Abstract
BACKGROUND AND PURPOSE
Transient receptor potential canonical 5 (TRPC5) channels are widely expressed, including in the CNS, where they potentiate fear responses. They also contribute to other non-selective cation channels that are stimulated by G-protein-coupled receptor agonists and lipid and redox factors. Steroids are known to modulate fear and anxiety states, and we therefore investigated whether TRPC5 exhibited sensitivity to steroids.
EXPERIMENTAL APPROACH
Human TRPC5 channels were conditionally expressed in HEK293 cells and studied using intracellular Ca2+ measurement, whole-cell voltage-clamp and excised patch techniques. For comparison, control experiments were performed with cells lacking TRPC5 channels or expressing another TRP channel, TRPM2. Native TRPC channel activity was recorded from vascular smooth muscle cells.
KEY RESULTS
Extracellular application of pregnenolone sulphate, pregnanolone sulphate, pregnanolone, progesterone or dihydrotestosterone inhibited TRPC5 activity within 1–2 min. Dehydroepiandrosterone sulphate or 17β-oestradiol had weak inhibitory effects. Pregnenolone, and allopregnanolone, a progesterone metabolite and stereo-isomer of pregnanolone, all had no effects. Progesterone was the most potent of the steroids, especially against TRPC5 channel activity evoked by sphingosine-1-phosphate. In outside-out patch recordings, bath-applied progesterone and dihydrotestosterone had strong and reversible effects, suggesting relatively direct mechanisms of action. Progesterone inhibited native TRPC5-containing channel activity, evoked by oxidized phospholipid.
CONCLUSIONS AND IMPLICATIONS
Our data suggest that TRPC5 channels are susceptible to relatively direct and rapid stereo-selective steroid modulation, leading to channel inhibition. The study adds to growing appreciation of TRP channels as non-genomic steroid sensors.
Keywords: cationic channel, transient receptor potential canonical 5, neurosteroid, progesterone
Introduction
The seven mammalian transient receptor potential canonical (TRPC) proteins assemble to form non-selective Ca2+-permeable channels (Birnbaumer, 2009; channel and receptor nomenclature follows Alexander et al., 2009). The human TRPC5 gene was identified in a region on the X-chromosome that contains loci for non-syndromic mental retardation (Sossey-Alaoui et al., 1999; Beech, 2007). Although its expression is not restricted to the nervous system, the only known phenotype of Trpc5 gene-disrupted mice is reduced innate fear responses to aversive stimuli (Riccio et al., 2009). Therefore, there is potential for TRPC5 channels to be the target of substances that promote or suppress anxiety. The channel activity is already known to be potentiated by acidosis and the toxic metal ion Pb2+ (Semtner et al., 2007; Sukumar and Beech, 2010), effects that may contribute to fear responses of suffocation and poisoning. However, inhibitors of the channels with anxiolytic activity are not known.
Neuroactive steroids are synthesized from cholesterol in the brain, adrenal glands and gonads (Compagnone and Mellon, 2000). They include pregnenolone sulphate, pregnenolone, dehydroepiandrosterone, progesterone, pregnanolone, allopregnanolone and testosterone (Charalampopoulos et al., 2008; Schumacher et al., 2008; Veleiro and Burton, 2009). Several of the steroids, including progesterone and testosterone, are anxiolytic, and have putative roles in anxiety disorders (Edinger and Frye, 2005). Progesterone is also a major sex hormone that can reach high serum concentrations in females. Female rats display reduced anxiety at pro-oestrus, when progesterone concentrations are high. Anxiolytic effects are mediated by GABAA receptor modulation (Toufexis et al., 2004), but may also involve additional mechanisms (Auger and Forbes-Lorman, 2008). Such steroids also have other wide-ranging effects that could potentially be explained, at least partially, by actions on widely distributed ion channels such as TRPC channels.
A small number of studies have suggested links between TRP channels and steroids. Endogenous capsaicin responses, presumably mediated by TRPV1, a member of the TRP Vanilloid (V) channels, are inhibited by pregnenolone sulphate and dehydroepiandrosterone (Chen and Wu, 2004), and a member of the melastatin (M) class of TRP channels, TRPM3, is stimulated by pregnenolone sulphate and dehydroepiandrosterone sulphate (Wagner et al., 2008; Majeed et al., 2010). There are no reports of other TRP channels being linked with neuroactive steroids, although TRPC2 (which is not expressed in humans) is stimulated by corticosterone 21-sulphate, a component of mouse urine (Nodari et al., 2008). TRPM3 is not stimulated by corticosterone 21-sulphate (Majeed et al., 2010). During studies of TRPM3 channels, we tested pregnenolone sulphate against TRPC5 in a counter screen and found activity. Here we describe these effects and report on an investigation of the steroid structure-activity relationships. Our data suggest that TRPC5 channels should be added to the list of non-genomic membrane steroid sensors.
Methods
Cell culture
Human TRPC5 and TRPM2 channels were expressed as described previously (McHugh et al., 2003; Zeng et al., 2004). TRP channel cDNA, stably incorporated into HEK293 cells, was under the control of a tetracycline-inducible promoter, such that addition of 1 µg·mL−1 tetracycline (Tet+)-induced expression of exogenous channels. Cells not treated with tetracycline (Tet-) were used as control. Cells were maintained in Dulbecco's modified Eagle's medium-F12+ GLUTAMAX (Cat No. 31331, Gibco, Paisley, UK) supplemented with 10% fetal calf serum, 100 units·mL−1 penicillin/streptomycin (Sigma, St. Louis, MO, USA) and the selection antibiotics (10 µg·mL−1 blasticidin and 400 µg·mL−1 zeocin; Invitrogen, Paisley, UK) at 37°C in a 5% CO2 incubator.
