Abstract
Adenosine-regulated glutamate signaling in astrocytes is implicated in many neurological and neuropsychiatric disorders. In this study, we examined whether adenosine A1 receptor regulates EAAT2 expression in astrocytes using pharmacological agents and siRNAs. We found that adenosine A1 receptor-specific antagonist DPCPX or PSB36 decreased EAAT2 expression in a dose-dependent manner. Consistently, knockdown of A1 receptor in astrocytes decreased EAAT2 mRNA expression while overexpression of A1 receptor upregulated EAAT2 expression and function. Since A1 receptor activation is mainly coupled to inhibitory G-proteins and inhibits the activity of adenylate cyclase, we investigated the effect of forskolin, which activates adenylate cyclase activity, on EAAT2 mRNA levels. Interestingly, we found that forskolin reduced EAAT2 expression in dose- and time-dependent manners. In contrast, adenylate cyclase inhibitor SQ22536 increased EAAT2 expression in dose- and time-dependent manners. In addition, forskolin blocked ethanol-induced EAAT2 upregulation. Taken together, these results suggest that A1 receptor-mediated signaling regulates EAAT2 expression in astrocytes.
1. Introduction
Adenosine signaling has been implicated in the pathophysiology of many central nervous system disorders including alcoholism [1,2]. Adenosine, an inhibitory neurotransmitter or a neuromodulator, exerts its function via four well-characterized G-protein coupled adenosine receptors, A1, A2A, A2B, and A3 [3]. Extracellular or synaptic adenosine levels are mainly regulated by nucleoside transporters [4]. Among several nucleoside transporters, type 1 equilibrative nucleoside transporter(ENT1) regulates extracellular adenosine levels in response to acute ethanol treatment in cultured cells [5]. Acute ethanol treatment increases extracellular adenosine in cultured cells by selectively inhibiting ENT1, while chronic ethanol exposure results in tolerance characterized by a decrease in ENT1 expression and extracellular adenosine levels are no longer increased [5]. Since mice lacking ENT1 exhibit reduced ataxic/hypnotic effects to acute ethanol exposure [6] and lowered initial ethanol sensitivity [7], ENT1 null mice mimic the status of chronic ethanol treatment. Consistently, ENT1 null mice consume more alcohol compared to wild-type littermates [6]. One of the neural mechanisms underlying these behaviors is attributed to increased glutamate neurotransmission in the nucleus accumbens (NAc) [6]. Our recent study indicates that increased tolerance to acute ethanol intoxication is possibly related to increased glutamate signaling in ENT1 null mice [7]. Moreover, we have also found that inhibition of ENT1 expression or activity reduces EAAT2 expression and glutamate activity in cultured astrocytes, which might contribute to increased extracellular glutamate levels in ENT1 null mice [8].
One of the important roles of astrocytes, which are the most abundant cell type in the mammalian central nervous system, is to uptake glutamate in the synaptic cleft to protect neurons from excessive stimulation [9]. Glutamate uptake is primarily mediated by excitatory amino acid transporters (EAATs including EAAT1-5) in the central nervous system. EAATs are sodium- and potassium-dependent members of solute carrier family 1 (SLC1) and are widely distributed throughout the mammalian brain [10]. Among the five mammalian EAATs, EAAT1 and EAAT2 are expressed in astrocytes [10]. However, EAAT2 is responsible for ~90% of glutamate uptake into astrocytes [11,12]. Since adenosine A1 receptor (A1R) is abundantly expressed in astrocytes [13,14] along with EAAT2, we investigated the role of A1R and its signaling pathway in regulating EAAT2 expression. Here we examined whether A1R expression or function is correlated with EAAT2 mRNA levels in astrocytes using A1R-selective antagonists, A1R-specific siRNAs, or overexpression. Our results suggest that A1R-mediated signaling regulates EAAT2 expression in astrocytes.
2. Materials and methods
2.1. Astrocyte culture and reagents
The astrocytic cell line C8-D1A was obtained from ATCC (American Type Culture Collection, VA), which was cloned from the mouse cerebellum [15]. As we previously described [8], cells were maintained in Dulbecco’s modified Eagle medium containing glucose (Invitrogen, Carlsbad, CA), 10% heat-inactivated fetal bovine serum (FBS; ATCC, American Type Culture Collection, VA), 1% L-glutamine (Gibco, Auckland, New Zealand), and 1% Antibiotic-Antimycotic (Invitrogen). Monolayers were cultured at 37°C in the presence of 5% CO2/95% O2 (normoxia) in a fully humidified atmosphere with medium replacement every 2–3 days. DPCPX (8-cyclopentyl-1,3-dipropylxanthine), PSB36, forskolin, and SQ22536 were purchased from Tocris Bioscience (Ellisville, MO) and ethanol was purchased from Sigma-Aldrich (St. Louis, MO).
