Abstract
Zebrafish provide unique opportunities for optogenetic studies of behavior. Here, we review the most recent work using optogenetic and imaging approaches to study the neuronal circuits controlling movements in the transparent zebrafish. Specifically, we focus on what we have learned from zebrafish about neuronal migration, network formation and behavioral control, and what the future may hold.
Introduction
Optogenetics is an emerging field that utilizes light and genes to interrogate the nervous system [1–3]. It is difficult to imagine a vertebrate animal better suited to optogenetic technology than the zebrafish (Danio rerio). A see-through, behaving animal provides at least two unique opportunities to understand how the vertebrate nervous system generates behavior. First, one can perform time-lapse imaging in developing animals to determine how neurons take shape and coalesce into functional networks. Second, one can monitor and manipulate neuronal activity in behaving animals to study the functional organization of these networks once they’re assembled.
The advantages of zebrafish for studies of neural circuits and behavior are often touted and are the subject of recent reviews [4–6]. Here, we will cover work over the past couple years that has used optogenetic and imaging strategies to study the development and operation of neuronal networks that control movements in zebrafish. Our goal is to highlight what we have learned by studying zebrafish motor networks and to what degree this reflects information of consequence beyond the zebrafish model.
Formation of neurons, nuclei and layers
The vertebrate nervous system is populated by a wide variety of neurons organized into nuclei, columns and layers. Our understanding of neuron formation, migration and organization is largely based on a series of static images collected at different time points. Several recent studies have focused their attention on these issues using more dynamic in vivo time-lapse approaches in zebrafish.
Neurons are spun out at different time periods, a process that contributes to neuronal diversity. To sustain neuronal proliferation, asymmetric cell division often occurs, where one cell becomes a neuron while the other remains a progenitor that can give rise to additional neurons. Until recently, it was thought that during asymmetric cell division, the more apical daughter cell (the closest to the progenitor pool) remained a progenitor, while the more basal daughter cell (located further away from the progenitor pool) became the neuron. A very recent study has overturned this idea by visualizing dividing progenitor cells in the caudal hindbrain and rostral spinal cord of embryonic zebrafish [7]. These observations reveal that during asymmetric cell division, the cell that maintains contact with the basal membrane of the neural tube becomes the progenitor, while neurons derive from the more apical daughter cell. This evidence contrasts with expectations based on prior work and reveals a previously unappreciated level of complexity to the replenishment of progenitor pools.
Another form of cell division that contributes to neuronal diversity is one in which a progenitor generates neurons with two different identities. In the spinal cord, earlier work showed that cells from one dorsoventral domain, the p2 progenitor domain, generate two distinct neuronal subtypes: excitatory V2a cells and inhibitory V2b cells. Both classes of neuron have axons that project on the same side of the body. What was not known, however, was how these cells were generated. A recent study used time-lapse imaging to investigate this issue and revealed that the final division of pair-producing progenitors in the zebrafish spinal cord produces one excitatory V2a neuron and one inhibitory V2b neuron [8]. Delta-Notch interactions between the two daughter cells play a critical role in the proportional generation of the two classes of spinal neurons [8,9]. This finding has interesting implications regarding the assembly of spinal motor networks, where excitatory and inhibitory interneurons with similar morphologies are generated in equal numbers. This might help to provide a rough balance between excitation and inhibition that seems functionally important in many networks.
Once neurons are generated, they very often migrate to their final destination where they form either nuclei or laminae. This process plays an important role in the organization of neuronal circuitry. Migration of hindbrain motor nuclei has been the source of recent interest in zebrafish [10–12]. For example, facial branchiomotor neurons (FBMNs) undergo a posterior migration from their site of birth in a more rostral region of hindbrain (rhombomere 4 or r4) into more caudal regions (rhombomere 6–7 or r6–7). Once there, FBMNs migrate laterally to their ultimate destination. Despite their more caudal location, axons from FBMNs still exit at r4 where they innervate muscles of the 2nd brachial arch (corresponding to the muscles that control facial expression, middle ear and upper neck in mammals). The advantage of migration for circuit function is unknown, but it might act to minimize the cost of wiring up networks [13]. The effects of perturbing migration on the wiring and subsequent function of motor nuclei should offer some insight into the role of migration, which occurs widely in nervous systems.
