Abstract
Background & Aims
Interferon reportedly decreases the incidence of hepatocellular carcinoma (HCC) in patients with chronic hepatitis C. The Hepatitis C anti-viral long-term treatment against cirrhosis (HALT-C) trial showed that 4 years of maintenance therapy with peginterferon does not reduce liver disease progression. We investigated whether peginterferon decreases the incidence of HCC in the HALT-C cohort over a longer post-treatment follow-up period.
Methods
The study included 1,048 patients with chronic Hepatitis C (Ishak fibrosis scores ≥3) who did not have a sustained virological response (SVR) to therapy. They were randomly assigned to groups given a half-dose of peginterferon or no treatment (controls) for 3.5 years and followed for a median 6.1 (maximum 8.7) years.
Results
Eighty-eight patients developed HCC (68 definite, 20 presumed): 37/515 that were given peginterferon (7.2%) and 51/533 controls (9.6%; P=0.24). There was a significantly lower incidence of HCC among patients given peginterferon therapy who had cirrhosis, but not fibrosis, based on analysis of baseline biopsy samples. After 7 years, the cumulative incidences of HCC in treated and control patients with cirrhosis were 7.8% and 24.2%, respectively (hazard ratio [HR]=0.45; 95% confidence interval [CI]: 0.24–0.83); in treated and control patients with fibrosis they were 8.3% and 6.8%, respectively (HR=1.44; 95% CI: 0.77–2.69). Treated patients with a ≥2-point decrease in the histologic activity index, based on a follow-up biopsy, had a lower incidence of HCC than those with unchanged or increased scores (2.9% vs. 9.4%; P=0.03).
Conclusions
Extended analysis of the HALT-C cohort showed that long-term peginterferon therapy does not reduce the incidence of HCC among patients with advanced hepatitis C who did not achieve SVRs. Patients with cirrhosis who received peginterferon treatment had a lower risk for HCC than controls.
Keywords: Interferon therapy, hepatitis C clinical trial, interferon non-responders, liver cancer
INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third most common cause of cancer death in the world.1 In the United States, hepatitis C is the greatest contributor to both the occurrence of HCC and its observed increased incidence over the last 20 years. Among persons with chronic hepatitis C, risk factors for the development of HCC are incompletely understood. HCC has been found primarily, but not exclusively, in patients with cirrhosis. In addition to the degree of liver fibrosis, biochemical markers of advanced liver disease (e.g., low platelet count, low albumin), presence of esophageal varices, diabetes, obesity, and use of tobacco and excessive alcohol have been associated with an increased risk for HCC. The risk of HCC is decreased in patients with chronic hepatitis C who achieved a sustained virological response (SVR) to interferon/ribavirin treatment;2, 3 however, the effectiveness of interferon therapy in reducing HCC among patients with hepatitis C virus (HCV)-related cirrhosis who did not achieve an SVR is unclear.
The Hepatitis C Antiviral Long-term Treatment against Cirrhosis (HALT-C) Trial was a randomized, multi-center trial to determine whether 3.5 years of half-dose pegylated interferon (peginterferon) treatment reduced liver disease progression among patients with hepatitis C and advanced fibrosis who were nonresponders to peginterferon and ribavirin. Analysis of the HALT-C Trial results after 3.5 years of treatment revealed that peginterferon did not reduce the overall risk of liver disease progression.4 A subsequent report focusing on HCC development also showed no difference between the treated and control groups during and immediately after the period of maintenance peginterferon therapy.5 We continued to follow the HALT-C Trial cohort off therapy for a total of up to 8.7 years to monitor for the development of decompensated liver disease and HCC. During the extended follow-up period, the number of patients with HCC increased progressively. The aims of the current analysis were: 1) to determine the incidence of HCC among the HALT-C Trial cohort after a longer duration of follow-up, 2) to identify factors associated with the development of HCC in the HALT-C Trial cohort, and 3) to ascertain whether maintenance peginterferon therapy had any delayed, post-treatment effect in preventing HCC.
PATIENTS AND METHODS
The design of the HALT-C Trial has been described previously.4, 6 Briefly, patients with chronic hepatitis C had to meet the following criteria for enrollment: failure to achieve SVR after previous interferon treatment with or without ribavirin, the presence of advanced hepatic fibrosis on liver biopsy (Ishak fibrosis score ≥3), no history of hepatic decompensation or HCC, and the absence of defined exclusion criteria.
All patients had been previously treated with one or more courses of interferon, with the most recent course being a combination of full-dose peginterferon and ribavirin. Patients who remained viremic during treatment and those who experienced breakthrough or relapse after initial response were randomized to maintenance therapy (peginterferon alfa-2a 90 μg weekly) or to no further treatment for the next 3.5 years. Following completion of the 3.5 years of the randomized trial, all patients were invited to continue follow-up without treatment until October 2009.
At entry, all patients were required to have an hepatic ultrasound, computed tomography (CT) or magnetic resonance imaging (MRI) with no evidence of hepatic mass lesions suspicious for HCC and to have a serum alpha fetoprotein (AFP) <200 ng/mL.
All patients had a liver biopsy performed prior to enrollment. Liver biopsies were repeated 1.5 and 3.5 years after randomization and reviewed by a panel of hepatic pathologists, blinded to randomization group. The Ishak scoring system was used to grade inflammation (histologic activity index [HAI] 0–18) and to stage fibrosis (0–6).7 Liver biopsies performed for the diagnosis of HCC and liver explants from patients who underwent transplantation for HCC were also reviewed centrally. Patients were stratified at the time of randomization into two groups, those with cirrhosis (Ishak fibrosis stages 5 or 6) and those with noncirrhotic fibrosis (Ishak fibrosis 3 or 4).
All patients underwent upper gastrointestinal endoscopy at the time of randomization and 3.5 years after randomization for assessment of the presence and size of esophageal varices. For the current analysis, patients were categorized as not having varices (absent) or as having varices, regardless of their size.
HCC Surveillance and Diagnostic Criteria
Patients were scheduled to be seen every 3 months during the 3.5 years of the randomized trial and every 6 months thereafter. A complete blood count, a liver panel (albumin, aspartate aminotransferase [AST], alanine aminotransferase [ALT], alkaline phosphatase, and bilirubin), international normalized ratio of prothrombin time (INR), and AFP level were obtained at the local clinical center at each visit. Ultrasound was repeated at the time of randomization, 6 months after randomization, and every 6–12 months thereafter. Patients with an elevated or rising AFP and those with new lesions on ultrasound were evaluated further with CT or MRI. Diagnostic liver biopsy and treatment for HCC were performed at the discretion of the investigators at each of the clinical sites.
