Abstract
Background
Primary sclerosing cholangitis results in elevated but fluctuating serum alkaline phosphatase levels that occasionally return to normal.
Aims
To investigate the frequency of normalization of alkaline phosphatase in newly diagnosed primary sclerosing cholangitis patients and the subsequent clinical outcomes.
Methods
Records of newly diagnosed primary sclerosing cholangitis patients were examined retrospectively for laboratory values and clinical end points (cholangiocarcinoma, liver transplantation and death) within 10 years of diagnosis. Data from a recent prospective ursodeoxycholic acid treatment trial were also studied.
Results
Eighty-seven patients met the inclusion criteria. Normalization of alkaline phosphatase was seen in 35 (40%) patients. Five (14%) patients with normalization reached an end point whereas 17 (33%) of the patients with persistent elevation reached an end point (P=0.02). Ursodeoxycholic acid was used similarly by both groups. When the investigative criteria were applied to a prospective trial, there was again a significant relationship between normalization of alkaline phosphatase and survival in patients receiving ursodeoxycholic acid (P<0.01) and the placebo group (P=0.02).
Conclusions
Serum alkaline phosphatase was found to normalize in a high proportion of newly diagnosed primary sclerosing cholangitis patients. This was significantly associated with a better prognosis in a retrospective cohort and when data from a prospective treatment trial was evaluated.
Keywords: alkaline phosphatase, primary sclerosing cholangitis, ursodeoxycholic acid
INTRODUCTION
Primary sclerosing cholangitis (PSC) is a chronic, progressive cholestatic liver disease. It is characterized by fibrosis of the intrahepatic and extrahepatic biliary tree leading to bile duct strictures in the absence of other causes.1 PSC currently has a median survival of approximately 17 years after diagnosis, primarily due to progression to end-stage liver disease.2 Medical therapy has proven disappointing, with no currently available agents shown to improve survival. Liver transplantation is the only treatment option to improve long-term prognosis, with 10-year survival rates of 70% after transplant.3 As such, natural history models have been developed and validated in order to predict prognosis of individual patients. The model developed at the Mayo Clinic incorporates age, bilirubin, albumin, aspartate aminotransferase (AST) and history of variceal bleeding.4
Ursodeoxycholic acid (UDCA) has been studied extensively for use in PSC and primary biliary cirrhosis (PBC). UDCA therapy has been correlated with improved 10-year survival in PBC.5 Recent work has shown that PBC patients with a biochemical response to UDCA, usually defined as a reduction in the alkaline phosphatase (ALP) level, have higher survival rates than non-responders.6,7 This improvement in survival has not been reported in PSC, despite UDCA improving laboratory values including ALP.8 This has been consistent at varying dosing levels.9,10
Persistent elevation of ALP is the laboratory finding that often prompts the investigations that result in the diagnosis of PSC, especially in the setting of inflammatory bowel disease. As such, it is routinely measured during clinical follow up and generally remains abnormal;11 however it will occasionally normalize spontaneously.12,13 It was unclear if this was meaningful in regards to prognosis. The aim of this study was to determine the frequency of normalization of ALP in patients with PSC and investigate whether this is associated with liver transplant-free survival.
METHODS
After approval from the appropriate institutional review board, electronic medical records from the Mayo Clinic in Rochester, MN were retrospectively reviewed for all patients coded for the first time with a diagnosis of “sclerosing cholangitis” from January 1, 1997 to December 31, 2001. Only patients that received a new diagnosis of PSC at our center within the time period and had at least 1 year of clinical follow-up were considered eligible for inclusion. The criteria used to diagnose PSC were the presence of cholestatic liver disease and characteristic findings of biliary duct dilatation and narrowing on a cholangiographic investigation that was reviewed by a radiologist or gastroenterologist at our institution. Other causes of elevated serum ALP and sclerosing cholangitis were excluded at the time of diagnosis.
Exclusion criteria included age less than 18 years at diagnosis, a previous diagnosis of a chronic liver disease or a prior liver transplant, evidence of cholangiocarcinoma or need for liver transplantation within 1 year of diagnosis and death prior to 1 year of follow-up.
