Abstract
While the variability of cardiovascular disease (CVD) mortality by geography, race and sex is well known, less is known about risk factor variation. We assessed 20-year incidence of hypertension, a CVD risk factor, across four US urban areas and by race-sex. Among 3,436 eligible adults 18–30 years old when recruited in 1985–86 in the community-based Coronary Artery Risk Development in Young Adults (CARDIA) cohort, we examined 20-year cumulative incidence of hypertension (SBP ≥140 mmHg or DBP ≥90 mmHg or anti-hypertensive medication use at any examination) by site and race-sex, adjusting for baseline and time-dependent covariates with Cox regression. Twenty-year incidence, when the mean ages were about 45 years, was 34.5% in black men (n=617), 37.6% in black women (n=965), 21.4% in white men (n=856), and 12.3% in white women (n=998)(P<0.001). Incidence was 33.6% in Birmingham, 23.4% in Chicago, 19% in Minneapolis, and 27.4% in Oakland, (P<0.001). After adjustment for age, race, sex, heart rate, BMI, smoking, family history, education, uric acid, alcohol use, physical activity, and baseline SBP, hazard ratios (95% CI) compared to Birmingham were 0.72 (0.59–0.87) for Chicago, 0.60 (0.50–0.74) for Minneapolis, and 0.73 (0.61–0.87) for Oakland. Race-sex differences persisted after adjustment for site, especially for black women. From young adulthood to middle age, hypertension incidence varies significantly across urban areas. Independently of geography, blacks, especially women, are at markedly higher risk of hypertension. Hypertension incidence may contribute to geographic and racial differences in CVD mortality including stroke.
Journal Subject Codes: hypertension, incidence, geography, demography, epidemiology
Introduction
Despite a 65% decline in cardiovascular disease (CVD) mortality from 1968 to 2006, significant geographic and demographic disparities in CVD mortality persist in the US and may be widening.1, 2 CVD mortality, including stroke and heart disease, tends to be higher and to have declined more slowly in the Southeastern US.1, 2 It is higher in blacks compared with whites and in men compared with women.1, 2 Although gender disparities have narrowed significantly, racial disparities have worsened, with smaller declines among blacks.1, 2 Observational studies suggest that CVD risk factors and socioeconomic factors may largely explain racial disparities in CVD mortality,3, 4 but have not established that CVD risk factors explain the geographic variations.5–8
Hypertension, a major risk factor for CVD, even in young adults,9 may contribute to geographic variations in CVD mortality10 and may account for 44% of the black-white disparity.11 However, the US data that show geographic differences are more consistent for hypertension prevalence6, 7, 12 and conflicting for hypertension incidence.13, 14 Although previous longitudinal studies have demonstrated that blacks have higher hypertension incidence than whites,15–17 it is not known whether these racial differences persist as young adults approach middle age17, 18 or whether US geographic variations in hypertension incidence differ across race-sex groups.
Currently, little is known about the magnitude of geographic and demographic variations in hypertension incidence in the US, how geographic and demographic variations in hypertension incidence change over time, and to what degree hypertension risk factors explain these geographic and demographic differences. Therefore, we examined 20-year hypertension incidence in a longitudinal community-based multi-site bi-racial cohort followed from early adulthood, exploring racial and geographic variability and whether risk factors for hypertension explain any observed variability. We hypothesized that 20-year hypertension incidence would be higher in the one site located in the Southeastern US (Birmingham, AL) compared to the other sites and in black men compared to the other race-sex groups. Known hypertension risk factors would not explain observed site and race-sex differences in hypertension incidence.
Methods
Study Design, Participants, and Measurements
In 1985–6, the Coronary Artery Risk Development in Young Adults (CARDIA) study enrolled 5,115 black and white men and women aged 18–30 years, recruited by random digit dialing in Birmingham, Alabama; Chicago, Illinois; and Minneapolis, Minnesota; and by random selection from a healthcare plan in Oakland, California. At the Birmingham, Minneapolis, and Chicago sites, participants were randomly selected from total communities or from specific census tracts. Within each site, the sample was designed to comprise approximately equal numbers of participants by sex, self-defined race (black, white), age (18–24 years, 25–30 years), and education (≤high school, >high school). The sampling plan and initial cohort characteristics are available elsewhere.19
Participants were contacted by telephone every year and examined in person 2, 5, 7, 10, 15, and 20 years after baseline. While some exam content varied, key data elements remained constant over time. Re-examination rates for survivors were 91%, 86%, 81%, 79%, 74%, and 72% at the six examinations. Of 5,115 CARDIA participants, we excluded 146 (2.9%) with hypertension at baseline, 1,393 (27.2%) not attending the year 20 examination, 173 who died (3.4%), and 212 (4.1%) without ≥1 additional follow-up examination (other than year 20), leaving 3,436 (67.2%) available for analysis. Some participants met several exclusion criteria. At year 20, follow-up was 68% in Minneapolis and 73–74% elsewhere; and was 60% for black men, 70% for black women, 79% for white men and 78% for white women (P<0.0001).
Participants presented fasting in the morning. Tobacco use, strenuous physical activity and intake of caffeine, food and alcohol were proscribed. Examinations included blood pressure (BP) and anthropometric measurements, phlebotomy, and structured questionnaires on socio-demographics, medical and family history, psychosocial characteristics, and nutrition, among others. Blood was drawn from an antecubital vein and, after serum separation, aliquots were stored at −70° C until shipped on dry ice to a central laboratory. The Young Adult Longitudinal Trends in Antioxidants (YALTA) study is ancillary to CARDIA and measured serum carotenoid concentrations in most CARDIA participants at years 0, 7, and 15. All data collection personnel were trained and certified using a standardized process. All examinations were approved by the institutional review boards at each institution. Each participant provided written informed consent.
BP Measurement and Hypertension Incidence
Following a 5-minute rest, BP was measured in the right arm of seated participants at three 1-minute intervals using an appropriately sized cuff and a specific protocol.20 The BP of record was the average of the last 2 measurements. For years 0 to 15, systolic and diastolic BP were recorded at the onset of phase I and phase V of Korotkoff sounds using a random zero sphygmomanometer. For year 20, BP was measured using a standard automated BP measurement monitor (OmROn model HEM907XL; Omron, Mannheim, Germany), calibrated to random zero sphygmomanometer values based on dual readings in 903 participants. The recalibrated year 20 systolic BP = 3.74 + 0.96 times the observed Omron systolic BP; the recalibrated year 20 diastolic BP = 1.30 + 0.97 times the observed Omron diastolic BP.21 BP was monitored by central examination of digit preference and retraining when necessary.13 Hypertension was defined as systolic BP ≥140 mm Hg, or diastolic BP ≥90 mm Hg, or self-reported use of anti-hypertensive medication. A participant was classified as hypertensive at year 20 if criteria were met at any exam. To assess the effect of the automated BP measurement at year 20, we repeated all analyses for 15-year hypertension incidence and results were similar.
