Abstract
One of the characteristic signs of retinitis pigmentosa (RP) is the progressive loss of night vision. We have previously shown that the gain of rod photoreceptor activation is moderately reduced in some patients with RP, but this decrease in activation kinetics is not sufficient to account for the night blindness. Recently, single rod recording from animal models of RP showed rods under degeneration remain saturated for shorter periods than normal rods; i.e. are less able to sustain the rod photoresponse. Using paired-flash ERG, here we determine whether rod phototransduction inactivation parameters might also be abnormal in patients with RP. Inactivation parameters were derived from 13 subjects with normal vision, 16 patients with adRP, and 16 patients with autosomal recessive/isolate (rec/iso) RP. The adRP cases included 9 patients with rhodopsin mutations and 7 patients with peripherin/RDS mutations. The inactivation phase was derived using a double-flash paradigm, with a test flash of 2.7 log scot td-sec followed at varying intervals by a 4.2 log scot td-sec probe flash. Derived rod photoresponses to this just-saturating test flash in normal subjects exhibit a critical time to the initiation of recovery (Tsat) of 525±90 (SD) msec. The values of Tsat were 336±104 (SD) msec in patients with adRP (P<0.001) and 271±45 (SD) msec (P<0.001) in patients with rec/iso RP. When Tsat values were categorized by mutations, the values were 294±91 (SD) msec (P<0.001) for rhodopsin mutations, and 389±100 (SD) msec (p=0.01) for peripherin/RDS mutations. Overall, Tsat in patients with RP was significantly correlated with the amplitude of ISCEV standard rod response (r = 0.56; P < 0.001) and the gain of the activation phase of phototransduction (r=0.6, P<0.001). Tsat may be a useful marker for therapeutic efficacy in future clinical trials in RP.
Keywords: Rod inactivation, ERG, phototransduction, RP, rhodopsin, peripherin/RDS
1. Introduction
Retinitis pigmentosa (RP) affects primarily the photoreceptors, especially the rods. Rods hyperpolarize to light, the biochemical basis of which is the rod phototransduction cascade. Phototransduction converts photonic energy into graded changes in rod membrane potential, which trigger electrical and chemical changes in other neuronal types in the retina. The resulting photoresponse is a representation of the electrical activities in the retina when a stimulus is presented. The human electroretinogram (ERG) is used clinically to record these photoresponses non-invasively. The ERG waveform represents the propagation of signal transduction in the retina and the a-wave in particular represents photoreceptor activities (Dowling, 1987; Hood and Birch, 1990a, b; Ogden, 1994; Steinberg, 1991). The kinetics of the rod a-wave reflects both the activation and inactivation stages of rod phototransduction.
Much is known about the biochemical basis of the activation steps in vertebrate rods (Pugh and Lamb, 1993). A mathematical model based on the known biochemical events has been shown to accurately index the gain of phototransduction from the membrane current of the outer segment of single rods (Kraft et al., 1993; Lamb and Pugh, 1992; Pugh and Lamb, 1993) as well as massed rod current indexed by the rod ERG a-wave (Breton et al., 1994; Cideciyan and Jacobson, 1996; Hagins et al., 1970; Hood and Birch, 1994). Reduction of the gain of rod activation (log S) has been found in a sub-population of RP and other retina degeneration diseases (Birch et al., 2002; Birch et al., 1995; Hood and Birch, 1996; Jacobson et al., 2000; Shady et al., 1995; Tzekov et al., 2003).
Recent studies reported that rate of rod inactivation can be shifted to longer or shorter than normal times by specific mutations. Mouse rods lacking guanylate cyclase activating proteins GCAP1 and GCAP2 (GCAPs(−/−)) showed delayed recovery of the membrane current recorded from single rod outer segments (Burns et al., 2002). Consistent with this result, we reported massed rod inactivation was delayed in patients of cone-rod dystrophy with mutations in guanylate cyclase-activating protein (GCAP1) (Jiang et al., 2008). In addition to GCAPs, slowed rod inactivation was reported in mice with a deletion in any component of the RGS9.Gbeta5.R9AP GTPase-activating (GAP) complex (Chen et al., 2000; Keresztes et al., 2004; Krispel et al., 2003; Krispel et al., 2006). On the contrary, upregulation of the expression of GAP complex lead to earlier-than normal photoreceptor recovery (Krispel et al., 2006; Nishiguchi et al., 2004). Recently, earlier-than-normal rod recovery was reported in multiple animal models of RP, including the dystrophic Royal College of Surgeons (RCS) rat (Niculescu, 2004), transgenic pigs expressing P347L or P347S rhodopsin (Kraft et al., 2005), and a mild light damage model using albino rat (Kraft et al., 2005; Niculescu, 2004; Wen, 2008).
The rate of rod inactivation is also determined by the intensity of the stimulating flash. A two-branched relationship between Tsat and ln (Itest) had been reported in salamander and mice rod recordings (Burns et al., 2002; Pepperberg et al., 1992) and in the human ERG (Birch et al., 1995).
We previously found that patients with pro-23-his rhodopsin mutation showed slower than normal recovery when tested with intense flashes (i.e. on the upper branch of the Tsat - ln (Itest) function (Birch et al., 1995). Here we sought to determine the extent to which abnormal rod phototransduction inactivation is present to test saturating flashes lie on the lower slope of the Tsat-ln (Itest) function. Moreover, we sought to quantify the relationships among rod inactivation kinetics, rod activation kinetics, and the degree of rod degeneration as indexed by International Society for Clinical Electrophysiology of Vision (ISCEV) standard rod response amplitudes in a sample of patients with different genetic forms of RP.
