Abstract
In this report, we have assessed the behavioral responses of mice missing the Ppif gene (CyPD-KO), encoding mitochondrial cyclophilin D (CyPD). Mitochondrial CyPD is a key modulator of the mitochondrial permeability transition which is involved in the regulation of calcium- and oxidative damage-induced cell death. Behavioural screening of CyPD-KO mice (ranging between 4 and 15 months of age) was accomplished using a battery of behavioral paradigms which included testing of motor functions, exploratory activity, anxiety/emotionality, as well as learning and memory skills. We found that, compared to wild-type mice, CyPD-KO mice were (i) more anxious and less explorative in open field and elevated plus maze and (ii) performed better in learning and memory of avoidance tasks, such as active and passive avoidance. However, the absence of CyPD did not alter the nociceptive threshold for thermal stimuli. Finally, deletion of CyPD caused also an abnormal accumulation of white adipose tissue resulting in adult-onset obesity, which was not dependent on increased food and/or water intake. Taken together, our results suggest a new fundamental role of mitochondrial CyPD in basal brain functions and body weight homeostasis.
Keywords: mitochondrial permeability transition, rotarod, open field, plus maze, conditioning avoidance, food/water intake
Cyclophilin D (CyPD) is a soluble protein located within the mitochondrial matrix, which belongs to the peptidyl-proplyl cis-trans isomerases cyclophilin chaperone family (Galat and Metcalfe, 1995). CyPD binds to undefined components of the mitochondrial inner membrane to regulate the activation of the mitochondrial permeability transition pore (mPTP); activation of the mPTP results in an increase the permeability of this membrane with resulting decreases in mitochondrial membrane potential, mitochondrial swelling, and mitochondrial release of apoptogenic proteins, like cytochrome-c. As a consequence, these events result in caspase activation and cell death by caspase-dependent apoptosis or, if the mPTP opening is sufficiently prolonged, by necrosis (Petronilli et al., 2001; Green & Kroemer, 2004; Bernardi et al., 2006; Armstrong, 2006; Tsujimoto et al., 2006).
Recent studies in which the expression of CyPD has been eliminated by inactivation of Ppif gene in mice (CyPD-KO) have confirmed an important role for CyPD as a key regulator of mPTP activation (Baines et al., 2005; Basso et al., 2005; Nakagawa et al., 2005; Schinzel et al., 2005). In these studies, CyPD-deficient cells from liver, heart and brain died normally in response to various apoptotic stimuli, but showed resistance to necrotic cell death induced by reactive oxygen species and calcium overload. In addition, CyPD-KO mice were protected from heart and brain ischemia/reperfusion-induced cell death in vivo and, as recently demonstrated (Forte et al., 2007), from axonal damage in a model of experimental autoimmune encephalomyelitis. Finally, a critical role for CyPD, and by extension the mPTP, has also been recently reported in platelet activation and thrombosis (Jobe et al., 2007) and in the pathogenesis of various genetic forms of muscular dystrophy (Merlini et al., 2008; Millay et al., 2008). From these and other findings (Yasuda et al., 2006; De Marchi et al., 2006), it has been suggested that CyPD-dependent regulation of the mitochondrial permeability transition (mPT) may play a crucial role in cellular responses to specific stresses of direct relevance to human disease such as those induced by calcium and oxidative damage.
CyPD-KO mice are born at the expected Mendelian frequency, develop normally, and have no obvious phenotype, with normal-appearing brain and cerebrovasculature (Schinzel et al., 2005). These findings have been taken as evidence that CyPD-dependent mPT is not involved in apoptotic processes, which are essential for embryonic development and viability of adult mice. However, the involvement of CyPD in regulating apoptosis is controversial. Indeed, it has been recently demonstrated that the expression of Apop-1, an apoptogenic protein, induces apoptosis through a CyPD-dependent pathway, and that cells from mice lacking CyPD were resistant to Apop-induced apoptosis (Yasuda et al., 2006).
Despite a number of studies of CyPD-KO mice, an accurate behavioral assessment of the effect of CyPD deletion on physiological and neurological functions is still lacking. In the present study, we examined the effect of CyPD deletion on health status, motor functions, exploratory activity and anxiety levels, as well as on learning and memory skills in avoidance tasks. We also measured the threshold for thermal nociception, and food and water intake. We find that CyPD-KO mice exhibit a striking behavioral phenotype, indicating an important role of CyPD in the age-induced depression of brain function.
EXPERIMENTAL PROCEDURES
Animals
Subjects were male mice in which the expression of CyPD protein was eliminated by specific deletion of the Ppif gene, encoding CyPD (Basso et al., 2005). F1 heterozygotes were back-crossed for eight generations into C57BL/6J genetic background. Heterozygous Ppif(+/−) mice were crossed to generate Ppif(−/−) (CyPD-KO) and control, homozygous wild-type (WT) littermates. The genotype of individual mice was established by PCR analysis of genomic DNA, as described elsewhere (Basso et al., 2005). Mice were housed in standard transparent plastic cages (4 per cage) under standard animal room conditions (free access to food and water; 12-h light/dark cycle; 23°C). Pellet food (Harlan Global Diet 2018, purchased from Harlan Italy, S. Pietro al Natisone, Italy) and tap-water were continuously available. Care and handling of the animals were in accordance with NIH ethical regulations and with the Italian National law (DL116/92, application of the European Communities Council Directive 86/609/EEC).
Preliminary observations
For the observation of general health status, a subset of tests from the Irwin Gross Neurological Screen (Irwin, 1968) was used. Gait and posture, tremors, palpebral closure and lacrimation, piloerection and whiskers appearance, grooming and defecation were taken as indexes of general health status. Gross inspection was performed by putting mice into a white plastic sheet surrounded by a clear acrylic cylindrical viewing jar (14 cm diameter; 18 cm height). After 5 min observation, righting reflex was also tested.
Assays of body weight and food/ water intake
Body weight was measured at the same hour (2:00 p.m.) at the beginning of each month, starting from 3 months of age. Food and water intakes were measured every day at 9:00 a.m. for 2 weeks. For this purpose, mice were housed one per cage. The food pellet (18.9% proteins, 6.0% fats, 3.8% fibers, 5.9% ash; 3.3 Kcal/g of metabolizable energy) was used to feed the entire mouse colony.
Motor coordination and locomotor activity
Motor coordination
Animals were tested using an accelerating rotarod (Rota-Rod Treadmill, Model 7650, Ugo Basile, Comerio, Italy). Rotarod tests were performed in two phases: training and testing. During both phases, mice were subjected to two daily trials separated by a 45 min interval. During training, mice were allowed to become accustomed to the stationary rod (day one), and followed by further training for the next two days to a rotating rod (6 rpm); fixed training was terminated after 3 min. During the four consecutive days of testing, mice were placed on the stationary rod for 30 s, after which rod rotation at 6 rpm was started, gradually increased to 30 rpm over a time course of 3 min. Trials were terminated after 3 min or when the mice fell off the rod. The time elapsing from the beginning of acceleration and to mouse fall was automatically recorded.
Locomotor activity
The locomotor activity was estimated both from activity in open-field and in shuttle-box cages using the same apparatus used for active avoidance learning (see below). The measure of locomotor activity in shuttle-boxes was performed with the shuttle-box lamps switched off and without electric shock on the floor (Sansone et al., 1999). Mice were subjected to a 60 min activity test and the number of crossings from one compartment to the other was recorded. Using this procedure, shuttle-boxes activity represents an estimation of the locomotor activity under dark conditions. Shuttle-box activity was analysed in two separate 0–30 and 30–60 min periods (Luvisetto et al., 2004).
Anxiety-related behaviors
Open field
This test was performed in a square arena (home-made, 40×40×20 cm) with opaque walls and with the floor divided into 25 squares (8×8 cm). According to their distance from the walls, the squares were classified as border (16 squares), intermediate (8 squares), and central (1 square). At the beginning of the test, the mouse was placed in the central square and, during 5 min test, the total time of moving and the time of moving into a defined zone (border, intermediate or central squares), together with number of entries into central square was recorded. Entry into a specific square was computed only if the mouse entered the square with all four paws (four paw criterion).
