Abstract
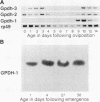
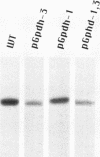
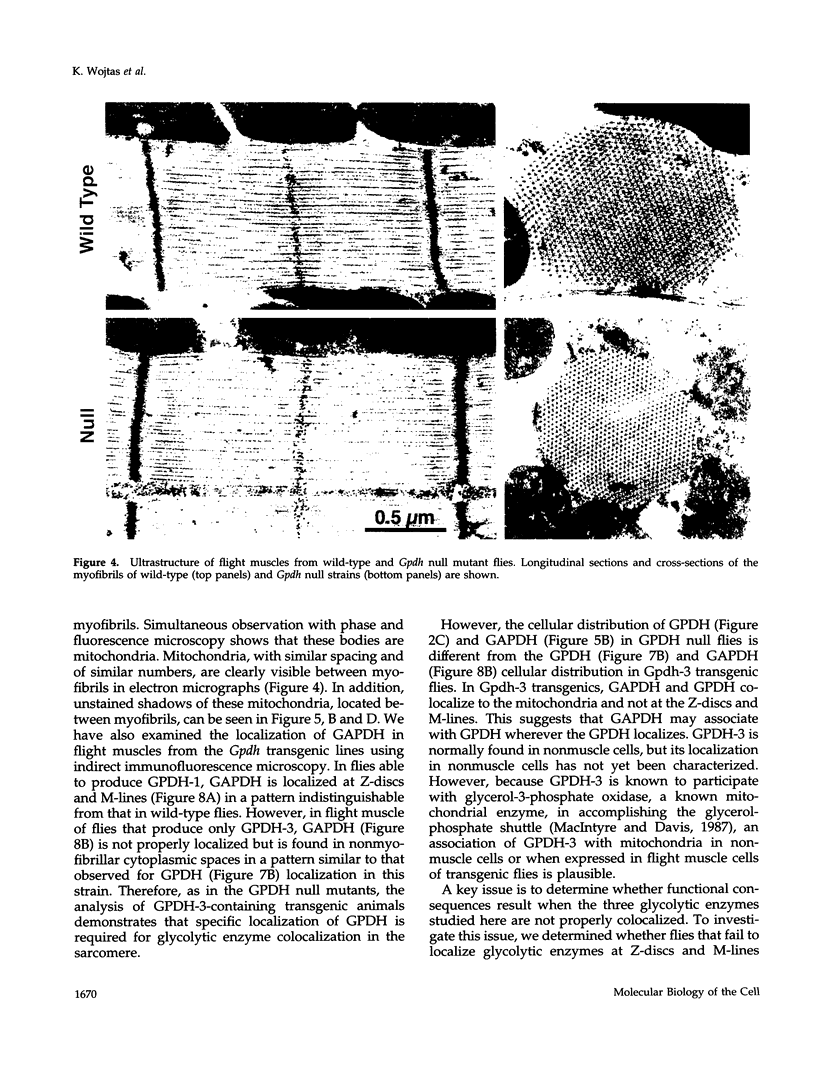
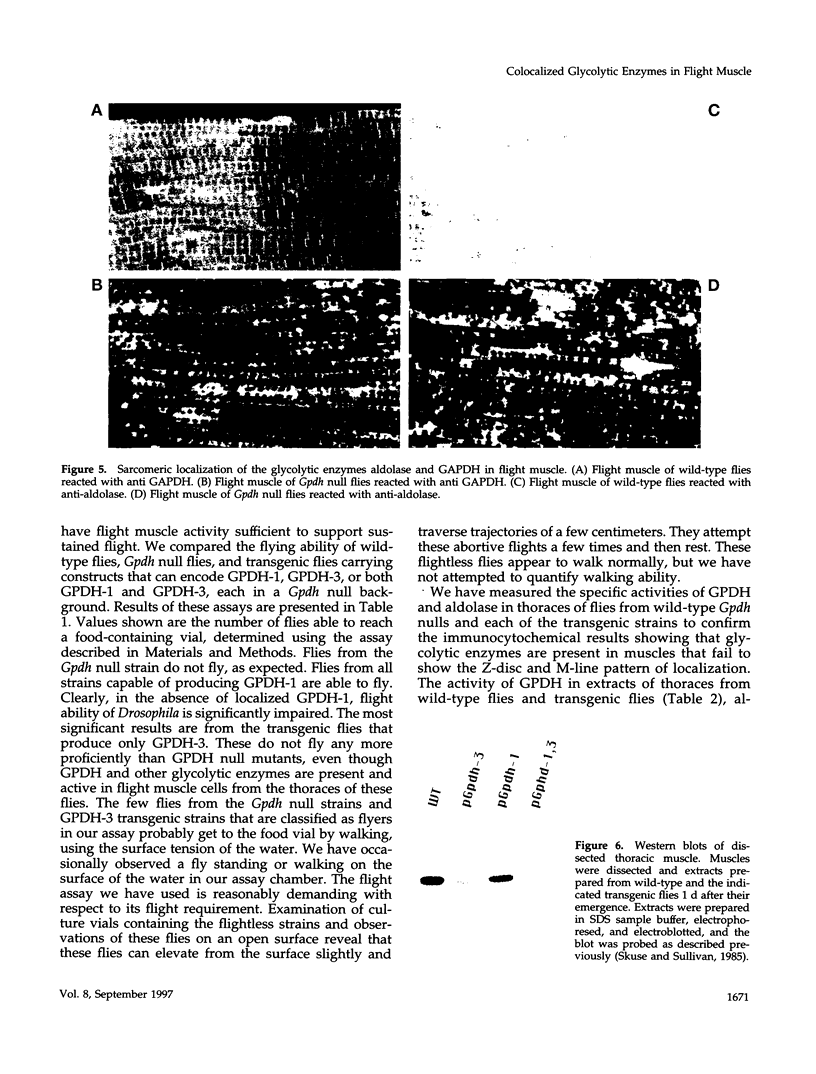
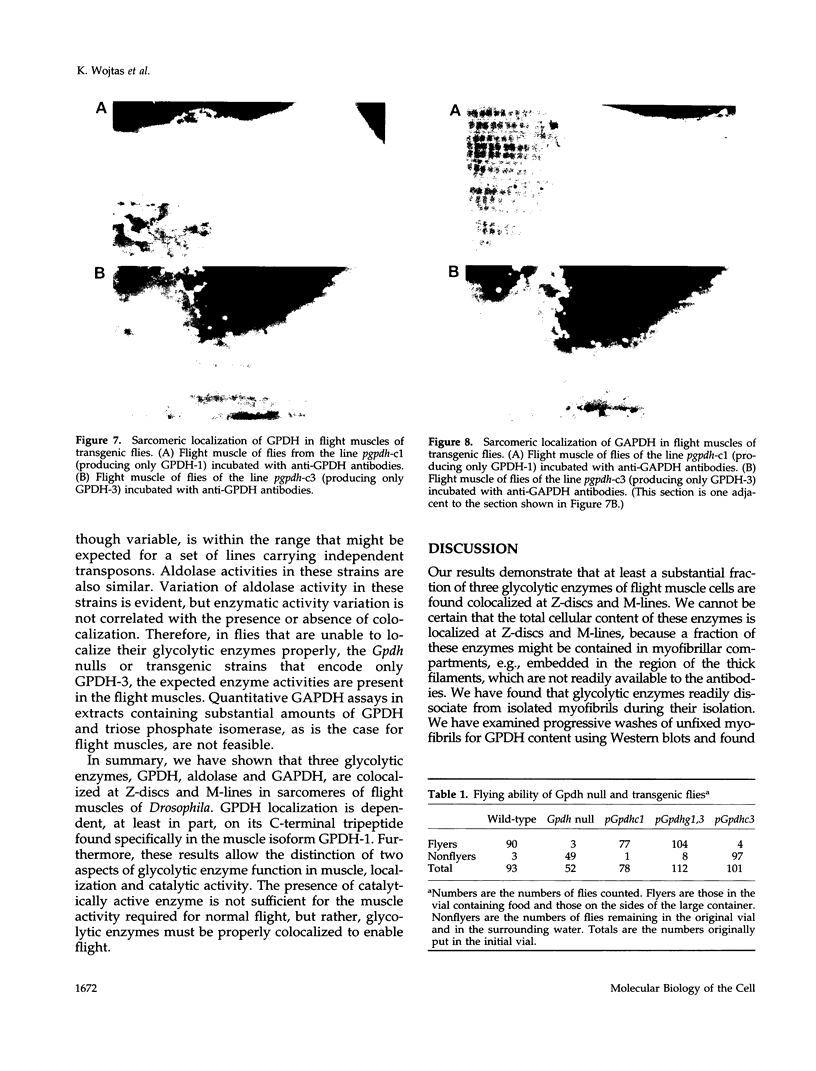
Structural relationships between the myofibrillar contractile apparatus and the enzymes that generate ATP for muscle contraction are not well understood. We explored whether glycolytic enzymes are localized in Drosophila flight muscle and whether localization is required for function. We find that glycerol-3-phosphate dehydrogenase (GPDH) is localized at Z-discs and M-lines. The glycolytic enzymes aldolase and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are also localized along the sarcomere with a periodic pattern that is indistinguishable from that of GPDH localization. Furthermore, localization of aldolase and GAPDH requires simultaneous localization of GPDH, because aldolase and GAPDH are not localized along the sarcomere in muscles of strains that carry Gpdh null alleles. In an attempt to understand the process of glycolytic enzyme colocalization, we have explored in more detail the mechanism of GPDH localization. In flight muscle, there is only one GPDH isoform, GPDH-1, which is distinguished from isoforms found in other tissues by having three C-terminal amino acids: glutamine, asparagine, and leucine. Transgenic flies that can produce only GPDH-1 display enzyme colocalization similar to wild-type flies. However, transgenic flies that synthesize only GPDH-3, lacking the C-terminal tripeptide, do not show the periodic banding pattern of localization at Z-discs and M-lines for GPDH. In addition, neither GAPDH nor aldolase colocalize at Z-discs and M-lines in the sarcomeres of muscles from GPDH-3 transgenic flies. Failure of the glycolytic enzymes to colocalize in the sarcomere results in the inability to fly, even though the full complement of active glycolytic enzymes is present in flight muscles. Therefore, the presence of active enzymes in the cell is not sufficient for muscle function; colocalization of the enzymes is required. These results indicate that the mechanisms by which ATP is supplied to the myosin ATPase, for muscle contraction, requires a highly organized cellular system.