In mammals, the cerebellum is a striking laminated structure whose role in motor coordination is intensely studied. The development of the cerebellum in zebrafish, including the migration and the genetic specification of identified cerebellar network components, has now become a focus of study [14–19]. Remarkably, even in 4–5 day old larval zebrafish, the equivalents of granule cells, Purkinje cells, and eurydendroid cells, in addition to climbing, parallel and mossy fiber pathways, are already present [14–18]. The several recent studies describe the neuronal types in larvae and adults [16], identify the patterns of proneural gene expression associated with the generation of these cell types, whose production persists into adulthood [17], and reveal that the continued proliferation into adulthood is correlated with the absence of the external germinal layer seen in amniotic vertebrates [18]. The new work also identifies mutants that alter granule and Purkinje cell numbers [16] and reveals the differentiation of granule neuron subtypes [14] and a role for cadherins in their migration [15]. While these developmental studies are fundamental in their own right, a simple, accessible, functional and intact cerebellum should also provide a wonderful opportunity to study the essentials of cerebellar network function with the array of tools available in zebrafish.
Identifying motor networks
To study neuronal networks, it helps if you can reproducibly identify them. The expression of reporters driven by promoters for specific genes, like transcription factors, has been a successful way to identify motor networks in zebrafish in the past [20–22]. Our own work has used transgenic lines of fish to investigate the contribution of different classes of spinal neuron to locomotion. Studies of excitatory spinal neurons have revealed that switches in interneuron class occur during changes in swimming speed [23]. So, it is likely that more than one spinal premotor network is responsible for producing the full range of locomotor movements in zebrafish, as also appears to be the case in mammals [24]. In addition, we recently found that there are inhibitory interneurons that are shared between different motor behaviors, while others are dedicated to particular movements [25]. Dedicated neurons playing roles in escape have segmental, especially powerful commissural inhibitory connections that block motor output on the side opposite and escape bend. Another dedicated class is a large ascending inhibitory neuron only activated during struggling that may shape the pattern of activity in the spinal cord to give rise to the bends that propagate from tail to head during struggling, opposite the direction found during swimming. The picture that is emerging is that a core network of shared spinal neurons may be shaped by more specialized interneurons to produce an assortment of motor behaviors in vertebrates [26].
As we mentioned above, neurons develop at different times, which plays a crucial role in their ultimate identity. We, like others, have used optogenetic birth-dating strategies to identify neurons and networks according to when they differentiated [20,27,28]. Work in the spinal cord suggests that networks differentiate in a very logical progression; those that ultimately control the fastest/strongest movements develop first, with those controlling increasingly slower/weaker movements added with time [20,28]. The sequential addition of neurons according to speed/force generates a topographic map of recruitment in the larval spinal cord [29]. This work is beginning to forge links between patterns of development and later function and is likely to impact studies of nervous system organization more broadly.
Another way to selectively light up neuronal networks in zebrafish is to use an enhancer/trap strategy [30–33]. Recently, a zebrafish enhancer/trap line was used to elucidate the contribution of a specific class of spinal interneuron to locomotion [34]. Enhancer/trap lines often have mosaic, random expression, however in this particular line of fish the only neurons labeled in the spinal cord were an identified class of commissural local (CoLo) glycinergic interneuron. Using a combination of electrophysiology, imaging, ablation and behavioral approaches, the authors determined the context in which CoLo neurons are active and their connectivity, and were able to selectively remove one population, and only that population, and test the behavioral consequences. This study represents a very compelling test of the contribution of a spinal neuron to a vertebrate behavior. The work demonstrated unambiguously that interneurons in spinal cord can play a critical role in filtering out conflicting commands from the brain.
Monitoring and manipulating motor networks
One way to begin to understand how identified networks contribute to behavior is to determine when they are active. Imaging neuronal activity in behaving animals has been possible in zebrafish for quite some time, but one drawback to even the most recent approaches is the requirement for the fish to be at least partially restrained [35–37]. While this is far better than some of the more invasive procedures required for electrophysiology, ideally you want to monitor neuronal activity in freely swimming fish when they can exercise their full behavioral repertoire.
