2). Forty-eight (7.3%) of the 659 infants with consent who were born in the GA window were not enrolled, usually because of lack of staffing, equipment, or time. Only 19% of the mothers who were screened delivered infants who were subsequently enrolled in the study. A neonatal consultation was performed in 69.7% of cases in which the parents were approached for consent. Mothers who received a neonatal consultation were more likely to consent (P < .001), but consent was obtained during the consultation in only 11.6% of cases.
FIGURE 2.
Modification of the Consolidated Standards for Reporting of Trials diagram, showing the flow of participants through the enrollment stage of a randomized trial using prenatal consent. IUFD indicates intrauterine fetal demise.
We estimated the cost of the consent process by using data from the questionnaire. Because the time needed to obtain consent was not recorded as a continuous variable, the costs were calculated as ranges. It took between 1735 and 2790 hours to obtain consent decisions from the 2228 mothers who were approached, to enroll 611 infants. On the basis of the standard coordinator salary for the NRN at the time of the trial, this represents between $65 945 and $106 029. If we add the time needed to screen 2826 mothers, estimating that screening required 5 to 10 minutes per mother, then the total time and cost estimates for screening and consent to enroll 611 infants range from 1971 to 3261 hours and from $74 894 to $123 927, respectively. With the assumption of equivalent enrollment for the part of the trial not covered by this secondary study, the total screening and consent costs for the trial would have been between $161 311 and $266 920.
Comparison of all infants in the GDB who were eligible for SUPPORT but whose mothers were not approached with infants whose mothers were approached revealed several important differences (Table 2). Approached mothers were significantly more likely to be older and to have a high school education, private medical insurance, and ≥1 prenatal visit. Infants of approached mothers were more likely to be non-Hispanic white. A significantly larger proportion of eligible infants whose mothers were not approached had no prenatal steroid exposure, compared with infants whose mothers were approached (20.0% vs 3.4%; P < .001). Maternal parity was lower among women who were approached, but gravidity was not significantly different between the groups. Eligible infants in the GDB who were not enrolled in the SUPPORT had mothers who were significantly less likely to have insurance and to have received prenatal care and were >4 times more likely to have received no prenatal steroid treatment, compared with enrolled infants (15.7% vs 3.8%) (Table 3).
TABLE 2.
Demographic Data Comparing Infants Whose Mothers Were Approached for Consent and Infants Whose Mothers Were Not Approached
| Infants Whose Mothers Were Approached (N = 2082) | Infants Whose Mothers Were Not Approached (N = 2290) | P | |
|---|---|---|---|
| Maternal age, mean ± SD, y | 27.5 ± 6.4 | 26.9 ± 6.8 | .0071 |
| White, non-Hispanic, n (%) | 853 (41.0) | 774 (33.9) | <.0001 |
| Maternal gravidity, mean ± SD | 2.7 ± 2.0 | 2.9 ± 2.1 | .0844 |
| Maternal parity, mean ± SD | 2.2 ± 1.4 | 2.3 ± 1.5 | .0463 |
| Maternal education more than high school, n (%) | 1134 (75.3) | 1090 (68.3) | <.0001 |
| Self-pay/uninsured, n (%) | 130 (6.3) | 228 (10.0) | <.0001 |
| ≥1 prenatal visit, n (%) | 2015 (96.8) | 2112 (92.4) | <.0001 |
| No antenatal steroid treatment, n (%) | 71 (3.4) | 457 (20.0) | <.0001 |
TABLE 3.