2.2. Real-time RT-PCR
To measure mRNA levels, real-time quantitative RT-PCR was performed with the iCycler IQ Real-Time PCR detection system (Bio-Rad Laboratories, Inc., Hercules, CA) using QuantiTect SYBR Green RT-PCR Kit (Qiagen, Valencia, CA). Total RNA was isolated using RNAeasy-Mini kit (Qiagen) and treated with the TURBO DNA-free RNA Kit (Ambion, Austin, TX) to remove residual DNA for analysis of gene expression levels using quantitative RT-PCR as described [8]. Gene-specific primers for ENT1, EAAT2, A1R, and GAPDH were purchased (Qiagen). The real-time RT-PCR experiment was performed as described [8]. The mRNA expressions of the genes were normalized by GAPDH as a housekeeping gene. Fold or percentage changes were calculated by subtracting GAPDH Ct values from Ct values for the gene of interest using the 2−ΔΔCt method [16].
2.3. A1 receptor and ENT1 knockdown in astrocytes
The target sequences of A1R-8 siRNA and A1R-10 siRNA for A1 receptors are 5′-CACTGTCTTCACCAAACTAAA-3′ and 5′-CTCCTTGGGTGTGAATATTGA-3′, respectively. The target sequences of ENT1-1 siRNA and ENT1-3 siRNA for ENT1 are 5′-CAGGACAGGTATAAGGCAGTA-3′ and 5′-AAGATTGTGCTCATCAATTCA-3′, respectively. siRNAs for A1R, ENT1 or control siRNA (10 nM) were transfected into 105 astrocytes in a 24-well plate using HiPerFect transfection reagent (Qiagen). Forty-eight hours after the transfection, total RNA was isolated and the expression levels of ENT1, A1R, and EAAT2 mRNA were measured by real-time RT-PCR.
2.4. A1 receptor overexpression
pCMV-SPORT6-A1R (OriGene, Rockville, MD) was used to overexpress mouse A1R in the astrocyte C8-D1A cell line. 1 μg DNA constructs (pCMV-SPORT6 as a control or pCMV-SPORT6-A1R) were transfected into 105 astrocytes in a 24-well plate or 5 × 105 astrocytes in 6-well plates using 4 μl Lipofectamine 2000 (Invitrogen). After the transfection, total RNA was isolated at different times and the expression levels of A1R mRNA, ENT1 mRNA and EAAT2 mRNA were measured by real-time RT-PCR. For A1R and EAAT2 protein expression levels, [3H] DPCPX binding and glutamate uptake assays were performed, respectively.
2.5. [3H] DPCPX binding assay
A1R binding of transfected astrocytes was measured using A1R antagonist [3H] DPCPX (specific radioactivity: 120 Ci/mmol, concentration: 1.0 mCi/ml; American Radiolabeled Chemicals, St. Louis, MO) as described [17]. Specific binding (A1R protein expression level) was defined as total minus nonspecific binding.
2.6. Glutamate uptake assay
Glutamate uptake activity of astrocytes using L-[G-3H]-glutamic acid (specific radioactivity: 29 Ci/mmol, concentration: 1.0 mCi/ml; Amersham Bioscience, Arlington Heights, IL) was measured as described [8]. Glutamate uptake was initiated by the addition of uptake buffer containing 2.5 μM glutamate with 2 μCi/ml radiolabeled [3H]-glutamate. The relative level of glutamate uptake was calculated using the ratio of radioactivity to protein quantity of cell extracts.
2.5. Statistical analysis
All data were expressed as mean ± SEM (standard error mean) and were analyzed by unpaired two-tailed t-tests or one-way ANOVA followed by a Tukey post-hoc test for individual comparisons. Results of comparisons were considered significantly different if the p value was < 0.05.