Freshly discarded human saphenous vein segments were obtained anonymously and with informed consent from patients undergoing open heart surgery in the General Infirmary at Leeds. Approval was granted by the Leeds Teaching Hospitals Local Research Ethics Committee. Proliferating vascular smooth muscle cells (VSMC) were prepared from these segments using an explant technique and grown in Dulbecco's modified Eagle's medium + GLUTAMAX (Cat # 31966, Gibco). The medium was supplemented with 10% fetal calf serum, 100 units·mL−1 penicillin/streptomycin (Sigma) at 37°C in a 5% CO2 incubator. Experiments were performed on cells passaged 3–5 times.
Ca2+ measurement
Twenty-four hours prior to intracellular Ca2+ measurements, HEK293 cells were plated at 80–90% confluence in clear-bottom poly-D lysine coated 96-well plates (Corning, NY, USA) or VSMCs were plated at 80–90% confluence in non-coated, square-bottomed clear plates (Cat No. 167008, Nunclon, Roskilde, Denmark). Immediately prior to recordings, cells were incubated for 1 h at 37°C in standard bath solution (SBS) containing 2 µM fura-2 acetoxymethyl ester (and 0.01% pluronic acid), and then washed with SBS once before adding the recording buffer (SBS with appropriate solvent). All experiments were at 23 ± 2°C. SBS in de-ionized water contained (in mM): NaCl 135, KCl 5, MgCl2 1.2, CaCl2 1.5, glucose 8 and HEPES 10; pH was titrated to 7.4 using 4 M NaOH. The osmolality of this solution was 290 mOsm·kg−1. Calcium-free SBS was SBS without added CaCl2. Measurements were made on a 96-well fluorescence plate reader (FlexStation II384, Molecular Devices, Sunnyvale, CA, USA). Fura-2 was excited by light of 340 and 380 nm and emitted light was collected at 510 nm. Wells of the 96-well plate were studied in a column format and loaded alternately for test and control conditions. Intracellular calcium (Cai2+) concentration is indicated as the ratio of fura-2 fluorescence (F) emission intensities for 340 and 380 nm (F ratio).
Electrophysiology
Recordings were made using the whole-cell or outside-out patch configuration of the patch-clamp technique. Borosilicate glass capillaries with an outside diameter of 1 mm and an inside diameter of 0.58 mm (Harvard Apparatus, Holliston, MA, USA) were used as the basis for patch pipettes. Pipettes were pulled using a PP-830 vertical two-stage pipette-puller (Narishige, Tokyo, Japan). Pipette resistances after fire polishing and filling with pipette solution were 3–5 MΩ. Pipettes were mounted either on a CV203BU or CV-4 head-stage (Molecular Devices, Sunnyvale, CA, USA) connected to a three-way coarse manipulator and micro-manipulator (Newport 300P, Newport Corporation, Irvine, CA or Mitutoyo, Japan). Electrodes comprised silver wires coated with chloride ions. Electrical signals were amplified and recorded using an Axopatch 200B or 1D amplifier and pCLAMP 8 software (Molecular Devices). Data were filtered at 1 kHz and sampled digitally at 2 kHz via a Digidata 1322A analogue to digital converter (Molecular Devices). Series resistances were <10 MΩ. The voltage protocol consisted of a step from a holding potential of 0 mV to −100 mV, followed by a 0.1 s ramp to +100 mV, before returning to 0 mV (repeated every 10 s). Analysis was performed offline using Clampfit 8.2 (Molecular Devices) and Origin 7.5 software (OriginLab, Northampton, MA, USA). The extracellular solution was SBS. The patch-pipette solution contained (in mM): CsCl 145, MgCl2 2, EGTA 1, HEPES 10, Na2ATP 5 and Na2GTP 0.1. The pH was titrated to 7.2 using 4 M CsOH, and the osmolality was 295 mOsm·kg−1. The solution was filtered using a 0.2 µm membrane filter (Minisart, Sartorius Stedim Biotech, Goettingen, Germany), divided into aliquots of ∼50 µL and stored at −20°C. Capacitance measurements in the whole-cell configuration before patch excision (18.2 ± 1.5 pF, n= 10) and then after patch excision (1.15 ± 0.13 pF, n= 10) confirmed the successful generation of outside-out patches, in addition to visual inspection using the microscope; pipette capacitance was not compensated.
Data analysis
Origin 7.5 software (OriginLab) was used for data analysis and presentation. Averaged data are expressed as mean ± SEmean. Control and test data were produced in pairs and compared using an independent Student's t-test (intracellular Ca2+ measurement) or paired Student's t-test (electrophysiology performed on the same cell). Probability (P) of less than 0.05 was considered statistically significant. Where indicated as %, test data were normalized to control (Ctrl) data. All intracellular Ca2+ measurement data are presented as n/N, where n is the number of independent experiments (i.e. on different 96-well plates) and N is the number of wells used in the 96-well plates. For patch-clamp recordings, n is the number of cells from which measurements were made.
Materials
Steroids were purchased from Sigma or Steraloids (Newport, RI, USA), and stock solutions were stored according to the suppliers' instructions. The following steroids were prepared as 10–100 mM stocks in 100% DMSO: pregnenolone sulphate, progesterone, dehydroepiandrosterone sulphate, pregnanolone sulphate, pregnanolone and allopregnanolone (3α-hydroxy-5α-pregnan-20-one or 3α,5α-tetrahydroprogesterone). Gadolinium was purchased from Sigma and prepared as 20–100 mM stock solutions in deionized water. Dihydrotestosterone, 17β-oestradiol and cortisol were prepared as 10 mM stocks in 100% ethanol. Sphingosine-1-phosphate (S1P) was from Sigma, and prepared as a 10 mM stock in 100% methanol. 1-palmitoyl-2-glutaroyl-phosphatidylcholine (PGPC) was purchased as a 16.4 mM stock (in ethanol) from Cayman Chemicals (Ann Arbor, MI, USA). All other reagents were from Sigma.