2. Materials and methods
2.1. Subjects
The subjects included 13 normal controls, 16 patients with autosomal dominant RP (adRP), and 16 patients with an autosomal recessive/isolate (rec/iso RP). The 16 patients with adRP consisted of 9 patients with rhodopsin (RHO) mutations and 7 patients with peripherin/RDS mutations. The mutations associated with the rec/iso cases are unknown. These patients were selected from a larger cohort referred for ERG testing by ophthalmologists specialized in retinal diseases. Genetic testing results were retrieved from the database of Southwest Eye Registry of the Retina Foundation of the Southwest. In order to ensure sufficient a-wave amplitude for reliable analysis, patients were included if their ISCEV standard rod response amplitude was greater than 10 μV. The tenets of the Declaration of Helsinki were followed and all subjects gave written informed consent after a full explanation of the procedures was given.
2.2. Evaluation of rod activation and inactivation kinetics
Human subjects were dark adapted for 45 minutes after pupil dilation (1% tropicamide and 2.5% phenylephrine hydrochloride). ERGs were recorded in a ganzfeld dome with bipolar contact lens (Burian-Allen electrode, Hansen Laboratories, Coralville, IA). ISCEV Standard rod ERGs were recorded immediately after dark adaptation, followed by high-intensity ERGs, paired-flash ERGs and lastly, light-adapted ERGs.
Methods of evaluating the rod activation process and detailed analysis procedures are described elsewhere (Hood and Birch, 1994; Shady et al., 1995; Tzekov et al., 2003). Briefly, after 45 minutes of dilation and dark adaptation, achromatic flash (Model 2150, Novatron, Dallas, TX) was presented with at least 45 seconds between flashes. Three responses were averaged at each retinal illuminance (3.2, 3.6, 4.1, and 4.4 log scot td-sec). Rod-only a-waves were obtained by subtracting cone-only a-waves, which were recorded to the same four illuminances in the presence of a rod-saturating background light (3.3 log scot td) in the ganzfeld dome. The leading edges of the a-waves were fitted simultaneously with a mathematical model based on the activation model of rod phototransduction (Breton et al., 1994; Lamb and Pugh, 1992). The sensitivity (log S) was derived from the fitting process to describe the phototransduction gain in the rod activation process (Hood and Birch, 1994, 1996; Shady et al., 1995; Tzekov et al., 2003)
Methods of pair-flash ERG recording and detailed analysis procedures are also described elsewhere (Birch et al., 1995; Pepperberg et al., 1997). Briefly, a test flash was paired with a probe flash on each trial, with test flash delivered first. The inter-stimulus-interval between the two flashes was variable. The test flash was an achromatic flash (2.7 log scot td-sec) delivered by the Grass Photostimulator (PS33 Plus, Grass Instruments, Warwick, RI). The probe flash (λcut-off = 470 nm, 4.2 log scot td-sec) was delivered by a high-intensity flash lamp (Model 2150, Novatron, Dallas, TX). Tsat, the period prior to recovery from saturation from the test flash, was a derived value from an exponential recovery function: A/Amax=1 − exp[ − (t − Tsat)/tau], where Amax is the saturating amplitude of rods, and A is the derived rod response amplitude to the test flash at various time points (msec) after the test flash was given, and tau is the time constant of recovery.
Three subjects (one patient with rec/iso RP, one blue cone monochromat, and one normal control) were tested with a range of test flash retinal illuminances (2.7, 3.2, 3.6, and 4.1 log scot td-sec). For these higher test flash intensities, a second Model 2150 Novatron stimulator replaced the Grass Photostimulator.
2.3. Statistical analysis
All two-sample hypothesis tests were preceded by serial randomness test and F test for equal variance. Unpaired independent two-sample Student’s t-test was used for samples with equal variance. The Wilcoxon Rank-Sum test was used for samples with unequal variance. All statistical analyses were performed using Igor Pro 6.12 (WaveMetrics, Inc. Lake Oswego, Oregon).
3. Results
3.1. Time to initiation of rod recovery (Tsat) to a fixed test flash
The “paired flash” ERG paradigm is used to determine Tsat, the value of the inter-stimulus interval (ISI), after which the rods start to respond to the probe flash. That is, it is a measure of the duration of rod saturation caused by the test flash.