3. Results
3.1. A1 receptor regulates EAAT2 expression and function
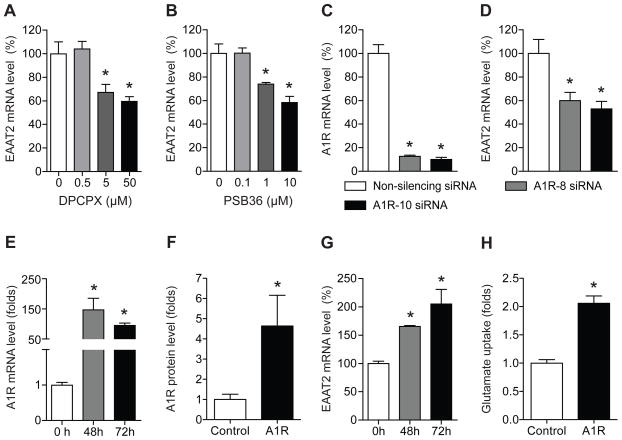
Since adenosine A1 receptor (A1R) is known to promote glutamate-mediated synaptic neurotransmission in the hippocampus [18] and striatum [19], we examined whether pharmacological inhibition of A1R alters EAAT2 expression in astrocytes. First, we treated astrocytes with a highly selective A1R antagonist, DPCPX, for 24 h and then analyzed EAAT2 mRNA expression using real-time RT-PCR. As shown in Fig. 1A, DPCPX treatment significantly decreased EAAT2 mRNA expression. One-way ANOVA analysis indicated a significant effect of DPCPX (F3, 18 = 9.52, p < 0.001). We also examined the effect of PSB36, another A1R antagonist. As shown in Fig. 1B, a 24 h treatment with PSB36 significantly reduced EAAT2 mRNA levels. Similarly, one-way ANOVA analysis revealed a significant effect of PSB36 (F3, 12 = 15.01, p < 0.001). We further examined whether inhibition of A1R regulates EAAT2 expression using siRNA. We confirmed that two different siRNA constructs against A1R significantly decreased A1R mRNA levels 48 h after transfection compared to non-silencing control siRNA (Fig. 1C). Both siRNAs for A1R significantly reduced EAAT2 mRNA levels (Fig. 1D). These results suggest that A1R regulates EAAT2 expression in astrocytes. Next, we examined whether overexpression of A1R increases EAAT2 expression. As shown in Fig. 1E and F, A1R mRNA levels were significantly increased (Fig. 1E) and A1R protein levels were also significantly increased by 4.6-fold (Fig. 1F) in astrocytes transfected with pCMV-A1R compared to those treated with vehicle plasmid. Overexpressed A1R significantly upregulated EAAT2 mRNA levels at 48 h and 72 h (Fig. 1G) and increased EAAT2 glutamate uptake activity by 2.1-fold (Fig. 1H). Together, these results demonstrate that A1R expression is causally related to EAAT2 expression and function in astrocytes.
Fig. 1.
Inhibition of A1 receptor decreases EAAT2 mRNA expression. (A) EAAT2 mRNA expression was decreased by DPCPX at 5 and 50 μM (n = 6). (B) EAAT2 mRNA expression was reduced by PSB36 at 1 and 10 μM (n = 4). *p < 0.05, compared to the control group by Tukey post-hoc tests. (C) A1R mRNA levels were knocked down after A1R siRNA transfection for 48 h (n = 4). (D) EAAT2 mRNA levels were reduced by A1R knockdown (n = 4). 10 nM non-silencing siRNA or A1R siRNAs (A1R-8 siRNA and A1R-10 siRNA) were used to treat cells. (E) A1R mRNA levels were increased after 48 h and 72 h of A1R cloned gene transfection (n = 4). (F) Similarly, A1R protein expression levels were increased by 4.6-fold after 48 h of gene transfection (n = 3~4). (G) EAAT2 mRNA levels were upregulated by cloned A1R (n = 4). (H) EAAT2 glutamate uptake activity were increased by A1R overexpression (n = 6). The levels of A1R mRNA or EAAT2 mRNA were determined by real time RT-PCR using GAPDH as an internal normalization control. The levels of A1R and EAAT2 protein were determined using A1R-specific radioligand binding assay and EAAT2 glutamate uptake assay, respectively. *p < 0.05, compared to non-silencing siRNA-treated cells by unpaired two-tailed t-test. All data were expressed as mean ± SEM.
