Abstract
BACKGROUND AND PURPOSE
P2X receptors mediate sympathetic control and autoregulation of the renal circulation triggering contraction of renal vascular smooth muscle cells (RVSMCs) via an elevation of intracellular Ca2+ concentration ([Ca2+]i). Although it is well-appreciated that the myocyte Ca2+ signalling system is composed of microdomains, little is known about the structure of the [Ca2+]i responses induced by P2X receptor stimulation in vascular myocytes.
EXPERIMENTAL APPROACHES
Using confocal microscopy, perforated-patch electrical recordings, immuno-/organelle-specific staining, flash photolysis and RT-PCR analysis we explored, at the subcellular level, the Ca2+ signalling system engaged in RVSMCs on stimulation of P2X receptors with the selective agonist αβ-methylene ATP (αβ-meATP).
KEY RESULTS
RT-PCR analysis of single RVSMCs showed the presence of genes encoding inositol 1,4,5-trisphosphate receptor type 1(IP3R1) and ryanodine receptor type 2 (RyR2). The amplitude of the [Ca2+]i transients depended on αβ-meATP concentration. Depolarization induced by 10 µmol·L−1αβ-meATP triggered an abrupt Ca2+ release from sub-plasmalemmal (‘junctional’) sarcoplasmic reticulum enriched with IP3Rs but poor in RyRs. Depletion of calcium stores, block of voltage-gated Ca2+ channels (VGCCs) or IP3Rs suppressed the sub-plasmalemmal [Ca2+]i upstroke significantly more than block of RyRs. The effect of calcium store depletion or IP3R inhibition on the sub-plasmalemmal [Ca2+]i upstroke was attenuated following block of VGCCs.
CONCLUSIONS AND IMPLICATIONS
Depolarization of RVSMCs following P2X receptor activation induces IP3R-mediated Ca2+ release from sub-plasmalemmal (‘junctional’) sarcoplasmic reticulum, which is activated mainly by Ca2+ influx through VGCCs. This mechanism provides convergence of signalling pathways engaged in electromechanical and pharmacomechanical coupling in renal vascular myocytes.
Keywords: renal vascular smooth muscle cells; αβ-meATP; NF 279; MRS 2578; Ca2+ signalling; P2X receptor; voltage-gated Ca2+ channels; inositol 1,4,5-trisphosphate receptor; ryanodine receptor; sarcoplasmic reticulum
Introduction
Renal blood flow (RBF) accounts for 20–25% of cardiac output at rest (Malpas and Leonard, 2000; Cupples and Braam, 2007). The mechanisms controlling contraction/relaxation of renal vascular smooth muscle cells (RVSMCs), which regulate the diameter of renal resistance arteries and hence RBF and glomerular filtration rate, play a fundamental role in the control of systemic blood pressure (DiBona, 2004; Navar, 2005).
Vascular myocytes are stimulated to contract by elevation of intracellular Ca2+ concentration ([Ca2+]i) resulting in activation of Ca2+/calmodulin-dependent myosin light chain kinase which causes force development via myosin light chain phosphorylation (Walsh, 1994). An increase in [Ca2+]i in smooth muscle cell (SMC) is caused by either depolarization of the myocyte membrane (electromechanical coupling; Somlyo and Somlyo, 1968) resulting from activation of ionotropic receptors or metabotropic receptor-coupled cationic channels and leading to Ca2+ influx via voltage-gated Ca2+ channels (VGCCs), or by activation of metabotropic receptors (pharmacomechanical coupling; Somlyo and Somlyo, 1968) coupled via Gq/11-protein to phospholipase C (PLC) activation followed by inositol 1,4,5-trisphosphate (IP3) liberation and IP3 receptor (IP3R)-mediated Ca2+ release, or by a combination of these mechanisms (Bolton et al., 1999; Davis and Hill, 1999; Wray and Burdyga, 2010). Either of these events, which cause an initial rise in [Ca2+]i, may be further augmented by ryanodine receptor (RyR)-mediated Ca2+ release activated via a Ca2+-induced Ca2+ release (CICR) mechanism (Fabiato and Fabiato, 1975). However, the recruitment of RyR-mediated Ca2+ release by voltage-gated Ca2+ entry and/or IP3R-mediated Ca2+ release in smooth muscles is still an area of extensive debate and controversy (reviewed in Laporte et al., 2004; Wray and Burdyga, 2010). Indeed, the relative contribution of RyRs and IP3Rs to intracellular [Ca2+]i mobilization varies in different types of SMCs, and often depends on the strengths and mechanism of SMC stimulation. In some phasic smooth muscles, for example, urinary bladder and vas deferens, Ca2+ entry through VGCCs is tightly coupled to RyR-mediated Ca2+ release. Electrical stimulation of these SMCs, in which close proximity of VGCCs and RyRs within the caveolar domains was confirmed with 3D-immunofluorescence and electron microscopy (Moore et al., 2004), triggers an abrupt RyR-mediated Ca2+ release at multiple sub-PM regions (‘hot spots’, Ohi et al., 2001; Morimura et al., 2006). The ability of Ca2+ entering the SMC via VGCCs to induce RyR-mediated Ca2+ release has also been demonstrated in voltage-clamp experiments performed on SMCs from pregnant myometrium (Shmigol et al., 1998), ileum (Kohda et al., 1997), mesenteric artery (Bolton and Gordienko, 1998), cerebral arteries (Kamishima and McCarron, 1997) and portal vein (Coussin et al., 2000). However, other research groups working on different or even the same SMC type failed to detect any evidence of CICR (Fleischmann et al., 1996; Kamishima and McCarron, 1996; Bradley et al., 2002). The ability of Ca2+ liberated via IP3Rs to trigger RyR-mediated Ca2+ release also varies among different types of smooth muscles and depends on receptor distribution and isoform expression (reviewed in Wray and Burdyga, 2010). Emerging evidence highlights the importance of RyRs and CICR in calcium signalling and arteriolar contraction in the renal microcirculation. It was demonstrated that in these arterioles RyR/CICR contribute to [Ca2+]i responses initiated by Ca2+ entry and by IP3R-mediated Ca2+ release (reviewed in Arendshorst and Thai, 2009).
Two major sympathetic cotransmitters, noradrenaline and ATP, mediate vasoconstriction of small arteries (Wier et al., 2009) by acting on metabotropic α1-adrenoceptors and ionotropic P2X receptors respectively. Thus, P2X receptors provide an important therapeutic target, mediating substantial vasoconstrictor drive resistant to adrenoceptor antagonists (Wier et al., 2009).
In the kidney, apart from sympathetic transmission, ATP is involved in paracrine regulation. On being released by apical macula densa cells in response to an increase in NaCl concentration in the distal tubular fluid (Bell et al., 2003), ATP triggers P2X receptor-mediated vasoconstriction of afferent arterioles (Inscho et al., 2003), thus decreasing RBF and glomerular filtration rate. This tubuloglomerular feedback mechanism (Nishiyama et al., 2000; Guan et al., 2007a; Surprenant and North, 2009) and myogenic mechanism are two important components of renal autoregulation (Cupples and Braam, 2007).
The respective role of RyRs and IP3Rs in smooth muscle contraction is an area of active investigation. Although RyRs and IP3Rs are both Ca2+-sensitive and can activate each other via CICR mechanism, leading to an all-or-none regenerative response, graded Ca2+ signals have been reported to be evoked by increasing stimuli targeting RyRs (Isenberg and Han, 1994) or IP3Rs (Bootman et al., 1994). The resolution of this paradox has been suggested to lie in the local control of Ca2+ microdomains that function and are regulated autonomously (Berridge, 1997), the gradual recruitment of which by gradually increasing stimuli would result in graded Ca2+ signals. Imaging microdomain Ca2+ in muscle cells has reshaped our understanding of Ca2+ signalling and provided direct evidence to validate the local control theory in skeletal, cardiac and smooth muscles (Wang et al., 2004; McCarron et al., 2006).
In smooth muscles, IP3R-mediated Ca2+ release is essential for [Ca2+]i mobilization and contraction, especially (but not exclusively) induced by stimulation with neurotransmitters and hormones (Boittin et al., 1999; Bayguinov et al., 2000; McCarron et al., 2002; Zhang et al., 2003; Lamont and Wier, 2004; MacMillan et al., 2005; Gordienko et al., 2008). The role of IP3Rs seems to be to facilitate CICR within and between RyRs domains (Boittin et al., 1998; Gordienko and Bolton, 2002; White and McGeown, 2002; Zhang et al., 2003). However, a cooperative relationship between IP3Rs and RyRs is not always the rule. When both receptor types share the same calcium store, the interplay between them can be regulated via alterations in the sarcoplasmic reticulum (SR) calcium load. For example, in SMCs from both the gastric antrum and vas deferens block of IP3Rs, which caused an elevation of the caffeine-releasable store content, increased spontaneous Ca2+ spark activity (White and McGeown, 2002, 2003).
Another important aspect of the SMC Ca2+ signalling system is the regulation of IP3R-mediated Ca2+ release by [Ca2+]i. In vascular SMCs of small arteries, Lamont and Wier (2004) concluded that if RyRs were blocked, cell-wide Ca2+ waves could still be evoked by strong activation of adrenoceptors leading to myocyte and vessel contraction. Thus, CICR apparently can occur among IP3Rs alone, although normally, at low levels of adrenoceptor activation RyRs are needed to trigger IP3R dependent Ca2+ waves (Wier and Morgan, 2003). Furthermore, activation of IP3Rs by a localized increase in [Ca2+]i was directly demonstrated in an elegant experiment conducted on rabbit urinary bladder myocytes; IP3R-mediated Ca2+ release was found to be induced by local Ca2+ uncaging (Ji et al., 2006).
We have previously demonstrated that following activation of muscarinic receptors in intestinal myocytes IP3R-mediated Ca2+ release is facilitated by Ca2+ influx via VGCCs (Gordienko et al., 2008). Although M3 receptors are linked to a Gq/11/PLC/IP3/Ca2+ signalling pathway, we hypothesized that in contrast to cardiac muscle, excitation–contraction (E–C) coupling in smooth muscle occurs by Ca2+ entry through VGCCs, which evokes IP3R-mediated Ca2+ release via CICR mechanism. This hypothesis needs to be tested in vascular myocytes regulated by ionotropic receptors, which are not coupled to the Gq/11-GTP/PLC/IP3 system.
Taking into account the importance of ionotropic P2X receptors (Burnstock, 2007; Surprenant and North, 2009) in the regulation of renal circulation (Malpas and Leonard, 2000; Inscho et al., 2003; Cupples and Braam, 2007; Guan et al., 2007a), we tested our hypothesis on RVSMCs, which, as we have recently demonstrated, express functional monomeric P2X1 and heteromeric P2X1/4 receptors (Harhun et al., 2010). We found that depolarization of RVSMCs following P2X receptor activation induces IP3R-mediated Ca2+ release from sub-plasmalemmal (‘junctional’) sarcoplasmic reticulum (jSR), which is activated mainly by Ca2+ influx through VGCCs. Our results support the notion that, in contrast to cardiac muscle, E–C coupling in vascular smooth muscle may occur by Ca2+ entry through VGCCs, which evokes an initial IP3R-mediated Ca2+ release activated via a CICR mechanism. A preliminary account of this study has been published in abstract form (Povstyan et al., 2009).
Methods
Isolation of renal vascular smooth muscle cells (RVSMCs)
Male Wistar Kyoto rats (180–250 g, 65 animals obtained from Charles Rivers laboratories) were humanely killed by decapitation after cervical dislocation as approved under Schedule 1 of the UK Animals (Scientific Procedures) Act 1986. Interlobar and arcuate arteries were dissected from the kidney as described previously (Gordienko et al., 1994). Following the dissection, the blood vessels with outer diameter of 50–150 µm were cut into 1–2 mm pieces and collected in ice-cold physiological salt solution (PSS) containing (in mmol·L−1): NaCl 120, KCl 6, CaCl2 2.5, MgCl2 1.2, glucose 12, HEPES 10; pH adjusted to 7.4 with NaOH. After a 10 min rinse in Ca2+-free PSS, the tissue samples were transferred into the same solution supplemented with (mg·mL−1): protease (Type X) 0.75, collagenase (Type 1A) 1.5, soybean trypsin inhibitor 1 and bovine serum albumin 1, and incubated for 20 min at 37 °C. The tissue samples were then rinsed for 10 min in an enzyme-free Ca2+-free solution and triturated using a glass Pasteur pipette. Isolated RVSMCs were obtained by several cycles of trituration, each followed by transfer to fresh solution with gradually increasing [Ca2+] (from 0.125 to 1.25 mmol·L−1). Transfer of the tissue samples to fresh solution before trituration facilitated removal of debris and damaged cells from the suspension, while gradually increasing [Ca2+] in the medium prevented the myocytes from ‘calcium shock’ and hypercontraction (Gordienko and Zholos, 2004). Small aliquots of the cell suspension were transferred to the experimental chambers and diluted with PSS. RVSMCs were then either collected for reverse transcription polymerase chain reaction (RT-PCR) experiments or used for electrical recording and confocal imaging within 8 h of isolation.
RNA isolation and RT-PCR analysis
Reverse transcription polymerase chain reaction analysis was performed using single isolated RVSMCs. The myocytes (pool of ∼500 cells per sample) were collected under a microscope using a wide-bore glass micropipette and frozen immediately after collection. Total RNA was extracted using the Qiagen RNeasy extraction kit (Qiagen, Crawley, UK). cDNA was obtained using Superscript II Reverse Transcriptase (Invitrogen, Paisley, UK) and used in RT-PCR. cDNA was used as a template in a 50 µL RT-PCR reaction containing 1.5 mmol·L−1 MgCl2, 0.2 mmol·L−1 deoxynucleoside triphosphates, 0.2 µmol·L−1 forward and reverse primers (Invitrogen), and 2.5 units of platinum Taq DNA polymerase (Invitrogen). Amplification was performed using a Touchgene Thermocycler (Bibby Scientific Ltd., Staffordshire, UK) according to the following schedule: 94°C for 2 min; 40 cycles (for brain and liver tissue preparations) or 45 cycles (for RVSMC samples) of 94°C for 30 s; 57°C for 60 s; and 72°C for 3 min, followed by a final elongation period of 10 min at 72°C. No-template control PCR was also performed simultaneously with every reaction. To avoid detecting genomic DNA contamination, primers were designed to span at least one intron of the genomic sequence for genes encoding proteins of interest. The experiments were repeated with at least four samples of RVSMCs obtained from different animals. The purity of the RVSMC sample was confirmed by the expression of the gene encoding the SMC marker, smooth muscle myosin heavy chain, but not the markers for other types of cell present in the vascular wall such as: fibroblasts and endothelial cells (CD34), neurones (PGP9.5) and pericytes (NG2), as previously described (Harhun et al., 2009; 2010;). PCR products were separated and visualized in ethidium bromide-stained 2% agarose gel by electrophoresis.
The following primers (the data in parentheses show: Genebank accession number, the sense bordering nucleotide position, the anti-sense bordering nucleotide position) were used in this study: β-actin (NM_031144, 306–325, 1007–1026), smooth muscle myosin heavy chain for SMCs (X16262, 447–466, 1182–1191), CD34 for endothelial cells and fibroblasts (NM_001107202, 50–69, 882–901), PGP9.5 for neurones (D10699, 54–73, 544–563), NG2 proteoglycan for pericytes (NM_031022, 1815–1834, 2791–2810), inositol 1,4,5-trisphosphate (IP3) receptor type 1 (NM_001007235, 6244–6263, 7030–7049), IP3 receptor type 3 (NM_013138, 6142–6161, 7088–7107), ryanodine receptor type 1 (XM_001078539, 8038–8057, 8811–8830), ryanodine receptor type 2 (NM_032078, 8513–8532, 9317–9336), ryanodine receptor type 3 (XM_342491, 8035–8054, 9101–9120).
