Abstract
It is widely accepted that microorganisms are social beings. Whereas communication via chemical signals (e.g. quorum sensing) has been the focus of most investigations, the use of physical signals for microbial cell-cell communication has received only limited attention. Here, I argue that physical modes of microbial communication could be widespread in nature. This is based on experimental evidence on the microbial emission and response to three physical signals: sound waves, electromagnetic radiation, and electric currents. These signals propagate rapidly and, even at very low intensities, they provide useful mechanisms when a rapid response is required. I also make some suggestions for promising future research avenues that could bring novel and unsuspected insights into the physical nature of microbial signaling networks.
Can microbial conversations get physical?
Communication is by definition a process of information (signal) exchange between a sender and a receiver through a common medium. Quorum sensing enables microorganisms to communicate chemically by responding coordinately to the accumulation of extracellular chemical signals (autoinducers) and reprogramming gene expression as a function of cell density [1]. Experimental evidence is also available that indicates that microorganisms can generate and respond to physical signals such as sound waves, electromagnetic radiation and electric currents [2–6]. However, the technical challenges associated with probing microbial physical signaling networks at the intensities and time scales required have for long limited this field of research. As a result, the role of physical signals as information carriers has received only limited attention.
Recent technological advances now enable the physical probing of microbial cells with unprecedented sensitivity, and the detection, characterization and quantification of physical signals that could function in cell-cell communication. These new approaches provide the intellectual framework to reinterpret early work on physical signaling and support the notion that physical modes of microbial communication are widespread. Here I describe the available evidence that supports the role of sound waves, electromagnetic radiation, and electric currents in cell-cell communication with a focus on microorganisms, both prokaryotic (bacteria) and eukaryotic (yeast and protozoa). I also provide my opinion about the limitations of these studies, outstanding questions, and what I consider the most promising directions to advance this field of research. Unlike chemical signals, physical signals are subjected less to diffusion constraints and can propagate through a wide range of media, including cells, which I propose can enable faster cellular responses. I also present evidence that links physical signaling to the metabolic status of the emitting and recipient cells, and speculate about the possibility that the energy carried in these signals is the real ‘language’ used in physical modes of microbial communication. With this Opinion, I hope to stimulate research in this controversial, yet exciting, field of research that has only been marginally explored.
‘If microorganisms could talk…’: cell-cell communication via sound waves
The ability of cells to communicate with sounds was suggested based on the observation that sound waves stimulated the growth of Bacillus carboniphilus under stress conditions [2]. Though unable to grow in high salt concentrations or at high temperature [7], growth was stimulated by neighboring cells of the same or different species grown on a separate plate stacked on top and regardless of the presence of a separating 2 mm iron barrier to prevent the exchange of volatile substances [8]. Growth under non-permissive conditions was also stimulated by specific sound frequencies applied with an external speaker [2] and by including in the growth medium carbon materials, such as graphite or activated charcoal, known to convert external electromagnetic radiation into sound [9]. These results suggested that the growth-stimulating signal was physical and, possibly, sonic in nature. Interestingly, although no sound waves were detected from dense lawns of B. carboniphilus and other bacteria and yeast, Bacillus subtilis produced reproducible sound spectra [2]. The average spectrum had three broad frequency peaks that matched well with the B. carboniphilus growth-promoting frequencies. Thus, these early studies suggested that some microbial cells might be able to communicate using sounds.
Intracellular motions and sound communication
Sound waves are generated when objects vibrate. Essential cellular processes, such as the activity of molecular motors and the cytoskeleton, enzymatic reactions, chromosome packaging and replication, transcription, and protein synthesis, folding or unfolding, generate forces that induce intracellular motions [10–19]. The polar oscillation of proteins during cell division [20] or cytoskeleton assembly [21] also contribute greatly to the dynamics of the cell interior and generate polarizing ionic currents inside the cell and charge-induced nanoscale motions. The global effect of intracellular motions is that cells and their components vibrate. Using the sensitivity of an atomic force microscope (AFM) to probe cellular nanomechanics in an acoustically insulated environment, Pelling et al. demonstrated that the cell wall of single, living cells of Saccharomyces cerevisiae exhibited local, periodic nanoscale motions of similar average amplitude (3.0 ± 0.5 nm), yet variable, temperature-dependent frequencies (0.9–1.6 kHz) [22]. These results not only demonstrated that intracellular motions were strong enough to propagate across the stiff yeast cell wall, they also showed they could generate reproducible acoustic signals. Furthermore, the motions disappeared after treatment with the metabolic inhibitor sodium azide, suggesting they had a metabolic origin. The oscillations of single cells had activation energies (58 kJ/mol) and velocities such as those reported for cytoskeleton motors. The magnitude of the forces (~10 nN) was such that it would have required the concerted action of several molecular motors, as expected of a dedicated system that transmitted the metabolic status of the cell as mechanical vibration and sound [22].
Can sounds carry information for cell-cell communication?
The transduction of intracellular motions into external sounds supports the idea that cells emit sound waves to carry information about their metabolic status. The metabolic status reflects the internal energy of the cell, and, therefore, is likely to modulate the intensity and frequency of the vibrations and acoustic signals that are generated (Figure 1a). The amplitude of the sound wave measures its intensity, i.e., the amount of energy in the wave, and correlates well with the intensity of the cell’s vibration. Intensity also determines how far sound waves can travel. With sufficient intensity (and adequate frequency), the sound signal could reach a receptive cell with enough force so as to induce it to vibrate. The induced vibrations could modulate the recipient’s metabolism. The same activation energies of the yeast’s cell wall oscillations [22] activate or deactivate DNA regulatory proteins [23]. Even low frequency conformational vibrations resulting from changes in DNA hydration and counter-ion binding can affect promoter structure and DNA-protein binding interactions and modulate gene expression [24, 25]. External sources of energy that, similar to sound waves, trigger nanoscale motions and polarization in the cell have also been linked to changes in gene expression and cell growth [26–28], enzymatic activity [29] and the expression of genes involved in energy metabolism [30]. Sound signals could also be transduced by specific membrane receptors. Mechanosensitive ion channels, widespread among microorganisms, allow the influx of ions and internal polarization in response to stretch forces in the lipid bilayer, thereby providing a mechanism for the reception and transduction of sound signals [31].