3.2. Effects of ENT1 and A1 receptor knockdown in decreasing EAAT2 expression
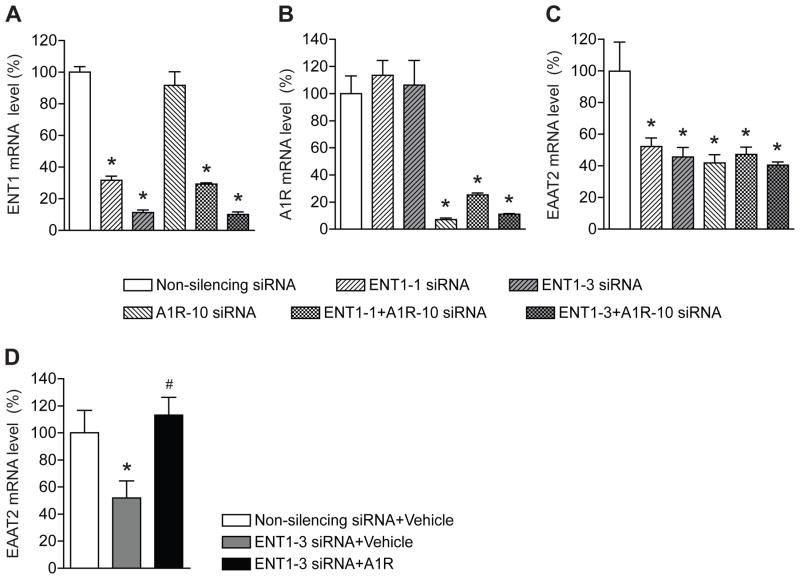
Since we previously reported that inhibition of ENT1 function or expression decreases EAAT2 mRNA expression [8], we examined the effect of simultaneous ENT1 and A1R inhibition by a combination of siRNAs. As we reported previously, two siRNAs against ENT1 significantly reduced ENT1 mRNA [8]. The treatment of siRNAs against A1R has no effect on ENT1 mRNA expression (Fig. 2A). In addition, combined treatments of siRNA against ENT1 and A1R showed a similar result as siRNA treatment against ENT1 alone (Fig. 2A). On the other hand, ENT1-specific siRNA has no effect on A1R mRNA expression (Fig. 2B). Interestingly, as shown in Fig. 2C, inhibition of both ENT1 and A1R reduced EAAT2 mRNA levels similarly to the inhibition of ENT1 or A1R alone, suggesting a threshold reduction of EAAT2 mRNA levels could have been achieved. Furthermore, ENT1 siRNA-mediated downregulation of EAAT2 mRNA expression was rescued by A1R overexpression (Fig. 2D), therefore, both ENT1 and A1R can regulate EAAT2 expression in astrocytes.
Fig. 2.
Both ENT1 and A1 receptor knockdown decrease EAAT2 expression. (A) ENT1 mRNA levels were specifically knocked down after ENT1 siRNA transfection for 48 h (n = 4). (B) A1R mRNA levels were specifically knocked down after A1R siRNA transfection for 48 h (n = 4). (C) EAAT2 mRNA levels were reduced by co-treatment of ENT1 knockdown and A1R knockdown. 10 nM non-silencing siRNA, ENT1 siRNAs (ENT1-1 and ENT1-3), and/or A1R-10 siRNA were treated together or separately in astrocytes for 48 h. (D) ENT1 siRNA-mediated downregulation of EAAT2 mRNA expression was rescued by A1R overexpression (n = 3~4). The levels of ENT1, A1R, and EAAT2 mRNA were determined by real time RT-PCR using GAPDH as an internal normalization control. n =4; *p < 0.05 compared to non-silencing siRNA-treated cells by unpaired two tailed t-test. All data were expressed as mean ± SEM.
3.3. Adenylate cyclase regulates EAAT2 and ENT1 mRNA expressions
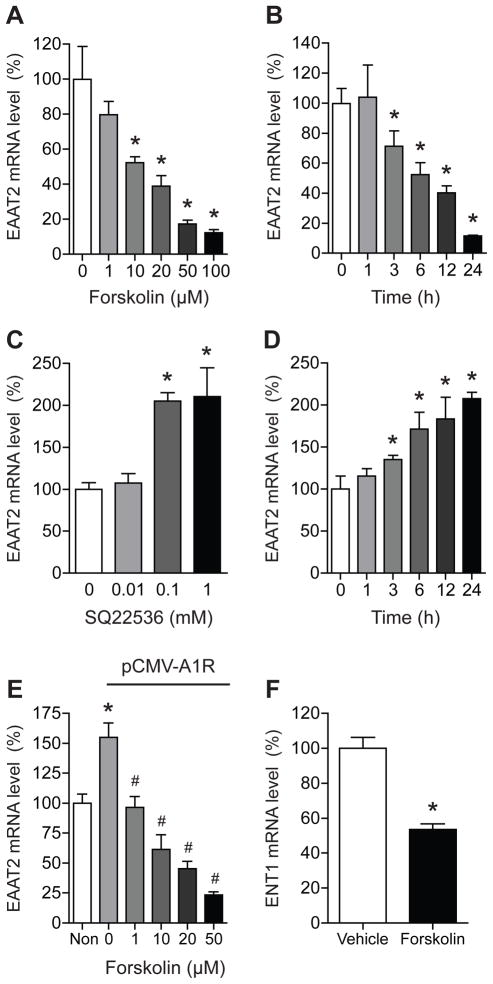
Since A1R is an inhibitory G-protein coupled receptor, its inhibition or knockdown may increase the activity of adenylate cyclase and subsequently elevate cAMP levels. Thus, we examined whether activation of adenylate cyclase by forskolin decreases EAAT2 mRNA expression. As shown in Fig. 3A, EAAT2 mRNA expression was reduced in a dose-dependent manner (one-way ANOVA, F5, 18 = 15.53, p < 0.0001) by forskolin. Also, EAAT2 mRNA expression was significantly reduced in a time-dependent manner during exposure to 100 μM forskolin (Fig. 3B). One-way ANOVA analysis displayed a significant effect of time (F5, 14 = 18.00, p < 0.0001). We then examined whether inhibition of adenylate cyclase increases EAAT2 mRNA expression. As shown in Fig. 3C, treatment with adenylate cyclase inhibitor SQ22536 increased EAAT2 mRNA expression. One-way ANOVA analysis showed a significant effect of dose (F3, 9 = 11.09, p < 0.01). As we expected, EAAT2 mRNA expression was significantly increased in a time-dependent manner in astrocytes exposed to 1 mM SQ22536. One-way ANOVA analysis indicated significant effect of time (F5, 25 = 6.703, p < 0.001). Also, forskolin was able to inhibit the A1R-mediated increase in EAAT2 expression in a dose-dependent manner (Fig. 3E). One-way ANOVA analysis indicated significant effect of dose (F4, 12 = 27.19, p < 0.0001). Furthermore, forskolin treatment (20 μM) was able to reduce ENT1 mRNA expression in astrocytes. These data suggest that adenylate cyclase regulates EAAT2 and ENT1 mRNA expressions in astrocytes.
