Abstract
Advanced age is associated with declines in brain structure and in cognitive performance, but it is unclear, which aspects of brain aging mediate cognitive declines. We inquired if individual differences in white matter integrity contribute to age differences in two cognitive domains with established vulnerability to aging: executive functioning and speed of processing. The participants were healthy volunteers age 50–81, some of whom had elevated blood pressure, a known vascular risk factor. Using latent variable analyses, we examined whether age differences in regional white matter integrity mediated age-related differences in executive functions and speed of processing. Although diffusion-related latent variables showed stronger age differences than white matter volumes and white matter hyperintensity volumes, only one of them was significantly associated with cognitive performance. Smaller linear anisotropy partially mediated age-related reduction in speed of processing. The effect was significant in posterior (temporal-parietal-occipital) but not anterior (frontal) region, and appeared stronger for cognitive rather than reaction time measures of processing speed. Presence of hypertensive participants did not affect the results. We conclude that in healthy adults, deterioration of axonal integrity and ensuing breech of connectivity may underpin age-related slowing of information processing.
Keywords: Aging, Cognition, White Matter, DTI, MRI
Introduction
Many cognitive skills decline with age, but executive functions and speed of processing are considered to be particularly vulnerable (Horn, 1986; Rhodes, 2004; Van der Elst et al., 2006a,b). However, neural mechanisms underpinning these functional declines remain unclear. In search of neural mediators of differential cognitive aging, many researchers have focused on the aging of the cerebral white matter. To date, several studies reported significant correlations between various indices of white matter integrity and executive performance (Brickman et al., 2006; Burns et al., 2005; Charlton et al., 2006; De Groot et al., 2000; Grieve et al., 2007; Gunning-Dixon and Raz, 2000, 2003; Kennedy and Raz, 2009; Madden et al., 2009b; Raz et al., 2003; Schiavone et al., 2009; Vernooij et al., 2009; Zahr et al., 2009; Ziegler et al., 2008) and between white matter integrity and speed of processing (e.g., Kennedy and Raz, 2009; Madden et al., 2009a; Penke et al., 2010; Vernooij et al., 2009). These findings are frequently interpreted as favouring the “disconnection hypothesis,” according to which cognitive declines stem from disruption of the information flow in neural networks following age-related deterioration of the white matter through axonal damage and demyelination (Hogan et al., 2006). Heterogeneity of measures of white matter integrity hampers interpretation of this literature, as it is unclear which aspects of age-related differences in white mater structure are particularly responsible for the age-related neuropsychological deficits. Almost all investigations to date have focused on a limited number of indicators of white matter integrity, attending either to white matter volume, its diffusion properties or the presence of local pathology. Hence, there is a need for a comparison among multiple indices of white matter integrity and determination of their relative utility for predicting cognitive declines.
Although it is impossible to assess the causal role of white matter deterioration in cognitive declines outside of the framework of a longitudinal study, cross-sectional comparisons can inform about the relative contribution of individual differences in mediators to age-related variability (Baron and Kenny, 1986; Lindenberger and Pötter, 1998; Preacher et al., 2007). To date, mediation analyses of putative white matter substrates of age-related declines in executive functioning or processing speed has been conducted in only a handful of studies that employed multiple regression methodology (Brickman et al., 2006; Charlton et al., 2008; Head et al., 2009; Head et al., 2008; Madden et al., 2009b; Zahr et al., 2009). Although supportive of the mediation hypothesis, those investigations focused only on a single index of white matter integrity at a time. Thus, it remains unclear which of the most frequently used indices of white matter integrity is the most sensitive in detecting a mediating effect. Only one study (Vernooij et al., 2009) compared the three indices of white matter integrity and their effect on executive functioning and speed. The authors concluded that gross volume of normally appearing white matter, volume of white matter hyperintensities, and indices of mean, radial, and axial diffusivity reflect different aspects of pathophysiology, and suggested that diffusivity measures might have an advantage in predicting cognitive performance over other measures of white matter integrity. In that study, conducted in a large community sample of typical elderly, contributions of multiple health factors could be confounded with age. Moreover, the cognitive constructs were operationalized by indicators from only two tasks (Stroop and Letter-Digit Substitution) that were used in constructing composite scores for several presumably distinct cognitive entities. Statistical methods of that study (multiple ordinary least squares models) were insufficient for testing complex relationships among cognitive and brain variables.
For testing such multivariate associations, structural equation models (SEM) provide a more suitable framework. Making a step in the right direction, several studies used SEM for analyses of mediation effects of brain variables in age-related differences in executive functions, speed of processing, and memory (Head et al., 2009; Head et al., 2008; Penke et al., 2010; Voineskos et al., 2010). However, in those studies only manifest measures or principal component analysis were used, thus precluding examination of associations on the level of constructs that is possible in the framework of SEM with latent variables. The latter approach involves extraction of common variance to form latent factors corresponding to theoretical constructs from multiple tests, while simultaneously removing measurement error and test-specific variance. Such analytic approach provides a significant conceptual as well as statistical advantage (Little, Lindenberger & Nesselroade, 1999).
The objective of the present study was to investigate in healthy adults, the mediating effects of age differences in white matter integrity on the relationship between age and age-sensitive cognitive functions, i.e., executive functioning and speed of processing. We compared three types of measures of white matter integrity: white matter volumes, white matter hyperintensities (WMH), and indices based on diffusion properties, which comprised fractional anisotropy (FA), linear (similar to axial) anisotropy (A) and planar (similar to radial) anisotropy (R). Executive functioning were assessed by multiple cognitive tasks covering the domains of inhibition, working memory, and set shifting. Speed of processing was measured by paper-and-pencil tasks that involved comparison of verbal and pictorial stimuli, as well as computerized measures of speed of processing in the working memory. We used confirmatory factor analysis to build latent factors from separate measurement models of brain and cognitive variables. To test hypotheses regarding mediation influence of brain variables on cognitive constructs, we validated the measurement models, and afterwards constructed structural models involving the latent factors that represented constructs measured by the specific tests and indices of white matter integrity.
We hypothesized that advanced age would be associated with significant reduction in speed of processing and decline in executive functions, and that those declines would be associated with reduction in integrity of the white matter. Based on previous analyses of brain measures in this sample, we hypothesized that diffusion-based indices of white matter integrity would fare better in predicting declines in age-sensitive cognitive skills than volume or WMH indices. Because hypertension has negative effect on white matter integrity in this sample (Burgmans et al., 2010), we hypothesized that hypertension, even treated and reasonably controlled, could be an important modifier of relationship between age-related differences in white matter and cognition.
Methods
Participants
The participants for this study were selected from the first wave of a longitudinal MRI study of 219 healthy community volunteers (aged 18–81 years) from the Metro Detroit area, who were recruited through advertisements in the local media and screened via a telephone interview and health questionnaire. The exclusion criteria were a history of cardiovascular, neurological and psychiatric conditions, history of stroke, head trauma with a loss of consciousness for more than 5 minutes, history of alcohol and drug abuse, or a diagnosis of diabetes or thyroid disorder. The items used to screen for cardiovascular disease included an open question on any kind of diseases or complaints related to the heart or the large blood vessels as well as taking specific medications prescribed for treatment of cardiovascular conditions. The participants had corrected visual acuity of 20/50 or better (Optic 2000, Stereo Optic) and adequate hearing acuity (hearing threshold levels 40 dB or better for frequencies of 500–4000 Hz; Maico, MA27). To screen for dementia and depression we used the MMSE (Folstein et al., 1975) and the Center for Epidemiologic Studies Depression Scale (CES-D; Radloff, 1977). Only persons who scored 26 or higher on MMSE and 15 or lower on CES-D were invited to participate. All participants provided written informed consent in accord with university and hospital review board guidelines.
Because the present study involved the phenomena that are extremely rare in younger adults (e.g., WMH and hypertension), we selected only participants of fifty years of age and older, 93 from the total sample of 219, including 36 hypertensive. Thirty-one of the hypertensive participants reported a history of hypertension diagnosed by their physician and were taking at least one antihypertensive medication, for the mean duration of 6.7 years. Five additional participants who were not diagnosed with hypertension were classified as hypertensive because they had an abnormally high average systolic (> 140 mmHg) or diastolic (> 90 mmHg) blood pressure measured at the research center. Blood pressure was averaged across the arms and at least three measurements on different days. Thus, presence of past or current hypertension is conceptualized in this study as a marker of vascular risk. Reduction of the blood pressure through medical intervention may reduce future risk, but does not eliminate the history of elevated blood pressure and possibility of its damaging influence on brain and cognition. The remaining 57 participants in this sample were normotensive, who did not differ from the hypertensive group in age, education and distribution of sex and ethnicity (see Table 1).
