Abstract
Expanding the variety of treatments available to aid smoking cessation will allow the treatments to be customized to particular types of smoker. The key is to understand which subpopulations of smokers with the appropriate treatment. This study used adult female Sprague-Dawley rats to evaluate the efficacy of D-cycloserine, a partial NMDA glutamate receptor agonist, in reducing nicotine self-administration. Rats were trained to self-administer nicotine (0.03 mg/kg/infusion, IV) via operant lever response (FR1) with a secondary visual reinforcer. Two studies of D-cycloserine effects on nicotine self-administration were conducted: an acute dose-effect study (0, 10, 20 and 40 mg/kg, sc) and a chronic study with 40 mg/kg given before each test session for two weeks. Effects on rats with low or high pretreatment baseline levels of nicotine self-administration were assessed. In the acute study there was a significant interaction of D-cycloserine x baseline level of nicotine self-administration. In the low baseline group, 10 mg/kg D-cycloserine significantly decreased nicotine self-administration. In the high baseline group, 40 mg/kg significantly increased nicotine self-administration. In the repeated injection study, there was also a significant interaction of D-cyloserine x baseline level of nicotine self-administration. Chronic D-cycloserine significantly reduced nicotine self-administration selectively in rats with low baseline nicotine use, but was ineffective with the rats with higher levels of baseline nicotine self-administration. NMDA glutamate treatments may be particularly useful in helping lighter smokers successfully quit smoking, highlighting the need for diverse treatments for different types of smokers.
Keywords: Nicotine, Self-administration, D-cycloserine, Rat
1. Introduction
Greater diversity of treatments to aid smoking cessation will provide increased opportunity for adapting treatment to best suit particular types of smokers to maximize the chance for successful and permanent cessation of tobacco use. The treatments that are currently approved for use including nicotine replacement products, as well as other nicotinic and non-nicotinic treatments, are effective only in a minority of smokers trying to quit. The search for treatments that are more effective overall has been slow and has produced only incremental improvement. Treatments that work for some smokers may be ineffective or even be counterproductive for others. Rather than searching for the better magic bullet for all smokers, it may be more useful to find treatments, which are effective for particular subpopulations of smokers.
Tobacco addiction is estimated to be responsible for five million premature deaths worldwide per year (Hatsukami et al., 2008). Therapy to aid smoking cessation, such as various forms of nicotine replacement as well as bupropion and varenicline treatment, have been found to significantly improve cessation rates. However, there is still much room for improvement. Even the best treatments are only successful long-term in about a third of the people trying to quit (Rovina et al., 2009). It has become clear that a greater diversity of treatments for smoking cessation is needed.
Nicotine indirectly affects many noncholinergic transmitter systems by its stimulating the efflux of a variety of neurotransmitters, including the biogenic amines: dopamine, norepinepherine, serotonin and histamine as well as GABA and glutamate (Wonnacott et al., 1989). The involvement of dopamine in nicotine reward has been shown (Pich et al., 1997) inasmuch as the dopaminergic projection from the ventral tegmental area to the nucleus accumbens is important for a wide variety of drug addictions. Given the complexity of addiction and the brain s essential interconnectedness other neural systems are also certainly involved. Drugs acting on some of these non-nicotinic receptor systems may be useful in promoting smoking cessation. Some of these non-nicotinic treatments are already in development and some have already been tried in humans. In previous studies we have found that serotonin 5HT2 receptor blockade with ketanserin (Levin et al., 2008) and histamine H1 blockade with pyrilamine (Levin et al., 2010) significantly reduce nicotine self-administration in the classic rat model. Others have found the treatments affecting dopamine D3 receptors (Andreoli et al., 2003), α1 adrenergic (Villegier et al., 2007); hypocretin (LeSage et al., 2010); GABA systems (Markou et al., 2004; Paterson et al., 2004; Paterson and Markou, 2002) and metabotropic glutamate receptors (Bespalov et al., 2005; Dravolina et al., 2007; Kenny et al., 2003; Markou et al., 2004; Paterson et al., 2005; Paterson and Markou, 2005; Paterson et al., 2003; Tessari et al., 2004) and NMDA glutamate antagonist treatment in the ventral tegmental area and the central nucleus of the amygdala (Kenny et al., 2009) reduce nicotine self-administration in rats. The key to the successful use of diverse treatments for combating nicotine self-administration is to develop a better understanding of which subpopulation of smokers would most benefit. The current study evaluated the efficacy of a partial agonist at NMDA glutamate receptors, D-cycloserine.