Elevated plus maze
The elevated plus maze was a home-made cross-maze constituted of four arms (30×5 cm), two of them enclosed within a 15 cm-high wall (closed arms) while the other two were open (open arms). The central platform between the arms measured 5×5 cm. The entire apparatus was 40 cm elevated above the floor. At the beginning of test, the mouse was placed on the central platform facing an open arm. The number of entries into open and closed arms (as measurement of activity) and the time spent on either type of arm excluding time in center (as measurement of anxiety) during 5 min of test period was recorded. Arm entry was computed with the four paw criterion. Total number of arm entries and time spent on each arm were the sum of entries and time onto open (Op) and closed (Cl) arms. The data collected were used to calculate percentage of time on open arms using the equation (File, 2001): (Op/ (Op+Cl)) ×100.
Object exploration
This test, based on a partial modification of a behavioral paradigms used in rats (Ennaceur & Delacour, 1988), was performed in three distinct phases: habituation, training and testing. During habituation (which was repeated twice separated by a 2 hour interval), the mouse was placed in a circular arena (60 cm diameter) clear of objects and left free to explore it for 5 min before being returned to the home cage. During training (24 hours after habituation), each mouse was transferred to the circular arena where two identical black-stained acrylic cylinders (2 cm diameter; 10 cm long) were placed equidistant from the walls. To avoid odor cues, duplicates of the objects were used. During testing (4 hours after training) the mouse was transferred again into the arena containing two objects, one of which was a duplicate of the training object (F, familiar) while the other was a novel object (N), different from the F object both in size and colour. N was a white-stained acrylic disc (5 cm diameter; 1 cm thickness). Care was taken to avoid place preference by randomly changing the position of the two objects. Both during training and testing, mice were allowed to explore the arena for 5 min, and approaches to the objects at a distance of 2 cm or less was scored as object exploration.
Learning and memory of avoidance tasks
Active avoidance
The active avoidance test evaluated associative learning and retention for conditioning events (Bovet et al., 1969). Briefly, mice learn to avoid a noxious stimulus by a specific locomotor response driven by a conditioning stimulus which is presented few seconds before the noxious stimulus. The apparatus was computer-controlled and consisted of 2 sets of 8 shuttle boxes (home-made acrylic boxes; 40×10 cm) divided into two 20×10 cm compartments connected by a 3×3 cm opening. A light (10W) was alternately switched on in the two compartments and used as conditioned stimulus (CS). The CS preceded the onset of the unconditioned stimulus (US) by 5 s, and overlapped it for 25 s. Using this procedure, the light was present in the compartment for 30 s (5 s alone and 25 s together with the US). After 30 s both CS and US were terminated and the cycle immediately began in the other compartment. The US was an electric shock (0.2 mA) continuously applied to the grid floor (stainless steel rods spaced 0.4 cm apart). Over extensive training, mice learn to associate CS and US, and to avoid US by running into the dark compartment. An avoidance response was recorded when mice avoided US by running into the dark compartment within 5 s of the onset of CS. If mice failed to avoid the US they could however escape it. In such case, mice responses were recorded as simple escape responses. Mice were subjected to 5 daily, 100-trial avoidance sessions. Failure of escape response seldom occurred.
Passive avoidance
The passive avoidance test is a two-day task which measures the memory of an aversive experience through the simple avoidance of a location in which the aversive experience occurred, using a step-through inhibitory avoidance apparatus as described by Castellano et al. (1999). The apparatus consisted of a box divided into two compartments of different size: the smaller one was illuminated while the larger one was equipped with a removable cover to allow it to be kept dark. A removable partition divided the two compartments. The floor of the larger compartment consisted of two oblique stainless steel plates folded at the bottom through which a continuous current could be delivered. On the training day, each mouse was placed in the lit compartment, facing away form the dark compartment. When the mouse turned around, the partition leading to the dark compartment was opened and the latency to move into the dark compartment was recorded. When the mouse stepped with all four paws into the dark compartment, the partition was closed and two mild footshocks (0.4 mA; 50 Hz; 2 s duration) were delivered with an interval of 5 s. The mouse was then removed from the apparatus and returned to its home cage. Retention was tested 30 min and 24 hr later following a similar procedure, except that no shock was administered. Latency of the mouse to move into the dark compartment (step-through latency) after the opening of the door was taken as a measure of memory retention. The footshock sensitivity was checked in a few WT and CyPD-KO naive mice by using the same cage as for passive avoidance. For this purpose, when the mouse was inside the dark compartment, an opening was left on the partition and an electric shock of increasing intensity was delivered to the floor cage. Using this procedure, we did not observe differences between WT and CyPD-KO mice, all mice immediately escaping from the electrified compartment when the current intensity reached 0.1 mA.
Nociceptive sensitivity
To assess nociceptive threshold toward thermal stimuli, mice were tested in hot-plate test. For this purpose. mice were placed on the center of an hot-plate apparatus (PanLab, Barcellona, Spain), consisting of a metal plate set at 50 ± 1 °C enclosed by a 19 cm diameter acrylic cylinder. Temperature was chosen in the range commonly reported in the literature (Mogil et al., 2006). Nociceptive response was assessed by scoring the latency before the first appearance of licking or shaking of fore- or hind-paws; testing was terminated after 60 sec. Each mouse was tested only once since repeated tests in this assay lead to profound latency alterations (Wilson & Mogil, 2001).
Experimental groups
For preliminary observations and food/water intake, WT and CypD-KO mice of different ages were chosen from the colony and assigned to different experimental groups for testing at 4 and 15 months of age (n = 6 for each age and genotype). Mice assigned to these experimental groups were not considered for other analyses.
For behavioral assessment, WT and CyPD-KO mice at 4 and 10 months of age (n=24 in each genotype-age group) were tested. Mice were naive for each behavioral test, i.e., mice were tested only once and mice which performed behavioral tests at 4 months were not further used for the test at 10 months. Within each genotype-age group, mice were subdivided in two subgroups (12 mice per group) which were subjected to 2 different sequences of behavioral paradigms. In one sequence, mice were subjected to rotarod, open field, plus maze, and object exploration/recognition tests, with 2 days of rest between each test. One week after the end of object exploration/recognition test, mice were scored for active avoidance learning. In the other sequence, mice were subjected to shuttle-box activity, hot-plate and passive avoidance learning, with 1 week of rest between each test.
All testing was carried out from 9:00 a.m. to 2:00 p.m. during the light phase of the light/dark cycle, in soundproof rooms with the investigator outside the room, except for rotarod and passive avoidance tests. The rooms were illuminated with a low intensity twilight lamp, except for shuttle-box locomotor activity and active avoidance learning tests which were performed with the light off. When appropriate, mice were video-recorded and data were collected and analysed by remote PC.
Statistical analysis
All values are expressed as mean±SEM. Statistics for open field, plus maze, and passive avoidance data were performed by parametric analysis of variance (ANOVA). Statistics for food and water intakes, rotarod, shuttle-box activity, and active avoidance data were performed by two-factors analysis of variance for repeated measures (RMANOVA), considering genotype-age group as between-subject factor and time periods, or daily sessions, as repeated measures (within-subject factor). When appropriate, pairwise comparisons between genotype-age groups were carried out using Bonferroni/Dunn test, considering WT (4) mice as control. Data from object exploration/recognition test were analyzed with paired t-test to determine significant difference in exploring old and new objects for each genotype-age group. Significance level was set to 5% in all the analyses.
RESULTS
Preliminary observations: CyPD-KO mice develop an adult-onset obesity
Careful visual inspection did not reveal any obvious difference between CyPD-KO and WT mice. CyPD-KO mice maintained a normal gait, posture and spontaneous movements were normal; no abnormal stereotyped behavior or signs of ataxia or dystonia were apparent. Defecation was present without differences in bolus number between CyPD-KO and WT mice. Eyes were normally open and lacrimation was absent. Fur appeared tidy and well groomed, piloerection was absent, and whiskers were intact in all mice. The righting reflex was comparable for the two genotypes. The only visible phenotype was the size of CyPD-KO mice, which appeared bigger than WT (Fig. 1) and at 10 months clearly developed adult-onset obesity (Fig. 1b). At age older than 12 months, body weight of individual CyPD-KO mice remained almost constant and began to slowly decrease after 20 months (Fig. 1a).
Figure 1. Body, organ and tissue weights.