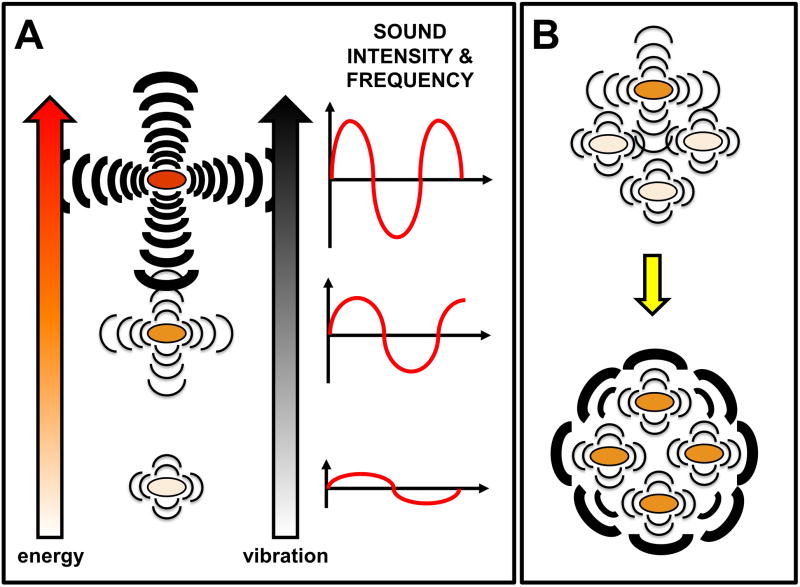
Figure 1.
Cell-cell communication via sound waves. (a) Correlation between the metabolic status (and, therefore, the energy available inside the cell), the cell’s vibration, and the emitted sound. The cell’s metabolic status determines the level of activity of cellular processes, such as transcription and translation, that result in the generation of internal motions. These motions appear to be able to produce a generalized vibration of the cell with characteristic intensity and frequency that, therefore, would reflect its unique metabolic status. The vibrations would propagate through the medium as sound waves. The intensity, or energy contained in the wave, would control how far the signal can travel. The frequency, or the ‘pitch’ of the sound, would control whether a cell can ‘sense’ the incoming signal or not. If the receiving cell is receptive to that particular frequency it will vibrate proportionally to the intensity of the signal, and the vibrations might induce a biological response (e.g. growth). This might also result in the emission of additional sound waves with unique characteristics of intensity and frequency that could propagate to reach other cells. (b) Energy dissipation and cell’s vibrational response as a result of sound communication enables the coordinately dissipation of the energy of the cells. A coherent collective vibrational mode (bottom) is reached when all the cells are ‘in tune’, which could amplify the signal.
Because sound waves spread by inducing the vibration of particles in the propagating medium, the energy of the sound wave is ‘diluted’ progressively. This creates gradients of energy analogous to the chemical gradients of autoinducers in quorum sensing, which ultimately determine the maximum ‘calling’ distance for effective communication [32]. Therefore, cells in communal settings, such as colonies, biofilms and microbial mats, are likely candidates to benefit from sound communication. Such close populations would allow the rapid propagation and detection of sounds, even at low intensities, and could cooperate to amplify the sound signal from individual cells (Figure 1b).
An interesting aspect of sound signaling that deserves further study is the role that sound frequency has in cell-cell communication. Every particle has a unique natural frequency of vibration and, therefore, produces a distinctive sound, very much like voice tonality and pitch in humans. Not surprisingly, the acoustic frequency ranges of signals emitted by yeast cells (0.9–1.6 kHz) [22] differed substantially from the signals emitted by colonies of B. subtilis (8–43 kHz) [2]. Furthermore, temperature, which modulates the metabolic activity of the cell and its intracellular vibrations, affected the specific frequency, but not the intensity, of the yeast’s oscillatory motions [22]. Hence, sound frequencies could provide information about the sender and its metabolic status. Furthermore, mechanical systems such as microbial cells absorb more energy when the frequency of the incoming vibration matches their natural frequency of vibration, a process known as mechanical resonance. This suggests that cells could be more receptive to the sounds generated by cells of the same species and could even sense their own frequencies in a noisy environment, thereby providing a mechanism to differentiate friends from foes.
Communication via biophotons
Many organisms, from bacteria to fish, can generate electromagnetic radiation or ‘light’ (Box 1) from exergonic chemical reactions involving specific substrates and enzymes, a process known as bioluminescence [33]. Yet a wide range of non-luminous microorganisms, including bacteria, yeast and protozoa, emit ‘biophotons’ as an ultraweak type of radiation covering the visible and/or near-infrared spectrum [34–37]. Remarkably, the reciprocal emission and response to biophotons has been demonstrated in a few microorganisms. Studies with Escherichia coli populations grown in a glass cylinder separated by a clear or opaque glass window (to permit, respectively, the passage of light or to filter visible and UV light) demonstrated reciprocal interactions between the neighboring populations only when separated by the glass window [3]. In general, growth was stimulated in cultures separated with a clear glass window compared to the controls, suggesting that wavelengths in the visible ranges or longer were necessary to induce a biological response. Light emissions between 450–800 nm were measured between the cultures using two identical photon-counting machines and showed a direct link between biophoton emission in the visible and/or near-infrared region and the growth parameters. Interestingly, the correlation between culture density and radiation was not linear, a phenomenon that has been observed in other organisms [38] and deserves further investigation. Nevertheless, these results are in agreement with studies in mammalian cells and other bacteria that suggest that visible and/or infrared radiation can function as an information carrier [36, 37, 39]. Although biophoton emission has been linked to intracellular increases in reactive radicals [34, 40, 41], similar reciprocal interactions were reportedly reproduced after treating the cultures with laser irradiation, which decreases the levels of radical formation inside the cells [3]. This suggests that other cellular mechanisms can generate biophotons as well.
Box 1. The electromagnetic radiation spectrum.