Drug application
Renal vascular smooth muscle cells were stimulated with αβ-methylene ATP (αβ-meATP) and caffeine which were applied as a 2 s pulse through a glass micropipette attached to the outlet of a pressure ejector Picospritzer III (Intracel Ltd, Royston, Hertfordshire, UK). Similar application of the control solution (without agonist) had no effect on [Ca2+]i. In the experiments where the same RVSMC was stimulated with αβ-meATP and caffeine or different concentrations of αβ-meATP, the agonist-containing micropipette was replaced in between successive agonist applications. Antagonists were superfused through the experimental bath.
Visualization of [Ca2+]i changes
Changes of [Ca2+]i in isolated RVSMCs were imaged using the high-affinity fluorescent Ca2+ indicators Fluo-4 or Fluo-3. Fluo-4 was loaded by incubating the RVSMCs for 20 min with 5 µmol·L−1 fluo-4 acetoxymethyl ester (Fluo-4 AM; diluted from a stock containing 2 mmol·L−1 Fluo-4 AM and 0.025% (w·v−1) pluronic F-127 in dimethyl sulphoxide) followed by a 40 min wash in PSS to allow time for de-esterification. Fluo-3 was loaded through the patch pipette by dialysis of the myocyte with a solution containing 0.1 mmol·L−1 of fluo-3 pentapotassium salt. The intensity of Fluo-4 or Fluo-3 fluorescence was normalized to the average fluorescence intensity in the images acquired before agonist application and colour coded. The temporal profiles of the agonist-induced [Ca2+]i transients are illustrated by the plots showing: (i) the time course of the normalized Fluo-4 or Fluo-3 fluorescence intensity (F/F0) averaged within multiple sub-plasmalemmal regions where F/F0 changes were initiated and rose above 1.5 or (ii) the time course of F/F0 averaged within entire confocal optical slice of RVSMC (Gordienko et al., 2008).
Visualization of intracellular calcium stores and the SR
The intracellular calcium stores in RVSMCs were visualized using the low-affinity (Kd(Ca)= 42 µmol·L−1) fluorescent Ca2+ indicator fluo-3FF, which was loaded by incubating the myocytes with 5 µmol·L−1 fluo-3FF AM at room temperature for 90 min followed by a 60 min wash in PSS (Gordienko and Zholos, 2004; Gordienko et al., 2008). The SR was visualized using: (i) ER-Tracker™ Blue-White DPX, which was loaded by incubating the myocytes with 5 µmol·L−1 of the dye for 40 min; and (ii) Brefeldin A BODIPY 558/568 (Abeele et al., 2006), which was loaded by incubating the myocytes with 2 µmol·L−1 of the dye for 20 min followed by 1.5 h wash.
Immunostaining of the SR Ca2+ release channels
Prior to immunostaining, the SR in RVSMCs was stained with Brefeldin A BODIPY 558/568, as described above. The myocytes were then fixed by incubating them in 4% (w·v−1) paraformaldehyde for 15 min. Non-specific binding was blocked by incubating the myocytes with 1% (w·v−1) bovine serum albumin (BSA) and 0.3% (w·v−1) Triton X-100 (a cell permeabilizing agent) for 1 h at room temperature. Primary and secondary antibodies were diluted in PSS supplemented with 0.3 (w·v−1) Triton X-100. To visualize the distribution of IP3 receptors (IP3Rs), we used an IP3R type 1 (IP3R1)-specific antibody, because IP3R1 has been shown to be ubiquitous in various tissues (Mackrill et al., 1997; Gordienko and Zholos, 2004; Gordienko et al., 2008) and RT-PCR analysis confirmed expression of gene encoding IP3R1 in RVSMCs. This antibody was developed (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) in goat and has been shown to selectively recognize IP3R1 in other types of SMCs (Taylor and Traynor, 1995; Gordienko and Zholos, 2004; Gordienko et al., 2008). RyRs were detected with a monoclonal anti-RyR antibody derived (Sigma-Aldrich Co., RBI, Natick, MA, USA) from the 34C hybridoma (produced by the fusion of P3X 63 Ag8.653 myeloma cells and spleen cells from Balb/c mice). This antibody reacts strongly with RyR1, 2 and 3 and was previously used for immunodetection of RyRs in single SMCs (Gordienko and Zholos, 2004; Gordienko et al., 2008). RVSMCs were incubated with primary anti-IP3R1 or anti-RyR antibodies (both at 1:200 dilution) overnight at 4°C. Following a 10 min rinse (4 times) in PSS, primary antibody-specific binding was detected by incubating the cells for 3 h at room temperature with either donkey anti-goat IgG or goat anti-mouse IgG, both conjugated to MFP 488 (both at 1:400 dilution; MoBiTec, Göttingen, Germany). Prior to confocal imaging the extracellular solution was replaced with Vectashield mounting medium (Vector Laboratories, Inc. Burlingame, CA, USA) containing DAPI for visualization of the cell nucleus. The spatial pattern of the Ca2+ release channel distribution was related to the spatial organisation of the SR and nucleus. In controls, incubation with primary antibody was omitted from the experimental protocol.
Confocal microscopy
Experimental chambers with RVSMCs were placed on the stage of either Axiovert 100 M or Axiovert 200 M inverted microscopes attached to a LSM 510 and LSM 510 META laser-scanning units respectively (Zeiss, Oberkochen, Germany). The SCSi interface of the confocal microscopes was hosted by a Pentium PC (32-bit Windows NT 4.0 operating system) running LSM 510 software (Zeiss, Oberkochen, Germany).
During time series protocol, the x-y confocal images of Fluo-4 or Fluo-3 fluorescence were acquired at 20–40 Hz using a Zeiss plan-Apochromat 40× 1.3 N.A. oil-immersion objective. Fluo-4 or Fluo-3 fluorescence was excited by the 488 nm line of a 30 mW argon ion laser (Laser-Fertigung, Hamburg, Germany) and was captured at wavelengths above 505 nm. The illumination intensity was attenuated to 0.6–0.7% with an acousto-optical tuneable filter (Zeiss, Oberkochen, Germany). To optimize signal quality, the pinhole was set to provide a confocal optical section below 1.2 µm (measured with 0.2 µm fluorescent beads). The focus was adjusted to acquire the images from the middle of the myocyte depth. In the images acquired in this way, the events initiated within 1 µm of the cell surface (edge) were considered to have sub-plasmalemmal origin and their temporal profiles were analysed (see above).
The imaging of the myocyte structures and spatial distribution of the SR Ca2+ release channels was performed using a Zeiss plan-Apochromat 63× 1.4 N.A. oil-immersion objective. The pinhole was set to provide an x-y resolution of 0.4 µm and a confocal optical section (z resolution) of 0.8–1 µm (measured with 0.2 µm fluorescent beads). Averaging of four frames was used to reduce a shot noise. To avoid any bleed-through of the fluorescence signal in multistaining experiments, fluorochromes with well-separated excitation and emission spectra were used and imaging was performed using the frame-by-frame multitrack mode of the confocal scanner: sequential acquisition via well-separated optical channels of the x-y images produced by fluorescence of different fluorochromes. In these experiments, fluo-4, fluo-3FF and MFP 488 fluorescence were excited by the 488 nm line of a 30 mW argon ion laser and detected at wavelengths of 505–550 nm; ER-Tracker™ Blue-White DPX and DAPI fluorescence were excited by the 405 nm line of a 25 mW laser diode and detected at wavelengths of 420–480 nm; Brefeldin A BODIPY 558/568 was excited by the 543 nm line of a 1 mW helium/neon ion laser and detected at wavelengths above 560 nm. The photomultiplier gain and offset in each optical channel were set individually to achieve similar signal intensity at each channel and remove subsignal noise from the images. The adequacy of the imaging protocol applied to the multilabelled myocytes was confirmed by control experiments on the monolabelled cells.
Electrophysiological recordings
Electrical recordings from single RVSMCs preloaded with fluo-4 AM (see above) were performed by using perforated-patch (200 µg·mL−1 amphotericin B) technique. This allowed low-resistance access to the cell while minimally interfering with [Ca2+]i (because pores formed by amphotericin B are Ca2+-impermeable). The myocytes were bathed in PSS, dialysed with solution composed of (in mmol·L−1): KCl 115; NaCl 6, HEPES 10 (pH adjusted to 7.4 with KOH), and stimulated with 2 s pulses of 10 µmol·L−1αβ-meATP applied at 20 min intervals (see above). Fire-polished borosilicate patch pipettes filled with pipette solution were connected to the head stage of an Axopatch 200A (Molecular Devices Co., Sunnyvale, CA, USA) input amplifier and had a free-tip resistance of 2–5 MΩ. Electrical recordings were synchronized with confocal [Ca2+]i imaging using a TTL synchronizing pulse generated by the confocal scanner at the beginning of the time series protocol. The electrical signals were filtered at 1 kHz (–3 dB frequency) by four-pole low-pass Bessel filter and digitized at 5 kHz using a DigiData 1200 hosted by a Pentium PC running pCLAMP 6.0 software (Molecular Devices Co., CA, USA).
Flash photolysis
Inositol 1,4,5-trisphosphate was rapidly and uniformly released within RVSMC from d-myo-inositol 1,4,5-trisphosphate, P4(5)-1-(2-nitrophenyl)ethyl ester trisodium salt (‘caged’ IP3) by 1 ms flash of light (300–380 nm) from a xenon arc lamp, as described previously (Gordienko and Zholos, 2004). In these experiments, the myocytes were bathed in PSS and dialysed with solution composed of (in mmol·L−1): KCl 115, NaCl 6, MgATP 5, Li3GTP 1, HEPES 10 (pH adjusted to 7.4 with KOH) and supplemented with 30 µmol·L−1‘caged’ IP3 and 100 µmol·L−1 fluo-3 pentapotassium salt. To ensure equilibration between the pipette solution and cytosol, confocal [Ca2+]i imaging was commenced at least 5 min after establishment of the whole-cell configuration.
Data analysis and statistical procedures
Image processing was carried out using an Indy workstation (Silicon Graphic, Inc., Mountain View, CA, USA) with custom routines written in IDL (Research Systems, Inc., Boulder, CO, USA). The final figures were produced using MicroCal Origin (MicroCal Software Inc., Northampton, MA, USA) and CorelDraw 7.0 (Corel Corporation, Ottawa, Ontario, Canada). The data are expressed as mean values ± SEM for the number of cells (n) analysed. Comparative analysis of the data groups was performed using Student's t-test with the threshold for statistical significance set at the 0.05 level.
Drug/molecular target nomenclature
Nomenclature of drugs/molecular targets used in this work conforms to BJP's Guide to Receptors and Channels, 4th edition (Alexander et al., 2009).
Drugs and chemicals
Fluo-4 AM, fluo-3 pentapotassium salt, ER-Tracker™ Blue-White DPX, Brefeldin A BODIPY 558/568 and pluronic F-127 were obtained from Invitrogen Ltd. (Paisley, UK). Fluo-3FF acetoxymethyl ester was from TefLabs (Austin, TA, USA). Donkey anti-goat IgG and goat anti-mouse IgG, both conjugated to MFP 488 were from MoBiTec (Göttingen, Germany). d-myo-inositol 1,4,5-trisphosphate, P4(5)-1-(2-nitrophenyl)ethyl ester, trisodium salt (‘caged’ IP3) was from Calbiochem-Novabiochem (UK) Ltd. (Beeston, Nottingham, UK). αβ-meATP trisodium salt, NF279, MRS2578 and (7R)-4-Hydroxy-7-methoxy-N,N,N-trimethyl-3,5,9-trioxa-4-phosphaheptacosan-1-aminium-4-oxide (edelfosine) were from Tocris (Bristol, UK). The RNA extraction kit was purchased from Qiagen (Crawley, UK); the primers and other reagents for RT-PCR experiments were purchased from Invitrogen (Paisley, UK). 1-[6-([(17β)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino)hexyl]-1H-pyrrole-2,5-dione (U-73122), 1-[6-([(17β)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino)hexyl]-2,5-pyrrolidinedione (U-73343), protease (Type X), collagenase (type 1A), soybean trypsin inhibitor (Type II-S), BSA, ATP (disodium salt), GTP (dilithium salt), creatine, HEPES, 1,3,7-trimethylxanthine (caffeine), dimethyl sulphoxide, paraformaldehyde and Triton X-100 were obtained from Sigma-Aldrich (Poole, UK). All other chemicals were from BDH Laboratory Supplies, AnalaR grade (Pool, UK).
Results
Dependence of [Ca2+]i transients on P2X receptor agonist concentration
Fluo-4-loaded RVSMCs were stimulated with gradually increasing concentrations of the P2X receptor agonist αβ-meATP applied through a glass micropipette as a 2 s pulse (Figure 1A). αβ-meATP was applied at 20 min intervals, during which the application pipette was replaced with a new one containing a different concentration of the agonist. The same RVSMC was stimulated with at least three different agonist concentrations followed by application of 100 µmol·L−1αβ-meATP. The intensity of fluo-4 fluorescence was normalized to that before agonist application and colour-coded. Traces of self-normalized fluo-4 fluorescence (F/F0) reflect the dynamics of [Ca2+]i changes induced by different αβ-meATP concentrations (Figure 1A; top left). Each of the four panels in Figure 1 (bottom) show 30 sequential images captured from the same RVSMC following application of 0.1, 0.25, 0.5 and 100 µmol·L−1αβ-meATP (i–iv, respectively) during the periods highlighted in the traces. To quantify the relationship between the response amplitude and αβ-meATP concentration, averaged peaks of ΔF/F0 transients were normalized to that induced by 100 µmol·L−1αβ-meATP in the same cell and plotted versus αβ-meATP concentrations (Figure 1A; top right). A dose–response curve fitted to the data using a non-linear least-squares minimization algorithm revealed EC50= 0.18 ± 0.01 µmol·L−1 (n= 5–10). On the one hand, this value is more than six times lower than that which we have recently reported for P2X receptor-mediated current in RVSMCs (Harhun et al., 2010), suggesting that some amplification mechanism contributes to Ca2+ mobilization. On the other hand, a gradual increase in the amplitude and rate of rise of the [Ca2+]i response with increasing αβ-meATP concentration suggests that either no regenerative mechanisms are involved or that these mechanisms are restricted to Ca2+ microdomains that function autonomously and are gradually recruited with increasing stimuli (Berridge, 1997). In support of the latter, localized Ca2+ events were observed following stimulation with low (≤0.5 µmol·L−1) concentrations of αβ-meATP (Figure 1A; panels i–iii). In response to high (≥1 µmol·L−1) concentrations of αβ-meATP, Ca2+ mobilisation was initiated by a sub-plasmalemmal [Ca2+]i upstroke (SPCU) (Gordienko et al., 2008), an abrupt increase in [Ca2+]i at multiple sub-plasmalemmal regions, which propagates through the entire cell volume within 300 ms (Figure 1A, panel iv and B, panels i–iii).
Figure 1.
