Abstract
BACKGROUND AND PURPOSE
Quercetin is anti-inflammatory in macrophages by inhibiting lipopolysaccharide (LPS)-mediated increases in cytokine and nitric oxide production but there is little information regarding the corresponding effect on the vasculature. We have examined the effect of quercetin, and its principal human metabolites, on inflammatory changes in the porcine isolated coronary artery.
EXPERIMENTAL APPROACH
Porcine coronary artery segments were incubated overnight at 37°C in modified Krebs-Henseleit solution with or without 1 µg·mL−1 LPS. Some segments were also co-incubated with quercetin-related flavonoids or Bay 11-7082, an inhibitor of NFκB. Changes in isometric tension of segments to vasoconstrictor and vasodilator agents were recorded. Nitrite content of the incubation solution was estimated using the Griess reaction, while inducible nitric oxide synthase was identified immunohistochemically.
KEY RESULTS
Lipopolysaccharide reduced, by 35–50%, maximal contractions to KCl and U46619, thromboxane A2 receptor agonist, and impaired endothelium-dependent relaxations to substance P. Nitrite content of the incubation medium increased 3- to 10-fold following exposure to LPS and inducible nitric oxide synthase was detected in the adventitia. Quercetin (0.1–10 µM) opposed LPS-induced changes in vascular responses, nitrite production and expression of inducible nitric oxide synthase. Similarly, 10 µM Bay 11-7082, 10 µM quercetin 3′-sulphate and 10 µM quercetin 3-glucuronide prevented LPS-induced changes, while myricetin (10 µM) was inactive. Myricetin (10 µM) prevented quercetin-induced modulation of LPS-mediated nitrite production.
CONCLUSION AND IMPLICATIONS
Quercetin, quercetin 3′-suphate and quercetin 3-glucuronide, exerted anti-inflammatory effects on the vasculature, possibly through a mechanism involving inhibition of NFκB. Myricetin-induced antagonism of the effect of anti-inflammatory action of quercetin merits further investigation.
Keywords: quercetin, quercetin sulphate, quercetin glucuronide, myricetin, porcine coronary artery, lipopolysaccharide, nitric oxide, CD31
Introduction
Numerous epidemiological studies indicate that flavonoid intake as part of a balanced diet confers beneficial health effects in man, including improved cardiovascular function, reduced incidence of cancer and amelioration of symptoms associated with inflammatory disorders (Boots et al., 2008). Quercetin is a major flavonoid found in various foods, such as apples, onions and broccoli, that is reported to possess a range of biological activities in isolated cells and tissues (Halliwell, 2007; Boots et al., 2008). When ingested as a supplement in man some of the effects noted for quercetin have been consistent with both the epidemiological and in vitro observations (Williamson and Manach, 2005). For example, Edwards et al. (2007) demonstrated that quercetin lowered blood pressure in hypertensive subjects, an effect potentially related to the reported direct vasodilator action of this flavonoid, and its metabolites, on isolated blood vessels (Perez-Vizcaino et al., 2002).
With respect to putative anti-inflammatory activity of quercetin, cytokine-induced or lipopolysaccharide (LPS)-induced production of nitric oxide and prostanoids in human macrophage cell lines have been reported to sensitive to high concentrations (>10 µM). of the flavonoid (Chen et al., 2005; Hamalainen et al., 2007). The mechanism for this protective effect of quercetin has been attributed to suppression of LPS-induced activation of NFκB, possibly linked to stabilization of the cytoplasmic NFκB/IκB complex (Hamalainen et al., 2007) and specific inhibition of IκB kinase (Chen et al., 2005). In mouse microglia, quercetin (at approx' 3 µM) exerted a greater inhibitory effect on LPS-induced nitrite production compared with that elicited by interleukin-1β (Chen et al., 2005), thereby highlighting the selective nature of this action. The significance of these observations has, however, been called into question because the concentrations used greatly exceeds plasma levels of the aglycone detected in man (approximately 30 nM) and there is no information regarding the biological activity of key metabolites (Kroon et al., 2004).
It is well recognized that the vasculature is a significant component in the development of inflammatory responses. Endothelial cells exhibit signs of altered expression of cell adhesion molecules (Read et al., 1994), while vasoconstrictor responses are reduced under the influence of locally generated dilator substances (Mitchell et al., 2007; van Gil et al., 2008). For example, nitric oxide production in blood vessels is increased by inflammatory stimuli and is recognized as a major contributor to increased local blood flow (Mitchell et al., 2007). While there is recent information concerning the effect of quercetin and its metabolites on the expression of endothelial cell adhesion molecules (Tribolo et al., 2008), there is no comparable study regarding the changes in contractions of vascular smooth muscle.
In the present study we have investigated the effect of quercetin, and two of the key metabolites in man, quercetin 3′-sulphate and quercetin 3-glucuronide (Kroon et al., 2004; Wang and Morris, 2005), against LPS-induced changes in contractions of the porcine isolated coronary artery (Qi et al., 2007) and the generation of nitric oxide. In addition, as many quercetin-rich foods also contain significant amounts of myricetin, a 5′ hydroxylated derivative of quercetin (Fusi et al., 2005; Hamalainen et al., 2007), this flavonoid was also examined.
Methods
Porcine hearts were obtained from a local abattoir and placed in modified Krebs-Henseleit (K-H) solution (composition (mM): NaCl, 118; KCl, 4.8; MgSO4.7H2O, 1.2; CaCl2.2H2O, 1.3; NaHCO3, 25.0; KH2PO4, 1.2.), maintained at 4°C before being transported to the laboratory. The anterior descending branch of the coronary artery was dissected from the hearts, cleaned of connective tissue and then divided into 4 mm long segments.
Contraction studies
For contraction experiments, single segments of the porcine coronary artery were incubated in 2 mL K-H solution (previously gassed with 95% O2 and 5% CO2 for 5 min.) containing 2% Ficoll and a combination of 60 µg·mL−1 benzyl penicillin and 20 µg·mL−1 streptomycin sulphate. The solution also contained 1 µg·mL−1 LPS, various concentrations of quercetin or a combination of LPS and the flavonoid (added 60 min before LPS) and then sealed in a sterilized glass vial and stored overnight at 37°C for 16–18 h. In some experiments the effect of 10 µM myricetin, 10 µM quercetin 3′-sulphate or 10 µM quercetin 3-glucuronide in the presence of LPS was examined. In addition, the effect of co-incubation with a selective inhibitor of NFκB, Bay 11-7082 (10 µM) (Pierce et al., 1997), against LPS-induced changes in the blood vessel was also assessed. Unless, indicated otherwise, all experiments were conducted on nominally endothelium-intact segments of the coronary artery. In a further set of experiments the effect of 1 µM quercetin was examined against LPS-induced changes in contractile responses in endothelium-denuded segments of the coronary artery, prepared as described by Suri et al. (2010). In all instances the segments were effectively paired so that each experimental condition had a control segment taken from the same animal
After overnight storage, segments were removed from the incubation solution and placed in K-H solution (maintained at 37°C and gassed with 95% O2 and 5% CO2) in a 15 mL isolated organ bath, prepared for isometric tension recordings as previously described (Qi et al., 2007) and allowed to equilibrate for 60 min. Contractions of the segment were measured using a Grass FT03 isometric force transducer connected to a MacLab unit coupled to a Macintosh LC4 computer running Chart 3.5. An initial resting tension of 80 mN was slowly applied to each segment at the end of the equilibration period and the recorded tension declined to 40–60 mN over the next 40 min. Segments were repeatedly exposed to 60 mM KCl for 15 min until reproducible contractions were observed. The preparations were exposed to cumulatively increasing concentration of either KCl (6–60 mM) or U46619 (a stable thromboxane-mimetic analogue, 9,11-dideoxy-9α,11α-methanoepoxyprostaglandin F2α, 1–200 nM). When each preparation was exposed to a maximally effective concentration of U46619, 10 nM substance P was added to assess the integrity of the endothelium. In some experiments, the involvement of nitric oxide in the effect of LPS on contractile responses was investigated by adding 10 µM 1400 W, a selective inhibitor of inducible nitric oxide synthase (iNOS) (Garvey et al., 1997), 30 min before constructing concentration-response curves to either KCl or U46619.