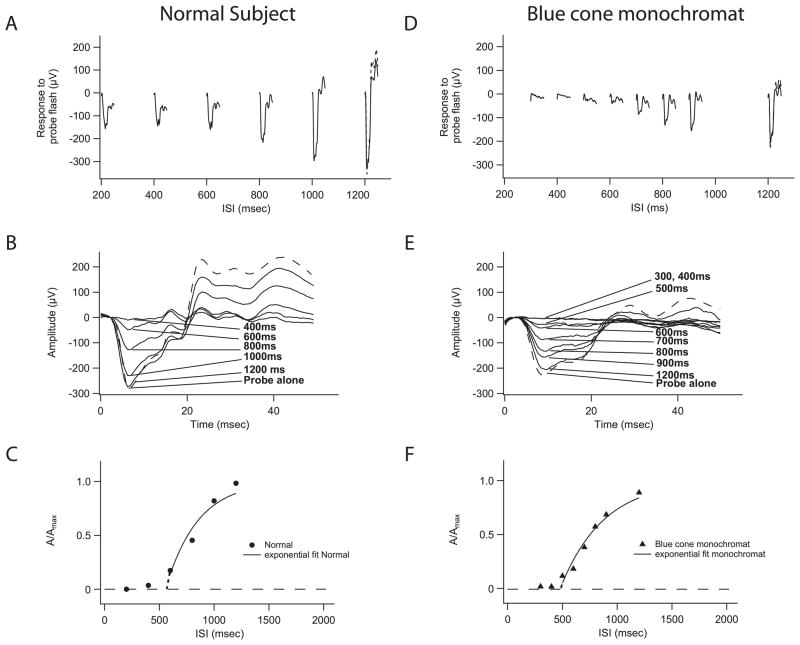
Figure 1A shows the ERG waveforms (solid traces) of a normal subject to probe flashes at different inter-stimulus-intervals (ISI)(0.2 sec to 1.2 sec) following the test flash. The probe response is small at shorter ISIs (0.2 sec to 0.6 sec). These small asymptotic probe response in Fig. 1A are cone mediated (Birch et al., 1995; Pepperberg et al., 1997), as the cones recover faster than the rods (Schnapf et al., 1987). However, as ISI lengthens, the probe responses grow in amplitude (Fig. 1A) due to rod recovery. When the ISI is 1.2 seconds (Fig. 1A), the amplitude of the rod response to the probe flash is equivalent to that when the probe flash is presented alone (Fig. 1A, broken line).
Figure 1.
Tsat values derived from a normal subject and a patient with blue cone monochromacy. (A) ERG waveforms of a normal subject to a probe flash after a fixed test flash at different inter-stimulus-interval (ISI). Response to probe alone is shown in broken line. (B) As rods recover from saturation, the rod-only response to probe grew in amplitude until it is equivalent to the probe presented alone. Rod-only responses were derived by subtracting the cone responses at 200 msec from responses at each ISI. (C) Tsat of 556 msec was derived from the rod photoresponse recovery to the test flash (A/Amax) in a normal subject, where A is the derived rod response amplitude of the test flash at a particular time point (t = ISI). A(t) = Amax− Aprobe (t) (Pepperberg et al., 1997). (D) For a blue-cone monochromat, every response to a light flash can be considered as pure rod response. The rods did not respond to probe flash at ISIs of 200 msec, 300 msec, or 400 msec, but grew in amplitude at more prolonged ISIs. Response to probe alone is shown in broken line. (E) ERG waveforms of a blue cone monochromat to a probe flash after a fixed test flash at different ISIs. (F) For a blue cone monochromat, the time course of photoresponse recovery to the 2.7 log sc td-sec test flash was equivalent to normal, with a Tsat value of 479 msec.
We assumed that the probe response at ISI of 200 msec (Fig. 1A) is a pure cone response to the probe flash and subtracted its waveform from all probe responses shown in Figure 1A. The resulting waveforms are shown in Figure 1B, which were taken as the pure rod responses at different ISIs to the probe flashes (Fig. 1B). As shown in Figure 1B, at ISI of 400 msec, the rods still had not responded to the probe flash; that is, the amplitude of the rod probe response was close to zero. However, the rods fully recovered and showed a maximum response to the probe flash when the ISI was 1.2 seconds. In other words, at ISIs of 200 msec and 400 msec, the rods were still saturated by the test flash, and therefore, could not respond to the probe flash. As the rods recovered from saturation, the response to the probe grew in amplitude until it was equivalent to the probe presented alone (Fig. 1B).
Figure 1C shows the data from this normal subject plotted as the relative recovery of the rod response to the test flash from saturation (A/Amax), where A is the derived rod response amplitude of the test flash at a particular time point (t = ISI) after the test flash (A(t) = Amax − Aprobe (t)) (Pepperberg et al., 1997). Thus, Figure 1C shows that rods were initially saturated by the test flash, but recovered completely over the next second or so. We fit this relationship with an exponential recovery function (A/Amax=1 − exp[− (t − Tsat)/tau]), where Tsat indicates the initiation of rod recovery from saturation from the test flash. In Figure 1C, the solid curve is the best fit of the exponential recovery function and the derived Tsat value for this normal subject was 556 msec. For 13 normal subjects, the mean Tsat value was 525 ± 90 (SD) msec, and the mean tau value was 535 ± 186 (SD) msec.
3.2. Normal Tsat in blue-cone monochromat validates cone subtraction procedure
The fundamental premise of the “paired-flash” paradigm is that pure rod response can be derived from mixture of rod and cone responses by computer-based linear subtraction. This premise has been verified in previous studies (Birch et al., 1995; Pepperberg et al., 1997). For a blue-cone monochromat, every response to the light flashes should be a pure rod response. Thus, Tsat should be within normal limits even without the subtraction procedure to isolate the rod probe response.
The blue-cone monochromat (RFSW#355; age 65) had a normal dark-adapted threshold and a normal rod ERG amplitude. However, light-adapted cone responses and the 31 Hz flicker response (above rod fusion frequency) were minimally detectable. The probe responses from this blue cone monochromat are shown in Figure 1D and 1E as a function of ISI. The rods did not respond to probe flashes at ISIs of 200 msec, 300 msec, or 400 msec, but grew in amplitude at more prolonged ISIs. The time course of photoresponse recovery to the 2.7 log sc td-sec test flash was equivalent to normal, with a Tsat value of 479 msec, and tau value of 392 msec (Fig. 1F).
