Abstract
Glucocorticoids influence organ functions through the glucocorticoid receptor, a protein acetylated and deacetylated by several histone acetyltransferases and deacetylases. We reported that the circadian rhythm-related transcription factor “Clock”, a key component of the biological CLOCK with inherent histone acetyltransferase activity, acetylates glucocorticoid receptor lysines within its hinge region -a “lysine cluster” containing a KXKK motif- and represses its transcriptional activity. This Clock-induced repression of the glucocorticoid receptor activity is inversely phased to the diurnally circulating glucocorticoids and may act as a local counter regulatory mechanism to the actions of these hormones. Importantly, uncoupling of the central CLOCK-regulated hypothalamic-pituitary-adrenal-axis and peripheral CLOCK-mediated alterations of glucocorticoid action, such as chronic stress and frequent trans-time zone travel or night-shift work, may cause functional hypercortisolism and contribute to various pathologies. Thus, acetylation-mediated epigenetic regulation of the glucocorticoid receptor may be essential for the maintenance of proper time-integrated glucocorticoid action, significantly influencing human well-being and longevity.
Keywords: acetylation, circadian rhythm, Clock, histone acetyltransferase (HAT), histone deacetylase (HDAC), hypothalamic-pituitary-adrenal (HPA) axis, Sirt1
1. Introduction
Mammalian organisms are influenced by unforeseen changes in the environment called “stressors”, and thus, have developed a highly sophisticated and conserved system, the Stress System, to help deal with them. This system is composed of the hypothalamic-pituitary-adrenal (HPA) axis and the locus caeruleus/norepinephrine-autonomic nervous systems (Chrousos, 1995; Miller and O'Callaghan, 2002; Sheridan, 2003; Chrousos, 2009; Chrousos, 2010). The HPA axis consists of the parvicellular corticotropin-releasing hormone (CRH)- and arginine vasopressin (AVP)-secreting neurons located in the hypothalamic paraventricular nucleus (PVN), the corticotrophs of the pituitary gland, and the adrenal gland cortices (Elenkov et al., 2000; Chrousos, 2009; Kudielka and Wust, 2010). The PVN neurons release CRH and AVP into the hypophyseal portal system located under the median eminence of the hypothalamus in response to signals from higher brain regulatory centers. Secreted CRH and AVP reach the pituitary gland and synergistically stimulate the secretion of adrenocorticotropic hormone (ACTH) (Bao et al., 2008; Chrousos, 2009; Aguilera, 2010). ACTH released into the systemic circulation finally stimulates production and secretion of glucocorticoids from the cortex of the adrenal glands (Chrousos, 1995). Secreted glucocorticoids in turn suppress higher regulatory centers, the PVN and the pituitary gland, forming a closed negative feedback loop that aims to reset the activated HPA axis and restore its homeostasis (Chrousos, 1995; Arafah, 2006; Chrousos, 2009; Aguilera, 2010).
The stress-responsive HPA axis is essential for survival in mammals and has strong and diverse actions on every aspect of their physiology (Kino and Chrousos, 2005; Aguilera, 2010; Chrousos, 2010). Indeed, its end-effector molecules, the glucocorticoids, are necessary for proper functioning of virtually all organs and tissues, including the central nervous system (CNS), and the respiratory, cardiovascular, immune, and musculoskeletal systems (Eskandari and Sternberg, 2002; Kino and Chrousos, 2005). Upon exposure to stress, glucocorticoids secreted into the systemic circulation in large amounts dramatically alter physiology, influencing behavior, shifting intermediary metabolism towards catabolism and modulating immune function (Chrousos, 1995; Chrousos, 2001; Kino and Chrousos, 2005; Kyrou and Tsigos, 2009).
The activity of the Stress System is diurnally linked to the rotation of the planet and appropriately connected with the daily activity/rest of the organism and circulating glucocorticoid levels, ie. cortisol in humans and corticosterone in rodents, are under the strong circadian influence of the suprachiasmatic nucleus (SCN) of the hypothalamus (Chrousos, 1995; Nader et al., 2010). In humans, the cortisol diurnal zenith is reached in the early morning and the nadir at midnight, with the purpose of helping adjust the body’s activities to the regular periodicity of day/night changes. The time-integrated daily secretion of glucocorticoids is tightly regulated; indeed, the circadian, negative feedback and stress-related activities of the HPA axis are integrated “rheostatically” by higher brain centers (Chrousos, 1995; Nader et al., 2010).
The overall sensitivity of tissues to glucocorticoids, on the other hand, is also regulated by various physiologic and pathologic processes (Kino et al., 2003; Chrousos and Kino, 2005). For example, glucocorticoid action in cells is specifically adjusted during the different phases of the cell cycle (Cidlowski and Michaels, 1977; Abel et al., 2002), while adrenomedullary cells exposed to very high concentrations of glucocorticoids directly diffusing from the adjacent adrenal cortex, are resistant to these hormones in a gene-specific fashion (Wurtman, 2002; Ehrhart-Bornstein and Bornstein, 2008). In addition, several autoimmune/allergic/inflammatory disorders, the metabolic syndrome, septic conditions and even infection with the human immunodeficiency virus type-1, have been associated with alterations in the responsiveness of specific organs and tissues to glucocorticoids (Kino et al., 2003; Webster et al., 2004; Chrousos and Kino, 2005; Chrousos and Kino, 2007). Underlying mechanisms(s) for the alterations of local glucocorticoid actions, however, have not been fully elucidated as yet.
2. Glucocorticoid Receptor (GR) and Regulation of its Activities
The diverse actions of glucocorticoids at their target tissues are mediated by a single intracellular protein molecule the glucocorticoid receptor (GR) (Chrousos, 1995; Nicolaides et al., 2010) (Figure 1). This receptor, also known as the “nuclear receptor superfamily 3, group C, member 1 (NR3C1)”, is expressed virtually in all organs and tissues of the human body, and belongs to the steroid/sterol/thyroid/retinoid/orphan nuclear receptor superfamily, which consists of 48 members in humans (O'Malley, 1990; Kino et al., 2003; Chrousos and Kino, 2005). The human GR gene, located in the short arm of chromosome 5 (5q31.3), is composed of 9 exons, and encodes two protein molecules GRα and GRβ through alternative use of specific exons 9α and 9β (Kino et al., 2009; van der Vaart and Schaaf, 2009) (Figure 1). GRα is the ubiquitously expressed classic receptor that binds to and mediates most of the known actions of glucocorticoids, while GRβ , although also expressed widely, does not bind glucocorticoids and its physiologic actions have not been fully elucidated as yet (Kino et al., 2009). It recently became evident that the GRα variant mRNA is translated from at least 8 initiation sites into multiple amino terminal GRα isoforms, termed A through D (A, B, C1-C3 and D1-D3), with distinct specific transcriptional activities on glucocorticoid-responsive genes (Lu and Cidlowski, 2005).