Two definitions of HCC were adopted.5 Definite HCC was defined by histologic confirmation or a new mass lesion on imaging with AFP levels increasing to ≥1,000 ng/mL. Presumed HCC was defined as a new mass lesion on ultrasound in the absence of histology and AFP <1,000 ng/mL in conjunction with one of the following characteristics: a) two liver imaging studies showing a mass lesion with characteristics of HCC (vascular enhancement with or without wash out), b) progressively enlarging lesion on ultrasound leading to death of the patient, or c) one additional imaging study showing a mass lesion with characteristics of HCC that either increased in size over time or was accompanied by increasing AFP levels. All cases of HCC (presumed and definite) were reviewed by an Outcomes Review Panel comprised of rotating panels of three trial investigators. An earlier report included all patients with definite or presumed HCC as judged by the Outcomes Review Panel prior to October 15, 2007.5 The current analysis included all cases of HCC up to the final visit on October 20, 2009.
Statistical Analyses
Statistical analyses were performed at the Data Coordinating Center (New England Research Institutes, Watertown, MA) with SAS® release 9.1 (SAS Institute, Cary, NC). Cumulative incidence of HCC was determined by Kaplan-Meier analysis, and differences were compared by Cox proportional hazards regression. Absolute risk reduction was calculated from estimates and standard errors in the Kaplan-Meier survival curves. We used multivariate Cox regression with forward and backward selection to evaluate predictors of HCC. Variables that were significant in univariate analysis (p<0.05) were included, except for those that were assumed to be highly correlated with other variables. We used logistic regression to evaluate predictors of adherence to peginterferon therapy and SAS® macro CRISKCOX for competing risk analysis among HCC, liver-related death, and liver transplantation.
Adherence to peginterferon was defined as remaining on peginterferon, regardless of dose, ≥2 years after randomization. For the analysis of adherence to peginterferon and risk of HCC, only patients without HCC up to 2.5 years and had regular follow-up were included; these criteria eliminated the possibility that peginterferon was discontinued because of the diagnosis of HCC. For analysis of viral suppression and risk of HCC, only treated patients who were tested for HCV RNA1.5 years after randomization and who also had no HCC up to that time point were included. Decrease in hepatic inflammation was defined as a reduction in HAI by ≥2 points between the year-1.5 biopsy and baseline biopsy. For analysis of decrease in hepatic inflammation and risk of HCC, only patients with a follow-up biopsy at year 1.5 and no HCC or liver transplantation before that time point were included.
RESULTS
Patient Characteristics
Of the 1,050 patients randomized, 90 met predefined criteria for HCC (presumed or definite), but two were excluded because they only met criteria for presumed HCC at the time of HCC diagnosis, did not receive HCC treatment, and did not meet criteria for definite HCC after more than 2 years of follow-up. Therefore, 1,048 patients were included in this analysis. The median duration of follow-up from randomization was 6.1 (range 0–8.7) years.
Table 1 summarizes the characteristics of the patients at the time of enrollment into the HALT-C Trial. Compared to patients without HCC, those in whom HCC subsequently developed were older, had lower body mass index (BMI), had laboratory values indicating more advanced liver disease, higher AFP, higher HAI, and higher fibrosis score, and were more likely to have esophageal varices and to have been smokers.
Table 1.
Baseline Characteristics of Patients with and without Subsequent HCC Development
| No HCC (n=960) | HCC (n=88) | ||||
|---|---|---|---|---|---|
| Mean/% | SD | Mean/% | SD | P-value* | |
| DEMOGRAPHICS | |||||
| Age | 49.9 | 7.2 | 52.5 | 7.0 | 0.003 |
| Female | 29% | 24% | 0.36 | ||
| Race | 0.08 | ||||
| White | 72% | 64% | |||
| Hispanic | 8% | 6% | |||
| Black | 18% | 24% | |||
| Others | 2% | 6% | |||
| METABOLIC FACTORS | |||||
| BMI | 30.0 | 5.6 | 28.2 | 4.1 | 0.005 |
| Diabetes mellitus | 24% | 20% | 0.51 | ||
| VIRAL FACTORS | |||||
| Genotype 1 | 94% | 91% | 0.17 | ||
| Genotype 1, no sub | 19% | 19% | (1 vs. not 1) | ||
| Genotype 1a | 40% | 33% | 0.29 | ||
| Genotype 1a/1b | 2% | 2% | (1a vs. 1b) | ||
| Genotype 1b | 32% | 36% | |||
| Log10 HCV RNA (IU/mL) | 6.44 | 0.52 | 6.37 | 0.51 | 0.15 |
| HCV RNA response during Lead-in | |||||
| <2 Log10 decrease | 55.1% | 66.7% | Reference | ||
| 2–4 Log10 decrease | 21.1% | 17.4% | 0.18 | ||
| >4 Log10 decrease | 23.8% | 15.9% | 0.18 | ||
| LABS | |||||
| Platelets ×1000/mm3 | 169 | 66 | 127 | 49 | <0.0001 |
| Albumin, g/dL | 3.9 | 0.4 | 3.7 | 0.4 | <0.0001 |
| AST, U/L | 85 | 58 | 111 | 65 | <0.0001 |
| ALT, U/L | 105 | 75 | 127 | 92 | 0.02 |
| Alkaline phosphatase, U/L | 98 | 44 | 120 | 59 | <.0001 |
| Total bilirubin, mg/dL | 0.78 | 0.4 | 0.88 | 0.41 | 0.03 |
| Prothrombin time, INR | 1.04 | 0.11 | 1.09 | 0.12 | <0.0001 |
| AFP ng/mL | 16.6 | 29.2 | 25.8 | 29.6 | 0.004 |
| DCP mAU/mL | 38.0 | 64.6 | 47.8 | 49.2 | 0.15 |
| SEVERITY OF LIVER DISEASE | |||||
| CTP score | 5.20 | 0.42 | 5.39 | 0.53 | <0.0001 |
| Esophageal varices | 24% | 43% | <0.0001 | ||
| LIVER HISTOLOGY | |||||
| Cirrhosis on biopsy (Ishak 5–6) | 39% | 55% | 0.002 | ||
| HAI | 7.5 | 2.1 | 8.0 | 2.1 | 0.01 |
| ALCOHOL USE | |||||
| Average consumption, gm/day | 25.5 | 40.5 | 24.1 | 31.2 | 0.77 |
| SMOKING | |||||
| Pack years of cigarettes | 14.62 | 16.75 | 17.16 | 16.81 | 0.03 |
| Ever smoked | 75% | 85% | 0.01 |
Data shown as mean and SD or percent
P values based on Cox regression analysis
Incidence of HCC
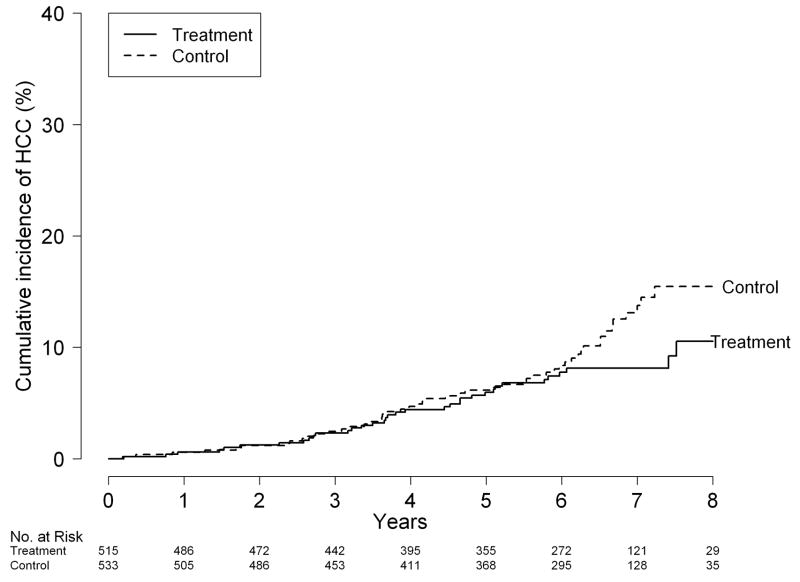
Of the 88 patients who met HALT-C Trial criteria for HCC, 68 had definite HCC (66 were histologically confirmed), while 20 had presumed HCC (Table 2). Sixty-six (75%) of these 88 patients had stage 1 or 2 HCC according to the United Network of Organ Sharing classification. Although treated patients were less likely to have HCC than controls, 7.2% (37/515) vs. 9.6% (51/533), this difference was not statistically significant (hazard ratio [HR] for HCC in patients randomized to peginterferon treatment 0.77, 95% confidence interval [CI] 0.51–1.18, P = 0.24) (Figure 1A).