The data compiled from the medical record included gender, age at diagnosis, cholangiogram results and whether stricture dilatation was performed, liver biopsy at diagnosis and the corresponding Ludwig stage14 if one was performed, concomitant inflammatory bowel disease and presence of symptoms attributable to PSC (pruritus, abdominal pain and fatigue severe enough to limit activities). Laboratory values performed at our institution were also collected; this included serum ALP, AST, total bilirubin, albumin and international normalized ratio (INR). The use of UDCA and the highest dosing level in mg/kg were recorded. End points considered were the development of cholangiocarcinoma, liver transplantation or death if they occurred within 10-years after the initial diagnosis. Times to end point were calculated from the date of the diagnostic cholangiogram to the date of the procedure that diagnosed the cholangiocarcinoma, the date of the liver transplant procedure or the date of death.
ALP levels were compared to the upper limits of normal (ULN) for all laboratory testing performed in the study period and calculated as a ratio. ALP was considered to be persistently abnormal if the levels were continuously greater than a ratio of 1 and considered to normalize if there were one or more values with a ratio of 1 or less.
These criteria were also applied to data collected in a recent PSC treatment trial.10 This was a prospective, double-blinded trial of high-dose UDCA (28 to 30 mg/kg/day). Liver enzymes were assessed every 3 months over 3 years in a standardized fashion. Similar primary endpoints were utilized as in the current study, with the additional consideration of development of varices and progression to cirrhosis as clinical endpoints
Statistics
Median and inter-quartile range was calculated for all initial characteristics and laboratory values of the included patients. When presented, median values are followed by the inter-quartile range parenthetically unless otherwise noted. As the ULN for ALP varied due to a change in assays during the study period, all values presented were calculated as a ratio with the test result compared to ULN. For comparison of baseline characteristics and biochemical values, Pearson chi-square tests were used for binomial values and Wilcoxon rank-sum tests were used for continuous variables where appropriate.
Event-free survival was estimated by the Kaplan-Meier method and compared between the group with normalization of ALP levels and the group with persistently abnormal levels using the Wilcoxon log-rank test. Data on patients that did not reach an end point were censored at the time of their last follow-up visit or 10-years after their diagnosis, dependent on which was earlier. Kaplan-Meier analysis was also used to determine time to ALP normalization and compared between the group that received UDCA and the group that did not using the Wilcoxon log-rank test. Cox proportional-hazards regression modelling was performed to evaluate covariates (initial levels of ALP, AST, total bilirubin and Mayo risk score) that may have influenced time until endpoint or normalization as indicated. Initial biopsy scores were not included in this analysis as the data were not available for more than 10% of patients. The association between normalization and survival in the UDCA treatment trial data was assessed statistically using Fischer's exact test.
All statistical testing was done at the conventional two-tailed level of 0.05.
RESULTS
A total of 87 patients met the inclusion criteria and were incorporated in the study. The clinical features and biochemical findings of the patient population at the time of entry are included in Table 1. The median follow-up time was 7.3 years (4.5 - 8.9). A total of 22 (25%) reached a clinical end point within the study period, with 5 (6%) developing cholangiocarcinoma, 10 (11%) undergoing a liver transplant and 7 (8%) dying without cholangiocarcinoma or transplantation.
Table 1.
Clinical characteristics and initial laboratory values of the study population. Alkaline phosphatase results are shown as a ratio compared to upper limit of normal range for each assay. Normal ranges for laboratory values were: aspartate aminotransferase 8-48 U/L, total bilirubin 0.1-1 mg/dL, international normalized ratio 0.9-1.2 and albumin 3.5-5 g/dL.