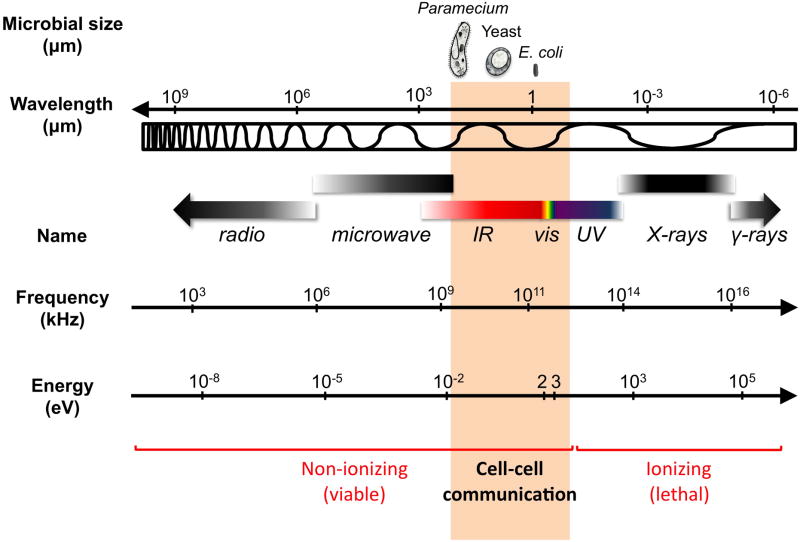
The electromagnetic spectrum spans a broad range of wavelengths, from X-rays to radio waves, with characteristic frequencies and energies (Figure I). As a result, each type of radiation has unique properties and must be considered independently. In general, radiation below the visible wavelengths carries enough energy to ionize molecules and atoms and be lethal to microorganisms. Except for a few, rare resistant microorganisms, ultraviolet (UV) irradiation leads to the formation of highly reactive radicals and directly damages DNA beyond repair. Because of the ionizing power of radiation below the visible spectrum, it is unlikely to be relevant in cell-cell communication. On the other hand, radiation above the low infrared has broader wavelengths than the average size of microbial cells to be relevant for signaling. This narrows down the electromagnetic radiation that could serve as an information carrier for microbial cell-cell communication to the visible (400–750 nm) and near-infrared (750–2500 nm) region of the spectrum, also known as biophoton emission. Ultraweak UV photon emission (mitogenetic radiation) has been reported as a microbial signal [80], yet these studies often used detection systems lacking the sensitivity required for measurements in the UV region. Thus, whether this type of radiation can function as an information carrier in microorganisms remains to be determined.
A recent study [4] with the protozoan Paramecium caudatum also showed a reciprocal response of large and small populations separated by materials that transmitted (quartz) or blocked (glass) UV wavelengths (250–340 nm). Because of the variability inherent to working with heterogeneous cultures, random combinations of cultures and controls were used for each separating material and the significance of the results was evaluated with statistic tests. Positive or negative effects in growth, cell division and energy-uptake were observed as a function of the separating material and the density of the cultures acting as senders or receivers. Although biophoton exchange was not directly measured nor was a particular biological response conclusively associated to a specific type of radiation, this study suggested that a cell-cell communication system via electromagnetic radiation of specific wavelengths and frequencies was operative.
Can biophoton communication be biologically relevant?
Spontaneous biophoton emission has, in general, been linked to metabolic disturbances that generate reactive oxygen species such as in stationary phase and under conditions of chemical or physical stress [34, 40–43]. Yet all microorganisms have some degree of emission, suggestive of an intrinsic physical property of the cell, rather than a metabolic aberration resulting from the tendency of the disturbed system to return to thermal equilibrium. An explanation that I favor is that biophoton emission is a consequence of a delocalized coherent electromagnetic field formed inside the cell [44]. Microbial cells are subjected to longitudinal polar oscillations of proteins, such as during cell division [20] and the assembly of asymmetric cellular appendages such as flagella, pili or stalks [45], that displace cytoplasmic ions from pole to pole. The continuous motion of charged particles inside the cell generates an electromagnetic field and, therefore, a potential source of electromagnetic radiation. Mechanical, thermal and chemical stresses, metabolic activity, and growth stage can modulate the intracellular ionic currents and, hence, biophotonic activity.
Unlike sound waves, electromagnetic radiation does not depend on vibrations for transmission. Hence, it does not require a medium for propagation and decays very slowly [38]. Because of this, biophotons can theoretically propagate without progressive dilution and as a function of the intensity of the signal. Disturbances of the cell’s thermal equilibrium could regulate the intensity of the signal and, therefore, how ‘loud’ it is. According to Kirchhoff's law, at thermal equilibrium, the emission of electromagnetic radiation by a system equals its absorption. This suggests that emitting cells also can absorb incoming radiation. The intensity and frequency of the radiation could also finely regulate how ‘receptive’ a cell is to the incoming radiation. Cells might also use specific membrane receptors to sense incoming biphotonic activity. The observed spectral ranges for biophoton emission are in the region of the spectrum (ca. 400–750 nm wavelengths) that provides the energy for microorganisms with light-dependent energy transduction mechanisms [46]. These organisms use dedicated photoreceptors for light transduction, yet genes encoding homologues of photosensory receptors are widespread in chemotrophic, non-phototrophic microorganisms [47] and could provide a conserved mechanism for the use of light in cell-cell communication.
‘Wired’ electronic networks for cell-cell communication
Extracellular electron transfer via microbial nanowires
The defining property of all life forms is the generation of energy through electron transfer processes such as respiration, photosynthesis, and elemental cycling. In dissimilatory metal-reducing microorganisms such as Geobacter and Shewanella the electrons are transferred outside the cell and to Fe(III) oxide minerals, using, respectively, a direct contact mechanism [48] and soluble mediators [49]. Electronic contact in Geobacter bacteria requires the expression of type IV pili that, remarkably, do not mediate common pili functions such as surface motility (twitching) and adhesion [5]. They are conductive and required for metal respiration, suggesting they are the electrical connection between the cell and the mineral. In the model organism Geobacter sulfurreducens, conductive pili are also expressed in the absence of Fe(III) oxides when grown under suboptimal growth conditions [50]. Thus, pili production is not specifically associated with the presence of metal oxides, but rather with the physiological state(s) associated with suboptimal growth, which occurs at lower temperatures, during growth transitions, and when G. sulfurreducens has to use insoluble electron acceptors. Expression of conductive pili in the absence of Fe(III) oxides also causes cells to agglutinate [50], a process that promotes direct exchange of electrons between cells [51]. Furthermore, the pili also serve as structural and electronic conduits between cells in biofilms formed on Fe(III) oxide coatings and electrodes [6, 50]. Substantial spacing also is observed among the biofilm cells, consistent with an electronic pathway in which the pili transfer electrons along their length.
One of the interesting features of Geobacter’s pili is that they are conductive even at the low voltages [5] that would be biologically relevant for electronic communication between cells [52]. Furthermore, the resistance to electron flow is higher at low voltages than at high voltages [52]. High voltages such as those found between a cell and the Fe(III) oxide mineral will, therefore, enable fast electron flow via pili. In contrast, the low (±300–400 mV) redox potentials of microbial cells [53] would favor the controlled flow of electrons via the high resistance pathway and as a function of the differential potential, and, therefore, the metabolic status, of the connected cells. The retractable nature of bacterial pili [54] also provides a mechanism to adjust the nanowire length and cell spacing for optimum electronic communication between the most electronegative (sender) and the most electropositive (recipient) cells [52] (Figure 2a).