Table 1.
Description of the sample and cognitive measures
| Variable; mean (SD) or % | Total | Men | Women | p-values of group comparisons; calculated by ANOVA (A) or χ2 tests | |||||
|---|---|---|---|---|---|---|---|---|---|
| Normotensive | Hypertensive | Normotensive | Hypertensive | Sex | HTN | Sex ×HTN | |||
| Number of participants | 93 | 25 | 15 | 32 | 21 | ||||
| Age | 62.0 (7.9) | 61.4 (7.7) | 61.1 (8.4) | 61.2 (7.8) | 64.7 (8.0) | A | 0.438 | 0.253 | 0.279 |
| Education in years | 15.8 (2.6) | 15.5 (2.6) | 16.8 (3.3) | 15.5 (2.6) | 15.9 (2.4) | A | 0.572 | 0.189 | 0.385 |
| Caucasian race | 68.8 % | 68.0 % | 80.0 % | 75.0 % | 52.4 % | χ2 | 0.505 | 0.415 | |
| Hypertensive medication | 33.3 % | 0.0 % | 86.7 % | 0.0 % | 85.7 % | χ2 | 0.882 | <0.001*** | |
| Years of treatment with medication | 2.2 (4.4) | 0.0 (0.0) | 6.1 (5.0) | 0.0 (0.0) | 5.5 (5.8) | A | 0.900 | <0.001*** | 0.671 |
| Systolic Pressure in mm Hg | 126.5 (12.6) | 122.7 (9.3) | 133.6 (13.0) | 120.8 (10.4) | 134.6 (12.6) | A | 0.844 | <0.001*** | 0.548 |
| Diastolic Pressure in mm Hg | 76.4 (7.2) | 74.4 (5.4) | 81.5 (9.4) | 73.1 (6.4) | 80.1 (4.9) | A | 0.447 | <0.001*** | 0.972 |
| MMSE | 28.6 (1.0) | 28.7 (1.2) | 28.4 (1.1) | 28.6 (1.0) | 28.7 (0.7) | A | 0.781 | 0.706 | 0.364 |
| Intracranial volume in ml | 1366.9 (160.9) | 1469.0 (147.2) | 1435.8 (186.3) | 1325.3 (111.3) | 1259.7 (134.5) | A | <0.001** | 0.107 | 0.594 |
| WCST, perseverative errors | 18.1 (11.1) | 14.9 (9.6) | 15.4 (11.4) | 21.9 (10.0) | 17.9 (12.9) | A | 0.025* | 0.411 | 0.349 |
| Stroop color naming task, seconds | 21.0 (7.8) | 21.7 (8.9) | 19.0 (8.0) | 21.6 (7.6) | 20.7 (6.8) | A | 0.738 | 0.321 | 0.601 |
| NBV3ER, errors for 3-back trials | 6.4 (4.1) | 6.5 (4.1) | 7.3 (4.6) | 6.1 (3.6) | 6.3 (4.5) | A | 0.455 | 0.591 | 0.724 |
| NBNV3ER, errors for 3-back trials | 11.6 (4.4) | 10.4 (4.5) | 11.4 (4.3) | 12.8 (4.4) | 11.1 (4.3) | A | 0.147 | 0.583 | 0.169 |
| NBV1RT, reaction time | 1483.2 (348.8) | 1551.7 (391.4) | 1466.4 (295.3) | 1417.7 (291.6) | 1510.1 (409.4) | A | 0.380 | 0.848 | 0.243 |
| NBV2RT, reaction time | 1792.6 (448.4) | 1696.9 (368.3) | 1653.3 (285.2) | 1763.3 (368.6) | 2049.0 (628.5) | A | 0.035* | 0.117 | 0.081 |
| NBNV1RT, reaction time | 3355.0 (704.8) | 3517.5 (881.1) | 3234.0 (554.4) | 3218.7 (637.4) | 3450.0 (645.5) | A | 0.482 | 0.928 | 0.097 |
| NBNV2RT, reaction time | 3977.2 (831.1) | 4100.3 (941.0) | 3634.8 (661.3) | 3836.6 (704.3) | 4282.7 (891.2) | A | 0.605 | 0.744 | 0.011* |
| LetComp, no of correct items | 18.4 (4.6) | 17.5 (4.5) | 19.1 (3.8) | 19.5 (4.8) | 17.5 (4.7) | A | 0.506 | 0.616 | 0.066 |
| PatComp, no of correct items | 30.6 (5.2) | 30.0 (5.7) | 31.0 (4.7) | 30.8 (5.7) | 30.6 (4.5) | A | 0.714 | 0.775 | 0.582 |
Note. Effects are from univariate ANOVAs (A); and χ2 tests. HTN – Hypertension, WCST = Wisconsin Card Sorting Test; NBV = N-back verbal; NBNV = N-back nonverbal; LetCom = Letter Comparison; PatComp = Pattern Comparison.
p<0.05;
p<0.001.
Measures of executive functioning
Wisconsin Card Sorting Test
In a computerized version of the Wisconsin Card Sorting Test (WCST), participants were asked to sort cards with geometric designs into categories by the shape, color or number of the designs that appear on the cards. The participants were asked to match a card appearing on the bottom of the screen with one of four key cards displayed at the top using a keypad. The computer provided accuracy feedback. The correct category switched after 10 consecutive correct responses. Participants continued until six categories were achieved, or the maximum 128 trials. The index of performance was the number of perseverative errors. The Spearman-Brown test-retest reliability for perseverative errors is .83 (Ingram et al., 1999).
Stroop task: Color naming
We used a paper-pencil version of the color Stroop test (Stroop, 1935), devised by Salthouse & Meinz (1995). This Stroop test includes three conditions (neutral, compatible and incompatible), with two trials collected for each condition. We included only the first trial in our analyses. Participants read color words presented in compatible ink (compatible condition), named the color of the ink in which X s were printed (neutral condition), and named the color of the ink in which color words were printed in an incompatible color (incompatible condition). When the interference score is computed as a difference between reading time for incongruent stimuli and color-naming time for neutral stimuli, the estimated split-half reliability is .72 (Salthouse and Meinz, 1995). Because difference scores are less reliable than their components (e.g., Lord, 1958), we defined the cost of interference as a studentized residual from regression of the reading time in the incompatible condition on color-naming time in the neutral condition. Thus, for every participant, the interference cost was defined as a deviation of reading speed for incongruent stimuli from what would be expected based on color-naming speed of the neutral stimuli. In addition, studentizing resulted in scores that were normalized by the standard deviation thus taking into account possible differences in variability in reading times across various stimuli.
Verbal N-back task
In this computerized task, participants viewed a randomly ordered sequence of digits between 1 and 9. The length of a string varied from five to nine digits, and the participants were required to report the digit in the nth position after the full string was presented. There were three blocks of trials, and for each block participants reported either the digit that was 1-back in the sequence, the digit 2-back in the sequence, or the digit 3-back in the sequence. Instructions were read aloud and displayed on the computer screen: “In this task you will see a series of digits presented one at a time. At the end of the presentation, you will use the number pad to indicate the last (next-to-last or third to-last) digit shown.” No directions are given regarding speed or accuracy.
Response requirements were a nonverbal key press on the keyboard using the number pad. The trials were of variable presentation length depending on how many stimuli (5–9) were presented in any given trial, but the stimuli were presented for a set time of 1 s. There was no time-out, as a key press was required before the next trial started. The inter-trial interval varied, depending on the duration of the participant s response, but the inter-stimulus interval was constant at 200 ms. There were 20 trials for each experimental block (1-, 2-, or 3-back), with the presentation order randomized across participants, but like experimental conditions were always presented together (i.e., the 20 trials for each block were presented consecutively). The index of performance was the number of errors in the 3-back condition, as the other conditions evidenced ceiling effects. The estimated test-retest reliability for this task is .91 (Salthouse et al., 1996).
Nonverbal N-back task
The procedure for the nonverbal N-back task (Dobbs and Rule, 1989; Hultsch et al., 1990) was the same as for the verbal version except that the stimuli were from Kroll and Potter (Kroll and Potter, 1984). In addition, the test phase consisted of a screen displaying all nine abstract figures paired with digits and participants indicated the figure in the nth position. The distinction between the verbal and non-verbal stimuli comes in because in the verbal task, one can meaningfully use language (digit names) in processing of the stimuli. The nonverbal task is comprised of abstract figures with no reference, and therefore has no similar verbal component. The index of performance was again the number of errors in the 3-back condition. The estimated reliability for this task is .88 (Salthouse et al., 1996).