| Patients | ||
|---|---|---|
| n = 87 | ||
| Male sex, n (%) | 64 | ( 74 ) |
| Follow-up years* | 7.3 | (4.5 - 8.9) |
| Age at diagnosis* | 44 | (33 - 52) |
| Symptomatic at diagnosis, n (%) | 37 | ( 43 ) |
| Inflammatory bowel disease, n (%) | 66 | ( 76 ) |
| Biopsy at diagnosis, n (%) | 65 | ( 75 ) |
| Biopsy stage* | 2 | (1 - 3) |
| Laboratory values at diagnosis: | ||
| Alkaline phosphatase (ULN ratio)* | 2.8 | (1.8 - 4.6) |
| AST (U/L)* | 74 | (44.5 - 125) |
| Total bilirubin (mg/dL)* | 1.1 | (0.7 - 1.8) |
| INR* | 1 | (0.9 - 1) |
| Albumin (g/dL)* | 4 | (3.7 - 4.3) |
| Initial MELD score* | 8 | (7 - 10.5) |
| Initial Mayo risk score* | 0.12 | (0 - 0.84) |
AST, aspartate aminotransferase; INR, international normalized ratio; MELD, model for end-stage liver disease; ULN, upper limit of normal.
Median with inter-quartile range included parenthetically.
Normalization of ALP was observed in 35 (40%) of the patients. The median time to normalization was 1.03 years (0.35 - 3.95). There were no significant differences in sex, age, concomitant inflammatory bowel disease or the presence of symptoms due to PSC between the groups (Table 2). Twenty-five (71%) of those that normalized had 3 or more serum ALP assays within normal range during their follow-up period, with a median of 5 (2 - 12). Six of the patients that normalized had persistently normal ALP values during the remainder of their follow-up period, with a median of 4 assays (4 - 7) over a median of 3.5 years (2.4 - 5.1).
Table 2.
Clinical characteristics and laboratory values at the initial visit of patients with and without alkaline phosphatase normalization. Significantly different P values are noted in bold. Alkaline phosphatase results are shown as a ratio compared to upper limit of normal range for each assay. Normal ranges for laboratory values were: aspartate aminotransferase 8-48 U/L, total bilirubin 0.1-1 mg/dL, international normalized ratio 0.9-1.2 and albumin 3.5-5 g/dL.
| Normalized | Persistently abnormal | P values | |||
|---|---|---|---|---|---|
| n = 35 | n = 52 | ||||
| Male sex, n (%) | 25 | ( 71 ) | 39 | ( 75 ) | 0.7 |
| Follow-up years* | 8.3 | (6.8 - 9.6) | 6.8 | (3.4 - 8.6) | |
| Age at diagnosis* | 43 | (33 - 51) | 45 | (33 - 52) | 0.98 |
| Symptomatic at diagnosis, n (%) | 18 | ( 51 ) | 19 | ( 37 ) | 0.16 |
| Inflammatory bowel disease, n (%) | 28 | ( 80 ) | 38 | ( 73 ) | 0.45 |
| Biopsy at diagnosis, n (%) | 26 | ( 74 ) | 39 | ( 75 ) | 0.94 |
| Biopsy stage* | 1 | (1- 2) | 2 | (2 - 3) | <0.01 |
| Laboratory values: | |||||
| Alkaline phosphatase (ULN ratio)* | 2 | (1.1 - 3.2) | 3.4 | (2.4 - 5) | <0.01 |
| AST (U/L)* | 47 | (33 - 100) | 102 | (60 - 140) | <0.01 |
| Total bilirubin (mg/dL)* | 0.8 | (0.6 - 1.5) | 1.2 | (0.8 - 2.2) | 0.04 |
| INR* | 1 | (0.9 - 1) | 1 | (0.9 - 1.1) | 0.41 |
| Albumin (g/dL)* | 4.1 | (3.8 - 4.4) | 3.9 | (3.6 - 4.2) | 0.13 |
| Initial MELD score* | 8 | (6 - 9) | 8 | (7 - 11) | 0.16 |
| Initial Mayo risk score* | 0 | (0 - 0.4) | 0.41 | (0 - 1.07) | <0.01 |
| Treated with UDCA, n (%) | 18 | ( 51 ) | 29 | ( 56 ) | 0.69 |
| Treated with high dose UDCA#, n (%) | 5 | ( 14 ) | 7 | ( 13 ) | 0.78 |
| Treated with stricture dilatation, n (%) | 15 | ( 43 ) | 30 | ( 58 ) | 0.17 |
AST, aspartate aminotransferase; INR, international normalized ratio; MELD, model for end-stage liver disease; UDCA, ursodeoxycholic acid; ULN, upper limit of normal.