A recent study demonstrated the feasibility of monitory neuronal activity in freely swimming fish by using the Gal4-UAS system to drive the expression of a bioluminescent fusion protein GFP-Aequorin in the nervous system of larval zebrafish, [38]. The approach involved targeting GFP-Aequorin to a small set of cells and then detecting photons emitted from the whole animal, which must have, by inference, arisen from the labeled neurons it contained. The authors went on to show that a ‘neuroluminescent’ signal could be detected from a small group of hypocretin-positive neurons in the hypothalamus, which correlated well with periods of increased locomotor activity, as would be predicted from their presumed role during sleep-like states in larval fish [39,40]. While the approach is dependent on very specific targeting of small subsets of neurons to be confident about the source of the emitted photons, nonetheless it offers a powerful way of correlating activity in identified neurons with natural behavior.
Once the neurons active during a behavior have been identified, a critical next step to revealing their role in behavior is to manipulate their activity to reveal how the behavior changes. Zebrafish embryos and larvae have often been used as a proving ground for constructs that manipulate neuronal activity with light. In most cases, movements are the functional read-out. One of the earliest in vivo demonstrations of channelrhodopsin-2 (ChR2) was an experiment performed in zebrafish [41]. Flashes of blue light caused spikes in somatosensory trigeminal and Rohon-Beard neurons expressing ChR2, which, as you’d expect, caused zebrafish embryos to twitch on cue. An in vivo demonstration of light-activated glutamate receptors (LiGluRs) was also provided by expression in groups of neurons in zebrafish larvae, which included somatosensory neurons [42]. Surprisingly, in this study activation of LiGluRs in sensory neurons with light did not elicit any motor activity, but rather prevented the normal response to tactile stimulation. This finding is difficult to interpret without some indication of what the activation of LiGluRs was doing to the zebrafish neurons.
More recently, methods to silence neuronal activity in vivo have been demonstrated in zebrafish. By expressing halorhodopsin (NpHR) throughout the larval nervous system, a recent study showed that activation of NpHR can suppress firing in as yet unidentified, but spontaneously active hindbrain neurons [43]. By systematically silencing different regions of the hindbrain, the authors revealed a region in which NpHR-mediated neuronal silencing elicited a curious ‘rebound’ activation of swimming. It is unclear why silencing neurons should lead to a delay in swimming. Future work examining the identity of networks in this region, their activation patterns and their connectivity, could provide some explanation. The same group also recently demonstrated the application of optical tools to examine an identified motor network in larval zebrafish. Pan-neuronal expression of both ChR2 and NpHR was used to optically activate and silence, respectively, different regions of the brain to probe the premotor circuitry responsible for the optokinetic response [44]. The conclusion reached was that the saccade-generating circuit in zebrafish was in many ways similar to its mammalian counterpart. In addition to NpHR, another recently proven way to silence neuronal activity in living zebrafish is via a potassium-selective ionotropic glutamate receptor, called HyLighter, which when expressed in motoneurons can reduce the probability of touch-evoked movements [45].
One recent optogenetic perturbation led to a rather unexpected result [46]. Kolmer-Agduhr (KA) neurons are GABAergic neurons in the spinal cord that contact the central canal, but whose function was unresolved. Using a series of Gal4-UAS driver lines, optical stimulation with LiGluRs, and genetic silencing with tetanus toxin light chain (TeTxLC), the authors investigated the role of KA cells during swimming behavior. A common result from the application of neuromodulatory chemicals to larval zebrafish is an effect on the occurrence of swim bouts, with no obvious effect on the patterning of the rhythm within bouts [47–49]. Consistent with this observation, the authors demonstrate that perturbing KA cells can modulate the occurrence of swim bouts in larval fish. However, in contrast to the expected inhibitory role of GABA, KA cells instead appear to play an excitatory role because their activation enhances the production of swimming, while their silencing reduces it. GABA can play excitatory roles early in embryonic development, but these studies of zebrafish larvae are well past these early embryonic stages, so how this excitatory effect on swimming comes about is a mystery. Future work determining the context in which these neurons are active, as well as the nature and patterns of their connectivity, should resolve this mystery.