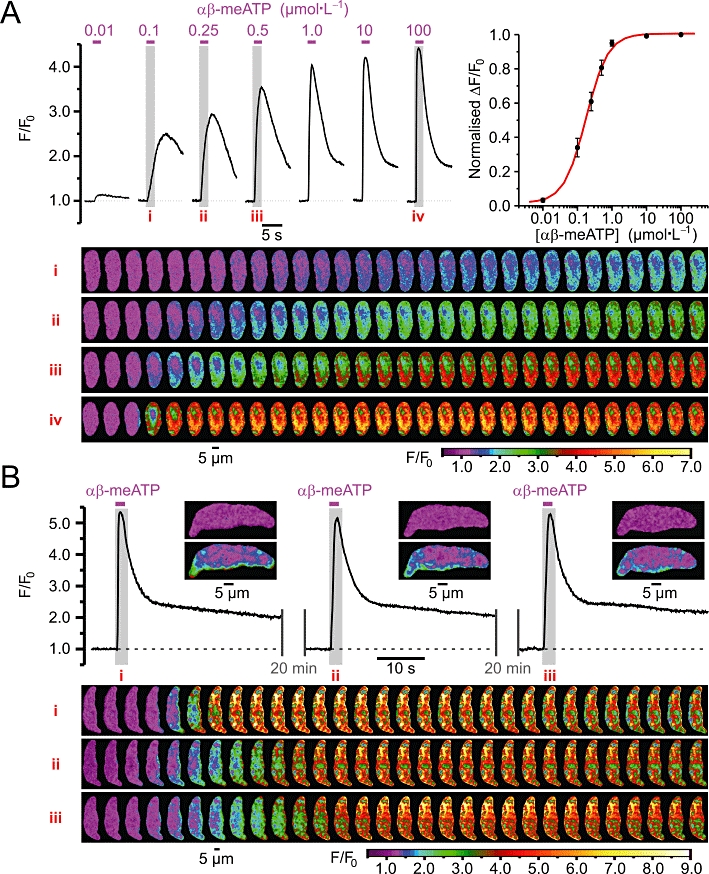
(A) Dependence of [Ca2+]i transients induced by P2X receptor stimulation in renal vascular smooth muscle cells (RVSMCs) on αβ-methylene ATP (αβ-meATP) concentration. Traces of self-normalized Fluo-4 fluorescence (F/F0) averaged within confocal (<1.2 µm) slice of the cell reflect [Ca2+]i transients induced by different αβ-meATP concentrations, as indicated (the drug applications are denoted by magenta bars above the traces, top left). Please note that the traces shown were obtained from two RVSMCs: cell 1 – the responses to 0.1, 0.25, 0.5 and 100 µmol·L−1αβ-meATP and cell 2 – the responses to 0.01, 1.0 and 10 µmol·L−1αβ-meATP. Averaged peaks of ΔF/F0 transients normalized to that induced by 100 µmol·L−1αβ-meATP in the same cell are plotted versus corresponding αβ-meATP concentrations (top right). Dose–response curve fitted to the data revealed EC50= 0.18 ± 0.01 µmol·L−1 (n = 5–10). The four panels (bottom) of 30 colour-coded confocal images were sequentially captured during the periods highlighted in the traces (top left), i–iv respectively. (B) Traces of self-normalized Fluo-4 fluorescence averaged within sub-plasmalemmal regions of initiations (insets) reflect high reproducibility of [Ca2+]i transients induced by repetitive (with 20 min interval) applications of 10 µmol·L−1αβ-meATP (top). The three panels (bottom) of 30 images were captured during the highlighted periods (top), i–iii respectively.
To compensate for cell-to-cell variations in Fluo-4 load and/or resting [Ca2+]i and, thereby, to permit the comparison of findings between different RVSMCs, we evaluated the effect of different drugs on SPCU by relating the fluorescent response (ΔF/F0) induced by 10 µmol·L−1αβ-meATP in the presence of a drug to that obtained in control in the same myocyte. In these experiments, the same Fluo-4-loaded RVSMCs were stimulated with 2 s pulses of αβ-meATP applied with a 20 min interval. The response to the succeeding stimulation (Test) was related to the response to the first stimulation (Control). The test response was obtained either in control conditions (to evaluate the response reproducibility) or following 20 min incubation with a drug or drug combination. This experimental strategy was validated by high reproducibility of [Ca2+]i transients induced in control conditions by 10 µmol·L−1αβ-meATP applied at 20 min intervals (Figure 1B, top). On average, the reproducibility of the response amplitude was 99 ± 1% (n= 20) (Figure 2E, ‘Untreated’ and 7A). Comparing images in the panels also revealed a remarkable constancy of the positions of the sites of initiation of Ca2+ mobilization associated with each αβ-meATP application (Figure 1B, panels i–iii). This allowed us to analyse SPCU in all subsequent experiments.
Figure 2.
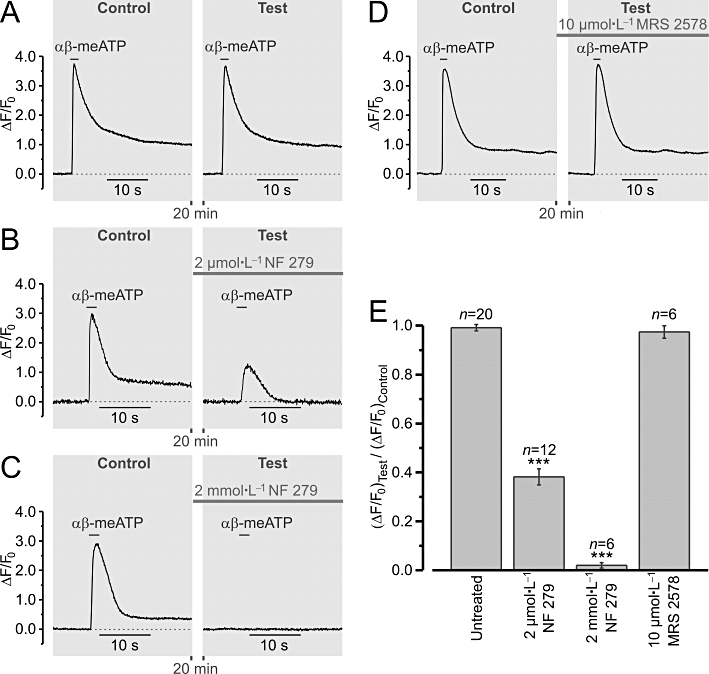
Stimulation of renal vascular smooth muscle cells (RVSMCs) with 10 µmol·L−1αβ-methylene ATP (αβ-meATP) selectively activates P2X receptors. Traces (A–D) of relative changes in Fluo-4 fluorescence (ΔF/F0) illustrate the experimental protocol. RVSMCs were stimulated with 2 s pulse of αβ-meATP applied twice at a 20 min interval. The peak amplitude of the response (ΔF/F0) to the second stimulation (Test) was normalized to that of the response to the first stimulation (Control). The Test response was obtained either in the absence an antagonist [A, ‘Untreated’ in (E)] or following incubation with 2 µmol·L−1 (B) or with 2 mmol·L−1 (C) of the P2X receptor antagonist NF 279, or with 10 µmol·L−1 of the P2Y receptor antagonist MRS 2578 (D). Statistical analysis (E) revealed that the αβ-meATP-induced responses were significantly reduced by NF 279 but were insensitive to MRS 2578. ***P < 0.001.
The ability of 10 µmol·L−1αβ-meATP to selectively activate P2X receptors was confirmed in separate experiments (Figure 2), where the effects of the P2X receptor antagonist NF 279 and P2Y6 receptor antagonist MRS 2578 on αβ-meATP-induced [Ca2+]i transients were tested. Of the eight recombinant P2Y receptor subtypes (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14), only four subtypes were found to be expressed in the kidney: P2Y1, P2Y2, P2Y4 and P2Y6 (reviewed in Guan et al., 2007b). Our RT-PCR analysis of the expression of the genes encoding P2Y receptors performed using single isolated RVSMCs (see Methods) revealed the expression of genes encoding only two P2Y receptor subtypes: P2Y2 and P2Y6 (data not shown). Although both of these P2Y receptor subtypes are uridine nucleotide-sensitive rather than adenine nucleotide-sensitive and should not be affected by 10 µmol·L−1αβ-meATP used in this study, we still tested the effect of the P2Y6 receptor antagonist MRS 2578 (as there is no commercially available selective P2Y2 receptor antagonist) on αβ-meATP-induced [Ca2+]i transients. We found that the peak amplitude of the response (ΔF/F0) of Fluo-4-loaded RVSMCs to 10 µmol·L−1αβ-meATP was reduced by 61 ± 3% (n= 12) following a 20 min incubation with 2 µmol·L−1 NF 279 and by 98 ± 1% (n= 6) following incubation with 2 mmol·L−1 NF 279, but was unaffected by 10 µmol·L−1 MRS 2578 (n= 6; P= 0.29).
SPCU and plasmalemmal electrical events
Confocal imaging of [Ca2+]i transients induced in RVSMCs by 10 µmol·L−1αβ-meATP was combined with electrical recordings performed using the perforated patch technique (Gordienko et al., 2008). Under current clamp (Figure 3A), SPCU was associated with a spike-like depolarization, which reached a peak of −2.8 ± 1.0 mV within 90 ± 15 ms (n= 14). Only three out of 14 RVSMCs revealed an overshoot (3.2 ± 1.1 mV). This suggests that the αβ-meATP-induced depolarization is mainly caused by the P2X receptor-mediated rather than VGCC-mediated current. Concurrent elevation of [Ca2+]i peaked within 792 ± 35 ms (n= 14) and persisted after recovery of the membrane potential to the resting level, thus, implying that mechanisms other than P2X-mediated Ca2+ entry contribute to the intracellular Ca2+ mobilization.
Figure 3.
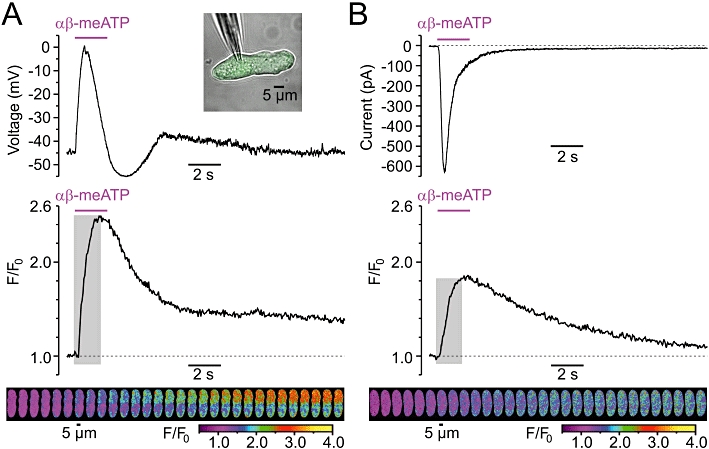
Relationship between the sub-plasmalemmal [Ca2+]i upstroke (SPCU) induced in renal vascular smooth muscle cells (RVSMCs) by 10 µmol·L−1αβ-methylene ATP (αβ-meATP) and the plasmalemmal electrical events. Changes in the cell membrane potential (A) and transmembrane current at Vh=−60 mV (B) were recorded in Fluo-4-loaded RVSMC using the perforated-patch technique (A, top inset) simultaneously with confocal imaging of [Ca2+]i changes. In (A) and (B): electrical recordings (top), traces of self-normalized Fluo-4 fluorescence averaged within sub-plasmalemmal regions of initiations (middle), gallery (bottom) of colour-coded (F/F0) images sequentially captured during the highlighted (middle) periods.
When the same RVSMC was voltage-clamped (at −60 mV), P2X receptor activation did not induce depolarization of the cell membrane, thus preventing a contribution of VGCCs to the αβ-meATP-induced response. Under these conditions, the peak of the Fluo-4 response (ΔF/F0) was reduced by 43% (Figure 3B). On average, the peak of the αβ-meATP-induced response (ΔF/F0) detected under voltage-clamp conditions was reduced by 37 ± 4% (n= 14) relative to that detected under current-clamp conditions. This statistically significant reduction (P < 0.00008; paired t-test) suggests that Ca2+ entry through VGCCs contributes to the αβ-meATP-induced SPCU. Under voltage-clamp conditions P2X-mediated inward current reached a peak within 106 ± 18 ms, while concurrent SPCU peaked within 1508 ± 40 ms (n= 14). This discrepancy suggests that Ca2+ release from the SR is recruited following P2X receptor activation. Indeed, even though VGCCs were not activated under these conditions, the elevation of [Ca2+]i continued to advance when the P2X-mediated current started to decline. Deceleration of the SPCU resulting from VGCC deactivation implies that the SR Ca2+ release can be speeded up by Ca2+ entry through VGCCs. Thus, under voltage-clamp conditions, when depolarization of the cell membrane by P2X receptor-mediated current does not occur, the link between P2X receptor activation and Ca2+ entry via VGCCs is lost and [Ca2+]i mobilization evoked by P2X receptor activation is substantially distorted. Therefore, all subsequent experiments were conducted on non-clamped myocytes.
Contribution of voltage-gated Ca2+ entry and the SR Ca2+ release to SPCU
In these experiments, nicardipine was applied 30 s before αβ-meATP to minimize the possible effect of VGCC block on the SR calcium load. The ability of the external application of 5 µmol·L−1 nicardipine to completely block VGCCs within 16 s was confirmed in separate voltage-clamp experiments (n= 5; data not shown). The effects of blocking VGCCs following calcium store depletion (Figure 4A) and calcium store depletion following block of VGCCs (Figure 4B) on SPCU induced by 10 µmol·L−1αβ-meATP were tested. Incubation of RVSMCs for 20 min with 50 µmol·L−1 cyclopiazonic acid (CPA), an inhibitor of the sarco-/endoplasmic reticulum Ca2+-ATPase (SERCA), which has no direct effect on VGCCs, Ca2+-dependent K+ channels or voltage-dependent K+ channels (Suzuki et al., 1992), completely depleted intracellular calcium stores in RVSMCs, as assessed with 5 mmol·L−1 caffeine (n= 9; data not shown). The fraction of the calcium store depletion induced by a single application of 5 mmol·L−1 caffeine was estimated in RVSMCs pre-incubated for 5 min with 10 µmol·L−1 CPA (to inhibit SERCA without the calcium store depletion) and was found to be 94 ± 2% (n= 12; data not shown), thus confirming that 5 mmol·L−1 is a sufficient concentration of caffeine to test the SR Ca2+ content in these cells. Depletion of calcium stores by a 20 min incubation with 50 µmol·L−1 CPA reduced the peak of the αβ-meATP-induced response (ΔF/F0) by 53%. Subsequent block of VGCCs with 5 µmol·L−1 nicardipine (while keeping calcium stores depleted) resulted in an additional reduction of the peak response by 20% (Figure 4A). The peak response was attenuated by 44% following block of VGCCs alone, and was reduced further by 10% following subsequent calcium store depletion (Figure 4B). These observations suggest that both Ca2+ entry through VGCCs and the SR Ca2+ release contribute to the SPCU. The response persisting in the presence of both nicardipine and CPA arose from Ca2+ entry though P2X receptors and had a peak amplitude 29 ± 3% of that in control (n= 20).
Figure 4.