Results
Pregnenolone sulphate inhibits TRPC5 channel activity
TRPC5 channels are Ca2+-permeable, allowing their functions to be detected using an intracellular Ca2+-indicator dye. Gadolinium ions (Gd3+) stimulated the channels and gave robust Ca2+-entry signals in cells expressing TRPC5 channels (Tet+), but not cells lacking these channels (Tet-) (Figure 1A). Treatment for 15 min with pregnenolone sulphate (Figure 1B) caused concentration-dependent inhibition of Gd3+-evoked TRPC5 channel activity (Figure 1C,D). The threshold concentration for effect was about 1 µM, and the IC50 was 19 µM (Figure 1D). Pregnenolone sulphate also inhibited TRPC5 channel function when assessed by whole-cell voltage-clamp recording (Figure 1E). The TRPC5 current–voltage relationship (I-V) showed inward rectification at negative voltages and outward rectification at positive voltages with a plateau around 0 mV, which gave an approximate inverted S-shape. This signature I-V was strongly suppressed, confirming that the steroid inhibited TRPC5 channels rather than background channel activity (Figure 1F, Figure S1A,B). Effects on TRPC5 channel-mediated currents were apparent at >1 µM pregnenolone sulphate (Figure 1G).
Figure 1.

Inhibition of Gd3+-stimulated TRPC5 channels by pregnenolone sulphate (PregS). All data were generated from cells over-expressing TRPC5 (Tet+) except for the control (Tet-) data shown in panel A, and by intracellular Ca2+ measurement (A,C,D) or whole-cell patch-clamp (E–G). A. Effect of application of 20 µM Gd3+. (B) Chemical structure of pregnenolone sulphate, including labelling of rings and carbon atom positions. (C) Effect of a 15 min treatment with 10 or 50 µM pregnenolone sulphate on responses to 20 µM Gd3+ (N= 4 for each concentration). (D) Concentration-dependent inhibition by pregnenolone sulphate (n/N= 3/12) with a fitted Hill equation (IC50 19 µM). Responses to Gd3+ were measured 80 s after its application, as indicated in panel C. (E) Time-series plot showing the effect of extracellular application of 1 or 10 µM pregnenolone sulphate on the outward (+95 mV) and inward currents (−95 mV) stimulated by 20 µM Gd3+. (F) Typical current-voltage relationships (I-Vs) in the presence of Gd3+ alone (20 Gd3+), and then plus 100 µM pregnenolone sulphate. (G) Mean data for effects of pregnenolone sulphate (µM) on outward and inward currents elicited by 20 µM Gd3+ (n= 3–4 for each concentration). TRPC5, transient receptor potential canonical 5.
Inhibition by pregnenolone sulphate was not restricted to Gd3+-stimulated TRPC5. Alternative TRPC5 channel stimulators are sphingosine-1-phosphate (S1P), which acts via endogenous Gi/o-coupled receptors (Xu et al., 2006), and lysophosphatidylcholine (LPC), which acts relatively directly (Flemming et al., 2006). Pregnenolone sulphate significantly inhibited S1P- and LPC-evoked TRPC5 channel activity (Figure 2A–D). Pregnenolone sulphate was not acting non-specifically to inhibit Ca2+-signalling because it did not affect Ca2+ signals due to TRPM2 channels (Figure 2E,F) or endogenous ATP-evoked Ca2+-release (Figure 2G,H). The data suggest that pregnenolone sulphate is a negative modulator of TRPC5 channels.
Figure 2.

General inhibition of TRPC5 channels and selectivity. Data were generated by intracellular Ca2+ measurement from TRPC5-expressing (Tet+) cells (A–D), TRPM2-expressing (Tet+) cells (E,F), or control cells not induced to express TRPC5 channels (Tet-) (G,H). (A) Typical effect of 10 µM pregnenolone sulphate on TRPC5 channels stimulated by 5 µM sphingosine-1-phosphate (S1P). (B) Mean data for experiments shown in panel A (n/N= 3/18). (C) Typical effect of 10 µM pregnenolone sulphate on TRPC5 stimulated by 5 µM lysophosphatidylcholine (LPC). (D) Mean data for experiments in panel C (n/N= 3/18). (E) Typical effect of 10 µM pregnenolone sulphate on TRPM2 stimulated by 1 mM hydrogen peroxide (H2O2). (F) Mean data for experiments in panel E (n/N= 3/24). (G) Typical effect of 10 µM pregnenolone sulphate on Ca2+ release elicited by 100 µM ATP. (H) Mean data for experiments in (G) (n/N= 3/12). Ctrl indicates the solvent control. TRPC5, transient receptor potential canonical 5; TRPM, transient receptor potential melastatin.
Chemical structure-activity relationships
Pregnenolone, the non-sulphated form of pregnenolone sulphate (Figure 3A), did not affect TRPC5-dependent Ca2+ entry (Figure 3B) or ionic current (Figure 3C,D) at 10 µM concentration. Pregnanolone sulphate is the same as pregnenolone sulphate, except it lacks the double bond of ring B, and the sulphate is in the mirrored (α) configuration (Figure 3E). It inhibited TRPC5 channel activity in Ca2+ measurement (Figure 3F) and patch-clamp (Figure S1C) experiments (I-Vs are shown in Figure S1 D). Pregnanolone contains the acetyl group at ring D, but lacks the sulphate group on ring A or the double bond of ring B (Figure 3I). It was an inhibitor of TRPC5 (Figure 3J,K) (I-Vs are shown in Figure S1D). The stereo isomer of pregnanolone, allopregnanolone (Figure 3I), lacked effect on TRPC5 channels (Figure 3J). Dehydroepiandrosterone sulphate contains the double bond of ring B and the β-sulphate, but the acetyl group of ring D is replaced by a ketone (Figure 3L). In contrast to pregnenolone sulphate, it was a poor inhibitor of TRPC5, even at 50 µM (Figure 3M). The data suggest a stereo-selective steroid binding site that is negatively coupled to TRPC5 channel activity.