Covariates
Covariates included age,17 race, sex, cigarette smoking (categorized as smoked never/in the past or currently),17, 22 education (<12 years, 12 years, 13–15 years, ≥16 years),7, 22 and family history of hypertension.17 Height and weight were measured without shoes or outer garments and body mass index (BMI) calculated17, 22 and categorized as: normal (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2) or obese (≥30 kg/m2). Heart rate was measured by palpating the radial artery before the first BP measurement.22 Heavy alcohol use22 was defined as >14 drinks weekly for men and >7 drinks weekly for women. Serum uric acid (UA)22(years 0, 10, 15, 20) was measured by the uricase method and hyperuricemia was defined as ≥7.0 mg/dL in men and ≥6.0 mg/dL in women.23 Serum insulin22(µU/mL) (years 0, 7, 10, 15, 20) was measured using a modified immunoassay technique (LINCO Research, St. Charles, MO).23 Leisure-time physical activity24 during the preceding year was measured at each examination using an interviewer-administered and validated CARDIA Physical Activity History.20
Diet was explored using circulating carotenoids, a dietary pattern score, and urine sodium/potassium ratio. Serum carotenoid concentrations were measured at years 0, 7, and 15 using a high performance liquid chromatography assay (Molecular Epidemiology and Biomarker Research Laboratory, University of Minnesota) and four serum carotenoid concentrations (α-carotene, β-carotene, lutein/zeaxanthin, cryptoxanthin) were summed.21 Dietary intake was measured at years 0, 7, 15, and 20 using an interviewer-administered CARDIA diet history.20 An a priori dietary pattern score was created by classifying 46 food groups as beneficial (n=20), adverse (n=13), or neutral (n=13) in terms of hypothesized health effects, following the principles in Lockheart et al.25 Food groups considered beneficial or adverse with respect to health effects were categorized by increasing consumption level with scores of 0 to 4 (for food groups considered beneficial) or 4 to 0 (for food groups considered adverse). The a priori dietary pattern score was the sum of category scores, with theoretical maximum 132. Urine sodium and potassium concentrations were measured using up to three 24-hour collections for a sub-study (n=1,100) in year 5 and the mean urine sodium/mean urine potassium ratio was calculated.
Data Analysis
Changes in characteristics from year 0 to year 20 and cumulative 20-year hypertension incidence were compared by site (Birmingham referent) or race-sex (black men referent) using logistic or linear regression or generalized estimating equations (PROC GENMOD) after adjusting for age and time as indicated.
Cox regression analyses examined the adjusted associations between 20-year cumulative hypertension incidence and the main independent variables, site and race-sex group, overall and within race-sex group. Model A included age. Model B added baseline values of heart rate, BMI, cigarette smoking, family history of hypertension, education, uric acid, alcohol use, and physical activity. Model C added baseline systolic BP to Model B.17 Model D adjusted for the same variables in Model B but treated heart rate, BMI, cigarette smoking, family history of hypertension, education, uric acid, alcohol use and physical activity as time-dependent variables. Model E added baseline systolic BP to Model D.
We examined the role of diet by adding sum of four carotenoids or dietary pattern score to Models B–E and allowing the variables to vary with time. We also developed models that included baseline diastolic BP (instead of or in addition to systolic BP) or log-transformed insulin (at baseline or time-dependent). To examine whether the geographic or demographic variations in hypertension incidence were changing over time, we introduced time and, a site*time interaction term (in the overall cohort and within race-sex groups) or a race-sex*time interaction term into Model E. Urine sodium/potassium ratio was added to models for eligible subjects without hypertension at year 5 (n=825).
Sensitivity Analyses
To assess for potential follow-up bias, we performed estimates of cumulative 20-year hypertension incidence and repeated the Cox regression analyses without requiring participants to attend the year 20 exam. To assess effects of non-response bias, we took a response propensity approach, using the probability of inclusion (attend year 0, 20 and ≥1 exam) based on all 5,115 participants as weights to compute point estimates, and bootstrapping to calculate 95% confidence intervals. All analyses used SAS software, version 9.1 (Research Triangle Institute, Research Triangle Park, NC).
Results
Baseline Characteristics and Their 20-Year Change by Site
Minneapolis had fewer black participants (35%) than Birmingham (53%), Chicago (46%) or Oakland (51%)(Table 1). BMI increases were similar in Birmingham and Oakland but lower in Chicago and Minneapolis. Cigarette smoking decreased more in Minneapolis than elsewhere. Elevated uric acid prevalence decreased in Minneapolis but increased elsewhere. Heavy alcohol use decreased in Chicago only. Physical activity and dietary scores decreased less but carotenoid scores increased more in Oakland than in Birmingham. Systolic BP increased similarly in the 4 sites (5–6.5 mmHg). Diastolic BP increases were lower in Minneapolis than elsewhere (2 vs. 4–5.5 mmHg).
Table 1.
Characteristics at Baseline and Change in Characteristics During 20-Year Study Period by Examination Site, among Adults Aged 18–30 Years at Baseline: The CARDIA Study, 1985–2005
| Birmingham (n=784) |
Chicago (n=778) |
Minneapolis (n=902) |
Oakland (n=972) |
|||||
|---|---|---|---|---|---|---|---|---|
| Variable | Year 0 (%) |
Change Year 0 to Year 20 (%) |
Year 0 (%) |
Change Year 0 to Year 20 (%) |
Year 0 (%) |
Change Year 0 to Year 20 (%) |
Year 0 (%) |
Change Year 0 to Year 20 (%) |
| Age, years, mean (SD) | 24.7 (3.7) | 20.1 (0.6) | 24.9 (3.5) | 20.1 (0.6) | 25.6 (3.5) | 20.1 (0.5)** | 25.0 (3.7) | 20.1 (0.6) |
| Black men | 21 | NA | 17 | NA | 15 | NA | 19 | NA |
| Black women | 32 | NA | 29 | NA | 20 | NA | 32 | NA |
| White men | 24 | NA | 26 | NA | 30 | NA | 19 | NA |
| White women | 22 | NA | 29 | NA | 35 | NA | 30 | NA |
| BMI, kg/m2, mean (SD) | 24.3 (4.9) | 5.7 (4.6) | 24.5 (5.0) | 4.4 (4.7)* | 24.2 (4.5) | 4.8 (4.9)* | 24.2 (4.7) | 5.3 (5.9) |
| <18.5 | 6 | −5 | 5 | −4 | 3 | −2 | 4 | −3 |
| 18.5–24.9 | 59 | −35 | 60 | −28 | 63 | −36 | 65 | −36 |
| 25–29.9 | 23 | 10 | 23 | 9 | 25 | 12 | 21 | 12 |
| ≥30 | 12 | 31 | 12 | 24 | 9 | 27 | 10 | 27 |
| Heart rate, per 30 seconds, mean (SD) | 34.5 (5.0) | −1.1 (5.8) | 33.8 (5.3) | −0.1 (6.1)* | 34.7 (5.6) | −1.5 (6.7) | 34.9 (5.1) | −1.6 (5.7) |
| Current cigarette smoking | 24 | −5 | 25 | −7 | 35 | −10** | 21 | −5 |
| Family history of hypertension | 54 | NA | 48 | NA | 49 | NA | 49 | NA |
| Education | ||||||||
| <12 years | 7 | −3 | 9 | −4 | 10 | −5 | 4 | −1 |
| 12 years | 31 | −11 | 23 | −6 | 31 | −5 | 24 | −9 |
| 13–15 years | 37 | −6 | 30 | −4 | 27 | −5 | 41 | −11 |
| ≥16 years | 26 | 20 | 38 | 13 | 32 | 15 | 32 | 21 |
| Elevated uric acid† | 15 | 4 | 12 | 3 | 13 | −1* | 12 | 4 |
| Heavy alcohol use‡ | 9 | 1 | 16 | −4** | 13 | 2 | 11 | 2 |
| Systolic BP, mean (SD) | 108.8 (10.1) | 6.5 (15.6) | 108.4 (10.2) | 6.5 (13.7) | 109.2 (10.2) | 5.2 (12.8) | 111.3 (9.8) | 5.5 (13.7) |
| Diastolic BP, mean (SD) | 68.2 (8.6) | 4.5 (12.4) | 66.2 (9.9) | 5.5 (13.0) | 68.0 (8.1) | 2.2 (11.0)* | 69.7 (8.4) | 4.0 (11.9) |
| Fasting insulin, µU/mL, mean (SD) | 11.9 (6.4) | 5.6 (10.8) | 11.2 (4.7) | 4.5 (9.3) | 10.7 (5.9) | 4.6 (10.0) | 11.4 (5.0) | 5.5 (10.0) |
| Physical activity per 300 exercise units | 1.2 (0.9) | −0.3 (0.9) | 1.6 (1.1) | −0.5 (1.0)* | 1.5 (0.9) | −0.2 (1.0) | 1.3 (1.0) | −0.1 (1.0)** |
| Sum of 4 carotenoids§ | 40.3 (18.2) | 11.7 (23.9) | 48.7 (25.1) | 14.9 (31.8) | 45.9 (25.2) | 20.2 (31.9)* | 50.3 (29.1) | 29.7 (39.2)* |
| Dietary pattern score║ | 60.6 (11.0) | −2.9 (11.4) | 64.5 (12.7) | −2.1 (12.1) | 66.9 (12.7) | −3.6 (12.5) | 67.5 (13.7) | −0.9 (11.7)* |
P≤0.01 compared with Birmingham.
