Abstract
This study was designed to determine the potential mechanism/mechanisms of previously observed enhanced fetal cortisol secretion following exposure to long-term hypoxia (LTH). Pregnant ewes were maintained at high altitude (3820 m) for approximately the last 100 days of gestation. Between the gestation days of 138 and 141, adrenal glands were collected from LTH and age-matched normoxic control fetuses. Cyclic adenosine monophosphate (cAMP), cortisol, and steroidogenic acute regulatory (StAR) protein were measured in response to adrenocorticotropic hormone (ACTH) stimulation. Cortisol responses to ACTH were also measured in the presence of the protein kinase (PKA) inhibitor H-89, proopiomelanocortin (POMC), or 22-kDa pro-ACTH. Cortisol output was higher in the LTH group compared to the control (P < .05), following ACTH treatment while the cAMP response was similar in both groups. Although PKA inhibition decreased cortisol production in both groups, however no differences were observed between groups. Western analysis revealed a significant increase in protein expression for StAR in the LTH group (P < .05, compared to control). Proopiomelanocortin and 22-kDa pro-ACTH did not alter the cortisol response to ACTH treatment. Results from the present study taken together with those of previous in vivo studies suggest that the enhanced cortisol output in the LTH group is not the result of differences in cAMP generation or PKA. We conclude that enhanced cortisol production in LTH adrenals is the result of enhanced protein expression of StAR and potential downstream signaling pathways.
Keywords: 22-kDa pro-ACTH, ACTH, cAMP, PKA, StAR, POMC
Introduction
The hypothalamic-pituitary-adrenal (HPA) axis is a key component of the stress response during fetal development governing the production of cortisol from the adrenal cortex. Cortisol plays a pivotal role in lipolysis, glycogenolysis, and protein catabolism, and in fetal sheep increased fetal adrenal cortisol biosynthesis during the last 3 weeks of gestation is essential for organ maturation. The classic studies of Liggins1 showed fetal cortisol also play an important role in the initiation of parturition in this species. However, prematurely elevated levels of glucocorticoids can have negative effects on the fetus by suppressing anabolic processes resulting in muscle atrophy and delayed maturation and organ growth.2 Therefore, regulation of adrenal cortisol biosynthesis must be well coordinated for effective responses to stress as well as to allow effective organ maturation and successful timing of delivery.
A wide range of stressors activates the fetal HPA axis, one of the most potent being hypoxia. Although much is known about the effects of acute hypoxia on fetal HPA function,3–6 very little is known about the effects of long-term hypoxia (LTH) on the fetal HPA axis including cortisol biosynthesis. Using a unique model of high-altitude hypoxia during gestation in sheep, our laboratory has demonstrated a significant number of adaptive changes in the fetal HPA axis response to LTH. We have shown that LTH increases the processing of proopiomelanocortin (POMC) to adrenocorticotropic hormone (ACTH) in the anterior pituitary of fetal sheep near term,7 resulting in elevated basal plasma ACTH1-39 in the LTH fetuses compared to the normoxic control group. In addition, the LTH fetuses also exhibited elevated circulating levels of the ACTH precursors (POMC and 22-kDa pro-ACTH). Despite higher levels of basal plasma ACTH1-39, basal plasma cortisol concentrations in the LTH group remained the same as control and were accompanied with a decreased adrenocortical expression of key steroidogenic enzymes, P450 cholesterol side chain cleavage (CYP11A1), and P450 17α-hydroxylase (CYP17), in the LTH fetuses when compared to the normoxic controls.8 Schwartz et al,9 showed that POMC and 22-kDa pro-ACTH exert a significant inhibitory effect on ACTH-induced cortisol synthesis in ovine fetal adrenal cortical cells (FACs) in vitro. These data suggest that under basal conditions, the elevated levels of ACTH precursors play a role in maintaining normal cortisol secretion despite elevated ACTH levels in the LTH fetus. However, despite these differences, LTH fetuses demonstrate an enhanced cortisol response to a secondary stressor such as umbilical cord occlusion10 or hypotension11 compared to normoxic controls and as such are able to overcome a putative inhibitory signal governing the adaptive basal adrenocortical function.
A key question arising from these studies is what is the mechanism that is responsible for this adaptation observed in the LTH fetus at the level of the adrenal gland? ACTH, via cyclic 3,5-adenosine mono phosphate (cAMP) and its protein kinase (PKA), initiates steroidogenesis by liberating cholesterol via cholesterol esterase. Protein kinase also activates steroidogenic acute regulatory (StAR) protein, transferring cholesterol to the inner mitochondrial membrane where CYP11A1 (P450 side chain cleavage) metabolizes cholesterol to pregnenolone, the initial limiting step in steroidogenesis.12,13 We designed the present study to test the hypothesis that the enhanced cortisol output following a secondary stressor in LTH fetuses is the result of enhanced activity of the cyclic cAMP), PKA, or the StAR protein pathway. We also determined whether the LTH alters FAC sensitivity to the ACTH precursors POMC and 22-kDa pro-ACTH.
Methods
Animals Procedures
All procedures were conducted with approval of the Institutional Animal Care and Use Committees (Loma Linda University School of Medicine, Loma Linda, California). Pregnant sheep were transported to Barcroft Laboratory White Mountain Research Station in Bishop, California, at an elevation of 3820 m at ~day 40 of gestation and maintained at this facility to near term (term = 146 days) at which time they were transferred to Loma Linda University. Upon arrival, hypoxia was maintained in these animals by nitrogen infusion through a maternal tracheal catheter to maintain maternal arterial Po 2 levels similar to that observed at high altitude (~60 mm Hg) as previously described.10,11 On days 138 to 141 of gestation both normoxic control and LTH ewes were sedated with pentobarbital sodium, intubated, and maintained under general anesthesia with 1.5% to 2% halothane in oxygen. Fetuses were then delivered through a midline laparoptomy, and the fetal adrenal glands were collected in ice-cold media M-199 (Sigma-Aldrich, St. Louis, MO), containing 2.2 g sodium bicarbonate, 2.0 g bovine serum albumin, and 0.1 g l-glutamine.