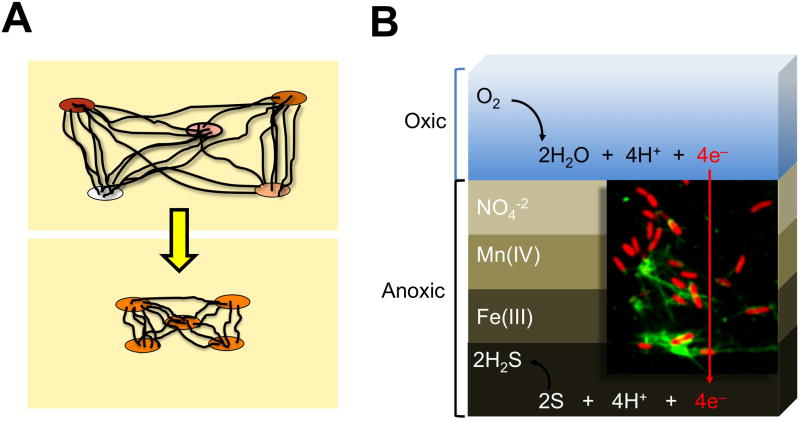
Figure 2.
Wired electronic networks. (a) The retractability of pilus nanowires could enable the control of nanowire length as a function of the redox status of the cells that are connected to the electronic grid, thereby promoting cooperative electronic dissipation. (b) The fast dissipation of electrons via nanowire networks has been hypothesized to couple spatially separated processes in the sediments, thus linking the reduction of oxygen (O2) in the upper, oxic layers to the anoxic production of hydrogen sulphide (H2S) in the bottom layer of the anoxic sediments [57]. Inset shows a fluorescence micrograph of Geobacter sulfurreducens cells (red) connected by pilus nanowires (green, detected with anti-pilin antibodies).
Similar to other bacterial pili, Geobacter pili are formed by polymerization of a single pilin peptide subunit. However, the Geobacter pilin gene is quite divergent and forms an independent line of descent within the family Geobacteraceae [5], suggesting that electrical communication via conductive pili might be widespread in this bacterial family. Other bacteria such as Shewanella oneidensis, which are phylogenetically distant from Geobacter, also produce conductive conduits between their cells that are composed of c-cytochromes [55], suggesting that, despite mechanistic differences, wired networks might operate in other bacteria.
Wired electronic communication in the environment
The discovery of microbial nanowires as cellular electronic conduits supported the notion that wired electronic networks could act as carriers of information between cells in the environment. Laboratory studies using saturated sand columns inoculated with S. oneidensis linked the production of nanowire-like structures to the appearance of electrical potentials corresponding to the chemical reactions carried out across the sediments [56]. As shown in Figure 2b, electric currents also couple discreet biogeochemical processes across marine sediments, from oxygen consumption in the aerobic layers to hydrogen sulphide and organic carbon oxidation in the deep, anaerobic sediment layers [57]. Despite the spatial separation of the two processes (ca. 12–19 mm), O2 consumption in the top layers was rapidly (less than 1 h) coupled to the oxidation of hydrogen sulphide and organic carbon in the sediment depths, thereby ruling out chemical diffusion. A distinct pH peak was also measured during O2 consumption, consistent with a process such as the transport of electrons that generates protons. The electric currents directed to the anoxic zone enabled the consumption of ca. 40% of the total O2 in the upper layers, demonstrating that electronic networks contribute greatly to the biogeochemical processes of the sediments. These studies help explain why burying electrodes in sediments harnesses an electric current [58], a process that also requires the electrical connections of Geobacter’s pili [6, 59]. The current densities measured across the sediments [57] also correlated well with the current densities measured for sediment electrodes [58], consistent with a similar biological mechanism for the propagation of the electric currents. Most importantly, these studies also suggest that many microorganisms could be connected in these natural power grids. However, we know very little about the microbial diversity of these electronic networks and the interactions among their integrants.
Is electronic communication a widespread form of microbial cell-cell communication?
Although knowledge about electronic communication is set to advance quickly thanks to studies of nanowire-producing bacteria, all microorganisms are likely to communicate electrically at some level due to their polarizable nature. Well known examples are the electrical reorientation of cells and voltage-directed swimming patterns (galvanotaxis or electrotaxis) [60] and cell growth reorientation (galvanotropism) [61, 62]. The plasma membrane gives cells a net electrostatic charge [63, 64] and a fluidic medium for the charge-dependent diffusion of membrane-associated proteins [64, 65]. Electrical stimulation can directly modulate the activity of membrane-bound ion channels and control intracellular ion fluxes and cell polarization [60, 62, 66]. Electrical signals also change the membrane potential and promote the reorganization of anionic phospholipids and formation of localized charge and permeability foci that attract membrane proteins, including ion channels, and regulate their activity [64, 67, 68]. Membrane phospholipids also have intrinsic curvature tendencies and preferentially localize to regions of the cell with the highest or lowest curvatures [69, 70]. These defined lipid domains anchor intracellular hyperstructures and provide a pathway that links changes in the membrane’s potential to essential biological processes such as DNA replication, chromosome segregation, and cytokinesis [69].
Voltage-activated ion channels also are widespread in the three domains of life [71] and could serve as specific membrane receptors for electrical signals. Similar to their eukaryotic counterparts, the prokaryotic voltage-dependent channels studied thus far are ion-specific and voltage-gated, but the gating speeds and kinetics differ significantly [72, 73]. This suggests that each receptor has evolved to respond to particular electrical signals. Once a specific threshold voltage is reached, the channels open and allow the passage of positively charged ions inside the cell. This makes the cell more electropositive and favors the opening of more channels. The polarization changes are coupled to transcriptional regulation [74] and provide yet another mechanism for cell reprogramming as a function of the electrical signal being received.
Thus, the polarizable nature of microbial cells provides mechanisms to control the cell’s electrical potential and its ability to act as sender or receiver of electric signals as a function of its redox status. The medium used to propagate the electric signal need not be restricted to nanowires. Other forms of signal propagation also might be possible, including ionic or metallic conductance through the extracellular medium, which could be relevant in biofilms and in sediments with conductive minerals, respectively, or direct cell-cell contact.
Why physical signaling?
The evolutionary significance of physical modes of communication can be best understood when compared to chemical communication. Regardless of the nature of the chemical signal, quorum sensing requires substantial energy expenditure for the synthesis of the signal and cognate receptor. The signal also must be synthesized in sufficient quantities to reach the minimum threshold of detection by the cognate receptor and account for its diffusion and dilution. In contrast, physical signals require minimum energy investment because they operate at low intensities and use the energy liberated from natural cellular processes. For example, the metabolic activity and growth rate of a cell increase in environments rich in nutrients. This also increases the oscillatory motions and polarization of the cell that lead to the production of sound waves, electromagnetic radiation or electrical signals. In this manner, the ‘excess’ energy can be used to generate and transmit physical signals. Once they reach a recipient cell, they induce its vibration and/or polarization. The intensity and frequency of the incoming signal as well as the metabolic status of the recipient cells will ultimately determine the recipient’s response and how ‘receptive’ it is to the incoming signal.