Glutamate is likely involved in the actions of nicotine given that glutamate is the predominant excitatory neurotransmitter in the brain and that nicotine simulates glutamate release (Wonnacott et al., 1989). D-cycloserine is a partial agonist at NMDA glutamate receptors via its interaction with the coupled glycine modulatory site (Hood et al., 1989). It reduces maximal activation of the receptor to only 40–50% of the maximal activation by glycine (Watson et al., 1990). Therefore D-cycloserine may be having its effects mediated via a net reduction in maximal activation of the NMDA receptor.
D-cycloserine has been found to have effects on cognitive function, enhancing reversal learning (Riekkinen et al., 1998). It also has effects on the behavioral actions of drugs of abuse. It facilitates extinction of conditioned place preference for cocaine (Paolone et al., 2009). D-cycloserine has significant interactions with tobacco smoking. In a recent clinical study, D-cycloserine was found to reduce reactivity of nicotine-dependent smokers to smoking cues, however it was not seen to significantly reduce smoking behavior (Santa Ana et al., 2009). It may be the case that not all smokers respond well to D-cycloserine.
The current study evaluated in young adult female Sprague-Dawley rats the efficacy of D-cycloserine for decreasing nicotine self-administration. The rats were trained to self-administer nicotine via operant lever response with a visual secondary reinforcer. An acute dose-effect study was conducted of D-cycloserine on nicotine self-administration in rats in relation to low or high baseline level of nicotine self-administration (below or above the median response during the baseline training) was undertaken. In a follow-up study, repeated injections of D-cycloserine were given for two weeks to evaluate chronic effects on nicotine self-administration in rats with high or low baseline levels of nicotine self-administration.
2. Methods
2.1. Subjects
Young adult female Sprague-Dawley rats (Taconic Labs, Germantown, NY, USA) were given access to IV nicotine self-administration. The studies were conducted in accordance with the regulations outlined by the Duke University Animal Care and Use Committee. The rats were housed in approved standard laboratory conditions in a Duke University Vivarium facility near the testing room to minimize any stress induced by transporting the rats. They were kept on a 12:12 reverse day/night cycle, so that they were in their active phase during behavioral testing. The rats in the drug i.v. self-administration studies were singly housed to prevent them from damaging each other s catheters. All rats were allowed access to water at all times and fed daily approximately 20–30 minutes after completing the sessions.
2.2. Behavioral Procedures
Solutions of nicotine bitartrate were prepared weekly in pyrogen-free glassware in sterilized isotonic saline. The dose used for self-administration (0.03 mg/kg/infusion) was calculated as a function of the nicotine base weight. The pH of the solutions was adjusted to 7.0 using NaOH and then the solutions were passed through a Nalgene filter (Nalgene Nunc International, Rochester, NY, USA) for sterilization. Between sessions, all solutions were kept refrigerated in the dark to prevent the decomposition of nicotine.
Rats had catheters surgically implanted into the jugular vein to enable them to receive nicotine infusions. Catheters were flushed daily, before the sessions began, with a 0.3 ml solution containing 100U/ml heparinized saline (Baxter Health Corporation, Deerfield, IL, USA). When sessions were over, the nicotine remaining in the ports was drawn out and replaced by a 0.25 ml sterile lock consisting of heparinized saline 500U/ml with 8-mg/ml gentamicin (American Pharmaceutical Partners, Schaumburg, IL, USA).
The rats were trained to self-administer nicotine (0.03 mg/kg/infusion, IV) via operant lever response (FR1) with a visual secondary reinforcer. Two levers were available to be pressed and only one caused the delivery of nicotine on an FR1 schedule. Pressing the lever on the active side resulted in the activation of the feedback tone for 0.5 s and the immediate delivery of one 50-μl infusion of nicotine in less than 1 s. Each infusion was immediately followed by a one-min period in which the cue lights went out, the house light came on and responses were recorded but not reinforced. Each session lasted for three hours.