Adrenocortical Cell Dispersion
The adrenal cortex was separated from the medulla, enzymatically dispersed in 0.4% collagenase solution containing 40 mg collagenase Type II (Worthington Biomedical, Lakewood, NJ), 40 mg of Polypep bovine protein digest (Sigma), and 100 µL of DNase I (Type IV) from Sigma-Aldrich (dissolved in 10 mL of sodium Krebs buffer). Following dispersion, FACs were aliquoted (2.5× 105 cells) into individual tubes or 24-well cell culture plates with media (M-199) and allowed to equilibrate for 2 hours prior to initiation of each study as required by each experimental protocol. Cell viability was confirmed by Trypan blue exclusion.
Cyclic 3,5-Adenosine Mono Phosphate and Cortisol Responses to ACTH
For assessment of cortisol responses to ACTH, FACs, in duplicate, were treated with 10 nmol/L ACTH, and cells and media were collected at 0 (baseline), 15, 30, and 60 minutes after treatment, frozen in liquid nitrogen, and stored at −80°C until determination of cAMP and cortisol, respectively.
Effects of PKA Inhibition on Cortisol Biosynthesis
The role of the cAMP-dependent PKA on cortisol biosynthesis was determined by pretreating FACs (2.5 × 105 cells/well; in triplicate) with the PKA inhibitor, H-89 (5 μmol/L) for 1 hour followed by ACTH treatment (100 pmol/L). Media samples were collected at 0 minutes (baseline) and 1 hour after addition of ACTH. All media samples were frozen in liquid nitrogen for determination of cortisol.
Effects of POMC and 22-kDa Pro-ACTH on Cortisol Biosynthesis
Recombinant ovine POMC and 22-kDa pro-ACTH were produced as previously described.14 Fetal adrenal cortical cells (2.5 × 105 cells/well; in triplicate) were either untreated or treated with ACTH (100 pmol/L), POMC (200 pmol/L), or 22-kDa pro-ACTH (200 pmol/L) alone or ACTH plus either POMC or 22-kDa pro-ACTH. Media samples were collected at time 0 (baseline) and 1 hour after treatments and then frozen in liquid nitrogen for determination of cortisol.
Cyclic 3,5-Adenosine Mono Phosphate Analysis
Upon retrieval from −80°C, tubes containing FACs were thawed in ice/water and the media was carefully removed from the pelleted cells. Tissue was homogenized using a pestle mortar for 3 minutes/sample, on ice, adding 100 µL ice-cold 6% trichloroacetic acid (TCA) in 1.5 mL polypropylene tubes. Following homogenization, the tube and pestle tip were rinsed with 200 µL 6% TCA (2×) to obtain a final total volume of 600 µL. Samples were incubated for an additional 45 minutes. At the end of the incubation step, the samples were centrifuged at 2000g for 15 minutes at 4°C, the supernatant recovered, and transferred to 50 mL conical tubes. The supernatant was extracted 3 times with 5 parts of water saturated diethyl ether. The upper ether layer was aspirated and discarded after each wash. After the ether was fully evaporated from the sample, 400 µL of the supernatant was transferred into polypropylene tubes and dried under a stream of nitrogen at 60°C. Samples were stored at −80°C until the day of analysis. Cyclic 3,5-adenosine mono phosphate was analyzed using a cAMP (125I) assay system nonacetylation procedure radioimmunoassay (RIA) from GE HealthCare (Piscataway, NJ). On the day of assay, the dried extracts were reconstituted in 1000 µL volume of assay buffer prior to analysis and the procedures for cAMP determination were followed per manufacture’s instructions.
Cortisol Assay
Cortisol was measured using a commercially available enzyme-linked immunosorbent assay (ELISA) cortisol Kit (Oxford Biomedical Research, Oxford, MI) that has been previously described and validated for use in our laboratory.15,16
Western Blot Analysis of StAR Protein