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbas J. A., Galceran J., Torroja L., Prado A., Ferrús A. Abnormal muscle development in the heldup3 mutant of Drosophila melanogaster is caused by a splicing defect affecting selected troponin I isoforms. Mol Cell Biol. 1993 Mar;13(3):1433–1439. doi: 10.1128/mcb.13.3.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batke J. Remarks on the supramolecular organization of the glycolytic system in vivo. FEBS Lett. 1989 Jul 17;251(1-2):13–16. doi: 10.1016/0014-5793(89)81419-x. [DOI] [PubMed] [Google Scholar]
- Brooks S. P., Storey K. B. Where is the glycolytic complex? A critical evaluation of present data from muscle tissue. FEBS Lett. 1991 Jan 28;278(2):135–138. doi: 10.1016/0014-5793(91)80101-8. [DOI] [PubMed] [Google Scholar]
- Collier G. E., Sullivan D. T., MacIntyre R. J. Purification of alpha-glycerophosphate dehydrogenase from Drosophila melanogaster. Biochim Biophys Acta. 1976 Apr 8;429(2):216–223. [PubMed] [Google Scholar]
- Cook J. L., Bewley G. C., Shaffer J. B. Drosophila sn-glycerol-3-phosphate dehydrogenase isozymes are generated by alternate pathways of RNA processing resulting in different carboxyl-terminal amino acid sequences. J Biol Chem. 1988 Aug 5;263(22):10858–10864. [PubMed] [Google Scholar]
- Cripps R. M., Becker K. D., Mardahl M., Kronert W. A., Hodges D., Bernstein S. I. Transformation of Drosophila melanogaster with the wild-type myosin heavy-chain gene: rescue of mutant phenotypes and analysis of defects caused by overexpression. J Cell Biol. 1994 Aug;126(3):689–699. doi: 10.1083/jcb.126.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. J., Keller G. A., Schneider M., Howell S. H., Garrard L. J., Goodman J. M., Distel B., Tabak H., Subramani S. Peroxisomal protein import is conserved between yeast, plants, insects and mammals. EMBO J. 1990 Jan;9(1):85–90. doi: 10.1002/j.1460-2075.1990.tb08083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S. J., Winzor D. J. Equilibrium partition studies of the myofibrillar interactions of glycolytic enzymes. Arch Biochem Biophys. 1989 Nov 15;275(1):185–191. doi: 10.1016/0003-9861(89)90363-9. [DOI] [PubMed] [Google Scholar]