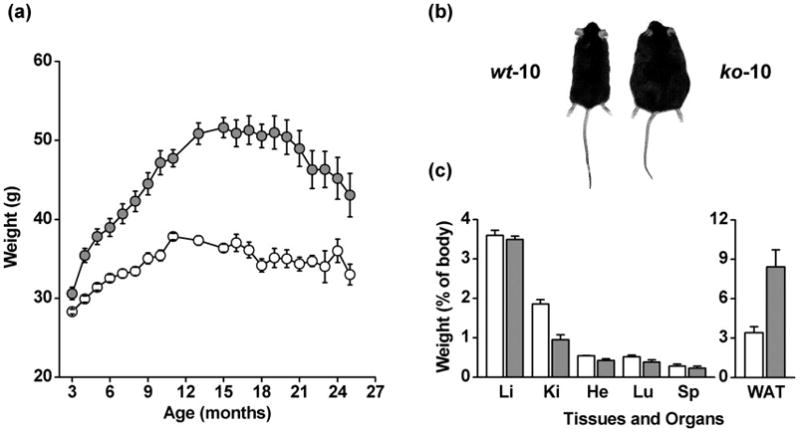
(a) Mean body weight of WT (○; n=64) and CyPD-KO ( ; n=60) mice at various ages. (b) Representative examples of one WT-10 and one CyPD-KO-10 mice. (c) Mean weight of organs or tissues, expressed as % of total body weight, of WT-15 (white bars; n=3) and CyPD-KO-15 (grey bars, n=3) mice. Li, liver; Ki, kidney (x2); He, heart; Lu, lungs; Sp, spleen; WAT, visceral white adipose tissue.
; n=60) mice at various ages. (b) Representative examples of one WT-10 and one CyPD-KO-10 mice. (c) Mean weight of organs or tissues, expressed as % of total body weight, of WT-15 (white bars; n=3) and CyPD-KO-15 (grey bars, n=3) mice. Li, liver; Ki, kidney (x2); He, heart; Lu, lungs; Sp, spleen; WAT, visceral white adipose tissue.
To characterize this adult-onset obesity, WT (15) (n=3) and CyPD-KO (15) (n=3) mice were sacrificed for examination of organs and tissues. Anatomical comparison revealed that size and weight of liver, heart, lung and spleen were proportional to the body weight while the kidneys appeared smaller in CyPD-KO (15) mice (Fig. 1c). Thymus and thyroid gland appeared of normal proportions and no differences were detected in size or number of lymphnodes (not shown). On the other hand, CyPD-KO (15) mice appeared full of white adipose tissue (WAT), which accumulated both subcutaneously (mainly around the hindpaw musculature) and intraperitoneally (surrounding all visceral organs with a large mass of white adipose tissue also infiltrating the thoracic cavity). Brown adipose tissue appeared normal and its mass was not increased in CyPD-KO (15) mice. Thus, the adult-onset obesity of CyPD-KO mice was essentially due to abnormal accumulation of WAT.
Food/water intake in WT and CyPD-KO mice
To establish if the abnormal accumulation of WAT in CyPD-KO mice was due to increased caloric intake, WT (4) (n=6), WT (15) (n=6), CyPD-KO (4) (n=6) and CyPD-KO (15) (n=6) mice were isolated from the colony, housed in single cages and food (Fig. 2a) and water (Fig. 2b) intake evaluated over 12 days period. Body weights of these mice were 30, 32, 31, 32, 33, and 30 gr for WT (4); 43, 35, 40, 41, 39, and 42 gr for CyPD-KO (4); 38, 35, 46, 39, 41, and 42 gr for WT (15); and 55, 50, 54, 53, 47, and 55 gr for CyPD-KO (15), respectively. Although the body weight of each CyPD-KO mice was higher than that of each WT mouse, no significant differences in food (RMANOVA; genotype-age groups: F3,20 = 2.459, p = 0.0770; days: F11,20 = 0.608, p = 0.8214) and water (RMANOVA; genotype-age groups: F3,20 = 1.431, p = 0.2634; days: F11,220 = 1.109, p = 0.3549) intake were observed. It should be noted that when corrected for food/water intake, assessment of body weight of each mouse (results not shown) reveals that CyPD-KO mice consume significantly less than other groups. Thus, the adult-onset obesity of CyPD-KO mice was not a consequence of increased food/water intake and therefore, obesity is likely due to differences in metabolism and/or energy expenditure leading to abnormal accumulation of WAT.
Figure 2. Food and water intake.
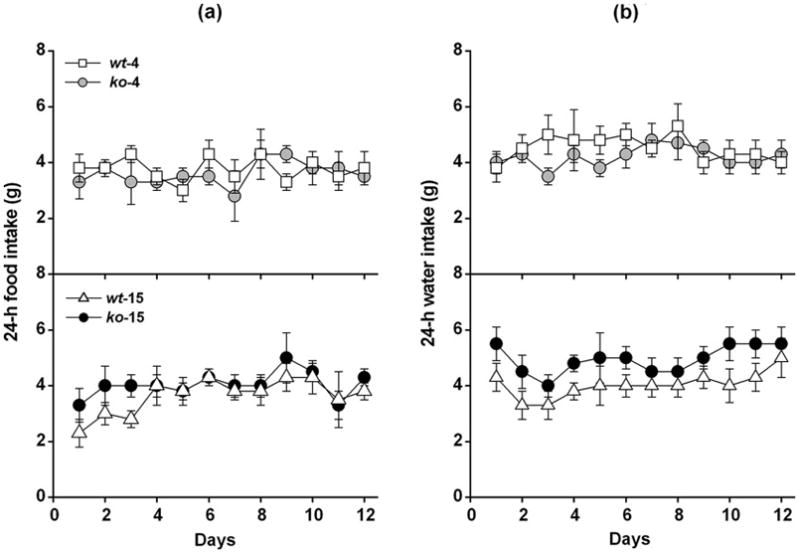
Daily food (a) and water (b) intakes of WT-4 (□), CyPD-KO-4 ( ), WT-15 (△) and CyPD-KO-15 (●) mice, during 12 consecutive days. Number of animals was n=6 for each genotype-age group.
), WT-15 (△) and CyPD-KO-15 (●) mice, during 12 consecutive days. Number of animals was n=6 for each genotype-age group.
Decreased motor coordination and locomotor activity in CyPD-KO mice
The mean rotarod performances recorded in WT (4) (n=12), CyPD-KO (4) (n=12), WT (10) (n=12) and CyPD-KO (10) (n=12) mice, and expressed as mean time on the rod before falling, are reported in Fig. 3a (six trials of training with rotarod at fixed rate and eight trials of testing with accelerated rotarod). WT (4), WT (10), and CyPD-KO (4) mice behaved identically: each quickly learned to stay in equilibrium for almost 2 min. during training, and for at least 1 min. during testing. On the other hand, CyPD-KO (10) mice were unable to stay in equilibrium onto rotarod during both training and testing sessions. RMANOVA for rotarod data, during training and testing sessions, showed significant main effect for genotype-age groups (training: F3,44 = 23,994, p < 0.0001; testing: F3,44 = 14.724, p < 0.0001), and sessions (training: F5,220 = 39.669, p < 0.0001; testing: F7,308 = 6.679, p < 0.0001), as well as a significant group × session interaction (training: F15,220 = 5.288, p < 0.0001; testing F21,308 = 3.403, p < 0.0001). Pairwise comparison showed significant differences between WT (4) mice and CyPD-KO (10) mice (training and testing: p < 0.0001) but not WT (10) (training: p = 0.0733; testing: p = 0.1649) or CyPD-KO (4) (training: p = 0.4928; testing: p = 0.0953) mice. These data indicate a loss of motor coordination in CyPD-KO (10) mice, but not in CyPD-KO (4) mice, which can be partially explained by the abnormal obesity observed in CyPD-KO (10) with age.
Figure 3. Rotarod, shuttle-box activity and open field.