Steroidogenic acute regulatory protein was analyzed from FACs collected at 0 (baseline) and 60 minutes for both normoxic control and LTH groups, as described above. Fetal adrenal cortical cells were lysed in 100 μL of lysis buffer (97.9% prelysis buffer [150 mmol/L NaCL, 50 mmol/L Tris-HCL, 10 mmol/L EDTA, 0.01% Tween-20, 0.01% β-mercaptoethanol], 1.67% phenylmethanesulfonylfluoride [PMSF], 0.2% leupeptin, 0.1% DTT, 0.04% aprotinin [Sigma, St Louis, Missouri]) and left on ice for 40 minutes. Homogenates were centrifuged at 15 000g for 15 minutes at 4°C and the resulting supernatants aliquoted and stored at –80°C. Protein concentration was determined using a bicinchoninic acid (BCA) protein assay (Pierce Bioreagents, Rockford, Illinois) with bovine serum albumin as the standard. Absorbance was measured at 595 nm on a Perkin Elmer (Watham, Massachusetts) bioassay plate reader. Steroidogenic acute regulatory protein expression was determined by Western blotting using methods that we have previously described and validated.7,8 Briefly, protein samples were denatured for 5 minutes at boiling temperature, then, a total of 10 µg of protein were loaded per lane. Protein samples were separated using 15% polyacrylamide gels (Bio-Rad, Hercules, CA) and subjected to electrophoresis (sodium dodecyl sulfate polyacrylamide gel electrophoresis [SDS–PAGE]) in running buffer containing 30 mL Tris/glycine/SDS stock, and 270 mL distilled water at room temperature. Electrophoresis was performed at 121 volts for 55 minutes and protein transferred to nitrocellulose membranes using a Transblot cell apparatus (Bio-Rad) at 4°C, 270 mA for 2 hours. The transfer buffer consisted of 80 mL of concentrated transfer buffer, 160 mL of methanol (analytical reagent grade), and 560 mL of distilled water. Nitrocellulose membranes were subsequently incubated with a rabbit polyclonal StAR primary antibody (Abcam, Product# ab3343). The primary antibody solution contained 5% nonfat dry milk (0.5 g) in 10 mL of Tris-Buffered Saline containing 0.1% Tween 20 (TTBS). The primary antibody was added at a 1:1000 dilution (10 µL of primary antibody in 10 mL blocking solution) and membranes incubated at 4°C overnight on a 3-dimensional (3D) rotator. The membranes were then washed 3×, 15 minutes each with TTBS washing buffer on a 3D rotator and incubated with anti-rabbit immunoglobulin G ([IgG] Goat), horseradish peroxidase (HRP)-labeled, secondary antibody (NEF812, PerkinElmer) solution at a 1:5000 dilution (10 µL of secondary antibody in 50 mL blocking solution) for 75 minutes on a 3D rotator at 4°C. The membranes were then washed 3×, 15 minutes each with TTBS washing buffer on a 3D rotator, treated with chemiluminescence solution (Chemi-Glow, Alpha Innotech, San Leandro, CA) prior to imaging with the FluorChem imaging system (Alpha Innotech), as we have previously described.16 The integrated density values (I.D.V.) of the bands were used to compare normoxic control and LTH StAR protein expression. An internal positive standard prepared from whole fetal adrenal tissue was used to normalize StAR protein.
Statistical Analysis
Differences between normoxic control and LTH FACs were compared using analysis of variance (ANOVA), with Bonferroni posttests used were appropriate and P < .05 was considered significant. All data are presented as means ± standard error of mean (SEM).
Results
Cortisol and cAMP Responses to ACTH
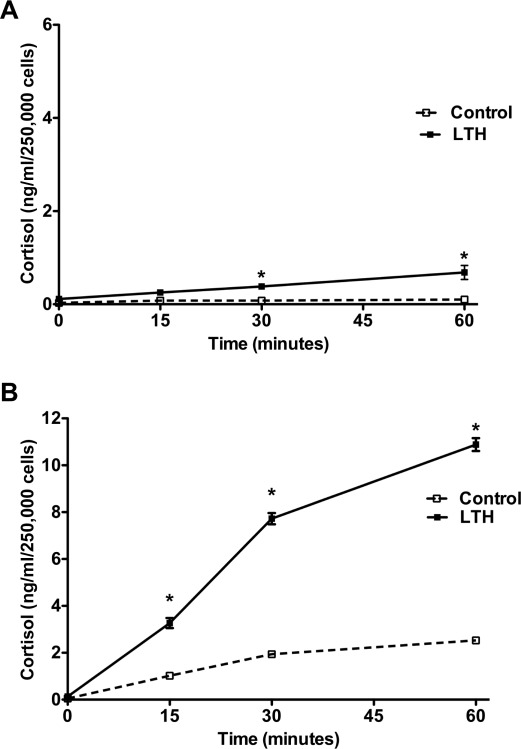
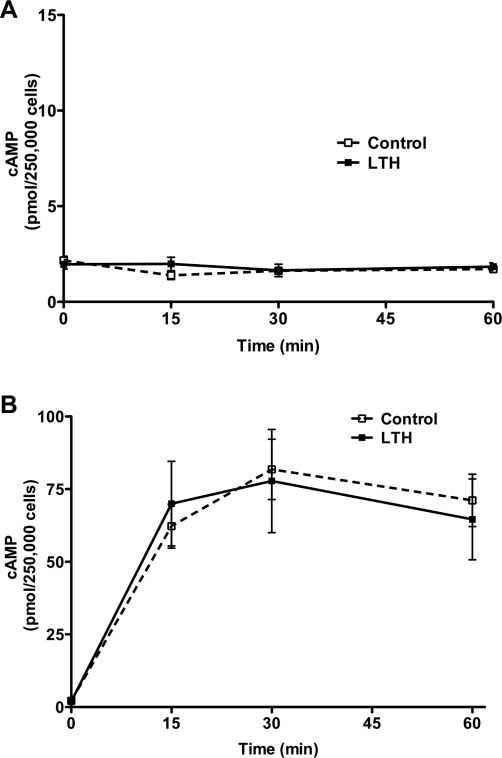
Basal cortisol secretion in control FACs remained relatively constant throughout the study period (Figure 1A). In contrast, in the LTH group, cortisol secretion increased steadily during the study period and was significantly greater than control (P < .05) at 30 and 60 minutes (Figure 1A). Following ACTH treatment, there was a significant increase in cortisol biosynthesis in both control and LTH FACs (Figure 1B). However cortisol output was significantly higher in the LTH group when compared to the control, (P < .05) at 15, 30, and 60 minutes. Basal cAMP levels were not different between groups (Figure 2A ). In response to ACTH, there was a marked increase in cAMP levels in both control and LTH FACs. However, there was no difference in cAMP levels between control and LTH FACs at any of the time points measured.
Figure 1.
Time course of basal (A) and ACTH-stimulated (B) cortisol production in control and LTH FACs. Under basal conditions, cortisol output was significantly greater in the LTH group compared with control (*P < .05). Although ACTH (10 nmol/L) increased cortisol biosynthesis in both groups (n = 6 for each group), the response was significantly greater in the LTH group compared to control (P < .05). LTH indicates long-term hypoxia; FACs, fetal adrenal cortical cells; ACTH, adrenocorticotropic hormone.