Speed of processing
Verbal and nonverbal N-back task
See above. The indices of performance were the reaction times (RT) on the 1-back and 2-back conditions. The RTs from the 3-back conditions were not used due to a substantial number of errors committed by many participants.
Letter Comparison and Pattern Comparison Task
Both tasks require participants to make rapid judgements about whether or not two sets of stimuli are the same or different. In the letter comparison task participants were represented with two pages that contain two columns of letter strings. There were a total of 21 pairs of three to nine letters. The participants task was to write an “S” (for same) of “D” (for different) on the line between the pair of stimuli. The pattern comparison task had the same structure, but consisted of 30 pairs of line patterns containing three to nine line segments. Participants were given 30 seconds per page and instructed to complete the task as rapidly and accurately as possible. The index of performance was the total number of correct items. Estimated reliability for letters is .77 and .87 for pattern comparison (Salthouse and Meinz, 1995).
Image acquisition
Four series of MRI images were acquired on a 4T MRI system (Bruker Biospin, Ettlingen, Germany) with an 8-channel RF coil. Magnetization-prepared rapid gradient echo (MPRAGE) T1-weighted images were acquired in the coronal plane with the following parameters: TR = 1600 ms, TE = 4.38 ms, TI = 800 ms, FOV = 256 mm ×256 mm, in plane resolution = 0.67 mm × 0.67 mm, slice thickness = 1.34 mm, matrix size = 384×384, number of slices = 176. Fluid-attenuated inversion recovery (FLAIR) images were acquired in the axial plane, with TR = 8440 ms, TE =112 ms, TI=2200 ms, FA = 150°, FOV = 256 mm ×256 mm, in plane resolution = 1 mm × 1 mm, contiguous slice thickness = 2 mm, matrix size = 256 × 256. A Turbo Spin-Echo (TSE) sequence was used to acquire 50 contiguous axial slices of Proton-density (PD)/T2-weighted images with TR = 3700 ms, TE = 19 ms/96 ms, FA = 150°, FOV = 256 × 256 mm2, in plane resolution = 1 × 1 mm2, slice thickness = 2 mm, matrix size = 256 × 256. Diffusion Tensor Images (DTI) were acquired with the following parameters: TR = 4900 ms, TE = 79 ms, 6 diffusion directions, 10 averages, 41 slices, FOV = 256 × 256 mm2, voxel size = 2 × 2 × 3 mm3, GeneRalized Autocalibrating Partially Parallel Acquisitions (GRAPPA) acceleration factor 2. The MR images used in this study were free of pathological findings.
Image analyses
Before processing, we used the MNI software (Montreal Neurological Institute, Montreal, USA) to correct the T1-weighted and FLAIR images for the effects of magnetic field inhomogeneities (Sled et al., 1998). All analyses were performed in native space, with an exception for the T1-weighted images. The latter were transformed into standardized MNI space via a linear transformation, which allowed transfer of the images and templates back into the native space (see Collins et al., 1994) for the linear transformation procedure). The DTI images were preprocessed with the BrainVoyagerQX software, version 1.10 (Brain Innovation, Maastricht, the Netherlands) in native space.
For several post-processing steps we used the custom software package GIANT (Gronenschild et al., 2009). Regions of interest (ROIs) were traced on the T1-weighted image of each participant, and five outcome measurements were calculated within each ROI: volume of the white matter ROIs, volume of the WMH, and DTI-derived indices: fractional anisotropy (FA), linear anisotropy (A) and planar anisotropy (R). The lobar (frontal, parietal, temporal, and occipital) ROI measures described in Burgmans et al. (2010) were used in construction of measurement models.
Volumetric image analysis
The individual ROI templates were overlaid on the T1-weighted images in standardized MNI space. To segment the T1-weighted images into gray matter, white matter and cerebro-spinal fluid, we used the MNI software (Zijdenbos et al., 2002). All voxels that were recognized as white matter were selected within each ROI, which resulted in a segmented ROI template. Finally, the segmented ROI template was transformed back into native space. The ROIs were verified slice-by-slice, and all instances of suboptimal segmentation were corrected manually. Finally, the volumes of each white matter ROI were calculated. To test the reliability of this method, the operator (SB) performed the whole procedure twice on ten randomly selected brains. This procedure yielded high test-retest reliability for each ROI, with intraclass correlation coefficients > 0.90 (ICC, formula 1,1: one-way random effects, Shrout and Fleiss, 1979). To avoid counting abnormal white matter in the volume measures, the volumes of WMH (see below) were removed, lobe-by-lobe, from the white matter volumes.
White Matter Hyperintensities
To quantify the volumes of WMH on the FLAIR images, we used a semi-automatic tool (GIANT). The tool has been validated by manual expert tracings and was used in previous publications (e.g., Henskens et al., 2008). First, the algorithm was trained to classify WMH correctly. For this purpose, the image intensity scale of the FLAIR images was standardized (Nyúl and Udupa, 1999). Next, five FLAIR scans with a substantial amount of white matter lesions were selected and the white matter lesions in these stacks were traced manually. These manual tracings were used to derive parameters for the automatic classification of the WMH. Second, the actual quantification of WMH was performed semi-automatically. Horizontal FLAIR and T2-weighted images were displayed and aligned side by side on the computer monitor. This allowed visual inspection of the scan and easy identification of WMH. In each slice, a WMH was indicated manually by clicking in its region in a FLAIR image, thus generating a seed point and providing starting parameters for region growing. Manual corrections were performed when necessary. Finally, the total volume of the WMH within each ROI was calculated. The WMH quantification was performed twice on ten randomly selected brains by the same rater (SB), and yielded high test-retest reliability: intraclass correlation coefficient = 0.99 (formula ICC 1,1, Shrout and Fleiss, 1979).
Diffusion Tensor Imaging
Diffusion tensor generated by DTI can be visualized as an ellipsoid and its asymmetry can be captured by a normalized index, Fractional Anisotropy (FA, Basser and Pierpaoli, 1996). In addition, the diffusion tensor can be decomposed into two basic geometric entities corresponding to linear and planar diffusion cases (Westin et al., 2002). As described by Westin and colleagues, in the linear case, the relationship among the eigenvalues of the diffusion tensor is λ1 ≫ λ2 ≈ λ3. In that case, diffusion is presumed to occur in the direction of the main axis. In the planar case, λ1 ≈λ2 ≫ λ3, the diffusion is in the plane defined by the first and second principal axes. Linear and planar anisotropy are to some extent comparable with axial and radial diffusivity. The difference is that linear and planar anisotropy are relative measures as they take into account all three eigenvalues, which therefore may be a more robust measure of the direction of water diffusion than each of individual eigenvalues may. We defined three indices of diffusivity: FA, Cl = 1− λ2/λ1, and Cp = (λ2−λ3)/λ1. The later two reflect linear and planar anisotropy, respectively. Decrease in Cl means decrease in linear anisotropy, i.e. reduced likelihood of diffusion in the principal direction or departure from the linear case. Lower Cp values imply reduced planar anisotropy or departure from the planar case. In some studies, eigenvalues of the diffusion tensor are used to compute axial (λ1) and radial (λ2+λ3/2) diffusivity indices. Axial and radial diffusivity increase with age and correlate negatively with FA (Sullivan et al., 2006; Vernooij et al., 2008); linear (Cl) and planar (Cp) anisotropy decrease with age and correlate positively with FA (Wang et al., 2009; Burgmans et al., 2010). In this sample, the correlations between axial diffusivity and linear anisotropy for individual lobes ranged between r=−.57 and −.69, all p <.001. For axial diffusivity and planar anisotropy, the correlations were r = −.67 and −.77, all p < .001.
We used BrainVoyagerQX to generate the FA maps from the DTI images. The images were inspected for relevant motion artifacts, but none were found. Since DTI is very sensitive to data transformation, we minimized manipulations of the original scan data, and performed the analyses in the native space. Each DTI scan was aligned to the T1-image in native space and an FA map was calculated without applying smoothing filters. The mean FA values of the individual brains in native space were used for the statistical analyses. To isolate the white matter and to exclude the gray matter and CSF voxels, we applied a threshold of FA > 0.20. The accuracy of this white matter FA map was verified by visual inspection after overlaying the FA map on the T1-weighted image and evaluating its match to the white matter. Finally, the individual ROI template was overlaid on the FA map and the mean FA of each ROI was calculated. The same procedure was followed in generating maps of the linear tensor component (Cl) that is comparable though not equivalent to axial anisotropy (and hence designated in this paper as A) and planar tensor component, Cp, that is comparable to radial anisotropy (and hence designated as R). However, no additional thresholding was applied to those maps. Reliability of all measures determined on 10 randomly sampled brains and is reported in detail elsewhere (Burgmans et al., 2010)
Statistical analyses
To compute the descriptive statistics and evaluate the effects of sex and hypertension we used an ordinary least-squares General Linear Model (GLM) approach. Group differences of the categorical variables were assessed with the χ2 test. For the main analyses and hypotheses testing, we used latent variable structural equation modeling (SEM) implemented in Mplus (Version 5.2; Muthén and Muthén, 1998–2010). SEM is a powerful analytical tool that allows testing theory-driven hypotheses about mutual influences of multiple variables and directionality of these effects in a wide variety of a priori models (Kline, 2005). A typical SEM model consists of a measurement model, representing the relationship of a number of observed variables to an unobserved construct, as well as a structural model that characterizes the flow of variance among the latent variables (Bollen, 1989, 2002). Using latent variables in mediation analysis allows accounting for measurement error in the observed variables that can otherwise potentially reduce mediation relations (MacKinnon, 2008). In the SEM framework, the focus is not on specific measurements but on the constructs that those measurements are presumed to assess.