Table 2.
Number of Patients with HCC
| Fibrosis | Cirrhosis | All patients | |||||
|---|---|---|---|---|---|---|---|
| Treatment | Control | Treatment | Control | Treatment | Control | Total | |
| Total Patients | 308 | 313 | 207 | 220 | 515 | 533 | 1048 |
| Presumed HCC only | 2 | 5 | 4 | 9 | 6 | 14 | 20 |
| Presumed HCC then Definite HCC | 8 | 2 | 3 | 11 | 11 | 13 | 24 |
| Definite HCC only | 13 | 10 | 7 | 14 | 20 | 24 | 44 |
| Total HCC cases (%) | 23 (7.5) | 17 (5.4) | 14 (6.8) | 34 (15.5) | 37 (7.2) | 51 (9.6) | 88 (8.4) |
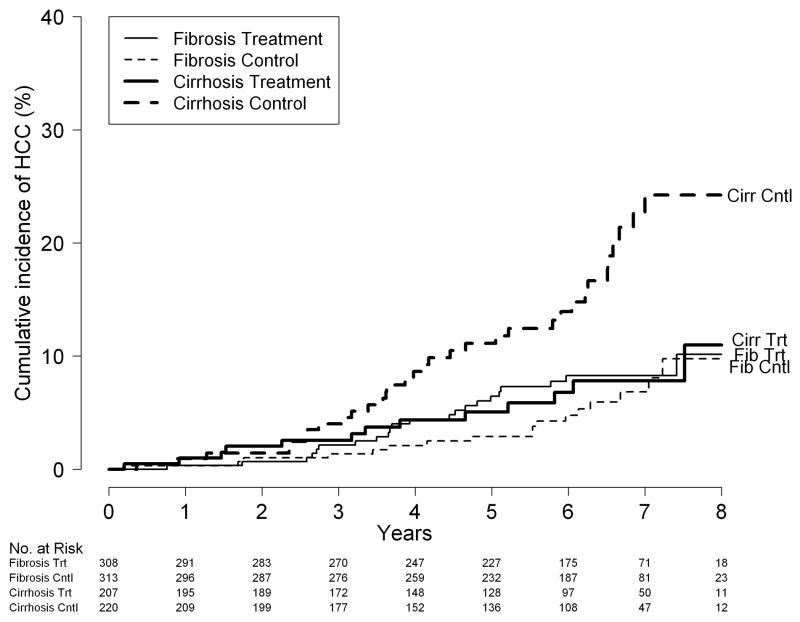
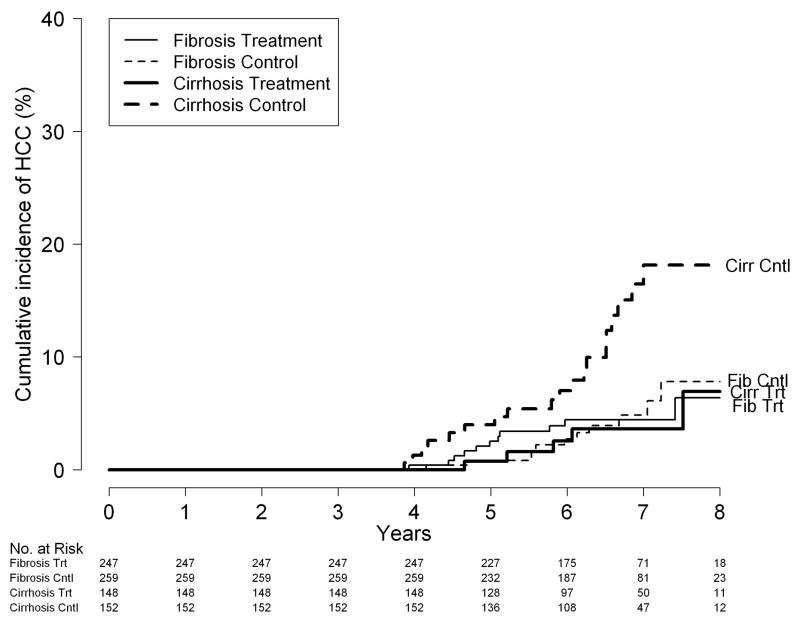
Figure 1.
Cumulative incidence of HCC; (A) by treatment assignment: peginterferon vs. control; (B) by treatment assignment and fibrosis strata (Ishak fibrosis stage 5 or 6 [cirrhosis] vs. Ishak 3 or 4 [fibrosis] at baseline); and (C) by treatment assignment and fibrosis strata for patients who were still at risk 3.75 years after randomization.
When patients with cirrhosis at baseline were analyzed separately, however, significantly fewer of those randomized to receive peginterferon had a diagnosis of HCC than those randomized to no treatment, 6.8% (14/207) vs.15.5% (34/220), respectively (HR for HCC in patients randomized to receive peginterferon 0.45 [95% CI 0.24–0.83], P = 0.01). The cumulative incidence of HCC at 3, 5 and 7 years in the peginterferon group was 2.6%, 5.1%, and 7.8%, and, in the untreated control group, 4.0%, 11.1%, and 24.2%, respectively (log-rank test, P = 0.009). The absolute risk difference was 1.5% (95% CI −2.1%–5.0%) at 3 years, 6.1% (95% CI 0.4%–11.7%) at 5 years, and 16.4% (95% CI 2.3%–24.1%) at 7 years; the incidence of HCC in the two treatment groups did not diverge until after year 4 (Figure 1B).