Figure 2.
Time course of basal (A) and ACTH-stimulated (B) cAMP production in control and LTH FACs. There was no difference in either basal or ACTH-stimulated cAMP generation between control and LTH FACs (n = 6 for each group). LTH indicates long-term hypoxia; FACs, fetal adrenal cortical cells; ACTH, adrenocorticotropic hormone; cAMP, cyclic adenosine monophosphate.
Effects of PKA Inhibition on Cortisol Biosynthesis
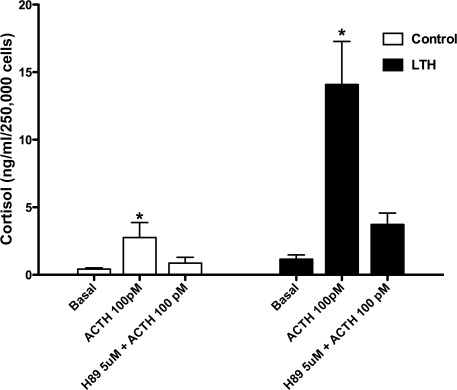
Similar to the studies presented in Figure 1, the cortisol response to ACTH was significantly greater in the FACs from the LTH group compared to control at 1 hour (Figure 3 ). In response to H-89 inhibition of PKA, cortisol biosynthesis was decreased to levels observed in the unstimulated FACs in both groups and the overall percentage decrease in cortisol response with H-89 was similar in both control (28.7 ± 3.4) and LTH (23.2 ± 3.9; P > .05).
Figure 3.
Effects of ACTH-stimulated cortisol production in response to PKA inhibition with H-89. In response to ACTH alone, cortisol secretion increased significantly in both groups compared to basal cortisol secretion (*P < .05). Pretreatment with H-89 returned cortisol secretion to basal levels. ACTH, adrenocorticotropic hormone; PKA, protein kinase. Control n = 6, LTH n = 7
Steroidogenic Acute Regulatory Protein Expression
Western analysis of StAR showed that the mature, inactive (30 kDa) form of StAR was significantly higher in the LTH group when compared to the normoxic control (P < .05) under unstimulated conditions (1.02 ± 0.07 vs 0.59 ± 0.05, LTH vs control, respectively), and at 60 minutes following ACTH treatment (1.26 ± 0.04 vs 0.65 ± 0.05, LTH vs control, respectively), I.D.V. (Figure 4 ).
Figure 4.
Steroidogenic acute regulatory (StAR) protein expression in control (n = 6) and LTH (n = 6). FACs before and after ACTH treatment. Protein expression was significantly greater in LTH versus control FACs under both basal conditions and ACTH stimulation (*P < .05 compared to control). LTH indicates long-term hypoxia; FACs, fetal adrenal cortical cells; ACTH, adrenocorticotropic hormone.
Proopiomelanocortin and 22-kDa Pro-ACTH
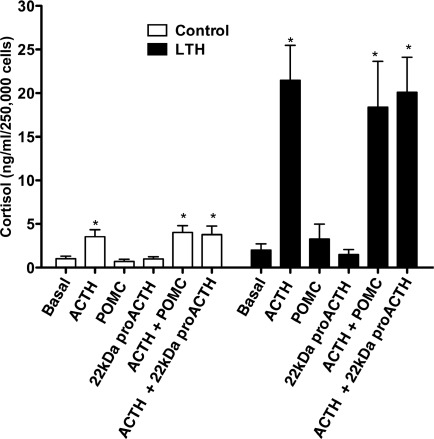
Proopiomelanocortin and 22-kDa pro-ACTH alone did not affect basal cortisol synthesis in either control or LTH FACs (Figure 5 ). In response to ACTH treatment, there was a significant increase in cortisol biosynthesis in both control and LTH FACs. However, the cortisol response in the LTH group was greater than control (P < .01). Simultaneous treatment with ACTH and either POMC or 22-kDa pro-ACTH did not affect cortisol synthesis compared to ACTH treatment alone.
Figure 5.
Adrenocorticotropic hormone (ACTH)-stimulated cortisol production in control (n = 5) and LTH (n = 5) FACs in response to ACTH alone or simultaneous treatment with ACTH and either POMC or 22-kDa pro-ACTH. ACTH treatment significantly increased cortisol production in both groups, which was unaffected by POMC or 22-kDa pro-ACTH (P < .05 compared to basal. LTH indicates long-term hypoxia; FACs, fetal adrenal cortical cells; POMC, proopiomelanocortin.
Discussion
Our laboratory has previously demonstrated that LTH results in a significant number of adaptive changes in the fetal HPA axis. Under basal conditions, despite higher plasma levels of immunoreactive ACTH1-39 in the LTH fetus, cortisol concentrations are similar to normoxic controls.7,11 However, in response to a secondary stressor such as umbilical cord occlusion, LTH fetuses exhibited an enhanced cortisol response compared to normoxic controls.10 Similarly, in response to a less severe secondary stressor (hypotension), the cortisol response was also enhanced in the LTH fetal sheep.11 We also showed that LTH increases plasma ACTH precursors (POMC and 22-kDa pro-ACTH).7 Taken together, these results suggest that the fetal adrenal sensitivity to ACTH decreases in response to development under conditions of LTH preventing overactivation or early-activation of the FAC maturation while the capacity to respond to acutely elevated ACTH during stress is enhanced. We designed the present study to address potential cellular mechanism/mechanisms of the enhanced cortisol secretion in response to elevated ACTH and the response to the putative inhibitory ACTH precursors.