Median with inter-quartile range included parenthetically.
High dose UDCA was dosing greater than 25 mg/kg.
Patients with normalization of ALP during their follow-up period had a median ALP ratio of 2 (1.1 - 3.2) at the time of diagnosis compared to 3.4 (2.4 - 5) in the group that did not normalize (P<0.01). Patients with normalization of ALP also had significantly lower AST (47 (33 - 100) vs. 102 (60 - 140), P<0.01) and total bilirubin values (0.8 (0.6 - 1.5) vs. 1.2 (0.8 - 2.2), P=0.04) at diagnosis. Mayo risk score was significantly lower in the normalization group (0 (0 - 0.4) vs. 0.41 (0 - 1.07), P<0.01) when calculated with the laboratory values obtained at the time of diagnosis. In those that underwent biopsy, the median Ludwig stage was lower in the normalization group than in the persistently abnormal group (1 (1 - 2) vs. 2 (2 - 3), P<0.01). Other parameters were not significantly different (Table 2).
Altogether, 5 (14%) patients with normalization reached an end point, with 1 (3%) developing cholangiocarcinoma, 1 (3%) receiving a liver transplant after progression to end-stage liver disease and 3 (9%) dying without cholangiocarcinoma or transplantation. The deaths were attributed to metastatic colon cancer in one patient, oligoastrocytoma in one patient and the cause of death was not available for one patient. None of the 6 patients with persistently normal ALP experienced an end point. Seventeen (33%) of the patients with persistent abnormalities of ALP levels reached an end point, with 4 (8%) developing cholangiocarcinoma, 9 (17%) receiving a liver transplant due to progression to end-stage liver disease and 4 (8%) dying without cholangiocarcinoma or transplantation. The deaths were attributed to hepatocellular carcinoma in one patient, adenocarcinoma of the lung in one patient and the cause of death was not available for two patients.
Kaplan Meier analysis revealed that patients with normalization have a significantly lower rate of reaching an end point than patients with persistent abnormalities (P= 0.02, Figure 1). Cox regression analysis demonstrated that this remained significant (hazard ratio 0.215, P<0.01) after adjustment for initial levels of ALP, AST, total bilirubin and Mayo risk score. When patients with biopsy-proven stage 3 or 4 fibrotic disease were excluded from analysis, patients with normalization of ALP maintained a significantly lower rate of reaching end point by Kaplan Meier analysis (P= 0.012).
Figure 1.
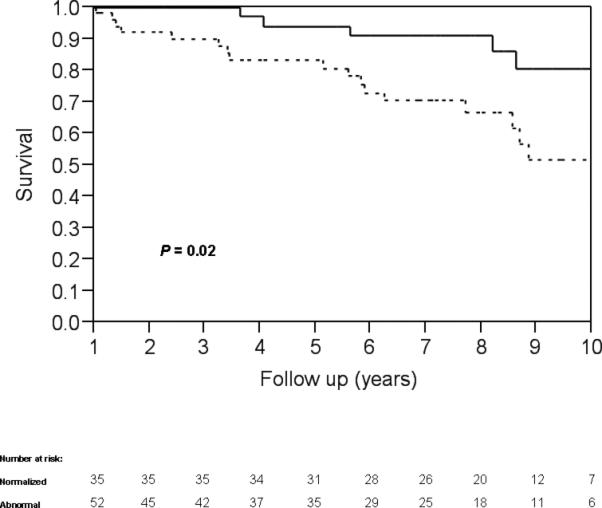
Kaplan-Meier analysis of end point-free survival for patients that experienced normalization of alkaline phosphatase (solid line) and patients with persistently abnormal alkaline phosphatase (dashed line) demarcated by years (P=0.02).