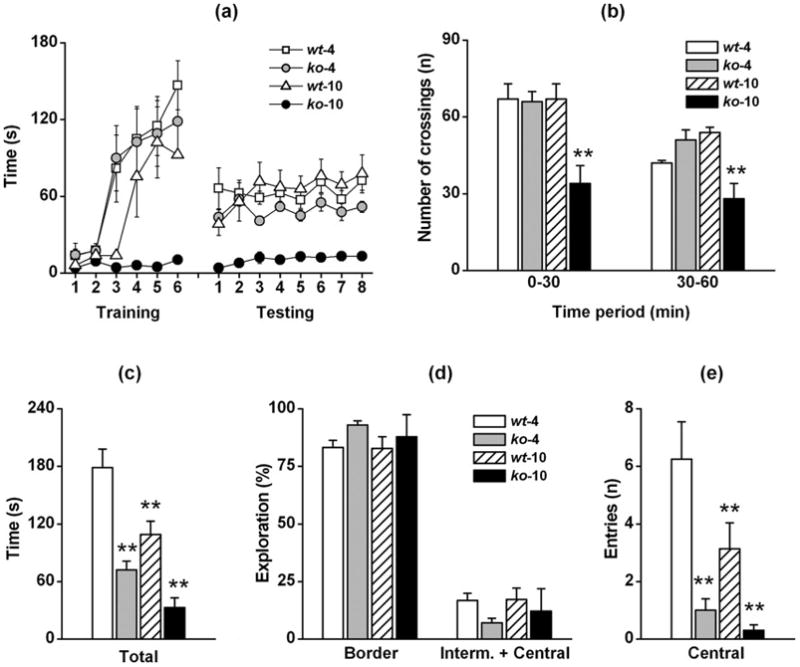
(a) Time before fall from rotarod at fixed (training) and accelerated (testing) rate as recorded in WT-4 (□), CyPD-KO-4 ( ), WT-10 (△), and CyPD-KO-10 (●) mice. (b) Number of crossings of WT-4, WT-10, CyPD-KO-4 and CyPD-KO-10 mice during 60 min activity in shuttle-box. Two periods are reported: 0–30 and 30–60 min. (c) Total exploring time, (d) percent of total exploration time spent to explore border or intermediate + central squares, and (e) number of entries into central squares of WT-4, CyPD-KO-4, WT-10 and CyPD-KO-10 mice during 5 min of open field test. White, grey, dashed and black bars refer to WT-4, CyPD-KO-4, WT-10, and CyPD-KO-10 mice, respectively. For each test, number of animals was n=12 for each genotype-age group. Statistical significance: p < 0.01 (**).
), WT-10 (△), and CyPD-KO-10 (●) mice. (b) Number of crossings of WT-4, WT-10, CyPD-KO-4 and CyPD-KO-10 mice during 60 min activity in shuttle-box. Two periods are reported: 0–30 and 30–60 min. (c) Total exploring time, (d) percent of total exploration time spent to explore border or intermediate + central squares, and (e) number of entries into central squares of WT-4, CyPD-KO-4, WT-10 and CyPD-KO-10 mice during 5 min of open field test. White, grey, dashed and black bars refer to WT-4, CyPD-KO-4, WT-10, and CyPD-KO-10 mice, respectively. For each test, number of animals was n=12 for each genotype-age group. Statistical significance: p < 0.01 (**).
Locomotor activity under dark condition was estimated by recording the activity in shuttle-box cages measured as mean number of crossings during 60 min test. The first (0–30 min) and the second (30–60 min) time periods were separately recorded (Fig 3b). Both WT (4) (n=12), WT (10) (n=12) and CyPD-KO (4) (n=12) mice behaved similarly with virtually the same number of crossings, while CyPD-KO (10) (n=12) mice showed reduced number of crossings indicating a reduced locomotor activity. RMANOVA showed significant effect for genotype-age group (F3,44 = 31.774, p = 0.0002), and time period (F1,44 = 23.918, p < 0.0001), as well as a significant group × time period interaction (F3,44 = 2.846, p = 0.0429). Compared to WT (4), pairwise comparison showed significant differences only for CyPD-KO (10) mice (p < 0.0001) but not for WT (10) (p = 0.8540) and CyPD-KO (4) (p = 0.6101) mice. These data confirm previous findings of rotarod test, and indicate that CyPD-KO (10) mice were impaired both in motor coordination and spontaneous locomotor activity, again perhaps due to the abnormal obesity exhibited by older CyPD-KO mice.
Reduced exploratory activity of CyPD-KO mice in open field test
To further analyze the activity of mice, we performed an open field test under light conditions. At difference from shuttle-boxes, the activity in open field represents a complex behavior which mimics the natural conflict in mice between their tendency to explore a novel environment and their tendency to avoid a lit open area. Mouse behavior in open field is the result of combined positive or negative stimuli, activity being mainly enhanced by exploratory drive and depressed by anxiety (Wilson et al., 1976). An anxious animal will tend to explore the open field by moving mostly close to the protective walls of the arena (border area), and will seldom move toward the intermediate regions or across the central areas of the arena.
During 5 min of open field test of mice previously tested on the rotarod, we measured the total moving time spent to explore the open field arena (Fig. 3c), the percent of total moving time spent to explore border or intermediate+central areas (Fig. 3d), and the number of entries into central area (Fig. 3e). WT (4) (n=12) mice actively explored the open field, with more than 80% of exploration along the borders and rarely through the central area of the arena. Compared to WT (4) mice, both WT (10) (n=12), CyPD-KO (4) (n=12), and CyPD-KO (10) (n=12) mice showed reduced exploratory activity with even less entries in the central area of the arena, while the percentage of time spent by moving around the border was similar. ANOVA showed a significant effect of genotype-age group for total time exploration (F3,44 = 21.114, p < 0.0001) and for entries in the central area of the open field arena (F3,44 = 16.416, p < 0.0001) but not for the percent time exploring the border or intermediate+central areas (F3,44 = 0.934, p = 0.4388). Compared to WT (4) mice, pairwise comparison showed significant differences both for WT (10) (total time: p = 0.0015; central entries: p = 0.0012), CyPD-KO (4) (total exploration time and central entries: p < 0.0001) and CyPD-KO (10) (total exploration time and central entries: p < 0.0001) mice. Moreover, pairwise comparison of CyPD-KO (4) and CyPD-KO (10) mice with WT (10) mice showed significant difference both for CyPD-KO (4) (total exploration time: p = 0.0218; central entries: p = 0.0070) and CyPD-KO (10) (total time: p = 0.0006; central entries: p = 0.0017). Considering that CyPD-KO (4) and WT (10) mice have almost the same weight, the observed significant differences in the open field data clearly indicate that weight is not a factor in this measure. Comparison of activity under dark (shuttle-box activity) or light (open field) conditions reveals an increased aversion toward open space also in older WT mice, and a particularly enhanced aversion in CyPD-KO mice, already evident at 4 months of age.
Increased anxiety/emotionality of CyPD-KO mice in elevated plus maze test
The anxiety level of mice, previously tested in rotarod and open field tests, was assessed by the elevated plus maze test (Lister, 1987). Due to the presence of the elevated open arms, which combine elements of unfamiliarity, openness and elevation, the elevated plus maze test is specifically designed to assess fear and anxiety-like behaviors evoked by the exploration of new and potentially dangerous places. Mice usually avoid exploration of the open arms and tend to enter, and spend time preferentially, on the closed arms. Fig. 4 shows exploration time (Fig. 4a) and number of entries (Fig. 4b) on to closed and open arms as recorded in WT (4), CyPD-KO (4), WT (10) and CyPD-KO (10) mice during 5 min of plus maze test. Mice tested in the plus maze were previously tested for rotarod and open field tests. Both genotype-age groups explored preferentially the closed arms, without significant differences in exploration time (ANOVA: F3, 44 = 1.787, p = 0.1254) and number of entries (ANOVA: F3, 44 = 0.850, p = 0.2071); mice spent only a small fraction of testing time exploring the open arms (see the inset of Fig. 4a showing the percentage of time spent exploring the open arms). However, ANOVA and pairwise comparison showed significant differences between WT (4) mice (time: F3,44 = 5.099, p = 0.0042; entries: F3,44 = 8.723, p = 0.0001), CyPD-KO (4) (time: p = 0.0078; entries: p = 0.0007) and CyPD-KO (10) (time: p = 0.0047; entries: p < 0.0001) but not for WT-10 (time: p = 0.5108; entries: p = 0.1145) mice in exploring the open arms. Together with the open field data, data from plus maze test confirm an enhanced anxiety in CyPD-KO mice.
Figure 4. Elevated plus-maze and object exploration/recognition.