In an effort to elucidate these adaptive changes, we utilized an in vitro, primary cell culture system. An initial step was to determine the response of the LTH FACs to ACTH in vitro. There was a significant increase in cortisol biosynthesis in both control and LTH FACs in response to ACTH. However, the overall response in the LTH group was greater than control. Importantly, this response mimics what we previously observed in vivo studies in response to secondary stressors.10,11 Since we previously showed that messenger RNA (mRNA) for the ACTH receptor and mRNA and protein expression for the key steroidogenic enzymes CYP11A1 and CYP17 involved in cortisol synthesis are actually reduced in response to LTH,8 it was apparent that other mechanisms are involved in this enhanced response to ACTH in the LTH group.
Adrenocorticotropic hormone mediates its signal transduction primarily via activation of adenylate cyclase resulting in cAMP generation and downstream protein kinase A activation.17,18 Since this is the first key step in this signaling pathway, we hypothesized that the LTH FACs would demonstrate an enhanced cAMP response. In the present study, however, cAMP levels increased during the first 30 minutes following ACTH stimulation and remained elevated throughout the remainder of the experiment with values similar between groups (Figure 2). Considering that we previously observed a nearly 50% decrease in ACTH receptor expression in the LTH FAC,19 the retained capacity to produce cAMP compared to control fetuses would suggest that some aspect of adenylate cyclase activation has been increased to compensate for the lower ACTH receptor expression. This could be via increases in either Gαs expression or increases in adenylate cyclase itself. Alternatively, phosphodiesterase activity could be suppressed in the LTH FACs providing for elevated cAMP levels despite lowered ACTH receptor expression. In the present study, we did not use a phosphodiesterase inhibitor since we wanted to focus on cortisol production associated with physiological levels of cAMP in a manner similar to other studies.20–22 Clearly, future studies will be needed to resolve differences in the expression and or coupling of Gαs/adenylate cyclase to the ACTH receptor and/or phosphodiesterase activity.
Since cAMP production was similar in LTH and control FACs, yet cortisol production elevated in LTH FACs, PKA itself might represent a site mediating the increased cortisol production in response to ACTH. In the present study, we chose to examine the role of PKA utilizing the PKA inhibitor, H-89. The results from this study showed that inhibition of PKA in ovine FACs plays an important role in the regulation of ACTH-induced cortisol synthesis consistent with adult adrenocortical cells. As observed in the cAMP study above, the cortisol response to ACTH alone was significantly greater in the LTH FACs compared to control (Figure 3). Importantly this same difference was observed when we used a more physiological dose of ACTH (100 pmol/L) and peak values were similar to when 10 nmol/L ACTH was used. In response to H89 inhibition of PKA, the net decrease was significantly greater in the LTH group compared to control. However, this was the result of the enhanced cortisol response to ACTH alone. However, the overall percentage inhibition of the cortisol response to ACTH was similar in both groups, suggesting that PKA activity is similar in control and LTH FACs. To our knowledge, these are the first studies to directly demonstrate the role of PKA in cortisol biosynthesis in the ovine fetal adrenal.
One of the initial steps in steroidogenesis is the transfer of cholesterol to the inner mitochondrial membrane via StAR23 and is regarded the first rate-limiting step in steroidogenesis. Hypoxia was previously shown to enhance StAR protein expression in neonatal rat adrenal glands.24 Recent studies from our laboratory showed that StAR expression was greater in the LTH FAC tissue when compared to control.8 This implicates StAR as a potential key target for the enhanced cortisol synthesis observed in the present study in the LTH FACs. These results suggested that StAR might play a more important role during the process of glucocorticoid synthesis under conditions of LTH. Since StAR protein is involved in the transfer of cholesterol from the outer to the inner mitochondrial membrane, elevated StAR may serve to provide more cholesterol for processing to cortisol, suggesting that enhanced cholesterol delivery may occur in these animals to compensate for lower CYP11A1 and CYP17 expression. In the present study, Western analysis showed that the mature, inactive (30 kDa) form of StAR was increased in the LTH group when compared to the normoxic control substantiating the in vivo findings. StAR expression was significantly elevated in LTH adrenocortical cells (both basal and after 1 hour of ACTH stimulation). In the absence of ACTH, there was elevated cortisol production in the LTH cells compared to control, likely reflecting basal cholesterol transport due to the higher StAR. In response to ACTH, the elevated StAR would facilitate the increased steroidogenesis observed in the LTH adrenocortical cells. Although phosphorylation of StAR via PKA is thought to activate StAR translocation and thus cholesterol transfer, we were unable to evaluate differences in StAR phosphorylation between LTH and control FACs since there are currently no antibodies available for detecting phospho-StAR in ruminants. A role for StAR in the enhanced cortisol biosynthesis in LTH FACs is also supported by our prior observation that there are no differences in expression of downstream steroidogenic enzymes, CYP218 and CYP11B1 (Myers DA and Ducsay CA, Unpublished observations.). This would likely implicate early steps in cortisol biosynthesis that are the target of LTH. An intriguing aspect for future studies would also be to examine whether differences exist in the LTH FACs in mitochondrial numbers and/or mitochondrial function.
An important characteristic of PKA is that its role in phosphorylation is short lived and reversible, with dephosphorylation mediated by phosphoprotein phosphatases.25 There are various studies that have shown that phosphoprotein phosphatases (PP1, PP2A, and PP2B) are highly expressed in the adrenal and luteal cells.26–28 Recent data suggest that phosphoprotein phosphatases may play a role in the regulation of StAR protein expression and cortisol biosynthesis.29–31 Studies have shown that inhibition of PP1 and PP2A activities with calyculin A and cantharin causes an inhibition of StAR protein expression, and a dose-response inhibition of steroidogenesis. Additionally, other reports have shown that inhibition of PP1 and PP2A activities with calyculin A, okadaic acid a selective inhibitor of PP2A, and cantharin causes an inhibition at the level of transcription of key enzymes such as CYP11A1.32 It is possible that phosphorylation and dephosphorylation events may play a dual-specificity role in the regulation of steroidogenesis under conditions of LTH.