2.3. D-cycloserine Treatment
After training for nicotine self-administration on an FR1 schedule (0.03 mg/kg/infusion) for five sessions the rats were tested for the effects of the D-cycloserine. First, there was an acute dose-effect study of D-cycloserine (0, 10, 20 and 40 mg/kg, sc, N=16) effects on nicotine self-administration. Each dose was administered twice in a repeated measures counterbalanced design. The doses were injected sc 10 minutes before the beginning of the nicotine self-administration session. Then, in a new set of rats the effects of repeated injections of D-cycloserine were studied. Repeated injections of 40 mg/kg of D-cycloserine were given for two weeks (5 days/week) to evaluate chronic effects on nicotine self-administration (N=14 controls, N=16 D-cycloserine-treated). After two weeks of treatment and access to nicotine, the rats had a one-week hiatus with no testing and no access to nicotine. Then they were given resumed access to nicotine self-administration. This was done to model enforced abstinence followed by resumed access to drug. Typically, smokers trying to quit do not go through extinction of self-administration (i.e. continued self-administration actions without drug delivery); rather they attempt to force themselves to be abstinent from both the act of self-administration and contingent nicotine delivery. However, for most the availability of cigarettes proves to be too much a temptation for continued self-enforced abstinence.
2.4. Data Analysis
The data were analyzed with analysis of variance. In both studies there was a between subjects factor of low or high baseline nicotine self-administration (below or above the median number of nicotine infusions self-administered over the four sessions prior to the onset of the D-cycloserine treatments). Cohort was used as a between subjects control variable. In the acute study, D-cycloserine doses and saline were administered in a repeated measures, counterbalanced design two times. The repeated measures were D-cycloserine dose and the repeated administration of each dose. In the chronic study with a separate set of rats, D-cycloserine (40 mg/kg) or saline was administered for 10 sessions 5 days/week for two weeks, following one week of nicotine self-administration without pre-session injections. Chronic D-cycloserine was a between-subjects factor. Week of administration was a repeated measure. Significant interactions were followed up by tests of the simple main effects. Alpha of p<0.05 (two-tailed) was used as the threshold for significance.
3. Results
The current study found in young adult female Sprague-Dawley rats that D-cycloserine, a partial agonist at NMDA glutamate receptors, significantly reduces nicotine self-administration in rats with lower levels of nicotine self-administration during baseline testing.
3.1. Acute D-cycloserine Effects on Nicotine Self-administration
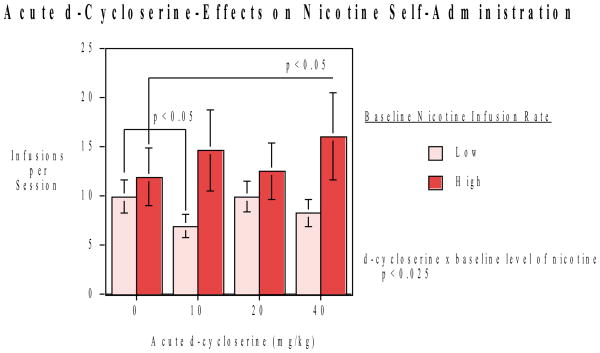
The rats were trained to self-administer nicotine (0.03 mg/kg/infusion, IV) via operant lever response (FR1) with a visual secondary reinforcer. An acute dose-effect study of D-cycloserine (0, 10, 20 and 40 mg/kg, sc, N=16) effects on nicotine self-administration showed that there was a significant (F(3,36)=3.49, p<0.025) interaction of D-cycloserine x low or high baseline level of nicotine self-administration. In the low baseline group the 10 mg/kg D-cycloserine dose significantly (p<0.05) decreased nicotine self-administration. With the high nicotine self-administration group 40 mg/kg dose significantly (p<0.05) increased nicotine self-administration (Fig. 1). No significant interaction of the D-cycloserine effect with phase (first or second) of administration was seen.
Figure 1.
Acute dose-effect functions of d-cycloserine on nicotine-self-administration (mean ±sem) (Total N=16). There was a significant d-cycloserine x pretreatment nicotine self-administration level interaction (p<0.05). Paired comparisons of d-cycloserine doses vs. control showed that after 10 mg/kg the rats with lower pretraining nicotine self-administration had significantly lower nicotine self-administration. The rats with the high baseline response showed a significant elevation of nicotine self-administration after 40 mg/kg d-cycloserine.