Nitrite ion determination
For the measurement of nitrite ion accumulation, a marker for nitric oxide production (Kelm, 1999), two segments were incubated in each vial for 24 h with aerated Dulbecco's modified Eagle's medium (DMEM) (without phenol red) containing 1 mM L-arginine, 60 µg·mL−1 benzyl penicillin and 20 µg·mL−1 streptomycin. The segments were exposed to either 1 µg·mL−1 LPS, a flavonoid, or combination of LPS and flavonoid, as described above. Some preparations were also exposed to either 10 µM 1400 W, 1 µM dexamethasone or 10 µM Bay 11-7082 for 24 h in the presence of 1 µg·mL−1 LPS. After the incubation period, the segments were removed, briefly blotted on paper cloth and weighed. Nitrite ion accumulation in the incubation medium was determined by the Greiss reaction as previously described (Ukil et al., 2006). Five hundred microlitres of Greiss reagent (1% sulphanilamide and 0.1% naphthylethylamine diamine in 5% hydrocholoric acid) was added to 500 µL of the incubation medium and optical density measured at 550 nm, using a UNICAM UV/VIS spectrophotometer. A standard concentration-optical density curve was calculated for every assay using sodium nitrite solution against a blank (500 µL DMEM and 500 µL Griess Reagent). In a separate experiment we established that incubation of DMEM containing either the flavonoid, LPS or a combination of both (without the arterial segments) produced an optical density reading similar to the blank. The minimum detectable amount of nitrite was 375 nmoles.
Immunohistochemistry
Sections of the coronary artery incubated under the above conditions were prepared on a cryostat (5 µm thick) and stored frozen at −80°C until required. The section were then warmed to room temperature for 20 min and fixed in cold acetone at −4°C for 20 min. To block endogenous peroxidase activity, sections were treated with blocking serum (two drops of vectastain in 5 mL phosphate buffer solution (pH 7.4) containing 2% w/v immunohistochemical grade bovine serum albumin) for 10 min at room temperature. The sections were then incubated for 1 h with primary mouse antibodies: porcine-CD31 (diluted 1:75) or rabbit iNOS (diluted 1:100). The sections were then washed in phosphate buffer solution, incubated for 10 min with biotinylated anti-mouse antibody in 10% NGS (Vectastain ABC Kit), washed in phosphate buffer solution, incubated for a further 5 min with ABC (avidin-biotin-peroxidase) reagent in phosphate buffer solution and, finally, washed again. Immunoreactive CD31 and iNOS were visualized by incubating the sections in Vector Red substrate (one drop of levamisole solution to 5 mL of 200 M Tris-HCl pH 8.2) for 30 min. Finally, sections were dehydrated and cover-slipped with DPX mounting medium. The observation and photographs were made using a light microscope (Leica DM4000B) and an imaging digital camera. Images were obtained using Openlab (improvision, UK).
Data analysis and statistics
Contractions produced by U46619 and KCl were measured as milliNewtons force (mN). Responses were expressed as the maximum contraction (Emax), and potency determined as the negative logarithm of the concentration causing 50% of the maximum response (-log EC50 or pD2) using a logistic equation (Kaleidagraph, version 3.6 Synergy Software). The values are shown as the mean (±SEM). The content of nitrite ions in the medium was calculated according to the equation deduced from the standard curve. The amount on nitrite ions in the medium was then divided by the wet weight of tissues to obtain the amount of nitrite ion production from each segment, as nmoles (mg wet weight)−1. In the majority of instances differences between mean force developed (mN) in segments from the same animal were assessed by a paired Student's t-test (two-tailed). When responses were normalized, relative to either the maximum contractile response in the corresponding control segment, or pre-existing tone (dilator responses), then difference were assessed using a Wilcoxon test. Where there was more than one treatment condition assessed differences were analysed by anova followed by post hoc Dunnett's test. A P-value < 0.05 was considered statistically significant.
Materials
Benzyl penicillin, streptomycin sulphate, Ficoll, LPS (Eschericha coli O III:B4), Bay 11-7082 ((E)-3(4-methylphenylsulfonyl)-2-propenenitrile), sulphanilamide, N-(1-napthyl)-ethylene-diamine dihydrochloride and quercetin dehydrate were all obtained from Sigma-Aldrich Company Ltd (Poole, Dorset, UK). Substance P was obtained from Bachem (UK). U46619 was obtained from Alexis Coporation (Nottingham, UK). 1400 W was obtained from Tocris Cookson Ltd (Avonmouth, UK). Dexamethasone sodium phosphate was purchased from Organon (Cambridge, UK). DMEM was supplemented with antibiotics (see above) and 2 mM L-glutamine (Gibco). The metabolites of quercetin, quercetin-3′-sulphate and quercetin-3-glucuronide, were prepared at the Institute of Food Research, Norwich (Needs and Kroon, 2006). Antibodies against rabbit iNOS (Santa Cruz Botechology, Santa Cruz, Califonia, USA) and mouse anti-porcine CD31 (MCA1747, Serotec, Kidlington, UK) were also obtained. Quercetin, Bay 11-7082 and quercetin metabolites were dissolved in 100% DMSO at a concentration of 10 mM (<0.1% DMSO in final incubation medium), whereas dexamethasone was dissolved in absolute ethanol at a concentration of 10 mM, all other drugs were dissolved in distilled water.
Results
Contraction studies
KCl and U46619 elicited concentration-dependent contractions of the porcine coronary artery (Figure 1A,B), with a potency (pD2) of 1.59 ± 0.01 (n= 11) and 7.96 ± 0.05 (n= 11) respectively. Overnight exposure of the porcine coronary artery to 1 µg·mL−1 LPS significantly reduced the maximum response KCl to 68.8. ± 3.1% (n= 11) of control without significantly altering the potency (pD2 1.55 ± 0.02). Similarly, the maximum response to U46619 was reduced to 71.4 ± 2.1% (n= 11) of control with no alteration in the potency (pD2− 7.89 ± 0.07). Following overnight co-incubation of segments with LPS and 10 µM Bay 11-7082, a selective inhibitor of NFκB (and subsequent removal), submaximal and maximal responses to both agonists were significantly increased (Figure 1), with the maximum contractions equivalent to 84.9 ± 3.1% (KCl) and 89.8 ± 2.6% (U46619) of the control responses (Figure 1). The potency of the agonists was not altered (data not shown). As shown in Figure 1C,D, LPS-induced suppression of the maximum responses to KCl (31.9 ± 4.5%, n= 12) and U46619-induced contractions (28.9 ± 12.4%, n= 12) were abolished following post-incubation exposure to 10 µM 1400 W, a selective inhibitor of iNOS.