Fig. 3.
Adenylate cyclase activity regulates EAAT2 expression in astrocytes. (A) EAAT2 mRNA expression levels were decreased by forskolin (adenylate cyclase activator) treatment for 24 h at 10 ~ 100 μM doses. (B) EAAT2 mRNA levels were significantly reduced in a time dependent manner in astrocytes exposed to 100 μM forskolin. (C) EAAT2 mRNA levels were upregulated by SQ22536 (adenylate cyclase inhibitor) at 0.1 or 1 mM doses in a dose-dependent manner in astrocytes after 24 h exposure. (D) EAAT2 mRNA levels were significantly upregulated at 3 ~ 24 h after 1 mM SQ22536 treatment. (E) A1R-mediated increase in EAAT2 expression was inhibited by forskolin in a dose-dependent manner. (F) ENT1 mRNA levels were significantly reduced after 20 μM forskolin treatment. The levels of EAAT2 mRNA were determined by real time RT-PCR using GAPDH as an internal normalization control. n = 4; *p < 0.05 compared to control group and #p < 0.05 compared to control group within pCMV- SPORT6-A1R-transfected groups by Tukey post-hoc tests. All data were expressed as mean ± SEM.
3.4. Forskolin inhibits ethanol-induced upregulation of EAAT2 expression
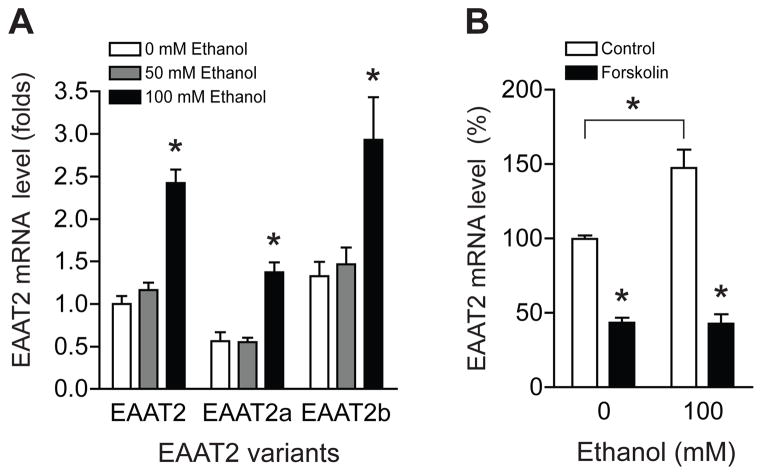
Since several EAAT2 splice variants also regulate glutamate uptake activity [20], we examined the effect of ethanol on two major EAAT2 splice variants (EAAT2a and EAAT2b) in astrocytes. As shown in Fig. 4A, mRNA expression of EAAT2 and its splicing variants were significantly increased by ethanol treatment (100 mM). One-way ANOVA analysis revealed a significant effect of ethanol dose (F2, 27 = 34.88, p < 0.0001). Interestingly, forskolin treatment inhibited ethanol-induced EAAT2 mRNA expression (Fig. 4B). These results indicate that cAMP signaling contributes to ethanol-regulated EAAT2 mRNA expression in astrocytes.
Fig. 4.