Figure 1. Genomic and complementary DNA, and protein structures of the human GR, functional domains distribution of GRα, and the GR isoforms produced through alternative splicing.
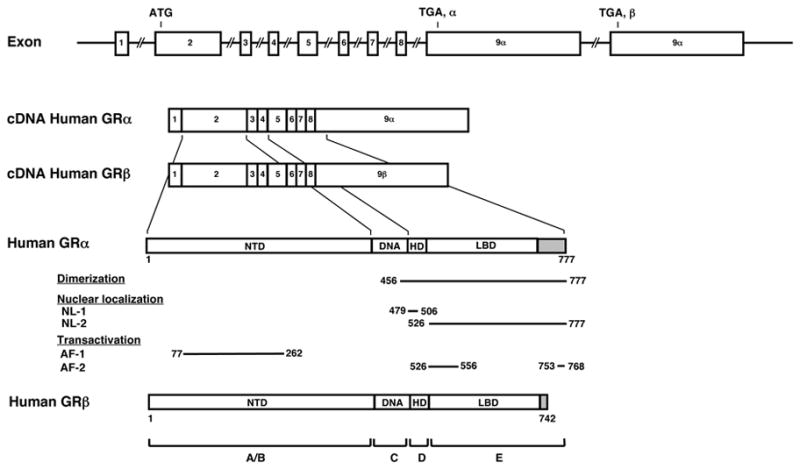
The human GR gene consists of 10 exons. Exon 1 is untranslated region, exon 2 codes for the immunogenic domain (A/B), exon 3 and 4 for the DNA-binding domain (C), and exons 5-9 for the hinge region (D) and the ligand-binding domain (E). GR does not contain an F region, in contrast to the other steroid hormone receptors. The human GR gene contains two terminal exons 9 (exon 9α and 9β ) alternatively spliced to produce the classic GRα and the nonligand-binding GRβ . C-terminal gray colored domains in GRα and GRβ show their specific portions. Locations of several functional domains are also indicated.
AF-1 and -2: activation function 1 and 2; DBD; DNA-binding domain; HD: hinge region; LBD: Ligand-binding domain; NTD: N-terminal region, NL1 and 2: Nuclear translocation signal 1 and 2.
The human GRα consists of 777 amino acids and has 3 major distinct functional domains, the N-terminal or immunogenic (NTD), the DNA-binding (DBD) and the ligand-binding (LBD) domains (Hollenberg et al., 1985; Kino and Chrousos, 2004) (Figure 1). GRα has also a hinge region (HD), located between the DBD and LBD and spanning amino acids 481 to 520 (Nader et al., 2009). GRα is located primarily in the cytoplasm in the absence of glucocorticoid ligand, as part of hetero-oligomeric complexes containing heat shock proteins (HSPs) 90, 70, 50, 20 and, possibly, other proteins as well (Pratt, 1993; Hager, 2002; Revollo and Cidlowski, 2009). After binding to its agonist ligand, GRα undergoes conformational changes, dissociates from the heat shock proteins, homo- or hetero-dimerizes, and translocates as a dimer and/or monomer into the nucleus through the nuclear pore, via an active ATP-dependent process mediated by its nuclear localization signals (NL)-1 and -2 (Savory et al., 1999). NL-1 is located in the junction of DBD and the hinge region, while NL-2 spans the entire LBD (Savory et al., 1999) (Figure 1).
Inside the nucleus, the ligand-activated GRα directly interacts as a homo- or hetero-dimer with specific DNA sequences, the glucocorticoid response elements (GREs), in the promoter regions of target genes, (Beato et al., 1989; Kino et al., 2003; Chrousos and Kino, 2005; So et al., 2007). GR contains two transactivation domains, activation function (AF)-1 and -2, located at its NTD and LBD, respectively (Figure 1), through which it interacts with many proteins and protein complexes, such as the nuclear receptor coactivator [p160, p300/CREB-binding protein (CBP) and p300/CBP-associated factor (p/CAF)] complexes and the SWI/SNF and vitamin D receptor-interacting protein/thyroid hormone receptor-associated protein (DRIP/TRAP) chromatin-remodeling complexes, eventually influencing the activity of the RNA polymerase II and its ancillary factors, modulating the transcription rates of glucocorticoid-responsive genes (McKenna and O'Malley, 2002; Kino and Chrousos, 2004; Chrousos and Kino, 2005; Rosenfeld et al., 2006).
In addition to transactivation or transrepression of the glucocorticoid-responsive genes explained above, GRα mutually modulates other signal transduction cascades through protein-protein interaction with specific transcription factors, by influencing the ability of these factors to stimulate or inhibit the transcription rates of their respective target genes (Barnes, 1998; Chrousos and Kino, 2005; Kino and Chrousos, 2005). This activity may be more important than the GRE-mediated one, granted that mice harboring a mutant GRα, which is active in terms of protein-protein interactions but inactive in terms of dimerization and transactivation via DNA GREs, survive and procreate, in contrast to mice with a deletion of the entire GR gene that die immediately after birth from severe respiratory distress syndrome (Cole et al., 1995; Reichardt et al., 1998).
3. Acetylation of GR
In addition to co-regulators and other transcription factors that modulate GR-induced transcriptional activity, several distinct signaling pathways influence the transcriptional activity of the GR via post-translational modifications of the receptor protein (Chrousos and Kino, 2005). These include methylation, nitrosylation, sumoylation and ubiquitination, and phosphorylation, the last of which has been studied best. Indeed, several kinases, such as the cell-cycle-related kinases, mitogen-activated kinases (MAPKs) and the glycogen synthase kinases, phosphorylate specific serine or threonine residues of the GR, while energy sensing AMP-activated protein kinase indirectly phosphorylates GR through activation of p38 MAPK (Rogatsky et al., 1998; Itoh et al., 2002; Wang and Garabedian, 2003; Ismaili and Garabedian, 2004; Szatmary et al., 2004; Miller et al., 2005; Kino, 2007; Kino et al., 2007; Kino et al., 2010; Nader et al., 2010).
GR is also acetylated and this epigenetic modulation of the GR has important regulatory roles on the biologic actions of glucocorticoids in target tissues. Indeed, acetylation is a general epigenetic modulation that controls activity of various cytoplasmic as well as nuclear proteins (Minucci and Pelicci, 2006). Further, acetylation of nuclear proteins, including histones, plays important roles in the regulation of gene expression and subsequent alteration of biologic activities of organisms. For example, acetylation of the N-terminal tail of chromatin-associated histones by HAT coactivators is essential for initiating transcription by exposing the promoter DNA to transcription factors, other chromatin-modifying molecules and the RNA polymerase II complex (An, 2007). HAT coactivators also acetylate various other molecules, such as the coactivators themselves, other transcription intermediate components, several transcription factors, including nuclear receptors and chaperone molecules like HSP90 (Kovacs et al., 2005; Murphy et al., 2005; Minucci and Pelicci, 2006).