Figure 1.
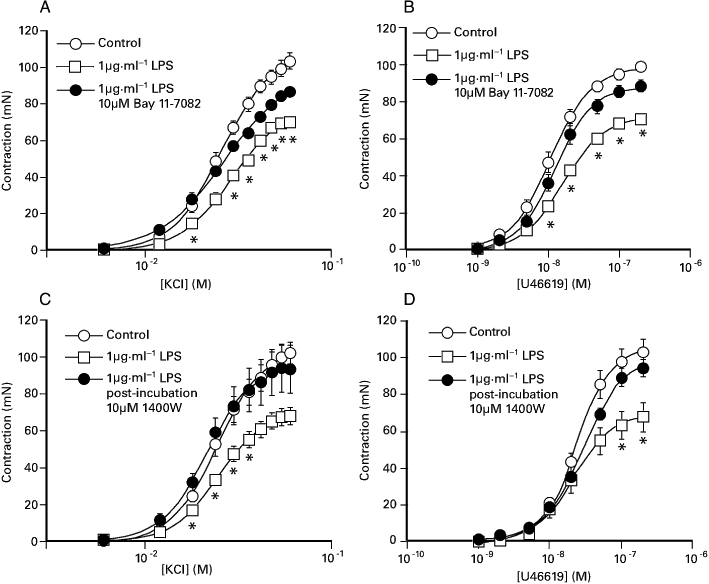
The effect of overnight exposure of the porcine coronary artery to 1 µg·mL−1 LPS with or without 10 µM Bay 11-7082 (with subsequent removal) on (A) KCl- and (B) U46619-induced contraction. In some experiments responses to (C) KCl and (D) U46619 were also examined in the absence and presence (‘post-incubation’) of 10 µM 1400 W. Bay 11-7082 was included in the incubation medium 1 hour before overnight exposure to LPS, while 1400 W was added to the organ bath 20 min prior to construction of the concentration response curves. The responses shown are as mean ± SEM of 11–12 observations. *P < 0.05, significant difference between the responses for the paired LPS-treated preparations. LPS, lipopolysaccharide.
Overnight co-incubation of porcine coronary artery segments with 1 µg·mL−1 LPS and 10 µM quercetin (and subsequent removal) increased responses to both KCl and U46619 compared with that of LPS alone (Figure 2). In contrast, overnight incubation with 10 µM myricetin did not affect LPS-induced inhibition of KCl and U46619-induded contractions (Figure 2). At the end of the U46619 concentration-response assay, the addition of 10 nM substance P produced a transient relaxation (25.9 ± 5.6%, n= 13) in control preparations (see also Table 1) that was significantly reduced (P < 0.01) following LPS treatment. As shown in Table 2, the inhibitory effect of LPS on substance P-induced relaxations was prevented by co-incubation with 1 µM and 10 µM quercetin. In contrast, substance P-induced relaxations were not significantly different between segments incubated overnight with either 1 µg·mL−1 LPS or 1 µg·mL−1 LPS and 10 µM myricetin (Table 2).
Figure 2.
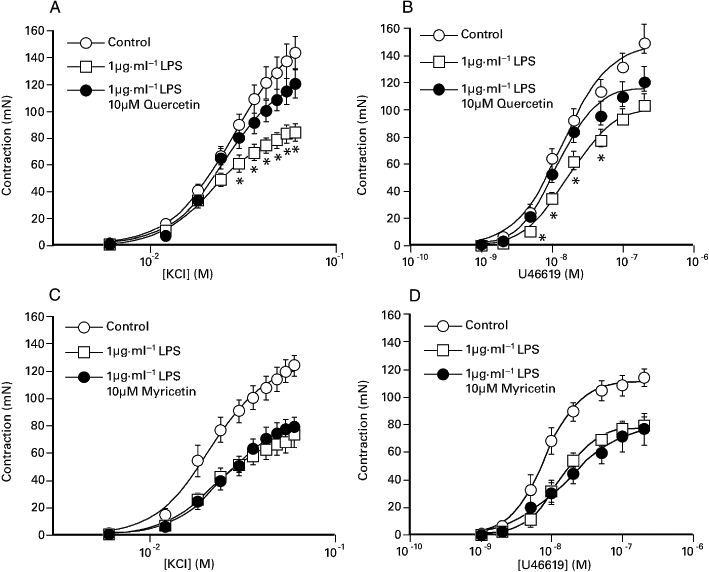
The effect of overnight exposure of the porcine coronary artery to 1 µg·mL−1 LPS, in the presence or absence of either (A, B) 10 µM quercetin or (C, D) 10 µM myricetin, on responses elicited by KCl and U46619. The responses shown are as mean ± SEM of 7–13 observations. *P < 0.05, significant difference between the responses for the paired LPS-treated preparations. LPS, lipopolysaccharide.
Table 1.
Effect of Bay 11-7082, quercetin and myricetin on the maximum response (mN) and potency (pD2) of KCl and U46619 contractions and substance P(SP)-induced relaxation in isolated porcine coronary arteries incubated for 16 h in modified Krebs-Henseleit solution
| KCl | U46619 | SP | |||
|---|---|---|---|---|---|
| Max (mN) | pD2 | Max (mN) | pD2 | % relaxation | |
| Control (n= 11) | 101.5 ± 4.3 | 1.59 ± 0.01 | 96.8 ± 2.3 | 7.96 ± 0.04 | ND |
| 10 µM Bay 11-7082 | 83.5 ± 3.3** | 1.57 ± 0.02 | 86.1 ± 2.0** | 7.91 ± 0.08 | ND |
| Control (n= 21) | 124.6 ± 9.2 | 1.54 ± 0.02 | 120.6 ± 9.6 | 7.67 ± 0.07 | 37.1 ± 4.9 |
| 10 µM quercetin | 98.1 ± 6.7** | 1.62 ± 0.03 | 109.8 ± 6.7 | 7.88 ± 0.06 | 24.1 ± 3.1** |
| Control (n= 7) | 121.6 ± 7.3 | 1.68 ± 0.04 | 110.8 ± 6.4 | 8.11 ± 0.08 | 16.7 ± 7.6 |
| 10 µM myricetin | 73.5 ± 5.2** | 1.68 ± 0.01 | 82.6 ± 7.2** | 7.97 ± 0.08 | 7.2 ± 1.4 |
| Control (n= 8) | 98.1 ± 9.3 | 1.54 ± 0.02 | 97.1 ± 1.6 | 8.01 ± 0.03 | 38.8 ± 2.0 |
| 1 µM quercetin | 94.4 ± 6.3 | 1.52 ± 0.02 | 93.2 ± 4.1 | 8.01 ± 0.03 | 35.2 ± 2.5 |
Values shown are the mean ± SEM of observations from paired segments taken from 7 to 21 different animals.
P < 0.05,
P < 0.01; significantly different from the paired control preparation.
ND, not done.
Table 2.