At low intensities physical signals provide a fine-tuning mechanism for cells to communicate their energy status and coordinately dissipate excess energy cooperatively to maximize the use of the available resources. However, above a particular intensity threshold, such as when the cell is under conditions of stress or high cell density, the signals could carry too much energy and be too ‘loud’. Such loud signals could even function as cellular alarm systems and induce dispersal or reprogrammed cell death similarly, for example, to how the frequency and intensity of ultrasonication can have growth-promoting effects or be a powerful particle dispersing or disinfecting agent depending on [75]. Dispersal enables the colonization of new, less adverse ecological niches, while specific frequencies could even be used to prey on more sensitive cells of the same or different species as a means to increase nutrient availability.
Physical signals also propagate faster than chemical signals because they are less limited by diffusion. This provides a superior mechanism for cell signaling when a rapid response is required. In addition, they can propagate across the cell envelope of a recipient cell and directly affect enzyme activity and gene expression, thereby bypassing the requirement for a cognate receptor on the plasma membrane. Mechanical deformations of the cell membrane and generalized vibrations induced by physical signals can, for example, be transmitted through intracellular protein matrices at the speed of sound [10], a rapid transmission pathway that could provide cells with a response advantage.
The lack of diffusion constraints and the rapid propagation of the signal also make physical signaling ideal for cell-cell communication over long distances. While the most frequent ‘calling distance’ reported for quorum sensing in the environment is in the low micrometer ranges (and only exceptionally reaching millimeter ranges) [32], physical signals have been reported to operate over millimeter to centimeter distances [2, 4, 57]. The distance that physical signals, such as sound and light, can travel is dependent on the intensity of emission and, in porous media, on its frequency. Because they can also to be sensed at very low intensities, signals from single cells, individual cellular processes or the activity of single molecules could produce an ‘audible’ signal for cells located at great distances.
Qualitative differences among the physical signals could also determine the mode of communication used by different microorganisms. Sounds and light, for example, propagate faster in non-porous media because scattering is minimized. They are therefore expected to be more relevant for cell-cell communication over shorter distances or in non-porous environments such as in colonies and biofilms. In contrast, electronic communication via nanowires would be more effective in porous environments such as the Fe(III) and Mn(IV) oxide-rich environments inhabited by dissimilatory metal reducers. This is because the nanowires protrude from the cell and penetrate through the pores of the minerals for increased access to the electron acceptors and other cells. Not surprisingly, nanowires have been proposed to couple biogeochemical reactions that are spatially separated by millimeter to centimeter distances [56, 57].
Conclusions and future directions
Growing experimental evidence supports the notion that microorganisms can generate and respond to signals that are physical in nature. However, many outstanding questions still remain (Box 2). More work is needed to conclusively demonstrate that physical signals act as carriers of specific information. Progress in this field can only advance as technical barriers are overcome. Microbial cultures are metabolically heterogeneous and the physical properties of individual cells or molecules are often masked in a culture or a colony. Probing the physical behavior of single cells would be advantageous yet technically difficult. However, the past years have witnessed the development of powerful methods and instruments that could be applied to the study of microbial physical signaling. Nanoscale voltmeters have been developed that enable the mapping of the electric field inside a cell [76] and could revolutionize investigations on biophoton and electrical communication. Ultra-low light detectors coupled to electron multiplier cameras are also available and have been used to demonstrate biophotonic conduction along neural fibers [77]. Such sensitive photonic sensing devices could greatly advance the field of biophoton communication and, when coupled to nanomicroscopes, they could enable the detection and visualization of electronic signals carried by microbial components in situ.
Box 2. Outstanding questions.
What specific information do physical signals carry?
Are physical signals transduced by specific cognate receptors on the membrane?
Do microbial cells use specific, intracellular signaling networks or rely on global, unspecific responses?
How related are all types of physical signaling? That is, are they all manifestations of the same physical phenomenon, such as the degree of polarization of the cell?
Could energy be the real ‘language’ in all modes of physical communication?
Because the cell envelope cannot function as a barrier for non-mechanical signals [78], the possibility of cells having specific membrane-associated cognate receptors has never been explored. Yet specific mechanosensors might have evolved to activate signaling cascades in response to sound-induced vibrations, ultraweak radiation could be sensed and transduced by dedicated photosensory receptors, and voltage-activated channels might participate in the transduction of electrical signals. Similarly, little is known about how specific intracellular physical signaling networks are. While global, unspecific responses such as intracellular motions and polarization are known to participate in the transduction of physical signals, microorganisms might employ dedicated signaling networks. Physical and chemical signaling networks are intimately connected in the cell and it is challenging to dissect the contribution of one and another with the sensitivity required to make meaningful conclusions. However, genetic approaches, such as the isolation of mutants deficient in the generation or response to the signal, coupled to sensitive methodologies for physical cellular probing could provide fundamental insights into physical transduction in microbial cells.
The most outstanding question is perhaps how related all types of physical modes of communication really are. The charge of the cell membrane, intracellular mechanical motions, and polar oscillations are all fundamental physical properties of biological systems that control the degree of ionic (electric) polarization inside the cell. The biological consequence of the polarizable nature of microbial cells is that physical signals can be interconverted similar to the conversion of physical signals in human telecommunication (Figure 3). Some authors, for example, have suggested that the growth-promoting effects that sound waves have on some microorganisms [2] can also be interpreted if electromagnetic radiation, rather than a sound signal, was used as a communication carrier [23]. Because all cells are charged systems and, therefore, electrically excitable, they could also display optical properties and emit electromagnetic radiation, a process known as electroluminescence [79]. This raises the question of how specific physical signals really are. Because the unifying property of all physical signals is the energy they carry, energy could be the specific information transmitted from cell to cell. After all, life is the interaction of matter and energy. Physical signaling could be an ancestral language of all living forms and, perhaps, a key code to decipher if we want to understand the microbial conversations that have for so long remained inaudible.