P≤0.05 compared with Birmingham. P values derived from logistic regression using fixed effect method for dichotomous variables and general linear models for continuous variables. Change Year 0 to Year 20 is the difference between the value (percentage or mean) of the characteristic in Year 20 minus the value of the characteristic in Year 0.
Elevated uric acid: ≥7 mg/dL for men and ≥6 mg/dL for women.
Heavy alcohol: >14 drinks/week (men) and >7 drinks/week (women).
Sum of four carotenoids indicates sum of serum concentrations of α-carotene, β-carotene, lutein/zeaxanthin, and cryptoxanthin. Change in sum of four carotenoids is change from years 0 to 15.
An a priori dietary pattern score was created by classifying 46 food groups as beneficial (n=20), adverse (n=13), or neutral (n=13). Food groups considered beneficial or adverse were categorized by increasing consumption level with scores of 0 to 4 (for food groups considered beneficial) or 4 to 0 (for food groups considered adverse). The a priori dietary pattern score was the sum of category scores, with theoretical maximum 132.
Baseline Characteristics and Their 20-Year Change by Race-Sex
BMI increases were higher in black women and lower in whites versus black men (Table 2). Mean heart rate increased in black men but decreased in black women and whites. Cigarette smoking decreased less in black men than in other groups. Elevated uric acid prevalence increased in blacks but decreased in whites. Compared to black men, physical activity decreased less in women, carotenoid scores increased more in whites, and dietary scores decreased less in black women. Systolic and diastolic BP increases were higher in black women (10/8 mmHg) and men (7/6 mmHg) than in white women (4/1 mmHg) or men (2/1 mmHg).
Table 2.
Characteristics at Baseline and Change in Characteristics During 20-Year Study Period by Race-Sex, among Adults Aged 18–30 Years at Baseline: The CARDIA Study, 1985–2005
| Black Men (n=617) |
Black Women (n=965) |
White Men (n=856) |
White Women (n=998) |
|||||
|---|---|---|---|---|---|---|---|---|
| Variable | Year 0 (%) |
Change Year 0 to Year 20 (%) |
Year 0 (%) |
Change Year 0 to Year 20 (%) |
Year 0 (%) |
Change Year 0 to Year 20 (%) |
Year 0 (%) |
Change Year 0 to Year 20 (%) |
| Age, years, mean (SD) | 24.4 (3.7) | 20.1 (0.6) | 24.5 (3.9) | 20.1 (0.6) | 25.5 (3.3) | 20.1 (0.6) | 25.6 (3.4) | 20.1 (0.6) |
| Birmingham, AL | 27 | NA | 26 | NA | 22 | NA | 17 | NA |
| Chicago, IL | 21 | NA | 23 | NA | 24 | NA | 22 | NA |
| Minneapolis, MN | 22 | NA | 18 | NA | 32 | NA | 32 | NA |
| Oakland, CA | 30 | NA | 32 | NA | 22 | NA | 29 | NA |
| BMI, kg/m2 Mean (SD) | 24.6 (4.3) | 5.0 (4.3) | 25.7 (6.1) | 6.5 (5.5)* | 24.2 (3.4) | 4.2 (4.9)* | 23.0 (4.1) | 4.4 (5.0)** |
| <18.5 | 3 | −2 | 7 | −6 | 2 | −1 | 6 | −5 |
| 18.5–24.9 | 61 | −37 | 49 | −32 | 64 | −40 | 73 | −29 |
| 25–29.9 | 26 | 9 | 24 | 2 | 29 | 18 | 15 | 14 |
| ≥30 | 10 | 30 | 21 | 35 | 5 | 23 | 6 | 20 |
| Heart rate, per 30 seconds, mean (SD) | 32.0 (4.5) | 1.3 (6.2) | 35.7 (5.2) | −1.5 (6.4)* | 33.5 (5.0) | −0.9 (5.5)* | 35.8 (5.3) | −2.4 (5.8)* |
| Current cigarette smoking | 31 | −3 | 28 | −5** | 23 | −8* | 24 | −10* |
| Family history of hypertension | 54 | NA | 59 | NA | 41 | NA | 46 | NA |
| Education | ||||||||
| <12 years | 12 | −4 | 10 | −5 | 5 | −2 | 3 | −2 |
| 12 years | 39 | −9 | 35 | −8 | 18 | −5 | 21 | −9 |
| 13–15 years | 34 | 2 | 41 | −6 | 31 | −9 | 30 | −10 |
| ≥16 years | 15 | 12 | 15 | 18 | 46 | 16 | 46 | 21 |
| Elevated uric acid | 20 | 4 | 7 | 8** | 21 | −1 | 7 | 0** |
| Heavy alcohol use | 12 | −1 | 6 | 1 | 15 | −2 | 15 | 2 |
| Systolic BP, mean (SD) | 114.8 (9.3) | 7.1 (13.5) | 107.5 (8.8) | 10.1 (16.3)* | 114.0 (9.9) | 2.4 (12.1)* | 104.4 (8.8) | 4.1 (11.9)* |
| Diastolic BP, mean (SD) | 70.3 (9.2) | 5.6 (12.0) | 67.0 (8.7) | 8.2 (13.0)* | 70.4 (8.7) | 0.9 (11.2)* | 66 (8.1) | 1.4 (10.7)* |
| Fasting insulin, mean (SD) | 11.7 (6.4) | 6.3 (12.2) | 13.2 (6.8) | 5.8 (11.7) | 10.3 (3.9) | 5.8 (9.7) | 10.2 (4.4) | 3.1 (6.6)* |
| Physical activity, exercise units | 1.8 (1.2) | −0.4 (1.2) | 0.9 (0.7) | −0.2 (0.9)* | 1.7 (1.0) | −0.3 (1.0) | 1.4 (0.9) | −0.2 (0.9)* |
| Sum of 4 carotenoids | 41.7 (21.2) | 14.8 (28.1) | 43.7 (20.9) | 15.1 (29.5) | 44.4 (23.1) | 22.5 (31.4)* | 53.6 (30.8) | 22.4 (38.6)* |
| Dietary pattern score | 57.9 (10.8) | −2.7 (12.9) | 59.6 (10.7) | −0.7 (11.9)* | 67.7 (11.9) | −3.7 (11.3) | 72.2 (12.4) | −2.6 (12.0) |
P≤0.01 compared with black men.