Among patients with noncirrhotic fibrosis at baseline, similar numbers of treated and control patients were found to have a diagnosis of HCC, 7.5% (23/308) vs. 5.4% (17/313), respectively (HR for HCC in these patients randomized to receive peginterferon 1.44 (95% CI, 0.77–2.69, P = 0.26). Seven of these 40 patients had no evidence of cirrhosis on any of the follow-up biopsies, surgically resected tumors or liver explants. In noncirrhotics, the cumulative incidence of HCC at 3, 5 and 7 years in the peginterferon group was 2.1%, 6.4%, and 8.3% and in the control group, 1.4%, 2.9%, and 6.8%, respectively; the incidence of HCC in the two treatment groups was similar throughout the duration of the study (Figure 1B). Cox regression with interaction confirmed an interaction between treatment group and fibrosis stratum (P = 0.01).
To determine whether the observed results could have been related to uneven distribution of patients with presumed HCC in the two treatment groups, we repeated the analysis after exclusion of the 20 patients who only met criteria for presumed HCC. The results were similar, HR for HCC in the treated group 0.43 (95% CI 0.21–0.89, P = 0.02) for patients with cirrhosis at baseline and 1.86 (95% CI 0.91–3.78, P = 0.09) for patients with bridging fibrosis at baseline.
Because a beneficial effect of peginterferon was not observed during the first 4 years, we repeated the analysis on 994 patients who were still alive, had not undergone liver transplantation, and did not have HCC up to 1400 days after randomization (roughly 3 months after completion of the randomized treatment phase or 3.75 years from randomization). The cumulative incidence of HCC at 5 and 7 years in the peginterferon vs. control groups was significant, 0.7% vs. 4.0% and 3.6% vs. 18.1%, respectively (log-rank test, P = 0.01) among patients with baseline cirrhosis and insignificant, 2.5% vs. 0.8% and 4.4% vs. 4.8%, respectively (log-rank test, P = 0.69) among patients with baseline bridging fibrosis (Figure 1C). The hazard ratios were 0.33 (95% CI 0.13–0.83, P = 0.02) for patients with baseline cirrhosis and 1.19 (95% CI 0.52–2.69, P = 0.69) for patients with baseline bridging fibrosis. For all patients combined, the HR was 0.64 (95% CI 0.36–1.14; P = 0.13).
Patients might succumb from a liver-related death or undergo liver transplantation and no longer be at risk for HCC; therefore, a competing risk analysis to estimate the effect of liver transplantation or liver-related death on the incidence of HCC was conducted. Similar numbers of patients in the peginterferon and control groups underwent liver transplantation or had liver-related deaths (31 and 35 among patients with cirrhosis at baseline and 14 and 13 among patients with bridging fibrosis at baseline, respectively). The differences in the incidence of HCC between the peginterferon and control groups did not change after adjusting for these competing risks (HR 0.45, 95% CI 0.24–0.83, P = 0.01 for patients with cirrhosis at baseline and HR 1.44, 95% CI 0.77–2.69, P = 0.26 for patients with bridging fibrosis at baseline).
Impact of Duration of Peginterferon Treatment on Incidence of HCC
To further confirm the potential beneficial effect of peginterferon in reducing the incidence of HCC, we assessed the association between adherence to maintenance peginterferon and the incidence of HCC. For this analysis, treated patients were stratified into two groups: patients who were still receiving peginterferon (regardless of dose) ≥2 years after randomization and those who had stopped peginterferon <2 years after randomization but continued to be followed. Patients who received peginterferon for ≥2 years had a significantly lower incidence of HCC compared to those who were treated for <2 years (HR 0.36, 95% CI 0.16–0.82, P = 0.02) (Table 3); however, this difference was observed only in the patients with cirrhosis at baseline (HR 0.10, 95% CI 0.03–0.44, P = 0.002). Because patients who tolerated peginterferon treatment had less advanced liver disease (supplementary table), we performed a multivariate analysis to determine whether the duration of peginterferon treatment was associated, independent of disease severity, with a reduced risk of HCC, which was, in fact, the case (see below).
Table 3.
Incidence of HCC by Treatment Duration and Fibrosis Stratum*
| Peginterferon Duration | No. of Patients | No. (%)With HCC | P value | HR | 95% CI |
|---|---|---|---|---|---|
| Fibrosis | |||||
| ≥2 yr | 234 | 17 (7.3%) | 0.6 | 0.72 | 0.21–2.47 |
| <2 yr | 36 | 3 (8.3%) | -- | 1.00 | -- |
| Cirrhosis | |||||
| ≥2 yr | 146 | 4 (2.7%) | 0.002 | 0.10 | 0.02–0.44 |
| <2 yr | 37 | 5 (13.5%) | -- | 1.00 | -- |
| Both Strata | |||||
| ≥2 yr | 380 | 21 (5.5%) | 0.02 | 0.36 | 0.16–0.82 |
| <2 yr | 73 | 8 (11.0%) | -- | 1.00 | -- |
This analysis included 453 of the 515 patients in peginterferon group. Patients were excluded if they were followed for less than 2.5 years, were diagnosed with HCC before 2.5 years, or missed either their 0.5 or 1.5 year visits.
Viral Suppression and Incidence of HCC
Of the 468 patients randomized to maintenance peginterferon and were evaluated for virologic response at year 1.5, 30/396 (7.6%), 1/36 (2.8%), and 1/36 (2.8%) patients with <2 log, 2–4 log, and ≥4 log decrease in HCV RNA were subsequently diagnosed to have HCC, HR for the patients with ≥2 vs. <2 log decrease in HCV RNA 0.38 (95% CI 0.09–1.59, P = 0.18).
Association of Decrease in HAI with Decreased Incidence of HCC
In an earlier analysis of the HALT-C Trial data, we showed that maintenance peginterferon reduced HAI compared to no treatment.4 To determine whether the effect of peginterferon on prevention of HCC was related to its effect in decreasing hepatic inflammation, we analyzed 426 patients in the peginterferon group and 416 in the control group who had repeat liver biopsies at 1.5 years. Treated patients who had an unchanged or increased HAI had a higher incidence of HCC than those who had a decrease in HAI by ≥2 points on the year-1.5 biopsy: 9.4% vs. 2.9% (HR 2.98 and 95% CI 1.14–7.81, P = 0.03) (Table 4). The incidence of HCC in the control group was similar regardless of changes in HAI on the year-1.5 biopsy and comparable to that in the treatment group that did not have a decrease in HAI.
Table 4.