Figure 3.
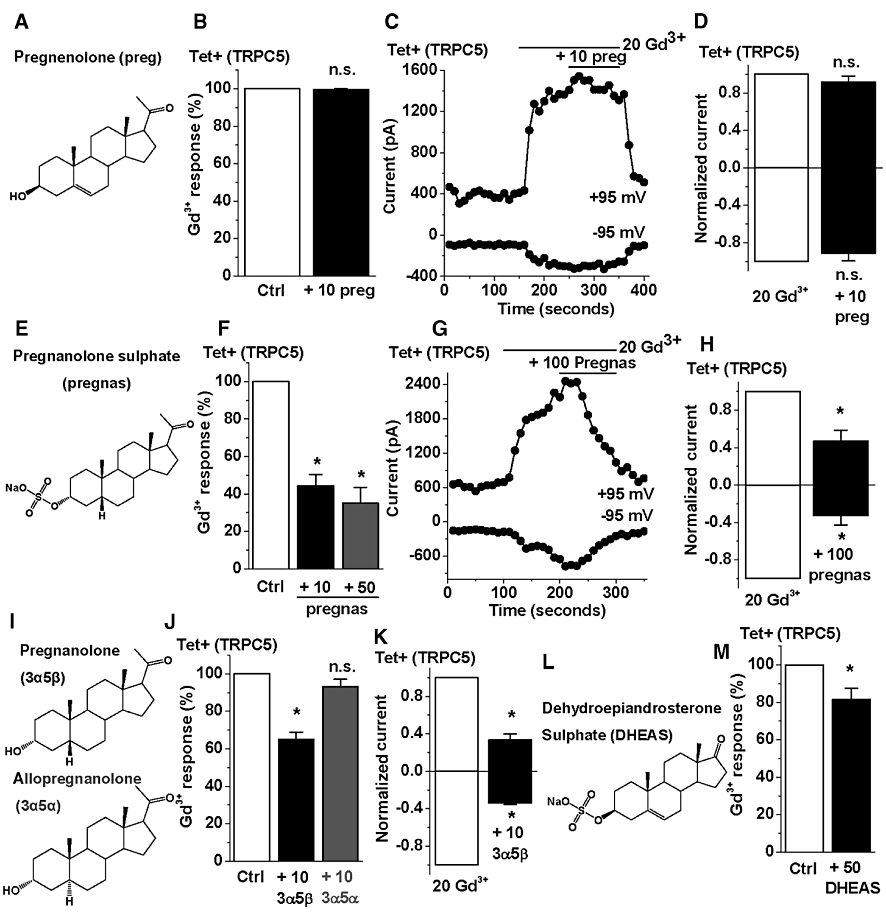
Steroid structure-activity relationships at TRPC5 channels. All data were generated from cells over-expressing TRPC5 channels (Tet+) by intracellular Ca2+ measurement (B,F,J,M) or whole-cell patch-clamp. (A,E,I,L) 2-dimensional structures of pregnenolone (preg), pregnanolone sulphate (pregnas), pregnanolone (3α5β), allopregnanolone (3α5α) and dehydroepiandrosterone sulphate (DHEAS). (B) Mean data for the effect of 10 µM pregnenolone on TRPC5 channels stimulated by 20 µM Gd3+ (n/N= 3/12). (C) Time-series plot showing the effect of 10 µM pregnenolone on current elicited by 20 µM Gd3+. (D) Mean data for experiments in panel C (n= 3). (F) Mean data for the effect of 10 or 50 µM pregnanolone sulphate on TRPC5 channels stimulated by 20 µM Gd3+ (n/N= 3/12). (G) Time-series plot from a whole-cell recording showing the effect of 100 µM pregnanolone sulphate on the current elicited by 20 µM Gd3+. (H) Mean data for experiments in panel G (n= 3). (J) Mean data for the effect of 10 µM pregnanolone or allopregnanolone on TRPC5 channels stimulated by 20 µM Gd3+ (n/N= 3/12). (K) Mean data for the effect of 10 µM pregnanolone on current elicited by 20 µM Gd3+ (n= 3). (M) Mean data for the effect of 50 µM dehydroepiandrosterone sulphate on TRPC5 channels stimulated by 20 µM Gd3+ (n/N= 3/24). Ctrl indicates the solvent control. TRPC5, transient receptor potential canonical 5; TRPM, transient receptor potential melastatin.
Inhibition by progesterone
An important endogenous steroid that meets the structural requirements outlined above is progesterone (Figure 4A). Progesterone inhibited Gd3+-stimulated TRPC5 channel activity in Ca2+-measurement (Figure 4B) and patch-clamp (Figure 4C–E) experiments, with marked effects at 10 µM (Figure 4B–E) and an IC50 of 5 µM (Figure 4B). Progesterone also inhibited S1P- and LPC-evoked TRPC5 channel activity (Figure 5A–D). The effect on responses to S1P was particularly marked, and so we investigated much lower concentrations of progesterone. Inhibitory effects of 10 and 100 nM progesterone were observed (Figure 5E,F). Such potent inhibition was not, however, observed against ATP-evoked TRPC5 channel activity (Figure 5G–J), suggesting that it is not a general phenomenon for all types of receptor-activated TRPC5 channels. The data suggest that progesterone is a relatively potent inhibitor of TRPC5 channels, particularly when stimulated by S1P.