- Holtham K. A., Slepecky N. B. A simplified method for obtaining 0.5-microns sections of small tissue specimens embedded in PEG. J Histochem Cytochem. 1995 Jun;43(6):637–643. doi: 10.1177/43.6.7769235. [DOI] [PubMed] [Google Scholar]
- Klemenz R., Weber U., Gehring W. J. The white gene as a marker in a new P-element vector for gene transfer in Drosophila. Nucleic Acids Res. 1987 May 26;15(10):3947–3959. doi: 10.1093/nar/15.10.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knull H. R., Walsh J. L. Association of glycolytic enzymes with the cytoskeleton. Curr Top Cell Regul. 1992;33:15–30. doi: 10.1016/b978-0-12-152833-1.50007-1. [DOI] [PubMed] [Google Scholar]
- Kotarski M. A., Pickert S., Leonard D. A., LaRosa G. J., MacIntyre R. J. The characterization of alpha-glycerophosphate dehydrogenase mutants in Drosophila melanogaster. Genetics. 1983 Oct;105(2):387–407. doi: 10.1093/genetics/105.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronert W. A., O'Donnell P. T., Bernstein S. I. A charge change in an evolutionarily-conserved region of the myosin globular head prevents myosin and thick filament accumulation in Drosophila. J Mol Biol. 1994 Feb 25;236(3):697–702. doi: 10.1006/jmbi.1994.1182. [DOI] [PubMed] [Google Scholar]
- Kronert W. A., O'Donnell P. T., Fieck A., Lawn A., Vigoreaux J. O., Sparrow J. C., Bernstein S. I. Defects in the Drosophila myosin rod permit sarcomere assembly but cause flight muscle degeneration. J Mol Biol. 1995 May 26;249(1):111–125. doi: 10.1006/jmbi.1995.0283. [DOI] [PubMed] [Google Scholar]
- Kvassman J., Pettersson G., Ryde-Pettersson U. Mechanism of glyceraldehyde-3-phosphate transfer from aldolase to glyceraldehyde-3-phosphate dehydrogenase. Eur J Biochem. 1988 Mar 1;172(2):427–431. doi: 10.1111/j.1432-1033.1988.tb13905.x. [DOI] [PubMed] [Google Scholar]