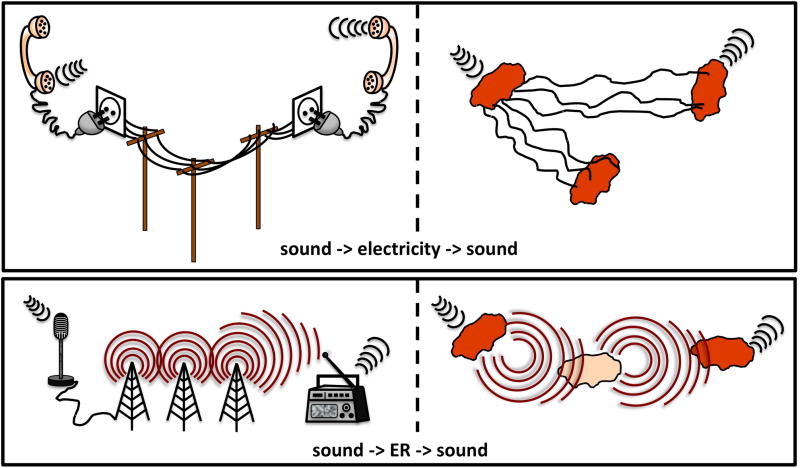
Figure 3.
Interconversion of physical signals in human communication networks (left) and microbial analogs (right). In all panels, the sender is on the left, the receiver on the right, and information carriers in the middle. In a manner analogous to the interconversion of sounds and electric signals in fixed telephone lines, microbial cells might be polarized by incoming sound waves of the correct frequency to control the flow of electrons and electric currents generated or received by the cell and vice versa (top panel). Furthermore, the degree of polarization could also control the electrical excitability of the cell and the intracellular nanoscale motions that could induce sound-generating vibrations. The interconversion of electromagnetic radiation (ER) [GT1]and sounds during radio broadcasting also could have a microbial analog (bottom panel). The intracellular movement of charged particles that is induced by sound waves could create an electromagnetic field and serve as a source of ER. The reverse would be also possible: ER might induce mechanical vibration and polarization of the cell, thereby enabling the generation of sounds and electric currents, respectively.
Figure I.
The electromagnetic spectrum. Microbial sizes (for the unicellular protozoan Paramecium, a Saccharomyces yeast cell and the bacterium E. coli) and radiation wavelengths (in micrometer, μm) are shown on top. The name of the radiation, its frequency (kiloHertzs, kHtz) and the energy of the photons (electron volts, eV) are shown below. Scales are approximate. The shaded area of the non-ionizing radiation shows the region of the spectrum that is likely to be relevant to microbial cell-cell communication.
Acknowledgments
I would like to express my gratitude to Cesar Sanchez for critical reading of this manuscript and helpful suggestions. This work was supported by grants R01 ES017052–01 from the National Institute of Environmental Health Science Superfund Program and MCB-1021948 from NSF, and a Strategic Partnership Grant from the Michigan State University Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ng WL, Bassler BL. Bacterial quorum-sensing network architectures. Annu Rev Genet. 2009;43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsuhashi M, et al. Production of sound waves by bacterial cells and the response of bacterial cells to sound. J Gen Appl Microbiol. 1998;44:49–55. doi: 10.2323/jgam.44.49. [DOI] [PubMed] [Google Scholar]
- 3.Trushin MV. Studies on distant regulation of bacterial growth and light emission. Microbiology. 2003;149:363–368. doi: 10.1099/mic.0.25825-0. [DOI] [PubMed] [Google Scholar]
- 4.Fels D. Cellular communication through light. PLOS One. 2009;4:1–8. doi: 10.1371/journal.pone.0005086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reguera G, et al. Extracellular electron transfer via microbial nanowires. Nature. 2005;435:1098–1101. doi: 10.1038/nature03661. [DOI] [PubMed] [Google Scholar]
- 6.Reguera G, et al. Biofilm and nanowire production lead to increased current in microbial fuel cells. Appl Environ Microbiol. 2006;72:7345–7348. doi: 10.1128/AEM.01444-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuhashi M, et al. Studies on carbon material requirements for bacterial proliferation and spore germination under stress conditions: a new mechanism involving transmission of physical signals. J Bacteriol. 1995;177:688–693. doi: 10.1128/jb.177.3.688-693.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuhashi M, et al. Bacillus carboniphilus cells respond to growth-promoting physical signals from cells of homologous and heterologous bacteria. J Gen Appl Microbiol. 1996;42:315–323. [Google Scholar]
- 9.Matsuhashi M, et al. Growth-promoting effect of carbon material upon bacterial cells propagating through a distance. J Gen Appl Microbiol. 1997;43:225–230. doi: 10.2323/jgam.43.225. [DOI] [PubMed] [Google Scholar]
- 10.Howard J. Mechanical signaling in networks of motor and cytoskeletal proteins. Annu Rev Biophys. 2009;38:217–234. doi: 10.1146/annurev.biophys.050708.133732. [DOI] [PubMed] [Google Scholar]
- 11.Bustamante C, et al. Mechanical processes in biochemistry. Annu Rev Biochem. 2004;73:705–748. doi: 10.1146/annurev.biochem.72.121801.161542. [DOI] [PubMed] [Google Scholar]
- 12.Bao G, Suresh S. Cell and molecular mechanics of biological materials. Nat Mater. 2003;2:715–725. doi: 10.1038/nmat1001. [DOI] [PubMed] [Google Scholar]
- 13.Bustamante C, et al. Ten years of tension: single-molecule DNA mechanics. Nature. 2003;421:423–427. doi: 10.1038/nature01405. [DOI] [PubMed] [Google Scholar]
- 14.Mazumder A, et al. Dynamics of chromatin decondensation reveals the structural integrity of a mechanically prestressed nucleus. Biophys J. 2008;95:3028–3035. doi: 10.1529/biophysj.108.132274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ibarra B, et al. Proofreading dynamics of a processive DNA polymerase. EMBO J. 2009;28:2794–2802. doi: 10.1038/emboj.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herbert KM, et al. Single-molecule studies of RNA polymerase: motoring along. Annu Rev Biochem. 2008;77:149–176. doi: 10.1146/annurev.biochem.77.073106.100741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tinoco I, Jr, et al. Determination of thermodynamics and kinetics of RNA reactions by force. Q Rev Biophys. 2006;39:325–360. doi: 10.1017/S0033583506004446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wen JD, et al. Following translation by single ribosomes one codon at a time. Nature. 2008;452:598–603. doi: 10.1038/nature06716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller DJ, et al. Force probing surfaces of living cells to molecular resolution. Nat Chem Biol. 2009;5:383–390. doi: 10.1038/nchembio.181. [DOI] [PubMed] [Google Scholar]