The human GR was first shown to be acetylated at lysines 494 and 495 located in its hinge region, while it was deacetylated by histone deacetylase 2 (HDAC2) (Ito et al., 2006) (Figure 2A). The acetylation of GR was demonstrated by employing an anti-acetylated lysine-specific antibody and the GR mutants defective in specific acetylation sites, but was not confirmed by methods directly detecting acetylated residues of the GR protein, such as the mass-spectrometry. Deacetylation of GR by this HDAC was required for efficient transrepression of nuclear factor of κ B (NF-κ B)-induced transcriptional activity by the GR (Ito et al., 2006). These findings indicate that acetylation of the GR at these lysine residues attenuates the repressive effect of this nuclear receptor on NF-κ B. These lysine residues of the GR are in the a common acetylation motif KXKK, where K is lysine and X is any amino acid (Figure 2A). This motif is shared by other steroid hormone receptors, the mineralocorticoid (MR), androgen (AR) and progesterone (PR) receptors, but not with the estrogen receptors (ER) α and β (Faus and Haendler, 2006), suggesting that acetylation is a general and conserved regulatory mechanism for several steroid hormone receptors. Indeed, the human androgen receptor is acetylated at lysines 632 and 633 located in the KXKK motif, while p300, p/CAF and the Tat-interacting protein 60 (Tip60) HAT coactivators are responsible for the acetylation of these residues (Fu et al., 2000; Gaughan et al., 2002). PR is also acetylated at lysines located in the KXKK motif (Gaviglio et al., 2010), whereas acetylation of the MR has not been shown as yet. Interestingly, the human ERα is acetylated by p300 at lysines 266, 268, 302 and 303, and this posttranslational modification increases binding to estrogen response elements (Faus and Haendler, 2006; Kim et al., 2006). Acetylation of AR can be inhibited by addition of the class I/II HDAC inhibitor tricostatin-A (TSA), while p300-mediated acetylation of ERα is inhibited by TSA and the class III HDAC inhibitor nicotinamide, suggesting that AR and ERα are deacetylated by these HDACs, including HDAC1/2 and the class III HDAC Sirt1 (Fu et al., 2000; Kim et al., 2006). Recently, AR was shown to interact directly with Sirt1, and this HDAC efficiently deacetylated this receptor (Fu et al., 2006).
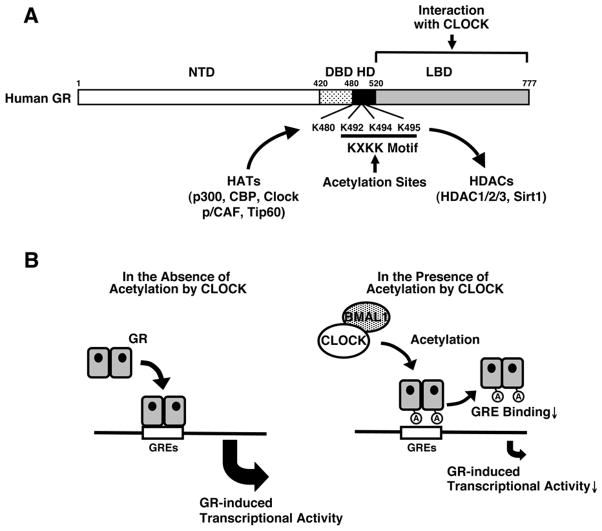
Figure 2. Acetylation sites of the GR and regulation of GR transcriptional activity by Clock.
A: Multiple acetylation sites in the human GR and possible acetylases and deacetylases for the GR.
The human GR has 4 acetylation sites in its hinge region; lysines, 480, 492, 494 and 495. These lysine residues may be acetylated by several HATs, such as p300, CBP, Clock, p/CAF and Tip60, while they are several potential deacetylates including class I to III HDACs, such as HDCA1, 2, 3 and Sirt1. Clock physically interacts with GR LBD through the domain enclosed in its C-terminal part and acetylates GR at all lysine residues located in a lysine cluster of the hinge region.
Modified from (Nader et al., 2009).
B: A heuristic model of the physiologic implications of this study.
Clock/Bmal1 acetylates GR via its intrinsic HAT activity through physical interaction with GR LBD, reduces affinity of GR to its cognate DNA GREs and ultimately suppresses GR-induced transcriptional activity.
Modified from (Nader et al., 2009).
A: acetylation, Bmal1: brain-muscle-arnt-like protein 1, CBP: CRE-binding protein-binding protein, Clock: circadian locomotor output cycle kaput, DBD: DNA-binding domain, GR: glucocorticoid receptor, GRE: glucocorticoid response element, HAT: histone acetyltransferase, HDAC: histone deacetylase, HD: hinge region, LBD: ligand-binding domain, NTD: N-terminal domain, p/CAF: p300/CBP-associated factor, Tip60: Tat-interacting protein 60
Temperature-activating factor (TAF)-Iβ and pp32, which are components of the inhibitor of histone acetyltransferases (INHAT), together with the other component TAF-Iα, also antagonize p300-mediated acetylation of ERα (Seo et al., 2001; Loven et al., 2004). Since GR can directly interact with p300 and TAF-Iβ , and is sensitive to TSA and Sirt1 (Ichijo et al., 2005; Amat et al., 2007; Ichijo et al., 2008), it is likely that GR is also acetylated by HAT coactivators, including p300, and deacetylated by class I/II HDACs and/or Sirt1 (Figure 2A). Acetylation of GR by HATs might also be influenced further by the INHAT components.
4. CLOCK-mediated Acetylation of GR: Implications to Circadian Rhythm-mediated Regulation of Glucocorticoid Action in Target Tissues
We recently found that the Clock transcription factor acetylated GR at its multiple lysine cluster in the hinge region, which includes lysines 494 and 495 located in the KXKK motif, and repressed GR-induced transcription of several glucocorticoid-responsive genes, by employing several GR mutants defective in acetylation sites and the anti-acetylated lysine-specific antibody (Nader et al., 2009). Clock appears to acetylate GR in the nucleus, after this receptor has bound glucocorticoids in the cytoplasm and has translocated into the nucleus. Clock, the “circadian locomotor output cycle kaput”, and its heterodimer partner “brain-muscle-arnt-like protein 1” (Bmal1) belong to the basic helix-loop-helix (bHLH)-PER-ARNT-SIM (PAS) superfamily of transcription factors, and play an essential role in the generation of the circadian oscillation rhythm of the CLOCK system that functions as an internal circadian time keeper (Takahashi et al., 2008). The Clock/Bmal1 heterodimer binds the E-box response elements located in the promoter region and stimulates the transcription of other essential clock genes, such as Periods (Per1, Per2 and Per3) and Cryptochromes (Cry1 and Cry2). Accumulated proteins Pers and Crys then form a complex with caseine kinase 1ε and δ, are phosphorylated, translocate into the nucleus and repress the transcriptional activity of the Clock/Bmal1 heterodimer by inhibiting its binding to the E-box response elements located in their own promoters, ultimately forming a negative feedback transcriptional loop that maintains an oscillation of their gene expression approximately every 24 hours (Kiyohara et al., 2006; Kondratov et al., 2006; Takahashi et al., 2008; Nader et al., 2010). In addition to the regulation of this principal transcriptional loop, Clock/Bmal1 stimulates expression of other CLOCK-related proteins, such as Rev-erbα, retinoic acid receptor-related orphan receptor α (RORα), Dec1, Dec2 and albumin gene D site-binding protein (Dbp), which form an auxiliary loop that stabilizes the main regulatory loop composed of Clock/Bmal1, Pers and Crys. Importantly, the transcription factors of the main and auxiliary loops control numerous “downstream” clock-responsive genes and influence a variety of biological activities (Ko and Takahashi, 2006; Ripperger and Schibler, 2006; Takahashi et al., 2008; Nader et al., 2010). The CLOCK transcription factor system located in the SCN of the brain hypothalamus, acts as the “master” oscillator and generator of the body’s circadian rhythm under the strong influence of the light/dark input from the eyes (Takahashi et al., 2008). The peripheral CLOCK system distributed in all organs and tissues, including the CNS outside the SCN, acts generally as a “slave” CLOCK under the regulation of the central SCN CLOCK by as yet unknown mechanisms; both neuronal and humoral connections have been implicated (Takahashi et al., 2008; Nader et al., 2010).