UDCA therapy was initiated in 47 (54%) patients in the study group. Twelve (14%) received high dose UDCA regimens of greater than 25 mg/kg. Neither UDCA use at any dose (18 (51%) vs. 29 (56%), P=0.69) nor the proportion on a high-dose regimen (5 (14%) vs. 7 (13%), P=0.78) was found to be different between the patients that normalized and those that did not (Table 2). There was not a significant difference in time to normalization with use of UDCA (P=0.48). Cox regression modeling supported this (hazard ratio 1.29, P=0.48). In addition, there was not a significant difference in survival between patients that spontaneously normalized or those that normalized with UDCA by Kaplan Meier analysis (P=0.54).
There were 104 endoscopic dilatations performed on patients with abnormal ALP, and 7 (7%) resulted in normalization of ALP within 3 months of the procedure. Endoscopic dilatation of strictures during cholangiogram was performed in a lower percentage of patients with normalization of ALP than in those with persistently abnormal values, but this did not reach statistical significance (15 (43%) vs. 30 (58%), P=0.17). Nine (26%) patients with normalization received combination UDCA therapy and endoscopic dilatation and 18 (35%) patients without normalization received combination therapy (P=0.38).
ALP levels were noted to improve in both the UDCA and placebo branches in the high-dose UDCA trial. The UDCA treatment group improved from mean and range ALP ratio of 3.3 (0.7 - 11.2) to 1.7 (0.6 - 16.5) and the placebo group improved from 3.2 (0.5 - 16.9) to 2.4 (0.4 - 12.1). Using the criteria of normalization of ALP, there was a significant association between normalization and decreased endpoints in both the UDCA and placebo branches. Within the UDCA treatment group, 4/20 (20%) with normalization of ALP reached an endpoint while 27/56 (48%) with persistent abnormality reached an endpoint (P<0.01). Similar findings were noted in the placebo group, where 1/12 (8%) with normalization reached an endpoint while 26/61 (43%) with persistent abnormality reached an end-point (P=0.02).
DISCUSSION
ALP is a routine laboratory test used to screen for liver disease and elevated levels are often the first indication of PSC. ALP values can follow a variable course during the progression of PSC, with values occasionally dipping below the upper limit of normal.12,13 To our knowledge, the clinical relevance of this has not previously been explored. We found that a high proportion of newly diagnosed patients will experience normalization of serum ALP and that this correlated with an improved 10-year prognosis.
Normalization of ALP occurred in 40% of the study cohort. This is much higher than a prior Mayo Clinic assessment, which found that 3% of the PSC population diagnosed between 1979 and 1987 had normal ALP levels.13 We found that normalization of ALP was more common soon after diagnosis. In the intervening years between the studies, there has been an increased awareness of PSC and an increased frequency of cholangiography performance. With this in mind, the overarching reason for the increased prevalence in the current study population is most likely the exclusion of previously defined PSC, thus focusing our study on patients earlier in their disease course than those included in the prior cohort.
Patients with normalization of ALP had a significantly lower incidence of adverse clinical end points during 10-years of clinical follow-up. This was primarily related to a lower number of liver transplantations for end-stage liver disease, although the subsets of endpoints were not large enough to compare statistically. When the initial patient characteristics were examined, the normalization group had significantly lower ALP, AST and bilirubin levels. Importantly, normalization of ALP was still an indicator of improved prognosis when the other laboratories were adjusted for statistically. Mayo risk scores were also lower at the time of diagnosis, indicating that patients with milder disease were more likely to have normal ALP levels. Thus, normalization of ALP seems to be a marker for patients diagnosed early in their disease course or that are less likely to rapidly progress to end-stage liver disease.