- Lang A. B., Wyss C., Eppenberger H. M. Localization of arginine kinase in muscles fibres of Drosophila melanogaster. J Muscle Res Cell Motil. 1980 Jun;1(2):147–161. doi: 10.1007/BF00711796. [DOI] [PubMed] [Google Scholar]
- Lynch R. M., Paul R. J. Compartmentation of glycolytic and glycogenolytic metabolism in vascular smooth muscle. Science. 1983 Dec 23;222(4630):1344–1346. doi: 10.1126/science.6658455. [DOI] [PubMed] [Google Scholar]
- Masters C. J., Reid S., Don M. Glycolysis--new concepts in an old pathway. Mol Cell Biochem. 1987 Jul;76(1):3–14. doi: 10.1007/BF00219393. [DOI] [PubMed] [Google Scholar]
- Masters C. Interactions of glycolytic enzymes. Trends Biochem Sci. 1989 Sep;14(9):361–361. doi: 10.1016/0968-0004(89)90003-0. [DOI] [PubMed] [Google Scholar]
- Maughan D., Wegner E. On the organization and diffusion of glycolytic enzymes in skeletal muscle. Prog Clin Biol Res. 1989;315:137–147. [PubMed] [Google Scholar]
- Méjean C., Pons F., Benyamin Y., Roustan C. Antigenic probes locate binding sites for the glycolytic enzymes glyceraldehyde-3-phosphate dehydrogenase, aldolase and phosphofructokinase on the actin monomer in microfilaments. Biochem J. 1989 Dec 15;264(3):671–677. doi: 10.1042/bj2640671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien S. J., Shimada Y. The alpha-glycerophosphate cycle in Drosophila melanogaster. IV. Metabolic, ultrastructural, and adaptive consequences of alphaGpdh-l "null" mutations. J Cell Biol. 1974 Dec;63(3):864–882. doi: 10.1083/jcb.63.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offer G., Starr R., Trinick J. Phosphofructokinase: a component of the thick filament? Adv Exp Med Biol. 1988;226:61–73. [PubMed] [Google Scholar]
- Opperdoes F. R. Glycosomes may provide clues to the import of peroxisomal proteins. Trends Biochem Sci. 1988 Jul;13(7):255–260. doi: 10.1016/0968-0004(88)90158-2. [DOI] [PubMed] [Google Scholar]
- Ovádi J. Old pathway--new concept: control of glycolysis by metabolite-modulated dynamic enzyme associations. Trends Biochem Sci. 1988 Dec;13(12):486–490. doi: 10.1016/0968-0004(88)90237-x. [DOI] [PubMed] [Google Scholar]