All structural models in this study were evaluated via maximum likelihood estimation with several indices of model fit. Models were considered an acceptable fit with χ2/df of less than 2, comparative fit index of more than .91 (CFI; Bentler, 1990), and a root-mean-square error of approximation of less than .08 provided that it included .05 within its 90% confidence interval (RMSEA; Browne and Cudeck, 1993; Steiger, 1990). Nested models were compared by testing the significance of the difference with χ2, with the degrees of freedom equal to the difference in the number of free parameters between the two models. The threshold of statistical significance was p < .05.
Results
Sample characteristics and cognitive measures
Descriptive data for all tasks are presented in Table 1. Intracranial volume (ICV) was used to correct white matter volumes for differences in head and body size via a linear equation: Volumeadji = Volumei − b(ICVi − Mean ICV) for each participant i. In this equation, Volumeadji is the adjusted ROI volume, Volumei is the raw ROI volume, b is the slope of the ROI volume regression on ICV, and Mean ICV is the sample mean of the intracranial volume.
We tested the skewness and kurtosis of all variables against zero by using the z-distribution with |z| > 3, p < .01 criterion for problematic values (Tabachnik & Fidell, 2006). Due to high skewness and kurtosis the WMH measures were log-transformed, although the distribution remained somewhat skewed even after the transformation. The distributions of five out of the ten cognitive variables, including the verbal 1-back and 2-back tasks, the STROOP reading speed and errors as well as the number of WCST perseverative errors were also skewed, but were normalized by log transformations. To avoid scaling discrepancies and to facilitate interpretation of results, all variables were z-transformed for subsequent analyses.
As evident from Table 1, intracranial volume was larger in men than in women and the blood pressure was significantly higher in the hypertensive participants compared to their normotensive counterparts, despite of the effects of anti-hypertensive medication. However, the normotensive and hypertensive participants did not differ with respect to age, education, MMSE, and intracranial volume, and distribution of sex and ethnicity. With regard to the cognitive measures, men performed significantly better on the WCST than women and had better reaction times on the N-back verbal task. In addition, there was a sex × hypertension interaction on the N-back nonverbal task.
Structural equation modeling analyses
Although the primary focus of this study was the test of mediation models of age-related differences in cognition, we first present separate analyses of white matter integrity measures and the indices of cognitive ability, as well as their relationship to age. After these preparatory analyses, we present the core findings of the study, i.e., a series of structural models that examine the extent to which age differences in white matter integrity mediate age differences in cognitive ability.
Cognitive performance variables
The ten cognitive tests were hypothesized to measure two different cognitive constructs: Speed of Processing (Speed) and Executive Functioning (EF). To examine the factor structure of the test battery, a latent-factor model with two independent factors was specified using confirmatory factor analysis approach (Bentler and Mooijaart, 1989) (Figure 1). That model reproduced the data quite well: χ2 = 52.43, df = 44, CFI = .97, RMSEA = .05 (90% CI = 0 to .09). A one-factor model fitted the data poorly, suggesting that EF and Speed are separate though correlated factors. The correlation between EF and Speed was r = .45 (p < .05).
Figure 1.
Measurement model for cognitive performance variables. * p < .05, standardized path coefficients. Abbreviations: NB – N-back, V – verbal, NV – nonverbal, ER – errors, RT – response time, WCST – Wisconsin Card Sorting Test, perseverative errors; LetCom and PatCom – letter and pattern comparison.
In the next step, age was included as an independent, error-free variable by regressing EF and Speed on age. The fit for this model was good, χ2 = 60.89, df = 52, CFI = .97, RMSEA = .04 (90% CI = .00 to .08). The regression paths between EF and age and between Speed and age were significant. Fixing these paths to zero resulted in significant loss in model fit, and they were retained in the model. Thus, the cognitive measures conformed well to a structure with two separate and moderately correlated constructs, both negatively associated with age.
Age differences in white matter integrity
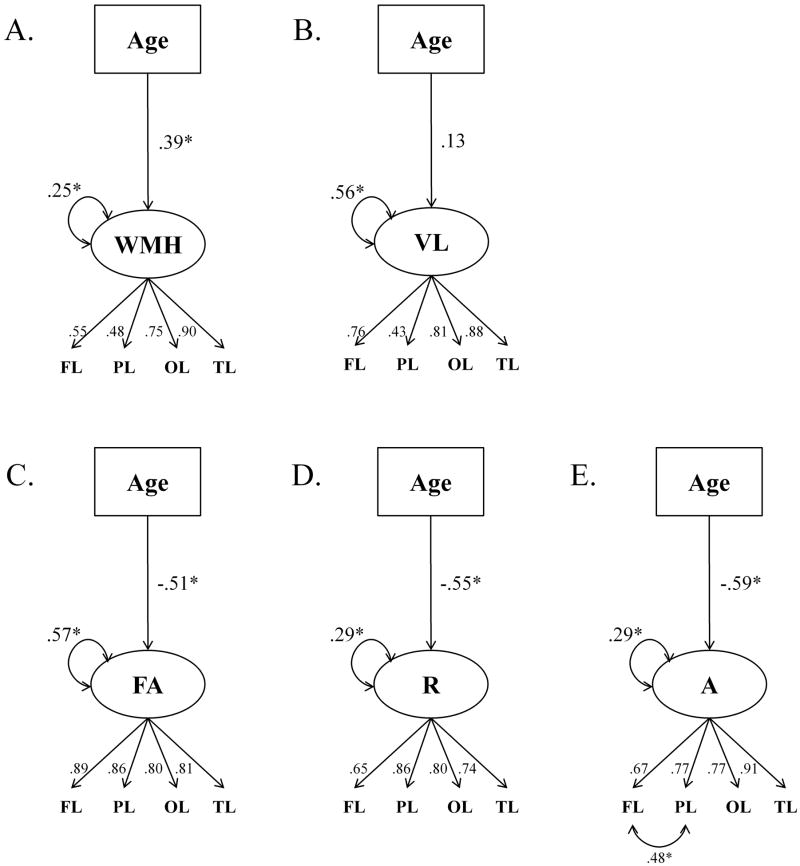
We obtained five different measures of white matter integrity within four lobes: frontal (FL), parietal (PL), temporal (TL) and occipital (OL)1. These measures included regional white matter volume, regional volume of WMH, fractional anisotropy (FA), linear anisotropy (A), and planar anisotropy (R). Thus, there were 20 white-matter measures, and with less than 100 participants, data reduction was in order. Because volume, WMH, and anisotropy indices reflect relatively independent properties of the cerebral white matter, and were uncorrelated in this sample (Burgmans et al., 2010), we specified and evaluated separate measurement models for each set of the indices. However, because FA, A, and R are believed to reflect different properties of the white matter and may exert differential effects on cognition (Song et al., 2003; Song et al., 2005; Sun et al., 2006; Zhang et al., 2008), we specified three separate2 models for each of the DTI-based measures of white matter integrity. Thus, we fitted five models to the white matter data. Although the findings pertaining to the white matter integrity measures in this sample have been reported in our previous publication (Burgmans et al., 2010), those data were not analyzed in SEM framework as presented here.
The model for white matter volume included one latent factor represented by its corresponding values for FL, PL, TL, OL. This model fitted the data very well, χ2 = 6.47, df = 6, CFI = 1.00, RMSEA = .03 (90% CI = .00 to .14). Similarly, in a separate model, WMH was represented by one latent factor measured by its corresponding WMH volumes in FL, PL, TL, OL. This model showed excellent fit, χ2 = 1.61, df = 6, CFI = 1.00, RMSEA = .00 (90% CI = .00 to .00). The similar model for FA fitted the data very well, χ2 = 5.14, df = 6, CFI = 1.00, RMSEA = .00 (90% CI = .00 to .13). The final models for R and A3 showed acceptable fit (for R: χ2 = 11.59, df = 6, CFI = .97 RMSEA = .10 (90% CI = .00 to .19), for A: χ2 = 8.13, df = 5, CFI = .99 RMSEA = .08 (90% CI = .00 to .18). Thus, all indices of white matter integrity yielded robust latent factors reflecting the respective white matter properties across the brain lobes.