Figure 4.

Inhibition of Gd3+-stimulated TRPC5 channels by progesterone. All data were generated from cells over-expressing TRPC5 channels (Tet+) by intracellular Ca2+ measurement (B,C) or whole-cell patch-clamp. (A) Two-dimensional structure of progesterone. (B) Mean data for the inhibitory effect of progesterone on TRPC5 channel-mediated Ca2+ signals elicited by 20 µM Gd3+, fitted using the Hill equation giving an IC50 of 5 µM (n/N= 6/30 for 1 µM, and 3/12 each for 10 and 50 µM progesterone). (C) Typical time-series plot showing the effects of application of 1 and 10 µM progesterone on current elicited by 20 µM Gd3+. (D) Typical I-Vs in the presence of Gd3+ alone (20 Gd3+), and then plus 1 and 10 µM progesterone. (E) Mean data for experiments in (C,D) (n= 13 and 7 for 1 and 10 µM progesterone, respectively). TRPC5, transient receptor potential canonical 5.
Figure 5.

General TRPC5 channel inhibition by progesterone and potency against S1P-evoked activity. Data were generated by intracellular Ca2+ measurement from TRPC5-expressing (Tet+) cells or cells not induced to express TRPC5 channels (Tet-) (H,J). (A) Experimental record showing the effect of 10 µM progesterone on TRPC5 channels stimulated by 5 µM sphingosine-1-phosphate (S1P). (B) Mean data for experiments in panel A (n/N= 3/18). (C) Typical trace showing the effect of 10 µM progesterone on TRPC5 channels stimulated by 5 µM lysophosphatidylcholine (LPC). (D) Mean data for experiments in panel C (n/N= 3/18). (E) Typical trace showing the effects of 0.01 and 0.1 µM progesterone (prog) on TRPC5 channels stimulated by 5 µM S1P. (F) Mean data for experiments in panel E (n/N= 4/18). (G,H) Typical traces showing the effect of 0.1 µM progesterone (prog) on Ca2+ signals elicited by 100 µM ATP in cells. Note the more sustained response in Tet+ cells, reflecting TRPC5 channel activity. (I,J) Mean data for the effect of 0.1 µM progesterone (+prog) on the transient (Trans.) and sustained (Sust.) responses evoked by ATP in TRPC5 channel-expressing or control (Tet-) cells (n/N= 3/16). Ctrl indicates the solvent control. S1P, sphingosine-1-phosphate. TRPC5, transient receptor potential canonical 5.
Effects of other sex steroids
Because of the effects of progesterone on TRPC5 channels, other primary sex steroids (dihydrotestosterone and 17β-oestradiol) were investigated, using cortisol as a comparison (Figure 6A). In Ca2+-measurement experiments on TRPC5-expressing cells, 10 µM dihydrotestosterone and 17β-oestradiol inhibited Gd3+ responses by about 30 and 15%, respectively, whereas cortisol had no effect (Figure 6B). The result was similar in whole-cell patch-clamp recordings except the effect of dihydrotestosterone was more striking, and effects of 17β-oestradiol were not reliably detected (Figure 6C,D). The data suggest that inhibition of TRPC5 channels by progesterone was mimicked by dihydrotestosterone.
Figure 6.

Inhibition of TRPC5 channels by dihydrotestosterone. (A) Chemical structures of dihydrotestosterone (DhT), 17β-oestradiol (17β) and cortisol (cort). (B–D) Data were generated by intracellular Ca2+ measurement (B) or whole-cell patch-clamp (C,D) from TRPC5-expressing (Tet+) cells B. Mean data for the effect of 15 min treatment with 10 µM each of dihydrotestosterone, 17β-oestradiol or cortisol on TRPC5 channels stimulated by 20 µM Gd3+ (n/N= 3/24). (C) Typical time-series plot showing the effect of extra-cellular application of 10 µM cortisol, dihydrotestosterone or 17β-oestradiol on currents at −95 mV and +95 mV elicited by 20 µM Gd3+. The durations of compound application are indicated by horizontal bars. (D) Mean normalized data for experiments in panel C (n= 4–5 for each steroid, each compared with its own control). TRPC5, transient receptor potential canonical 5.
Reversible inhibition in excised outside-out membrane patches
In order to investigate if sex steroids were acting relatively directly on TRPC5 channels, we performed excised outside-out patch recordings and bath-applied the steroids; that is to the outer membrane surface. Gd3+ was used to activate the TRPC5 channels, avoiding complications of receptor-coupling mechanisms (Figure 7A). Quite large macroscopic ionic currents were evoked by Gd3+, which consistently had similar but less rectifying I-Vs compared with whole-cell currents (Figure 7B,C). Within 100 s of steroid application, the TRPC5 channel currents were strongly suppressed by 10 µM dihydrotestosterone or progesterone (Figure 7A–D). Full recovery of currents was observed on wash-out of the steroids. The data suggest that these sex steroids are direct and reversible negative modulators of TRPC5 channel activity at the membrane.
Figure 7.

Inhibition of TRPC5 channels in excised outside-out patches. Data were generated from patches excised from cells over-expressing TRPC5 channels (Tet+). (A) Typical time-series plot from an outside-out patch recording showing the effects of extra-cellular application of 10 µM dihydrotestosterone (DhT) or progesterone (prog) on current elicited by 20 µM Gd3+. Arrows indicate the time points (i–iv) at which I-Vs were sampled for panels B and C. (B,C) Typical I-Vs in the presence of Gd3+ alone (20 Gd3+), and then plus 10 µM dihydrotestosterone (B) and progesterone (C). (D) Mean data for experiments in (A–C) (n= 4–6). TRPC5, transient receptor potential canonical 5.