3.2. Repeated D-cycloserine Injections and Nicotine Self-administration
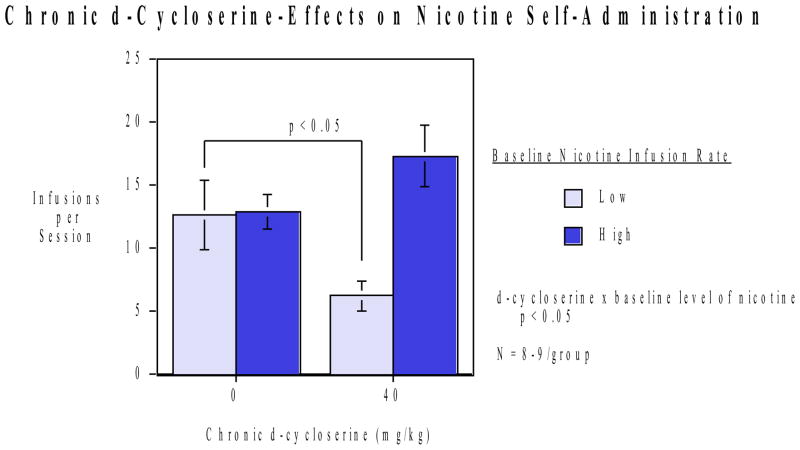
In the follow-up study with a different set of rats, repeated injections of 40 mg/kg of d-cycloserine were given for two weeks to evaluate chronic effects on nicotine self-administration (N=16 controls, N=14 D-cycloserine-treated). As with the acute study, there was a significant (p<0.05) interaction of D-cyloserine x baseline levels of nicotine self-administration. D-cycloserine significantly (p<0.025) reduced nicotine self-administration selectively in rats with low baseline levels of response over the two weeks of treatment. No significant effect of 40 mg/kg D-cycloserine was seen in the fires session of the chronic study. This is congruent with the first study. However, during the rest of the chronic study there was overall a significant reduction in nicotine self-administration in the low baseline group. D-cycloserine was ineffective with the rats with higher levels of baseline nicotine self-administration, which showed signs of increased response for nicotine (Fig. 2). No significant interaction of the D-cycloserine effect with week (first or second) of administration was seen. During the week of resumed access after the week of enforced abstinence similar mean differences were seen. The low baseline controls averaging 9.80±1.76 infusions per session and the low baseline D-cycloserine-treated rats averaging 7.30±2.04 infusions per session. The high baseline controls averaged 9.89±1.80 infusions/session and the D-cycloserine treated rats averaged 16.88±6.33 infusions per session. However during the resumption week these differences were not statistically significant.
Figure 2.
Repeated d-cycloserine injections and nicotine self-administration (mean±sem). There was a significant (p<0.05) interaction of d-cycloserine x baseline nicotine self-administration level (N=14 controls, N=16 D-cycloserine-treated). In the low-level nicotine self-administration group, chronic d-cycloserine treatment significantly (p<0.05) reduced nicotine self-administration relative to the rats with the same baseline level of nicotine self-administration that were treated with the saline vehicle. In contrast, rats with higher than median levels of baseline nicotine self-administration did not decrease nicotine self-administration relative to controls with high pretreatment nicotine self-administration but rather showed trend toward increased nicotine self-adminsitration.
4. Discussion
Experience with the development and use of treatments for smoking cessation has shown that although there are some treatments such as the various forms of nicotine replacement, bupropion and varenicline that significantly increase cessation rates, none of these treatments routinely help the majority of smokers trying to quit. Some of this lack of success of single treatments may result from the variety of different types of smokers and the potential variety of optimal treatments to fit their diverse needs. Tailoring treatments to subgroups of smokers may provide greater overall cessation success. Greater diversity of treatments to aid smoking cessation will provide greater opportunity for adapting treatment to best suit particular types of smokers to maximize the chance for successful cessation. Nicotine indirectly affects many noncholinergic transmitter systems by stimulating the efflux of a variety of neurotransmitters. Drugs acting on some of these non-nicotinic receptor systems may be useful in promoting smoking cessation.