3.3 Premature photoresponse recovery is linked to rod degeneration in RP
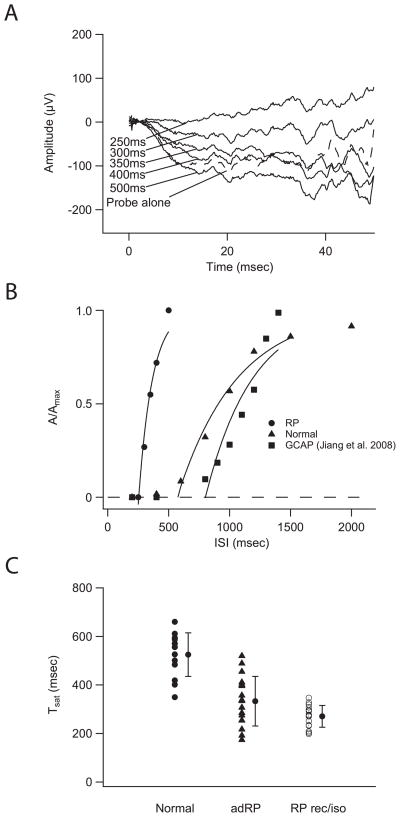
Fig. 2A shows rod-only responses from a 37 year-old patient with adRP (RHO pro-23-his mutation). The derived time course of recovery is shown in Fig 2B (filled circles) with a Tsat of 254 msec and tau of 113 msec. The representative normal subject (triangles) shows slower recovery, with a Tsat of 571 msec and tau of 485 msec. Also shown is the recovery function from a previously reported patient with cone-rod dystrophy (squares) due to a GCAP1 mutation (Jiang et al., 2008) and a Tsat of 802 msec and tau of 581 msec. Compared to the representative normal subject, the rod photoresponse recovers earlier in the RP patient with a RHO mutation and later in the patient with CRD (GCAP1 mutation).
Figure 2.
Tsat for patients with RP. (A) Rod-only response from a 37 year-old patient with adRP (RHO pro-23-his mutation) (Fig. 2A). The Tsat derived from this patient was 254 msec with tau of 113 msec (filled circles, Fig. 2B). (B) Figure 2B compares the full time course of the rod photoresponse recovery in a representative patient with RP (RHO pro-23-his mutation) and a normal subject. Also shown is the recovery function from a previously reported patient with cone-rod dystrophy due to a GCAP1 mutation (squares) (Jiang et al., 2008). Compared to the representative normal subject, the rod photoresponse recovers earlier in the RP patient with RP and later in the CRD patient with a GCAP1 mutation. (C) Figure 2C shows Tsat results from 16 patients with autosomal dominant RP and 16 patients with rec/iso RP. Average Tsat derived from 16 patients with adRP was 336±104 (SD) msec, a 36% reduction from the normal 525±90 (SD) msec (P < 0.001). The mean Tsat in the 16 rec/iso RP cases was 271±45 (SD) msec, 48% lower than the normal average (P<0.001). In summary, Tsat were shorter in patients with RP. Statistical results of Tsat measure in all RPs are presented in Table 1.
The Tsat values for 16 patients with autosomal dominant RP and 16 patients with rec/iso RP are shown in Figure 2C. Tsat, derived from 16 patients with adRP, was 336±104 (SD) msec, a 36% reduction from the normal 525±90 (SD) msec. The reduction was statistically significant (P < 0.001). The 16 patients with adRP include 9 patients with rhodopsin mutations (four cases of pro-23-his, single cases of pro-170-arg, pro-210-arg, phe-9-val, ala-164-val, and cys-185-arg). The mean Tsat value for these patients was 294±91(SD) msec, as compared to 525±90 (SD) msec for the 13 normal subjects (Fig. 2C). The 44% decrease from normal mean Tsat value was statistically significant (P<0.001).
We next asked whether mutations in peripherin/RDS are associated with changes in Tsat. Peripherin is present in the rim of mature disks of rod and cone outer segments (Arikawa et al., 1992). The product of the peripherin/rds gene is a photoreceptor disc membrane-associated glycoprotein (Travis et al., 1991). Five of the seven patients with RDS/peripherin mutation had manifested RP phenotype, but the other two were asymptomatic at the time of testing. The mean Tsat in 7 patients with peripherin/RDS was 389±100 (SD) msec, which was 26% smaller than the normal value of 525±90 (SD) msec. This difference was statistically significant (P=0.01). The Tsat values in the peripherin/RDS group ranged from 217 msec to 520 msec (Table 1). The two highest Tsat values (489 msec and 520 msec) were derived from the two patients who carried a peripherin/RDS mutation but were asymptomatic.
Table 1.
Characterization of rod recovery kinetics with Tsat and Tau in normal subjects and patients with RP.
| Count | Tsat (msec) | Reduction | Significance | Tau (msec) | Reduction | Significance | |
|---|---|---|---|---|---|---|---|
| Normal | 13 | 525 ± 90 (SD) | 535±186 (SD) | ||||
| adRP | 16 | 336±104 | 36% | P<0.001 | 340±173 | 36% | P=0.007 |
| RHO | 9 | 294±91 | 44% | P<0.001 | 347±203 | 35% | P=0.04 |
| RDS | 7 | 389±100 | 26% | P=0.01 | 331±140 | 38% | P=0.01 |
| rec/iso RP | 16 | 271±45 | 48% | P<0.001 | 422±160 | 21% | P=0.09 |
Table 1 presents the statistical results of Tsat and tau in patients with RP and associated mutations. Significance differences and percent reductions are relative to the normal average.