Effect of quercetin, myricetin and quercetin metabolites on the maximum response (g weight) and potency (pD2) of KCl and U46619 contractions and SP-induced relaxation in segments of the porcine isolated coronary artery incubated for 16 h in modified Krebs-Henseleit solution in the presence of 1 µg·mL−1 LPS
| KCl | U46619 | SP | |||
|---|---|---|---|---|---|
| Max (% control) | pD2 | Max (% control) | pD2 | % relaxation | |
| LPS (n= 13) | 60.1 ± 3.5 | 1.62 ± 0.03 | 71.2 ± 3.4 | 7.72 ± 0.06 | 9.35 ± 1.9 |
| LPS10 µM quercetin | 85.8 ± 4.7** | 1.57 ± 0.04 | 83.5 ± 6.5 | 7.87 ± 0.08* | 22.5 ± 3.9** |
| LPS (n= 7) | 58.6 ± 5.9 | 1.64 ± 0.04 | 70.3 ± 4.4 | 7.89 ± 0.06 | 5.4 ± 0.6 |
| LPS10 µM myricetin | 65.9 ± 8.2 | 1.59 ± 0.04 | 66.3 ± 7.8 | 7.97 ± 0.08 | 9.1 ± 3.0 |
| LPS (n= 26) | 62.6 ± 3.0 | 1.59 ± 0.03 | 77.6 ± 3.4 | 7.72 ± 0.03 | 8.6 ± 1.4 |
| LPS1 µM quercetin | 95.0 ± 4.7** | 1.60 ± 0.02 | 110.0 ± 7.4** | 7.84 ± 0.03* | 23.6 ± 3.4** |
| LPS (n= 13) | 64.0 ± 4.8 | 1.53 ± 0.04 | 75.5 ± 3.4 | 7.73 ± 0.04 | 7.9 ± 2.0 |
| LPS0.1 µM quercetin | 83.7 ± 5.8** | 1.57 ± 0.03 | 90.8 ± 5.6* | 7.92 ± 0.05* | 12.0 ± 3.4 |
| Endothelium-denuded segments | |||||
| LPS (n= 8) | 78.6 ± 7.6 | 1.62 ± 0.02 | 70.3 ± 4.7 | 7.71 ± 0.09 | 0 |
| LPS1 µM quercetin | 105.3 ± 10.6** | 1.64 ± 0.01 | 100.8 ± 2.5** | 7.91 ± 0.06 | 0 |
| LPS (n= 8) | 47.5 ± 2.3 | 1.60 ± 0.02 | 58.7 ± 3.2 | 7.84 ± 0.03 | 20.2 ± 5.7 |
| LPS10 µM quercetin3′-sulphate | 83.6 ± 3.6** | 1.65 ± 0.01 | 87.0 ± 4.1** | 7.98 ± 0.03* | 49.5 ± 4.9** |
| LPS (n= 10) | 58.5 ± 2.1 | 1.58 ± 0.02 | 62.6 ± 3.6 | 7.84 ± 0.03 | 10.0 ± 4.6 |
| LPS10 µM quercetin3-glucuronide | 83.6 ± 3.6** | 1.65 ± 0.01* | 99.0 ± 5.7** | 7.86 ± 0.01 | 30.8 ± 8.7* |
| LPS (n= 10)1 µM quercetin | 85.3 ± 2.0 | 1.62 ± 0.02 | 87.3 ± 3.6 | 7.99 ± 0.03 | 32.5.0 ± 5.2 |
| LPS1 µM quercetin10 µM Bay 11-7082 | 79.5 ± 5.3 | 1.65 ± 0.02 | 79.5 ± 5.7 | 7.96 ± 0.04 | 25.4 ± 4.0 |
Values shown are the mean ± SEM of observations in paired segments from 7 to 26 different animals.
* P < 0.05,
P < 0.01; significant difference in the response between paired segments and Wilcoxon test. Unless indicated otherwise all segments were endothelium-intact.
LPS, lipopolysaccharide.
Surprisingly, overnight incubation of segments with either 10 µM quercetin alone or 10 µM myricetin alone (followed by subsequent removal) was associated with a significant reduction, of the contractions elicited by KCl (see Table 1). Responses to U46619 were also significantly reduced, following overnight exposure to 10 µM myricetin alone (Table 1). Although exposure to 10 µM quercetin did not significantly affect U46619-induced contractions (Table 1), substance P-induced relaxations were significantly reduced. Table 1 also shows that overnight exposure to 1 µM quercetin did not affect either KCl or U46619-induced contractions or substance P-induced relaxations.
Figure 3 shows that porcine coronary artery segments exposed overnight to a combination of 0.1 µM quercetin with 1 µg·mL−1 LPS, or 1 µM quercetin with 1 µg·mL−1 LPS, elicited larger contractions to both KCl and U46619 compared with segments exposed to 1 µg·mL−1 LPS alone. Prior exposure to 1 µM or 10 µM quercetin prevented the inhibitory effect of 1 µg·mL−1 LPS on substance P-induced relaxation and was associated with a small increase in the potency of U46619 (Table 2). The impairment of KCl and U46619 responses caused by 1 µg·mL−1 LPS was also significantly reduced by prior exposure to either 10 µM quercetin-3′-sulphate or 10 µM quercetin-3-glucuronide (Table 2). Similarly, substance P-induced relaxations following exposure to LPS were significantly larger when preparations were co-incubated with the metabolites (Table 2). Figure 4 shows that while both 1 µM quercetin and a combination of 1 µM quercetin and 10 µM Bay K 11-7082 was able to oppose the inhibitory effect of LPS on constrictor responses and endothelium-dependent relaxations, there was no difference between the two conditions (see Table 2).
Figure 3.
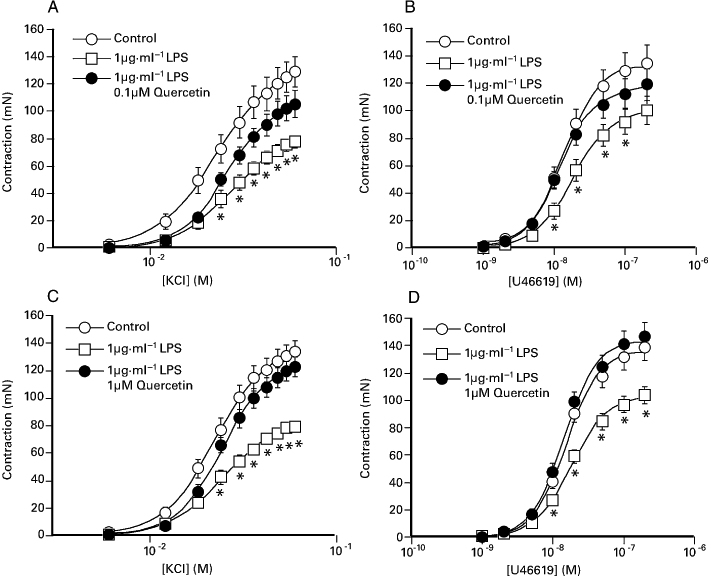
The effect of overnight exposure of the porcine coronary artery to 1 µg·mL−1 LPS, in the presence or absence of either (A, B) 0.1 µM quercetin or (C, D) 1 µM quercetin, on responses elicited by KCl and U46619. The responses shown are as mean ± SEM of 13–26 observations. *P < 0.05, significant difference between responses for the paired LPS-treated preparations. LPS, lipopolysaccharide.
Figure 4.