P≤0.05 compared with black men. P values derived from logistic regression using fixed effect method for dichotomous variables and general linear models for continuous variables. Change Year 0 to Year 20 is the difference between the value (percentage or mean) of the characteristic in Year 20 minus the value of the characteristic in Year 0.
Cumulative 20-Year Hypertension Incidence, Before Adjustment
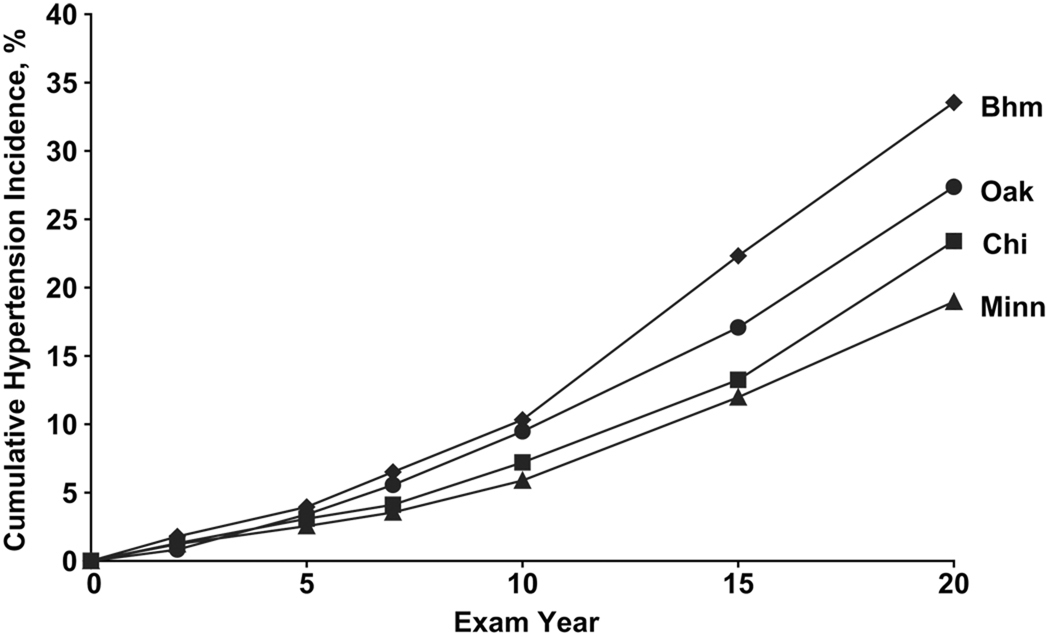
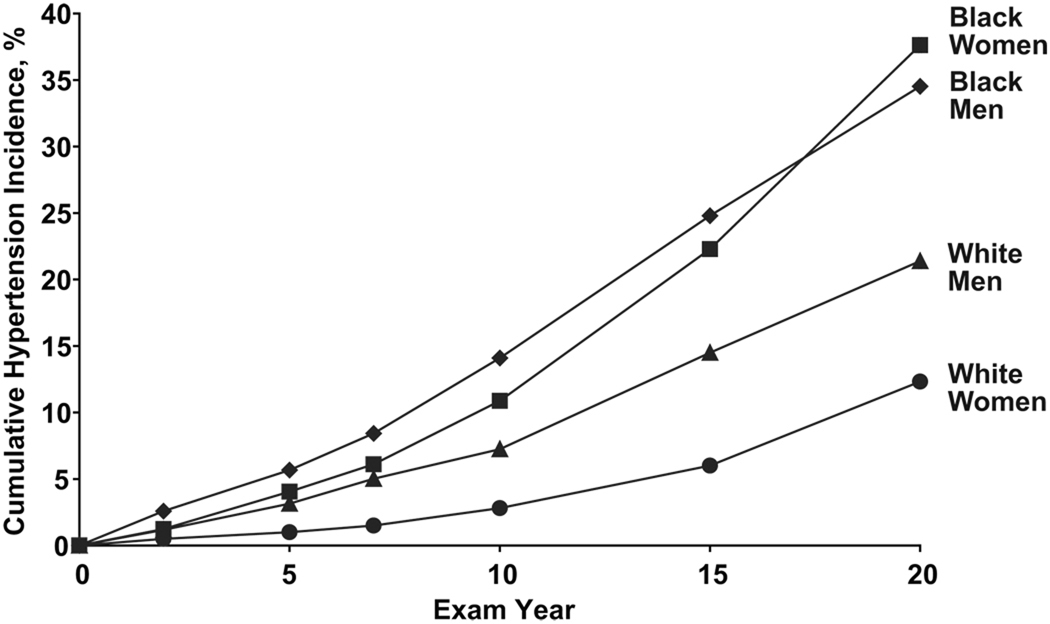
Cumulative 20-year hypertension incidence was 25.7% overall. Participants in Birmingham had greater incident hypertension (33.6%) than Chicago (23.4%; P<0.001), Minneapolis (19%; P<0.001), or Oakland (27.4%; P=0.002)(Figure 1). 20-year hypertension incidence was higher among black men (34.5%) and black women (37.6%; P=0.045) compared to white men (21.4%; P<0.001) or white women (12.3%; P<0.001)(Figure 2).
Figure 1.
Cumulative 20-Year Hypertension Incidence by Examination Site, among Adults Aged 18–30 Years at Baseline: The CARDIA Study, 1985–2005
Figure 2.