Finally, the mean Tsat in the 16 rec/iso RP cases was 271±45 (SD) msec, 48% lower than the normal average (P<0.001) (Fig. 2C). Thus, Tsat reductions appear to be common within the RP phenotype. The average Tsat in rec/iso RP (271±45 msec) was shorter than that in the autosomal dominant RP (336±104 msec) (P=0.03). This difference probably resulted from the inclusion of the two asymptomatic patients with peripherin/RDS mutation in the adRP group. After removal of these two patients, no significant difference could be detected between the 16 patients with rec/iso RP (271±45 msec) and the 14 patients with symptomatic adRP (311±87 msec) (P=0.08).
Table 1 also showed that tau values were significantly lower (P=0.007) in patients with adRP (36% reduction), either associated with RHO mutation (35% reduction, P=0.04) or peripherin/RDS mutation (38% reduction, P=0.01). For patients with rec/iso RP, the average tau values were 21% lower than normal average, although the difference was not statistically significant (P = 0.09).
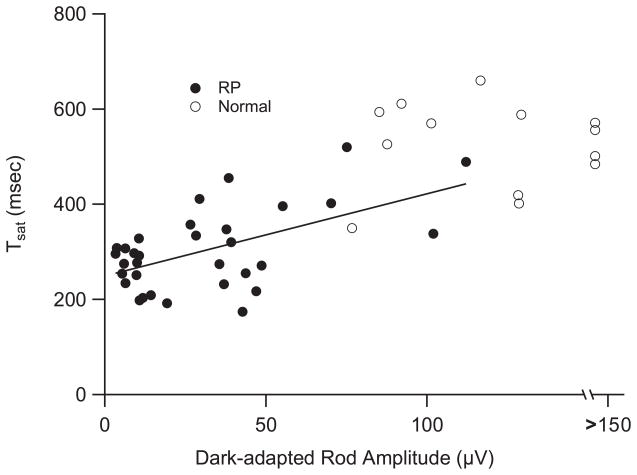
3.4 Tsat decreases when dark-adapted rod response amplitude decreases
The human ERG b-wave reflects the activity of bipolar cells within the nuclear layer (Hood and Birch, 1990a, 1992). With the assumption that rod-bipolar cell synaptic transmission remains unaffected by retinal degeneration, the dark-adapted ERG rod response (ISCEV standard) has been recognized as a surrogate marker for rod activity (Marmor et al., 2009). We examined the relationship between Tsat and the ISCEV standard rod response amplitude. As shown in Figure 3, there is a significant correlation between these two indices of rod dysfunction in 32 RP patients (Pearson’s Correlation Coefficient = 0.56, P<0.001). Thus, as ISCEV rod response diminishes, Tsat generally becomes shorter.
Figure 3.
Relationship between Tsat and the ISCEV standard rod response amplitude in RP. There is a significant correlation between these two indices of rod dysfunction in 32 patients with RP (filled circles) (Pearson’s Correlation Coefficient = 0.56, P<0.001). Thus, as ISCEV rod response diminishes, Tsat generally become shorter. The relationship between Tsat and rod amplitude in 13 normals is also shown (unfilled circles).
3.5 Effect of test flash intensity on Tsat
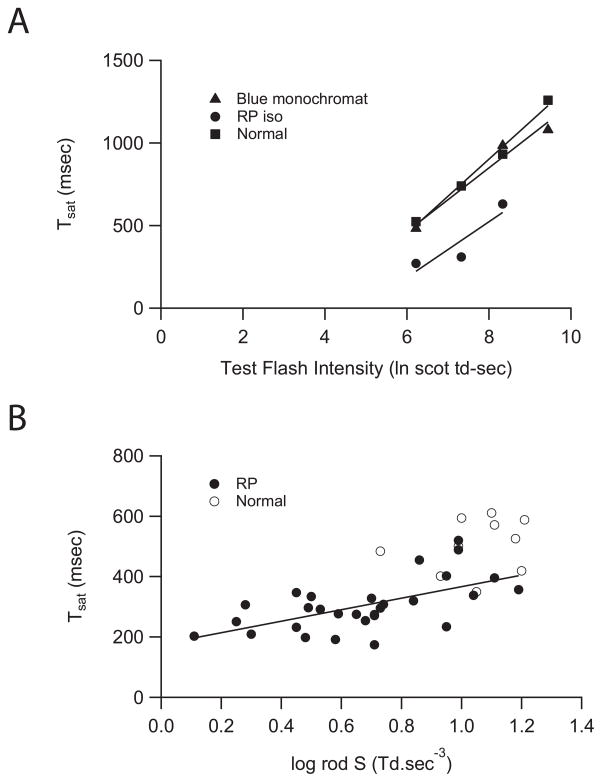
In single rod recordings, the relationship between Tsat and the natural logarithm (ln) of test flash intensity (Itest) can be divided into two branches (Pepperberg et al., 1992). The slope of the linear regression of the lower branch is the dominant time constant and index as the rate-limiting step of photoresponse inactivation (Krispel et al., 2006). Similarly, a two-branched relationship between Tsat and ln (Itest) was observed when Tsat is derived from paired-flash ERG in normal subjects and patients with pro-23-his associated adRP (Birch et al., 1995). In Figure 4A, we examined the lower branch of the Tsat and ln (Itest) function (6.2–9.4 ln scot td-sec) derived from paired-flash ERG. Figure 4A shows the linear relationship between the Tsat and the natural logarithm (ln) of test flash intensity (Itest) when Itest is between 6.2 and 9.4 ln scot td-sec, which is equivalent to 2.7 to 4.1 log scot td-sec. Decreasing the test flash intensity by a factor of 10 decreased Tsat by approximately 400 msec.