Adenylate cyclase activator forskolin inhibits ethanol-induced EAAT2 upregulation. (A) mRNA levels of EAAT2 and two EAAT2 variants (EAAT2a and EAAT2b) were upregulated in astrocytes exposed to 100 mM ethanol treatment for 24 h. (B) Ethanol-mediated EAAT2 upregulation was completely blocked by 20 μM forskolin treatment for 24 h. The levels of EAAT2 mRNA were determined by real time RT-PCR using GAPDH as an internal normalization control. n = 4; *p < 0.05 compared to control group by unpaired two tailed t-tests. All data were expressed as mean ± SEM.
4. Discussion
One of the main functions of astrocytes is to regulate glutamate levels in the brain. Rapid removal of extracellular or synaptic glutamate plays an important role in neuronal activity and dysregulation of this process can result in various neuronal diseases including addictive and psychiatric disorders [21]. Glutamate-mediated excitatory signaling contributes to the pathogenesis of alcohol abuse and dependence [22,23]. We previously reported that EAAT2 expression is causally related to glutamate uptake activity in astrocytes [8] and our present study suggests that adenosine A1R and its downstream signaling regulate EAAT2 mRNA expression and function.
Interestingly, inhibition of the presynaptic adenosine A1R is known to promote glutamate-mediated synaptic neurotransmission in the hippocampus [18]. Similarly, inhibition of adenosine A1R in the striatum increases glutamate-evoked postsynaptic activity [19]. Our previous data demonstrated that diminished A1R function might be causally related to increased glutamate release in the nucleus accumbens of ENT1 null mice [6]. Our new findings in this study demonstrate that inhibition of adenosine A1R expression decreases EAAT2 expression, suggesting that A1R might regulate ethanol-sensitive EAAT2. Thus, it appears that the observed increased extracellular glutamate of ENT1 null mice could be a result of not only increased glutamate release but also reduced glutamate uptake. Additionally, we found that overexpression of A1R increased EAAT2 mRNA and protein expression, suggesting that A1R expression is positively correlated with EAAT2 expression. Overexpression of A1R was able to rescue ENT1 siRNA-mediated downregulation of EAAT2 mRNA expression. Previously, we demonstrated that A1R agonist, N6-CPA (cyclopentyladenosine), reduces ethanol consumption in ENT1 null mice [6], further supporting the assertion that A1R as well as EAAT2 might regulate ethanol intake.
The expression pattern of ENT1 in the human brain closely overlaps that of A1R [24] and adenosine contributes to ethanol-induced ataxia primarily through activation of A1 rather than A2A receptors [25]. Therefore, A1R-regulated EAAT2 mRNA expression appears to contribute to ethanol sensitivity and tolerance. A1R is coupled to Gi or Go, which inhibit cAMP production and PKA activity, possibly leading to a decrease of CREB activity [26]. In contrast, A2A receptors are coupled to Gs or Golf, and stimulate adenylate cyclase [27]. Our data showed that activating adenylate cyclase activity by forskolin decreases EAAT2 mRNA expression, suggesting that Gi-coupled A1R is most likely responsible for regulating EAAT2 mRNA expression in astrocytes. However, since A2A receptor is also expressed in astrocytes, further studies are required to determine whether A2A receptor activity or an interaction between A1 and A2A receptors contributes to EAAT2 expression and function.
Interestingly, EAAT2 expression seems to be regulated by CREB in primary cultured cells [28]. Since our current study demonstrated that decreased adenylate cyclase activity elevates EAAT2 expression, reduced cAMP-dependent protein kinase A (PKA) and CREB activity might increase EAAT2 expression. This possibility suggests that PKA-driven CREB might regulate EAAT2 expression indirectly by regulating other transcription factors of EAAT2 expression. Thus far, we have not found a putative CREB binding site in the proximal mouse EAAT2 promoter region (data not shown). Interestingly, a recent study showed that transcription factors NF-κB [29] and kappa B-motif binding phosphoprotein (KBBP) [30] regulate EAAT2 expression. Thus, further studies are warranted to uncover downstream transcription factors of adenosine A1R signaling, which could interact with the EAAT2 promoter region. \
Mouse EAAT2 (Slc1a2) gene is located in a central part of chromosome 2 (E2) [31], near quantitative trait loci that modulate neuro-excitability and seizure frequency in mouse models of alcohol withdrawal and epilepsy [32]. One genetic variant of EAAT2, G603A, is known to be associated with antisocial alcoholics [33] and cirrhotic alcoholics [34]. EAAT2 expression has also been implicated in morphine and methamphetamine addiction [35]. Microinjection of EAAT2 expression vector into the nucleus accumbens is known to attenuate morphine and methamphetamine-induced conditioned place preference [35]. Interestingly, systemic administration of ceftriaxone, a β-lactam antibiotic, which is known to increase brain EAAT2 expression levels [36], decreases cocaine self-administration in rats [37,38].