Inhibition of endogenous TRPC channel activity
The above data on TRPC5 were generated from HEK 293 cells that conditionally over-expressed human TRPC5 channels. To investigate the relevance to endogenous TRPC5 channels, we measured a Ca2+-influx signal evoked by an oxidized phospholipid (PGPC), which we have previously shown is mediated by channels containing TRPC5 and TRPC1 subunits (Al-Shawaf et al., 2010). Progesterone (50 µM) inhibited the PGPC-evoked Ca2+-entry events (Figure 8A,B), consistent with inhibition of the TRPC channels occurring. Simultaneously, there was lowering of the basal Ca2+ concentration by progesterone (Figure 8C), which was probably not related to TRPC1/5 channels because they lack basal activity in these cells (Al-Shawaf et al., 2010). The data suggested that endogenous TRPC5-containing channels were sensitive to inhibition by progesterone.
Figure 8.
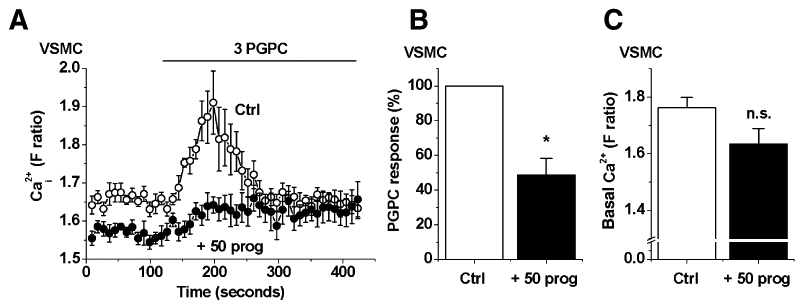
Inhibition by progesterone of Ca2+-entry through endogenous TRPC5-containing channels. Data were generated by intracellular Ca2+ measurement from human saphenous vein vascular smooth muscle cells (VSMC). (A) Effect of 15 min treatment with 50 µM progesterone (prog) on the Ca2+ response elicited by 3 µM 1-palmitoyl-2-glutaroyl-phosphatidylcholine (PGPC). (B,C) Mean data for the effects of progesterone on the amplitude of the PGPC response (B, normalized to control) and basal Ca2+ level (C, absolute fura-2 ratio) in VSMC (n/N= 5/54). TRPC5, transient receptor potential canonical 5.
Discussion and conclusions
The results of this study suggest that TRPC5 channels are negatively modulated by certain neuroactive steroids acting via a rapid non-genomic mechanism. The channels were inhibited by pregnenolone sulphate, pregnanolone (or its β-sulphated form), progesterone or dihydrotestosterone. There was slight inhibition by 17β-oestradiol, but no effect of pregnenolone, allopregnanolone or cortisol. Progesterone was the strongest inhibitor, and, like dihydrotestosterone, had a rapid and reversible effect in excised membrane patches, suggesting the possibility of direct steroid binding to the channel. The effects of pregnanolone, but not its stereo-isomer, allopregnanolone, indicate a requirement for a specific binding site. Progesterone also inhibited endogenous channels that contain TRPC5. The data strengthen the connection between steroids and TRP channels, suggesting unique sensing capabilities for specific steroidal moieties.
The TRP channels that have been linked to neuroactive steroids are TRPV1 (Chen and Wu, 2004) and TRPM3 (Wagner et al., 2008; Majeed et al., 2010). In the case of TRPV1, stimulatory and inhibitory effects occur, whereas for TRPM3, all reported effects are stimulatory. In contrast, none of the steroids stimulated TRPC5; only inhibitory effects were observed. Differences are also evident in the specific steroids that have effects: Progesterone has strong effects on TRPC5, but no effect on TRPV1 (even at 100 µM progesterone) and fails to stimulate TRPM3 (Wagner et al., 2008; Majeed et al., 2010) or affect TRPM2 (data not shown); and pregnanolone sulphate has a strong effect on TRPC5, but only a weak effect on TRPM3. Differences are also found when making comparisons with other types of steroid-regulated ion channels: Notably, there is absence of effect of allopregnanolone on TRPC5 that contrasts markedly with GABAA ligand-gated ion channels, which are potently potentiated by this steroid (Schumacher et al., 2007). Overall, the findings suggest that steroid binding sites exist on TRP channels, as they do on other ion channel types, such as GABAA receptors. Analysis of the steroid structure-activity relationships for GABAA receptors has been extensive (Veleiro and Burton, 2009), whereas comparable information for TRP channels is limited. Nevertheless, there are indications that the steroid sites on, or near, TRP channels are different between classes of TRP channel and different compared with other types of ion channel, allowing responses to different steroids. Notably, small changes in steroid chemistry, including stereo-isomerism, make the difference between effect and no effect. Inspection of 3D structures of steroids that are active against TRPC5 compared with allopregnanolone, which is inactive, suggests that subtle changes in rings A and B are critical in determining modulation of TRPC5 channels (Figure S2).
The effect of progesterone on TRPC5 was relatively rapid in onset and occurred in excised membrane patches, which means that it is independent of the channel expression mechanism or intracellular organelles, and argues for a membrane-delimited effect. While it is tempting to conclude that the steroids bind directly to TRPC5, there are other possibilities to consider: Relatively high concentrations of steroids could modify the structure of the bilayer, and thus indirectly modulate protein function; however, this is unlikely to be relevant to action on TRPC5 channels because of the observed structure–activity relationships, including an effect of pregnanolone but not allopregnanolone. We also found no effect on other membrane events, including TRPM2 activity and ATP receptor-coupling to Ca2+-release. G protein-coupled receptors exist for progesterone, which are linked to Gi/o proteins (Schumacher et al., 2007), and TRPC5 channels are well recognized to be modulated by agonists at G protein-coupled receptors; however, the TRPC5 channel is stimulated by such agonists, including when acting through a Gi/o pathway (Xu et al., 2006; Beech, 2007), thus contrasting with the universal inhibitory actions of the steroids, including progesterone presented here. Progesterone also binds to the progesterone receptor membrane component-1, a protein with a relatively low affinity for progesterone, and this interaction accounts for some membrane-initiated effects of progesterone (Peluso, 2007). However, we are not aware of any evidence that this protein can couple to ion channel function.