Figure 4.
Reduced Tsat derived from paired-flash ERG is correlated to reduced phototransduction gain (S). (A) Tsat is known to vary with the intensity of the test flash and the Tsat-intensity function can be divided into upper and lower branches (Birch et al., 1995). Figure 4A shows the linear relationships in the lower branch between 6.2 and 9.4 ln scot td-sec (2.7 to 4.1 log scot td-sec). (B) Earlier findings suggest that the dystrophic rods in patients with RP may have reduced phototransduction gain (log S) (Birch et al., 1995; Shady et al., 1995; Hood and Birch, 1996; Jacobson et al., 2000; Birch et al., 2002; Tzekov et al., 2003). Figure 4B shows a positive and significant correlation was found between Tsat and log S in the 29 patients with RP (r = 0.6, P<0.001) where both measures were available. Lines show linear fit to data.
Earlier findings suggest that the dystrophic rods in patients with RP may have reduced phototransduction gain (log S) (Birch et al., 2002; Birch et al., 1995; Hood and Birch, 1996; Jacobson et al., 2000; Shady et al., 1995; Tzekov et al., 2003). In other words, the dystrophic rods may not be as sensitive to the test stimulus as the healthy rods. Therefore, we determined the relationship between Tsat and phototransduction gain (log S). Within the sample of 32 patients with RP tested for inactivation parameters, phototransduction gain (log S) values were also available for 29 RP patients. A positive and significant correlation was found between Tsat and log rod S in these 29 patients with RP (r=0.6, P<0.001) (Fig. 4B). However, even patients with log rod S within the normal range showed lower than normal values of Tsat.
4. Discussion
We found that the rod photoresponse duration, as indexed by Tsat, is shorter in patients with RP than in normal subjects. Previously, we found the gain of the activation mechanism, as measured by the value of log S, is reduced in a majority of patients with rhodopsin mutations (Birch et al., 1995). Biochemically, reduced gain and premature inactivation will result in decreased synaptic input to bipolar cells. Together with rod loss, this helps to explain the loss of sensitivity of the ISCEV standard rod b-wave response and the elevated dark-adapted threshold in patients with rod degeneration. Studies of single rod recording support this argument. Pro-347-leu and pro-347-ser mutations in rhodopsin, like the rhodopsin mutations included in this study, lead to autosomal dominant RP in human. By direct recording from single rod photoreceptors in pigs, Kraft et al. (Kraft et al., 2005)showed that transgenic rods with pro-347-leu and pro-347-ser rhodopsin have earlier-than-normal time-to-peak, diminished sensitivity to light, and reduced integration time. Degenerating rods associated with the Mertk mutation (Gal et al., 2000) in Royal College of Surgeons (RCS) rat showed earlier than normal recovery without changes in gain (Niculescu, 2004). Similar results were found in albino rat rods after mild light damage (Wen, 2008).
In contrast to the findings in RP, we previously found that rod inactivation was significantly delayed in two patients with CRD associated with mutations in guanylate cyclase-activating protein (GCAP1) (Jiang et al., 2008). The delayed rod inactivation possibly resulted from the inability of mutant GCAP1 to respond to intracellular [Ca2+] drop when cGMP-gated cation channel is closed (Jiang et al., 2008). In transgenic mouse models, mutations in other genes directly involved in inactivation steps can also lead to delayed recovery from activation (Chen et al., 2000; Keresztes et al., 2004; Krispel et al., 2003). Thus, it seems that delayed inactivation kinetics is specific to a small number of mutations and may be useful in guiding mutation screening.
Although a correlation exists between shortened Tsat and rod b-wave amplitude (r = 0.56), Tsat measures cannot be replaced by rod b-wave measures. Because the rod b-wave reflects the massed rod response, response amplitude depends on not just sensitivity of rods, but also surviving rod count. Being a kinetic measure, Tsat is not affected by surviving rod count in retinal degeneration.
Tsat and log S are correlated since both reflect severity of the disease. However, they are not interchangeable since inactivation kinetics is faster than normal even in patients with normal sensitivity (Fig. 4B).
Furthermore, kinetic measures such as Tsat may be a more sensitive marker for rod health than sensitivity measures. Recent studies show that rod photoresponse recovery kinetics shorten to degenerative stress (Niculescu, 2004; Wen, 2008). However, the shortened rod recovery reverts back to normal timing during recovery in a normal environment. Recovery is less pronounced in sensitivity measures (Wen, 2008).