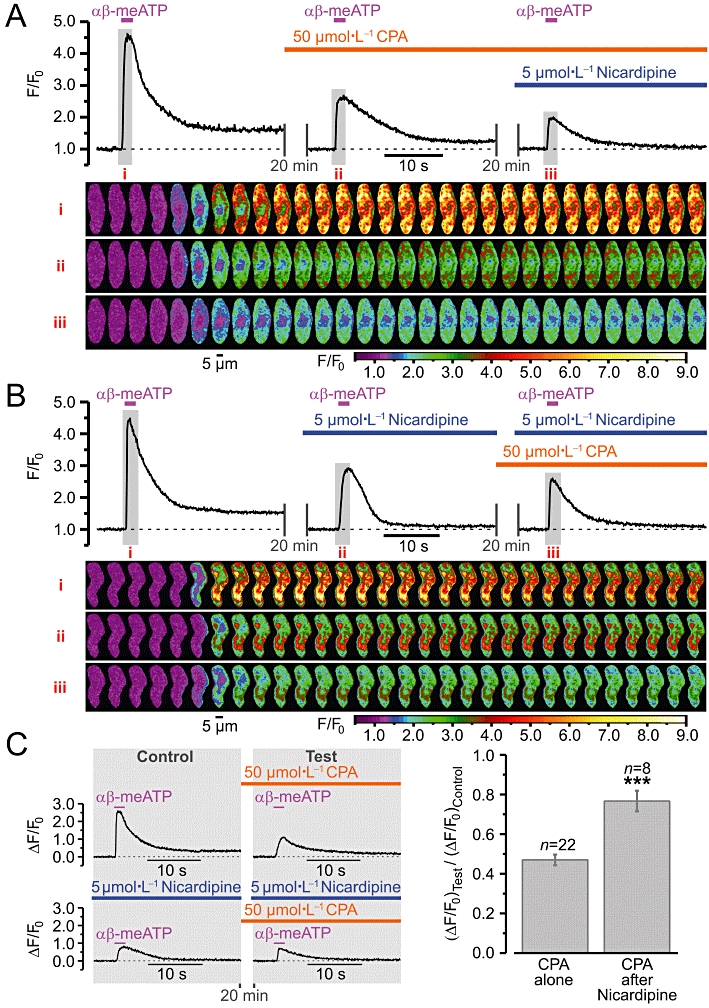
Ca2+ entry via voltage-gated Ca2+ channels (VGCCs) following P2X receptor activation in renal vascular smooth muscle cells (RVSMCs) induces Ca2+ release from intracellular calcium stores. Effect of (A) block of VGCCs (with 5 µmol·L−1 nicardipine) following calcium store depletion (by 20 min incubation with 50 µmol·L−1 cyclopiazonic acid, CPA) and (B) calcium store depletion following block of VGCCs on SPCU triggered by repetitive (with 20-min interval) applications of 10 µmol·L−1αβ-methylene ATP (αβ-meATP). The panels below the traces of self-normalized Fluo-4 fluorescence show colour-coded (F/F0) confocal images sequentially captured during the periods highlighted in the traces, i–iii respectively. (C) The amplitude of ΔF/F0 transients detected following calcium store depletion (Test) was normalized to that detected before incubation with CPA (Control) before and following block of VGCCs (left) and compared (right). ***P < 0.001.
To assess the role of Ca2+ entry through VGCCs in the activation of the SR Ca2+ release, we compared the effect of calcium store depletion following block of VGCCs with that observed in the absence of nicardipine (Figure 4C). This experimental strategy (illustrated by traces obtained from two other RVSMCs) revealed that in control conditions calcium store depletion reduced the response (ΔF/F0) to αβ-meATP by 53 ± 3% (n= 22), while when VGCCs were blocked, the calcium store depletion decreased the response by only 23 ± 5% (n= 8). Significant attenuation of the effect of the calcium store depletion on the SPCU following block of VGCCs implies that Ca2+ release from the SR following P2X receptor activation is induced mainly by Ca2+ entry through VGCCs.
Contribution of RyRs and IP3Rs to the SR Ca2+ release following P2X receptor activation
Ryanodine receptors activity was assessed with 100 µmol·L−1 ryanodine, a highly selective agent, which at micromolar concentrations blocks RyR-mediated Ca2+ release without calcium store depletion (Sutko et al., 1997; Janiak et al., 2001; Gordienko and Bolton, 2002) and does not affect IP3Rs (Janiak et al., 2001) or VGCCs (Balke and Wier, 1991). IP3R activity was assessed with 30 µmol·L−1 2-aminoethoxydiphenyl borate (2-APB). Because it was previously suggested that 2-APB may affect mechanisms of intracellular Ca2+ homeostasis other than those mediated by IP3R, including inhibition of store-operated Ca2+ entry and SERCA (reviewed by Bootman et al., 2002), we tested the effect of 30 µmol·L−1 2-APB on the SR calcium load and Ca2+ entry mechanisms evoked by P2X receptor activation in RVSMCs (Figure 5).
Figure 5.
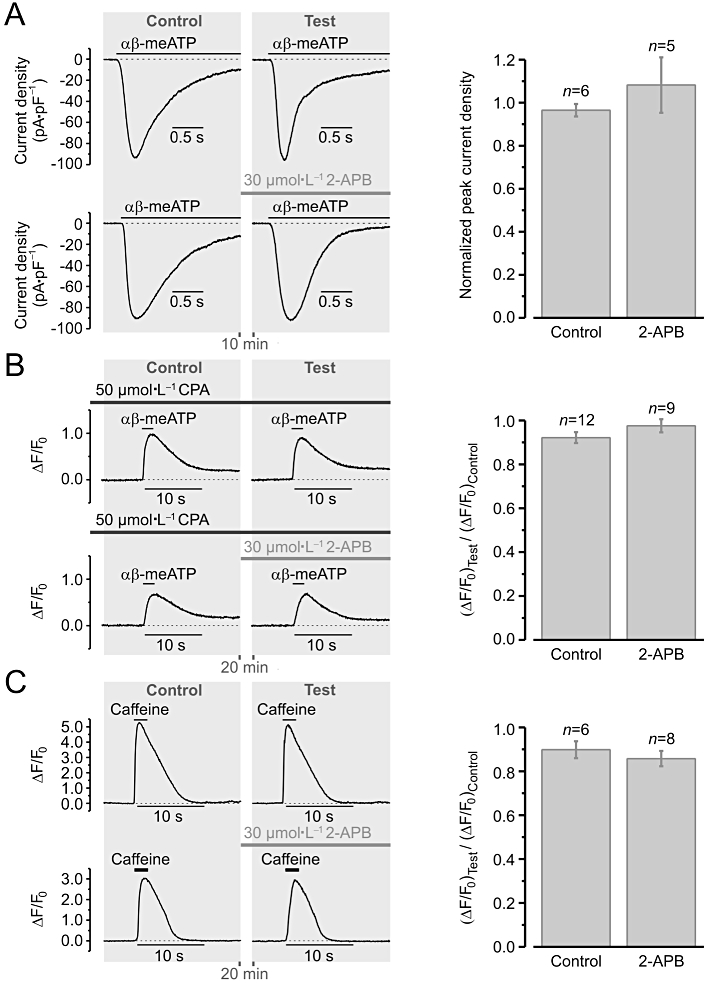
2-aminoethoxydiphenyl borate (2-APB) (30 µmol·L−1) exerts no effect on (A) the P2X receptor-mediated cationic current (B) the Ca2+ entry mechanisms induced by P2X receptor activation and (C) the sarcoplasmic reticulum (SR) calcium load. Left panels (in A–C) illustrate experimental protocols. Renal vascular smooth muscle cells were stimulated with 2 s pulses of 10 µmol·L−1αβ-methylene ATP (αβ-meATP) (A, B) or 5 mmol·L−1 caffeine (C) applied twice from a glass micropipette at 10-min (A) or 20-min (B, C) intervals and the peak amplitude of the responses was measured: (A) current density and (B, C) relative changes in Fluo-4 fluorescence (ΔF/F0). Depleting the calcium store by at least 20 min incubation with 50 µmol·L−1 CPA unmasked Ca2+ entry induced by P2X receptor stimulation (B). The peak amplitude of the response (ΔF/F0) to caffeine reflects the SR calcium load (C). The peak amplitude of the response to the second application of the agonist (Test) was normalized to that of the response to the first application of the agonist (Control) in each case (A–C). The Test response was obtained either in the absence of 2-APB (to asses reproducibility of the response to the agonist) or following incubation with 30 µmol·L−1 2-APB (to assess the effect of the drug). Statistical analysis (right panels in A–C), revealed no significant effect of 30 µmol·L−1 2-APB: P= 0.36 (A), P= 0.28 (B) and P= 0.45 (C).
P2X receptor-mediated current (Figure 5A) was unaffected by a 10 min incubation with 30 µmol·L−1 2-APB (n= 5). To unmask Ca2+ entry induced by P2X receptor stimulation in RVSMCs, the intracellular calcium stores were depleted by at least 20 min incubation with 50 µmol·L−1 CPA prior to stimulation with 10 µmol·L−1αβ-meATP (Figure 5B). Under these experimental conditions, αβ-meATP-induced [Ca2+]i transients were insensitive to 30 µmol·L−1 2-APB (n= 9). The SR calcium load, assessed with 5 mmol·L−1 caffeine (Figure 5C), was also unaffected by the 20 min incubation with 30 µmol·L−1 2-APB (n= 8). These observations validate 30 µmol·L−1 2-APB as a selective probe for IP3R-mediated Ca2+ release in RVSMCs.
To analyse the contribution of RyRs and IP3Rs to the SR Ca2+ release evoked by P2X receptor activation, the effects of successive cumulative inhibition of IP3Rs after RyRs (Figure 6A) and RyRs after IP3Rs (Figure 6B) on SPCU induced by 10 µmol·L−1αβ-meATP were tested. Block of RyRs reduced the peak of the response (ΔF/F0) to αβ-meATP by only 18%. Subsequent inhibition of IP3Rs resulted in an additional reduction of the response by 57% (Figure 6A). The response was attenuated by 60% following inhibition of IP3Rs alone, and reduced further by 8% following subsequent block of RyRs (Figure 6B).
Figure 6.
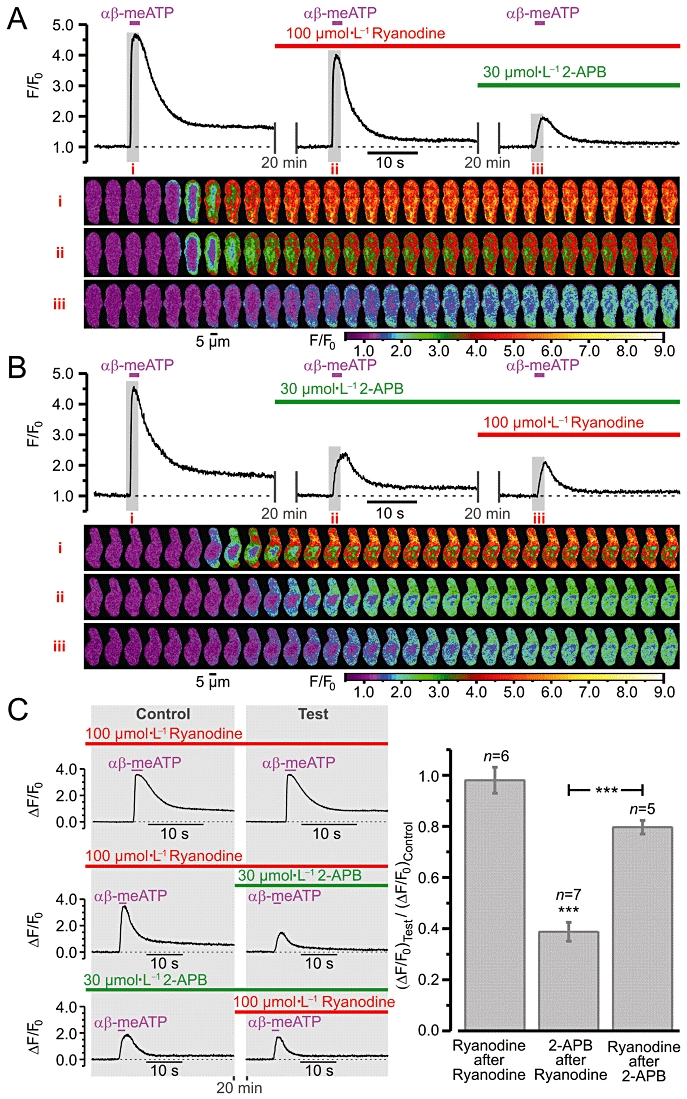
Relative contribution of ryanodine receptor (RyR)- and inositol 1,4,5-trisphosphate receptor (IP3R)-mediated Ca2+ release to sub-plasmalemmal [Ca2+]i upstroke (SPCU) induced by P2X receptor activation in renal vascular smooth muscle cells. Effect of successive cumulative inhibition of (A) IP3Rs [with 30 µmol·L−1 2-aminoethoxydiphenyl borate (2-APB) ] after RyRs (with 100 µmol·L−1 ryanodine) and (B) RyRs after IP3Rs on SPCU triggered by repetitive applications of 10 µmol·L−1αβ-methylene ATP (αβ-meATP). The cells were incubated with the Ca2+ release channel inhibitors for 20 min before the application of αβ-meATP. The panels below the traces of self-normalized Fluo-4 fluorescence show colour-coded confocal images sequentially captured during the periods highlighted in the traces, i–iii respectively. (C) The peak amplitude of the response to the second application of the agonist (Test) was normalized to that of the response to the first application of the agonist (Control). The Test response was obtained either in the presence of ryanodine alone (to assess possible effect of ryanodine on the calcium store load; left: top panel) or in the presence of both 2-APB and ryanodine (to asses the cumulative effect of the drugs; left: middle and bottom panels). The amplitudes of ΔF/F0 transients detected in the presence of the two inhibitors were normalized to that detected in the presence of one of them and in both cases were compared (right). ***P < 0.001.
An important condition of the above strategy is the ability of 100 µmol·L−1 ryanodine to block RyRs without calcium store depletion. This was tested in separate experiments. In these experiments (Figure 6C, left: top panel), after 20 min incubation with 100 µmol·L−1 ryanodine the RVSMCs were stimulated with 10 µmol·L−1αβ-meATP twice. The αβ-meATP pulses were applied at 20 min intervals. The amplitude of the [Ca2+]i transient induced by the second αβ-meATP application (Test) was related to that induced by the first one (Control). On average, the reproducibility of the amplitude of the response to 10 µmol·L−1αβ-meATP in the presence of 100 µmol·L−1 ryanodine was found to be 98 ± 5% (n= 6) (Figure 6, right); this was not statistically different (P= 0.778) from that observed in RVSMCs not treated with ryanodine, 99 ± 1% (n= 20) (Figure 2E). This observation indicates that, with the experimental protocol used in this study, 100 µmol·L−1 ryanodine blocks RyR-mediated Ca2+ release without depleting the calcium store.
Ryanodine receptors and IP3Rs are both Ca2+-sensitive and may activate each other via CICR mechanism (Fill and Copello, 2002; Foskett et al., 2007). Thus, by blocking one receptor type the effect of CICR on the other receptor type will also be lost. To eliminate the effect of possible cross-activation of these receptors from the analysis, the responses (ΔF/F0) to αβ-meATP in the presence of both blockers were normalized to the responses in the presence of each blocker alone and compared (Figure 6C). This revealed that when RyRs were initially blocked, inhibition of IP3Rs reduced the response by 61 ± 4% (n= 7). In contrast, when IP3Rs were initially blocked, inhibition of RyRs reduced the response by only 20 ± 3% (n= 5). This suggests that Ca2+ release from the SR following P2X receptor activation is mediated mainly by IP3Rs.