Finally, age was included as independent, error-free variable in each of the measurement models by regressing each of the latent white matter factors on age. The fit for the resulting model of white matter volume was good, χ2 = 7.69, df = 9, CFI = 1.00, RMSEA = .00 (90% CI = .00 to .10). The regression path between age and the Volume factor was not significant and age did not explain a significant amount of variance in the Volume factor (R2 = .02). For WMH, inclusion of age resulted in excellent model fit, χ2 = 3.88, df = 9, CFI = 1.00, RMSEA = .00 (90% CI = .00 to .04). The regression path between age and WMH was significant and the amount of variance in the WMH factor explained by age was R2 = .15 (p = .05). For FA, the inclusion of age resulted in a good model fit, χ2 = 15.30, df = 9, CFI = .98, RMSEA = .09 (90% CI = .00 to .16). FA was negatively related to age and the variance in FA explained by age was R2 = .26 (p < .05). Age also accounted for a significant proportion of variance in R, R2 = .31 (p < .05), χ2 = 13.29, df = 9, CFI = .98, RMSEA = .07 (90% CI = .00 to .15). Including age in the model for A resulted in a good model fit, χ2 = 14.65, df = 8, CFI = .97, RMSEA = .10 (90% CI = .00 to .17). The regression path between age and A was significant and the proportion of variance in A explained by age was R2 = .35 (p < .05). Thus, all indices of white matter integrity formed coherent constructs, but evidenced differential relation with age. As previously demonstrated in a different type of statistical analyses, diffusion-based measures showed substantially stronger association with age than WMH burden, whereas gross volume of the white matter exhibited no discernable age trends.
After establishing the pattern of age differences in various indices of white matter integrity, we investigated what if any role those differences played in mediating age differences in cognition. Furthermore, we examined whether hypertension affected white matter integrity, cognition, and their relationships with age.
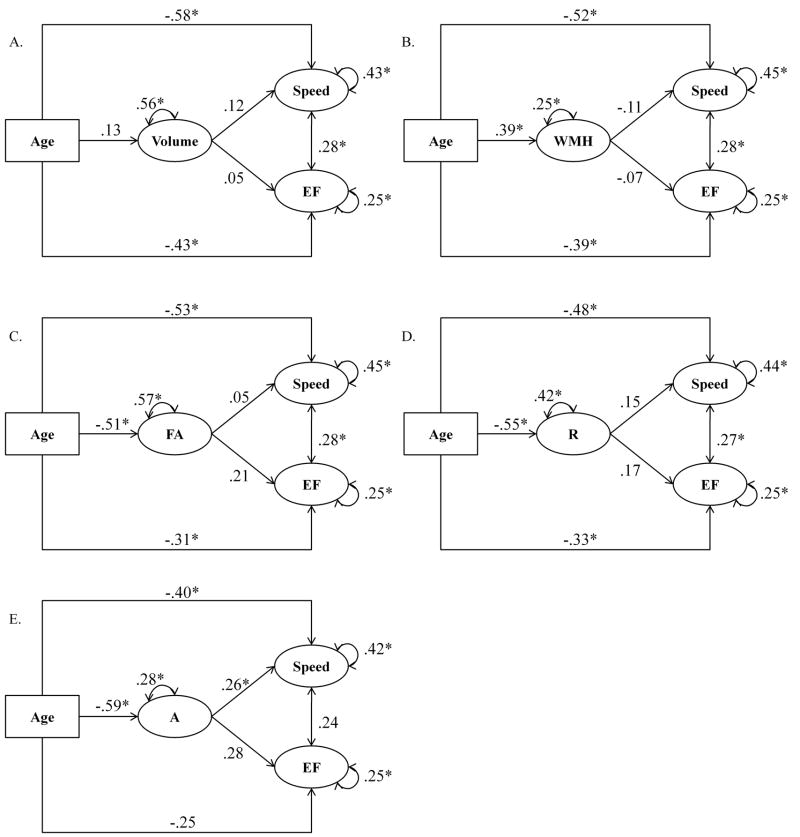
Mediation of age differences in cognition by white matter integrity: Structural models
Figure 3 depicts the hypothesized structural model for white matter volume as a mediator of age effects. This model reproduced the data well: χ2 = 130.69, df = 99; CFI = .94; RMSEA = .06 (90% CI = .03 to .08). However, none of the regression paths between Volume and Speed and Volume and EF were significant. The mediation model for WMH also reproduced the variances-covariances structure of the data very well, χ2 = 113.11, df = 99; CFI = .97; RMSEA = .04 (90% CI = .00 to .07), but did not mediate the effect of age on Speed or EF.
Figure 3.
Mediation of age differences in cognition by white matter integrity: structural models for A. white matter volume, B. volume of the white matter hyperintensities, WMH, C. fractional anisotropy, FA, D. planar (≈radial) anisotropy, R, and E. linear (≈axial) anisotropy, A. * p < .05, standardized path coefficients.
With regard to the DTI-based measures, the structural model including age effect on FA and FA effect on Speed and EF resulted in a good model fit (χ2 = 107.10, df = 99; CFI = .99; RMSEA = .03 (90% CI = .0 to .06) but FA did not mediate the relationship between age and Speed or age and EF. Similarly, the regression of Speed and EF on R was not significant: χ2 = 129.57.71, df = 99; CFI = .94; RMSEA = .06 (90% CI = .02 to .08). Finally, the structural model for A was specified so that age affected A, which in turn affected Speed and EF. This model showed a good fit: χ2 = 111.66, df = 98; CFI = .98; RMSEA = .04 (90% CI = .00 to .07). Moreover, the relationship between Speed and A was positive and significant (β = .26, p = .040), indicating that older adults with lower linear anisotropy (greater deviation from the canonical linear case) exhibited lower processing speed. In addition, the relationship between EF and A approached statistical significance, (β = .28, p = .052). Setting the path between A and Speed to zero significantly decreased the model fit: Δχ2 = 4.07, df = 1, p < .05. In contrast, when the path between A and EF was set to zero, the decrease in model fit was not significant: Δχ2 = 3.5, df = 1, p = .06.
We tested the indirect effect of age on Speed using bootstrapping approach (Shrout and Bolger, 2002), 2002; 95 % confidence interval with 10000 samples as implemented in Mplus 5.2). In an indirect effect a × b, a denotes the path between age and A, and b denotes the path between A and Speed. The 95% confidence interval for that effect estimate did not include zero: a × b = −.16 (p < .05, 95% CI = −.31 to −.003). Thus, the mediation effect of A was robust. Taken together, these results indicate that reduced linear anisotropy partially mediates the negative effect of age on speed of processing. In contrast, the effect of A on EF was not robust. In addition, the regression path from A to EF did not survive the bootstrap test.
To examine the contribution of different brain regions to the mediation effect more closely, we tested a mediation model in which instead of a common A factor, we included posterior and anterior A factors as mediators. The anterior A factor was represented by the FL measure, whereas the posterior A factor was represented by the PL, TL, and OL measures (Figure 4). This model showed good fit (χ2 = 69.996, df = 50; CFI = .96; RMSEA = .07 (90% CI = .02 to .10)). Although the posterior and anterior factors were highly correlated (r = .66, p < .05), we found a significant relationship between posterior A and Speed (β = .44, p = .020), but not between anterior A and Speed (β = −.21, p = .188).
Figure 4.

Mediation of age differences in cognition by posterior and anterior structural models for linear (≈axial) anisotropy, A. * p < .05, standardized path coefficients.
Two potentially different (though related) sets of manifest variables indexed the latent construct of Speed: more cognitive paper-and-pencil measures (Letter and Pattern Comparisons) and reaction time indices (low-load n-back conditions). To examine the relationships between the A factor and different speed measures more closely, we conducted two subsidiary mediation analyses. In the first, the Speed factor was formed only from LetComp and PatComp (the “cognitive measures”), with A and EF included in the model, as discussed above. This model showed good fit (χ2 = 51.16, df = 48; CFI = .99; RMSEA = .03 (90% CI = .00 to .07)) and the loading of Speed on A was significant (β = .41, p = .013). In the second subsidiary analysis, the Speed factor was indexed only by the reaction time measures, with A and EF in the model. That model also showed good fit (χ2 = 80.76, df = 71; CFI = .98; RMSEA = .04 (90% CI = .00 to .07)) but the loading of Speed on A was lower than for the cognitive speed index: β = .22, p = .09. Thus, the mediation effect seems to be stronger for the cognitive rather than reaction time tasks.