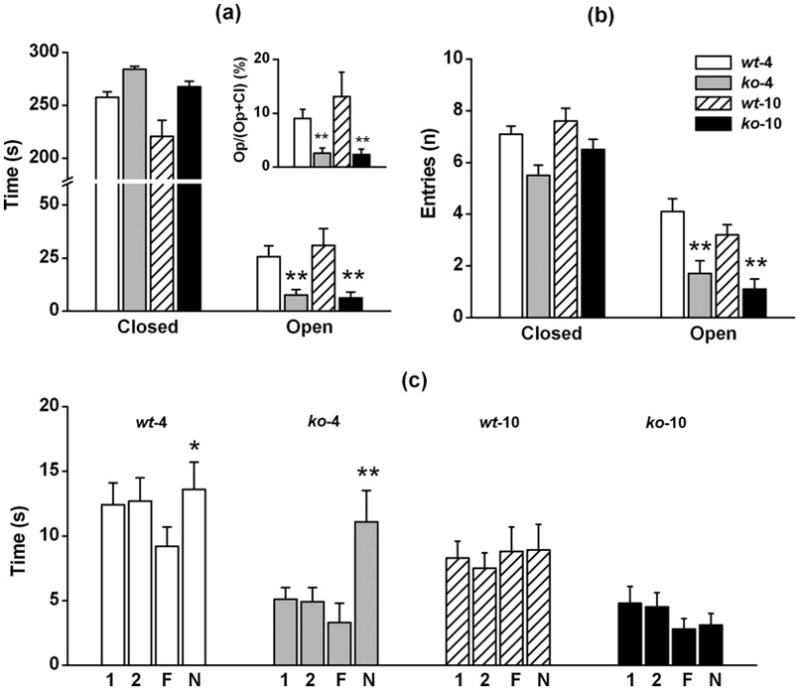
(a) Time and (b) number of entries into open and closed arms during 5 min of elevated plus maze test with WT- 4, CyPD-KO-4, WT-10 and CyPD-KO-10 mice. Inset of panel (a) shows the percentage of time spent in open arms compared to the total time in open and closed arms, excluding time in center. White, grey, dashed and black bars refer to WT-4, CyPD-KO-4, WT-10, and CyPD-KO-10 mice respectively. (c) Exploration time of two identical objects (1, 2) during training and of two different objects (F, N) during testing phase of object exploration/recognition with WT-4, CyPD-KO-4, WT-10 and CyPD-KO-10 mice. For each test, number of animal was n=12 for each genotype-age groups. Statistical significance: p < 0.05 (*); p < 0.01 (**).
Exploratory activity of CyPD-KO mice in object exploration/recognition test
As in the open field and elevated plus maze, mice behavior as assessed in the object exploration test is a combined result of various inputs, such as the exploratory drive, the anxiety/fear and/or the curiosity/attraction for novelty. In addition, this task investigates also the ability to recognize the novelty into a familiar environment. Fig. 4c shows the results of the object exploration test performed in WT (4) (n=12), CyPD-KO (4) (n=12), WT (10) (n=12) and CyPD-KO (10) (n=12) mice. Mice tested for object exploration/recognition were also studied in the rotarod, open field and plus maze tests. In keeping with previous results from the open field test, during the habituation phase of free exploration without objects, CyPD-KO mice explored the arena for a shorter time than WT mice (results not shown). During training performed with two identical objects, WT (4) mice explored the two objects (1, 2) for approximately the same time. Again, CyPD-KO (4) mice were less explorative than WT mice. During testing performed with two different objects (F, familiar; N, novel), two unusual behaviors became evident. First, both WT (4) and CyPD-KO (4) mice spent significantly more time exploring the novel object [WT (4): p = 0.0394; CyPD-KO (4): p = 0.0078; paired t-test] and second, CyPD-KO (4) mice explored the two objects for a longer time than during training. A completely different pattern was observed in tests of WT (10) and CyPD-KO (10) mice. Both WT (10) and CyPD-KO (10) mice were unable to recognize the novel object placed into a familiar environment and explored the novel and familiar objects for almost an equal time [WT (10): p = 0.9452; CyPD-KO (10): p = 0.6809; paired t-test]. These results suggest that, although CyPD-KO mice were more anxious and less explorative than WT mice, they behaved similarly in discriminating a novel object placed into a familiar environment, skills that were progressively lost with age in both WT and CyPD-KO mice.
Improved avoidance behavior of CyPD-KO mice in active avoidance test
With the aim of assessing avoidance behaviors, mice were subjected to two operant conditioning tests. We used two-way (active) and one-trial inhibitory (passive) avoidance paradigms since they are reliable and complementary tests for measuring the integrity of the learning and memory processes. Fig. 5a shows the cumulative avoidance responses during 5 daily sessions of active avoidance for WT (4) (n=12), CyPD-KO (4) (n=12), WT (10) (n=12) and CyPD-KO (10) (n=12) mice. Mice tested for active avoidance were previously tested in rotarod, open field, plus maze and object recognition tests. Both WT and CyPD-KO mice carried out a number of avoidance responses, progressively increasing from the first (I) to the fifth (V) session. RMANOVA analysis of avoidance responses yielded significant effects of genotype-age group (F3,44 = 25.700, p < 0.0001), and daily session (F4,176 = 135.856, p < 0.0001), as well as a significant group × daily session interaction (F12, 176 = 11.146, p < 0.0001). Pairwise comparisons showed significant differences between WT (4), CyPD-KO (4) (p = 0.0003) and CyPD-KO (10) (p < 0.0001) mice but not for WT (10) (p = 0.3375) mice. Compared to WT (4) mice, pairwise comparison for each daily session showed significantly increased avoidance responses for CyPD-KO (4) mice starting from the III session, and for CyPD-KO (10) mice starting from the I session.
Figure 5. Learning and memory of avoidance tasks and hot plate test.
(a–b) Active avoidance behavioral responses, calculated as (a) cumulative number of shock avoidance during 100 trials over 5 days or (b) consecutive blocks of 20 trials during each daily session in WT-4, CyPD-KO-4, WT-10 and CyPD-KO-10 mice. Number of mice was n=12 for each genotype-age group. (c) Step-through latency for untrained (first exposed to the apparatus) or trained (probed 30 min or 24 hr after training) WT-4, CyPD-KO-4, WT-10 and CyPD-KO-10 mice subjected to passive avoidance test. Total number of mice was n=12 for each genotype-age group. Six mice were probed for 30 min and six mice for 24 hr memory retention. Cutoff was set at 300 s. (d) Latency before the first licking/shaking of hindpaw of either WT-4 and CyPD-KO-4 (4M), or WT-10 and CyPD-KO-10 (10M) mice subjected to hot plate test. White, grey, dashed and black bars refer respectively to WT-4, CyPD-KO-4, WT-10, and CyPD-KO-10 mice. Number of mice was n=12 for each genotype-age group. Statistical significance: p < 0.01 (**).
The enhanced ability of CyPD-KO mice to avoid shock was not only quantitative but also qualitative. Fig. 5b shows the learning curves determined for each genotype-group. Each curve represents the rate of acquisition within each daily session and each point in the curves represents the mean avoidance response calculated in blocks of 20 trials (10 min). Analysis of the shape of the learning curves within each session enables a distinction between learning processes (which occur during the session) and the consolidation/retention processes (which occur in the 24-h interval between two daily sessions). Increasing avoidance within a session indicates good learning acquisition, while the difference between the last avoidance block of a session and the first avoidance block of the following session indicates memory consolidation and retention (Oliverio and Bovet, 1966). Learning curves for WT (4) mice show that the first avoidance blocks (beginning of each daily session) were slightly increased (starting from the III) and remained almost constant at the IV and V sessions, while the last avoidance blocks (end of each daily session) appeared strongly enhanced from the IV session. This pattern indicates that WT (4) mice were effective in learning, but only from the IV session, and rather ineffective in consolidation. Normally the learning and memory consolidation worsens with aging (Oliverio & Bovet, 1966). The worsening of both learning and consolidation appears evident in WT (10) mice during the IV and V session; whereas, starting from the III [CyPD-KO (4)] and the I session [CyPD-KO (10)], CyPD-KO mice showed enhanced learning and memory consolidation compared to WT mice. Taken together, data from active avoidance clearly indicate that the avoidance behaviors in CyPD-KO mice were not only more efficient than in WT mice, but also improved during aging.