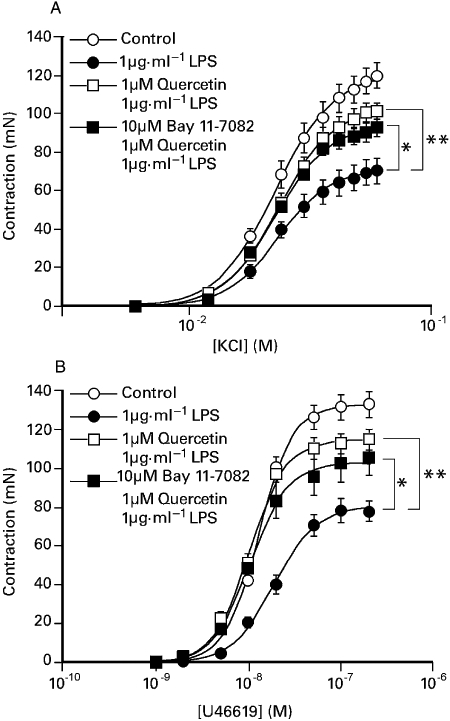
The effect of overnight exposure of the porcine coronary artery to 1 µg·mL−1 LPS in the presence or absence of either 1 µM quercetin, or 1 µM quercetin and 10 µM Bay K 11-7082 on responses to either (A) KCl or (B) U46619. The responses are shown as the mean ± SEM of 10 observations. *P < 0.05, **P < 0.01 significant differences between the maximum responses in preparations treated with LPS: anova followed by Dunnett's post hoc test. LPS, lipopolysaccharide.
In a separate set of experiments we established that following removal of the endothelium, overnight exposure to 1 µg·mL−1 LPS was still associated with a significant reduction in the maximum contractions to KCl (76.8 ± 3.2% of control segments, n= 8) and U46619 (71.2 ± 4.6% of the control segments, n= 8). Successful removal of the endothelium was verified at the end of the experiment by the failure of substance P to cause relaxation of U46619-induced tone. As shown in Table 2, incubation with 1 µM quercetin in endothelium-denuded segments afforded significant protection against the inhibitory effect of 1 µg·mL−1 LPS on maximal responses to both agonists.
Nitrite ion accumulation
Following overnight storage in DMEM control segments of the porcine coronary artery produced between 3 and 30 nmol nitrite (mg wet weight)−1. Exposure to 1 µg·mL−1 LPS was associated with a 5- to 20-fold increase in the production of nitrite ions by the coronary artery (Table 3). Co-incubation of the porcine coronary artery with either 10 µM 1400 W or 1 µM dexamthasone reduced the LPS response by 77.6 ± 5.3% (n= 12) and 70.9 ± 4.8% (n= 12), respectively, while 10 µM Bay 11-7082 abolished the response (Table 3). Evidence for the induction of NOS was provided by immunohistochemical examination of the porcine coronary artery. Figure 5 shows that control preparations express CD31 on endothelial cells but no evidence of iNOS. Segments treated overnight with 1 µg·mL−1 LPS exhibited increased expression of endothelial CD31 and the appearance of iNOS in the tunica adventitia (but not the tunica media). Co-incubation with 10 µM quercetin reduced LPS-induced expression of endothelial CD31 and adventitial NOS (Figure 5). Similar observations were noted in preparations of the coronary artery from three other animals.
Table 3.
Effect of inhibitors of nitric oxide synthase and various flavonoids on nitrite ion production of the porcine isolated coronary artery segments incubated for 16 h in modified Krebs-Henseleit solution in the absence or presence of 1 µg·mL−1 LPS
| Nitrite (nmol·mg−1 wet weight) | |||||
|---|---|---|---|---|---|
| n= 12 | n= 12 | n= 9 | n= 16 | n= 11 | |
| Control | 7.4 ± 3.2 | 5.5 ± 0.9 | 17.8 ± 7.6 | 21.1 ± 6.7 | 20.5 ± 3.8 |
| LPS | 99.8 ± 10.6 | 32.5 ± 0.5 | 138 ± 29.1 | 105 ± 18.8 | 274 ± 46.5 |
| LPS and flavonoid or inhibitor | 30.8 ± 3.3**(10 µM 1400 W) | 6.4 ± 0.9**(10 µM Bay 11-7082) | 70.6 ± 20.0**(0.1 µM quercetin) | 37.4 ± 10.2**(1 µM quercetin) | 80.1 ± 21.5**(10 µM quercetin 3′-sulphate) |
| LPS and flavonoid or inhibitor | 33.1 ± 5.6**(1 µM dexameth') | 40.8 ± 12.3**(10 µM quercetin 3-glucuronide) | |||
Values shown are the mean ± SEM of 9–16 observations.
*P < 0.05,
P < 0.01; denote a statistically significantly different from the LPS preparation (paired Student's t-test) or anova with a post hoc Dunnett test.
dexmeth, dexamethasone.
Figure 5.
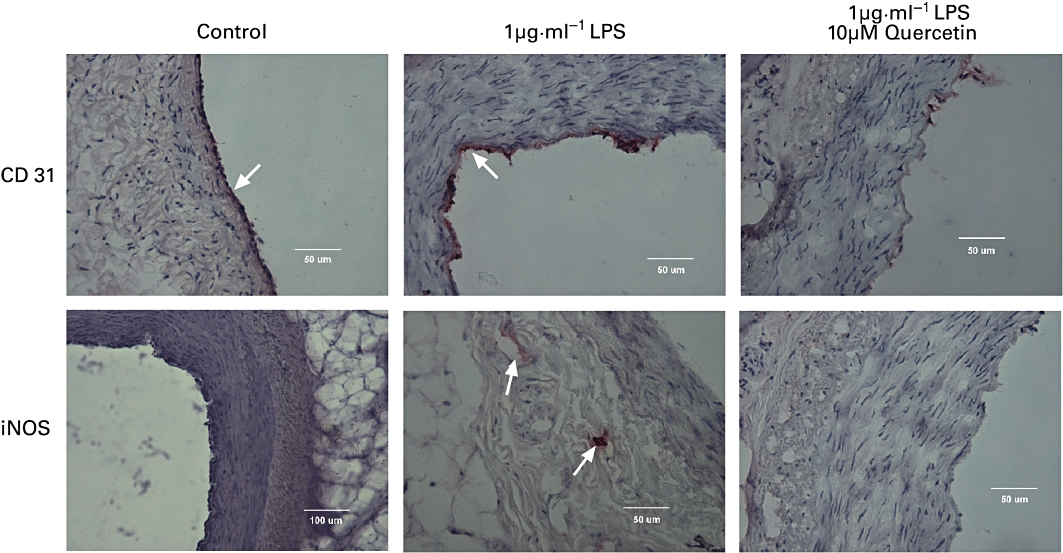
Immunohistochemical localization of (upper panels) PECAM-1 (CD31) and (lower panels) inducible nitric oxide synthase (iNOS) in the porcine isolated coronary artery following incubation in modified Krebs-Henseleit solution for 16–18 h at 37°C without (Control) or with either 1 µg·mL−1 LPS or 1 µg·mL−1 LPS and 10 µM quercetin (quercetin added 60 min before LPS). Evidence for the presence of these proteins in either the endothelium or tunica adventitia is shown by the presence of red staining and the bar on each panel represents either 100 µm or 50 µm. LPS, lipopolysaccharide.
While prior exposure to 10 µM quercetin caused a significant 89.6 ± 4.4% (n= 12) reduction in LPS-induced nitrite production (Figure 6A), exposure to 10 µM myricetin did not affect LPS-induced increase in nitrite production (Figure 6B). Table 3 shows that prior incubation with either 0.1 µM and 1 µM quercetin reduced LPS-induced nitrite production by about 50% and 70% respectively. Similarly, exposure to 10 µM quercetin 3′-sulphate and 10 µM quercetin 3-glucuronide significantly reduced LPS-induced production of nitrite (Table 3).