We tested the effect of hypertension on the white matter and cognition by entering hypertension status as a dummy variable in the measurement models for white matter volume, WMH, DTI-based measures and cognitive performance. No effect of hypertension was present for any of the latent factors.
Discussion
The main finding in this study is that a specific age-sensitive property of white matter microstructure mediated, in part, age differences in a specific cognitive characteristic. Specifically, reduced linear anisotropy believed to reflect breakdown of axonal organization (Harris et al., 2008; Song et al., 2003; Wang et al., 2009) mediated age-related slowing of information processing. In contrast, fractional and planar anisotropy that were just as much affected by age, evidenced no similar influence on cognitive performance.
As we observed the A and R factors are highly collinear in the model of white matter integrity (see footnote 2), these results suggest that the mediator effect of A extends beyond the common variance shared with the R factor. These results support our assumption that linear (approximately axial) and planar (approximately radial) anisotropy, although closely related to each other, might play different roles in cognitive aging.
In an apparent contradiction to some of the earlier correlational findings (Burns et al., 2005; Charlton et al., 2006; De Groot et al., 2000; Grieve et al., 2007; Gunning-Dixon and Raz, 2000, 2003; Kennedy and Raz, 2009; Madden et al., 2009b; Raz et al., 2003; Schiavone et al., 2009; Vernooij et al., 2009; Zahr et al., 2009; Ziegler et al., 2008), we observed no associations between other aspects of white matter integrity and cognitive performance. The reasons for that discrepancy may be manifold but it is important to note that those studies employed statistical methods that provide only partial account of the complex relationships among age, brain and cognition. Moreover, the present results are in accord with previous studies that used structural equation modeling, albeit without latent variable analyses. For example, Charlton et al. (2008) did not find a mediating effect of a global index of white matter integrity, white matter mean diffusivity, on processing speed and mental flexibility. Similarly, Head et al. (2008, 2009) did not find evidence for a mediating effect of white matter volume on speed and executive functioning. More recently, an association between diffusion properties of the white matter and speed of processing operationalized as reaction time was observed in a sample of older adults (about 72 yeas of age) along with the lack of association between white matter integrity and general intelligence or memory (Penke et al., 2010). Almost none of those studies (except the latter) examined the influence of a specific diffusion measures such as linear anisotropy on cognitive constructs.
The finding of an association between radial but not axial diffusivity and reaction time (Penke et al., 2010) differs from observations reported here. However, in that study, only reaction time indices formed the Speed factor, and only persons in a narrow age range were examined. The reasons for the observed discrepancy are unclear. It is possible that in that group, motor slowing exerted strong influence on the reaction time, and different types of barriers to diffusion of brain water dominated the anisotropy values. For example, one may speculate that in septuagenarians, the influence of myelin wrapping the axons overshadows the effect of disrupted axonal integrity. Combining DTI with imaging methods that are more sensitive to myelin content may allow testing that proposition. However, the results of SEM analyses presented here in the extant literature reinforce the need for multivariate assessment of structure – function relationships in the aging brain and caution regarding the findings that emerge from examining correlations between single measures of brain and cognition. Latent variable methods that have been applied to study associations between structural brain data and cognition (McArdle et al, 2004; Raz et al., 2005; Raz et al., 2008) may prove especially fruitful in that regard.
In comparing speed measures derived from RT tasks (1- and 2-back) and those obtained from pattern and letter comparisons we found that the association of speed factor with axonal integrity was dominated by the latter. This difference could not be attributed to differential reliability, as the reliability coefficients for all measures were comparable. However, we cannot definitively conclude that age-related differences in axonal anisotropy measures play more important role in tasks with ostensibly higher perceptual-cognitive demands. Standard instructions for n-back measures did not contain any indication of speed or accuracy demands, whereas pattern comparison tasks occurred under explicit speed-accuracy instruction. Thus, lesser constraints on respondent behavior could have produced responses with a greater variance thus weakening the association between RT and axial anisotropy. However, the coefficients of variation (mean/standard deviation) did not vary substantially across tasks and ranged from .17 for pattern comparison to .25 for letter comparison with a median of .22. Thus, differences in variance among tasks are unlikely to explain the observed pattern of results.
Notably, we observed no direct effects of linear anisotropy (or any other index of white matter integrity) on executive functions. Although some trends suggested that such association might exist and moreover, that the effect of linear axonal integrity on age differences in executive functions may be mediated via its effect on speed of processing, the obtained results were not sufficiently robust. Nonetheless, the fundamental role of speed of processing in executive functions and specifically in their age-related deterioration has been proposed in the past (Salthouse 1996), and that hypothesis is certainly worth pursuing in future studies.
The lack of associations between white matter volume and cognitive performance in this study adds to the literature marked by inconsistent findings (e.g. Gunning-Dixon and Raz, 2003; Head et al., 2008; Raz et al., 1998; see Raz, 2005 for a review). Moreover, the likelihood of finding age differences in white matter volume depends on multiple factors: the age range of the sample, the precision of regional demarcation, and presence of vascular risk, to name a few. With regard to WMH, one should keep in mind that although the literature suggests a moderate association between WMH burden and age-sensitive cognitive measures (Gunning-Dixon and Raz, 2000), it is based mainly on samples of older adults with significant WMH burden. In our sample of relatively healthy middle-aged and older adults, the load of WMH could have been too small to play a role in predicting executive performance or processing speed. It is possible that in a sample with more typical age-related increase vascular risk and related expansion of WMH, one could observe a more significant contribution of the latter.
Diffusion characteristics of the white matter may tap into more subtle alterations in the white matter than WMH do. Indeed, studies of normally appearing white matter in the vicinity of bona fide white matter lesions demonstrated impaired diffusion properties in comparison to more distant and presumably intact white matter regions (Engelter et al., 2000; Guo et al., 2001). Altered FA as well as radial and axial anisotropy can distinguish normal aging from even mild age-related cognitive pathology (Huang et al., 2007). Reduction in orderly structure of the white matter, through axonal damage and demyelination may be a harbinger of gradual disruption (Taylor et al., 2007) that degrades computational capacity and reduces redundancy of a massive parallel network that supports high processing speed. If this is the case, our findings may support for disconnection hypothesis of age-related cognitive declines.
In the prior analysis of the white matter measures in this sample (Burgmans et al., 2010), we found that vascular risk (hypertension) exacerbates age differences in white matter diffusion properties. Here, however, we found no significant effect of hypertension on relationship between white matter integrity (linear anisotropy) and age-sensitive cognitive skills. The reasons for that outcome are unclear, but relatively small sample and significant power demands on testing hypotheses about interactions probably played a role.
The findings presented here should be viewed in the context of several limitations. First, the cross-sectional design precludes assessment of causality. Although we estimated mediating effects and evaluated the contribution of individual differences in white matter integrity to age-related differences in cognitive functions, only longitudinal design can separate the effects of age and correlated variables on cognitive functioning (e.g., Lindenberger and Pötter, 1998). Second, the sample size and the proportion of older participants could have been too small to reveal subtle differences in white matter integrity that tend to emerge in later decades of the normal life span, and the links between those differences and cognitive performance. Third, age-related differences in white matter volume may be limited to specific circumscribed locales, and the analytic methods employed in this study could have been too coarse to detect such associations. Relatively coarse partitioning of the brain into lobes was dictated by our objective to compare volume, WMH and DTI-based assessments of white matter integrity, in which we had to adopt a level of analysis that was permitted by the “weakest link,” i.e. WMH. In any case, with only six diffusion directions we did not have enough data for reliable application of more sophisticated methods of DTI analyses such as tractography and tract-based statistics. It is possible that such tractographic analyses will lead to discovery associations between white matter diffusion properties and cognition that were not observed here. However, in a recent study of septuagenarians, no specific tract effects on reaction time were found beyond the general effect of white matter integrity (Penke et al., 2010). Fourth, we included four executive tests to measure executive functioning. Although some properties of the white matter were associated with performance on all of those tests, for others such links were limited to selected test scores. Because of significant construct heterogeneity of executive functions, it is possible that white matter deterioration might have a different effect on other measures that tap into different executive processes.