Improved avoidance behavior of CyPD-KO mice in passive avoidance test
The passive avoidance test takes advantage of the natural tendency of mice to prefer dark over lit spaces. Mice tested for the passive avoidance were previously tested for shuttle-box activity and hot-plate test. Untrained mice (i.e., mice which were first exposed to the apparatus) quickly entered the dark box within few seconds of door opening (Fig. 5c). No significant effect of genotpye-age group in untrained mice was observed (ANOVA: F3,44= 0.877, p = 0.4603; n=12 mice for each genotype-age group). A single training session is normally sufficient to learn the task (i.e. mice learn not to enter the dark box to avoid receiving the footshock). When the test was repeated 30 min after training, step-through latencies were strongly increased for each genotype-age group without significant differences (ANOVA: F3,20 = 0.068, p = 0.9764; n=6 mice for each genotype-age group), indicating that, independent of age, both WT and CyPD-KO mice successfully learned to avoid punishment in the dark box. Accordingly, the processes of memory formation and short term retention would be expected to be similar for each genotype-age group. However, as demonstrated by the higher step-through latencies at 24 hr, long term retention was enhanced in CyPD-KO mice compared to WT mice regardless of age. ANOVA showed significant effect of genotype-age group (F3,20 = 6.372, p = 0.0033; n=6 mice for each genotype-age group) and pairwise comparison showed significant differences between WT (4) and both CyPD-KO (4) (p = 0.0009) and CyPD-KO (10) (p = 0.0015) mice but not for WT (10) (p = 0.1077) mice. These data, together with data from active avoidance, suggest enhanced avoidance behavior in CyPD-KO (4) mice, an improvement that usually deteriorates with age, was maintained in CyPD-KO (10) mice.
Nociceptive threshold for thermal stimuli in CyPD-KO mice
Finally, the same mice used for activity in shuttle-box were scored in the hot plate test (Fig. 5d). The nociceptive responses during hot plate test (i.e., the latency before first paw licking or shaking) were not significantly different in WT and CyPD-KO mice (ANOVA: F3,44 = 0.453, p = 0.6754; n=12 mice for each genotype-age group). This reveals similar nociceptive thresholds for WT and CyPD-KO mice, independent of age.
DISCUSSION
In this report, we demonstrated that the lack of CyPD expression in mice confers a complex phenotype characterized by three main traits: (i) high levels of anxiety/emotionality in open field and elevated plus maze tests; (ii) facilitation of the learning in tasks of active and passive avoidance; and (iii) increased incidence of adult-onset obesity. All these traits are age-dependent in that they become more evident in adult (10 months of age) than in young (4 months of age) mice. On the other hand, no alteration has been observed in the nociceptive threshold to thermal stimuli. Overall, this behavioral and physiological profile points to an important role for mitochondria and mitochondrial homeostatic mechanisms in the generation of complex phenotypes in mammals.
In terms of the enhancement of anxiety/emotionality levels, CyPD-KO mice used in this study were isogenic to C57BL/6J (WT) mice. Compared to other inbred lines, C57BL/6J mice demonstrated variable levels of anxiety depending on specific behavioral paradigm used (Mathis et al., 1994; Crawley et al., 1997; van Gaalen and Steckler, 2000; Voikar et al., 2001; Rodgers et al., 2002; Yilmazer-Hanke et al., 2003). Here, data obtained from open field and elevated plus maze tests clearly underline discrete levels of anxiety/emotionality in WT mice, which were enhanced during aging from 4 to 10 months. Compared to WT mice, CyPD-KO mice showed further enhancement of anxiety/emotionality levels, both in the elevated plus maze and in the open field tests. Avoidance behaviors are also strongly determined by genetic background. The C57BL/6J strain is generally a poor learner of avoidance tasks, and rather ineffective in memory consolidation (Bovet et al., 1969; Sprott, 1974; Weinberger et al, 1992). Data obtained from active and passive avoidance tests confirm the very low avoidance responses of WT mice, which progressively worsened with age (from 4 to 10 months), consistent with the well-established deterioration of memory skills during aging. In contrast, the lack of CyPD expression resulted in enhanced avoidance behaviours, as assessed both in active and passive avoidance tasks, that, surprisingly, were not only more efficient than WT mice in young mice but also did not decline during aging from 4 to 10 months of age.
Given the parallel enhancement of anxiety/emotionality levels and avoidance behaviors observed in CyPD-KO mice, we are unable to establish whether the improvement in avoidance tasks we document in CyPD-KO mice are based in cognitive improvements or in the enhanced anxiety/emotionality levels. An univocal relationship between learning, memory and anxiety has never been clearly established in rodents, however, numerous behavioral, physiological, pharmacological, and genetic studies have outlined the existing overlap between these processes which closely interact at different levels in the CNS. Since anxiety and memory both require brain arousal, a condition which has been often considered as anxiogenic and promnestic, it is reasonable to assume that higher anxiety could be coupled to a better memory. Indeed, many examples of mice (including mutant mice lacking various receptors or other brain proteins) and rat strains (see Kalueff, 2007, for review) have been shown to exhibit contrasting differences related to their scores in tests of anxiety/emotionality and avoidance. For example, senescence-accelerated mice and colecystokinin-null mice (Kawamata et al., 1997; Brandeweide et al., 2005; Lo et al., 2007) as well as some rat strains (Ho et al., 2002; Escorihuela et al., 1999; but see also Ribeiro et al., 1999, for contrasting results) show high anxiety/emotionality levels in open field and plus maze tests and low avoidance behaviors in tests active and/or passive avoidance. Furthermore, it has also been shown that several kinds of anxiolytic drugs facilitate acquisition and increase the performances of both mice and rats in two-way active avoidance (Sansone, 1975; Fernandez-teruel et al., 1991). However, other studies have shown that different inbred lines of mice display different anxiety behavior in the elevated plus maze but showed no significant differences in active (Moragrea et al., 2005) and passive avoidance (Podhorna and Brown, 2002), or good retention scores in active avoidance (Crawley et al., 1997). Therefore, we cannot exclude the possibility that the high avoidance behavior of our CyPD-KO mice is due to their high anxiety/emotionality levels rather than their improved cognition functions. However, independent of the mechanisms involved (either emotional or cognitive or an interplay of both) in the high avoidance behavior of CyPD-KO mice, this phenotype appears particularly evident during aging and is suggestive of an important role of CyPD in aging processes.
Although biological studies have clearly established that many tissues in CyPD-KO mice (i.e., heart, brain, liver, skeletal muscle) are resistant to damage generated by reactive oxygen species (ROS) and calcium overload (Baines et al., 2005; Basso et al., 2005; Nakagawa et al., 2005; Schinzel et al., 2005; Forte et al., 2007; Jobe et al., 2007; Merlini et al., 2008; Millay et al., 2008), the involvement of CyPD protein in the physiological processes of aging has never been demonstrated. Aging is characterized by accumulated damage occurring over time from exposure to ROS and oxidative stress. Mitochondria, the major intracellular source of ROS, are particularly vulnerable to oxidative damage since ROS generated within mitochondria can potentially feed back on the organelles and directly damage mitochondrial components (Pollack and Leeuwenburg, 2001; Wei and Lee, 2002). Mitochondrial dysfunction, caused by chronic exposure to oxidants, increases the probability of mPTP opening, a downstream target of oxidant stress (Petronilli et al., 1994; Toescu et al., 2000) leading to cell death. Although the precise structure of the mPTP is as yet resolved, CyPD is a key activator of the mPTP (see Bernardi et al., 2006 for review). Interestingly, Mather and Rottenberg (2000) reported an enhanced activation of the mPTP in the brain of old versus young mice.
In the mature nervous system, mitochondria are present both in glial and neuronal cells where they are localized in axons, dendrites, growth cones and pre- and post-synaptic terminals. Brown et al. (2006) analyzed the synaptic and non-synaptic fraction of brain mitochondria and found an increased susceptibility to Ca2+ overload and an increased propensity to undergo mPT in synaptic mitochondria when compared to non-synaptic mitochondria. Recently, Naga et al. (2007) suggested that the increased occurrence of permeability transition in synaptic mitochondria is due to their high content of CyPD, since higher levels of CyPD were observed in synaptic mitochondrial fractions. These differences in mPT activation between synaptic and non-synaptic mitochondria were greatly reduced in mitochondria isolated from brains of CyPD-KO mice (Naga et al., 2007). If Ca2+ overload in synaptic mitochondria initiates cell death processes through activation of the mPTP, neurons from CyPD-KO mice, which do not contain CyPD (Naga et al., 2007), should be less prone to brain injury due to reduced activation of mPTP-dependent cell death pathways than neurons of WT mice. Moreover, given the postulated role of CyPD in mitochondrial changes associated with cell death, different levels of expression of CyPD may lead to a differential modulation of mPTP, resulting in changes in the release of cytochrome-c. Mitochondrial release of cytochrome-c is responsible for activation of the caspase cascade in apoptosis, proteins with a demonstrated role in many complex neuronal processes, including learning and memory (Glazner et al., 2000; Gilman and Matteson, 2002). Indeed, memory improvements after inhibition of caspase proteins has been recently demonstrated (Glazner et al., 2000; Gilman and Mattson, 2002; Gemma et al., 2005; Lu et al., 2006). A reduced activation of the caspase cascade in specific populations of neurons in CyPD-KO mice may partially explain their improvement in the avoidance behavior tasks where the integrity of synaptic plasticity is pivotal for learning processes.