Cumulative 20-Year Hypertension Incidence by Race-Sex, among Adults Aged 18–30 Years at Baseline: The CARDIA Study, 1985–2005
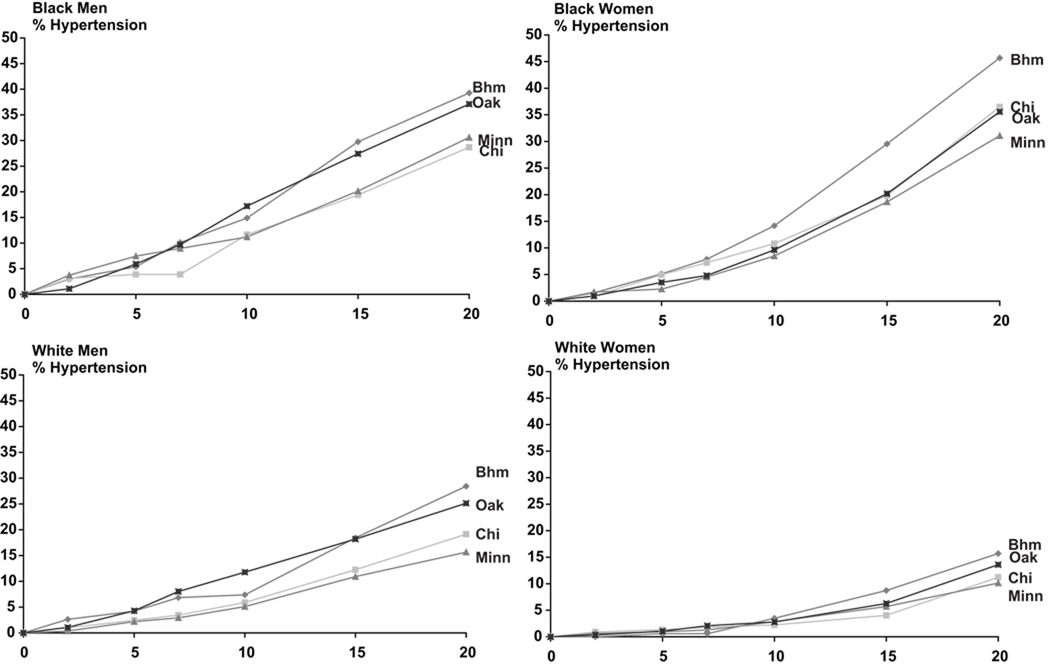
By year 10, Birmingham and Oakland had higher hypertension incidence than Chicago or Minneapolis (Figure 1). From years 10 to 20, hypertension incidence increased more rapidly in Birmingham than Oakland. Geographic differences in hypertension incidence were evident by year 10 for blacks and white men and year 15 for white women (Figure 3). For numerical data in the Figures, please see http://hyper.ahajournals.org.
Figure 3.
Cumulative 20-Year Hypertension Incidence for each of Four Race-Sex Groups, by Examination Site: The CARDIA Study, 1985–2005
Effect of Adjustment for Hypertension Risk Factors
After adjustment for covariate baseline values and demographics, Birmingham participants had a higher risk of hypertension incidence than those in Chicago or Minneapolis (Model B, Table 3). Black men had higher risk than white men or white women but similar to black women. After additional adjustment for baseline systolic BP, risk of hypertension remained lower in Chicago and Minneapolis and became lower in Oakland (Model C, Table 3). Risk of hypertension remained lower in whites but became higher in black women versus black men (Model C, Table 3). Adjustment for baseline diastolic BP did not change results (data not shown). There was no significant systolic BP*race-sex interaction in Model C (P=0.36).
Table 3.
Adjusted Hazard Ratios (95% Confidence Intervals) for Cumulative 20-Year Hypertension Incidence, among Adults Aged 18–30 Years at Baseline: The CARDIA Study, 1985–2005
| Variable | Model A (n=3,436) |
Model B (n=3,360) |
Model C (n=3,360) |
Model D (n=3,426) |
Model E (n=3,426) |
|---|---|---|---|---|---|
| Examination site | |||||
| Birmingham, AL | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Chicago, IL | 0.70 (0.58, 0.85) | 0.69 (0.57, 0.84) | 0.69 (0.57, 0.84) | 0.73 (0.61, 0.89) | 0.72 (0.59, 0.87) |
| Minneapolis, MN | 0.60 (0.50, 0.73) | 0.58 (0.47, 0.70) | 0.56 (0.46, 0.69) | 0.64 (0.53, 0.78) | 0.60 (0.50, 0.74) |
| Oakland, CA | 0.81 (0.68, 0.96) | 0.85 (0.71, 1.02) | 0.70 (0.59, 0.84) | 0.88 (0.74, 1.05) | 0.73 (0.61, 0.87) |
| Race-sex | |||||
| Black men | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Black women | 1.05 (0.88, 1.24) | 0.85 (0.70, 1.03) | 1.37 (1.12, 1.69) | 0.91 (0.76, 1.09) | 1.46 (1.20, 1.76) |
| White men | 0.55 (0.45, 0.68) | 0.65 (0.52, 0.80) | 0.64 (0.52, 0.80) | 0.70 (0.57, 0.86) | 0.70 (0.57, 0.87) |
| White women | 0.30 (0.24, 0.38) | 0.37 (0.29, 0.48) | 0.63 (0.49, 0.82) | 0.42 (0.33, 0.54) | 0.71 (0.56, 0.91) |
| Age (per year) | 1.07 (1.05, 1.09) | 1.06 (1.04, 1.09) | 1.07 (1.05, 1.09) | 1.07 (1.05, 1.09) | 1.07 (1.05, 1.09) |
| Heart rate (per beat) | 1.02 (1.01, 1.03) | 1.01 (1.00, 1.03) | 1.00 (1.00, 1.01) | 1.00 (1.00, 1.00) | |
| Body mass index (per kg/m2) | 1.06 (1.05, 1.07) | 1.04 (1.03, 1.05) | 1.03 (1.03, 1.04) | 1.03 (1.02, 1.04) | |
| Current cigarette smoking | 1.12 (0.96. 1.31) | 1.13 (0.97, 1.32) | 1.06 (0.90, 1.26) | 1.05 (0.89, 1.24) | |
| Family history of hypertension | 1.50 (1.30, 1.72) | 1.43 (1.24, 1.65) | 1.54 (1.33, 1.79) | 1.46 (1.26, 1.69) | |
| Education | |||||
| <12 years | 1.00 | 1.00 | 1.00 | 1.00 | |
| 12 years | 0.74 (0.58, 0.95) | 0.77 (0.61, 0.99) | 0.71 (0.53, 0.94) | 0.77 (0.58, 1.02) | |
| 13–15 years | 0.64 (0.50, 0.81) | 0.64 (0.50, 0.81) | 0.63 (0.48, 0.84) | 0.66 (0.50, 0.87) | |
| ≥16 years | 0.53 (0.41, 0.70) | 0.54 (0.41, 0.72) | 0.46 (0.34, 0.61) | 0.50 (0.37, 0.66) | |
| Elevated uric acid | 1.32 (1.10, 1.59) | 1.14 (0.95, 1.38) | 1.78 (1.55, 2.05) | 1.62 (1.40, 1.86) | |
| Heavy alcohol use | 0.89 (0.71, 1.12) | 0.86 (0.69, 1.08) | 1.09 (0.88, 1.35) | 1.14 (0.92, 1.41) | |
| Physical activity (per 300 exercise units) | 0.96 (0.88, 1.03) | 0.94 (0.87, 1.02) | 0.86 (0.79, 0.94) | 0.87 (0.80, 0.95) | |
| Baseline systolic blood pressure (per 1 mm Hg) | 1.06 (1.06, 1.07) | 1.06 (1.06, 1.07) |
Bolded figures indicate 95% CI not containing one, P<0.05. Heart rate, BMI, cigarette smoking, family history of hypertension, education, uric acid, alcohol use and physical activity are baseline values in Models B and C and time-dependent in Models D and E.