In conclusion, our results indicate that reduced linear anisotropy believed to reflect breakdown of axonal organization mediates age-related slowing of processing. Thus, our findings provide partial support for disconnection hypothesis of cognitive aging. No other aspects of white matter integrity were significantly associated with cognitive performance. Our results suggest that in healthy adults, the mediating role of white matter integrity on age-related cognitive decline is limited. On the other hand, our results show that DTI measures, in particular linear anisotropy, are more likely to detect the effects of white matter differences on age differences in cognition than volume measures and WMH do.
Supplementary Material
Figure 2.
Measurement models for the indicators of white matter integrity: A. Volume of white matter hyperintensities (WMH) B. Gross volume (VL), C. Fractional anisotropy (FA), D. Planar (≈radial) anisotropy (R), and E. Linear (≈axial) anisotropy (A). * p < .05, standardized path coefficients.
Acknowledgments
We thank Kristen Kennedy, Karen Rodrigue, Cheryl Dahle, Andrew Bender, Awantika Deshmukh, Yiqin Yang, and Peng Yuan, Wayne State University (Detroit, MI) for preparation of the MRI images and Pim Pullens, Maastricht University (Netherlands), for help in DTI analyses. We also thank Claude Lepage (Montreal Neurological Institute) for assistance in the use of the MNI software. The study was supported by a National Institutes of Health grant R37 AG-011230 to NR.
Footnotes
Disclosure statement
The authors have no actual or potential conflicts of interest associated with this research.
Although addition regions (genu, body, and splenium of the corpus callosum) were available in this data set (Burgmans et al., 2010), we decided not include them in the models: there were no WMH in those regions, their inclusion into volume and DTI models worsen the fit, and their inclusion in the mediation models did not affect the results.
When all three diffusion-base indices (A, R, FA) were entered simultaneously in one model, the resulting model fit was poor, probably due to multicollinearity among the DTI-based indices (Kline, 2005).
The initial model fit of the A model was relatively poor, χ2 = 27.31, df = 6, CFI = .90, RMSEA = .20 (90% CI = .13 to .28). Therefore, following the modification indices for the model we allowed for the residual correlations between the A measures for PL and FL to be estimated (Figure 2).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Bentler PM, Mooijaart A. Choice of structural model via parsimony: a rationale based on precision. Psychol Bull. 1989;106:315–317. doi: 10.1037/0033-2909.106.2.315. [DOI] [PubMed] [Google Scholar]
- Bollen KA. Structural Equations with Latent Variables. Wiley; New York, NY: 1989. [Google Scholar]
- Bollen KA. Latent Variables in Psychology and the Social Sciences. Annual Review of Psychology. 2002;53:605–634. doi: 10.1146/annurev.psych.53.100901.135239. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Zimmerman ME, Paul RH, Grieve SM, Tate DF, Cohen RA, Williams LM, Clark CR, Gordon E. Regional white matter and neuropsychological functioning across the adult lifespan. Biol Psychiatry. 2006;60:444–453. doi: 10.1016/j.biopsych.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing structural equation models. Sage Publications; Newbury Park, CA: 1993. [Google Scholar]
- Burgmans S, van Boxtel MP, Gronenschild EH, Vuurman EF, Hofman P, Uylings HB, Jolles J, Raz N. Multiple indicators of age-related differences in cerebral white matter and the modifying effects of hypertension. Neuroimage. 2010;49:2083–2093. doi: 10.1016/j.neuroimage.2009.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JM, Church JA, Johnson DK, Xiong C, Marcus D, Fotenos AF, Snyder AZ, Morris JC, Buckner RL. White matter lesions are prevalent but differentially related with cognition in aging and early Alzheimer disease. Arch Neurol. 2005;62:1870–1876. doi: 10.1001/archneur.62.12.1870. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Barrick TR, McIntyre DJ, Shen Y, O’Sullivan M, Howe FA, Clark CA, Morris RG, Markus HS. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006;66:217–222. doi: 10.1212/01.wnl.0000194256.15247.83. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Landau S, Schiavone F, Barrick TR, Clark CA, Markus HS, Morris RG. A structural equation modeling investigation of age-related variance in executive function and DTI measured white matter damage. Neurobiol Aging. 2008;29:1547–1555. doi: 10.1016/j.neurobiolaging.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- De Groot JC, De Leeuw FE, Oudkerk M, Van Gijn J, Hofman PAM, Jolles J, Breteler MM. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol. 2000;47:145–151. doi: 10.1002/1531-8249(200002)47:2<145::aid-ana3>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- Dobbs AR, Rule BG. Adult age differences in working memory. Psychol Aging. 1989;4:500–503. doi: 10.1037//0882-7974.4.4.500. [DOI] [PubMed] [Google Scholar]
- Engelter ST, Provenzale JM, Petrella JR, DeLong DM, MacFall JR. The effect of aging on the apparent diffusion coefficient of normal-appearing white matter. AJR Am J Roentgenol. 2000;175:425–430. doi: 10.2214/ajr.175.2.1750425. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive aging, executive function, and fractional anisotropy: A diffusion tensor MR imaging study. Am J Neuroradiol. 2007;28:226–235. [PMC free article] [PubMed] [Google Scholar]
- Gronenschild EHBM, Burgmans S, Smeets F, Vuurman EFPM, Uylings HBM, Jolles J. A time-saving and facilitating approach for segmentation of anatomically defined cortical regions: MRI volumetry. Psychiatry Research: Neuroimaging. 2009 doi: 10.1016/j.pscychresns.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: A quantitative review. Neuropsychology. 2000;14:224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: a prospective MRI study. Neuropsychologia. 2003;41:1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Guo AC, Jewells VL, Provenzale JM. Analysis of normal-appearing white matter in multiple sclerosis: comparison of diffusion tensor MR imaging and magnetization transfer imaging. AJNR Am J Neuroradiol. 2001;22:1893–1900. [PMC free article] [PubMed] [Google Scholar]
- Haris M, Kumar S, Raj MK, Das KJ, Sapru S, Behari S, Rathore RK, Narayana PA, Gupta RK. Serial diffusion tensor imaging to characterize radiation-induced changes in normal-appearing white matter following radiotherapy in patients with adult low-grade gliomas. Radiat Med. 2008;26:140–150. doi: 10.1007/s11604-007-0209-4. [DOI] [PubMed] [Google Scholar]
- Head D, Kennedy KM, Rodrigue KM, Raz N. Age differences in perseveration: Cognitive and neuroanatomical mediators of performance on the Wisconsin Card Sorting Test. Neuropsychologia. 2009;47:1200–1203. doi: 10.1016/j.neuropsychologia.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head D, Rodrigue KM, Kennedy KM, Raz N. Neuroanatomical and cognitive mediators of age-related differences in episodic memory. Neuropsychology. 2008;22:491–507. doi: 10.1037/0894-4105.22.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henskens LH, Kroon AS, van Oostenbrugge RJ, Gronenschild EH, Fuss-Lejeune MM, Hofman PA, Lodder J, de Leeuw PW. Increased aortic pulse wave velocity is associated with silent cerebral small-vessel disease in hypertensive patients. Hypertension. 2008;52:1120–1126. doi: 10.1161/HYPERTENSIONAHA.108.119024. [DOI] [PubMed] [Google Scholar]
- Hogan AM, Vargha-Khadem F, Saunders DE, Kirkham FJ, Baldeweg T. Impact of frontal white matter lesions on performance monitoring: ERP evidence for cortical disconnection. Brain. 2006;129:2177–2188. doi: 10.1093/brain/awl160. [DOI] [PubMed] [Google Scholar]
- Horn JL. Intellectual stability concepts. In: Steinberg RJ, editor. Advances in the Psychology of Human Intelligence. Erlbaum; Hillsdale, NJ: 1986. pp. 35–77. [Google Scholar]
- Huang J, Friedland RP, Auchus AP. Diffusion tensor imaging of normal-appearing white matter in mild cognitive impairment and early Alzheimer disease: preliminary evidence of axonal degeneration in the temporal lobe. AJNR Am J Neuroradiol. 2007;28:1943–1948. doi: 10.3174/ajnr.A0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultsch DF, Hertzog C, Dixon RA. Ability correlates of memory performance in adulthood and aging. Psychol Aging. 1990;5:356–368. doi: 10.1037//0882-7974.5.3.356. [DOI] [PubMed] [Google Scholar]
- Ingram F, Greve KW, Ingram PT, Soukup VM. Temporal stability of the Wisconsin Card Sorting Test in an untreated patient sample. Br J Clin Psychol. 1999;38 (Pt 2):209–211. doi: 10.1348/014466599162764. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. Aging white matter and cognition: Differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47:916–927. doi: 10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. 2. Guilford Press; New York, NY: 2005. [Google Scholar]