Finally, a novel finding of this manuscript is the increased incidence of adult-onset obesity in CyPD-KO mice. In contrast to rotarod tests, in which a loss of equilibrium in CyPD-KO (10) mice is likely due to their obesity, the adult-onset obesity of CyPD-KO (10) mice did not appear to interfere with their performance in the other behavioral tests, where good locomotor activity was a prerequisite. For example, although being heavier than WT mice, CyPD-KO (10) mice performed better than WT mice in active avoidance tests that require rapid activation of escape responses to avoid the punishment. The adult-onset obesity of our CyPD-KO (10) mice clearly suggests a specific role for CyPD, and possibly the mPTP, in energy metabolism and points to the general effect of mitochondrial manipulation on body energy expenditure. It should be noted that these mice became obese despite being fed a relatively low-fat diet. Alternatively, the increased body weight and obesity may be a consequence of reduced physical activity, as seems to be suggested by the reduced exploratory activity of CyPD-KO mice in open field test. In addition, the increased adiposity of CyPD-KO mice may be potentially due to diminished apoptosis of adipose cells. More directed studies will be necessary to clarify the origin of this adult-onset obesity and distinguish between these, and other alternatives. Although the adult-onset obesity of CyPD-KO mice needs further examination, our data clearly demonstrate that the lack of CyPD cannot be compensated for by other mechanisms, and that a fundamental link exists between CyPD and the large variety of processes that control the caloric balance and adipogenesis.
CONCLUSION
Our findings show that the absence of CyPD in C57BL/6J mice results in the modification of complex behavior phenotypes with consequent higher levels of anxiety/emotionality and the facilitation of the learning in tasks of active and passive avoidance. In each case, these traits are age-dependent in that they become more evident in adult (10 months of age) than in young (4 months of age) mice. In addition, mice lacking CyPD show an increased incidence of adult-onset obesity. Although further studies are needed to better identify the mechanisms underlying the behavioral changes documented in this report, our data point to a novel and unexpected link between CyPD, a mitochondrial matrix protein and a key regulator of mitochondrial homeostasis, and the physiological regulation of a large variety of complex responses, such as behavior and metabolism.
Abbreviations
- CyPD
cyclophilin D
- mPT
mitochondrial permeability transition
- mPTP
mitochondrial permeability transition pore
- ROS
reactive oxygen species
- WT (n) and CyPD-KO (n)
wild-type mice and mice lacking CyPD at n months of age
References
- Armstrong JS. The role of the mitochondrial permeability transition in cell death. Mitochondrion. 2006;6:225–234. doi: 10.1016/j.mito.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of CyPD reveals a critical role dor mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Basso E, Fante L, Fowlkes J, Petronilli V, Forte M, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of CyPD. J Biol Chem. 2005;280:18558–18661. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachy-Dyson E, Di Lisa F, Forte M. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 2006;273:2077–2099. doi: 10.1111/j.1742-4658.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- Bovet D, Bovet-Nitti F, Oliverio A. Genetic aspects of learning and memory in mice. Science. 1969;163:139–149. doi: 10.1126/science.163.3863.139. [DOI] [PubMed] [Google Scholar]
- Brandeweide J, Schachner M, Morellini F. Ethological analysis of the senescence-accelerated P/8 mouse. Behav Brain Res. 2005;158:109–121. doi: 10.1016/j.bbr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Brown MR, Sullivan PG, Geddes JW. Synaptic mitochondria are more susceptible to Ca2+ overload than nonsynaptic mitochondria. J Biol Chem. 2006;281:11658–11668. doi: 10.1074/jbc.M510303200. [DOI] [PubMed] [Google Scholar]
- Castellano C, Cestari V, Ciamei A, Pavone F. MK-801-Induced disruptions of one-trial inhibitory avoidance are potentiated by stress and reversed by naltrexone. Neurobiol Learn Mem. 1999;72:215–229. doi: 10.1006/nlme.1999.3908. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and reccomendations for molecular studies. Psychopharmacology (Berl) 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- De Marchi U, Basso E, Szabo I, Zoratti M. Electrophysiological characterization of the CyPD-deleted mitochondrial permeability transition pore. Mol Membr Biol. 2006;23:521–530. doi: 10.1080/09687860600907644. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats: 1. Behavioural data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Escorihuela RM, Fernandez-Teruel A, Gil L, Aguilar R, Tobena A, Driscoll P. Inbred Roman high- and low-avoidance rats: differences in anxiety, novelty-seeking, and shuttlebox behaviors. Physiol Behav. 1999;67:19–26. doi: 10.1016/s0031-9384(99)00064-5. [DOI] [PubMed] [Google Scholar]
- Fernandez-teruel A, Escorihuela RM, Boix F, Tobena A. Effects of different handling-stimulation procedures and benzodiazepines on two-way active avoidance acquisition in rats. Pharmacol Res. 1991;24:273–281. doi: 10.1016/1043-6618(91)90091-b. [DOI] [PubMed] [Google Scholar]
- File SE. Factors controlling measures of anxeity and responses to novelty in the mouse. Behav Brain Res. 2001;125:151–157. doi: 10.1016/s0166-4328(01)00292-3. [DOI] [PubMed] [Google Scholar]
- Forte M, Gold BG, Marracci G, Chaudhary P, Basso E, Johnsen D, Yu X, Fowlkes J, Bernardi B, Bourdette D. CyPD inactivation protects axons in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. Proc Natl Acad Sci USA. 2007;104:7558–7563. doi: 10.1073/pnas.0702228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galat A, Metcalfe SM. Peptidylproline cis/trans isomerases. Prog Biophys Mol Biol. 1995;63:67–118. doi: 10.1016/0079-6107(94)00009-x. [DOI] [PubMed] [Google Scholar]
- Gemma C, Fister M, Hudson C, Bickford PC. Improvement of memory for context by inhibition of caspase-1 in aged rats. Eur J Neurosci. 2005;22:1751–1756. doi: 10.1111/j.1460-9568.2005.04334.x. [DOI] [PubMed] [Google Scholar]
- Gilman CP, Mattson MP. Do apoptotic mechanisms regulate synaptic plasticity and growth-cone motility? Neuromolecular Med. 2002;20:197–214. doi: 10.1385/NMM:2:2:197. [DOI] [PubMed] [Google Scholar]
- Glazner GW, Chan SL, Lu C, Mattson MP. Caspase-mediated degradation of AMPA receptor subunits: a mechanism for preventing excitotoxic necrosis and ensuring apoptosis. J Neurosci. 2000;20:3641–3649. doi: 10.1523/JNEUROSCI.20-10-03641.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- Ho YJ, Eichendorff J, Schwarting RK. Individual response profiles of male Wistar rats in animal models of anxiety and depression. Behav Brain Res. 2002;136:1–12. doi: 10.1016/s0166-4328(02)00089-x. [DOI] [PubMed] [Google Scholar]
- Irwin S. Comprehensive observational assessment: Ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacologia. 1968;13:222–257. doi: 10.1007/BF00401402. [DOI] [PubMed] [Google Scholar]
- Jobe SM, Wilson KM, Leo L, Raimondi A, Molkentin JD, Lentz SR, Di Paola J. Critical role for the mitochondrial permeability transition pore and CypD in platelet activation and thrombosis. Blood. 2007;111:1257–1265. doi: 10.1182/blood-2007-05-092684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff AV. Neurobiology of memory and anxiety: from genes to behavior. Neural Plasticity. 2007;2007 doi: 10.1155/2007/78171. Article ID 78171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata T, Akiguchi I, Yagi H, Irino M, Sugiyama H, Akiyama H, Shimada A, Takemura M, Ueno M, Kitabayashi T, Ohnishi K, Seriu N, Higuchi K, Hosokawa M, Takeda T. Neuropathological studies on strains of senescence-accelerated mice (SAM) with age related deficits in learning and memory. Exper Gerontology. 1997;32:161–169. doi: 10.1016/s0531-5565(96)00063-0. [DOI] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Lo CM, Samuelson LC, Chambers JB, King A, Heiman J, Jandacek RJ, Sakai RR, Benoit SC, Raybould HE, Wood SC, Tso P. Characterization of mice lacking the gene for cholecystokinin. Am J Physiol Regul Integr Comp Physiol. 2007;294:R803–R810. doi: 10.1152/ajpregu.00682.2007. [DOI] [PubMed] [Google Scholar]