Outlook
The usefulness of any model system is its ability to establish principles that apply broadly, well beyond the model itself. For studies of neuronal network differentiation, assembly and function, the zebrafish is an extremely valuable model. To make strong links to other vertebrate systems, regardless of level of analysis, the key is to use genetic and/or anatomical homologies that unite vertebrates. Conserved genetic programs underlie much of the construction of the vertebrate nervous system, from spinal cord to cortex. In addition, zebrafish have simpler, yet homologous brain structures to other vertebrates, including mammals [50–52]. Studies in the future using zebrafish will hopefully throw some light on the neural mechanisms underlying more complex behaviors. If we are to do so, however, one key lesson from studies of zebrafish motor networks is that simply perturbing neurons, without being able to place perturbations in the context of the activity patterns and connectivity of neurons, can be more puzzling than informative. A rigorous approach to circuit/behavior busting requires detailed information about activity and connectivity to complement the elegant optogenetic approaches that now allow us to perturb discrete, identifiable neuronal classes with such precision.
Table 1.
Selected recent results from the zebrafish model. Column at the left shows images from the relevant papers. Row 1: Live Images of neurons in the neural tube during cell division from Fig. 1 in ref. 7. Yellow cell has adopted a neuronal fate. Apical side is at the left. Row 2: Horizontal view of pairs of neurons that arose from the same neuronal precursors, immunolabeled for the markers Chx10 (red) and Scl (green). From Fig. 2 in ref. 8. Row 3: Horizontal view of the brainstem motor pools (green) from a perturbation of Hox expression to explore the migration of motor pools. Red is 3A10 staining of neurons including the Mauthner cell (asterisk). From Fig. 3 in ref. 10. Row 4: Visualization of cerebellar structure in 5 day old zebrafish with antibodies to Parvalbumin7 and VGlut1. From Fig. 13 in ref. 16. Row 5: Recordings from two spinal interneurons in larval fish showing switches in activity from one to the other as swimming slows. From Fig. 7 in ref. 23. Row 6: Recordings form a spinal nerve (top) and an inhibitory interneuron showing the interneuron is activated during a struggling motor pattern. From Fig. 2 in ref. 26. Row 7: Missing escape tail bend after selective elimination of one class of spinal neuron. From Fig. 7 in ref. 34. Row 8: Left, Image of hypocretin neurons in a horizontal view of the brain (rostral at the top) along with the distribution of photons captured from the neurons, which are expressing GFP-Aequorin. From Fig. 5 in ref. 38.
| Discovery | Approach | Ref | |
|---|---|---|---|
 |
Neurons derive from the apical rather than the basal daughter cell in asymmetric cell divisions | Direct imaging of cell division in the intact nervous system | 7 |
 |
Pair producing progenitors can give rise to one excitatory and one inhibitory neuron, possibly achieving an initial excitatory and inhibitory balance in the CNS | Direct imaging of the generation of cell types in the intact spinal cord | 8 |
 |
Facial motoneurons migrate caudally, so the somata end up far away from the exit site of their motor axons. This puzzling movement may have implications for ideas of wiring minimization. | Imaging of migration of neurons in vivo | 10–12 |
 |
Even very young 4–5 day old zebrafish have the major cerebellar neuronal classes, offering a simple model used to examine the development and, perhaps eventually, the function of these networks. | Developmental studies of cerebellum | 14–19 |
 |
Increases in the speed of locomotion are accompanied by shifts in the neuronal networks, so that there is not one network for all speeds. Spinal neurons differentiate from those driving the fastest movements to those driving the slowest ones. | Functional imaging and physiology of spinal cord | 23,28 |
 |
Some spinal inhibitory neurons are shared by different motor behaviors while others are involved in just one, suggesting that the output of a core network is shaped by specialized neurons to produce different behaviors | Electrophysiology of spinal cord | 26 |
 |
The functional role of a spinal interneuron in generating behavior was conclusively demonstrated. | Enhancer trapping, imaging, physiology, and behavior | 34 |