The current study showed that D-cycloserine significantly reduced nicotine self-administration selectively in rats with low baseline levels of response. It was ineffective with the rats with higher levels of baseline nicotine self-administration, which showed signs of increased response for nicotine. It was encouraging that chronic D-cycloserine treatment continued to be effective in reducing nicotine self-administration, because clinical treatment would necessarily use chronic treatment. These results suggest that NMDA glutamate treatments may be useful in helping people successfully quit smoking. In particular, lighter smokers may benefit, highlighting the need for diverse treatments for different types of smokers.
The dose of 40 mg/kg was followed up for the chronic to see if the increase in nicotine self-administration in the high baseline group persisted. There was a modest increase in nicotine self-administration in the high baseline group given 40 mg/kg of D-cycloserine in the chronic study but this did not reach significance. However, this did contrast with the significant decrease in nicotine self-administration shown by the low baseline group given chronic treatment with D-cycloserine. With the first administration of 40 mg/kg in the chronic study the low baseline group did not show a decrease relative to control much like the acute experiment. However, as the chronic study continued there was an overall significant reduction in nicotine in the low baseline group given 40 mg/kg of D-cycloserine.
The differences in response of the high and low baseline groups to the effects of D-cycloserine on nicotine-self-administration may have been due to the differences in nicotine the groups had self-administered during the baseline training. Averaged over the four sessions prior to the drug study the high self-administering group self-dosed 18.4±1.4 (0.55 mg/kg total nicotine per session), while the low group averaged 7.0±0.8 infusions per session (0.21 mg/kg total nicotine per session). The high group averaged 2.6 times the nicotine self-administration than the low group. On each of the four sessions prior to the D-cycloserine the low group averaged 9.9, 5.9, 6.5, and 5.8 infusions per session while the high group averaged 17.0, 20.6, 18.0 and 17.8 infusions per session.
There are at least a couple of other possibilities why D-cycloserine selectively lowers nicotine self-administration in rats with lower baseline self-administration rates and if anything raises nicotine self-administration in those with higher baseline self-administration rates. It may be the case that those in the low baseline group are not very well conditioned to self-administer nicotine and this is easily disrupted by the D-cycloserine treatment, while the high baseline group is better conditioned and with the D-cycloserine treatment they work to overcome the disruption. The reinforcing value of nicotine may be small in the low baseline group and easily blocked by D-cycloserine, while the reinforcing value of nicotine may be greater in the high baseline group and with a bit of attenuation of reinforcement they work to restore the greater reinforcement they previously had.
The differences between the animals that self-administered greater or less amount of nicotine during the pre-training sessions may extend beyond just the actions of nicotine itself. There may be other pre-existing differences that caused them to self-administer greater or lesser amounts nicotine. There have been identified individual differences in the incentive motivation of animals some of which are attracted to the drug goal itself and others more to the cues associated with the drug delivery (Flagel et al., 2009). Given that with nicotine self-administration in particular the roles of conditioned cues is quite important for self-administration in humans (Rose et al., 1993) as well as rats (Caggiula et al., 2002) the difference between incentive motivation for drug vs. cues for drug could be quite important.
The partial agonist effects of D-cycloserine may be important for the divergent effects on high and low responding rats. It may be the case that baseline differences in NMDA involvement in the neural systems underlying nicotine reinforcement could explain the differential effects of this partial agonist of NMDA receptors on nicotine self-administration in these two groups. Further studies using full agonist and antagonists of NMDA receptors will be key in determining these differential effects.
For use clinically as a smoking cessation aid, it is useful that D-cycloserine is already approved for human use. D-cycloserine has been studied with smokers and was found to significantly reduce reactivity to smoking cues (Santa Ana et al., 2009). However, it was not seen to significantly reduce smoking behavior. The current study indicates that D-cycloserine may not be generally useful for all types of smokers. Lighter smokers may benefit more from D-cycloserine than heavy smokers who may actually increase smoking in reaction to D-cycloserine treatment.
In the pursuit of a greater diversity of treatments to promote smoking cessation, it is important to determine how subpopulations of smokers respond to various treatments. The most elementary measure of the different types of smokers is how many cigarettes per day the people smoke. The current study showed with acute and chronic treatment D-cycloserine selectively lowered nicotine self-administration in rats with lower but not higher baseline levels of nicotine self-administration.