- Lu C, Wang Y, Furukawa K, Fu W, Ouyang X, Mattson MP. Evidence that caspase-1 is a negative regulator of AMPA receptor-mediated long-term potentiation at hippocampal synapses. J Neurochem. 2006;97:1104–1110. doi: 10.1111/j.1471-4159.2006.03800.x. [DOI] [PubMed] [Google Scholar]
- Luvisetto S, Marinelli S, Rossetto O, Montecucco C, Pavone F. Central injection of botulinum neurotoxins: behavioural effects in mice. Behav Pharmacol. 2004;15:233–240. [PubMed] [Google Scholar]
- Mather M, Rottenberg H. Aging enhances the activation of the permeability transition pore in mitochondria. Biochem Biophys Res Commun. 2000;273:603–608. doi: 10.1006/bbrc.2000.2994. [DOI] [PubMed] [Google Scholar]
- Mathis C, Paul SM, Crawley JN. Characterization of benzodiazepine-sensitive behaviors in the A/J and C57BL/6J inbred strains of mice. Behav Gen. 1994;24:171–180. doi: 10.1007/BF01067821. [DOI] [PubMed] [Google Scholar]
- Merlini L, Angelin A, Tiepolo T, Braghetta P, Sabatelli P, Zamparelli A, Ferlini A, Maraldi NM, Bonaldo P, Bernardi P. Cyclosporin A corrects mitochondrial dysfunction and muscle apoptosis in patients with collagen VI myopathies. Proc Natl Acad Sci USA. 2008;105:5225–5229. doi: 10.1073/pnas.0800962105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millay DP, Sargent MA, Osinska H, Baines CP, Barton ER, Vuagniaux G, Sweeney HL, Robbins J, Molkentin JD. Genetic and pharmacologic inhibition of mitochondrial-dependent necrosis attenuates muscular dystrophy. Nat Med. 2008;14:442–447. doi: 10.1038/nm1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS, Ritchie J, Sotocinal SG, Smith SB, Croteau S, Levitin DJ, Naumova AK. Screening for pain phenotypes: analysis of three congenic mouse strain on a battery of nine nociceptive assays. Pain. 2006;126:24–34. doi: 10.1016/j.pain.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Moragrega J, Carmen Carrasco M, Redolat R. Effects of housing and nicotine on shuttle-box avoidance in male NMRI mice. Behav Brain Res. 2005;164:178–187. doi: 10.1016/j.bbr.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Naga KK, Sullivan PG, Geddes JW. High CyPD content of synaptic mitochondria results in increased vulnerability to permability transition. J Neurosci. 2007;27:7469–7475. doi: 10.1523/JNEUROSCI.0646-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. CyPD-dependent mitochondrial permeability transtion regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- Oliverio A, Bovet D. Effects of age on maze learning and avoidance conditioning of mice. Life Sci. 1966;5:1317–1324. [Google Scholar]
- Petronilli V, Costantini P, Scorrano L, Colonna R, Passamonti S, Bernardi P. The voltage sensor of the mitochondrial permeability transition pore is tuned by the oxidation-reduction state of vicinal thiols. Increase of the gating potential by oxidants and its reversal by reducing agents. J Biol Chem. 1994;269:16638–16642. [PubMed] [Google Scholar]
- Petronilli V, Penzo D, Scorrano L, Bernardi P, Di Lisa F. The mitochondrial permeability transition, release of cytochrome c and cell death. Correlation with the duration of pore openings in situ. J Biol Chem. 2001;276:12030–12034. doi: 10.1074/jbc.M010604200. [DOI] [PubMed] [Google Scholar]
- Podhorna J, Brown RE. Strain differences in activity and emotionality do not account for differences in learning and memory performance between C57BL/6 and DBA/2 mice. Genes Brain Behav. 2002;1:96–110. doi: 10.1034/j.1601-183x.2002.10205.x. [DOI] [PubMed] [Google Scholar]
- Pollack M, Leeuwenburgh C. Apoptosis and aging: role of mitochondria. J Gerontol. 2001;56A:B475–B482. doi: 10.1093/gerona/56.11.b475. [DOI] [PubMed] [Google Scholar]
- Ribeiro RL, Andreatini R, Wolfman C, Viola H, Medina JH, Da Cunha C. The “anxiety state” and its relation with rat models of memory and habituation. Neurobiol Learn Mem. 1999;72:78–94. doi: 10.1006/nlme.1998.3891. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Boullier E, Chatzimichalaki P, Cooper GD, Shorten A. Contrasting phenotypes of C57BL/6JOlaHsd, 129S2/SvHsd and 129/SvEv mice in two exploration-based tests of anxiety-related behaviour. Physiol Behav. 2002;77:301–310. doi: 10.1016/s0031-9384(02)00856-9. [DOI] [PubMed] [Google Scholar]
- Sansone M. Benzodiazepines and amphetamine on avoidance behaviour in mice. Arch Int Pharmacodyn Ther. 1975;218:125–132. [PubMed] [Google Scholar]
- Sansone M, Battaglia M, Pavone F. Attenuation by nimodipine of amitrityline-induced avoidance impairment in mice. Pharmacol Biochem Behav. 1999;62:613–618. doi: 10.1016/s0091-3057(98)00202-0. [DOI] [PubMed] [Google Scholar]
- Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. CyPD is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. PNAS. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprott RL. Passive-avoidance performance in mice: evidence for single-locus inheritance. Behav Biol. 1974;11:231–237. doi: 10.1016/s0091-6773(74)90401-5. [DOI] [PubMed] [Google Scholar]
- Toescu EC, Myronova N, Verkhratsky A. Age-related structural and functional changes of brain mitochondria. Cell Calcium. 2000;28:329–338. doi: 10.1054/ceca.2000.0167. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Nakagawa T, Shimizu S. Mitochondrial membrane permeability transition and cell death. Biochim Biophys Acta. 2006;1757:1297–1300. doi: 10.1016/j.bbabio.2006.03.017. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, Steckler T. Behavioural analysis of four mouse strains in an anxiety test battery. Behav Brain Res. 2000;115:95–106. doi: 10.1016/s0166-4328(00)00240-0. [DOI] [PubMed] [Google Scholar]
- Voikar V, Koks S, Vasar E, Rauvala H. Strain and gender differences in the behavior of mouse line commonly used in transgenic studies. Physiol Behav. 2001;72:271–281. doi: 10.1016/s0031-9384(00)00405-4. [DOI] [PubMed] [Google Scholar]
- Yasuda O, Fukuo K, Sun X, Nishitani M, Yotsui T, Higuchi M, Suzuki T, Rakugi H, Smithies O, Maeda N, Ogihara T. Apop-1 a novel protein inducing CyPD-dependent but Bax/Bak-related channel-independent apoptosis. J Biol Chem. 2006;281:23899–238907. doi: 10.1074/jbc.M512610200. [DOI] [PubMed] [Google Scholar]
- Yilmazer-Hanke DM, Roskoden T, Zilles K, Schwegler H. Anxiety-related behavior and densities of glutamate, GABAA, acetylcholine and serotonin receptors in the amygdala of seven inbred mouse strains. Behav Brain Res. 2003;145:145–159. doi: 10.1016/s0166-4328(03)00107-4. [DOI] [PubMed] [Google Scholar]
- Wei Y-H, Lee H-C. Oxidative stress, mitochondria DNA mutation, and impairment of antioxidant enzymes in aging. Exp Biol Med. 2002;227:671–682. doi: 10.1177/153537020222700901. [DOI] [PubMed] [Google Scholar]
- Weinberger SB, Koob GF, Martinez JL., Jr Differences in oneway active avoidance learning in mice of three inbred strains. Behav Genet. 1992;22:177–188. doi: 10.1007/BF01066996. [DOI] [PubMed] [Google Scholar]
- Wilson RC, Vacek T, Lanier DL, Dewsbury DA. Open-field behavior in muroid rodents. Behav Biol. 1976;17:495–506. doi: 10.1016/s0091-6773(76)90901-9. [DOI] [PubMed] [Google Scholar]
- Wilson SC, Mogil JS. Measuring pain in the knockout mouse: big challenges in a small mammal. Behav Brain Res. 2001;125:65–73. doi: 10.1016/s0166-4328(01)00281-9. [DOI] [PubMed] [Google Scholar]