Demographic Data Comparing Infants Who Were Enrolled in SUPPORT With Infants Who Were Eligible But Not Enrolled
| Enrolled Infants (N = 1316) | Nonenrolled Infants (N = 3056) | P | |
|---|---|---|---|
| Maternal age, mean ± SD, y | 27.1 ± 6.4 | 27.2 ± 6.7 | .7107 |
| White, non-Hispanic, n (%) | 521 (39.7) | 1106 (36.3) | .0329 |
| Maternal gravidity, mean ± SD | 2.8 ± 2.0 | 2.8 ± 2.0 | .9438 |
| Maternal parity, mean ± SD | 2.3 ± 1.5 | 2.3 ± 1.5 | .7725 |
| Maternal education more than high school, n (%) | 708 (74.1) | 1516 (70.61) | .0490 |
| Self-pay/uninsured, n (%) | 81 (6.2) | 227 (9.1) | .0012 |
| ≥1 prenatal visit, n (%) | 1263 (96.0) | 2864 (93.9) | .0052 |
| No antenatal steroid treatment, n (%) | 50 (3.8) | 478 (15.7) | <.0001 |
DISCUSSION
To our knowledge, this is the first study to evaluate the time and effort required to approach and to obtain prenatal consent from parents for the enrollment of their unborn infants. Our results revealed that 5 families needed to be identified and screened for every 1 infant enrolled successfully. Only 47.7% of women who consented proceeded to deliver infants in the study window. Although the time needed to obtain consent was recorded as a discrete variable, we found that the median was between 30 minutes and 1 hour. Multiplication of these bounds by the number of women approached for each enrolled infant (3.6 women) yielded a median value for coordinator time required to enroll 1 infant of 1.8 to 3.6 hours, compared with the original trial design of 1.5 to 2 hours. With the assumption that the SUPPORT enrollment rate was equivalent before data collection for the prenatal consent study began, the findings suggest that >4200 hours were spent screening >6000 women and approaching nearly 4800 women for consent to enroll 1316 infants in the SUPPORT. This estimate includes the time spent screening women who were not approached for consent, approaching women who did not consent, and obtaining consent from women who did not deliver in the GA window.
As can be seen in Fig 1, there was significant variation between centers in the process of enrollment. We have found this to be true in every trial in which we have participated, and we think that further analysis of why some centers enroll subjects at faster rates is a question worthy of further research.
Morley et al1 reviewed parents’ willingness to participate in multiple trials in the neonatal period and found that most parents (74%) were willing to allow their infants to participate in multiple trials. We had concerns that the burden of being approached for multiple trials would affect SUPPORT enrollment, especially because 5 centers also belonged to a maternal/fetal research network. We found that only 8.4% of women reported having been approached for another trial at the time of the SUPPORT consent discussion, probably because this involved prenatal consent and the window was early in gestation.
Because of the unique attributes of the NRN, we were able to obtain demographic data from the GDB for infants who were delivered in the SUPPORT GA window but were not enrolled in the study. Therefore, we were able to compare infants who were or were not enrolled and infants whose mothers were or were not approached. The approached versus nonapproached data help us to understand what part of the population never had an opportunity to participate in the enrollment process, as well as the reasons. The enrollment data allowed us to compare the subjects who were enrolled in the trial with those who were eligible but not enrolled. The result of the consent process was very inefficient and costly and biased the trial enrollment, such that the mothers and their infants differed in important ways from the available eligible population of very pre-term infants. Infants born to women who were not approached for consent were almost 6 times more likely not to have been exposed to prenatal steroid treatment, which is an intervention known to be associated with better short- and longer-term outcomes for preterm infants.2 They also were more likely to be more immature, and their mothers were significantly less likely to have insurance and to have received prenatal care.
The Consolidated Standards for Reporting of Trials is a document created with the intent of standardizing items reported in trial publications, including a checklist of necessary items.3,4 The standards require a flowchart depicting participants to quantify eligible populations, but they do not require identification of the demographic features of the nonenrolled group or the similarity of that group to the enrolled or consent-providing populations. In a review of National Institutes of Health (NIH)-funded interventional, randomized, controlled trials, Charlson and Horwitz5 found that only one-half of such trials collected any data on subjects who were eligible but not enrolled; for the trials that collected data, only 27% of nonenrollment of eligible subjects was the result of subject refusal. Following the Consolidated Standards for Reporting of Trials standards in all prenatal consent trials and obtaining basic demographic information for all eligible infants should help us understand whether similar reduction biases in enrollment occur in other studies.
Blinding has always been the standard method for protecting trials from biased enrollment. However, these controls protect the trial only from internal bias and do not provide protection against potential selection bias, as noted in our experience. Blinding does not guarantee the external validity of the findings or their generalizability to nonstudy patients. Manning6 and Silverman7 voiced concerns about this type of selection bias, stating that a disproportionate number of vulnerable, deprived families are involved in clinical research. Harth and Thong8 questioned parents who had volunteered in an asthma trial in Australia and concluded that volunteering parents were “significantly more socially disadvantaged and emotionally vulnerable.” In a study of recruitment and retention of premature infants in an early intervention trial, Constantine et al9 found significant enrollment biases related to race, weight, and site. Mitchell and Kline10 noted significantly greater proportions of black patients, uninsured patients, and Medicaid recipients in the nonparticipant group in a minimal-risk emergency department study. Aagaard-Tillery et al11 found that black subjects and Hispanic subjects were significantly less likely to allow use of their genetic samples for future studies. In our study, the sociodemographic variables tended to favor the enrolled group.