Change in HAI between Baseline and Year-1.5 Biopsies and Incidence of HCC*
| HAI decrease by ≥2 points | No. of Patients | No. (%) of patients with HCC | HR | 95% CI | P value | |
|---|---|---|---|---|---|---|
| Peginterferon | Yes | 171 | 5 (2.9%) | 1.00 | ||
| No | 255 | 24 (9.4%) | 2.98 | 1.14–7.81 | 0.03 | |
| No Biopsy | 42 | 3 (7.1%) | ||||
| Control | Yes | 104 | 10 (9.6%) | 2.90 | 1.13–7.50 | 0.03 |
| No | 312 | 30 (9.6%) | 3.12 | 1.07–9.14 | 0.04 | |
| No Biopsy | 57 | 5 (8.8%) |
Patients had to have a follow-up biopsy 1.5 years after randomization and to have no HCC up to the time of the biopsy
Multivariate Analyses for Predictors of HCC
We undertook stepwise Cox regression analysis to determine whether maintenance peginterferon had an independent effect in preventing HCC. Selected factors that were significant on univariate analysis: age, BMI, platelet, albumin, AST, alkaline phosphatase, total bilirubin, prothrombin time, esophageal varices, HAI, having ever smoked cigarettes, and other relevant factors including gender, black race, cirrhosis on baseline biopsy and treatment assignment were included in the model. To avoid over-fitting, several related laboratory markers that were significant on univariate analysis (Table 1) were not included in the model. Patients with cirrhosis on baseline biopsy assigned to peginterferon treatment had half the rate of HCC outcomes as those randomized to no treatment (HR 0.50, 95% CI 0.27–0.95) (Table 5). In contrast, the rate of HCC outcomes in patients with fibrosis on baseline biopsy, when analyzed separately, was similar regardless of treatment assignment (p = 0.38). Older age, lower BMI, lower platelet, higher AST, higher alkaline phosphatase, and history of smoking were also associated with increased risk of HCC. When the analysis was limited to patients in the treatment group, ability to remain on low-dose peginterferon for ≥2 years was an independent predictor that HCC would not develop (HR 0.39, 95% CI 0.16–0.92).
Table 5.
Stepwise Cox Regression Analysis to Predict Risk of HCC
| Stepwise** | ||
|---|---|---|
| HR | 95% CI | |
| Cirrhosis, Control | 1.00 | 00 |
| Cirrhosis, Peginterferon | 0.50 | 0.27–0.95 |
| Fibrosis, Control | 0.64 | 0.34–1.18 |
| Fibrosis, Peginterferon | 0.86 | 0.48–1.53 |
| Female gender | 0.88 | 0.52–1.48 |
| Black race | 1.58 | 0.94–2.66 |
| Age | 1.05 | 1.02–1.09 |
| BMI | 0.92 | 0.88–0.97 |
| Platelets/10 | 0.89 | 0.84–0.93 |
| AST/10 | 1.03 | 1.004–1.06 |
| Alkaline phoshatase/10 | 1.06 | 1.02–1.09 |
| Ever smoked, % | 2.37 | 1.28–4.37 |
Results of forward and backward selection of variables. Age, BMI, platelet, albumin, AST, alkaline phosphatase, total bilirubin, prothrombin time, esophageal varices, HAI, ever smoked, were selected from variables significant on univariate analysis, and cirrhosis, treatment, gender, and black race were “forced” into the model.
Because presence of esophageal varices at randomization was a strong risk factor for HCC in a previous analysis of HALT-C Trial outcomes,5 we examined the effect of their presence and development more closely, based on results from the baseline and year-3.5 upper endoscopies. Of the 781 patients who underwent a second endoscopy, 185 had varices noted at randomization, 148 had varices first noted at the second endoscopy (incident varices), and 448 did not have varices on either examination. Relative to patients without varices on either examination, subsequent HCC was more likely for patients with varices at randomization (HR 2.54, 95% CI 1.32–4.88) and for patients with incident varices (HR 2.68, 95% CI 1.35–5.32).
DISCUSSION
Previously, we reported that maintenance peginterferon in the HALT-C Trial was not effective in preventing clinical or histological outcomes.4 It has been suggested that the limited duration of follow-up in these reports may not have been sufficient to observe a clinical benefit of peginterferon as chemoprevention for HCC;8 in this vein, retrospective studies have suggested that a benefit of interferon in reducing the occurrence of HCC may not be observed for 5 years or more.9 Supporting this notion is a recent meta-analysis demonstrating that patients with hepatitis C who received interferon therapy had a significantly lower incidence of HCC but significant heterogeneity in the results limited confidence in this conclusion, and the duration of follow-up was an independent contributor to the heterogeneity.3 The process of hepatocarcinogenesis is well recognized to take place over many years, and, even after HCC has developed, many months to years may elapse before the tumor can be detected clinically. The HALT-C Trial investigators acknowledged the possibility that the initially planned follow-up period of 4 years from randomization (6 months after completion of randomized treatment) may have been too short to detect a benefit.10 Therefore, the protocol was amended to extend the duration of follow-up.
With continued observation of this large cohort of patients with advanced hepatitis C for up to 8.7 (median 6.1) years, we found that the number of patients with HCC increased to 88 from 53 (including 5 prevalent cases diagnosed in the first year) in an earlier report.5 Continued follow-up of the HALT-C Trial cohort for up to 5 years after the end of therapy did not show a statistically significant benefit of low dose peginterferon in reducing the incidence of HCC in patients with advanced hepatitis C who did not achieve SVR. In sub-group analysis, however, patients with cirrhosis at baseline assigned to treatment had lower risk of HCC.
Because of the unexpected difference among patients with cirrhosis between our previous and the current findings, we performed additional analyses to verify the consistency of the results. A beneficial effect of peginterferon was observed even when the analysis was restricted to patients with definite HCC (i.e., after exclusion of 20 patients with presumed HCC as defined by the protocol). In addition, a dose-response effect of peginterferon was apparent, as suggested by three findings. First, among patients randomized to receive peginterferon, multivariate analysis showed that those who stopped treatment before 2 years did not have a reduction in the risk of HCC after adjusting for laboratory markers of advanced liver disease. Second, among patients receiving peginterferon, those experiencing a ≥2-point reduction in HAI had an approximately 3-fold reduction in HCC compared to those who did not have a reduction in HAI on follow-up liver biopsy. Third, in a regression analysis of factors associated with the development of HCC, including treatment assignment, peginterferon therapy was associated with an approximate 50% reduction in the incidence of HCC over a median of 6.1 year observation period among patients with cirrhosis at baseline even when adjusted for other risk factors including age, race, smoking history, and laboratory tests associated with advanced liver disease. Nevertheless, while analysis by the two pre-specified strata of histological severity (bridging fibrosis and cirrhosis) was warranted, why the effect of peginterferon was confined to patients with cirrhosis is unclear, and the possibility that this is a chance finding cannot be excluded.