Figure 7 summarizes the effects of VGCC/SERCA/RyR/IP3R/PLC inhibitors on the SPCU induced in RVSMCs by 10 µmol·L−1αβ-meATP. The analysis strategy was the same as described above. The response to the succeeding stimulation (Test) was related to the response to the first stimulation (Control). The test response was obtained either in control conditions (to evaluate the response reproducibility) or following 20 min incubation with a drug or drug combination. Figure 7A shows sample traces illustrating this experimental protocol. Relative changes in the response peak amplitude (Figure 7B) and time-to-peak (Figure 7C) were analysed. In control conditions (‘Untreated’), the amplitude and time-to-peak of the test response constituted 99 ± 1% and 109 ± 5% of the control response respectively (n= 20). Block of RyRs (100 µmol·L−1 ryanodine) reduced the amplitude by only 18 ± 4% (n= 7) and had no significant effect on time-to-peak (P= 0.08). The effect of IP3R inhibition (30 µmol·L−1 2-APB) was significantly stronger: reduction of the amplitude by 48 ± 6% and increase of the time-to-peak by 98 ± 15% (n= 10). The effect of calcium store depletion (20 min incubation with 50 µmol·L−1 CPA) was also significantly stronger than that of RyR inhibition: reduction of the amplitude by 53 ± 3% and increase of the time-to-peak by 51 ± 10% (n= 22). The effect of simultaneous block of RyRs and IP3Rs was similar to the effect of calcium store depletion: reduction of the amplitude by 60 ± 4% and increase of the time-to-peak by 48 ± 11% (n= 12). Block of VGCCs (5 µmol·L−1 nicardipne) reduced the amplitude by 39 ± 2% and increased time-to-peak by 81 ± 11% (n= 18). Block of VGCCs following calcium store depletion reduced the amplitude by 70 ± 3% and decreased time-to-peak by 21 ± 8% (n= 20). It should be noted that calcium store depletion (with CPA) reduced the αβ-meATP-induced response significantly (P < 0.0006) more than block of VGCCs, suggesting that Ca2+ release from the SR is partially induced by Ca2+ entering the cell via P2X receptors. Calcium store depletion with simultaneous block of VGCCs reduced the response significantly (P < 0.0002) more than calcium store depletion alone, suggesting that Ca2+ entry via VGCCs makes a significant contribution to the αβ-meATP-induced Ca2+ mobilization. Comparison of mean amplitudes of the responses (ΔF/F0) to αβ-meATP in RVSMCS with depleted calcium stores obtained in the absence and in the presence of nicardipine suggests that the Ca2+ influx via P2X receptors is ∼1.8 times larger than that via VGCCs. Thus, Ca2+ influx following P2X receptor activation induces Ca2+ release from the SR, which is mediated mainly (but not solely) via IP3Rs and accelerates intracellular Ca2+ mobilization.
Figure 7.
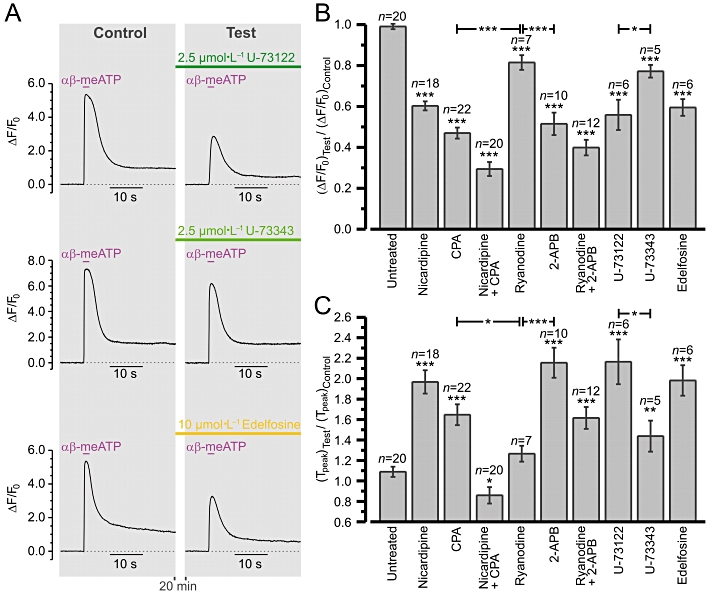
Summary of the effects of voltage-gated Ca2+ channel/sarco-/endoplasmic reticulum Ca2+-ATPase/ryanodine receptor/inositol 1,4,5-trisphosphate receptor/phospholipase C inhibitors on sub-plasmalemmal [Ca2+]i upstroke in renal vascular smooth muscle cells (RVSMCs). Experimental protocol is illustrated in (A). Fluo-4 loaded RVSMCs were stimulated with 2 s pulses of 10 µmol·L−1αβ-methylene ATP (αβ-meATP) applied with a 20 min interval. The response (ΔF/F0 averaged at sub-plasmalemmal regions of initiation) to the second stimulation (Test) was related to the response to the first stimulation (Control). The Test response was obtained either in control conditions (‘Untreated’) or following incubation with a drug or drug combination. Two parameters were examined and summarized: (B) relative change in peak amplitude and (C) relative change in time-to-peak. *P < 0.05, **P < 0.01 and ***P < 0.001 versus control, unless denoted otherwise. 2-APB, 2-aminoethoxydiphenyl borate; CPA, cyclopiazonic acid.
While P2X receptors do not signal via phospholipase C-β (PLC-β), basal levels of [IP3]i produced by spontaneous activity of PLC (Prestwich and Bolton, 1991; Gordienko and Bolton, 2002; Horowitz et al., 2005; Peng et al., 2007) may play a permissive role in activation of IP3R-mediated Ca2+ release by voltage-gated Ca2+ entry. Although the rates of hydrolysis by Gq-activated PLC-β are accelerated by elevation of [Ca2+]i, the results of experiments on permeabilized cells have suggested that [Ca2+]i levels achieved during cell signalling do not activate PLC-β by themselves but are required for PLC-β activation by Gq (Fisher et al., 1989; Horowitz et al., 2005). Thus, it is unlikely thatαβ-meATP-induced [Ca2+]i mobilization could per se activate PLC-β. Activation of any Gq/11-coupled receptors in RVSMCs by 10 µmol·L−1αβ-meATP is also unlikely, because the αβ-meATP-induced [Ca2+]i transients were completely abolished by selective P2X antagonist NF 279, but were unaffected by P2Y antagonist MRS 2578 (see Figure 2). Nevertheless, we tested the effect of commercially available PLC inhibitors on the αβ-meATP-induced [Ca2+]i transients. The aminosteroid compound U-73122 and its relatively inactive analogue U-73343 (Smith et al., 1990) both significantly reduced the amplitude and increased the time-to-peak of the response (Figure 7B and C). However, the effect of 2.5 µmol·L−1 U-73122 (the reduction of the amplitude by 44 ± 7% and increase of the time-to-peak by 99 ± 22%; n= 6) was significantly stronger than that of 2.5 µmol·L−1 U-73343 (the reduction of the amplitude by 22 ± 3% and increase of the time-to-peak by 32 ± 15%; n= 5). Although these compounds are known to have many side effects attributable to alkylation of various proteins (Horowitz et al., 2005), the difference between the effect of U-73122 and that of U-73343 suggests a permissive role of IP3 in activation of IP3Rs by Ca2+ in RVSMCs. To test this further, we examined the effect of another PLC inhibitor, the ether lipid analogue edelfosine (Powis et al., 1992; Horowitz et al., 2005). Pre-incubation with 10 µmol·L−1 edelfosine (Figure 7) reduced the amplitude of the αβ-meATP-induced [Ca2+]i transients by 40 ± 4% and increased time-to-peak by 82 ± 11% (n= 6). We therefore concluded that the ability of Ca2+ entering the RVSMCs following P2X receptor stimulation to induce IP3R-ediated Ca2+ release depends on [IP3]i which is determined by spontaneous basal activity of PLC.
To assess the role of Ca2+ entry through VGCCs in IP3R activation, we compared the effect of IP3R inhibition following block of VGCCs with that observed in the absence of nicardipine (Figure 8). This experimental strategy was validated by high reproducibility of the responses to 10 µmol·L−1αβ-meATP in the presence of 30 µmol·L−1 2-APB (Figure 8A). The effect of IP3R inhibition on the SPCU was attenuated by block of VGCCs (Figure 8B). To quantify this, the responses (ΔF/F0) in the presence of 2-APB were normalized to the responses before IP3R inhibition, both in control and in the presence of nicardipine, and compared (Figure 8C). This revealed that, when VGCCs were initially blocked, IP3R inhibition decreases the response by only 16 ± 4% (n= 12), while in control conditions (without pretreatment with nicardipine) IP3R inhibition reduced the response (ΔF/F0) to αβ-meATP by 48 ± 6% (n= 10). Significant attenuation of the effect of IP3R inhibition on SPCU by block of VGCCs implies that IP3R-mediated Ca2+ release is induced mainly (but not solely; see above) by Ca2+ influx via VGCCs.
Figure 8.
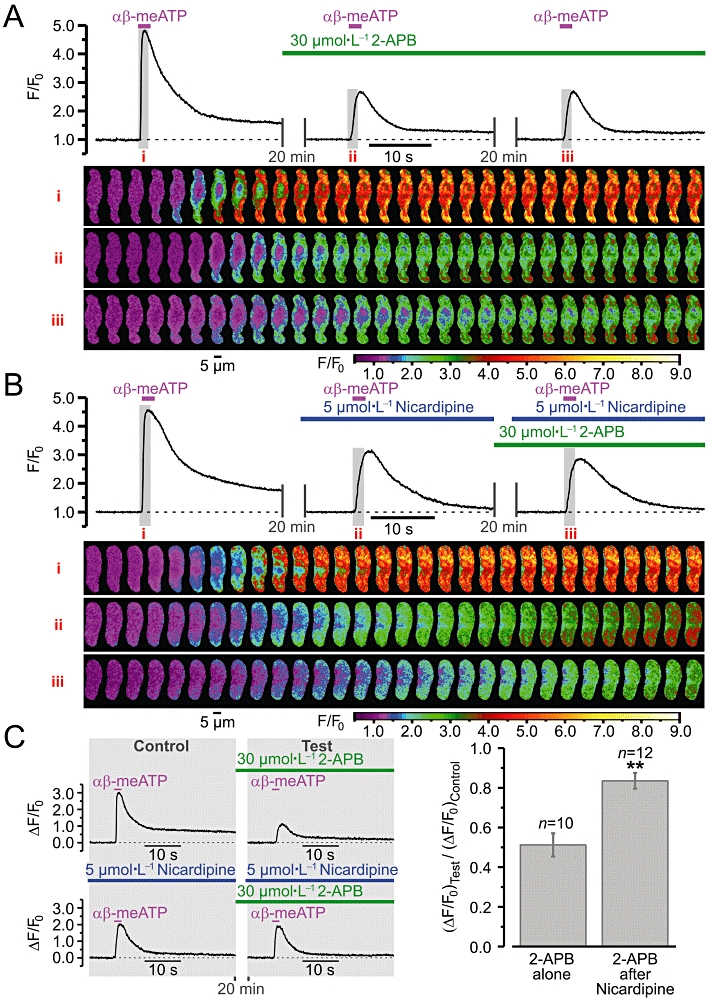
Relative contribution of P2X receptor- and voltage-gated Ca2+ channel (VGCC)-mediated Ca2+ entry to activation of inositol 1,4,5-trisphosphate receptor (IP3R)-mediated Ca2+ release in renal vascular smooth muscle cells. Effect of IP3R inhibition (A) and IP3R inhibition after block of VGCCs (B) on sub-plasmalemmal [Ca2+]i upstroke triggered by repeated applications of 10 µmol·L−1αβ-methylene ATP (αβ-meATP). The panels below the traces of self-normalized Fluo-4 fluorescence show colour-coded (F/F0) confocal images sequentially captured during the periods highlighted in the traces, i–iii respectively. (C) The amplitude of ΔF/F0 transients detected following IP3R inhibition (Test) was normalized to that detected before incubation with 2-aminoethoxydiphenyl borate (2-APB) (Control), both without (left, top panel) and in the presence of nicardipine (left, bottom panel), and compared (right). **P < 0.01.
Sub-plasmalemmal SR is enriched with IP3Rs
Tight functional coupling between VGCCs and IP3Rs suggests the expression of IP3Rs in jSR. Intracellular calcium stores, visualized in RVSMCs with the low-affinity Ca2+ indicator Fluo-3FF, coincided with SR elements, visualized with ER-Tracker Blue-White or Brefeldin A BODIPY (n= 27), and consisted of some central elements and a sub-plasmalemmal SR network (Figure 9A). Similar organization of the SR has been reported in other SMC types (Gordienko et al., 2001; 2008; Gordienko and Zholos, 2004). RT-PCR analysis of single RVSMCs revealed the expression of genes encoding IP3R type 1 and RyR type 2 (Figure 9B). Immunodetection of IP3Rs and RyRs in RVSMCs stained with Brefeldin A BODIPY and DAPI (Figure 9C) revealed that type 1 IP3Rs are primarily expressed in sub-plasmalemmal SR elements (n= 21), while RyRs predominate in deeper SR, particularly in the perinuclear region of the myocyte (n= 16). Based on this distribution of IP3Rs and RyRs, Ca2+ responses to stimuli selectively targeting IP3Rs and RyRs are expected to initiate from different positions within the cell. In the same RVSMC loaded with Fluo-4 and stained with ER-Tracker (Figure 9D), the sites of initiation of αβ-meATP-induced response coincide with positions of subplasmalemmal SR elements, while the site of initiation of the response to caffeine (which selectively activates RyR-mediated Ca2+ release) coincides with the position of deeper central SR elements (n= 8), as evident from a comparison of the images number 2 in the panels (Figure 9D, bottom) showing sequential images captured during the initial phase of the response to αβ-meATP (left) and caffeine (middle). Furthermore, flash release of IP3 (which selectively activates IP3Rs) within the entire cell volume (Figure 9D) initiated Ca2+ release at the cell periphery (n= 5), similar to P2X receptor stimulation.
Figure 9.