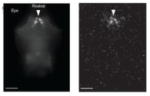 |
Two important approaches were demonstrated in zebrafish - one to monitor neuronal activity in freely swimming fish by using GFP-Aequorin and the other to excite and inhibit neurons with light-activated tools. | Using light to visualize and perturb neuronal activity. | 38,41–46 |
Acknowledgments
We are grateful to Shin-ichi Higashijima and Sandeep Kishore for comments on the manuscript. Work in the McLean Lab is supported by the Esther A. & Joseph Klingenstein Fund, the Searle Scholars Program, and the National Institute of Neurological Disorders and Stroke (R01NS067299). Work in the Fetcho Lab is supported by the National Institute of Neurological Disorders and Stroke (R01NS26539), a National Institutes of Health Pioneer Award (DP1OD006411) and the McKnight Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
* of special interest
** of outstanding interest
- 1.Miller G. Optogenetics. Shining new light on neural circuits. Science. 2006;314(5806):1674–1676. doi: 10.1126/science.314.5806.1674. [DOI] [PubMed] [Google Scholar]
- 2.Miesenbock G. The optogenetic catechism. Science. 2009;326(5951):395–399. doi: 10.1126/science.1174520. [DOI] [PubMed] [Google Scholar]
- 3.Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: Optical technologies for probing neural signals and systems. Nat Rev Neurosci. 2007;8(8):577–581. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
- 4.Friedrich RW, Jacobson GA, Zhu PX. Circuit neuroscience in zebrafish. Curr Biol. 2010;20(8):R371–R381. doi: 10.1016/j.cub.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 5.Baier H, Scott EK. Genetic and optical targeting of neural circuits and behavior--zebrafish in the spotlight. Curr Opin Neurobiol. 2009;19(5):553–560. doi: 10.1016/j.conb.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Portugues R, Engert F. The neural basis of visual behaviors in the larval zebrafish. Curr Opin Neurobiol. 2009;19(6):644–647. doi: 10.1016/j.conb.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *7.Alexandre P, Reugels AM, Barker D, Blanc E, Clarke JD. Neurons derive from the more apical daughter in asymmetric divisions in the zebrafish neural tube. Nat Neurosci. 2010;13(6):673–679. doi: 10.1038/nn.2547. This article examined a long-standing issue: once progenitor cells have undergone asymmetric cell division, which of the cells remains a progenitor and which one becomes a neuron? Only by performing time lapse imaging was it possible to demonstrate that the apical-most one of the two became a neuron, while the basal-most one remained a progenitor. Given the differences in apical versus basal signaling events, this distinction is quite important for understanding the basis of neuronal proliferation. [DOI] [PubMed] [Google Scholar]
- **8.Kimura Y, Satou C, Higashijima S. V2a and v2b neurons are generated by the final divisions of pair-producing progenitors in the zebrafish spinal cord. Development. 2008;135(18):3001–3005. doi: 10.1242/dev.024802. This study also takes advantage of time lapse imaging to demonstrate a remarkable phenomenon, that pair-producing progenitors in the spinal cord generate inhibitory and an excitatory neuron pairs. It is unclear if this will always be the case for every progenitor zone, but it certainly provides a simple, elegant means to balance the contribution of excitation and inhibition to network activity during development and also later on. [DOI] [PubMed] [Google Scholar]
- 9.Batista MF, Jacobstein J, Lewis KE. Zebrafish v2 cells develop into excitatory cid and notch signalling dependent inhibitory veld interneurons. Dev Biol. 2008;322(2):263–275. doi: 10.1016/j.ydbio.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Bingham SM, Sittaramane V, Mapp O, Patil S, Prince VE, Chandrasekhar A. Multiple mechanisms mediate motor neuron migration in the zebrafish hindbrain. Dev Neurobiol. 2010;70(2):87–99. doi: 10.1002/dneu.20761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohata S, Kinoshita S, Aoki R, Tanaka H, Wada H, Tsuruoka-Kinoshita S, Tsuboi T, Watabe S, Okamoto H. Neuroepithelial cells require fucosylated glycans to guide the migration of vagus motor neuron progenitors in the developing zebrafish hindbrain. Development. 2009;136(10):1653–1663. doi: 10.1242/dev.033290. [DOI] [PubMed] [Google Scholar]