A major obstacle in developing treatment for heterogeneous genetic forms of RP is the prolonged time course of the disease. The annual rate of decline in b-wave amplitude is slow so that trials typically lasts years (Berson et al., 1993; Hoffman et al., 2004). Here we have shown that Tsat is abnormally short in patients with different forms of RP. Further studies are needed to determine whether recovery of Tsat might be a useful biomarker for treatment efficacy.
Acknowledgments
We thank Dr. Dianna Hughbanks-Wheaton and Kaylie Clark at the Southwest Eye Registry and the Daiger lab at UT Houston for coordinating and performing genetic testing. This investigation was supported by US National Institutes of Health grant (NEI R01 09076) to D.G.B and D.C.H. and the Foundation Fighting Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arikawa K, Molday LL, Molday RS, Williams DS. Localization of peripherin/rds in the disk membranes of cone and rod photoreceptors: relationship to disk membrane morphogenesis and retinal degeneration. J Cell Biol. 1992;116:659–667. doi: 10.1083/jcb.116.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson EL, Rosner B, Sandberg MA, Hayes KC, Nicholson BW, Weigel-DiFranco C, Willett W. A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Arch Ophthalmol. 1993;111:761–772. doi: 10.1001/archopht.1993.01090060049022. [DOI] [PubMed] [Google Scholar]
- Birch DG, Hood DC, Locke KG, Hoffman DR, Tzekov RT. Quantitative electroretinogram measures of phototransduction in cone and rod photoreceptors: normal aging, progression with disease, and test-retest variability. Arch Ophthalmol. 2002;120:1045–1051. doi: 10.1001/archopht.120.8.1045. [DOI] [PubMed] [Google Scholar]
- Birch DG, Hood DC, Nusinowitz S, Pepperberg DR. Abnormal activation and inactivation mechanisms of rod transduction in patients with autosomal dominant retinitis pigmentosa and the pro-23-his mutation. Invest Ophthalmol Vis Sci. 1995;36:1603–1614. [PubMed] [Google Scholar]
- Breton ME, Schueller AW, Lamb TD, Pugh EN., Jr Analysis of ERG a-wave amplification and kinetics in terms of the G-protein cascade of phototransduction. Invest Ophthalmol Vis Sci. 1994;35:295–309. [PubMed] [Google Scholar]
- Burns ME, Mendez A, Chen J, Baylor DA. Dynamics of cyclic GMP synthesis in retinal rods. Neuron. 2002;36:81–91. doi: 10.1016/s0896-6273(02)00911-x. [DOI] [PubMed] [Google Scholar]
- Chen CK, Burns ME, He W, Wensel TG, Baylor DA, Simon MI. Slowed recovery of rod photoresponse in mice lacking the GTPase accelerating protein RGS9-1. Nature. 2000;403:557–560. doi: 10.1038/35000601. [DOI] [PubMed] [Google Scholar]
- Cideciyan AV, Jacobson SG. An alternative phototransduction model for human rod and cone ERG a-waves: normal parameters and variation with age. Vision Res. 1996;36:2609–2621. doi: 10.1016/0042-6989(95)00327-4. [DOI] [PubMed] [Google Scholar]
- Dowling JE. The Retina: An Approachable Part of the Brain. Belknap Press of Harvard University Press; Cambridge, Massachusets: 1987. [Google Scholar]
- Gal A, Li Y, Thompson DA, Weir J, Orth U, Jacobson SG, Apfelstedt-Sylla E, Vollrath D. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat Genet. 2000;26:270–271. doi: 10.1038/81555. [DOI] [PubMed] [Google Scholar]
- Hagins WA, Penn RD, Yoshikami S. Dark current and photocurrent in retinal rods. Biophys J. 1970;10:380–412. doi: 10.1016/S0006-3495(70)86308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DR, Locke KG, Wheaton DH, Fish GE, Spencer R, Birch DG. A randomized, placebo-controlled clinical trial of docosahexaenoic acid supplementation for X-linked retinitis pigmentosa. Am J Ophthalmol. 2004;137:704–718. doi: 10.1016/j.ajo.2003.10.045. [DOI] [PubMed] [Google Scholar]
- Hood DC, Birch DG. A quantitative measure of the electrical activity of human rod photoreceptors using electroretinography. Vis Neurosci. 1990a;5:379–387. doi: 10.1017/s0952523800000468. [DOI] [PubMed] [Google Scholar]
- Hood DC, Birch DG. The A-wave of the human electroretinogram and rod receptor function. Invest Ophthalmol Vis Sci. 1990b;31:2070–2081. [PubMed] [Google Scholar]
- Hood DC, Birch DG. A computational model of the amplitude and implicit time of the b-wave of the human ERG. Vis Neurosci. 1992;8:107–126. doi: 10.1017/s0952523800009275. [DOI] [PubMed] [Google Scholar]
- Hood DC, Birch DG. Rod phototransduction in retinitis pigmentosa: estimation and interpretation of parameters derived from the rod a-wave. Invest Ophthalmol Vis Sci. 1994;35:2948–2961. [PubMed] [Google Scholar]