Because UDCA has been shown to decrease ALP levels in PSC,8-10 it was important to assess the effect of this medication on the study subjects. UDCA therapy was used by a similar proportion of patients in both retrospective groups; this included a similar number of patients on high-dose regimens. The normalization of ALP was not greatly affected by UDCA treatment. Only 51% of those that normalized were treated with UDCA and there was not a significant difference in the rate of normalization with or without treatment. As expected when past studies of UDCA treatment in PSC are considered,8-10 UDCA was not shown to affect survival between patients that normalized spontaneously and those that normalized with UDCA therapy. This suggests that the association observed between ALP normalization and prognosis was not being driven by treatment with UDCA.
Fluctuations of ALP have been shown to occur independently of the presence of dominant strictures on cholangiogram.15 In one study, however, dilatation of dominant strictures decreased ALP values by 17% in PSC patients being treated with UDCA.16 In our population, the finding of strictures and subsequent dilatation was rarely associated with normalization of ALP. This suggests that endoscopic dilatations may have decreased ALP levels slightly, but the effect was not of sufficient magnitude to result in detected normalization. Thus, endoscopic therapy of strictures was not the underlying cause of the normalization of ALP in our study population.
The combination of UDCA and endoscopic dilatation has been shown to improve survival compared to predicted survival rates.17 In our population, both the patients with and without normalization of ALP had similar rates of UDCA therapy, endoscopic dilatation and combination of UDCA therapy and endoscopic dilatation. It does not seem that these therapies alone or in combination were directly responsible for the improved prognosis in patients with ALP normalization.
This study was limited by its retrospective nature, which did not allow for all subjects to have standardized appointment schedules or laboratory testing intervals. Limiting the study population to patients that were followed at a single center for at least 1 year should help diminish this concern.
To bolster the results of this study, the investigative criteria were also applied to data compiled from a recent prospective, double-blinded treatment trial with standard assessment of liver enzymes over a 3 year period.10 The primary analysis of this data did not reveal an association between a biochemical response and survival when laboratories were compared between those that reached endpoints and those that did not at the conclusion of the trial. However, when the data were reassessed using the protocol of normalization of ALP investigated in the current study, there was a significant association between normalization and better prognosis in both the UDCA treatment group and the placebo group. This supports the relationship observed in our retrospective cohort and should further alleviate concern regarding difference in laboratory or follow-up appointment frequency.
The association between normalization of ALP and better prognosis should be considered when designing and analyzing future trials in PSC. This may be a marker of disease diagnosed early in its course that is likely to remain quiescent. It may also be indicative of a treatment end point for medical therapy, showing benefit if a normal serum ALP can be achieved. This deserves to be investigated further as new regimens or therapies are developed and studied. In addition, the finding that the normalization of ALP levels were associated with improved prognosis is beneficial for patient care. While the Mayo risk score has been validated as a surrogate marker of survival, it is a complex formula that results in a number that may be difficult to understand for the average patient. However, a laboratory value returning to the normal range is intuitive and easy to explain to patients. This will hopefully allow physicians to improve their ability to counsel patients in a manner that is easy to understand and retain.
In summary, we found that a high proportion of newly diagnosed PSC patients experienced normalization of their ALP. This was associated with a better prognosis, likely secondary to decreased indications for liver transplantation. These findings were supported by similar results from a prospective trial patient cohort. This should be considered in the design and analysis of future studies involving PSC clinical outcomes and therapies.
Acknowledgments
Disclosures: This work was partially supported by NIH grant DK56924 and Axcan Pharma. The authors’ have no relevant conflicts of interest to disclose.