- 12.Mapp OM, Wanner SJ, Rohrschneider MR, Prince VE. Prickle1b mediates interpretation of migratory cues during zebrafish facial branchiomotor neuron migration. Dev Dyn. 2010;239(6):1596–1608. doi: 10.1002/dvdy.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen BL, Hall DH, Chklovskii DB. Wiring optimization can relate neuronal structure and function. Proc Natl Acad Sci U S A. 2006;103(12):4723–4728. doi: 10.1073/pnas.0506806103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volkmann K, Rieger S, Babaryka A, Koster RW. The zebrafish cerebellar rhombic lip is spatially patterned in producing granule cell populations of different functional compartments. Dev Biol. 2008;313(1):167–180. doi: 10.1016/j.ydbio.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 15.Rieger S, Senghaas N, Walch A, Koster RW. Cadherin-2 controls directional chain migration of cerebellar granule neurons. PLoS Biol. 2009;7 (11):e1000240. doi: 10.1371/journal.pbio.1000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **16.Bae YK, Kani S, Shimizu T, Tanabe K, Nojima H, Kimura Y, Higashijima S, Hibi M. Anatomy of zebrafish cerebellum and screen for mutations affecting its development. Dev Biol. 2009;330(2):406–426. doi: 10.1016/j.ydbio.2009.04.013. This is a beautiful study that begins the systematic examination of cell classes in the zebrafish cerebellum. Remarkably, a layered organization is already apparent by day 5. In addition, it also identifies zebrafish mutants with defects in cerebellar organization. This work, along with Ref. 17, will provide a foundation for future studies of cerebellum development and function using zebrafish. [DOI] [PubMed] [Google Scholar]
- *17.Kani S, Bae YK, Shimizu T, Tanabe K, Satou C, Parsons MJ, Scott E, Higashijima S, Hibi M. Proneural gene-linked neurogenesis in zebrafish cerebellum. Dev Biol. 2010;343(1–2):1–17. doi: 10.1016/j.ydbio.2010.03.024. This study examines the genetic specification of cerebellar neurons in zebrafish, which appear to be conserved among vertebrates. In combination with Ref. 16, this work will allow for links to be made between studies in zebrafish and homologous cerebellar networks in other vertebrates, including mammals. [DOI] [PubMed] [Google Scholar]
- 18.Chaplin N, Tendeng C, Wingate RJ. Absence of an external germinal layer in zebrafish and shark reveals a distinct, anamniote ground plan of cerebellum development. J Neurosci. 2010;30(8):3048–3057. doi: 10.1523/JNEUROSCI.6201-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elsen GE, Choi LY, Prince VE, Ho RK. The autism susceptibility gene met regulates zebrafish cerebellar development and facial motor neuron migration. Dev Biol. 2009;335(1):78–92. doi: 10.1016/j.ydbio.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimura Y, Okamura Y, Higashijima S. Alx, a zebrafish homolog of chx10, marks ipsilateral descending excitatory interneurons that participate in the regulation of spinal locomotor circuits. J Neurosci. 2006;26(21):5684–5697. doi: 10.1523/JNEUROSCI.4993-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higashijima S, Masino MA, Mandel G, Fetcho JR. Engrailed-1 expression marks a primitive class of inhibitory spinal interneuron. J Neurosci. 2004;24 (25):5827–5839. doi: 10.1523/JNEUROSCI.5342-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suster ML, Kania A, Liao M, Asakawa K, Charron F, Kawakami K, Drapeau P. A novel conserved evx1 enhancer links spinal interneuron morphology and cis-regulation from fish to mammals. Dev Biol. 2009;325(2):422–433. doi: 10.1016/j.ydbio.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 23.McLean DL, Masino MA, Koh IY, Lindquist WB, Fetcho JR. Continuous shifts in the active set of spinal interneurons during changes in locomotor speed. Nat Neurosci. 2008;11(12):1419–1429. doi: 10.1038/nn.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crone SA, Zhong G, Harris-Warrick R, Sharma K. In mice lacking v2a interneurons, gait depends on speed of locomotion. J Neurosci. 2009;29 (21):7098–7109. doi: 10.1523/JNEUROSCI.1206-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao JC, Fetcho JR. Shared versus specialized glycinergic spinal interneurons in axial motor circuits of larval zebrafish. J Neurosci. 2008;28 (48):12982–12992. doi: 10.1523/JNEUROSCI.3330-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berkowitz A, Roberts A, Soffe SR. Roles for multifunctional and specialized spinal interneurons during motor pattern generation in tadpoles, zebrafish larvae, and turtles. Front Behav Neurosci. 