Clock physically interacts with the GR LBD at its nuclear receptor-interacting domain (NRID) located in its middle portion, and acetylates human GR at amino acid 480, 492, 494 and 495 (Nader et al., 2009; Nader et al., 2010) (Figure 2A). Acetylation of GR attenuates binding of the receptor to GREs, and hence, represses GR-induced transactivation of GRE-driven promoters (Nader et al., 2009) (Figure 2B). The lysine residues acetylated by Clock are positioned in the C-terminal extension (CTE) located in the N-terminal portion of GR NTD, which is known to play a role in DNA recognition by steroid hormone receptors (Melvin et al., 2004). Thus, it is likely that acetylation of these residues reduces binding of GR to GREs in part by altering the action of CTE. The portion of the hinge region acetylated by Clock overlaps with NL-1 that spans the C-terminus of DBD to the hinge region and plays an essential role in the cytoplasmic to nuclear translocation of the receptor (Savory et al., 1999; Nader et al., 2009). Therefore, it is also possible that acetylation of GR alters nuclear translocation of this receptor. In addition to CTE and NL-1, the part having the KXKK acetylation motif in the AR hinge region suppresses the N/C interaction, which plays an important role in AR- and MR-induced transcriptional activity (Thompson et al., 2001; He et al., 2002; Rogerson and Fuller, 2003; Pippal et al., 2009). This portion of AR also has suppressive effect on p160 coactivator-mediated potentiation of AR transcriptional activity (Haelens et al., 2007). Thus, it appears that Clock-mediated acetylation may reduce GR transcriptional activity via multiple different modes of actions.
Phosphorylation of the N-terminal tail (serine residues) of histone H3 is a prerequisite for acetylation of the lysine residues of this molecule, indicating a functional link between phosphorylation and acetylation on the same target molecule (Cheung et al., 2000; Thomson et al., 2001; Clayton and Mahadevan, 2003). This concerted action of two major chemical modifications, which may represent molecular inputs from various extracellular and intracellular pathways, has been called “phosphoacetylation”. As the GR is sensitive to both phosphorylation and acetylation, it is possible that the former influences Clock-mediated acetylation and hence GR activity. This may provide another level of interaction between biological pathways that activate GR-targeting kinases, such as cell cycle-regulating kinases, p38 MAPK and AMPK, and the CLOCK-mediated circadian system, ultimately influencing the activity of glucocorticoid-responsive genes at target tissues in a complex fashion.
Circulating glucocorticoids fluctuate naturally in a circadian fashion, reaching their zenith in the early morning and their nadir in the late evening in diurnal animals, including humans (Chrousos, 1995; Chrousos, 2001). The light-activated central master CLOCK located in the SCN orchestrates this daily rhythmic release of glucocorticoids by influencing the central activity of the HPA axis through efferent connections from the SCN to the CRH/AVP-containing neurons of the PVN (Ishida et al., 2005; Kalsbeek et al., 2006; Bao et al., 2008). This central CLOCK-mediated diurnal regulation of circulating glucocorticoids is extremely important for adjusting the body’s daily activities. In addition to this central regulation of the HPA axis for glucocorticoid secretion by the adrenal glands, the central CLOCK system modulates glucocorticoid release from the adrenal cortex by altering its sensitivity to ACTH neurally through SCN-mediated activation of the autonomic nervous system (Ishida et al., 2005; Kalsbeek et al., 2006; Oster et al., 2006; Ulrich-Lai et al., 2006) (Figure 3). Since the circadian rhythm of the peripheral CLOCK system is synchronized to that of the central CLOCK and acetylation of GR by the peripheral CLOCK represses GR-induced transcriptional activity, acetylation-mediated regulation of GR transcriptional activity by Clock appears to function as a local counter regulatory mechanism to diurnally oscillating circulating glucocorticoids (Nader et al., 2010) (Figure 3).
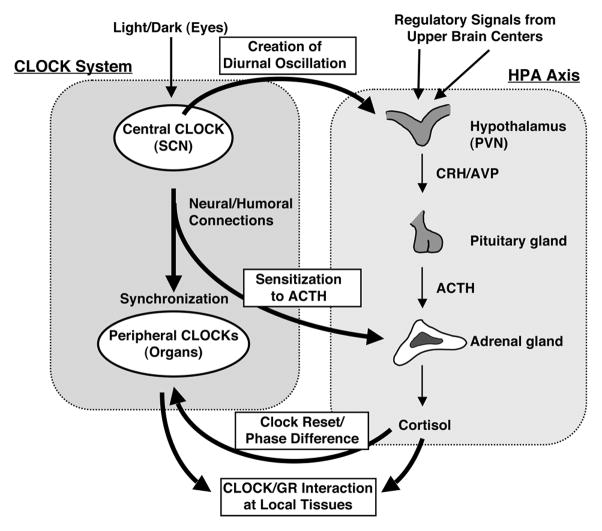
Figure 3. The circadian CLOCK system and the HPA axis influence each other’s activity at multiple levels.
The central CLOCK under the regulation of the light input controls and overrides the HPA axis and produces regular diurnal secretion of glucocorticoid hormones from the adrenal glands, while the peripheral CLOCKs, which are located in the adrenal glands and other components of the HPA axis and are regulated by the central CLOCK through neural and humoral pathways, also contribute to the rhythmic glucocorticoid secretion from these organs. Secreted glucocorticoids in turn reset and phase-delay circadian rhythm of the peripheral CLOCKs by stimulating the expression of several CLOCK-related genes. The peripheral CLOCKs also regulate glucocorticoid effect in local tissues through interaction between Clock/Bmal1 and GR, providing a local counter regulatory feedback loop to the effect of central CLOCK on the HPA axis.