- Ovádi J., Srere P. A. Channel your energies. Trends Biochem Sci. 1992 Nov;17(11):445–447. doi: 10.1016/0968-0004(92)90485-r. [DOI] [PubMed] [Google Scholar]
- Pierce G. N., Philipson K. D. Binding of glycolytic enzymes to cardiac sarcolemmal and sarcoplasmic reticular membranes. J Biol Chem. 1985 Jun 10;260(11):6862–6870. [PubMed] [Google Scholar]
- Roberts S. J., Somero G. N. Binding of phosphofructokinase to filamentous actin. Biochemistry. 1987 Jun 16;26(12):3437–3442. doi: 10.1021/bi00386a028. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Saide J. D., Chin-Bow S., Hogan-Sheldon J., Busquets-Turner L., Vigoreaux J. O., Valgeirsdottir K., Pardue M. L. Characterization of components of Z-bands in the fibrillar flight muscle of Drosophila melanogaster. J Cell Biol. 1989 Nov;109(5):2157–2167. doi: 10.1083/jcb.109.5.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
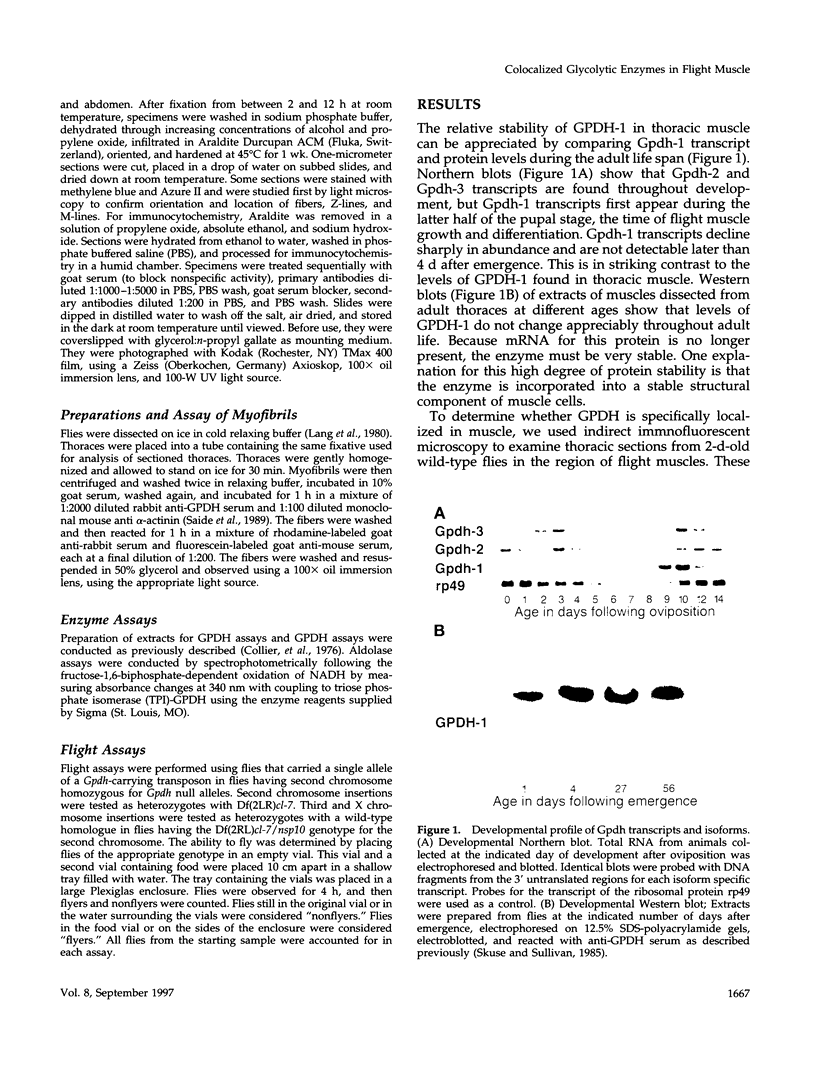
- Skuse G. R., Sullivan D. T. Developmentally regulated alternate modes of expression of the Gpdh locus of Drosophila. EMBO J. 1985 Sep;4(9):2275–2280. doi: 10.1002/j.1460-2075.1985.tb03926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer J. M., Wang C. C. Targeting proteins to the glycosomes of African trypanosomes. Annu Rev Microbiol. 1994;48:105–138. doi: 10.1146/annurev.mi.48.100194.000541. [DOI] [PubMed] [Google Scholar]
- Srivastava D. K., Bernhard S. A. Metabolite transfer via enzyme-enzyme complexes. Science. 1986 Nov 28;234(4780):1081–1086. doi: 10.1126/science.3775377. [DOI] [PubMed] [Google Scholar]
- Sullivan D. T., Carroll W. T., Kanik-Ennulat C. L., Hitti Y. S., Lovett J. A., Von Kalm L. Glyceraldehyde-3-phosphate dehydrogenase from Drosophila melanogaster. Identification of two isozymic forms encoded by separate genes. J Biol Chem. 1985 Apr 10;260(7):4345–4350. [PubMed] [Google Scholar]
- Sullivan D. T., Donovan F. A., Skuse G. Developmental regulation of glycerol-3-phosphate dehydrogenase synthesis in Drosophila. Biochem Genet. 1983 Feb;21(1-2):49–62. doi: 10.1007/BF02395391. [DOI] [PubMed] [Google Scholar]