Figure 6.
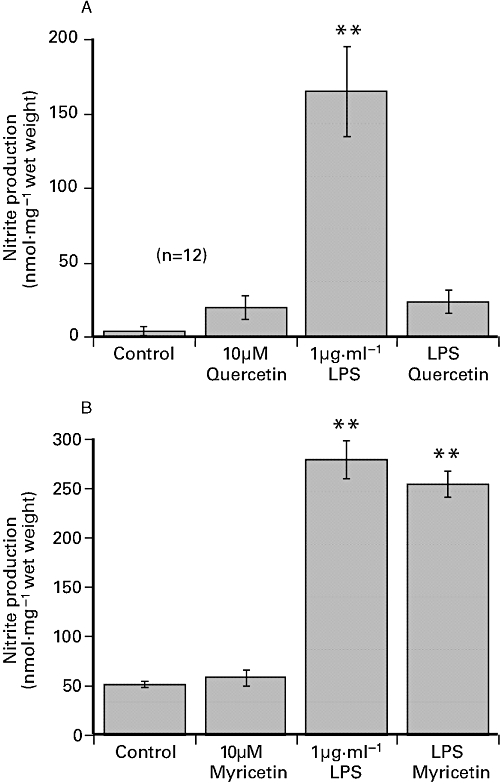
The effect of 24 h exposure to 1 µg·mL−1 LPS (A) 1 µg·mL−1 LPS plus 10 µM quercetin and (B) 1 µg·mL−1 LPS plus 10 µM myricetin on nitrite ion production in porcine coronary artery segments incubated in DMEM. The flavonoids were added to DMEM 60 min before LPS. The values shown are the mean ± SEM of 8–12 observations. **P < 0.01 significant difference from control, anova. DMEM, Dulbecco's modified Eagle's medium; LPS, lipopolysaccharide.
In light of the observations that quercetin, but not myricetin, reduced LPS-induced nitrite/nitrate production in the coronary artery (Figure 6), we also examined the effect of prior exposure to 10 µM myricetin against the inhibitory effect of 1 µM quercetin. Figure 7 shows that 1 µM quercetin alone markedly reduced LPS-induced nitrite production but was much less inhibitory in the presence of 10 µM myricetin.
Figure 7.
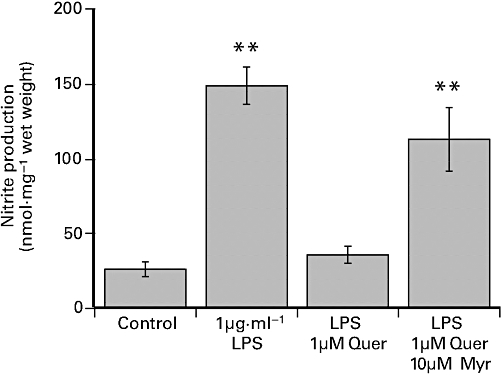
The effect of 24 h exposure to 1 µg·mL−1 LPS, 1 µg·mL−1 LPS plus 1 µM quercetin (Quer) and 1 µg·mL−1 LPS with a combination of 10 µM myricetin (Myr) and 1 µM quercetin on nitrite ion production in porcine coronary artery segments incubated in DMEM. Myricetin was added to DMEM 60 min before quercetin, which in turn was added 60 min prior to the addition of LPS. The values shown are the mean ± SEM of 12 observations. **P < 0.01 significant difference from control by anova. DMEM, Dulbecco's modified Eagle's medium; LPS, lipopolysaccharide.
Discussion
The principal observation in this study is that quercetin and two of its metabolites, quercetin 3′-sulphate and quercetin 3-glucuronide, inhibit key inflammatory changes in the porcine isolated coronary artery induced by prolonged exposure to LPS. The basis of this potentially beneficial effect of the flavonoid can be largely attributed to prevention of the induction of NOS.
Lipopolysaccharide-induced changes in the porcine coronary artery
Prolonged exposure of the coronary artery to LPS induced a significant reduction in contractile responses to both KCl and U46619, with a slightly greater effect against the former, and an impairment of endothelium-dependent relaxations to substance P. The effect of LPS on contractile responses is qualitatively similar to that noted in other studies (Gibreal et al., 2000; Piepot et al., 2000), but was not accompanied by a reduction in the potency of either agonist. In the case of substance P-induced relaxations, suppression of endothelium-dependent responses by LPS has been previously reported for the porcine coronary artery (Qi et al., 2007). The inhibitory effects of LPS on contractile responses was also observed in endothelium-denuded segments, which suggests that these changes are a function of independent actions on endothelial and non-endothelial cells in the blood vessel.
These changes in vascular responsiveness were accompanied by increased production of nitrite ions (as determined by the Griess reaction) and immunohistochemical evidence for induction of NOS in the blood vessel. The adventitial location for iNOS in the coronary artery is similar to that reported for the rat aorta (Kleschyov et al., 1998), where exposure to LPS caused a 4- to 10-fold greater activity for nitric oxide production in the tunica adventitia compared with the tunica media. A strong pharmacological link between biochemical and functional events was provided by the finding that exposure to 1400 W, a selective inhibitor of iNOS (Garvey et al., 1997), prevented LPS-induced changes in contractile responses and nitric oxide formation in the porcine coronary artery. As the effect of 1400 W on LPS-induced changes in the coronary artery were mimicked by inhibition of NFκB (with Bay 11-7082) it would appear that activation of this pathway precedes induction of NOS, as described in macrophages (Chen et al., 2005).
The effect of quercetin and quercetin metabolites
Quercetin, quercetin 3′-sulphate and quercetin 3-glucuronide suppressed the LPS-induced changes in endothelial and vascular responses, and the elevation in nitrite ion production, with the aglycone exhibiting activity at concentrations as low as 0.1 µM. As the effect of quercetin on LPS-induced changes was also noted in endothelium-denuded segments of the coronary artery, it would appear that the endothelium is not the primary site of action for this flavonoid. A direct action of quercetin on iNOS appears unlikely as immunohistochemical evidence clearly implicates suppression of the induction of the enzyme in the tunica adventitia, in a manner comparable with that reported for the anti-inflammatory agent dexamethasone (Korhonen et al., 2002). Quercetin has been reported to inhibit LPS-induced nitric oxide production in various non-vascular cells, including RAW 264.7 macrophages (Chen et al., 2001), J774.1 macrophages (Raso et al., 2001; Hamalainen et al., 2007)) and mouse BV microglia (Chen et al., 2005). Crucially, however, the potency of quercetin in the porcine isolated coronary artery is approximately 10- to 30-fold greater than that noted in cultured cells, where it is typically >3 µM. The efficacy of quercetin and its metabolites in this vascular model stands in marked contrast to that of myricetin, which failed to modify LPS-induced suppression of contractile responses.