A small randomized controlled trial by Nishiguchi et al11 was the first to suggest that interferon may reduce the risk of HCC in patients with HCV-related cirrhosis. This report was followed by many other reports, mostly from Japan and Europe. The majority of these studies were retrospective or cohort studies comparing the incidence of HCC in patients who had received interferon for 6–12 months with historical or concurrent patients who had not received treatment. The median duration of follow-up ranged from 2 to 15 years. Most studies showed a benefit of treatment, although the effect was predominantly seen in patients who achieved SVR. In addition, three independent meta-analyses suggested that interferon therapy decreased the incidence of HCC in patients with chronic hepatitis C3, 12, 13 In the most recent of these meta-analyses, the relative risk (RR) of HCC for treated vs. untreated patients was 0.43 (95% CI 0.33–0.56, P<0.00001) and for patients with SVR vs. without SVR was 0.35 (95% CI 0.26–0.46, P<0.0001); however, only four of the studies included were randomized controlled trials.
Few studies have compared the incidence of HCC in interferon nonresponders and untreated patients. In two large retrospective studies, the incidence of HCC in these two groups was similar. HCC developed in 14% (20/148) of nonresponders and 12% (19/144) of untreated patients in the study by Imai et al14 and in 11% (39/342) of nonresponders and 10% (54/562) of untreated patients in the study by Yu et al.15 As was true for the 3.5-year randomized phase of the HALT-C Trial, two other studies of long-term, low-dose peginterferon (COPILOT and EPIC3) in patients with advanced fibrosis or cirrhosis showed no benefit of treatment on overall clinical outcomes or HCC.16, 17 To date, however, only preliminary data on outcomes up to 4 years have been reported in these two studies.
The mechanisms by which peginterferon might prevent HCC are not clear. Certainly, achieving an SVR is associated with a reduction in HCC. Indeed, patients in HALT-C trial who achieved SVR after 48 weeks of combination therapy had lower risk of HCC compared to lead-in phase nonresponders, HR 0.19, 95% CI 0.04–0.80.18 Patients who had >2 log decline in HCV RNA after 1.5 years of low-dose peginterferon also had a lower incidence of HCC compared to those with <2 log decline but the difference was not significant and only 15.4% of the patients receiving low-dose peginterferon had >2 log decrease in HCV RNA after 18 months of treatment. Interferon has been shown to have antitumor effects in some malignancies and may have other beneficial effects including antiproliferative and antiangiogenic effects.19, 20 Chronic inflammation has been postulated to play a role in hepatitis B virus-(HBV) and HCV-related HCC. The observation that reduction in HCC incidence in the HALT-C Trial cohort was greater among the treated patients experiencing a reduction in HAI suggests that interferon may have acted through this pathway; however, absence of benefit among control patients with a reduction in hepatic inflammation indicates that interferon may exert its effects through other mechanisms and that a reduction in HAI on follow-up biopsies in interferon-treated patients may be a surrogate of other interferon effects.
The reasons for the apparent delay in the chemopreventive effect of peginterferon are also not known, but we speculate that the delay is related to the slow growth rate of HCC, estimated to have a median doubling time of 117 days.21 In fact, the Gompertzian model would predict that an HCC may have been present for several years before reaching a detectable size.22 Therefore, the HCCs diagnosed in the first 3 to 5 years may well have been present at enrollment into the HALT-C Trial. If, indeed, peginterferon prevented HCC, several years would have been required to detect this effect. In this study, the divergence in HCC incidence between the treated and control patients with cirrhosis at baseline was not apparent until after year 4. Unfortunately, with the termination of this study, we could not determine whether the protective effect disappears or increases with longer follow-up.
Several other interesting observations emerged during this study. Severity of liver disease and portal hypertension, as reflected by more abnormal biochemical tests, lower platelet counts, and presence of esophageal varices were associated with an increased risk of HCC. On the other hand, several studies have shown that patients with increased BMI have a higher rate of HCC;23, 24 while patients in the HALT-C Trial with higher BMI had a significantly lower incidence of HCC. An association between obesity and HCC in other studies may be related to the inclusion of patients with cirrhosis secondary to non-alcoholic fatty liver disease or the inclusion of patients with earlier stage liver disease in whom obesity and steatosis may have accelerated fibrosis progression thereby increasing the risk of HCC. The HALT-C Trial excluded patients with severe steatohepatitis and all patients had advanced fibrosis or cirrhosis. Alcohol and smoking have been reported to be associated with HCC. In the HALT-C Trial, although the average life-time alcohol consumption prior to entry into the HALT-C Trial was high, neither duration of regular alcohol consumption nor life-time alcohol consumption was associated with an increased risk of HCC. Smoking is a risk factor for many cancers, but its association with HCC is not as well described. Several prior reports suggested an association between smoking and histological severity of liver disease,25 and a few reports have appeared of an association between smoking and HCC.24, 26 In this study, smoking was a significant risk factor for HCC, even after adjustment for other risk factors. These data highlight the importance of counseling patients with liver disease on smoking cessation. Esophageal varices and more recently increase in hepatic venous pressure gradient had been shown to be predictors of HCC.27, 28 We found that both presence of varices at baseline and incident varices were associated with increased risk of HCC underscoring the need to evaluate for portal hypertension in patients with advanced liver disease.
In an earlier analysis of the incidence of HCC in the HALT-C Trial cohort, we found that 17% of patients with HCC had no evidence of cirrhosis on serial histology.5 In the current analysis, 8% of HCC developed in patients who had no evidence of cirrhosis. The lower rate of HCC in noncirrhotic patients in the current analysis is related to the subsequent finding of cirrhosis on explant livers in some of the earlier patients and a higher probability that patients diagnosed with HCC during the last two years of the HALT-C study had progressed from bridging fibrosis to cirrhosis. These data confirmed that HCC can occur in non-cirrhotic patients but the rate is low and the cost-effectiveness of HCC surveillance in these patients is unclear.
In conclusion, extended follow-up of the HALT-C Trial cohort showed a modest benefit of long-term peginterferon therapy in reducing the incidence of HCC in patients with hepatitis C and cirrhosis but not in those with advanced precirrhotic fibrosis, an effect that took several years after completion of therapy to become apparent. Although the results were corroborated in multiple sub-analyses, the clinical implications are not clear. Given the marginal beneficial effect and its restriction to only part of the HALT-C Trial cohort, that no overall long-term benefit on mortality was observed in this cohort,29 and given the side effects of peginterferon, necessitating dose reduction or discontinuation in a substantial number of patients, the utility of maintenance peginterferon therapy to prevent HCC in patients with HCV-related cirrhosis is doubtful.
Supplementary Material
Acknowledgments
This study was supported by the National Institute of Diabetes & Digestive & Kidney Diseases (contract numbers are listed below). Additional support was provided by the National Institute of Allergy and Infectious Diseases (NIAID), the National Cancer Institute, the National Center for Minority Health and Health Disparities and by General Clinical Research Center and Clinical and Translational Science Center grants from the National Center for Research Resources, National Institutes of Health (grant numbers are listed below). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. Additional funding to conduct this study was supplied by Hoffmann-La Roche, Inc., through a Cooperative Research and Development Agreement (CRADA) with the National Institutes of Health.