ACTH: adrenocorticotropic hormone, AVP: arginine vasopressin, CRH: corticotropin-releasing hormone, PVN: paraventricular nucleus, SCN: Suprachiasmatic nucleus. Modified from (Nader et al., 2010).
Dysregulation as well as uncoupling of the cortisol circadian rhythm under the influence of the SCN CLOCK system, and acetylation-mediated circadian changes in local tissue sensitivity to glucocorticoids by the peripheral CLOCK, may lead to functional hypercortisolism in target tissues, which could be associated with development of pathologic conditions (Nader et al., 2010). For example, rotating shift workers, whose circadian system is repetitively reset by night-time activity/day-time sleep, and persons exposed to frequent jet lag due to frequent travels over time zones, are at an increased risk for cardiometabolic disease and stroke, as well as early mortality that might result from such time-integrated increased target tissue exposure to glucocorticoids (Ekstrand et al., 1996; Davidson et al., 2006; Scheer et al., 2009; Harrington). In experimental jet lag in mice, the HPA axis plays a critical role in adjusting the behavioral activity of these animals against let lag, while the peripheral CLOCK in the adrenal glands is important for resetting diurnal changes of circulating corticosterone secreted from these organs (Kiessling et al., 2010). Accordingly, chemical blocking of glucocorticoid secretion from the adrenal glands could either accelerate or prolong jet lag, depending on the timing of treatment (Kiessling et al., 2010). Thus, the aceylation-mediated negative regulation of GR transcriptional activity by the peripheral circadian CLOCK systems, including that of the adrenal glands, together with circadian regulation of CRH/AVP/ACTH/circulating glucocorticoids by the SCN, appear to play significant roles in physical as well as mental activities of humans, and dysregulation in any of these components could lead to development of various behavioral and/or somatic disorders.
5. Concluding Remarks
Acetylation of the GR appears to be fundamental for the regulation of its transcriptional activity, as the KXKK motif is conserved phylogenetically in most of the steroid hormone receptors including the GRs of all vertebrates after lampreys, including mammals, in comparison to the regulation via phosphorylation of serine residues by the serine/threonine kinases, based on the evidence that residues susceptible to phosphorylation are conserved only in mammals. In agreement with these observations, circadian rhythm transcription factor Clock, which is functional in the adaptive response to day/night changes that is essential for survival of virtually all organisms living on earth, acetylates GR at several lysines, including those in the KXKK acetylation motif located in the hinge region, and regulates GR-induced transcriptional activity. Thus, GR acetylation, including that by the CLOCK circadian system, play several important roles in the regulation of glucocorticoid action in target tissues. Consequently, disruption in the coupling between the HPA axis and circadian GR acetylation or dysregulation in GR acetylation may lead to development of various pathologic conditions, such as components of the metabolic syndrome and cardiovascular disorders, which strongly influence well-being and longevity in humans. Sirt1, a class III HDAC and one of the 7 human sirtuins, deacetylates several nuclear receptors and influences longevity; this HDAC might also deacetylate the GR, counteracting Clock-mediated regulation by decreasing GR-induced transcriptional activity (Blander and Guarente, 2004; Fu et al., 2006; Amat et al., 2007; Li et al., 2007). We suggest that the balance between acetylation and deacetylation of the GR promoted by Clock and, possibly, Sirt1, respectively, might be critical for the maintenance of proper biologic functioning of the GR, ultimately influencing well-being and longevity. This potentially important regulation of GR actions and its physiologic and pathophysiologic significance in humans begs for further studies.
Acknowledgments
Literary work of this article was funded partly by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD and the University of Athens, Athens, Greece.
Abbreviation list
- ACTH
Adrenocorticotropic hormone
- Dbp
Albumin gene D site-binding protein
- AMPK
AMP-activated protein kinase
- AR
Androgen receptor
- AVP
Arginine vasopressin
- bHLH
Basic helix-loop-helix
- Bmal1
Brain-muscle-arnt-like protein 1
- CNS
Central nervous system
- Clock
Circadian locomotor output cycle kaput
- CRH
Corticotropin-releasing hormone
- Cry
Cryptochrome
- CTE
C-terminal extension
- DBD
DNA-binding domain
- ER
Estrogen receptor
- GR
Glucocorticoid receptor
- GRE
Glucocorticoid response element
- HSP
Heat shock protein
- HAT
Histone acetyltransferase
- HDAC
Histone deacetylase
- HPA axis
Hypothalamic-pituitary-adrenal axis
- INHAT
Inhibitor of histone acetyltransferases
- LBD
Ligand-binding domain
- MR
Mineralocorticoid receptor
- MAPK
Mitogen-activated kinase
- NTD
N-terminal or immunogenic domain
- NF-κ B
Nuclear factor of κ B
- NL
Nuclear localization signal
- NRID
Nuclear receptor-interacting domain
- NR3C1
Nuclear receptor superfamily 3, group C, member 1
- p/CAF
p300/CBP-associated factor
- CBP
p300/CREB-binding protein
- PVN
Paraventricular nucleus
- PAS
PER-ARNT-SIM
- Per
Period
- PR
Progesterone receptor
- RORα
Retinoic acid receptor-related orphan receptor α
- SCN
Suprachiasmatic nucleus
- Tip60
Tat-interacting protein 60
- TAF-Iβ
Temperature-activating factor-Iβ
- TSA
Tricostatin-A
- DRIP/TRAP
Vitamin D receptor-interacting protein/thyroid hormone receptor-associated protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel GA, Wochnik GM, Ruegg J, Rouyer A, Holsboer F, Rein T. Activity of the GR in G2 and mitosis. Mol Endocrinol. 2002;16:1352–1366. doi: 10.1210/mend.16.6.0842. [DOI] [PubMed] [Google Scholar]
- Aguilera G. HPA axis responsiveness to stress: Implications for healthy aging. Exp Gerontol. 2010 doi: 10.1016/j.exger.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat R, Solanes G, Giralt M, Villarroya F. SIRT1 is involved in glucocorticoid-mediated control of uncoupling protein-3 gene transcription. J Biol Chem. 2007;282:34066–34076. doi: 10.1074/jbc.M707114200. [DOI] [PubMed] [Google Scholar]
- An W. Histone acetylation and methylation: combinatorial players for transcriptional regulation. Subcell Biochem. 2007;41:351–369. [PubMed] [Google Scholar]