Quercetin-induced changes of nitric oxide production superficially mirrors the effects observed on LPS-induced suppression of contractile response. While this point is reinforced by the lack of effect of myricetin on LPS-induced nitrite production, the precise relationship between biochemical events and functional changes is clearly complex. The highest concentration of quercetin examined (10 µM) significantly impaired vasoconstrictor responses and substance P-mediated relaxations per se (see Table 1). Thus, the overall effect of 10 µM quercetin on LPS-induced changes in contractile responses is the sum of two opposing actions and less than that observed with 1 µM quercetin – but comparable with that noted for the overall effect of 0.1 µM quercetin. Also, it remains a possibility that the overall effect of LPS on contractile responses is product of the induction of several inflammatory mediators rather than just nitric oxide (Qi et al., 2007). Overall, it is noteworthy that quercetin modified the coronary actions of LPS in a similar manner to that reported for eritoran (a Toll-like receptor 4 antagonist) against endotoxin in rat aortic segments (Ehrentraut et al., 2007), which suggests that this flavonoid may be beneficial in inflammatory conditions.
It is well recognized that a key mediator of inflammatory responses in cells is the translocation of nuclear factor-κB (NF-κB) from the cytoplasm to the nucleus and activation of numerous genes, including those for NOS and pro-inflammatory cytokines (Liu and Malik, 2006). In the case of endothelial and vascular smooth muscle cells NFκB has been linked to increased expression of cell adhesion molecules (Read et al., 1994), the induction of NOS (Hattori et al., 2003) and associated with development of early atherosclerotic lesions (Hajra et al., 2000). Thus, stabilization of the NF-κB/IκB complex in the coronary artery could explain the protective effect of quercetin against LPS-induced changes in contractile responses, nitric oxide production and the expression of PECAM-1 (CD31) found in this study. This possibility is reinforced by the observation that a combination of quercetin and Bay K 11-7082, a known inhibitor of NFκB (Pierce et al., 1997) was no more effective against LPS-induced changes in the coronary artery than quercetin alone (see Figure 4), yet neither condition completely prevented the inhibitory effect of LPS. While the precise molecular target for this beneficial effect of quercetin was not investigated, the failure of myricetin to mimic the effect of quercetin on LPS-induced nitric oxide production, and to also ‘antagonise’ the effect of quercetin (see Figure 7), indicates that these structurally related flavonoids may be useful in future studies. It is noteworthy that the lack of effect of myricetin on inflammatory responses in the coronary artery is not a selective effect for the vasculature. Blonska et al. (2003) noted that quercetin and kaempferol were capable of inhibiting LPS-induced production of IL-1β in RAW 264.7 cells but myricetin was inactive.
Although the potency of the flavonoids against inflammatory events in the coronary artery is greater than that reported in macrophages, suggesting a selective action on the vasculature, the physiological relevance remains unclear. Quercetin is extensively metabolized in man and the concentrations used in this study exceeded the peak plasma levels of the aglycone (0.03 µM) and the metabolites (3 µM) detected following dietary ingestion (Kroon et al., 2004; Wang and Morris, 2005). However, two recent studies raise the possibility that flavanoids can be either generated in situ from metabolites accumulated in activated cells (Kawai et al., 2008a) or even preferentially concentrated in cells (Kawai et al., 2008b), potentially the threshold for physiological significance. Nonetheless, further studies on human blood vessels with lower concentrations of quercetin and the metabolites are warranted. In light of the striking difference between the effect of quercetin and myricetin on the porcine coronary artery, there is also a need to establish whether myricetin can inhibit the effect of quercetin in human vascular cells, effectively behaving as an ‘antagonist’.
The finding that quercetin can oppose the proinflammatory effect of LPS on the vasculature may also hold therapeutic significance. Recently, quercetin has been shown to attenuate both the release of pro-inflammatory cytokines in response to LPS in mice and the associated lethality (Teng et al., 2009). Significantly, this effect of quercetin was manifest even when administered several hours after exposure to LPS, raising the possibility that a similar mechanism may occur in the vasculature.
In conclusion, we have demonstrated that one of the major dietary flavonoids, quercetin and its principal human metabolites oppose pro-inflammatory events in both endothelial cells and vascular smooth muscle cells. These effects of quercetin are evident at lower concentrations than previously reported in studies using other cell types and suggests a selective action on the vasculature, particularly against the induction of NOS. Further studies on human blood vessels are warranted to establish whether these observations are relevant to the well-documented beneficial effects of dietary flavonoids (Halliwell, 2007; Boots et al., 2008).
Acknowledgments
This work was supported by a grant (BB/C508418/1) from the BBSRC and the Libyan Government (to SA). We are grateful to G Woods and Sons (Clipstone, Nottinghamshire) for the supply of pig hearts and to Lyndon Cochrane for assistance with preparation of the figures.
Glossary
Abbreviations
- DMEM
Dulbecco's modified Eagle's medium
- K-H
Krebs-Henseleit
- LPS
lipopolysaccharide
- NOS
nitric oxide synthase
Conflicts of interests
There are no conflicts of interest for the authors.
Supplementary material
Supporting Information: Teaching Materials; Figs 1–7 as PowerPoint slide.
References
- Blonska M, Czuba ZP, Krol W. Effects of flavone derivatives on interleukin-1β (IL-1β) mRNA expression and IL-1β protein synthesis in stimulated RAW 264.7 macrophages. Scand J Immunol. 2003;57:162–166. doi: 10.1046/j.1365-3083.2003.01213.x. [DOI] [PubMed] [Google Scholar]
- Boots AW, Haenen GRMM, Bast A. Health effects of quercetin: from antioxidant and nutraceutical. Eur J Pharmacol. 2008;585:325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Chen Y, Shen S, Lee W, Hou W, Yang L, Lee F. Inhibition of nitric oxide synthase inhibitors and lipopolysaccharides induced inducible NOS and cyclo-oxygenase-2 gene expressions by rutin, quercetin, and quercetin pentaacetate in RAW 264.7 macrophages. J Cell Biochem. 2001;28:537–548. doi: 10.1002/jcb.1184. [DOI] [PubMed] [Google Scholar]
- Chen J, Feng-Ming F, Chao P, Chen C, Jeng K, Hsu H, et al. Inhibition of iNOS gene expression by quercetin is mediated by inhibition of Ik B kinase, nuclear factor-kappa B and STAT1 and depends on heme oxygenase-1 induction in mouse BV-2 microglia. Eur J Pharmacol. 2005;521:9–20. doi: 10.1016/j.ejphar.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Edwards R, Lyon T, Litwin E, Rabovesky A, Jalili T. Quercetin reduces blood pressure in hypertensive subjects. J Nutr. 2007;137:2405–2411. doi: 10.1093/jn/137.11.2405. [DOI] [PubMed] [Google Scholar]