Our analysis suggested that underprivileged subjects were less likely to participate. Because this trial included subjects from 18 academic centers with long-term experience in randomized trials and a broad racial, ethnic, and socioeconomic mixture of populations, it is not likely that the bias represents an intention to include or to exclude any group. It seems more likely that subjects who did not receive prenatal care and thus did not have access to prenatal steroid treatment were more likely to come to the hospital on an emergency basis to deliver their infants, which did not allow time for the research team to obtain informed consent. The logical conclusion is that the nonenrollment of many subjects in this trial, despite the significant time and effort spent by the coordinators to include as many infants as possible, represents the nature of the prenatal consent process itself, which requires that women be approached at some time between admission and delivery.
Title 45 of the Code of Federal Regulations allows institutional review boards to waive some or all elements of consent.12 Our current observations and previous experience suggest that allowing trials comparing routinely used interventions to defer the consent process until after birth may increase the inclusion of the sickest and most at-risk populations. This would ensure that the infants enrolled in such trials would not differ significantly from the overall eligible population unless there was an actual difference in postdelivery consent rates among different social or ethnic groups. Trials involving an experimental drug or device or rising above the level of minimal risk obviously would not be appropriate for this type of enrollment.
CONCLUSIONS
Our prospective analysis of the process of prenatal consent in the SUPPORT has shown that prenatal screening and consent are labor-intensive, that enrollment numbers may be significantly lower than screening and consent rates, and that the population enrolled may not be representative of the total eligible population. In this complex interventional trial involving preterm infants, nearly 5 women were identified as being likely to deliver an infant in the GA window for every 1 infant enrolled in the trial. Rates of prenatal steroid exposure were significantly higher among infants enrolled in the SUPPORT, compared with infants who were eligible but not enrolled, with a similar difference between infants born to women who were approached or not approached. The present technique of obtaining prenatal informed consent is very difficult, time-consuming, and costly and has the potential to enroll selectively a group that is not representative of the available population. Comparative trials that examine the outcomes of 2 currently accepted practices and that have been identified as minimal risk should be considered favorably for waivers of consent.
Acknowledgments
The NIH, the Eunice Kennedy Shriver NICHD, and the National Heart, Lung, and Blood Institute provided grant support for the NRN SUPPORT (recruitment in 2005–2009).
The following investigators, in addition to those listed as authors, participated in this study: NRN Steering Committee Chairs: Alan H. Jobe, MD, PhD, University of Cincinnati (2003–2006); Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine (2006–2011); Case Western Reserve University: Rainbow Babies & Children’s Hospital (NIH grants GCRC M01 RR80 and U10 HD21364), Michele C. Walsh, MD, MS; Avroy A. Fanaroff, MD; Nancy S. Newman, RN; Bonnie S. Siner, RN; Cincinnati Children’s Hospital Medical Center: University of Cincinnati Hospital and Good Samaritan Hospital (NIH grants GCRC M01 RR8084 and U10 HD27853), Kurt Schibler, MD; Edward F. Donovan, MD; Vivek Narendran, MD, MRCP; Kate Bridges, MD; Barbara Alexander, RN; Cathy Grisby, BSN, CCRC; Marcia Worley Mersmann, RN, CCRC; Holly L. Mincey, RN, BSN; Jody Hessling, RN; Duke University School