The physiological relevance of neuroactive steroid effects on TRPC5 is currently uncertain. Endogenous concentrations of serum or brain pregnenolone sulphate in humans almost certainly do not become high enough to affect these channels (Schumacher et al., 2008), although we cannot exclude that local concentrations may be higher and regulated by local enzyme systems. Greater concentrations may be achieved, for example through oral administration of pregnenolone or DHEA as a dietary supplement, but this is considered to be pharmacology (Johnson et al., 2002). Progesterone was more potent, but was not a general inhibitor of all types of TRPC5 channel activity at concentrations of progesterone detected during the menstrual cycle (Havlikova et al., 2006). In pregnancy, however, sufficient progesterone may circulate to affect all modes of TRPC5 channel activity (Frye, 2007). More potent and potentially physiologically relevant actions of progesterone were observed, but they were restricted to S1P-evoked TRPC5 channel activity. Whether these more potent effects are relevant to other types of receptor-activated TRPC5 channel is unknown, but we did not reproduce them for ATP-evoked activity. It is possible that they could be explained by effects of progesterone on the S1P receptor or its Gi/o signalling pathway that couples to TRPC5 channels (Xu et al., 2006).
Our study revealed that the TRPC class of TRP channels is modulated by neuroactive steroids, adding to data published previously on TRPV (TRPV1) and TRPM (TRPM3) channels. It is the first report of an effect of the important hormone, progesterone, on TRP channels. The physiological significance of the steroid effects on TRPC5 has yet to be revealed but there is, for example potential relevance to anxiety states (Toufexis et al., 2004; Schumacher et al., 2007; Auger and Forbes-Lorman, 2008), growth cone formation and neuroprotection (Davare et al., 2009), and vascular remodelling in response to oxidized phospholipids (Al-Shawaf et al., 2010). Our study strengthens the association between steroids and TRP channels and suggests that the channels are non-genomic steroid sensors that enable membrane-initiated events.
Acknowledgments
Supported by a Wellcome Trust programme grant to DB, a University studentship to YM, an Egyptian Ministry of Higher Education scholarship to MA, and a JSPS International Collaboration Program grant to DB and KM.
Glossary
Abbreviations
- HEK 293 cells
human embryonic kidney cells
- LPC
lysophosphatidylcholine
- PGPC
1-palmitoyl-2-glutaroyl-phosphatidylcholine
- S1P
sphingosine-1-phosphate
- TRPC
transient receptor potential canonical
- TRPM
transient receptor potential melastatin
Conflict of interest
The authors state no conflict of interest.
Supplementary material
Additional Supporting Information may be found in the online version of this article:
Supporting Information: Teaching Materials; Figs 1–8 as PowerPoint slide.
Figure S1 Example I-Vs for steroid effects on TRPC5. Data were generated by whole-cell voltage-clamp in cells over-expressing TRPC5 (Tet +). (A) Characteristic multi-rectifying current–voltage relationship (I-V) for TRPC5 activity elicited by 20 µM Gd3 +. The I-V for background (basal, pre Gd3 +) current is shown for comparison. (B–D) Typical I-Vs for TPRC5 activity in the presence of 20 µM Gd3 +, and then plus 10 µM PregS (B), 100 µM pregnanolone sulphate (C), and 10 µM pregnanolone (D).
Figure S2 3D structures of steroid modulators of TRPC5. (A) Two-dimensional structures of allopregnanolone (3α5α), pregnanolone (3α5β), progesterone (prog), and dihydrotestosterone (DhT). (B–D) 3D-overlap of 3α5α with 3α5β (B), prog (C) and DhT (D).