Geographic and racial differences in risk of hypertension did not change in models with time-varying covariates (Models D and E, Table 3). Within race-sex groups, risk of incident hypertension was higher in Birmingham but some geographic contrasts were no longer statistically significant, please see http://hyper.ahajournals.org. Additional adjustment for carotenoids, dietary pattern score, or log-transformed fasting insulin did not change geographic or racial contrasts (data not shown). After full adjustment, there was evidence of a significant race-sex*time interaction (P<0.0001 for interaction term) suggesting that the race-sex differences in cumulative hypertension incidence (seen in Figure 2) changed significantly over time, even after controlling for variables in Model E. By examining the specific race-sex group*time interaction terms, we see that hypertension incidence changed over time for black or white women but not white men compared to black men [P<0.0001 for black women*time; P=0.46 for white men*time; P=0.03 for white women*time; black men referent]. However, there were no significant site*time interactions in models performed with the overall cohort or within race-sex groups suggesting that the geographic differences in cumulative hypertension incidence (seen in Figures 1 or 3) did not expand or narrow over time, even after controlling for variables in Model E. After adjusting for urine sodium/potassium ratio in the sub-sample, geographic and racial differences persisted but some racial contrasts were no longer statistically significant, please see http://hyper.ahajournals.org. Urine sodium/potassium had a significant interaction with site (P=0.03) but not race-sex (P=0.39).
Sensitivity Analyses
Cumulative 20-year hypertension incidence estimates were lower if participants were not required to attend the year 20 exam but geographic differences were similar, please see http://hyper.ahajournals.org. The magnitude of differences between cumulative 20-year hypertension incidence among CARDIA participants who did and did not attend the year 20 exam were greatest for blacks particularly those in non-Birmingham sites. Excluded participants were more likely than included participants to be current smokers, obese, to have ≤high school education, elevated uric acid, higher fasting insulin levels, lower dietary pattern and carotenoid scores, and higher systolic and diastolic BP. Analyses assessing the effects of non-response bias yielded similar results. Geographic and racial variations in risk of elevated blood pressure (systolic BP ≥130 mm Hg or diastolic BP ≥85 mm Hg or on anti-hypertensive medication) incidence were comparable except reduced in black women, please see http://hyper.ahajournals.org.
Discussion
We observed large differences in 20-year hypertension incidence across four US urban areas with 30–40% lower incidence in Chicago or Minneapolis compared with Birmingham. These differences were observed in blacks and whites, in men and women, and persisted after adjustment for major hypertension risk factors. Independent of geography and hypertension risk factors, blacks, particularly black women, had significantly higher rates of 20-year hypertension incidence. As hypertension incidence increased over time, racial differences also increased, whereas geographic differences persisted.
Although some7, 12, 13 but not all14 studies have shown greater hypertension prevalence or elevated BP incidence in the Southeastern US, few studies have assessed geographic differences in hypertension incidence over prolonged time periods. In one National Health and Nutrition Examination Surveys (NHANES I) analysis, region was not associated with incident hypertension between 1971 and 1984 for 14,235 adults aged 25–74 years perhaps owing to higher death rate, survival bias, masking of important geographic differences within the 4 large US census regions studied, and adjustment for diabetes, which may be on the causal pathway.14 Among younger adults, we found that differences in 20-year hypertension incidence between US cities were not fully accounted for by socio-demographic, behavioral, familial, and clinical correlates. Our findings are consistent with some26–28 but not all research17 showing geographic differences in BP even after adjusting for risk factors.
Potential causes of geographic differences in hypertension incidence include socioeconomic factors not captured by adjustment for education,29 dietary or lifestyle factors,30–32 or environmental-gene interactions.32 Our finding of a significant urine sodium/potassium*site interaction suggests that dietary sodium and potassium intake may modify the association between geography and hypertension incidence with greater effect in Minneapolis and Chicago; however, urine sodium/potassium was measured at year 5 only and the numbers in this sub-sample are small. Environmental exposures including air temperature33 and particulate air pollution34 may contribute to geographic differences with effect modification by obesity and genotypic or phenotypic indicators of systemic inflammation and oxidative stress.35 Given that geographic variations in BP appear at young ages,13, 27 differences in exposure to these or other potential risk factors may occur in early life.
Similar to some17 but not all18 research, we observed marked racial differences in hypertension incidence between US blacks and whites at middle age, in men and women, and after adjusting for major hypertension risk factors. A Baltimore study (n=377, mean age 44 years) may not have found racial differences because the participants had relatively high socioeconomic status, a factor associated with lower hypertension risk,29 most blacks were female (72%), most whites were male (73%), and follow-up was only 7 years.18 Our finding of some non-significant racial contrasts after adjusting for urine sodium/potassium in the sub-study may be due to decreased power or may reflect a true attenuation of the association between race-sex and hypertension incidence by dietary sodium and potassium intake. In addition, we found that black women had higher hypertension incidence after adjusting for baseline systolic BP; this masking of race-sex differences in hypertension by risk factors is consistent with prior research.36
Racial differences in hypertension incidence may result from racial differences in sympathetic reactivity to stress,37 salt sensitivity,37 cumulative effects of psychosocial factors and stress,38 responsiveness of the renin-angiotensin-aldosterone system,39 dietary or lifestyle factors,40 or environmental-gene interactions.41 We found striking BP increases in blacks compared to whites, and in women compared to men, changes supported by previous studies.18, 29 Given recent research showing that, among participants aged 7–16 years, boys had a greater increase in BP over 15 years than girls,41 our results suggest that sex trajectories in BP may vary from adolescence to young adulthood. Moreover, our observed race-sex trajectories in hypertension incidence may continue into middle-age, more so in disadvantaged subgroups.29
Our cohort includes four urban areas only and we used the baseline recruitment site for geographic assignment. Analyses including participants (80%) residing within 50 miles of their baseline recruitment site at year 20 produced similar results. Although the method for BP measurement changed from year 15 to year 20, results of analyses for cumulative 15-year hypertension incidence were similar. Despite high retention of the cohort, incomplete follow-up may bias our results; reassuringly, analyses that did not require the year 20 examination or that adjusted for the probability of missing variables did not change our results significantly. Although excluded participants had higher prevalence of hypertension risk factors than included participants, hypertension incidence was similar or lower in analyses which included many of the excluded participants, please see http://hyper.ahajournals.org.
Geographic variations in hypertension management or anti-hypertension medication use could contribute to our observed differences; however, we focus on incident hypertension, thus our differences would have to be influenced by medical management preceding the diagnosis of hypertension. Further, geographic variations in anti-hypertensive medication use did not change results in a previous CARDIA analysis.13 Some factors (e.g., lifestyle, family history) were self-reported and subject to recall bias. Although BMI may predict hypertension less than measures of central obesity, we obtained similar results when we replaced BMI with waist circumference in models. Importantly, our lifestyle measures may not capture lifestyle factors adequately given that we observed site differences in diet and physical activity but these factors did not explain variations in hypertension incidence. Moreover, our findings of robust associations between hypertension incidence and physical activity or BMI in fully adjusted models confirm that exercise and weight management are important modifiable risk factors for hypertension.