4:36. doi: 10.3389/fnbeh.2010.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caron SJ, Prober D, Choy M, Schier AF. In vivo birthdating by baptism reveals that trigeminal sensory neuron diversity depends on early neurogenesis. Development. 2008;135(19):3259–3269. doi: 10.1242/dev.023200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLean DL, Fetcho JR. Spinal interneurons differentiate sequentially from those driving the fastest swimming movements in larval zebrafish to those driving the slowest ones. J Neurosci. 2009;29:13566–13577. doi: 10.1523/JNEUROSCI.3277-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLean DL, Fan J, Higashijima S, Hale ME, Fetcho JR. A topographic map of recruitment in spinal cord. Nature. 2007;446(7131):71–75. doi: 10.1038/nature05588. [DOI] [PubMed] [Google Scholar]
- 30.Asakawa K, Suster ML, Mizusawa K, Nagayoshi S, Kotani T, Urasaki A, Kishimoto Y, Hibi M, Kawakami K. Genetic dissection of neural circuits by tol2 transposon-mediated gal4 gene and enhancer trapping in zebrafish. Proc Natl Acad Sci U S A. 2008;105(4):1255–1260. doi: 10.1073/pnas.0704963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *31.Koide T, Miyasaka N, Morimoto K, Asakawa K, Urasaki A, Kawakami K, Yoshihara Y. Olfactory neural circuitry for attraction to amino acids revealed by transposon-mediated gene trap approach in zebrafish. Proc Natl Acad Sci U S A. 2009;106(24):9884–9889. doi: 10.1073/pnas.0900470106. This innovative study used gene-trapping technology to identify and subsequently perturb discrete olfactory circuits in zebrafish that are involved attractive behavioral responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott EK, Mason L, Arrenberg AB, Ziv L, Gosse NJ, Xiao T, Chi NC, Asakawa K, Kawakami K, Baier H. Targeting neural circuitry in zebrafish using gal4 enhancer trapping. Nat Methods. 2007;4(4):323–326. doi: 10.1038/nmeth1033. [DOI] [PubMed] [Google Scholar]
- 33.Zhu P, Narita Y, Bundschuh ST, Fajardo O, Scharer YP, Chattopadhyaya B, Bouldoires EA, Stepien AE, Deisseroth K, Arber S, Sprengel R, et al. Optogenetic dissection of neuronal circuits in zebrafish using viral gene transfer and the tet system. Front Neural Circuits. 2009;3:21. doi: 10.3389/neuro.04.021.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **34.Satou C, Kimura Y, Kohashi T, Horikawa K, Takeda H, Oda Y, Higashijima S. Functional role of a specialized class of spinal commissural inhibitory neurons during fast escapes in zebrafish. J Neurosci. 2009;29(21):6780–6793. doi: 10.1523/JNEUROSCI.0801-09.2009. This is a technical tour-de-force that uses much of the zebrafish toolbox. The authors use transgenic animals, electrophysiology, calcium and confocal imaging, laser ablations and high-speed cinematography to study a single class of spinal interneuron. It is difficult to imagine a more comprehensive or convincing demonstration of the function of a neuronal class in vertebrates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orger MB, Kampff AR, Severi KE, Bollmann JH, Engert F. Control of visually guided behavior by distinct populations of spinal projection neurons. Nat Neurosci. 2008;11(3):327–333. doi: 10.1038/nn2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kohashi T, Oda Y. Initiation of mauthner- or non-mauthner-mediated fast escape evoked by different modes of sensory input. J Neurosci. 2008;28(42):10641–10653. doi: 10.1523/JNEUROSCI.1435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sankrithi NS, O’Malley DM. Activation of a multisensory, multifunctional nucleus in the zebrafish midbrain during diverse locomotor behaviors. Neuroscience. 2010;166(3):970–993. doi: 10.1016/j.neuroscience.2010.01.003. [DOI] [PubMed] [Google Scholar]