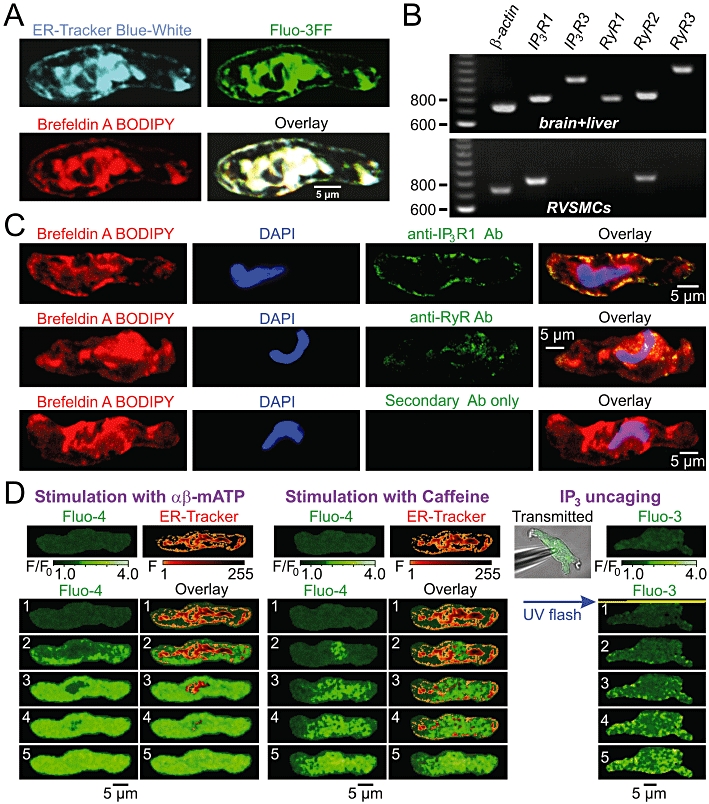
Spatial distribution of the sarcoplasmic reticulum (SR) Ca2+ release channels in renal vascular smooth muscle cells (RVSMCs). (A) Confocal images of the ER-tracker Blue-White, Fluo-3FF and Brefeldin A BODIPY fluorescence, and their overlay illustrate spatial organization of the SR in RVSMC. (B) RT-PCR analysis of inositol 1,4,5-trisphosphate receptor (IP3R) and ryanodine receptor (RyR) expression in RVSMCs. Primers designed to amplify genes encoding IP3R and RyR subtypes: IP3R1 (806 bp), IP3R3 (966 bp), RyR1 (793 bp), RyR2 (824 bp), RyR3 (1086 bp) were tested for their specificity using cDNA preparations of rat brain and liver tissue (top) before using them on RVSMC preparations (bottom). RVSMCs were found to express genes encoding IP3R1 and RyR2. (C) Immunodetection of IP3R1 (top) and RyR (middle) in RVSMC stained with Brefeldin A BODIPY and DAPI. Primary antibody binding was detected with MFP488-conjugated secondary antibody, which shows no non-specific binding in the absence of primary antibody (bottom). Confocal images highlight localization of IP3R1 in sub-plasmalemmal SR and RyR in deeper SR. (D) RVSMCs were either pre-incubated with Fluo-4AM and ER-tracker Blue-White (pseudocolour confocal images) and stimulated sequentially with 10 µmol·L−1αβ-methylene ATP (αβ-meATP) (left) or 5 mmol·L−1 caffeine (middle), or loaded with Fluo-3 and ‘caged’ IP3 via patch pipette (transmitted light and confocal images) and stimulated by whole-cell UV flash (right). Galleries of five sequential images (labelled 1–5, bottom) highlight spatial patterns of the initial phase of the responses.
Discussion
The nature of mechanisms coupling excitation to [Ca2+]i elevation, as well as the spatial organization and molecular composition of intracellular Ca2+ release units are important determinants of the contractile response in all types of muscles (Berridge, 1997; Wier and Balke, 1999; Lamb, 2002; Blatter et al., 2003; Wang et al., 2004; McCarron et al., 2006). In skeletal muscles, excitation triggers Ca2+ release from the SR via protein–protein interactions between VGCCs and RyRs (Lamb, 2002). In cardiac muscles, Ca2+ entry through VGCCs triggers RyR-mediated Ca2+ release via CICR mechanism (Wier and Balke, 1999; Blatter et al., 2003; Wang et al., 2004). In both cases, the structural basis for E–C coupling is close juxtaposition of the SR RyRs and VGCCs. In smooth muscles, elevation of [Ca2+]i is caused either by cell membrane depolarization (electromechanical coupling; Somlyo and Somlyo, 1968) leading to opening of VGCCs, which are manifested by the generation of action potentials or allow Ca2+ to ‘leak’ into the cell giving rise to ‘sparklets’ (Amberg et al., 2007), or by activation of metabotropic receptors (pharmacomechanical coupling; Somlyo and Somlyo, 1968) usually linked to Gq/11-GTP/PLC/IP3/IP3R-mediated Ca2+ release, or by a combination of these mechanisms (Bolton et al., 1999; Davis and Hill, 1999). The initial Ca2+ mobilization may be augmented by RyR-mediated Ca2+ release recruited via a CICR mechanism (Bolton and Gordienko, 1998; Bayguinov et al., 2000; White and McGeown, 2002; Kotlikoff, 2003; Zhang et al., 2003; Hotta et al., 2007; Gordienko et al., 2008). The contribution of these mechanisms to intracellular Ca2+ mobilization may vary in different SMC types, depending on the type and strength of stimuli, SMC excitability and spatial organisation of intracellular Ca2+ release units (Walsh, 1994; Bolton and Gordienko, 1998; Bolton et al., 1999; Davis and Hill, 1999; Bayguinov et al., 2000; Gordienko et al., 2001, 2008; Janiak et al., 2001; Gordienko and Bolton, 2002; White and McGeown, 2002; Kotlikoff, 2003; Wier and Morgan, 2003; Zhang et al., 2003; Lamont and Wier, 2004; McCarron et al., 2006; Amberg et al., 2007; Hotta et al., 2007; Wier et al., 2009).
We hypothesized that in vascular myocytes Ca2+ entry following activation of ionotropic P2X receptors may induce IP3R-mediated Ca2+ release. Our hypothesis was based on the following findings reported in visceral and vascular myocytes: (i) a localized increase in [Ca2+]i induced by local flash release of Ca2+ from a ‘caged’ precursor may trigger IP3R-mediated Ca2+ release (Ji et al., 2006); (ii) Ca2+ entry via VGCCs facilitates IP3R-mediated Ca2+ release following stimulation of metabotropic receptors (Gordienko et al., 2008); (iii) the stoichiometric ratio of RyRs to IP3Rs in SMCs is 1:9–10, suggesting the existence of a Ca2+-storage compartment devoid of RyRs but equipped with IP3Rs (Wibo and Godfraind, 1994); (iv) caffeine/ryanodine-releasable and IP3-releasable calcium stores in the SR of vascular myocytes are at least partially distinct with about 56% of the SR being solely IP3-sensitive (Blaustein et al., 2002); and (v) visualization of Ca2+ release sites recruited by selective IP3R stimulation and IP3R immunodetection in single SMCs suggests that they are predominantly expressed in sub-plasmalemmal SR (Bayguinov et al., 2000; Zhang et al., 2003; Gordienko and Zholos, 2004; Gordienko et al., 2008).
It should be noted, however, that there is no general agreement about the calcium store arrangement in SMCs. Indeed, the results of early experiments conducted on permeabilized strips of portal vein, pulmonary artery and taenia caeci suggested the existence of two separate calcium stores: Sα expressing both RyRs and IP3Rs and Sβ expressing only IP3Rs. The contribution of the Sβ store to IP3-induced Ca2+ release was estimated to be 60% in taenia caecum, 40% in pulmonary artery and only 6% in portal vein (reviewed in Wray and Burdyga, 2010). In contrast, the results of more recent experiments conducted on isolated pulmonary artery myocytes suggested that IP3-releasable and caffeine-releasable calcium stores are virtually completely separate (Janiak et al., 2001). A similar conclusion was made from the experiments performed on in intact precapillary vessels, where agonist-induced [Ca2+]i oscillations were insensitive to the combined action of caffeine and ryanodine (Borisova et al., 2009). An elegant study recently conducted by Rainbow et al. (2009) on guinea-pig hepatic portal vein myocytes provided strong evidence for the existence of a single luminally continuous SR where the opening of IP3Rs and RyRs is regulated by luminal [Ca2+], so that when the luminal [Ca2+] declines, the receptor opening also declines and stops. This model therefore suggests that the appearance of multiple stores in some functional studies may arise from the regulation of luminal [Ca2+] by RyRs and IP3Rs expressed in a single luminally continuous SR.
We tested our hypothesis that Ca2+ entry following P2X activation may induce IP3R-mediated Ca2+ release on RVSMCs, because: (i) P2X receptors mediate sympathetic control and autoregulation of the renal circulation (Malpas and Leonard, 2000; Nishiyama et al., 2000; Bell et al., 2003; Inscho et al., 2003; Cupples and Braam, 2007; Guan et al., 2007a; Surprenant and North, 2009); and (ii) RVSMCs express functional monomeric P2X1 and heteromeric P2X1/4 receptors (Harhun et al., 2010).
We found that depolarization of RVSMC induced by selective stimulation of P2X receptors with αβ-meATP triggers Ca2+ release from sub-plasmalemmal SR enriched with IP3Rs but poor in RyRs, similar to direct stimulation of IP3Rs by uniform cell-wide release of IP3 from its ‘caged’ precursor. The αβ-meATP-induced SPCU was significantly reduced by block of VGCCs or depletion of the intracellular calcium stores, indicating that activation of P2X receptors in RVSMCs recruits both voltage-gated Ca2+ entry and the SR Ca2+ release to intracellular Ca2+ mobilization. Activation of voltage-gated Ca2+ influx through L-type Ca2+ channels following P2X receptor stimulation was previously demonstrated in preglomerular microvascular SMCs of rat kidney (White et al., 2001). However, the involvement of the SR Ca2+ release mechanisms was not addressed in that study. Also, [Ca2+]i transients induced by 10 µmol·L−1αβ-meATP in preglomerular microvascular SMCs were completely abolished by 20 µmol·L−1 NF 279, while in RVSMCs from interlobar and arcuate arteries 2 mmol·L−1 NF 279 reduced the [Ca2+]i transients by 98%. This discrepancy may arise from variations in the distribution of P2X receptor subtypes in SMCs from different regions of renal vascular bed and may reflect the contribution of heteromeric P2X1/4 and monomeric P2X4 receptors to intracellular Ca2+ mobilization in RVSMCs from interlobar and arcuate arteries (Harhun et al., 2010). Here we not only demonstrated that the SR Ca2+ release is recruited following P2X receptor activation, but also provided strong evidence suggesting that this release is mediated mainly by IP3Rs. Indeed, depletion of calcium stores, block of VGCCs or IP3Rs reduced the amplitude and rate of rise of αβ-meATP-induced SPCU significantly more than block of RyRs. This implies that Ca2+ entry following P2X receptor activation induces IP3R-mediated Ca2+ release which serves to accelerate [Ca2+]i elevation and is initiated at sub-plasmalemmal SR enriched with IP3Rs but poor in RyRs.
It is unlikely that stimulation of RVSMCs with 10 µmol·L−1αβ-meATP recruited any metabotropic receptors coupled to a Gq/11/PLC/IP3 signalling pathway, because the αβ-meATP-induced [Ca2+]i transients were completely abolished by the selective P2X antagonist NF 279, but were unaffected by the P2Y antagonist MRS 2578. Even though activation of PLC-β by Gq is accelerated at [Ca2+]i levels achieved during cell signalling, direct activation of PLC-β by Ca2+ is also unlikely (Fisher et al., 1989; Horowitz et al., 2005). Nevertheless, the effect of PLC-β inhibition with either U-73122 or edelfosine on αβ-meATP-induced [Ca2+]i transients in RVSMCs was not statistically different from that of IP3R inhibition with 2-APB. This suggests that basal levels of [IP3]i produced by spontaneous activity of PLC (Prestwich and Bolton, 1991; Gordienko and Bolton, 2002; Horowitz et al., 2005; Peng et al., 2007) may play a permissive role in the activation of IP3R-mediated Ca2+ release by elevated [Ca2+]i achieved following P2X receptor activation. It should be noted, however, that U-73122 is known for its side effects attributable to alkylation of various proteins (Horowitz et al., 2005), while edelfosine appears to be cytotoxic causing cell swelling and eventual lysis, and therefore may well have its own side effects (Horowitz et al., 2005). Indeed, out of the16 RVSMCs tested, edelfosine caused lysis of four myocytes and induced substantial elevation of [Ca2+]i in five cells.
A comparison of the effect of the calcium store depletion with the cumulative effect of the calcium store depletion and block of VGCCs revealed that, following stimulation of RVSMCs with 10 µmol·L−1αβ-meATP, P2X receptors contributed more than VGCCs to Ca2+ entry. This is consistent with the high density of P2X receptor-mediated currents in RVSMCs (Harhun et al., 2010) and high Ca2+ permeability of P2X1 and P2X4 receptors: a relative Ca2+ over Na+ permeability (PCa/PNa) of 4.8 and 4.2 was reported for P2X1 and P2X4 receptors respectively (North, 2002; Egan et al., 2006). Nevertheless, IP3R-mediated Ca2+ release was activated mainly by Ca2+ entering the cell via VGCCs. Indeed, the effect of IP3R inhibition on the SPCU was reduced by threefold following block of VGCCs. This suggests the co-localization of plasmalemmal VGCCs and the jSR IP3Rs, and ‘local control’ (Berridge, 1997; McCarron et al., 2006) of Ca2+ release mechanisms in RVSMCs. The latter is also supported by the gradual dependence of [Ca2+]i transients on αβ-meATP concentration, despite the fact that a regenerative CICR mechanism was recruited (Arendshorst and Thai, 2009). The validation of this hypothesis, however, requires a separate electron microscopy study of ‘Ca2+ release units’ (Moore et al., 2004) in RVSMCs.
It is now generally accepted that jSR (which lies within 12–15 nm of plasmalemma), the overlying plasmalemma microdomains and the intervening, tiny volume of cytosol form junctional complexes that serve as the Ca2+‘buffer barrier’, through which Ca2+ can move directly between the extracellular fluid and jSR (Blaustein et al., 2002; Poburko et al., 2008). This may facilitate (i) local elevation of luminal [Ca2+] in jSR; and (ii) accumulation of molecules, including Ca2+, Na+ (Blaustein et al., 2002; Poburko et al., 2008), and IP3, in the cytosolic microvolume from which the diffusion into the bulk cytoplasm is markedly restricted (Poburko et al., 2008). Taking this into account, the following factors may favour activation of IP3Rs by Ca2+ influx: (i) a robust increase in [Ca2+]i in the junctional cytosolic microvolume (Blaustein et al., 2002; Poburko et al., 2008); (ii) spontaneous basal activity of PLC (Prestwich and Bolton, 1991; Gordienko and Bolton, 2002; Horowitz et al., 2005; Peng et al., 2007); (iii) Ca2+ activation of type 1 IP3Rs with positive co-operativity (Foskett et al., 2007); (iv) regulation of IP3Rs by the SR luminal [Ca2+] (Rainbow et al., 2009); and (v) IP3R clustering (Rahman and Taylor, 2009).
It should be noted, however, that the precise evaluation of the relative contribution of Ca2+ entry (P2X receptors and VGCCs) and Ca2+ release (IP3Rs and RyRs) mechanisms to αβ-meATP-induced [Ca2+]i transients is limited by: (i) a non-linear relationship between the intensity of the indicator fluorescence and [Ca2+]I; and (ii) the possible effects of pharmacological agents targeting the SR Ca2+ release mechanisms (either via the SR calcium load or via inhibition of IP3Rs/RyRs) on the cell membrane potential and, hence, the population of VGCCs available for activation by P2X receptor-mediated depolarization and/or the initial driving force for Ca2+ entry. The ability of calcium store modulators (CPA, ryanodine and 2-APB) to alter the cell membrane potential is usually attributed to their effect on spontaneous RyR- and/or IP3R-mediated Ca2+ release events, which modulate the cell membrane potential via activation of Ca2+-dependent K+ channels and/or Ca2+-dependent Cl- channels (Burdyga and Wray, 1999; Gordienko et al., 1999; Bayguinov et al., 2000; Hashitani and Brading, 2003; Hashitani et al., 2006; Zizzo et al., 2006; von der Weid et al., 2008; ZhuGe et al., 1998; 2010;). Since spontaneous Ca2+ release events were observed in less than 5% of RVSMCs used in this study, it is rather unlikely that inhibition of the SR Ca2+ release by ryanodine, 2-APB or CPA had any significant effect on resting membrane potential in these myocytes. Also, there is no evidence in the literature for a direct effect (which can be evaluated only from the single channel activity in isolated patches) of the calcium store modulators on potassium and/or chloride channels. On the contrary, it was demonstrated that neither ryanodine (Sakai et al., 1988) nor CPA (Suzuki et al., 1992) had any direct effect on Ca2+-dependent K+ channels in SMCs. In this study we have demonstrated that in RVSMCs 2-APB exerts no effect on the Ca2+ entry mechanisms induced by P2X receptor activation and the SR calcium load (Figure 5). Because the effect of 2-APB on αβ-meATP-induced [Ca2+]i transient was significantly attenuated in the presence of nicardipine, we concluded that in RVSMCs IP3R-mediated Ca2+ release following P2X receptor stimulation is activated mainly by Ca2+ entering the cell via VGCCs.