- Kroll JF, Potter MC. Recognizing words, pictures, and concepts: A comparison of lexical, object, and reality decisions. Journal of Verbal Learning and Verbal Behavior. 1984;93:39–66. [Google Scholar]
- Lindenberger U, Pötter U. The Complex Nature of Unique and Shared Effects in Hierarchical Linear Regression: Implications for Developmental Psychology. Psychological Methods. 1998;3:218–230. [Google Scholar]
- Little TD, Lindenberger U, Nesselroade JR. On selecting indicators for multivariate measurement and modeling with latent variables: When “good” indicators are bad and “bad” indicators are good. Psychological Methods. 1999;4(2):192–211. [Google Scholar]
- Lord FM. The Utilization of Unreliable Difference Scores. Journal of Educational Psychology. 1958;49:150–152. [Google Scholar]
- MacKinnon DP. Introduction to Statistical Mediation Analysis. Erlbaum; Mahwah, NJ: 2008. [Google Scholar]
- Madden DJ, Bennett IJ, Song AW. Cerebral White Matter Integrity and Cognitive Aging: Contributions from Diffusion Tensor Imaging. Neuropsychol Rev. 2009a doi: 10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, Davis SW, Dennis NA, Provenzale JM, Huettel SA. Cerebral white matter integrity mediates adult age differences in cognitive performance. J Cogn Neurosci. 2009b;21:289–302. doi: 10.1162/jocn.2009.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle JJ, Hamagami F, Jones K, Jolesz F, Kikinis R, Spiro R, Albert MS. Structural modeling of dynamic changes in memory and brain structure using longitudinal data from the Normative Aging Study. J Gerontol B Psychol Sci Soc Sci. 2004;59:P294–304. doi: 10.1093/geronb/59.6.p294. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 6. Muthén & Muthén; Los Angeles, CA: 1998–2010. [Google Scholar]
- Nyúl LG, Udupa JK. On standardizing the MR image intensity scale. Magn Reson Med. 1999;42:1072–1081. doi: 10.1002/(sici)1522-2594(199912)42:6<1072::aid-mrm11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Penke L, Munoz Maniega S, Murray C, Gow AJ, Hernandez MC, Clayden JD, Starr JM, Wardlaw JM, Bastin ME, Deary IJ. A general factor of brain white matter integrity predicts information processing speed in healthy older people. J Neurosci. 2010;30:7569–7574. doi: 10.1523/JNEUROSCI.1553-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Rucker DD, Hayes AF. Addressing moderated mediation hypotheses: Theory, methods, and prescriptions. Multivariate Behavioral Research. 2007;42:185–227. doi: 10.1080/00273170701341316. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measures. 1977;1:385–401. [Google Scholar]
- Raz N. The aging brain observed in vivo: Differential changes and their modifiers. In: Cabeza R, Nyberg L, Park DC, editors. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging. Oxford University Press; New York: 2005. pp. 17–55. [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12:95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: Vulnerability of the prefrontal regions and executive functions. Behav Neurosci. 2003;117:1169–1180. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: General trends, individual differences, and modifiers. Cerebral Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Ghisletta P, Rodrigue KM, Kennedy KM, Acker JD. Neuroanatomical correlates of fluid intelligence in healthy adults and persons with vascular risk factors. Cerebral Cortex. 2008;18:718–726. doi: 10.1093/cercor/bhm108. 2007 Jul 5; [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes MG. Age-related differences in performance on the Wisconsin card sorting test: a meta-analytic review. Psychol Aging. 2004;19:482–494. doi: 10.1037/0882-7974.19.3.482. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Hancock HE, Meinz EJ, Hambrick DZ. Interrelations of age, visual acuity, and cognitive functioning. J Gerontol B Psychol Sci Soc Sci. 1996;51:P317–330. doi: 10.1093/geronb/51b.6.p317. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Meinz EJ. Aging, inhibition, working memory, and speed. J Gerontol B Psychol Sci Soc Sci. 1995;50:P297–306. doi: 10.1093/geronb/50b.6.p297. [DOI] [PubMed] [Google Scholar]
- Schiavone F, Charlton RA, Barrick TR, Morris RG, Markus HS. Imaging age-related cognitive decline: A comparison of diffusion tensor and magnetization transfer MRI. J Magn Reson Imaging. 2009;29:23–30. doi: 10.1002/jmri.21572. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods. 2002;7:422–445. [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychological Bulletin. 1979;2:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Steiger JH. Structural model evaluation and modification: an interval estimation approach. Multivariate Behavioral Research. 1990;25:173–180. doi: 10.1207/s15327906mbr2502_4. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Sullivan EV, Adalsteinsson E, Pfefferbaum A. Selective age-related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cereb Cortex. 2006;16:1030–1039. doi: 10.1093/cercor/bhj045. [DOI] [PubMed] [Google Scholar]
- Sun SW, Liang HF, Trinkaus K, Cross AH, Armstrong RC, Song SK. Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magn Reson Med. 2006;55:302–308. doi: 10.1002/mrm.20774. [DOI] [PubMed] [Google Scholar]
- Tabachnik BC, Fidell LS. Cleaning up your act: Screening data prior to analysis. In: Tabachnik BC, Fidell LS, editors. Using Multivariate Statistics. 5. Needham Heights, MA: Allyn & Bacon; 2006. pp. 61–116. [Google Scholar]
- Taylor WD, Bae JN, MacFall JR, Payne ME, Provenzale JM, Steffens DC, Krishnan KR. Widespread effects of hyperintense lesions on cerebral white matter structure. AJR Am J Roentgenol. 2007;188:1695–1704. doi: 10.2214/AJR.06.1163. [DOI] [PubMed] [Google Scholar]
- Van der Elst W, Van Boxtel MPJ, Van Breukelen GJ, Jolles J. The Concept Shifting Test: adult normative data. Psychol Assess. 2006a;18:424–432. doi: 10.1037/1040-3590.18.4.424. [DOI] [PubMed] [Google Scholar]
- Van der Elst W, Van Boxtel MPJ, Van Breukelen GJ, Jolles J. The Stroop color-word test: influence of age, sex, and education; and normative data for a large sample across the adult age range. Assessment. 2006b;13:62–79. doi: 10.1177/1073191105283427. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, de Groot M, van der Lugt A, Ikram MA, Krestin GP, Hofman A, Niessen WJ, Breteler MM. White matter atrophy and lesion formation explain the loss of structural integrity of white matter in aging. NeuroImage. 2008;43:470–477. doi: 10.1016/j.neuroimage.2008.07.052. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, Ikram MA, Vrooman HA, Wielopolski PA, Krestin GP, Hofman A, Niessen WJ, Van der Lugt A, Breteler MM. White matter microstructural integrity and cognitive function in a general elderly population. Arch Gen Psychiatry. 2009;66:545–553. doi: 10.1001/archgenpsychiatry.2009.5. [DOI] [PubMed] [Google Scholar]
- Voineskos AN, Rajji TK, Lobaugh NJ, Miranda D, Shenton ME, Kennedy JL, Pollock BG, Mulsant BH. Age-related decline in white matter tract integrity and cognitive performance: A DTI tractography and structural equation modeling study. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.02.009. [Electronic publication ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Wu EX, Qiu D, Leung LH, Lau HF, Khong PL. Longitudinal diffusion tensor magnetic resonance imaging study of radiation-induced white matter damage in a rat model. Cancer Res. 2009;69:1190–1198. doi: 10.1158/0008-5472.CAN-08-2661. Epub 2009 Jan 20. [DOI] [PubMed] [Google Scholar]
- Westin CF, Maier SE, Mamata H, Nabavi A, Jolesz FA, Kikinis R. Processing and visualization for diffusion tensor MRI. Med Image Anal. 2002;6:93–108. doi: 10.1016/s1361-8415(02)00053-1. [DOI] [PubMed] [Google Scholar]
- Zahr NM, Rohlfing T, Pfefferbaum A, Sullivan EV. Problem solving, working memory, and motor correlates of association and commissural fiber bundles in normal aging: a quantitative fiber tracking study. Neuroimage. 2009;44:1050–1062. doi: 10.1016/j.neuroimage.2008.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Du AT, Hayasaka S, Jahng GH, Hlavin J, Zhan W, Weiner MW, Schuff N. Patterns of age-related water diffusion changes in human brain by concordance and discordance analysis. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.10.009. [Electronic publication ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DA, Piguet O, Salat DH, Prince K, Connally E, Corkin S. Cognition in healthy aging is related to regional white matter integrity, but not cortical thickness. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.10.015. [Electronic publication ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijdenbos AP, Forghani R, Evans AC. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imag. 2002;21:1280–1291. doi: 10.1109/TMI.2002.806283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.