- Ehrentraut S, Frede S, Stapel H, Mengden T, Grohe C, Fandrey J, et al. Antagonism of lipopolysaccharide-induced blood pressure attenuation and vascular contractility. Arterioscler Thromb Vasc Biol. 2007;27:2170–2176. doi: 10.1161/ATVBAHA.107.146100. [DOI] [PubMed] [Google Scholar]
- Fusi F, Sgargali G, Saponara S. Mechanism of myricetin stimulation of vascular L-type Ca2+ currents. J Pharmacol Exp Ther. 2005;313:790–797. doi: 10.1124/jpet.104.080135. [DOI] [PubMed] [Google Scholar]
- Garvey EP, Oplinger JA, Furfine ES, Kiff RJ, Laszlo F, Whittle B, et al. 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric oxide synthase in vitro and in vivo. J Biol Chem. 1997;272:4959–4963. doi: 10.1074/jbc.272.8.4959. [DOI] [PubMed] [Google Scholar]
- Gibreal H, Dittrich P, Saleh S, Mayer B. Inhibition of endotoxin-induced vascular hyporeactivity by 4-amino-tetrahydrobiopterin. Br J Pharmcol. 2000;131:1757–1765. doi: 10.1038/sj.bjp.0703752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gil JM, Zwaginga JJ, Hordijk PL. Molecular and functional interactions among monocyctes, platelets, and endothelial cells and their relevance for cardiovascular disease. J Leukoc Biol. 2008;85:195–204. doi: 10.1189/jlb.0708400. [DOI] [PubMed] [Google Scholar]
- Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, Cybulsky MI. The NF-κB signal transduction pathway in aortic endothelial cells is primed for activation in regions pre-disposed to atherosclerotic lesion formation. Proc Natl Acad Sci. 2000;97:9052–9057. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Dietary polyphenols: good, bad or indifferent for your health. Cardiovasc Res. 2007;73:341–347. doi: 10.1016/j.cardiores.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Hamalainen M, Nieminen R, Vuorela P, Heinonen M, Mollanen E. Anti-inflammatory effects of flavonoids: genistein, kaemperfol, quercetin and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effects on iNOS expression and NO production in activated macrophages. Mediators Inflamm. 2007;2007:45673–45683. doi: 10.1155/2007/45673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y, Hattori S, Kasai K. Lipopolysaccharide activates Akt in vascular smooth muscle cells resulting in induction of inducible nitric oxide synthase through nuclear factor-kappa B activation. Eur J Pharmacol. 2003;481:153–158. doi: 10.1016/j.ejphar.2003.09.034. [DOI] [PubMed] [Google Scholar]
- Kawai Y, Nishikawa T, Shiba Y, Saito S, Murota K, Shibata N, et al. Macrophage as a target of quercetin glucuronide in human atherosclerotic arteries. J Biol Chem. 2008a;283:9424–9434. doi: 10.1074/jbc.M706571200. [DOI] [PubMed] [Google Scholar]
- Kawai Y, Tanaka H, Murota K, Naito M, Terao J. (-)-Epicatechin gallate accumulates in foamy macrophages in human atherosclerosis aorta: implication in the anti-atherosclerosis action of tea catechins. Biochem Biophys Res Commun. 2008b;374:527–532. doi: 10.1016/j.bbrc.2008.07.086. [DOI] [PubMed] [Google Scholar]
- Kelm M. Nitric oxide metabolism and breakdown. Biochim Biophys Acta. 1999;5:273–289. doi: 10.1016/s0005-2728(99)00020-1. [DOI] [PubMed] [Google Scholar]
- Kleschyov AL, Muller B, Schott C, Stoclet J-C. Role of adventitial nitric oxide in vascular hyporeactivity induced by lipopolysaccharide in rat aorta. Br J Pharmacol. 1998;124:623–626. doi: 10.1038/sj.bjp.0701916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen R, Lahti A, Hamalainen M, Kankaaranta H, Moilanen E. Dexamethasone inhibits inducible nitric oxide synthase by destabilising mRNA in lipopolysaccharide-treated macrophages. Mol Pharmacol. 2002;62:698–704. doi: 10.1124/mol.62.3.698. [DOI] [PubMed] [Google Scholar]
- Kroon PA, Clifford MN, Crozier A, Day AJ, Donovan JL, Manach C. How should we assess the effects of exposure to dietary polyphenols in vitro? Am J Clin Nutr. 2004;80:15–21. doi: 10.1093/ajcn/80.1.15. [DOI] [PubMed] [Google Scholar]
- Liu SF, Malik AB. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol. 2006;290:622–645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- Mitchell JA, Ali F, Bailey L, Moreno L, Harrington LS. Role of nitric oxide and prostacyclin as vasoactive hormones released by the endothelium. Exp Physiol. 2007;93:141–147. doi: 10.1113/expphysiol.2007.038588. [DOI] [PubMed] [Google Scholar]
- Needs P, Kroon P. Convenient synthesis of metabolically important glucuronides and sulfates. Tetrahedron. 2006;62:6862–6868. [Google Scholar]
- Perez-Vizcaino F, Ibarra M, Angel L, Duarte J, Moreno L, Lopez G, et al. Endothelium-independent vasodilator effects of flavonoid quercetin and its metabolites in rat conductance and resistance arteries. J Pharmacol Exp Ther. 2002;302:66–72. doi: 10.1124/jpet.302.1.66. [DOI] [PubMed] [Google Scholar]
- Piepot HA, Boer C, Groenevald AB, Van Lamabalgen AA, Sipkema P. Lipopolysaccharide impairs endothelial nitric oxide synthesis in rat renal arteries. Kidney Int. 2000;57:2502–2510. doi: 10.1046/j.1523-1755.2000.00109.x. [DOI] [PubMed] [Google Scholar]
- Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, et al. Novel inhibitors of cytokine-induced Ikappaβalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem. 1997;272:21096–21003. doi: 10.1074/jbc.272.34.21096. [DOI] [PubMed] [Google Scholar]
- Qi W, Wei JX, Dorairaj I, Wilson VG, Mahajan R. Evidence that a prostanoid produced by cyclo-oxygenase-2 enhances contractile responses of the porcine isolated coronary artery following exposure to LPS. Br J Anaesth. 2007;98:1577–1588. doi: 10.1093/bja/ael378. [DOI] [PubMed] [Google Scholar]
- Raso GM, Meli R, Di Carlo G, Pacilio M, Di Carlo R. Inhibition of inducible nitric oxide synthase and cyclooxygenase expression by flavonoids in macrophage J774.1. Life Sci. 2001;68:921–931. doi: 10.1016/s0024-3205(00)00999-1. [DOI] [PubMed] [Google Scholar]
- Read MA, Whitley MZ, Williams AJ, Collins T. NF-?B and I?Bα: an inducible regulatory system in endothelial activation. J Exp Med. 1994;194:503–512. doi: 10.1084/jem.179.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri S, Liu X-H, Rayment S, Hughes D, Kroon P, Needs P, et al. Quercetin and its major metabolite selectively modulate cyclic GMP-dependent relaxations and associated tolerance in pig isolated coronary artery. Br J Pharmacol. 2010;159:566–575. doi: 10.1111/j.1476-5381.2009.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng D, Kang R, Xiao W, Zhang H, Lotze MT, Wang H, et al. Quercetin prevents lipopolysaccharide-induced HMGB1 release and pro-inflammatory function. Am J Respir Cell Mol Biol. 2009;41:651–660. doi: 10.1165/rcmb.2008-0119OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribolo S, Lodi F, Connor C, Suri S, Wilson VG, Taylor MA, et al. Comparative effects of quercetin and its predominant human metabolites on adhesion molecule expression in activated human vascular endothelial cells. Atherosclerosis. 2008;197:50–60. doi: 10.1016/j.atherosclerosis.2007.07.040. [DOI] [PubMed] [Google Scholar]
- Ukil A, Maity S, Das P. Protection from experimental colitis by theafalvin-3,3′-digallate correlates with inhibition of IKK and NFkappaB activation. Br J Pharmacol. 2006;149:121–131. doi: 10.1038/sj.bjp.0706847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Morris ME. Liquid chromatography-tandem mass spectroscopy assay for quercetin and conjugated quercetin metabolites in human plasma and urine. J Chromatogr B. 2005;821:194–201. doi: 10.1016/j.jchromb.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am J Clin Nutr. 2005;81:243S–255S. doi: 10.1093/ajcn/81.1.243S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