In summary, our findings provide a possible correlation between A1R expression or function and EAAT2 mRNA expression in astrocytes. Since glutamate signaling is involved in several aspects of alcohol use disorders, the current study of adenosine-mediated glutamate signaling will be helpful in understanding the etiology of alcohol use disorders in humans as well as in developing novel therapeutic strategies.
Acknowledgments
We thank D. Frederixon and D. Hinton for preparing the manuscript. This project was funded by the Samuel Johnson Foundation for Genomics of Addiction Program at Mayo Clinic to D.-S. C. and in parts by grants from the National Institutes of Health (NIH) to D.-S. C (AA015164, AA018779).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Asatryan L, Nam HW, Lee MR, Thakkar MM, Dar MS, Davies DL, Choi DS. Implication of the Purinergic System in Alcohol Use Disorders. Alcohol Clin Exp Res. 2011 doi: 10.1111/j.1530-0277.2010.01379.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruby CL, Adams C, Knight EJ, Nam HW, Choi DS. An Essential Role for Adenosine Signaling in Alcohol Abuse. Curr Drug Abuse Rev. 2010;3:163–174. doi: 10.2174/1874473711003030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM. Adenosine and brain function. Int Rev Neurobiol. 2005;63:191–270. doi: 10.1016/S0074-7742(05)63007-3. [DOI] [PubMed] [Google Scholar]
- 4.Sawynok J, Liu XJ. Adenosine in the spinal cord and periphery: release and regulation of pain. Prog Neurobiol. 2003;69:313–340. doi: 10.1016/s0301-0082(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 5.Nagy LE, Diamond I, Casso DJ, Franklin C, Gordon AS. Ethanol increases extracellular adenosine by inhibiting adenosine uptake via the nucleoside transporter. J Biol Chem. 1990;265:1946–1951. [PubMed] [Google Scholar]
- 6.Choi DS, Cascini MG, Mailliard W, Young H, Paredes P, McMahon T, Diamond I, Bonci A, Messing RO. The type 1 equilibrative nucleoside transporter regulates ethanol intoxication and preference. Nat Neurosci. 2004;7:855–861. doi: 10.1038/nn1288. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Nam HW, Lee MR, Hinton DJ, Choi S, Kim T, Kawamura T, Janak PH, Choi DS. Altered glutamatergic neurotransmission in the striatum regulates ethanol sensitivity and intake in mice lacking ENT1. Behav Brain Res. 2010;208:636–642. doi: 10.1016/j.bbr.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu J, Lee MR, Choi S, Kim T, Choi DS. ENT1 regulates ethanol-sensitive EAAT2 expression and function in astrocytes. Alcohol Clin Exp Res. 2010;34:1110–1117. doi: 10.1111/j.1530-0277.2010.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miguel-Hidalgo JJ. Withdrawal from free-choice ethanol consumption results in increased packing density of glutamine synthetase-immunoreactive astrocytes in the prelimbic cortex of alcohol-preferring rats. Alcohol Alcohol. 2006;41:379–385. doi: 10.1093/alcalc/agl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amara SG, Fontana AC. Excitatory amino acid transporters: keeping up with glutamate. Neurochem Int. 2002;41:313–318. doi: 10.1016/s0197-0186(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 11.Robinson MB. The family of sodium-dependent glutamate transporters: a focus on the GLT-1/EAAT2 subtype. Neurochem Int. 1998;33:479–491. doi: 10.1016/s0197-0186(98)00055-2. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- 13.Swanson RA, Liu J, Miller JW, Rothstein JD, Farrell K, Stein BA, Longuemare MC. Neuronal regulation of glutamate transporter subtype expression in astrocytes. J Neurosci. 1997;17:932–940. doi: 10.1523/JNEUROSCI.17-03-00932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- 15.Alliot F, Pessac B. Astrocytic cell clones derived from established cultures of 8-day postnatal mouse cerebella. Brain Res. 1984;306:283–291. doi: 10.1016/0006-8993(84)90377-9. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan GB, Bharmal NH, Leite-Morris KA, Adams WR. Role of adenosine A1 and A2A receptors in the alcohol withdrawal syndrome. Alcohol. 1999;19:157–162. doi: 10.1016/s0741-8329(99)00033-6. [DOI] [PubMed] [Google Scholar]
- 18.Manzoni OJ, Manabe T, Nicoll RA. Release of adenosine by activation of NMDA receptors in the hippocampus. Science. 1994;265:2098–2101. doi: 10.1126/science.7916485. [DOI] [PubMed] [Google Scholar]