- Arafah BM. Hypothalamic pituitary adrenal function during critical illness: limitations of current assessment methods. J Clin Endocrinol Metab. 2006;91:3725–3745. doi: 10.1210/jc.2006-0674. [DOI] [PubMed] [Google Scholar]
- Bao AM, Meynen G, Swaab DF. The stress system in depression and neurodegeneration: focus on the human hypothalamus. Brain research reviews. 2008;57:531–553. doi: 10.1016/j.brainresrev.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci (Lond) 1998;94:557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- Beato M, Chalepakis G, Schauer M, Slater EP. DNA regulatory elements for steroid hormones. J Steroid Biochem. 1989;32:737–747. doi: 10.1016/0022-4731(89)90521-9. [DOI] [PubMed] [Google Scholar]
- Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- Cheung P, Tanner KG, Cheung WL, Sassone-Corsi P, Denu JM, Allis CD. Synergistic coupling of histone H3 phosphorylation and acetylation in response to epidermal growth factor stimulation. Mol Cell. 2000;5:905–915. doi: 10.1016/s1097-2765(00)80256-7. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. Glucocorticoid therapy. In: Felig P, Frohman LA, editors. Endocrinology & Metabolism. McGraw-Hill; New York: 2001. pp. 609–632. [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nature reviews. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. Stress and sex versus immunity and inflammation. Sci Signal. 2010;3:pe36. doi: 10.1126/scisignal.3143pe36. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Kino T. Intracellular glucocorticoid signaling: a formerly simple system turns stochastic. Sci STKE 2005. 2005:pe48. doi: 10.1126/stke.3042005pe48. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Kino T. Glucocorticoid action networks and complex psychiatric and/or somatic disorders. Stress. 2007;10:213–219. doi: 10.1080/10253890701292119. [DOI] [PubMed] [Google Scholar]
- Cidlowski JA, Michaels GA. Alteration in glucocorticoid binding site number during the cell cycle in HeLa cells. Nature. 1977;266:643–645. doi: 10.1038/266643a0. [DOI] [PubMed] [Google Scholar]
- Clayton AL, Mahadevan LC. MAP kinase-mediated phosphoacetylation of histone H3 and inducible gene regulation. FEBS Lett. 2003;546:51–58. doi: 10.1016/s0014-5793(03)00451-4. [DOI] [PubMed] [Google Scholar]
- Cole TJ, Blendy JA, Monaghan AP, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schutz G. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 1995;9:1608–1621. doi: 10.1101/gad.9.13.1608. [DOI] [PubMed] [Google Scholar]
- Davidson AJ, Sellix MT, Daniel J, Yamazaki S, Menaker M, Block GD. Chronic jet-lag increases mortality in aged mice. Curr Biol. 2006;16:R914–916. doi: 10.1016/j.cub.2006.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhart-Bornstein M, Bornstein SR. Cross-talk between adrenal medulla and adrenal cortex in stress. Ann N Y Acad Sci. 2008;1148:112–117. doi: 10.1196/annals.1410.053. [DOI] [PubMed] [Google Scholar]
- Ekstrand K, Bostrom PA, Arborelius M, Nilsson JA, Lindell SE. Cardiovascular risk factors in commercial flight aircrew officers compared with those in the general population. Angiology. 1996;47:1089–1094. doi: 10.1177/000331979604701109. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- Eskandari F, Sternberg EM. Neural-immune interactions in health and disease. Ann N Y Acad Sci. 2002;966:20–27. doi: 10.1111/j.1749-6632.2002.tb04198.x. [DOI] [PubMed] [Google Scholar]
- Faus H, Haendler B. Post-translational modifications of steroid receptors. Biomed Pharmacother. 2006;60:520–528. doi: 10.1016/j.biopha.2006.07.082. [DOI] [PubMed] [Google Scholar]
- Fu M, Liu M, Sauve AA, Jiao X, Zhang X, Wu X, Powell MJ, Yang T, Gu W, Avantaggiati ML, Pattabiraman N, Pestell TG, Wang F, Quong AA, Wang C, Pestell RG. Hormonal control of androgen receptor function through SIRT1. Mol Cell Biol. 2006;26:8122–8135. doi: 10.1128/MCB.00289-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, Wang C, Reutens AT, Wang J, Angeletti RH, Siconolfi-Baez L, Ogryzko V, Avantaggiati ML, Pestell RG. p300 and p300/cAMP-response element-binding protein-associated factor acetylate the androgen receptor at sites governing hormone-dependent transactivation. J Biol Chem. 2000;275:20853–20860. doi: 10.1074/jbc.M000660200. [DOI] [PubMed] [Google Scholar]
- Gaughan L, Logan IR, Cook S, Neal DE, Robson CN. Tip60 and histone deacetylase 1 regulate androgen receptor activity through changes to the acetylation status of the receptor. J Biol Chem. 2002;277:25904–25913. doi: 10.1074/jbc.M203423200. [DOI] [PubMed] [Google Scholar]
- Gaviglio AL, Daniel AR, Czaplicki LM, Hillard CJ, Lange CA. Progesterone receptor hinge region regulates the kinetics of transcriptional response through phosphorylation, acetylation, and nuclear retention. City. 2010:114. doi: 10.1210/me.2010-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haelens A, Tanner T, Denayer S, Callewaert L, Claessens F. The hinge region regulates DNA binding, nuclear translocation, and transactivation of the androgen receptor. Cancer Res. 2007;67:4514–4523. doi: 10.1158/0008-5472.CAN-06-1701. [DOI] [PubMed] [Google Scholar]
- Hager GL. The dynamics of intranuclear movement and chromatin remodeling by the glucocorticoid receptor. Ernst Schering Res Found Workshop; 2002. pp. 111–129. [DOI] [PubMed] [Google Scholar]
- Harrington M. Location, location, location: important for jet-lagged circadian loops. J Clin Invest. 2010;120:2265–2267. doi: 10.1172/JCI43632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Lee LW, Minges JT, Wilson EM. Dependence of selective gene activation on the androgen receptor NH2- and COOH-terminal interaction. J Biol Chem. 2002;277:25631–25639. doi: 10.1074/jbc.M202809200. [DOI] [PubMed] [Google Scholar]
- Hollenberg SM, Weinberger C, Ong ES, Cerelli G, Oro A, Lebo R, Thompson EB, Rosenfeld MG, Evans RM. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985;318:635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichijo T, Chrousos GP, Kino T. Activated glucocorticoid receptor interacts with the INHAT component Set/TAF-Iβ and releases it from a glucocorticoid-responsive gene promoter, relieving repression: implications for the pathogenesis of glucocorticoid resistance in acute undifferentiated leukemia with Set-Can translocation. Mol Cell Endocrinol. 2008;283:19–31. doi: 10.1016/j.mce.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichijo T, Voutetakis A, Cotrim AP, Bhattachryya N, Fujii M, Chrousos GP, Kino T. The Smad6-histone deacetylase 3 complex silences the transcriptional activity of the glucocorticoid receptor: potential clinical implications. J Biol Chem. 2005;280:42067–42077. doi: 10.1074/jbc.M509338200. [DOI] [PubMed] [Google Scholar]
- Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, Tsujimoto G, Okamura H. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell metabolism. 2005;2:297–307. doi: 10.1016/j.cmet.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Ismaili N, Garabedian MJ. Modulation of glucocorticoid receptor function via phosphorylation. Ann N Y Acad Sci. 2004;1024:86–101. doi: 10.1196/annals.1321.007. [DOI] [PubMed] [Google Scholar]
- Ito K, Yamamura S, Essilfie-Quaye S, Cosio B, Ito M, Barnes PJ, Adcock IM. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-κ B suppression. J Exp Med. 2006;203:7–13. doi: 10.1084/jem.20050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Adachi M, Yasui H, Takekawa M, Tanaka H, Imai K. Nuclear export of glucocorticoid receptor is enhanced by c-Jun N-terminal kinase-mediated phosphorylation. Mol Endocrinol. 2002;16:2382–2392. doi: 10.1210/me.2002-0144. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Palm IF, La Fleur SE, Scheer FA, Perreau-Lenz S, Ruiter M, Kreier F, Cailotto C, Buijs RM. SCN outputs and the hypothalamic balance of life. Journal of biological rhythms. 2006;21:458–469. doi: 10.1177/0748730406293854. [DOI] [PubMed] [Google Scholar]
- Kiessling S, Eichele G, Oster H. Adrenal glucocorticoids have a key role in circadian resynchronization in a mouse model of jet lag. J Clin Invest. 2010;120:2600–2609. doi: 10.1172/JCI41192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MY, Woo EM, Chong YT, Homenko DR, Kraus WL. Acetylation of estrogen receptor α by p300 at lysines 266 and 268 enhances the deoxyribonucleic acid binding and transactivation activities of the receptor. Mol Endocrinol. 2006;20:1479–1493. doi: 10.1210/me.2005-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino T. Tissue glucocorticoid sensitivity: beyond stochastic regulation on the diverse actions of glucocorticoids. Horm Metab Res. 2007;39:420–424. doi: 10.1055/s-2007-980193. [DOI] [PubMed] [Google Scholar]
- Kino T, Chrousos GP. Glucocorticoid and mineralocorticoid receptors and associated diseases. Essays Biochem. 2004;40:137–155. doi: 10.1042/bse0400137. [DOI] [PubMed] [Google Scholar]
- Kino T, Chrousos GP. Glucocorticoid effect on gene expression. In: Steckler T, Kalin NH, Reul JMHM, editors. Handbook on Stress and the Brain. Elsevier BV; Amsterdam: 2005. pp. 295–312. [Google Scholar]
- Kino T, De Martino MU, Charmandari E, Mirani M, Chrousos GP. Tissue glucocorticoid resistance/hypersensitivity syndromes. J Steroid Biochem Mol Biol. 2003;85:457–467. doi: 10.1016/s0960-0760(03)00218-8. [DOI] [PubMed] [Google Scholar]
- Kino T, Ichijo T, Amin ND, Kesavapany S, Wang Y, Kim N, Rao S, Player A, Zheng YL, Garabedian MJ, Kawasaki E, Pant HC, Chrousos GP. Cyclin-dependent kinase 5 differentially regulates the transcriptional activity of the glucocorticoid receptor through phosphorylation: clinical implications for the nervous system response to glucocorticoids and stress. Mol Endocrinol. 2007;21:1552–1568. doi: 10.1210/me.2006-0345. [DOI] [PubMed] [Google Scholar]
- Kino T, Jaffe H, Amin ND, Chakrabarti M, Zheng YL, Chrousos GP, Pant HC. Cyclin-dependent kinase 5 modulates the transcriptional activity of the mineralocorticoid receptor and regulates expression of brain-derived neurotrophic factor. Mol Endocrinol. 2010;24:941–952. doi: 10.1210/me.2009-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino T, Su YA, Chrousos GP. Human glucocorticoid receptor isoform β: recent understanding of its potential implications in physiology and pathophysiology. Cell Mol Life Sci. 2009;66:3435–3448. doi: 10.1007/s00018-009-0098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyohara YB, Tagao S, Tamanini F, Morita A, Sugisawa Y, Yasuda M, Yamanaka I, Ueda HR, van der Horst GT, Kondo T, Yagita K. The BMAL1 C terminus regulates the circadian transcription feedback loop. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10074–10079. doi: 10.1073/pnas.0601416103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15:R271–277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- Kondratov RV, Kondratova AA, Lee C, Gorbacheva VY, Chernov MV, Antoch MP. Post-translational regulation of circadian transcriptional CLOCK(NPAS2)/BMAL1 complex by CRYPTOCHROMES. Cell cycle (Georgetown, Tex) 2006;5:890–895. doi: 10.4161/cc.5.8.2684. [DOI] [PubMed] [Google Scholar]
- Kovacs JJ, Murphy PJ, Gaillard S, Zhao X, Wu JT, Nicchitta CV, Yoshida M, Toft DO, Pratt WB, Yao TP. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell. 2005;18:601–607. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Wust S. Human models in acute and chronic stress: assessing determinants of individual hypothalamus-pituitary-adrenal axis activity and reactivity. Stress. 2010;13:1–14. doi: 10.3109/10253890902874913. [DOI] [PubMed] [Google Scholar]
- Kyrou I, Tsigos C. Stress hormones: physiological stress and regulation of metabolism. Curr Opin Pharmacol. 2009;9:787–793. doi: 10.1016/j.coph.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell. 2007;28:91–106. doi: 10.1016/j.molcel.2007.07.032. [DOI] [PubMed] [Google Scholar]
- Loven MA, Davis RE, Curtis CD, Muster N, Yates JR, Nardulli AM. A novel estrogen receptor α-associated protein alters receptor-deoxyribonucleic acid interactions and represses receptor-mediated transcription. Mol Endocrinol. 2004;18:2649–2659. doi: 10.1210/me.2003-0195. [DOI] [PubMed] [Google Scholar]
- Lu NZ, Cidlowski JA. Translational regulatory mechanisms generate N-terminal glucocorticoid receptor isoforms with unique transcriptional target genes. Mol Cell. 2005;18:331–342. doi: 10.1016/j.molcel.2005.03.025. [DOI] [PubMed] [Google Scholar]
- McKenna NJ, O'Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002;108:465–474. doi: 10.1016/s0092-8674(02)00641-4. [DOI] [PubMed] [Google Scholar]
- Melvin VS, Harrell C, Adelman JS, Kraus WL, Churchill M, Edwards DP. The role of the C-terminal extension (CTE) of the estrogen receptor alpha and β DNA binding domain in DNA binding and interaction with HMGB. J Biol Chem. 2004;279:14763–14771. doi: 10.1074/jbc.M313335200. [DOI] [PubMed] [Google Scholar]
- Miller AL, Webb MS, Copik AJ, Wang Y, Johnson BH, Kumar R, Thompson EB. p38 Mitogen-activated protein kinase (MAPK) is a key mediator in glucocorticoid-induced apoptosis of lymphoid cells: correlation between p38 MAPK activation and site-specific phosphorylation of the human glucocorticoid receptor at serine 211. Mol Endocrinol. 2005;19:1569–1583. doi: 10.1210/me.2004-0528. [DOI] [PubMed] [Google Scholar]