Given that we do not fully understand the mechanisms of the demographic and racial differences we demonstrated, action-oriented recommendations based on our findings may be premature. Other large epidemiologic studies42, 43 are contributing to our understanding of the pattern of end-organ damage attributable to hypertension in older populations as mechanistic studies40, 44 of pathophysiology are being actively pursued. In the meantime, we believe that the groups known to be at highest risk of hypertension (e.g., black women in the South) need especially high surveillance and aggressive management of known hypertension risk factors, such as obesity.
Perspectives
We found that hypertension incidence between young adulthood and middle-age varies significantly across four urban areas and race-sex with higher rates in the Southeast and in blacks, especially black women. Geography and demography have strong, independent effects that persist after adjustment for many hypertension risk factors. Our findings suggest that hypertension incidence may contribute to geographic and racial differences in CVD mortality including stroke. Potential biological, environmental, and genetic mechanisms of these findings warrant further investigation.
Supplementary Material
Acknowledgments
Sources of Funding
Work on this manuscript was partially supported by contracts: N01-HC-95095, N01-HC-48047, N01-HC-48048, N01-HC-48049, and N01-HC-48050; from the National Heart, Lung and Blood Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Deborah A. Levine: None
Cora E. Lewis: None
O. Dale Williams: None
Monika M. Safford: None
Kiang Liu: None
David A. Calhoun: None
Yongin Kim: None
David R. Jacobs, Jr.: None
Catarina I. Kiefe: None
References
- 1.National Heart, Lung, and Blood Institute. Morbidity and Mortality: 2009 Chart Book on Cardiovascular, Lung, and Blood Diseases. Bethesda, MD: National Institutes of Health; 2009. [Google Scholar]
- 2.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 3.Hozawa A, Folsom AR, Sharrett AR, Chambless LE. Absolute and attributable risks of cardiovascular disease incidence in relation to optimal and borderline risk factors: comparison of African American with white subjects--Atherosclerosis Risk in Communities Study. Arch Intern Med. 2007;167:573–579. doi: 10.1001/archinte.167.6.573. [DOI] [PubMed] [Google Scholar]
- 4.Thomas AJ, Eberly LE, Davey Smith G, Neaton JD, Stamler J. Race/ethnicity, income, major risk factors, and cardiovascular disease mortality. Am J Public Health. 2005;95:1417–1423. doi: 10.2105/AJPH.2004.048165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howard G, Cushman M, Prineas RJ, Howard VJ, Moy CS, Sullivan LM, D'Agostino RB, Sr, McClure LA, Pulley L, Safford MM. Advancing the hypothesis that geographic variations in risk factors contribute relatively little to observed geographic variations in heart disease and stroke mortality. Prev Med. 2009;49:129–132. doi: 10.1016/j.ypmed.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ezzati M, Oza S, Danaei G, Murray CJ. Trends and cardiovascular mortality effects of state-level blood pressure and uncontrolled hypertension in the United States. Circulation. 2008;117:905–914. doi: 10.1161/CIRCULATIONAHA.107.732131. [DOI] [PubMed] [Google Scholar]
- 7.Stolley PD, Kuller LH, Nefzger MD, Tonascia S, Lilienfeld AM, Miller GD, Diamond EL. Three-area epidemiological study of geographic differences in stroke mortality. II. Results. Stroke. 1977;8:551–557. doi: 10.1161/01.str.8.5.551. [DOI] [PubMed] [Google Scholar]
- 8.Filate WA, Johansen HL, Kennedy CC, Tu JV. Regional variations in cardiovascular mortality in Canada. Can J Cardiol. 2003;19:1241–1248. [PubMed] [Google Scholar]
- 9.Miura K, Daviglus ML, Dyer AR, Liu K, Garside DB, Stamler J, Greenland P. Relationship of blood pressure to 25-year mortality due to coronary heart disease, cardiovascular diseases, and all causes in young adult men: the Chicago Heart Association Detection Project in Industry. Arch Intern Med. 2001;161:1501–1508. doi: 10.1001/archinte.161.12.1501. [DOI] [PubMed] [Google Scholar]
- 10.Perry HM, Roccella EJ. Conference report on stroke mortality in the southeastern United States. Hypertension. 1998;31:1206–1215. doi: 10.1161/01.hyp.31.6.1206. [DOI] [PubMed] [Google Scholar]
- 11.Wong MD, Shapiro MF, Boscardin WJ, Ettner SL. Contribution of major diseases to disparities in mortality. N Engl J Med. 2002;347:1585–1592. doi: 10.1056/NEJMsa012979. [DOI] [PubMed] [Google Scholar]
- 12.Obisesan TO, Vargas CM, Gillum RF. Geographic variation in stroke risk in the United States. Region, urbanization, and hypertension in the Third National Health and Nutrition Examination Survey. Stroke. 2000;31:19–25. doi: 10.1161/01.str.31.1.19. [DOI] [PubMed] [Google Scholar]
- 13.Kiefe CI, Williams OD, Bild DE, Lewis CE, Hilner JE, Oberman A. Regional disparities in the incidence of elevated blood pressure among young adults: the CARDIA study. Circulation. 1997;96:1082–1088. doi: 10.1161/01.cir.96.4.1082. [DOI] [PubMed] [Google Scholar]
- 14.Gillum RF, Mussolino ME, Madans JH. Relation between region of residence in the United States and hypertension incidence--the NHANES I epidemiologic follow-up study. J Natl Med Assoc. 2004;96:625–634. [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson AL, Cornoni JC, Cassel JC, Tyroler HA, Heyden S, Hames CG. Influence of race, sex and weight on blood pressure behavior in young adults. Am J Cardiol. 1975;35:523–530. doi: 10.1016/0002-9149(75)90835-8. [DOI] [PubMed] [Google Scholar]
- 16.Apostolides AY, Cutter G, Daugherty SA, Detels R, Kraus J, Wassertheil-Smoller S, Ware J. Three-year incidence of hypertension in thirteen U.S. communities. On behalf of the Hypertension Detection and Follow-up Program cooperative group. Prev Med. 1982;11:487–499. doi: 10.1016/0091-7435(82)90063-9. [DOI] [PubMed] [Google Scholar]
- 17.Rywik SL, Williams OD, Pajak A, Broda G, Davis CE, Kawalec E, Manolio TA, Piotrowski W, Hutchinson R. Incidence and correlates of hypertension in the Atherosclerosis Risk in Communities (ARIC) study and the Monitoring Trends and Determinants of Cardiovascular Disease (POL-MONICA) project. J Hypertens. 2000;18:999–1006. doi: 10.1097/00004872-200018080-00002. [DOI] [PubMed] [Google Scholar]
- 18.He J, Klag MJ, Appel LJ, Charleston J, Whelton PK. Seven-year incidence of hypertension in a cohort of middle-aged African Americans and whites. Hypertension. 1998;31:1130–1135. doi: 10.1161/01.hyp.31.5.1130. [DOI] [PubMed] [Google Scholar]
- 19.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr., Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 20. [Accessed September 22, 2009];CARDIA Exam Materials Manuals of Operation. Available at: http://www.cardia.dopm.uab.edu/em_moop.htm.