of Medicine: University Hospital, Alamance Regional Medical Center, and Durham Regional Hospital (NIH grants GCRC M01 RR30 and U10 HD40492), Ronald N. Goldberg, MD; C. Michael Cotten, MD, MHS; Kathy J. Auten, MSHS; Kimberly A. Fischer, PhD, FNP-BC, IBCLC; Katherine A. Foy, RN; Gloria Siaw, BSN, CRA; Emory University: Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory Crawford Long Hospital (NIH grants CTSA UL1 RR25008, GCRC M01 RR39, and U10 HD27851), Barbara J. Stoll, MD; Susie Buchter, MD; Anthony Piazza, MD; David P. Carlton, MD; Ellen C. Hale, RN, BS, CCRC; Eunice Kennedy Shriver NICHD: Rosemary D. Higgins, MD; Stephanie Wilson Archer, MA; Indiana University: Indiana University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (NIH grants CTSA UL1 RR25761, GCRC M01 RR750, and U10 HD27856), Brenda B. Poindexter, MD, MS; James A. Lemons, MD; Diana D. Appel, RN, BSN; Faithe Hamer, BS; Dianne E. Herron, RN; Lucy C. Miller, RN, BSN, CCRC; Leslie D. Wilson, BSN, CCRC; RTI International (NIH grant U01 HD36790): Abhik Das, PhD; W. Kenneth Poole, PhD; Marie Gantz, PhD; Margaret Cunningham, BS; Betty K. Hastings; Amanda R. Irene, BS; Jeanette O’Donnell Auman, BS; Carolyn Petrie Huitema, MS; James W. Pickett II, BS; Kristin M. Zaterka-Baxter, RN, BSN; Stanford University: Lucile Packard Children’s Hospital (NIH grants CTSA UL1 RR25744, GCRC M01 RR70, and U10 HD27880), Krisa P. Van Meurs, MD; David K. Stevenson, MD; M. Bethany Ball, BS, CCRC; Melinda S. Proud, RCP; Tufts Medical Center: Floating Hospital for Children (NIH grants GCRC M01 RR54 and U10 HD53119), Ivan D. Frantz III, MD; John M. Fiascone, MD; Anne Furey, MPH; Brenda L. MacKinnon, RNC; Ellen Nylen, RN, BSN; University of Alabama at Birmingham: Health System and Children’s Hospital of Alabama (NIH grants GCRC M01 RR32 and U10 HD34216), Waldemar A. Carlo, MD; Namasivayam Ambalavanan, MD; Monica V. Collins, RN, BSN, MEd; Shirley S. Cosby, RN, BSN; Vivien A. Phillips, RN, BSN; University of California, San Diego: Medical Center and Sharp Mary Birch Hospital for Women (NIH grant U10 HD40461), Neil N. Finer, MD; Maynard R. Rasmussen, MD; Paul R. Wozniak, MD; Kathy Arnell, RNC; Clarence Demetrio, RN; Wade Rich, BSHS, RRT; University of Iowa: Children’s Hospital (NIH grants CTSA UL1 RR24979, GCRC M01 RR59, and U10 HD53109), Edward F. Bell, MD; John A. Widness, MD; Jonathan M. Klein, MD; Karen J. Johnson, RN, BSN; University of Miami: Holtz Children’s Hospital (NIH grants GCRC M01 RR16587 and U10 HD21397), Shahnaz Duara, MD; Ruth Everett-Thomas, RN, MSN; University of New Mexico: Health Sciences Center (NIH grants GCRC M01 RR997 and U10 HD53089), Kristi L. Watterberg, MD; Robin K. Ohls, MD; Julie Rohr, MSN, RNC, CNS; University of Rochester: Golisano Children’s Hospital at Strong (NIH grants GCRC M01 RR44 and U10 HD40521), Nirupama Laroira, MD; Dale L. Phelps, MD; Linda J. Reubens, RN, CCRC; Erica Burnell, RN; University of Texas Southwestern Medical Center at Dallas: Parkland Health and Hospital System and Children’s Medical Center Dallas (NIH grants GCRC M01 RR633 and U10 HD40689), Pablo J. Sánchez, MD; Charles R. Rosenfeld, MD; Walid A. Salhab, MD; Alicia Guzman; Gaynelle Hensley, RN; Melissa H. Lepps, RN; Nancy A. Miller, RN; University of Texas Health Science Center at Houston: Medical School and Children’s Memorial Hermann Hospital (NIH grant U10 HD21373), Kathleen A. Kennedy, MD, MPH; Jon E. Tyson, MD, MPH; Brenda H. Morris, MD; Beverly Foley Harris, RN, BSN; Anna E. Lis, RN, BSN; Sarah Martin, RN, BSN; Georgia E. McDavid, RN; Patti Pierce Tate, RCP; Sharon L. Wright, MT (ASCP); University of Utah: University Hospital, Intermountain Medical Center, LDS Hospital, and Primary Children’s Medical Center (NIH grants CTSA UL1 RR25764, GCRC M01 RR64, and U10 HD53124), Roger G. Faix, MD; Bradley A. Yoder, MD; Jill Burnett, RN; Jennifer J. Jensen, RN, BSN; Karen A. Osborne, RN, BSN, CCRC; Cynthia Spencer, RNC; Kimberlee