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edition. Br J Pharmacol. 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shawaf E, Naylor J, Taylor H, Riches K, Milligan CJ, O'Regan D, et al. Short-term stimulation of calcium-permeable transient receptor potential canonical 5-containing channels by oxidized phospholipids. Arterioscler Thromb Vasc Biol. 2010;30:1453–1459. doi: 10.1161/ATVBAHA.110.205666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger CJ, Forbes-Lorman RM. Progestin receptor-mediated reduction of anxiety-like behavior in male rats. PLoS One. 2008;3:e3606. doi: 10.1371/journal.pone.0003606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech DJ. Canonical transient receptor potential 5. Handb Exp Pharmacol. 2007;179:109–123. doi: 10.1007/978-3-540-34891-7_6. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L. The TRPC class of ion channels: a critical review of their roles in slow, sustained increases in intracellular Ca2+ concentrations. Annu Rev Pharmacol Toxicol. 2009;49:395–426. doi: 10.1146/annurev.pharmtox.48.113006.094928. [DOI] [PubMed] [Google Scholar]
- Charalampopoulos I, Remboutsika E, Margioris AN, Gravanis A. Neurosteroids as modulators of neurogenesis and neuronal survival. Trends Endocrinol Metab. 2008;19:300–307. doi: 10.1016/j.tem.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Chen SC, Wu FS. Mechanism underlying inhibition of the capsaicin receptor-mediated current by pregnenolone sulfate in rat dorsal root ganglion neurons. Brain Res. 2004;1027:196–200. doi: 10.1016/j.brainres.2004.08.053. [DOI] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- Davare MA, Fortin DA, Saneyoshi T, Nygaard S, Kaech S, Banker G, et al. Transient receptor potential canonical 5 channels activate Ca2+/calmodulin kinase Igamma to promote axon formation in hippocampal neurons. J Neurosci. 2009;29:9794–9808. doi: 10.1523/JNEUROSCI.1544-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Testosterone's anti-anxiety and analgesic effects may be due in part to actions of its 5alpha-reduced metabolites in the hippocampus. Psychoneuroendocrinology. 2005;30:418–430. doi: 10.1016/j.psyneuen.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Flemming PK, Dedman AM, Xu SZ, Li J, Zeng F, Naylor J, et al. Sensing of lysophospholipids by TRPC5 calcium channel. J Biol Chem. 2006;281:4977–4982. doi: 10.1074/jbc.M510301200. [DOI] [PubMed] [Google Scholar]
- Frye CA. Progestins influence motivation, reward, conditioning, stress, and/or response to drugs of abuse. Pharmacol Biochem Behav. 2007;86:209–219. doi: 10.1016/j.pbb.2006.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havlikova H, Hill M, Kancheva L, Vrbikova J, Pouzar V, Cerny I, et al. Serum profiles of free and conjugated neuroactive pregnanolone isomers in nonpregnant women of fertile age. J Clin Endocrinol Metab. 2006;91:3092–3099. doi: 10.1210/jc.2005-2785. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Bebb RA, Sirrs SM. Uses of DHEA in aging and other disease states. Ageing Res Rev. 2002;1:29–41. doi: 10.1016/s0047-6374(01)00369-4. [DOI] [PubMed] [Google Scholar]
- McHugh D, Flemming R, Xu SZ, Perraud AL, Beech DJ. Critical intracellular Ca2+ dependence of transient receptor potential melastatin 2 (TRPM2) cation channel activation. J Biol Chem. 2003;278:11002–11006. doi: 10.1074/jbc.M210810200. [DOI] [PubMed] [Google Scholar]
- Majeed Y, Agarwal AK, Naylor J, Seymour VA, Jiang S, Muraki K, et al. Cis-isomerism and other chemical requirements of steroidal agonists and partial agonists acting at TRPM3 channels. Br J Pharmacol. 2010;161:430–441. doi: 10.1111/j.1476-5381.2010.00892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodari F, Hsu FF, Fu X, Holekamp TF, Kao LF, Turk J, et al. Sulfated steroids as natural ligands of mouse pheromone-sensing neurons. J Neurosci. 2008;28:6407–6418. doi: 10.1523/JNEUROSCI.1425-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso JJ. Non-genomic actions of progesterone in the normal and neoplastic mammalian ovary. Semin Reprod Med. 2007;25:198–207. doi: 10.1055/s-2007-973432. [DOI] [PubMed] [Google Scholar]
- Riccio A, Li Y, Moon J, Kim KS, Smith KS, Rudolph U, et al. Essential role for TRPC5 in amygdala function and fear-related behavior. Cell. 2009;137:761–772. doi: 10.1016/j.cell.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher M, Guennoun R, Ghoumari A, Massaad C, Robert F, El-Etr M, et al. Novel perspectives for progesterone in hormone replacement therapy, with special reference to the nervous system. Endocr Rev. 2007;28:387–439. doi: 10.1210/er.2006-0050. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Liere P, Akwa Y, Rajkowski K, Griffiths W, Bodin K, et al. Pregnenolone sulfate in the brain: a controversial neurosteroid. Neurochem Int. 2008;52:522–540. doi: 10.1016/j.neuint.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Semtner M, Schaefer M, Pinkenburg O, Plant TD. Potentiation of TRPC5 by protons. J Biol Chem. 2007;282:33868–33878. doi: 10.1074/jbc.M702577200. [DOI] [PubMed] [Google Scholar]
- Sossey-Alaoui K, Lyon JA, Jones L, Abidi FE, Hartung AJ, Hane B, et al. Molecular cloning and characterization of TRPC5 (HTRP5), the human homologue of a mouse brain receptor-activated capacitative Ca2+ entry channel. Genomics. 1999;60:330–340. doi: 10.1006/geno.1999.5924. [DOI] [PubMed] [Google Scholar]
- Sukumar P, Beech DJ. Stimulation of TRPC5 cationic channels by low micromolar concentrations of lead ions (Pb2+) Biochem Biophys Res Commun. 2010;393:50–54. doi: 10.1016/j.bbrc.2010.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toufexis DJ, Davis C, Hammond A, Davis M. Progesterone attenuates corticotropin-releasing factor-enhanced but not fear-potentiated startle via the activity of its neuroactive metabolite, allopregnanolone. J Neurosci. 2004;24:10280–10287. doi: 10.1523/JNEUROSCI.1386-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veleiro AS, Burton G. Structure-activity relationships of neuroactive steroids acting on the GABAA receptor. Curr Med Chem. 2009;16:455–472. doi: 10.2174/092986709787315522. [DOI] [PubMed] [Google Scholar]
- Wagner TF, Loch S, Lambert S, Straub I, Mannebach S, Mathar I, et al. Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat Cell Biol. 2008;10:1421–1430. doi: 10.1038/ncb1801. [DOI] [PubMed] [Google Scholar]
- Xu SZ, Muraki K, Zeng F, Li J, Sukumar P, Shah S, et al. A sphingosine-1-phosphate-activated calcium channel controlling vascular smooth muscle cell motility. Circ Res. 2006;98:1381–1389. doi: 10.1161/01.RES.0000225284.36490.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F, Xu SZ, Jackson PK, McHugh D, Kumar B, Fountain SJ, et al. Human TRPC5 channel activated by a multiplicity of signals in a single cell. J Physiol. 2004;559:739–750. doi: 10.1113/jphysiol.2004.065391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