- 20.Huang KC, et al. Dynamic structures in Escherichia coli: spontaneous formation of MinE rings and MinD polar zones. Proc Natl Acad Sci USA. 2003;100:12724–12728. doi: 10.1073/pnas.2135445100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graumann PL. Dynamics of bacterial cytoskeletal elements. Cell Motil Cytoskeleton. 2009;66:909–914. doi: 10.1002/cm.20381. [DOI] [PubMed] [Google Scholar]
- 22.Pelling AE, et al. Local nanomechanical motion of the cell wall of Saccharomyces cerevisiae. Science. 2004;305:1147–1150. doi: 10.1126/science.1097640. [DOI] [PubMed] [Google Scholar]
- 23.Norris V, Hyland GJ. Do bacteria sing? Sonic intercellular communication between bacteria may reflect electromagnetic intracellular communication involving coherent collective vibrational modes that could integrate enzyme activities and gene expression. Mol Microbiol. 1997;24:879–880. doi: 10.1046/j.1365-2958.1997.3951756.x. [DOI] [PubMed] [Google Scholar]
- 24.Volkov SN, Kosevich AM. Theory of low-frequency vibrations in DNA macromolecules. J Biomol Struct Dyn. 1991;8:1069–1083. doi: 10.1080/07391102.1991.10507866. [DOI] [PubMed] [Google Scholar]
- 25.Lisy V, et al. On a simple model of low-frequency vibrations in DNA macromolecules. J Biomol Struct Dyn. 1996;13:707–716. doi: 10.1080/07391102.1996.10508883. [DOI] [PubMed] [Google Scholar]
- 26.Alipov ED, et al. Cell-density dependent effects of low-dose ionizing radiation on E. coli cells. Radiats Biol Radioecol. 2003;43:167–171. [PubMed] [Google Scholar]
- 27.Shcheglov VS, et al. Cell-to-cell communication in response of E. coli cells at different phases of growth to low-intensity microwaves. Biochim Biophys Acta. 2002;1572:101–106. doi: 10.1016/s0304-4165(02)00283-0. [DOI] [PubMed] [Google Scholar]
- 28.Berteaud AJ, et al. The effect of electromagnetic radiation of wavelength in the millimeter range on bacterial growth. C R Acad Sci Hebd Seances Acad Sci D. 1975;281:843–846. [PubMed] [Google Scholar]
- 29.Liu Y, et al. Magnetic field effect on singlet oxygen production in a biochemical system. Chem Commun (Camb) 2005:174–176. doi: 10.1039/b413489c. [DOI] [PubMed] [Google Scholar]
- 30.Gao W, et al. Effects of a strong static magnetic field on bacterium Shewanella oneidensis: an assessment by using whole genome microarray. Bioelectromagnetics. 2005;26:558–563. doi: 10.1002/bem.20133. [DOI] [PubMed] [Google Scholar]
- 31.Anishkin A, Kung C. Microbial mechanosensation. Curr Opin Neurobiol. 2005;15:397–405. doi: 10.1016/j.conb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Gantner S, et al. In situ quantitation of the spatial scale of calling distances and population density-independent N-acylhomoserine lactone-mediated communication by rhizobacteria colonized on plant roots. FEMS Microbiol Ecol. 2006;56:188–194. doi: 10.1111/j.1574-6941.2005.00037.x. [DOI] [PubMed] [Google Scholar]
- 33.Widder EA. Bioluminescence in the ocean: origins of biological, chemical, and ecological diversity. Science. 2010;328:704–708. doi: 10.1126/science.1174269. [DOI] [PubMed] [Google Scholar]
- 34.Niggli HJ. Ultraweak photons emitted by cells: biophotons. J Photochem Photobiol B. 1992;14:144–146. doi: 10.1016/1011-1344(92)85090-h. [DOI] [PubMed] [Google Scholar]
- 35.Devaraj B, et al. Biophotons: Ultraweak light emission from living systems. Curr Opin Sol St Mat Sc. 1997;2:188–193. [Google Scholar]
- 36.Albrecht-Buehler G. Rudimentary form of cellular 'vision'. Proc Natl Acad Sci USA. 1992;89:8288–8292. doi: 10.1073/pnas.89.17.8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albrecht-Buehler G. A long-range attraction between aggregating 3T3 cells mediated by near-infrared light scattering. Proc Natl Acad Sci USA. 2005;102:5050–5055. doi: 10.1073/pnas.0407763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang JJ. Physical properties of biophotons and their biological functions. Indian J Exp Biol. 2008;46:371–377. [PubMed] [Google Scholar]
- 39.AlbrechtBuehler G. Autofluorescence of live purple bacteria in the near infrared. Exp Cell Res. 1996;236:43–50. doi: 10.1006/excr.1996.3688. [DOI] [PubMed] [Google Scholar]
- 40.Tilbury RN. The effect of stress factors on the spontaneous photon-emission from microorganisms. Experientia. 1992;48:1030–1041. doi: 10.1007/BF01947991. [DOI] [PubMed] [Google Scholar]
- 41.Quickenden TI, Tilbury RN. Luminescence spectra of exponential and stationary phase cultures of respiratory deficient Saccharomyces cerevisiae. J Photochem Photobiol B: Biol. 1991;8:169–174. doi: 10.1016/1011-1344(91)80055-m. [DOI] [PubMed] [Google Scholar]
- 42.Godlewski M, et al. Spectra of the formaldehyde-induced ultraweak luminescence from yeast cells. J Photochem Photobiol B-Biol. 1993;21:29–35. doi: 10.1016/1011-1344(93)80160-b. [DOI] [PubMed] [Google Scholar]
- 43.Maccarrone M, et al. Ultraweak light emission is a common response of bacterial cells to chemicophysical stress. J Biolumin Chemilumin. 1998;13:287–293. doi: 10.1002/(SICI)1099-1271(1998090)13:5<287::AID-BIO489>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 44.Popp FA. Some essential questions of biophoton research and probable answers. In: Popp FA, et al., editors. Recent advances in biophoton research and its applications. World Scientific Publishing Co; 1992. pp. 1–46. [Google Scholar]
- 45.Shapiro L, et al. Generating and exploiting polarity in bacteria. Science. 2002;298:1942–1946. doi: 10.1126/science.1072163. [DOI] [PubMed] [Google Scholar]
- 46.Purcell EB, Crosson S. Photoregulation in prokaryotes. Curr Opin Microbiol. 2008;11:168–178. doi: 10.1016/j.mib.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 47.van der Horst MA, et al. Photosensing in chemotrophic, non-phototrophic bacteria: let there be light sensing too. Trends Microbiol. 2007;15:554–562. doi: 10.1016/j.tim.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Weber KA, et al. Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat Rev Microbiol. 2006;4:752–764. doi: 10.1038/nrmicro1490. [DOI] [PubMed] [Google Scholar]