- Miller DB, O'Callaghan JP. Neuroendocrine aspects of the response to stress. Metabolism. 2002;51:5–10. doi: 10.1053/meta.2002.33184. [DOI] [PubMed] [Google Scholar]
- Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- Murphy PJ, Morishima Y, Kovacs JJ, Yao TP, Pratt WB. Regulation of the dynamics of hsp90 action on the glucocorticoid receptor by acetylation/deacetylation of the chaperone. J Biol Chem. 2005;280:33792–33799. doi: 10.1074/jbc.M506997200. [DOI] [PubMed] [Google Scholar]
- Nader N, Chrousos GP, Kino T. Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: potential physiological implications. FASEB J. 2009;23:1572–1583. doi: 10.1096/fj.08-117697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader N, Chrousos GP, Kino T. Interactions of the circadian CLOCK system and the HPA axis. Trends Endocrinol Metab. 2010;21:277–286. doi: 10.1016/j.tem.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader N, Ng SS, Lambrou GI, Pervanidou P, Wang Y, Chrousos GP, Kino T. AMPK regulates metabolic actions of glucocorticoids by phosphorylating the glucocorticoid receptor through p38 MAPK. Mol Endocrinol. 2010;24:1748–1764. doi: 10.1210/me.2010-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaides NC, Galata Z, Kino T, Chrousos GP, Charmandari E. The human glucocorticoid receptor: molecular basis of biologic function. Steroids. 2010;75:1–12. doi: 10.1016/j.steroids.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley B. The steroid receptor superfamily: more excitement predicted for the future. Mol Endocrinol. 1990;4:363–369. doi: 10.1210/mend-4-3-363. [DOI] [PubMed] [Google Scholar]
- Oster H, Damerow S, Kiessling S, Jakubcakova V, Abraham D, Tian J, Hoffmann MW, Eichele G. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell metabolism. 2006;4:163–173. doi: 10.1016/j.cmet.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Pippal JB, Yao Y, Rogerson FM, Fuller PJ. Structural and functional characterization of the interdomain interaction in the mineralocorticoid receptor. Mol Endocrinol. 2009;23:1360–1370. doi: 10.1210/me.2009-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt WB. The role of heat shock proteins in regulating the function, folding, and trafficking of the glucocorticoid receptor. J Biol Chem. 1993;268:21455–21458. [PubMed] [Google Scholar]
- Reichardt HM, Kaestner KH, Tuckermann J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angel P, Schutz G. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93:531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- Revollo JR, Cidlowski JA. Mechanisms generating diversity in glucocorticoid receptor signaling. Ann N Y Acad Sci. 2009;1179:167–178. doi: 10.1111/j.1749-6632.2009.04986.x. [DOI] [PubMed] [Google Scholar]
- Ripperger JA, Schibler U. Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nature genetics. 2006;38:369–374. doi: 10.1038/ng1738. [DOI] [PubMed] [Google Scholar]
- Rogatsky I, Waase CL, Garabedian MJ. Phosphorylation and inhibition of rat glucocorticoid receptor transcriptional activation by glycogen synthase kinase-3 (GSK-3). Species-specific differences between human and rat glucocorticoid receptor signaling as revealed through GSK-3 phosphorylation. J Biol Chem. 1998;273:14315–14321. doi: 10.1074/jbc.273.23.14315. [DOI] [PubMed] [Google Scholar]
- Rogerson FM, Fuller PJ. Interdomain interactions in the mineralocorticoid receptor. Mol Cell Endocrinol. 2003;200:45–55. doi: 10.1016/s0303-7207(02)00413-6. [DOI] [PubMed] [Google Scholar]
- Rosenfeld MG, Lunyak VV, Glass CK. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 2006;20:1405–1428. doi: 10.1101/gad.1424806. [DOI] [PubMed] [Google Scholar]
- Savory JG, Hsu B, Laquian IR, Giffin W, Reich T, Hache RJ, Lefebvre YA. Discrimination between NL1- and NL2-mediated nuclear localization of the glucocorticoid receptor. Mol Cell Biol. 1999;19:1025–1037. doi: 10.1128/mcb.19.2.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo SB, McNamara P, Heo S, Turner A, Lane WS, Chakravarti D. Regulation of histone acetylation and transcription by INHAT, a human cellular complex containing the set oncoprotein. Cell. 2001;104:119–130. doi: 10.1016/s0092-8674(01)00196-9. [DOI] [PubMed] [Google Scholar]
- Sheridan J. The HPA axis, SNS, and immunity: a commentary. Brain Behav Immun. 2003;17(Suppl 1):S17. doi: 10.1016/s0889-1591(02)00061-2. [DOI] [PubMed] [Google Scholar]
- So AY, Chaivorapol C, Bolton EC, Li H, Yamamoto KR. Determinants of cell- and gene-specific transcriptional regulation by the glucocorticoid receptor. PLoS Genet. 2007;3:e94. doi: 10.1371/journal.pgen.0030094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmary Z, Garabedian MJ, Vilcek J. Inhibition of glucocorticoid receptor-mediated transcriptional activation by p38 mitogen-activated protein (MAP) kinase. J Biol Chem. 2004;279:43708–43715. doi: 10.1074/jbc.M406568200. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J, Saatcioglu F, Janne OA, Palvimo JJ. Disrupted amino- and carboxyl-terminal interactions of the androgen receptor are linked to androgen insensitivity. Mol Endocrinol. 2001;15:923–935. doi: 10.1210/mend.15.6.0647. [DOI] [PubMed] [Google Scholar]
- Thomson S, Clayton AL, Mahadevan LC. Independent dynamic regulation of histone phosphorylation and acetylation during immediate-early gene induction. Mol Cell. 2001;8:1231–1241. doi: 10.1016/s1097-2765(01)00404-x. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Arnhold MM, Engeland WC. Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. American journal of physiology. 2006;290:R1128–1135. doi: 10.1152/ajpregu.00042.2003. [DOI] [PubMed] [Google Scholar]
- van der Vaart M, Schaaf MJ. Naturally occurring C-terminal splice variants of nuclear receptors. Nucl Recept Signal. 2009 doi: 10.1621/nrs.07007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Garabedian MJ. Modulation of glucocorticoid receptor transcriptional activation, phosphorylation, and growth inhibition by p27Kip1. J Biol Chem. 2003;278:50897–50901. doi: 10.1074/jbc.M310297200. [DOI] [PubMed] [Google Scholar]
- Webster JI, Moayeri M, Sternberg EM. Novel repression of the glucocorticoid receptor by anthrax lethal toxin. Ann N Y Acad Sci. 2004;1024:9–23. doi: 10.1196/annals.1321.003. [DOI] [PubMed] [Google Scholar]
- Wurtman RJ. Stress and the adrenocortical control of epinephrine synthesis. Metabolism. 2002;51:11–14. doi: 10.1053/meta.2002.33185. [DOI] [PubMed] [Google Scholar]