- 21.Hozawa A, Jacobs DR, Jr., Steffes MW, Gross MD, Steffen LM, Lee DH. Circulating carotenoid concentrations and incident hypertension: the Coronary Artery Risk Development in Young Adults (CARDIA) study. J Hypertens. 2009;27:237–242. doi: 10.1097/HJH.0b013e32832258c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dyer AR, Liu K, Walsh M, Kiefe C, Jacobs DR, Jr., Bild DE. Ten-year incidence of elevated blood pressure and its predictors: the CARDIA study. Coronary Artery Risk Development in (Young) Adults. J Hum Hypertens. 1999;13:13–21. doi: 10.1038/sj.jhh.1000740. [DOI] [PubMed] [Google Scholar]
- 23.Rathmann W, Funkhouser E, Dyer AR, Roseman JM. Relations of hyperuricemia with the various components of the insulin resistance syndrome in young black and white adults: the CARDIA study. Coronary Artery Risk Development in Young Adults. Ann Epidemiol. 1998;8:250–261. doi: 10.1016/s1047-2797(97)00204-4. [DOI] [PubMed] [Google Scholar]
- 24.Parker ED, Schmitz KH, Jacobs DR, Jr., Dengel DR, Schreiner PJ. Physical activity in young adults and incident hypertension over 15 years of follow-up: the CARDIA study. Am J Public Health. 2007;97:703–709. doi: 10.2105/AJPH.2004.055889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lockheart MS, Steffen LM, Rebnord HM, Fimreite RL, Ringstad J, Thelle DS, Pedersen JI, Jacobs DR., Jr. Dietary patterns, food groups and myocardial infarction: a case-control study. Br J Nutr. 2007;98:380–387. doi: 10.1017/S0007114507701654. [DOI] [PubMed] [Google Scholar]
- 26.Shaper AG, Pocock SJ, Walker M, Cohen NM, Wale CJ, Thomson AG. British Regional Heart Study: cardiovascular risk factors in middle-aged men in 24 towns. Br Med J (Clin Res Ed) 1981;283:179–186. doi: 10.1136/bmj.283.6285.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruce NG, Cook DG, Shaper AG, Thomson AG. Geographical variations in blood pressure in British men and women. J Clin Epidemiol. 1990;43:385–398. doi: 10.1016/0895-4356(90)90124-8. [DOI] [PubMed] [Google Scholar]
- 28.Tell GS, Rutan GH, Kronmal RA, Bild DE, Polak JF, Wong ND, Borhani NO. Correlates of blood pressure in community-dwelling older adults. The Cardiovascular Health Study. Cardiovascular Health Study (CHS) Collaborative Research Group. Hypertension. 1994;23:59–67. doi: 10.1161/01.hyp.23.1.59. [DOI] [PubMed] [Google Scholar]
- 29.Diez Roux AV, Chambless L, Merkin SS, Arnett D, Eigenbrodt M, Nieto FJ, Szklo M, Sorlie P. Socioeconomic disadvantage and change in blood pressure associated with aging. Circulation. 2002;106:703–710. doi: 10.1161/01.cir.0000025402.84600.cd. [DOI] [PubMed] [Google Scholar]
- 30.Hajjar I, Kotchen T. Regional variations of blood pressure in the United States are associated with regional variations in dietary intakes: the NHANES-III data. J Nutr. 2003;133:211–214. doi: 10.1093/jn/133.1.211. [DOI] [PubMed] [Google Scholar]
- 31.Zhao L, Stamler J, Yan LL, Zhou B, Wu Y, Liu K, Daviglus ML, Dennis BH, Elliott P, Ueshima H, Yang J, Zhu L, Guo D. Blood pressure differences between northern and southern Chinese: role of dietary factors: the International Study on Macronutrients and Blood Pressure. Hypertension. 2004;43:1332–1337. doi: 10.1161/01.HYP.0000128243.06502.bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kotchen TA, Kotchen JM. Regional variations of blood pressure: environment or genes? Circulation. 1997;96:1071–1073. doi: 10.1161/01.cir.96.4.1071. [DOI] [PubMed] [Google Scholar]
- 33.Modesti PA, Morabito M, Bertolozzi I, Massetti L, Panci G, Lumachi C, Giglio A, Bilo G, Caldara G, Lonati L, Orlandini S, Maracchi G, Mancia G, Gensini GF, Parati G. Weather-related changes in 24-hour blood pressure profile: effects of age and implications for hypertension management. Hypertension. 2006;47:155–161. doi: 10.1161/01.HYP.0000199192.17126.d4. [DOI] [PubMed] [Google Scholar]
- 34.Auchincloss AH, Diez Roux AV, Dvonch JT, Brown PL, Barr RG, Daviglus ML, Goff DC, Kaufman JD, O'Neill MS. Associations between recent exposure to ambient fine particulate matter and blood pressure in the Multi-ethnic Study of Atherosclerosis (MESA) Environ Health Perspect. 2008;116:486–491. doi: 10.1289/ehp.10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz J, Park SK, O'Neill MS, Vokonas PS, Sparrow D, Weiss S, Kelsey K. Glutathione-S-transferase M1, obesity, statins, and autonomic effects of particles: gene-by-drug-by-environment interaction. Am J Respir Crit Care Med. 2005;172:1529–1533. doi: 10.1164/rccm.200412-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winkleby MA, Ragland DR, Syme SL, Fisher JM. Heightened risk of hypertension among black males: the masking effects of covariables. Am J Epidemiol. 1988;128:1075–1083. doi: 10.1093/oxfordjournals.aje.a115050. [DOI] [PubMed] [Google Scholar]
- 37.Calhoun DA, Oparil S. Racial differences in the pathogenesis of hypertension. Am J Med Sci. 1995;310 Suppl 1:S86–S90. doi: 10.1097/00000441-199512000-00016. [DOI] [PubMed] [Google Scholar]
- 38.Yan LL, Liu K, Matthews KA, Daviglus ML, Ferguson TF, Kiefe CI. Psychosocial factors and risk of hypertension: the Coronary Artery Risk Development in Young Adults (CARDIA) study. JAMA. 2003;290:2138–2148. doi: 10.1001/jama.290.16.2138. [DOI] [PubMed] [Google Scholar]
- 39.He FJ, Markandu ND, Sagnella GA, MacGregor GA. Importance of the renin system in determining blood pressure fall with salt restriction in black and white hypertensives. Hypertension. 1998;32:820–824. doi: 10.1161/01.hyp.32.5.820. [DOI] [PubMed] [Google Scholar]
- 40.Dekkers JC, Snieder H, Van Den Oord EJ, Treiber FA. Moderators of blood pressure development from childhood to adulthood: a 10-year longitudinal study. J Pediatr. 2002;141:770–779. doi: 10.1067/mpd.2002.128113. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Poole JC, Treiber FA, Harshfield GA, Hanevold CD, Snieder H. Ethnic and gender differences in ambulatory blood pressure trajectories: results from a 15-year longitudinal study in youth and young adults. Circulation. 2006;114:2780–2787. doi: 10.1161/CIRCULATIONAHA.106.643940. [DOI] [PubMed] [Google Scholar]
- 42.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 43.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 44.Voors AW, Webber LS, Berenson GS. Time course study of blood pressure in children over a three-year period. Bogalusa Heart Study. Hypertension. 1980;2:102–108. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.