In addition to the authors of this manuscript, the following individuals were instrumental in the planning, conduct and/or care of patients enrolled in this study at each of the participating institutions as follows:
University of Massachusetts Medical Center, Worcester, MA: (Contract N01-DK-9-2326) Gyongyi Szabo, MD, Barbara F. Banner, MD, Maureen Cormier, RN, Donna Giansiracusa, RN
University of Connecticut Health Center, Farmington, CT: (Grant M01RR-06192) Gloria Borders, RN, Michelle Kelley, RN, ANP
Saint Louis University School of Medicine, St Louis, MO: (Contract N01-DK-9-2324) Bruce Bacon, MD, Brent Neuschwander-Tetri, MD, Elizabeth M. Brunt, MD, Debra King, RN
Massachusetts General Hospital, Boston, MA: (Contract N01-DK-9-2319, Grant M01RR-01066; Grant 1 UL1 RR025758-01, Harvard Clinical and Translational Science Center) Raymond T. Chung, MD, Andrea E. Reid, MD, Atul K. Bhan, MD, Wallis A. Molchen, David P. Lundmark
University of Colorado Denver, School of Medicine, Aurora, CO: (Contract N01-DK-9-2327, Grant M01RR-00051, Grant 1 UL1 RR 025780-01), Thomas Trouillot, MD, Marcelo Kugelmas, MD, S. Russell Nash, MD, Jennifer DeSanto, RN, Carol McKinley, RN
University of California - Irvine, Irvine, CA: (Contract N01-DK-9-2320, Grant M01RR-00827) John C. Hoefs, MD, John R. Craig, MD, M. Mazen Jamal, MD, MPH, Muhammad Sheikh, MD, Choon Park, RN
University of Texas Southwestern Medical Center, Dallas, TX: (Contract N01-DK-9-2321, Grant M01RR-00633, Grant 1 UL1 RR024982-01, North and Central Texas Clinical and Translational Science Initiative) Thomas E. Rogers, MD, Peter F. Malet, MD, Janel Shelton, Nicole Crowder, LVN, Rivka Elbein, RN, BSN, Nancy Liston, MPH
University of Southern California, Los Angeles, CA: (Contract N01-DK-9-2325, Grant M01RR-00043) Sugantha Govindarajan, MD, Carol B. Jones, RN, Susan L. Milstein, RN
University of Michigan Medical Center, Ann Arbor, MI: (Contract N01-DK-9-2323, Grant M01RR-00042, Grant 1 UL1 RR024986, Michigan Center for Clinical and Health Research) Robert J. Fontana, MD, Joel K. Greenson, MD, Pamela A. Richtmyer, LPN, CCRC, R. Tess Bonham, BS
Virginia Commonwealth University Health System, Richmond, VA: (Contract N01-DK-9-2322, Grant M01RR-00065) Mitchell L. Shiffman, MD, Melissa J. Contos, MD, A. Scott Mills, MD, Charlotte Hofmann, RN, Paula Smith, RN
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD: T. Jake Liang, MD, David Kleiner, MD, PhD, Yoon Park, RN, Elenita Rivera, RN, Vanessa Haynes-Williams, RN
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition, Bethesda, MD: Leonard B. Seeff, MD, Patricia R. Robuck, PhD, Jay H. Hoofnagle, MD
University of Washington, Seattle, WA: (Contract N01-DK-9-2318) David R. Gretch, MD, PhD, Minjun Chung Apodaca, BS, ASCP, Rohit Shankar, BC, ASCP, Natalia Antonov, M. Ed.
New England Research Institutes, Watertown, MA: (Contract N01-DK-9-2328) Kristin K. Snow, MSc, ScD, Anne M. Stoddard, ScD, Margaret C. Bell, MS, MPH
Inova Fairfax Hospital, Falls Church, VA: Zachary D. Goodman, MD, PhD, Fanny Monge, Michelle Parks
Data and Safety Monitoring Board Members: (Chair) Gary L. Davis, MD, Guadalupe Garcia-Tsao, MD, Michael Kutner, PhD, Stanley M. Lemon, MD, Robert P. Perrillo, MD
Footnotes
The HALT-C Trial was registered with clinicaltrials.gov (#NCT00006164).
Authors’ contributions to the manuscript
| Name | Study design | Execution of study and Data collection | Data analyses | Drafting of manuscript | Critical review of manuscript |
|---|---|---|---|---|---|
| Anna Lok | x | x | x | x | x |
| James Everhart | x | x | x | x | |
| Elizabeth Wright | x | x | x | x | x |
| Adrian Di Bisceglie | x | x | x | x | x |
| Hae-young Kim | x | x | |||
| Richard Sterling | x | x | x | ||
| Gregory Everson | x | x | x | ||
| Karen Lindsay | x | x | x | ||
| William Lee | x | x | x | ||
| Herbert L. Bonkovsky | x | x | x | ||
| Jules Dienstag | x | x | x | ||
| Marc Ghany | x | x | x | ||
| Chihiro Morishima | x | x | x | ||
| Timothy Morgan | x | x | x | x | x |
Financial Disclosures Paragraph for Insertion into Manuscript
Financial relationships of the authors with Hoffmann-La Roche, Inc., are as follows: A.S. Lok is a consultant and receives research support; T.R. Morgan receives research support; A.M. Di Bisceglie is a consultant and receives research support; R.K. Sterling is a consultant and receives research support; G.T. Everson is a consultant and receives research support; K.L. Lindsay was a consultant and received research support from Hoffmann-La Roche, Inc. during this study and is now an employee of Tibotec, Inc. (a subsidiary of Johnson and Johnson), Titusville, NJ; W.M. Lee receives research support; and H.L. Bonkovsky receives research support. Authors with no financial relationships related to this project are: J.E. Everhart, E.C. Wright, H.-Y. Kim, J.L. Dienstag, M.G. Ghany, and C. Morishima.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Anna S. Lok, Email: ASLok@umich.edu.
James E. Everhart, Email: EverhartJ@extra.niddk.nih.gov.
Elizabeth C. Wright, Email: wrightel@niddk.nih.gov.
Adrian M. Di Bisceglie, Email: dibiscam@slu.edu.
Hae-Young Kim, Email: hkim@neriscience.com.
Richard K. Sterling, Email: RKSterli@vcu.edu.
Gregory T. Everson, Email: greg.everson@ucdenver.edu.
Karen L. Lindsay, Email: klindsay@usc.edu.
William M. Lee, Email: William.Lee@UTSouthwestern.edu.
Herbert L. Bonkovsky, Email: Herbert.bonkovsky@carolinashealthcare.org.