Although the study of phosphoprotein phosphatases was beyond the scope of the present study, this may be an area worthy of future investigation. It is possible that since StAR protein is increased in the LTH adrenocortical cells in vitro, then the phosphorylation state of StAR may have been higher. An increase in the amount of StAR protein would require addition of phosphate groups to drive the activity of the StAR protein. Several studies point to posttranslational modifications such as phosphorylation as being involved in the events leading to steroidogenesis particularly suggesting a regulatory event at the level of StAR protein.33–36 For instance, Arakane et al33 demonstrated in vitro that mutating potential sites of phosphorylation in the StAR protein reduces the capacity of COS-1 cell extracts to incorporate 32P into StAR protein. The study showed the phosphorylation sites were located at serine 194/195. Furthermore, the work of Orme-Johnson37 led to the discovery that StAR phosphorylation is initiated as a result of activating the ACTH-cAMP signaling pathway and subsequent phosphorylation of the protein.38
A unique and unexpected finding of the present studies was that while basal cortisol production in control FACs was relatively constant over the 1 hour incubation period as expected, cortisol production increased in the LTH FACs during the 1 hour incubation period (Figure 1A). This observation suggested removal of an inhibitory factor found in vivo, which limits basal cortisol production in these fetuses. Previous studies from Schwartz et al9 showed that the ACTH precursors POMC and 22-kDa pro-ACTH exert a significant inhibitory action on cortisol synthesis in fetal but not adult adrenocortical cells from sheep. This study provided an indication that the capacity to respond to the ACTH precursors is regulated and unique to the FAC. Since our previous in vivo studies demonstrated enhanced basal plasma concentrations of ACTH1-39 as well as POMC and 22-kDa pro-ACTH in LTH fetuses, it seemed possible that the capacity to respond to the inhibitory ACTH precursors is enhanced in the LTH FACs. Thus, we hypothesized that POMC and 22-kDa pro-ACTH were the inhibitory factors that were removed in the in vitro setting. 7
To test this hypothesis, we treated FACs from control and LTH fetuses with either ACTH alone or in combination with POMC or 22-kDa pro-ACTH in a manner similar to that previously described by Schwartz et al.9 In marked contrast to results from the Schwartz study,9 we found that neither of the peptides had an effect on cortisol biosynthesis in either control or LTH FACs (Figure 5). It is important to point out 2 key differences in their study and the present data. The first is that the majority of adrenals in their studies were collected from animals at an earlier gestational age than those used in the present study (138-141 dG). Given the noted lack of effect of the precursors on cortisol production in the adult adrenocortical cell as noted by Schwartz et al,9 it is likely that the ovine fetal adrenal becomes insensitive to the inhibitory effects of the ACTH precursors during the final maturational processes that occur in the adrenal cortex as term gestation approaches. Second, the ACTH precursors used in Schwartz’s study were purified from pituitaries; while in the present study, the precursors were recombinant ovine POMC and 22-kDa pro-ACTH. Perhaps the purification process used by Schwartz et al,9 changed the biological activity from the original native molecules or that differences in glycosylation state in the recombinant proteins compared to those of pituitary origin may have altered their bioactivity. In either case, the ACTH precursors had no inhibitory effect in our study. It does not appear, therefore, that the observed enhanced precursor levels in LTH fetuses play a role in the regulation of cortisol biosynthesis during late gestation.
Other factors found in the circulation inhibit glucocorticoid biosynthesis. Yuen et al39 found that leptin infusion into the ovine fetus had an inhibitory effect on cortisol secretion in vivo. In vitro studies using rat and human adrenal cortical cells also demonstrated that leptin inhibited glucocorticoid production.40 We previously showed that in vivo, LTH ovine fetuses had plasma leptin levels approximately 3-fold higher than normoxic controls.41 Perhaps the removal of the higher level of leptin inhibition in vitro allowed greater basal expression of cortisol in the LTH group. This hypothesis is currently under investigation.
In summary, the developing LTH sheep fetus has adapted to the adverse conditions of chronic hypoxia. Data from the present study indicate that the ability of the LTH fetal adrenal to respond to a secondary stressor with enhanced cortisol production is not the result of changes in cAMP, PKA or potential effects of POMC, or 22-kDa pro-ACTH. Steroidogenic acute regulatory protein expression, however, is a likely candidate mediating at least in part, the observed enhanced cortisol biosynthesis in the LTH FACs. Interestingly, the extracellular regulated kinases (ERK1/2) have been shown to regulate cAMP-dependent cortisol production at the level of StAR gene transcription. We have recently shown that inhibition of the MEK/ERK pathway results in significant inhibition of cortisol biosynthesis in FACs.42 We are currently investigating the role of ERK1/2 in the regulation of StAR in the LTH ovine fetal adrenal.
Footnotes
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
The authors disclosed receipt of the following financial support for the research and/or authorship of this article: National Institutes of Health Grants PO1HD31226, R01HD51951 and IMSD 2R 25GM060501-05.