- 19.Harvey J, Lacey MG. A postsynaptic interaction between dopamine D1 and NMDA receptors promotes presynaptic inhibition in the rat nucleus accumbens via adenosine release. J Neurosci. 1997;17:5271–5280. doi: 10.1523/JNEUROSCI.17-14-05271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shachnai L, Shimamoto K, Kanner BI. Sulfhydryl modification of cysteine mutants of a neuronal glutamate transporter reveals an inverse relationship between sodium dependent conformational changes and the glutamate-gated anion conductance. Neuropharmacology. 2005;49:862–871. doi: 10.1016/j.neuropharm.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Spanagel R, Kiefer F. Drugs for relapse prevention of alcoholism: ten years of progress. Trends Pharmacol Sci. 2008;29:109–115. doi: 10.1016/j.tips.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Tsai G. Glutamatergic neurotransmission in alcoholism. J Biomed Sci. 1998;5:309–320. doi: 10.1007/BF02253441. [DOI] [PubMed] [Google Scholar]
- 24.Jennings LI, Hao C, Cabrita MA, Vickers MF, Baldwin SA, Young JD, Cass CE. Distinct regional distribution of human equilibrative nucleoside transporter proteins 1 and 2 (hENT1 and hENT2) in the central nervous system. Neuropharmacology. 2001;10:722–731. doi: 10.1016/s0028-3908(00)00207-0. [DOI] [PubMed] [Google Scholar]
- 25.Dar MS. Modulation of ethanol-induced motor incoordination by mouse striatal A(1) adenosinergic receptor. Brain Res Bull. 2001;55:513–520. doi: 10.1016/s0361-9230(01)00552-4. [DOI] [PubMed] [Google Scholar]
- 26.Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 27.Fredholm BB, Chen JF, Masino SA, Vaugeois JM. Actions of adenosine at its receptors in the CNS: insights from knockouts and drugs. Annu Rev Pharmacol Toxicol. 2005;45:385–412. doi: 10.1146/annurev.pharmtox.45.120403.095731. [DOI] [PubMed] [Google Scholar]
- 28.Schluter K, Figiel M, Rozyczka J, Engele J. CNS region-specific regulation of glial glutamate transporter expression. Eur J Neurosci. 2002;16:836–842. doi: 10.1046/j.1460-9568.2002.02130.x. [DOI] [PubMed] [Google Scholar]
- 29.Lee SG, Su ZZ, Emdad L, Gupta P, Sarkar D, Borjabad A, Volsky DJ, Fisher PB. Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes. J Biol Chem. 2008;283:13116–13123. doi: 10.1074/jbc.M707697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Gozen O, Watkins A, Lorenzini I, Lepore A, Gao Y, Vidensky S, Brennan J, Poulsen D, Won Park J, Li Jeon N, Robinson MB, Rothstein JD. Presynaptic regulation of astroglial excitatory neurotransmitter transporter GLT1. Neuron. 2009;61:880–894. doi: 10.1016/j.neuron.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirschner MA, Copeland NG, Gilbert DJ, Jenkins NA, Amara SG. Mouse excitatory amino acid transporter EAAT2: isolation, characterization, and proximity to neuroexcitability loci on mouse chromosome 2. Genomics. 1994;24:218–224. doi: 10.1006/geno.1994.1609. [DOI] [PubMed] [Google Scholar]
- 32.Crabbe JC, Belknap JK. Behavior genetic analyses of drug withdrawal. Alcohol Alcohol Suppl. 1993;2:477–482. [PubMed] [Google Scholar]
- 33.Sander T, Ostapowicz A, Samochowiec J, Smolka M, Winterer G, Schmidt LG. Genetic variation of the glutamate transporter EAAT2 gene and vulnerability to alcohol dependence. Psychiatr Genet. 2000;10:103–107. doi: 10.1097/00041444-200010030-00001. [DOI] [PubMed] [Google Scholar]
- 34.Foley PF, Loh EW, Innes DJ, Williams SM, Tannenberg AE, Harper CG, Dodd PR. Association studies of neurotransmitter gene polymorphisms in alcoholic Caucasians. Ann N Y Acad Sci. 2004;1025:39–46. doi: 10.1196/annals.1316.005. [DOI] [PubMed] [Google Scholar]
- 35.Fujio M, Nakagawa T, Sekiya Y, Ozawa T, Suzuki Y, Minami M, Satoh M, Kaneko S. Gene transfer of GLT-1, a glutamate transporter, into the nucleus accumbens shell attenuates methamphetamine- and morphine-induced conditioned place preference in rats. Eur J Neurosci. 2005;22:2744–2754. doi: 10.1111/j.1460-9568.2005.04467.x. [DOI] [PubMed] [Google Scholar]
- 36.Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 37.Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2009;29:9239–9243. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]