- Hood DC, Birch DG. Assessing abnormal rod photoreceptor activity with the a-wave of the electroretinogram: applications and methods. Doc Ophthalmol. 1996;92:253–267. doi: 10.1007/BF02584080. [DOI] [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Iannaccone A, Weleber RG, Fishman GA, Maguire AM, Affatigato LM, Bennett J, Pierce EA, Danciger M, Farber DB, Stone EM. Disease expression of RP1 mutations causing autosomal dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2000;41:1898–1908. [PubMed] [Google Scholar]
- Jiang L, Wheaton D, Bereta G, Zhang K, Palczewski K, Birch DG, Baehr W. A novel GCAP1(N104K) mutation in EF-hand 3 (EF3) linked to autosomal dominant cone dystrophy. Vision Res. 2008;48:2425–2432. doi: 10.1016/j.visres.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keresztes G, Martemyanov KA, Krispel CM, Mutai H, Yoo PJ, Maison SF, Burns ME, Arshavsky VY, Heller S. Absence of the RGS9.Gbeta5 GTPase-activating complex in photoreceptors of the R9AP knockout mouse. J Biol Chem. 2004;279:1581–1584. doi: 10.1074/jbc.C300456200. [DOI] [PubMed] [Google Scholar]
- Kraft TW, Allen D, Petters RM, Hao Y, Peng YW, Wong F. Altered light responses of single rod photoreceptors in transgenic pigs expressing P347L or P347S rhodopsin. Mol Vis. 2005;11:1246–1256. [PubMed] [Google Scholar]
- Kraft TW, Schneeweis DM, Schnapf JL. Visual transduction in human rod photoreceptors. J Physiol. 1993;464:747–765. doi: 10.1113/jphysiol.1993.sp019661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krispel CM, Chen C-K, Simon MI, Burns ME. Prolonged Photoresponses and Defective Adaptation in Rods of G{beta}5−/− Mice. J Neurosci. 2003;23:6965–6971. doi: 10.1523/JNEUROSCI.23-18-06965.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krispel CM, Chen D, Melling N, Chen YJ, Martemyanov KA, Quillinan N, Arshavsky VY, Wensel TG, Chen CK, Burns ME. RGS expression rate433 limits recovery of rod photoresponses. Neuron. 2006;51:409–416. doi: 10.1016/j.neuron.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Lamb TD, Pugh EN., Jr A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J Physiol. 1992;449:719–758. doi: 10.1113/jphysiol.1992.sp019111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmor MF, Fulton AB, Holder GE, Miyake Y, Brigell M, Bach M. ISCEV Standard for full-field clinical electroretinography (2008 update) Doc Ophthalmol. 2009;118:69–77. doi: 10.1007/s10633-008-9155-4. [DOI] [PubMed] [Google Scholar]
- Niculescu DM. Physiological Optics. University of Alabama at Birmingham; Birmingham, AL: 2004. Dissertation: Physiological Characterization of the Light Response of Rod Photoreceptors in the Dystrophic Royal College of Surgeons Rat. [Google Scholar]
- Nishiguchi KM, Sandberg MA, Kooijman AC, Martemyanov KA, Pott JW, Hagstrom SA, Arshavsky VY, Berson EL, Dryja TP. Defects in RGS9 or its anchor protein R9AP in patients with slow photoreceptor deactivation. Nature. 2004;427:75–78. doi: 10.1038/nature02170. [DOI] [PubMed] [Google Scholar]
- Ogden TE. Clinical electroretinography. 2. Mosby; St. Louis, Missouri: 1994. [Google Scholar]
- Pepperberg DR, Birch DG, Hood DC. Photoresponses of human rods in vivo derived from paired-flash electroretinograms. Vis Neurosci. 1997;14:73–82. doi: 10.1017/s0952523800008774. [DOI] [PubMed] [Google Scholar]
- Pepperberg DR, Cornwall MC, Kahlert M, Hofmann KP, Jin J, Jones GJ, Ripps H. Light-dependent delay in the falling phase of the retinal rod photoresponse. Vis Neurosci. 1992;8:9–18. doi: 10.1017/s0952523800006441. [DOI] [PubMed] [Google Scholar]
- Pugh EN, Jr, Lamb TD. Amplification and kinetics of the activation steps in phototransduction. Biochim Biophys Acta. 1993;1141:111–149. doi: 10.1016/0005-2728(93)90038-h. [DOI] [PubMed] [Google Scholar]
- Schnapf JL, Kraft TW, Baylor DA. Spectral sensitivity of human cone photoreceptors. Nature. 1987;325:439–441. doi: 10.1038/325439a0. [DOI] [PubMed] [Google Scholar]
- Shady S, Hood DC, Birch DG. Rod phototransduction in retinitis pigmentosa. Distinguishing alternative mechanisms of degeneration. Invest Ophthalmol Vis Sci. 1995;36:1027–1037. [PubMed] [Google Scholar]
- Steinberg RH, Frishman LJ, Sieving PA. Negative components of the electroretinogram from proximal retina and photoreceptors. Oxford: Pergamon; 1991. [Google Scholar]
- Travis GH, Sutcliffe JG, Bok D. The retinal degeneration slow (rds) gene product is a photoreceptor disc membrane-associated glycoprotein. Neuron. 1991;6:61–70. doi: 10.1016/0896-6273(91)90122-g. [DOI] [PubMed] [Google Scholar]
- Tzekov RT, Locke KG, Hood DC, Birch DG. Cone and rod ERG phototransduction parameters in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2003;44:3993–4000. doi: 10.1167/iovs.02-1104. [DOI] [PubMed] [Google Scholar]
- Wen Y. Neurobiology. University of Alabama at Birmingham; Birmingham, Alabama: 2008. Dissertation: Physiological Characterization of the Light Response of Rod Photoreceptors in the Light Damaged Rat. [Google Scholar]