- Tompa P., Bär J., Batke J. Interaction of enzymes involved in triosephosphate metabolism. Comparison of yeast and rabbit muscle cytoplasmic systems. Eur J Biochem. 1986 Aug 15;159(1):117–124. doi: 10.1111/j.1432-1033.1986.tb09840.x. [DOI] [PubMed] [Google Scholar]
- Turner D. C., Wallimann T., Eppenberger H. M. A protein that binds specifically to the M-line of skeletal muscle is identified as the muscle form of creatine kinase. Proc Natl Acad Sci U S A. 1973 Mar;70(3):702–705. doi: 10.1073/pnas.70.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Waarde A., Van den Thillart G., Erkelens C., Addink A., Lugtenburg J. Functional coupling of glycolysis and phosphocreatine utilization in anoxic fish muscle. An in vivo 31P NMR study. J Biol Chem. 1990 Jan 15;265(2):914–923. [PubMed] [Google Scholar]
- Vigoreaux J. O. Alterations in flightin phosphorylation in Drosophila flight muscles are associated with myofibrillar defects engendered by actin and myosin heavy-chain mutant alleles. Biochem Genet. 1994 Aug;32(7-8):301–314. doi: 10.1007/BF00555832. [DOI] [PubMed] [Google Scholar]
- Wallimann T., Kuhn H. J., Pelloni G., Turner D. C., Eppenberger H. M. Localization of creatine kinase isoenzymes in myofibrils. II. Chicken heart muscle. J Cell Biol. 1977 Nov;75(2 Pt 1):318–325. doi: 10.1083/jcb.75.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallimann T., Turner D. C., Eppenberger H. M. Localization of creatine kinase isoenzymes in myofibrils. I. Chicken skeletal muscle. J Cell Biol. 1977 Nov;75(2 Pt 1):297–317. doi: 10.1083/jcb.75.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X. M., Gutfreund H., Lakatos S., Chock P. B. Substrate channeling in glycolysis: a phantom phenomenon. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):497–501. doi: 10.1073/pnas.88.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kalm L., Weaver J., DeMarco J., MacIntyre R. J., Sullivan D. T. Structural characterization of the alpha-glycerol-3-phosphate dehydrogenase-encoding gene of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5020–5024. doi: 10.1073/pnas.86.13.5020. [DOI] [PMC free article] [PubMed] [Google Scholar]