References
- 1. Liggins GC, Fairclough RJ, Grieves SA, Kendall JZ, Knox BS. The mechanism of initiation of parturition in the ewe. Recent Prog Horm Res. 1973;29:111–159 [DOI] [PubMed] [Google Scholar]
- 2. Meaney MJ, Viau V, Bhatnagar S, et al. Cellular mechanisms underlying the development and expression of individual differences in the hypothalamic-pituitary-adrenal stress response. J Steroid Biochem Mol Biol. 1991;39(2):265–274 [DOI] [PubMed] [Google Scholar]
- 3. Challis JR, Richardson BS, Rurak D, Wlodek ME, Patrick JE. Plasma adrenocorticotropic hormone and cortisol and adrenal blood flow during sustained hypoxemia in fetal sheep. Am J Obstet Gynecol. 1986;155(6):1332–1336 [DOI] [PubMed] [Google Scholar]
- 4. Fletcher AJ, Gardner DS, Edwards CM, Fowden AL, Giussani DA. Development of the ovine fetal cardiovascular defense to hypoxemia towards full term. Am J Physiol Heart Circ Physiol. 2006;291(6):H3023–H3034 [DOI] [PubMed] [Google Scholar]
- 5. Raff H, Wood CE. Effect of age and blood pressure on the heart rate, vasopressin, and renin response to hypoxia in fetal sheep. Am J Physiol. 1992;263(4 pt 2):R880–R884 [DOI] [PubMed] [Google Scholar]
- 6. Towell ME, Figueroa J, Markowitz S, Elias B, Nathanielsz P. The effect of mild hypoxemia maintained for twenty-four hours on maternal and fetal glucose, lactate, cortisol, and arginine vasopressin in pregnant sheep at 122 to 139 days' gestation. Am J Obstet Gynecol. 1987;157(6):1550–1557 [DOI] [PubMed] [Google Scholar]
- 7. Myers DA, Bell PA, Hyatt K, Mlynarczyk M, Ducsay CA. Long-term hypoxia enhances proopiomelanocortin processing in the near-term ovine fetus. Am J Physiol Regul Integr Comp Physiol. 2005;288(5):R1178–R1184 [DOI] [PubMed] [Google Scholar]
- 8. Myers DA, Hyatt K, Mlynarczyk M, Bird IM, Ducsay CA. Long-term hypoxia represses the expression of key genes regulating cortisol biosynthesis in the near-term ovine fetus. Am J Physiol Regul Integr Comp Physiol. 2005;289(6):R1707–R1714 [DOI] [PubMed] [Google Scholar]
- 9. Schwartz J, Kleftogiannis F, Jacobs R, Thorburn GD, Crosby SR, White A. Biological activity of adrenocorticotropic hormone precursors on ovine adrenal cells. Am J Physiol. 1995;268(4 pt 1):E623–E629 [DOI] [PubMed] [Google Scholar]
- 10. Imamura T, Umezaki H, Kaushal KM, Ducsay CA. Long-term hypoxia alters endocrine and physiologic responses to umbilical cord occlusion in the ovine fetus. J Soc Gynecol Investig. 2004;11(3):131–140 [DOI] [PubMed] [Google Scholar]
- 11. Adachi K, Umezaki H, Kaushal KM, Ducsay CA. Long-term hypoxia alters ovine fetal endocrine and physiological responses to hypotension. Am J Physiol Regul Integr Comp Physiol. 2004;287(1):R209–R217 [DOI] [PubMed] [Google Scholar]
- 12. Sewer MB, Waterman MR. ACTH modulation of transcription factors responsible for steroid hydroxylase gene expression in the adrenal cortex. Microsc Res Tech. 2003;61(3):300–307 [DOI] [PubMed] [Google Scholar]
- 13. Stocco DM. Star protein and the regulation of steroid hormone biosynthesis. Annu Rev Physiol. 2001;63:193–213 [DOI] [PubMed] [Google Scholar]
- 14. Bell ME, McDonald TJ, Myers DA. Proopiomelanocortin processing in the anterior pituitary of the ovine fetus after lesion of the hypothalamic paraventricular nucleus. Endocrinology. 2005;146(6):2665–2673 [DOI] [PubMed] [Google Scholar]
- 15. Ducsay CA, Mlynarczyk M, Kaushal KM, Hyatt K, Hanson K, Myers DA. Long-term hypoxia enhances ACTH response to arginine vasopressin but not corticotropin-releasing hormone in the near-term ovine fetus. Am J Physiol Regul Integr Comp Physiol. 2009;297(3):R892–R899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Monau TR, Vargas VE, Zhang L, Myers DA, Ducsay CA. Nitric oxide inhibits ACTH-induced cortisol production in nearterm, long term hypoxic ovine fetal adrenocortical cells. Reprod Sci. 2010;17(10):955–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Daniel PB, Walker WH, Habener JF. Cyclic AMP signaling and gene regulation. Annu Rev Nutr. 1998;18:353–383 [DOI] [PubMed] [Google Scholar]
- 18. Kamenetsky M, Middelhaufe S, Bank EM, Levin LR, Buck J, Steegborn C. Molecular details of camp generation in mammalian cells: A tale of two systems. J Mol Biol. 2006;362(4):623–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Myers DA, Hyatt K, Mlynarczyk M, Bird IM, Ducsay CA. Long-term hypoxia represses the expression of key genes regulating cortisol biosynthesis in the near-term ovine fetus. Am J Physiol Regul Integr Comp Physiol. 2005;289(6):R1707–R1714 [DOI] [PubMed] [Google Scholar]
- 20. Darbeida H, Durand P. Glucocorticoid enhancement of adrenocorticotropin-induced 3’,5'-cyclic adenosine monophosphate production by cultured ovine adrenocortical cells. Endocrinology. 1987;121(3):1051–1055 [DOI] [PubMed] [Google Scholar]