- **38.Naumann EA, Kampff AR, Prober DA, Schier AF, Engert F. Monitoring neural activity with bioluminescence during natural behavior. Nat Neurosci. 2010;13(4):513–520. doi: 10.1038/nn.2518. A very exciting and technically highly innovative study that demonstrated the feasibility of using GFP-Aeqorin to monitor neuronal activity in freely moving fish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prober DA, Rihel J, Onah AA, Sung RJ, Schier AF. Hypocretin/orexin overexpression induces an insomnia-like phenotype in zebrafish. J Neurosci. 2006;26(51):13400–13410. doi: 10.1523/JNEUROSCI.4332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Appelbaum L, Wang GX, Maro GS, Mori R, Tovin A, Marin W, Yokogawa T, Kawakami K, Smith SJ, Gothilf Y, Mignot E, et al. Sleep-wake regulation and hypocretin-melatonin interaction in zebrafish. Proc Natl Acad Sci U S A. 2009;106(51):21942–21947. doi: 10.1073/pnas.906637106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Douglass AD, Kraves S, Deisseroth K, Schier AF, Engert F. Escape behavior elicited by single, channelrhodopsin-2-evoked spikes in zebrafish somatosensory neurons. Curr Biol. 2008;18(15):1133–1137. doi: 10.1016/j.cub.2008.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szobota S, Gorostiza P, Del Bene F, Wyart C, Fortin DL, Kolstad KD, Tulyathan O, Volgraf M, Numano R, Aaron HL, Scott EK, et al. Remote control of neuronal activity with a light-gated glutamate receptor. Neuron. 2007;54(4):535–545. doi: 10.1016/j.neuron.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 43.Arrenberg AB, Del Bene F, Baier H. Optical control of zebrafish behavior with halorhodopsin. Proc Natl Acad Sci U S A. 2009;106(42):17968–17973. doi: 10.1073/pnas.0906252106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *44.Schoonheim PJ, Arrenberg AB, Del Bene F, Baier H. Optogenetic localization and genetic perturbation of saccade-generating neurons in zebrafish. J Neurosci. 2010;30(20):7111–7120. doi: 10.1523/JNEUROSCI.5193-09.2010. This study is an early use of optogenetic approaches to activate and silence neurons in order to elucidate the broad organization of zebrafish saccadic eye movement circuitry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Janovjak H, Szobota S, Wyart C, Trauner D, Isacoff EY. A light-gated, potassium-selective glutamate receptor for the optical inhibition of neuronal firing. Nat Neurosci. 2010;13:1027–1032. doi: 10.1038/nn.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wyart C, Del Bene F, Warp E, Scott EK, Trauner D, Baier H, Isacoff EY. Optogenetic dissection of a behavioural module in the vertebrate spinal cord. Nature. 2009;461(7262):407–410. doi: 10.1038/nature08323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brustein E, Drapeau P. Serotoninergic modulation of chloride homeostasis during maturation of the locomotor network in zebrafish. J Neurosci. 2005;25 (46):10607–10616. doi: 10.1523/JNEUROSCI.2017-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thirumalai V, Cline HT. Endogenous dopamine suppresses initiation of swimming in prefeeding zebrafish larvae. J Neurophysiol. 2008;100(3):1635–1648. doi: 10.1152/jn.90568.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *49.Burgess HA, Schoch H, Granato M. Distinct retinal pathways drive spatial orientation behaviors in zebrafish navigation. Curr Biol. 2010;20(4):381–386. doi: 10.1016/j.cub.2010.01.022. This work describes phototaxis in larval zebrafish and investigates the neural mechanisms underlying the behavior using mutants and laser ablations. Larvae turn away from darkness (a behavior mediated by the OFF pathway) and once oriented swim more frequently toward the light (ON pathway). One of the interesting findings from this study is that serotonin is involved in the increase in swimming bout occurrence characterized once the larva is properly oriented. This increase was specific to only larvae facing the target light, meaning it is likely released in a context dependent manner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amo R, Aizawa H, Takahoko M, Kobayashi M, Takahashi R, Aoki T, Okamoto H. Identification of the zebrafish ventral habenula as a homolog of the mammalian lateral habenula. J Neurosci. 2010;30(4):1566–1574. doi: 10.1523/JNEUROSCI.3690-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lillesaar C, Stigloher C, Tannhauser B, Wullimann MF, Bally-Cuif L. Axonal projections originating from raphe serotonergic neurons in the developing and adult zebrafish, danio rerio, using transgenics to visualize raphe-specific pet1 expression. J Comp Neurol. 2009;512(2):158–182. doi: 10.1002/cne.21887. [DOI] [PubMed] [Google Scholar]
- 52.Mueller T, Wullimann MF, Guo S. Early teleostean basal ganglia development visualized by zebrafish dlx2a, lhx6, lhx7, tbr2 (eomesa), and gad67 gene expression. J Comp Neurol. 2008;507(2):1245–1257. doi: 10.1002/cne.21604. [DOI] [PubMed] [Google Scholar]