Abbreviations
- ALP
Alkaline phosphatase
- AST
Aspartate aminotransferase
- PBC
Primary biliary cirrhosis
- PSC
Primary sclerosing cholangitis
- UDCA
Ursodeoxycholic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thorpe MEC, Scheuer PJ, Sherock S. Primary sclerosing cholangitis, the biliary tree and ulcerative colitis. Gut. 1967;8:435–448. doi: 10.1136/gut.8.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bambha K, Kim WR, Talwalkar J, et al. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology. 2003 Nov;125(5):1364–1169. doi: 10.1016/j.gastro.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Graziadei IW, Wiesner RH, Marotta PJ, et al. Long-term results of patients undergoing liver transplantation for primary sclerosing cholangitis. Hepatology. 1999;30:1121–1127. doi: 10.1002/hep.510300501. [DOI] [PubMed] [Google Scholar]
- 4.Kim WR, Therneau TM, Wiesner RH, et al. A revised natural history model for primary sclerosing cholangitis. Mayo Clinic Proceedings. 2000;75:688–694. doi: 10.4065/75.7.688. [DOI] [PubMed] [Google Scholar]
- 5.Poupon RE, Bonnand AM, Chrétien Y, et al. Ten-year survival in ursodeoxycholic acid-treated patients with primary biliary cirrhosis. The UDCA-PBC Study Group. Hepatology. 1999 Jun;29(6):1668–1671. doi: 10.1002/hep.510290603. [DOI] [PubMed] [Google Scholar]
- 6.Pares A, Caballeria L, Rodes J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology. 2006;130:715–720. doi: 10.1053/j.gastro.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 7.Kuiper EM, Hansen BE, de Vries RA, et al. Improved prognosis of patients with primary biliary cirrhosis that have a biochemical response to ursodeoxycholic acid. Gastroenterology. 2009;136(4):1281–1287. doi: 10.1053/j.gastro.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. N Engl J Med. 1997;336:691–695. doi: 10.1056/NEJM199703063361003. [DOI] [PubMed] [Google Scholar]
- 9.Olsson R, Boberg KM, de Muckadell OS, et al. High-dose ursodeoxycholic acid in primary sclerosing cholangitis: a 5-year multicenter, randomized, controlled study. Gastroenterology. 2005;129:1464–1472. doi: 10.1053/j.gastro.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 10.Lindor KD, Kowdley KV, Luketic VA, et al. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology. 2009;50(3):808–814. doi: 10.1002/hep.23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiesner RH, LaRusso NF. Clinicopathologic features of the syndrome of primary sclerosing cholangitis. Gastroenterology. 1980;79:200–206. [PubMed] [Google Scholar]
- 12.Olsson R, Broomé U, Danielsson A, et al. Spontaneous course of symptoms in primary sclerosing cholangitis: relationships with biochemical and histological features. Hepatogastroenterology. 1999;46(25):136–141. [PubMed] [Google Scholar]
- 13.Balasubramaniam K, Wiesner RH, LaRusso NF. Primary sclerosing cholangitis with normal serum alkaline phosphatase activity. Gastroenterology. 1990;98(6):1567–1571. doi: 10.1016/0016-5085(88)90378-2. [DOI] [PubMed] [Google Scholar]
- 14.Ludwig J, Dickson ER, McDonald GSA. Staging of chronic nonsuppurative destructive cholangitis (syndrome of primary biliary cirrhosis). Virchows Arch A Pathol Anat Histol. 1978;379:103–112. doi: 10.1007/BF00432479. [DOI] [PubMed] [Google Scholar]
- 15.Björnsson E, Lindqvist-Ottosson J, Asztely M, et al. Dominant strictures in patients with primary sclerosing cholangitis. Am J Gastroenterol. 2004 Mar;99(3):502–8. doi: 10.1111/j.1572-0241.2004.04106.x. [DOI] [PubMed] [Google Scholar]
- 16.Stiehl A, Rudolph G, Klöters-Plachky P, et al. Development of dominant bile duct stenoses in patients with primary sclerosing cholangitis treated with ursodeoxycholic acid: outcome after endoscopic treatment. J Hepatol. 2002 Feb;36(2):151–6. doi: 10.1016/s0168-8278(01)00251-3. [DOI] [PubMed] [Google Scholar]
- 17.Stiehl A, Rudolph G, Sauer P, et al. Efficacy of ursodeoxycholic acid treatment and endoscopic dilation of major duct stenoses in primary sclerosing cholangitis. An 8-year prospective study. J Hepatol. 1997 Mar;26(3):560–6. doi: 10.1016/s0168-8278(97)80421-7. [DOI] [PubMed] [Google Scholar]