Weaver-Lewis, RN, BSN; Wake Forest University Baptist Medical Center: Brenner Children’s Hospital and Forsyth Medical Center (NIH grants GCRC M01 RR7122 and U10 HD40498), T. Michael O’Shea, MD, MPH; Nancy J. Peters, RN, CCRP; Wayne State University: Hutzel Women’s Hospital and Children’s Hospital of Michigan (NIH grant U10 HD21385), Seetha Shankaran, MD; Beena Sood, MD, MS; Rebecca Bara, RN, BSN; Elizabeth Billian, RN, MBA; Women and Infants’ Hospital of Rhode Island (NIH grant U10 HD27904), Abbot R. Laptook, MD; William Oh, MD; Angelita M. Hensman, RN, BSN; Dan Gingras, RRT; Kim Francis, RN; Dawn Andrews, RN; Kristen Angela, RN; Yale University: Yale-New Haven Children’s Hospital and Bridgeport Hospital (NIH grants CTSA UL1 RR24139, GCRC MO1 RR125, GCRC M01 RR6022, and U10 HD27871), Richard A. Ehrenkranz, MD; Vineet Bhandari, MD; Harris C. Jacobs, MD; Pat Cervone, RN; Patricia Gettner, RN; Monica Konstantino, RN, BSN; JoAnn Poulsen, RN; Janet Taft, RN, BSN.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study.
Funded by the National Institutes of Health (NIH).
ABBREVIATIONS
- GDB
Generic Database
- NRN
Neonatal Research Network
- SUPPORT
Surfactant Positive Airway Pressure and Pulse Oximetry Randomized Trial in Extremely Low Birth Weight Infants
- GA
gestational age
- NIH
National Institutes of Health
- NICHD
National Institute of Child Health and Human Development
Footnotes
This trial has been registered at www.clinicaltrials.gov (identifier NCT 00233324).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
References
- 1.Morley C, Lau R, Davis P, Morse C. What do parents think about enrolling their premature babies in several research studies? Arch Dis Child Fetal Neonatal Ed. 2005;90(3):F225–F228. doi: 10.1136/adc.2004.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;(3):CD004454. doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Begg C, Cho M, Eastwood S, et al. Improving the quality of reporting of randomized controlled trials: the CONSORT statement. JAMA. 1996;276(8):637–639. doi: 10.1001/jama.276.8.637. [DOI] [PubMed] [Google Scholar]
- 4.Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA. 2001;285(15):1987–1991. doi: 10.1001/jama.285.15.1987. [DOI] [PubMed] [Google Scholar]
- 5.Charlson ME, Horwitz RI. Applying results of randomised trials to clinical practice: impact of losses before randomisation. Br Med J (Clin Res Ed) 1984;289(6454):1281–1284. doi: 10.1136/bmj.289.6454.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manning JD. Presumed consent in emergency neonatal research. J Med Ethics. 2000;26(4):249–253. doi: 10.1136/jme.26.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silverman WA. The myth of informed consent: in daily practice and in clinical trials. J Med Ethics. 1989;15(1):6–11. doi: 10.1136/jme.15.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harth SC, Thong YH. Sociodemographic and motivational characteristics of parents who volunteer their children for clinical research: a controlled study. BMJ. 1990;300(6736):1372–1375. doi: 10.1136/bmj.300.6736.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Constantine WL, Haynes C, Spiker W, Kendall-Tackett K, Constantine N. Recruitment and retention in a clinical trial for low birth weight, premature infants. J Dev Behav Pediatr. 1993;14(1):1–7. [PubMed] [Google Scholar]
- 10.Mitchell AM, Kline JA. Systematic bias introduced by the informed consent process in a diagnostic research study. Acad Emerg Med. 2008;15(3):225–230. doi: 10.1111/j.1553-2712.2008.00066.x. [DOI] [PubMed] [Google Scholar]
- 11.Aagaard-Tillery K, Sibai B, Spong CY, et al. Sample bias among women with retained DNA samples for future genetic studies. Obstet Gynecol. 2006;108(5):1115–1120. doi: 10.1097/01.AOG.0000241536.19539.14. [DOI] [PubMed] [Google Scholar]
- 12.US Department of Health and Human Services. General requirements for informed consent, 45 CFR §46. p. 116d. [Google Scholar]