Jules L. Dienstag, Email: jdienstag@partners.org.
Marc G. Ghany, Email: MarcG@bdg10.niddk.nih.gov.
Chihiro Morishima, Email: chihiro@u.washington.edu.
Timothy R. Morgan, Email: timothy.morgan@va.gov.
References
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Bruno S, Stroffolini T, Colombo M, et al. Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology. 2007;45:579–87. doi: 10.1002/hep.21492. [DOI] [PubMed] [Google Scholar]
- 3.Singal AK, Singh A, Jaganmohan S, et al. Antiviral Therapy Reduces Risk of Hepatocellular Carcinoma in Patients with Hepatitis C Virus-Related Cirrhosis. Clinical Gastroenterology and Hepatology. 2010;8:192–199. doi: 10.1016/j.cgh.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 4.Di Bisceglie AM, Shiffman ML, Everson GT, et al. Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon. N Engl J Med. 2008;359:2429–41. doi: 10.1056/NEJMoa0707615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lok AS, Seeff LB, Morgan TR, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138–48. doi: 10.1053/j.gastro.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee WM, Dienstag JL, Lindsay KL, et al. Evolution of the HALT-C Trial: pegylated interferon as maintenance therapy for chronic hepatitis C in previous interferon nonresponders. Control Clin Trials. 2004;25:472–92. doi: 10.1016/j.cct.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–9. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 8.Hoshida Y, Golub TR. Prolonged therapy for hepatitis C with low-dose peginterferon (letter) N Engl J Med. 2009;360:1152. author reply 1152–3. [PubMed] [Google Scholar]
- 9.International Interferon-alpha Hepatocellular Carcinoma Study Group. Effect of interferon-alpha on progression of cirrhosis to hepatocellular carcinoma: a retrospective cohort study. Lancet. 1998;351:1535–9. [PubMed] [Google Scholar]
- 10.Di Bisceglie A, Wright E, Dienstag J. Prolonged Therapy for Hepatitis C with Low-Dose Peginterferon (response to letter) New England Journal of Medicine. 2009;360:1152–1153. [PubMed] [Google Scholar]
- 11.Nishiguchi S, Kuroki T, Nakatani S, et al. Randomised trial of effects of interferon-alpha on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet. 1995;346:1051–5. doi: 10.1016/s0140-6736(95)91739-x. [DOI] [PubMed] [Google Scholar]
- 12.Papatheodoridis GV, Papadimitropoulos VC, Hadziyannis SJ. Effect of interferon therapy on the development of hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis: a meta-analysis. Aliment Pharmacol Ther. 2001;15:689–98. doi: 10.1046/j.1365-2036.2001.00979.x. [DOI] [PubMed] [Google Scholar]
- 13.Craxi A, Camma C. Prevention of hepatocellular carcinoma. Clin Liver Dis. 2005;9:329–46. viii. doi: 10.1016/j.cld.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Imai Y, Kawata S, Tamura S, et al. Relation of interferon therapy and hepatocellular carcinoma in patients with chronic hepatitis C. Osaka Hepatocellular Carcinoma Prevention Study Group. Ann Intern Med. 1998;129:94–9. doi: 10.7326/0003-4819-129-2-199807150-00005. [DOI] [PubMed] [Google Scholar]
- 15.Yu ML, Lin SM, Chuang WL, et al. A sustained virological response to interferon or interferon/ribavirin reduces hepatocellular carcinoma and improves survival in chronic hepatitis C: a nationwide, multicentre study in Taiwan. Antivir Ther. 2006;11:985–94. [PubMed] [Google Scholar]
- 16.Afdhal NH, Levine R, Brown R, Jr, et al. ColchicineVersus Peg-Interferon Alfa 2B Long Term Therapy: Results of the 4 Year Copilot Trial (abstract) Journal of Hepatology. 2008;48:S4. [Google Scholar]
- 17.Bruix J, Poynard T, Colombo M, et al. Pegintron Maintenance Therapy in Cirrhotic (Metavir F4) HCV Patients, Who Failed to Respond to Interferon/Ribavirn (IR) Therapy: Final Results of the EPIC3 Cirrhosis Maintenance Trial (abstract) Journal of Hepatology. 2009;50:S22. [Google Scholar]
- 18.Morgan TR, Ghany MG, Kim HY, et al. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology. 2010 doi: 10.1002/hep.23744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merle P, Barraud L, Lefrancois L, et al. Long-term high-dose interferon-alpha therapy delays Hepadnavirus-related hepatocarcinogenesis in X/myc transgenic mice. Ocogene. 2003;22:2762–71. doi: 10.1038/sj.onc.1206375. [DOI] [PubMed] [Google Scholar]
- 20.Indraccolo S. Interferon-alpha as angiogenesis inhibitor: learning from tumor models. Autoimmunity. 43:244–7. doi: 10.3109/08916930903510963. [DOI] [PubMed] [Google Scholar]
- 21.Sheu JC, Sung JL, Chen DS, et al. Growth rate of asymptomatic hepatocellular carcinoma and its clinical implications. Gastroenterology. 1985;89:259–66. doi: 10.1016/0016-5085(85)90324-5. [DOI] [PubMed] [Google Scholar]
- 22.Cheng SJ, Freeman RB, Jr, Wong JB. Predicting the probability of progression-free survival in patients with small hepatocellular carcinoma. Liver Transpl. 2002;8:323–8. doi: 10.1053/jlts.2002.31749. [DOI] [PubMed] [Google Scholar]
- 23.Saunders D, Seidel D, Allison M, et al. Systematic review: the association between obesity and hepatocellular carcinoma - epidemiological evidence. Aliment Pharmacol Ther. 2010;31:1051–63. doi: 10.1111/j.1365-2036.2010.04271.x. [DOI] [PubMed] [Google Scholar]
- 24.Marrero JA, Fontana RJ, Fu S, et al. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J Hepatol. 2005;42:218–24. doi: 10.1016/j.jhep.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Zein CO. Clearing the smoke in chronic liver diseases. Hepatology. 2010;51:1487–90. doi: 10.1002/hep.23694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chuang SC, Lee YC, Hashibe M, et al. Interaction between cigarette smoking and hepatitis B and C virus infection on the risk of liver cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 19:1261–8. doi: 10.1158/1055-9965.EPI-09-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giannini EG, Risso D, Testa R, et al. Prevalence and prognostic significance of the presence of esophageal varices in patients with hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2006;4:1378–84. doi: 10.1016/j.cgh.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Ripoll C, Groszmann RJ, Garcia-Tsao G, et al. Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J Hepatol. 2009;50:923–8. doi: 10.1016/j.jhep.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Bisceglie AM, Dienstag JL, Shiffman ML, et al. Patient Mortality and Liver Transplantation during the HALT-C Trial: Relationship to Chronic Liver Disease and Interferon Therapy (abstract) DDW. 2010 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.