Although activation of the SR Ca2+ release by Ca2+ entering the cell via VGCCs has been demonstrated in voltage-clamp experiments performed on different types of visceral and vascular SMCs (Kamishima and McCarron, 1997; Kohda et al., 1997; Bolton and Gordienko, 1998; Shmigol et al., 1998; Coussin et al., 2000), there is a number of studies (e.g. Bradley et al., 2002; 2004;) demonstrating that complete depletion of the SR of Ca2+ (including IP3-sensitive store) does not reduce [Ca2+]i transients induced by step-like depolarization of the cell membrane. The latter results suggest that CICR is not recruited. An alternative explanation given by Bradley et al. (2004) suggests that the SR and sarcolemma may form a passive physical barrier to Ca2+ influx (‘a Ca2+ trap’), which normally limits the [Ca2+]i rise occurring during depolarization. The drugs, which open the SR Ca2+ release channels and facilitate the SR Ca2+ leak, diminish the influence of ‘the Ca2+ trap’ and may, thereby, increase the amplitude of [Ca2+]i transients resulting from Ca2+ entry via VGCCs even when the SR contains little or no Ca2+ (Bradley et al., 2004).
In cardiac myocytes, abnormal intracellular Ca2+ handling arising from altered expression/function of RyRs and/or IP3Rs promotes arrhythmias, atrial fibrillation and heart failure (George and Lai, 2007; Kockskämper et al., 2008). In vascular myocytes, mechanisms coupling P2X receptor activation to IP3R-mediated Ca2+ release may be altered in hypertension. Indeed, it was demonstrated that impaired Ca2+ signalling attenuates P2X receptor-mediated vasoconstriction of afferent arterioles in angiotensin II hypertension (Zhao et al., 2005). On the other hand, elevated PLC-β levels and activity were reported in renal arterioles of young spontaneously hypertensive rats (Peng et al., 2007) and were suggested to account for previously demonstrated exaggerated renal vasoconstriction and Ca2+ signalling in preglomerular arterioles induced by angiotensin II, thromboxane A2 and vasopressin in spontaneously hypertensive versus normotensive rats (Feng and Arendshorst, 1997; Iversen and Arendshorst, 1999; Vagnes et al., 2005).
To conclude, we demonstrated that P2X receptor activation evokes IP3R-mediated Ca2+ release in RVSMCs, which is induced mainly by voltage-gated Ca2+ entry. This mechanism provides convergence of signalling pathways engaged in electromechanical and pharmacomechanical coupling in smooth muscles of renal vasculature and should be taken into consideration when developing new therapeutic strategies. Further work, however, is needed to examine whether and how this mechanism is altered in hypertension.
Acknowledgments
Supported by British Heart Foundation grants (PG/08/062/25382 and FS/06/077).
Glossary
Abbreviations
- 2-APB
2-aminoethoxydiphenyl borate
- αβ-meATP
αβ-methylene ATP
- CICR
Ca2+-induced Ca2+ release
- CPA
cyclopiazonic acid
- IP3
inositol 1,4,5-trisphosphate
- IP3R
inositol 1,4,5-trisphosphate receptor
- jSR
sub-plasmalemmal (‘junctional’) sarcoplasmic reticulum
- MRS2578
N,N''-1,4-butanediylbis[N'-(3-isothiocyanatophenyl)thio urea
- NF279
8,8'-[carbonylbis(imino-4,1-phenylenecarbonylimino-4,1-phenylenecarbonyl-imino)] bis(1,3,5-naphthalene-trisulphonic acid)
- PLC
phospholipase C
- RBF
renal blood flow
- RSNA
renal sympathetic nerve activity
- RT-PCR
reverse transcription polymerase chain reaction
- RVSMC
renal vascular smooth muscle cells
- RyR
ryanodine receptor
- SERCA
sarco-/endoplasmic reticulum Ca2+-ATPase
- SPCU
sub-plasmalemmal [Ca2+]i upstroke
- SR
sarcoplasmic reticulum
- U-73122
1-[6-([(17β)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino)hexyl]-1H-pyrrole-2,5-dione
- U-73343
1-[6-([(17β)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino)hexyl]-2,5-pyrrolidinedione
- edelfosine
(7R)-4-hydroxy-7-methoxy-N,N,N-trimethyl-3,5,9-trioxa-4 -phosphaheptacosan-1-aminium-4-oxide
- VGCC
voltage-gated Ca2+ channel
Conflicts of interest
None.
Supplementary material
Supporting Information: Teaching Materials; Figs 1–9 as PowerPoint slide.
References
- Abeele FV, Bidaux G, Gordienko D, Beck B, Panchin YV, Baranova AV, et al. Functional implications of calcium permeability of the channel formed by Pannexin 1. J Cell Biol. 2006;174:535–546. doi: 10.1083/jcb.200601115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th Edition. Br J Pharmacol. 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amberg GC, Navedo MF, Nieves-Cintron M, Molkentin JD, Santana LF. Calcium sparklets regulate local and global calcium in murine arterial smooth muscle. J Physiol. 2007;579:187–201. doi: 10.1113/jphysiol.2006.124420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendshorst WJ, Thai TL. Regulation of the renal microcirculation by ryanodine receptors and calcium-induced calcium release. Curr Opin Nephrol Hypertens. 2009;18:40–49. doi: 10.1097/MNH.0b013e32831cf5bd. [DOI] [PubMed] [Google Scholar]
- Balke CW, Wier WG. Ryanodine does not affect calcium current in guinea pig ventricular myocytes in which Ca2+ is buffered. Circ Res. 1991;68:897–902. doi: 10.1161/01.res.68.3.897. [DOI] [PubMed] [Google Scholar]
- Bayguinov O, Hagen B, Bonev AD, Nelson NT, Sanders KM. Intracellular calcium events activated by ATP in murine colonic myocytes. Am J Physiol. 2000;279:C126–C135. doi: 10.1152/ajpcell.2000.279.1.C126. [DOI] [PubMed] [Google Scholar]
- Bell PD, Lapointe JY, Sabirov R, Hayashi S, Peti-Peterdi J, Manabe K, et al. Macula densa cell signaling involves ATP release through a maxi anion channel. Proc Natl Acad Sci USA. 2003;100:4322–4327. doi: 10.1073/pnas.0736323100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. Elementary and global aspects of calcium signalling. J Physiol. 1997;499:291–306. doi: 10.1113/jphysiol.1997.sp021927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatter LA, Kockskämper J, Sheehan KA, Zima AV, Hüser J, Lipsius SL. Local calcium gradients during excitation-contraction coupling and alternans in atrial myocytes. J Physiol. 2003;546:19–31. doi: 10.1113/jphysiol.2002.025239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein MP, Golovina VA, Song H, Choate J, Lencesova L, Robinson SW, et al. Organization of Ca2+ stores in vascular smooth muscle: functional implications. In: Chadwick DJ, Goode JA, editors. Role of the Sarcoplasmic Reticulum in Smooth Muscle. Sussex: John Wiley & Sons Ltd.; 2002. pp. 125–137. [PubMed] [Google Scholar]
- Boittin FX, Coussin F, Macrez N, Mironneau G, Mironneau J. Inositol 1,4,5-trisphosphate and ryanodine-sensitive Ca2+ release channel-dependent Ca2+ signalling in rat portal vein. Cell Calcium. 1998;23:303–311. doi: 10.1016/s0143-4160(98)90026-4. [DOI] [PubMed] [Google Scholar]
- Boittin FX, Macrez N, Halet G, Mironneau J. Norepinephrine-induced Ca2+ waves depend on InsP3 and ryanodine receptor activation in vascular myocytes. Am J Physiol. 1999;277:C139–C151. doi: 10.1152/ajpcell.1999.277.1.C139. [DOI] [PubMed] [Google Scholar]
- Bolton TB, Gordienko DV. Confocal imaging of calcium release events in single smooth muscle cells. Acta Physiol Scand. 1998;164:567–575. doi: 10.1046/j.1365-201X.1998.00464.x. [DOI] [PubMed] [Google Scholar]
- Bolton TB, Prestwich SA, Zholos AV, Gordienko DV. Excitation-contraction coupling in gastrointestinal and other smooth muscles. Annu Rev Physiol. 1999;61:85–115. doi: 10.1146/annurev.physiol.61.1.85. [DOI] [PubMed] [Google Scholar]
- Bootman MD, Cheek TR, Moreton RB, Bennett DL, Berridge MJ. Smoothly graded Ca2+ release from inositol 1,4,5-trisphosphate-sensitive Ca2+ stores. J Biol Chem. 1994;269:24783–24791. [PubMed] [Google Scholar]
- Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002;16:1145–1150. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- Borisova L, Wray S, Burdyga T. How structure, Ca signals and cellular communications underlie function in precapillary vessels. Circ Res. 2009;105:803–810. doi: 10.1161/CIRCRESAHA.109.202960. [DOI] [PubMed] [Google Scholar]
- Bradley KN, Flynn ER, Muir TC, McCarron JG. Ca2+ regulation in guinea-pig colonic smooth muscle: the role of the Na+-Ca2+ exchanger and the sarcoplasmic reticulum. J Physiol. 2002;538:465–482. doi: 10.1113/jphysiol.2001.013039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KN, Craig JW, Muir TC, McCarron JG. The sarcoplasmic reticulum and sarcolemma together form a passive Ca2+ trap in colonic smooth muscle. Cell Calcium. 2004;36:29–41. doi: 10.1016/j.ceca.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Burdyga TV, Wray S. The effect of cyclopiazonic acid on excitation-contraction coupling in guinea-pig ureteric smooth muscle: role of the sarcoplasmic reticulum. J Physiol. 1999;517:855–865. doi: 10.1111/j.1469-7793.1999.0855s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Coussin F, Macrez N, Morel JL, Mironneau J. Requirement of ryanodine receptor subtypes 1 and 2 for Ca2+-induced Ca2+ release in vascular myocytes. J Biol Chem. 2000;275:9596–9603. doi: 10.1074/jbc.275.13.9596. [DOI] [PubMed] [Google Scholar]
- Cupples WA, Braam B. Assessment of renal autoregulation. Am J Physiol. 2007;292:F1105–F1123. doi: 10.1152/ajprenal.00194.2006. [DOI] [PubMed] [Google Scholar]
- Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- DiBona GF. The sympathetic nervous system and hypertension: recent developments. Hypertension. 2004;43:147–150. doi: 10.1161/01.HYP.0000113047.47711.fa. [DOI] [PubMed] [Google Scholar]
- Egan TM, Samways DS, Li Z. Biophysics of P2X receptors. Pflugers Arch. 2006;452:501–512. doi: 10.1007/s00424-006-0078-1. [DOI] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Contractions induced by a calcium-triggered release of calcium from the sarcoplasmic reticulum of single skinned cardiac cells. J Physiol. 1975;249:469–495. doi: 10.1113/jphysiol.1975.sp011026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng JJ, Arendshorst WJ. Calcium signaling mechanisms in renal vascular responses to vasopressin in genetic hypertension. Hypertension. 1997;30:1223–1231. doi: 10.1161/01.hyp.30.5.1223. [DOI] [PubMed] [Google Scholar]
- Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- Fisher SK, Domask LM, Roland RM. Muscarinic receptor regulation of cytoplasmic Ca2+ concentrations in human SK-N-SH neuroblastoma cells: Ca2+ requirements for phospholipase C activation. Mol Pharmacol. 1989;35:195–204. [PubMed] [Google Scholar]
- Fleischmann BK, Wang YX, Pring M, Kotlikoff MI. Voltage-dependent calcium currents and cytosolic calcium in equine airway myocytes. J Physiol. 1996;492:347–358. doi: 10.1113/jphysiol.1996.sp021313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George CH, Lai FA. Developing new anti-arrhythmics: clues from the molecular basis of cardiac ryanodine receptor (RyR2) Ca2+-release channel dysfunction. Curr Pharm Des. 2007;13:3195–3211. doi: 10.2174/138161207782341259. [DOI] [PubMed] [Google Scholar]
- Gordienko DV, Bolton TB. Crosstalk between ryanodine receptors and IP3 receptors as a factor shaping spontaneous Ca2+-release events in rabbit portal vein myocytes. J Physiol. 2002;542:743–762. doi: 10.1113/jphysiol.2001.015966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordienko DV, Zholos AV. Regulation of muscarinic cationic current in myocytes from guinea-pig ileum by intracellular Ca2+ release: a central role of inositol 1,4,5-trisphosphate receptors. Cell Calcium. 2004;36:367–386. doi: 10.1016/j.ceca.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Gordienko DV, Clausen C, Goligorsky MS. Ionic currents and endothelin signaling in smooth muscle cells from rat renal resistance arteries. Am J Physiol. 1994;266:F325–F341. doi: 10.1152/ajprenal.1994.266.2.F325. [DOI] [PubMed] [Google Scholar]
- Gordienko DV, Zholos AV, Bolton TB. Membrane ion channels as physiological targets for local Ca2+ signalling. J Microsc. 1999;196:305–316. doi: 10.1046/j.1365-2818.1999.00599.x. [DOI] [PubMed] [Google Scholar]
- Gordienko DV, Greenwood IA, Bolton TB. Direct visualization of sarcoplasmic reticulum regions discharging Ca2+ sparks in vascular myocytes. Cell Calcium. 2001;29:13–28. doi: 10.1054/ceca.2000.0180. [DOI] [PubMed] [Google Scholar]
- Gordienko DV, Harhun MI, Kustov MV, Pucovský V, Bolton TB. Sub-plasmalemmal [Ca2+]i upstroke in myocytes of the guinea-pig small intestine evoked by muscarinic stimulation: IP3R-mediated Ca2+ release induced by voltage-gated Ca2+ entry. Cell Calcium. 2008;43:122–141. doi: 10.1016/j.ceca.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Z, Osmond DA, Inscho EW. P2X receptors as regulators of the renal microvasculature. Trends Pharmacol Sci. 2007a;28:646–652. doi: 10.1016/j.tips.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Guan Z, Osmond DA, Inscho EW. Purinoceptors in the kidney. Exp Biol Med. 2007b;232:715–726. [PubMed] [Google Scholar]
- Harhun MI, Szewczyk K, Laux H, Prestwich SA, Gordienko DV, Moss RF, et al. Interstitial cells from rat middle cerebral artery belong to smooth muscle cell type. J Cell Mol Med. 2009;13:4532–4539. doi: 10.1111/j.1582-4934.2008.00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harhun MI, Povstyan OV, Gordienko DV. Purinoreceptor-mediated current in myocytes from renal resistance arteries. Br J Pharmacol. 2010;160:987–997. doi: 10.1111/j.1476-5381.2010.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Brading AF. Ionic basis for the regulation of spontaneous excitation in detrusor smooth muscle cells of the guinea-pig urinary bladder. Br J Pharmacol. 2003;140:159–169. doi: 10.1038/sj.bjp.0705320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Yanai Y, Kohri K, Suzuki H. Heterogeneous CPA sensitivity of spontaneous excitation in smooth muscle of the rabbit urethra. Br J Pharmacol. 2006;148:340–349. doi: 10.1038/sj.bjp.0706729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz LF, Hirdes W, Suh BC, Hilgemann DW, Mackie K, Hille B. Phospholipase C in living cells: activation, inhibition, Ca2+ requirement, and regulation of M current. J Gen Physiol. 2005;126:243–262. doi: 10.1085/jgp.200509309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta S, Yamamura H, Ohya S, Imaizumi Y. Methyl-beta-cyclodextrin prevents Ca2+-induced Ca2+ release in smooth muscle cells of mouse urinary bladder. J Pharmacol Sci. 2007;103:121–126. doi: 10.1254/jphs.sc0060213. [DOI] [PubMed] [Google Scholar]
- Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Physiological role for P2X1 receptors in renal microvascular autoregulatory behaviour. J Clin Invest. 2003;112:1895–1905. doi: 10.1172/JCI18499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G, Han S. Gradation of Ca2+-induced Ca2+ release by voltage-clamp pulse duration in potentiated guinea-pig ventricular myocytes. J Physiol. 1994;480:423–438. doi: 10.1113/jphysiol.1994.sp020372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen BM, Arendshorst WJ. Exaggerated Ca2+ signaling in preglomerular arteriolar smooth muscle cells of genetically hypertensive rats. Am J Physiol. 1999;276:F260–F270. doi: 10.1152/ajprenal.1999.276.2.F260. [DOI] [PubMed] [Google Scholar]
- Janiak R, Wilson SM, Montague S, Hume JR. Heterogeneity of calcium stores and elementary release events in canine pulmonary arterial smooth muscle cells. Am J Physiol. 2001;280:C22–C33. doi: 10.1152/ajpcell.2001.280.1.C22. [DOI] [PubMed] [Google Scholar]
- Ji G, Feldman M, Doran R, Zipfel W, Kotlikoff MI. Ca2+-induced Ca2+ release through localized Ca2+ uncaging in smooth muscle. J Gen Physiol. 2006;127:225–235. doi: 10.1085/jgp.200509422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamishima T, McCarron JG. Depolarization-evoked increases in cytosolic calcium concentration in isolated smooth muscle cells in rat portal vein. J Physiol. 1996;492:61–74. doi: 10.1113/jphysiol.1996.sp021289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamishima T, McCarron JG. Regulation of cytosolic Ca2+ concentration by Ca2+ stores in single smooth muscle cells from rat cerebral arteries. J Physiol. 1997;501:497–508. doi: 10.1111/j.1469-7793.1997.497bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kockskämper J, Zima AV, Roderick HL, Pieske B, Blatter LA, Bootman MD. Emerging roles of inositol 1,4,5-trisphosphate signaling in cardiac myocytes. J Mol Cell Cardiol. 2008;45:128–147. doi: 10.1016/j.yjmcc.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohda M, Komori S, Unno T, Ohashi H. Characterization of action potential-triggered [Ca2+]i transients in single smooth muscle cells of guinea-pig ileum. Br J Pharmacol. 1997;122:477–486. doi: 10.1038/sj.bjp.0701407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlikoff MI. Calcium-induced calcium release in smooth muscle: the case for loose coupling. Prog Biophys Mol Biol. 2003;83:171–191. doi: 10.1016/s0079-6107(03)00056-7. [DOI] [PubMed] [Google Scholar]
- Lamb GD. Excitation-contraction coupling and fatigue mechanisms in skeletal muscle: studies with mechanically skinned fibres. J Muscle Res Cell Motil. 2002;23:81–91. doi: 10.1023/a:1019932730457. [DOI] [PubMed] [Google Scholar]
- Lamont C, Wier WG. Different roles of ryanodine receptors and inositol (1,4,5)-trisphosphate receptors in adrenergically stimulated contractions of small arteries. Am J Physiol. 2004;287:617–625. doi: 10.1152/ajpheart.00708.2003. [DOI] [PubMed] [Google Scholar]
- Laporte R, Hui A, Laher I. Pharmacological modulation of sarcoplasmic reticulum function in smooth muscle. Pharmacol Rev. 2004;56:439–513. doi: 10.1124/pr.56.4.1. [DOI] [PubMed] [Google Scholar]
- McCarron JG, Craig JW, Bradley KN, Muir TC. Agonist-induced phasic and tonic responses in smooth muscle are mediated by InsP3. J Cell Sci. 2002;115:2207–2218. doi: 10.1242/jcs.115.10.2207. [DOI] [PubMed] [Google Scholar]
- McCarron JG, Chalmers S, Bradley KN, MacMillan D, Muir TC. Ca2+ microdomains in smooth muscle. Cell Calcium. 2006;40:461–493. doi: 10.1016/j.ceca.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Mackrill JJ, Challiss RAJ, O'Connell DA, Lai FA, Nahorski SR. Differential expression and regulation of ryanodine receptor and myo-inositol 1,4,5-trisphosphate receptor Ca2+ release channels in mammalian tissues and cell lines. Biochem J. 1997;327:251–258. doi: 10.1042/bj3270251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan D, Chalmers S, Muir TC, McCarron JG. IP3-mediated Ca2+ increases do not involve the ryanodine receptor, but ryanodine receptor antagonists reduce IP3-mediated Ca2+ increases in guinea-pig colonic smooth muscle. J Physiol. 2005;569:533–544. doi: 10.1113/jphysiol.2005.096529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpas SC, Leonard BL. Neural regulation of renal blood flow: a re-examination. Clin Exp Pharmacol Physiol. 2000;27:956–964. doi: 10.1046/j.1440-1681.2000.03386.x. [DOI] [PubMed] [Google Scholar]
- Moore ED, Voigt T, Kobayashi YM, Isenberg G, Fay FS, Gallitelli MF, et al. Organization of Ca2+ release units in excitable smooth muscle of the guinea-pig urinary bladder. Biophys J. 2004;87:1836–1847. doi: 10.1529/biophysj.104.044123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimura K, Ohi Y, Yamamura H, Ohya S, Muraki K, Imaizumi Y. Two-step Ca2+ intracellular release underlies excitation-contraction coupling in mouse urinary bladder myocytes. Am J Physiol. 2006;290:C388–C403. doi: 10.1152/ajpcell.00409.2005. [DOI] [PubMed] [Google Scholar]
- Navar LG. The role of the kidneys in hypertension. J Clin Hypertens. 2005;7:542–549. doi: 10.1111/j.1524-6175.2005.04130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Majid DS, Taher KA, Miyatake A, Navar LG. Relation between renal interstitial ATP concentrations and autoregulation-mediated changes in renal vascular resistance. Circ Res. 2000;86:656–662. doi: 10.1161/01.res.86.6.656. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Ohi Y, Yamamura H, Nagano N, Ohya S, Muraki K, Watanabe M, et al. Local Ca2+ transients and distribution of BK channels and ryanodine receptors in smooth muscle cells of guinea-pig vas deferens and urinary bladder. J Physiol. 2001;534:313–326. doi: 10.1111/j.1469-7793.2001.t01-3-00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Dang A, Arendshorst WJ. Increased expression and activity of phospholipase C in renal arterioles of young spontaneously hypertensive rats. Am J Hypertens. 2007;20:38–43. doi: 10.1016/j.amjhyper.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Poburko D, Fameli N, Kuo KH, van Breemen C. Ca2+ signaling in smooth muscle: TRPC6, NCX and LNats in nanodomains. Channels. 2008;2:10–12. doi: 10.4161/chan.2.1.6053. [DOI] [PubMed] [Google Scholar]
- Povstyan OV, Harhun MI, Gordienko DV. Electrical events and mechanisms of [Ca2+]i mobilisation induced by P2X receptor stimulation in rat renal resistance artery myocytes. Proc Physiol Soc. 2009;15:PC18. [Google Scholar]
- Powis G, Seewald MJ, Gratas C, Melder D, Riebow J, Modest EJ. Selective inhibition of phosphatidylinositol phospholipase C by cytotoxic ether lipid analogues. Cancer Res. 1992;52:2835–2840. [PubMed] [Google Scholar]
- Prestwich SA, Bolton TB. Measurement of picomole amounts of any inositol phosphate isomer separable by h.p.l.c. by means of a bioluminescence assay. Biochem J. 1991;274:663–672. doi: 10.1042/bj2740663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman T, Taylor CW. Dynamic regulation of IP3 receptor clustering and activity by IP3. Channels. 2009;3:226–232. doi: 10.4161/chan.3.4.9247. [DOI] [PubMed] [Google Scholar]
- Rainbow RD, Macmillan D, McCarron JG. The sarcoplasmic reticulum Ca2+ store arrangement in vascular smooth muscle. Cell Calcium. 2009;46:313–322. doi: 10.1016/j.ceca.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Sakai T, Terada K, Kitamura K, Kuriyama H. Ryanodine inhibits the Ca-dependent K current after depletion of Ca stored in smooth muscle cells of the rabbit ileal longitudinal muscle. Br J Pharmacol. 1988;95:1089–1100. doi: 10.1111/j.1476-5381.1988.tb11743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmigol A, Eisner DA, Wray S. Properties of voltage-activated [Ca2+] transients in single smooth muscle cells isolated from pregnant rat uterus. J Physiol. 1998;511:803–811. doi: 10.1111/j.1469-7793.1998.803bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Sam LM, Justen JM, Bundy GL, Bala GA, Bleasdale JE. Receptor-coupled signal transduction in human polymorphonuclear neutrophils: effects of a novel inhibitor of phospholipase C-dependent processes on cell responsiveness. J Pharmacol Exp Ther. 1990;253:688–697. [PubMed] [Google Scholar]
- Somlyo AV, Somlyo AP. Electromechanical and pharmacomechanical coupling in vascular smooth muscle. J Pharm Exp Ther. 1968;159:129–145. [PubMed] [Google Scholar]
- Surprenant A, North RA. Signaling at purinergic P2X receptors. Annu Rev Physiol. 2009;71:333–359. doi: 10.1146/annurev.physiol.70.113006.100630. [DOI] [PubMed] [Google Scholar]
- Sutko JL, Airey JA, Welch W, Ruest L. The pharmacology of ryanodine and related compounds. Pharmacol Rev. 1997;49:53–98. [PubMed] [Google Scholar]
- Suzuki M, Muraki K, Imaizumi Y, Watanabe M. Cyclopiazonic acid, an inhibitor of the sarcoplasmic reticulum Ca2+-pump, reduces Ca2+-dependent K+ currents in guinea-pig smooth muscle cells. Br J Pharmacol. 1992;107:134–140. doi: 10.1111/j.1476-5381.1992.tb14475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CW, Traynor D. Calcium and inositol trisphosphate receptors. J Mem Biol. 1995;145:109–118. doi: 10.1007/BF00237369. [DOI] [PubMed] [Google Scholar]
- Vagnes OB, Hansen FH, Feng JJ, Iversen BM, Arendshorst WJ. Enhanced Ca2+ response to AVP in preglomerular vessels from rats with genetic hypertension during different hydration states. Am J Physiol. 2005;288:F1249–F1256. doi: 10.1152/ajprenal.00363.2004. [DOI] [PubMed] [Google Scholar]
- Walsh MP. Regulation of vascular smooth muscle tone. Can J Physiol Pharmacol. 1994;72:919–936. doi: 10.1139/y94-130. [DOI] [PubMed] [Google Scholar]
- Wang SQ, Wei C, Zhao G, Brochet DX, Shen J, Song LS, et al. Imaging microdomain Ca2+ in muscle cells. Circ Res. 2004;95:798–806. doi: 10.1161/01.RES.0000125883.68447.A1. [DOI] [PubMed] [Google Scholar]
- von der Weid PY, Rahman M, Imtiaz MS, van Helden DF. Spontaneous transient depolarizations in lymphatic vessels of the guinea pig mesentery: pharmacology and implication for spontaneous contractility. Am J Physiol. 2008;290:H813–H822. doi: 10.1152/ajpheart.00007.2008. [DOI] [PubMed] [Google Scholar]
- White C, McGeown JG. Carbachol triggers RyR-dependent Ca2+ release via activation of IP3 receptors in isolated rat gastric myocytes. J Physiol. 2002;542:725–733. doi: 10.1113/jphysiol.2002.020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C, McGeown JG. Inositol 1,4,5-trisphosphate receptors modulate Ca2+ sparks and Ca2+ store content in vas deferens myocytes. Am J Physiol. 2003;285:C195–C204. doi: 10.1152/ajpcell.00374.2002. [DOI] [PubMed] [Google Scholar]
- White SM, Imig JD, Kim TT, Hauschild BC, Inscho EW. Calcium signaling pathways utilized by P2X receptors in freshly isolated preglomerular MVSMC. Am J Physiol. 2001;280:F1054–F1061. doi: 10.1152/ajprenal.2001.280.6.F1054. [DOI] [PubMed] [Google Scholar]
- Wibo M, Godfraind T. Comparative localization of inositol 1,4,5-trisphosphate and ryanodine receptors in intestinal smooth muscle: an analytical subfractionation study. Biochem J. 1994;297:415–423. doi: 10.1042/bj2970415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wier WG, Balke CW. Ca2+ release mechanisms, Ca2+ sparks, and local control of excitation-contraction coupling in normal heart muscle. Circ Res. 1999;85:770–776. doi: 10.1161/01.res.85.9.770. [DOI] [PubMed] [Google Scholar]
- Wier WG, Morgan K. α1-adrenergic signalling mechanisms in contraction of resistance arteries. Rev Physiol Biochem Pharmacol. 2003;150:91–139. doi: 10.1007/s10254-003-0019-8. [DOI] [PubMed] [Google Scholar]
- Wier WG, Zang WJ, Lamont C, Raina H. Sympathetic neurogenic Ca2+ signalling in rat arteries: ATP, noradrenaline and neuropeptide Y. Exp Physiol. 2009;94:31–37. doi: 10.1113/expphysiol.2008.043638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray S, Burdyga T. Sarcoplasmic reticulum function in smooth muscle. Physiol Rev. 2010;90:113–178. doi: 10.1152/physrev.00018.2008. [DOI] [PubMed] [Google Scholar]
- Zhang WM, Yip KP, Lin MJ, Shimoda LA, Li WH, Sham JS. ET-1 activates Ca2+ sparks in PASMC: local Ca2+ signaling between inositol trisphosphate and ryanodine receptors. Am J Physiol. 2003;285:L680–L690. doi: 10.1152/ajplung.00067.2003. [DOI] [PubMed] [Google Scholar]
- Zhao X, Cook AK, Field M, Edwards B, Zhang S, Zhang Z, et al. Impaired Ca2+ signaling attenuates P2X receptor-mediated vasoconstriction of afferent arterioles in angiotensin II hypertension. Hypertension. 2005;46:562–568. doi: 10.1161/01.HYP.0000179584.39937.41. [DOI] [PubMed] [Google Scholar]
- ZhuGe R, Sims SM, Tuft RA, Fogarty KE, Walsh JV., Jr Ca2+ sparks activate K+ and Cl- channels, resulting in spontaneous transient currents in guinea-pig tracheal myocytes. J Physiol. 1998;512:711–718. doi: 10.1111/j.1469-7793.1998.711ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZhuGe R, Bao R, Fogarty KE, Lifshitz LM. Ca2+ sparks act as potent regulators of excitation-contraction coupling in airway smooth muscle. J Biol Chem. 2010;285:2203–2210. doi: 10.1074/jbc.M109.067546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zizzo MG, Mulè F, Serio R. Mechanisms underlying hyperpolarization evoked by P2Y receptor activation in mouse distal colon. Eur J Pharmacol. 2006;544:174–180. doi: 10.1016/j.ejphar.2006.06.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