Acknowledgments
This research was supported by a National Institute on Drug Abuse P50 Center grant (DA027840) and an unrestricted grant from Philip Morris USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreoli M, Tessari M, Pilla M, Valerio E, Hagan JJ, Heidbreder CA. Selective antagonism at dopamine D3 receptors prevents nicotine-triggered relapse to nicotine-seeking behavior. Neuropsychopharmacology. 2003;28:1272–1280. doi: 10.1038/sj.npp.1300183. [DOI] [PubMed] [Google Scholar]
- Bespalov AY, Dravolina OA, Sukhanov I, Zakharova E, Blokhina E, Zvartau E, Danysz W, van Heeke G, Markou A. Metabotropic glutamate receptor (mGluR5) antagonist MPEP attenuated cue- and schedule-induced reinstatement of nicotine self-administration behavior in rats. Neuropharmacology. 2005;49(Suppl 1):167–178. doi: 10.1016/j.neuropharm.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Chaudhri N, Perkins KA, Evans-Martin FF, Sved AF. Importance of nonpharmacological factors in nicotine self-administration. Physiol Behav. 2002;77:683–687. doi: 10.1016/s0031-9384(02)00918-6. [DOI] [PubMed] [Google Scholar]
- Dravolina OA, Zakharova ES, Shekunova EV, Zvartau EE, Danysz W, Bespalov AY. mGlu1 receptor blockade attenuates cue- and nicotine-induced reinstatement of extinguished nicotine self-administration behavior in rats. Neuropharmacology. 2007;52:263–269. doi: 10.1016/j.neuropharm.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56 (Suppl 1):139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Stead LF, Gupta PC. Tobacco addiction. Lancet. 2008;371:2027–2038. doi: 10.1016/S0140-6736(08)60871-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood WF, Comptona RP, Monahan JB. d-Cycloserine: A ligand for the N-methyl-d-aspartate coupled glycine receptor has partial agonist characteristics. Neurosci Lett. 1989;98:91–95. doi: 10.1016/0304-3940(89)90379-0. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Chartoff E, Roberto M, Carlezon WA, Jr, Markou A. NMDA receptors regulate nicotine-enhanced brain reward function and intravenous nicotine self-administration: role of the ventral tegmental area and central nucleus of the amygdala. Neuropsychopharmacology. 2009;34:266–281. doi: 10.1038/npp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Paterson NE, Boutrel B, Semenova S, Harrison AA, Gasparini F, Koob GF, Skoubis PD, Markou A. Metabotropic glutamate 5 receptor antagonist MPEP decreased nicotine and cocaine self-administration but not nicotine and cocaine-induced facilitation of brain reward function in rats. Ann N Y Acad Sci. 2003;1003:415–418. doi: 10.1196/annals.1300.040. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Perry JL, Kotz CM, Shelley D, Corrigall WA. Nicotine self-administration in the rat: Effects of hypocretin antagonists and changes in hypocretin mRNA. Psychopharmacology. 2010;209:203–212. doi: 10.1007/s00213-010-1792-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Pruitt M, Cousins V, Slade S, Wells C, Cauley M, Hampton D, Rose JE. Histamine H1 antagonist treatment with pyrilamine reduces nicotine self-administration in rats. Eur J Pharmacol. 2010;650:256–260. doi: 10.1016/j.ejphar.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Levin ED, Slade S, Johnson M, Petro A, Horton K, Williams P, Rezvani AH. Ketanserin, a 5-HT2 antagonist, decreases nicotine self-administration in rats. Eur J Pharmacol. 2008;600:93–97. doi: 10.1016/j.ejphar.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Paterson NE, Semenova S. Role of gamma-aminobutyric acid (GABA) and metabotropic glutamate receptors in nicotine reinforcement: potential pharmacotherapies for smoking cessation. Ann N Y Acad Sci. 2004;1025:491–503. doi: 10.1196/annals.1316.061. [DOI] [PubMed] [Google Scholar]