- 49.Shi L, et al. Respiration of metal (hydr)oxides by Shewanella and Geobacter: a key role for multihaem c-type cytochromes. Mol Microbiol. 2007;65:12–20. doi: 10.1111/j.1365-2958.2007.05783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reguera G, et al. Possible nonconductive role of Geobacter sulfurreducens pilus nanowires in biofilm formation. J Bacteriol. 2007;189:2125–2127. doi: 10.1128/JB.01284-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Summers ZM, et al. Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria. Science. 2010;330:1413–1415. doi: 10.1126/science.1196526. [DOI] [PubMed] [Google Scholar]
- 52.Reguera G. Are microbial conversations being lost in translation? Microbe. 2009;4:506–512. [Google Scholar]
- 53.Brasca M, et al. Redox potential to discriminate among species of lactic acid bacteria. J Appl Microbiol. 2007;103:1516–1524. doi: 10.1111/j.1365-2672.2007.03392.x. [DOI] [PubMed] [Google Scholar]
- 54.Skerker JM, Berg HC. Direct observation of extension and retraction of type IV pili. Proc Natl Acad Sci USA. 2001;98:6901–6904. doi: 10.1073/pnas.121171698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.El-Naggar MY, et al. Electrical transport along bacterial nanowires from Shewanella oneidensis MR-1. Proc Natl Acad Sci USA. 2010;107:18127–18131. doi: 10.1073/pnas.1004880107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ntarlagiannis D, et al. Microbial nanowires: Is the subsurface 'hardwired'? Geophys Res Lett. 2007;34:1–5. [Google Scholar]
- 57.Nielsen LP, et al. Electric currents couple spatially separated biogeochemical processes in marine sediment. Nature. 2010;463:1071–1074. doi: 10.1038/nature08790. [DOI] [PubMed] [Google Scholar]
- 58.Bond DR, et al. Electrode-reducing microorganisms that harvest energy from marine sediments. Science. 2002;295:483–485. doi: 10.1126/science.1066771. [DOI] [PubMed] [Google Scholar]
- 59.Gorby YA, et al. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc Natl Acad Sci USA. 2006;103:11358–11363. doi: 10.1073/pnas.0604517103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ogawa N, et al. A physical model for galvanotaxis of Paramecium cell. J Theor Biol. 2006;242:314–328. doi: 10.1016/j.jtbi.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 61.Rajnicek AM, et al. Electric fields induce curved growth of Enterobacter cloacae, Escherichia coli, and Bacillus subtilis cells: implications for mechanisms of galvanotropism and bacterial growth. J Bacteriol. 1994;176:702–713. doi: 10.1128/jb.176.3.702-713.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Minc N, Chang F. Electrical control of cell polarization in the fission yeast Schizosaccharomyces pombe. Curr Biol. 2010;20:710–716. doi: 10.1016/j.cub.2010.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Epand RM, Epand RF. Domains in bacterial membranes and the action of antimicrobial agents. Mol Biosyst. 2009;5:580–587. doi: 10.1039/b900278m. [DOI] [PubMed] [Google Scholar]
- 64.Goldenberg NM, Steinberg BE. Surface charge: a key determinant of protein localization and function. Cancer Res. 2010;70:1277–1280. doi: 10.1158/0008-5472.CAN-09-2905. [DOI] [PubMed] [Google Scholar]
- 65.Shapiro L, et al. Why and how bacteria localize proteins. Science. 2009;326:1225–1228. doi: 10.1126/science.1175685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shanley LJ, et al. Influx of extracellular Ca2+ is necessary for electrotaxis in Dictyostelium. J Cell Sci. 2006;119:4741–4748. doi: 10.1242/jcs.03248. [DOI] [PubMed] [Google Scholar]
- 67.Poo M. In situ electrophoresis of membrane components. Annu Rev Biophys Bioeng. 1981;10:245–276. doi: 10.1146/annurev.bb.10.060181.001333. [DOI] [PubMed] [Google Scholar]
- 68.Tucker SJ, Baukrowitz T. How highly charged anionic lipids bind and regulate ion channels. J Gen Physiol. 2008;131:431–438. doi: 10.1085/jgp.200709936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mileykovskaya E, Dowhan W. Visualization of phospholipid domains in Escherichia coli by using the cardiolipin-specific fluorescent dye 10-N-nonyl acridine orange. J Bacteriol. 2000;182:1172–1175. doi: 10.1128/jb.182.4.1172-1175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kawai F, et al. Cardiolipin domains in Bacillus subtilis marburg membranes. J Bacteriol. 2004;186:1475–1483. doi: 10.1128/JB.186.5.1475-1483.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pohorille A, et al. The origin and early evolution of membrane channels. Astrobiology. 2005;5:1–17. doi: 10.1089/ast.2005.5.1. [DOI] [PubMed] [Google Scholar]
- 72.Koishi R, et al. A superfamily of voltage-gated sodium channels in bacteria. J Biol Chem. 2004;279:9532–9538. doi: 10.1074/jbc.M313100200. [DOI] [PubMed] [Google Scholar]
- 73.Ren D, et al. A prokaryotic voltage-gated sodium channel. Science. 2001;294:2372–2375. doi: 10.1126/science.1065635. [DOI] [PubMed] [Google Scholar]
- 74.Barbado M, et al. Gene regulation by voltage-dependent calcium channels. Biochim Biophys Acta. 2009;1793:1096–1104. doi: 10.1016/j.bbamcr.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 75.Chisti Y. Sonobioreactors: using ultrasound for enhanced microbial productivity. Trends Biotechnol. 2003;21:89–93. doi: 10.1016/s0167-7799(02)00033-1. [DOI] [PubMed] [Google Scholar]
- 76.Tyner KM, et al. “Nanosized voltmeter” enables cellular-wide electric field mapping. Biophys J. 2007;93:1163–1174. doi: 10.1529/biophysj.106.092452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun Y, et al. Biophotons as neural communication signals demonstrated by in situ biophoton autography. Photochem Photobiol Sci. 2010;9:315–322. doi: 10.1039/b9pp00125e. [DOI] [PubMed] [Google Scholar]
- 78.Hellingwerf KJ, et al. Current topics in signal transduction in bacteria. Antonie Van Leeuwenhoek. 1998;74:211–227. doi: 10.1023/a:1001738419877. [DOI] [PubMed] [Google Scholar]
- 79.Chen J, et al. Bright infrared emission from electrically induced excitons in carbon nanotubes. Science. 2005;310:1171–1174. doi: 10.1126/science.1119177. [DOI] [PubMed] [Google Scholar]
- 80.Tilbury RN. The effect of stress factors on the spontaneous photon emission from microorganisms. Experientia. 1992;48:1030–1041. doi: 10.1007/BF01947991. [DOI] [PubMed] [Google Scholar]