- 21. Su Y, Rose JC. The impact of acth receptor knockdown on fetal and adult ovine adrenocortical cell function. Reprod Sci. 2008;15(3):253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Valego NK, Rose JC. A specific crh antagonist attenuates acth-stimulated cortisol secretion in ovine adrenocortical cells. Reprod Sci. 2010;17(5):477–486 [DOI] [PubMed] [Google Scholar]
- 23. Manna PR, Dyson MT, Eubank DW, et al. Regulation of steroidogenesis and the steroidogenic acute regulatory protein by a member of the camp response-element binding protein family. Mol Endocrinol. 2002;16(1):184–199 [DOI] [PubMed] [Google Scholar]
- 24. Raff H, Hong JJ, Oaks MK, Widmaier EP. Adrenocortical responses to acth in neonatal rats: Effect of hypoxia from birth on corticosterone, star, and pbr. Am J Physiol Regul Integr Comp Physiol. 2003;284(1):R78–R85 [DOI] [PubMed] [Google Scholar]
- 25. Gorostizaga A, Cornejo Maciel F, Brion L, Maloberti P, Podesta EJ, Paz C. Tyrosine phosphatases in steroidogenic cells: regulation and function. Mol Cell Endocrinol. 2007;265-266:131–137 [DOI] [PubMed] [Google Scholar]
- 26. Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508 [DOI] [PubMed] [Google Scholar]
- 27. Ford SL, Abayasekara DR, Persaud SJ, Jones PM. Role of phosphoprotein phosphatases in the corpus luteum: I Identification and characterisation of serine/threonine phosphoprotein phosphatases in isolated rat luteal cells. J Endocrinol. 1996;150(2):205–211 [DOI] [PubMed] [Google Scholar]
- 28. Sayed SB, Whitehouse BJ, ones PM. Phosphoserine/threonine phosphatases in the rat adrenal cortex: a role in the control of steroidogenesis?. J Endocrinol. 1997;154(3):449–458 [DOI] [PubMed] [Google Scholar]
- 29. Burns CJ, Gyles SL, Persaud SJ, Sugden D, Whitehouse BJ, Jones PM. Phosphoprotein phosphatases regulate steroidogenesis by influencing star gene transcription. Biochem Biophys Res Commun. 2000;273(1):35–39 [DOI] [PubMed] [Google Scholar]
- 30. Jones PM, Sayed SB, Persaud SJ, Burns CJ, Gyles S, Whitehouse BJ. Cyclic amp-induced expression of steroidogenic acute regulatory protein is dependent upon phosphoprotein phosphatase activities. J Mol Endocrinol. 2000;24(2233–239 [DOI] [PubMed] [Google Scholar]
- 31. Poderoso C, Maciel FC, Gorostizaga A, Bey P, Paz C, Podesta EJ. The obligatory action of protein tyrosine phosphatases in acth-stimulated steroidogenesis is exerted at the level of star protein. Endocr Res. 2002;28(4):413–417 [DOI] [PubMed] [Google Scholar]
- 32. Sewer MB, Waterman MR. cAMP-dependent transcription of steroidogenic genes in the human adrenal cortex requires a dual-specificity phosphatase in addition to protein kinase a. J Mol Endocrinol. 2002;29(1):163–174 [DOI] [PubMed] [Google Scholar]
- 33. Arakane F, King SR, Du Y, et al. Phosphorylation of steroidogenic acute regulatory protein (star) modulates its steroidogenic activity. J Biol Chem. 1997;272(51):32656–32662 [DOI] [PubMed] [Google Scholar]
- 34. Fleury A, Mathieu AP, Ducharme L, Hales DB, LeHoux JG. Phosphorylation and function of the hamster adrenal steroidogenic acute regulatory protein (star). J Steroid Biochem Mol Biol. 2004;91(4):259–271 [DOI] [PubMed] [Google Scholar]
- 35. LeHoux JG, Fleury A, Ducharme L, Hales DB. Phosphorylation of the hamster adrenal steroidogenic acute regulatory protein as analyzed by two-dimensional polyacrylamide gel electrophoreses. Mol Cell Endocrinol. 2004;215(1-2):127–134 [DOI] [PubMed] [Google Scholar]
- 36. Manna PR, Stocco DM. Regulation of the steroidogenic acute regulatory protein expression: functional and physiological consequences. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5(1):93–108 [DOI] [PubMed] [Google Scholar]
- 37. Orme-Johnson NR. Distinctive properties of adrenal cortex mitochondria. Biochim Biophys Acta. 1990;1020(3):213–231 [DOI] [PubMed] [Google Scholar]
- 38. Pon LA, Hartigan JA, Orme-Johnson NR. Acute ACTH regulation of adrenal corticosteroid biosynthesis. Rapid accumulation of a phosphoprotein. J Biol Chem. 1986;261(28):13309–13316 [PubMed] [Google Scholar]
- 39. Yuen BS, Owens PC, Symonds ME, et al. Effects of leptin on fetal plasma adrenocorticotropic hormone and cortisol concentrations and the timing of parturition in the sheep. Biol Reprod. 2004;70(6):1650–1657 [DOI] [PubMed] [Google Scholar]
- 40. Pralong FP, Roduit R, Waeber G, et al. Leptin inhibits directly glucocorticoid secretion by normal human and rat adrenal gland. Endocrinology. 1998;139(10):4264–4268 [DOI] [PubMed] [Google Scholar]
- 41. Ducsay CA, Hyatt K, Mlynarczyk M, Kaushal KM, Myers DA. Long-term hypoxia increases leptin receptors and plasma leptin concentrations in the late-gestation ovine fetus. Am J Physiol Regul Integr Comp Physiol. 2006;291(5):R1406–R1413 [DOI] [PubMed] [Google Scholar]
- 42. Vargas VE, Myers DA, Kaushal KM, Ducsay CA. ACTH induced cortisol synthesis in ovine fetal adrenocortical cells is mediated in part by extracellular signal regulated kinase (erk) 1 and 2: effect of long term hypoxia (lth). Reprod Sci. 2009;16(3):250A [Google Scholar]