- Paolone G, Botreau F, Stewart J. The facilitative effects of D-cycloserine on extinction of a cocaine-induced conditioned place preference can be long lasting and resistant to reinstatement. Psychopharmacology. 2009;202:403–409. doi: 10.1007/s00213-008-1280-y. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Froestl W, Markou A. The GABAB receptor agonists baclofen and CGP44532 decreased nicotine self-administration in the rat. Psychopharmacology. 2004;172:179–186. doi: 10.1007/s00213-003-1637-1. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Froestl W, Markou A. Repeated administration of the GABAB receptor agonist CGP44532 decreased nicotine self-administration, and acute administration decreased cue-induced reinstatement of nicotine-seeking in rats. Neuropsychopharmacology. 2005;30:119–128. doi: 10.1038/sj.npp.1300524. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Increased GABA neurotransmission via administration of gamma-vinyl GABA decreased nicotine self-administration in the rat. Synapse. 2002;44:252–253. doi: 10.1002/syn.10073. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. The metabotropic glutamate receptor 5 antagonist MPEP decreased break points for nicotine, cocaine and food in rats. Psychopharmacology. 2005;179:255–261. doi: 10.1007/s00213-004-2070-9. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Semenova S, Gasparini F, Markou A. The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice. Psychopharmacology. 2003;167:257–264. doi: 10.1007/s00213-003-1432-z. [DOI] [PubMed] [Google Scholar]
- Pich EM, Pagliusi SR, Tessari M, Talabot-Ayer D, Hooft van Huijsduijnen R, Chiamulera C. Common neural substrates for the addictive properties of nicotine and cocaine. Science. 1997;275:83–86. doi: 10.1126/science.275.5296.83. [DOI] [PubMed] [Google Scholar]
- Riekkinen P, Ikonen S, Riekkinen M. D-cycloserine, a partial NMDA receptor-associated glycine- B site agonist, enhances reversal learning, but a cholinesterase inhibitor and nicotine has no effect. Neuroreport. 1998;9:3647–3651. doi: 10.1097/00001756-199811160-00016. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Levin ED. Role of nicotine dose and sensory cues in the regulation of smoke intake. Pharmacol Biochem Behav. 1993;44:891–900. doi: 10.1016/0091-3057(93)90021-k. [DOI] [PubMed] [Google Scholar]
- Rovina N, Nikoloutsou I, Katsani G, Dima E, Fransis K, Roussos C, Gratziou C. Effectiveness of pharmacotherapy and behavioral interventions for smoking cessation in actual clinical practice. Ther Adv Respir Dis. 2009;3:279–287. doi: 10.1177/1753465809350653. [DOI] [PubMed] [Google Scholar]
- Santa Ana EJ, Rounsaville BJ, Frankforter TL, Nich C, Babuscio T, Poling J, Gonsai K, Hill KP, Carroll KM. D-Cycloserine attenuates reactivity to smoking cues in nicotine dependent smokers: a pilot investigation. Drug Alcohol Depend. 2009;104:220–227. doi: 10.1016/j.drugalcdep.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessari M, Pilla M, Andreoli M, Hutcheson DM, Heidbreder CA. Antagonism at metabotropic glutamate 5 receptors inhibits nicotine- and cocaine-taking behaviours and prevents nicotine-triggered relapse to nicotine-seeking. Eur J Pharmacol. 2004;499:121–133. doi: 10.1016/j.ejphar.2004.07.056. [DOI] [PubMed] [Google Scholar]
- Villegier AS, Lotfipour S, Belluzzi JD, Leslie FM. Involvement of alpha1-adrenergic receptors in tranylcypromine enhancement of nicotine self-administration in rat. Psychopharmacology. 2007;193:457–465. doi: 10.1007/s00213-007-0799-7. [DOI] [PubMed] [Google Scholar]
- Watson GB, Bolanowski MA, Baganoff MP, Deppeler CL, Lanthorn TH. d-Cycloserine acts as a partial agonist at the glycine modulatory site of the NMDA receptor expressed in Xenopus oocytes. Brain Res. 1990;510:158–160. doi: 10.1016/0006-8993(90)90745-w. [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Irons J, Rapier C, Thorne B, Lunt GG. Presynaptic modulation of transmitter release by nicotinic receptors. In: Nordberg A, Fuxe K, Holmstedt B, Sundwall A, editors. Progress In Brain Research. Vol. 79. Elsevier Science Publishers B.V; 1989. pp. 157–163. [DOI] [PubMed] [Google Scholar]