Abstract
Aggrecan degradation in articular cartilage occurs predominantly through proteolysis and has been attributed to the action of members of the matrix metalloproteinase (MMP) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) families. Both families of enzymes cleave aggrecan at specific sites within the aggrecan core protein. One cleavage site within the interglobular domain (IGD), between Glu373–374Ala and five additional sites in the chondroitin sulfate-2 (CS-2) region of aggrecan were characterized as “aggrecanase” (ADAMTS) cleavage sites, while cleavage between Ser341–342Phe within the IGD of bovine aggrecan is attributed to MMP action. The objective of this study was to assess the cleavage efficiency of MMPs relative to ADAMTS and their contribution to aggrecan proteolysis in vitro. The analysis of aggrecan IGD degradation in bovine articular cartilage explants treated with catabolic cytokines over a 19-day period showed that MMP-mediated degradation of aggrecan within the IGD can only be observed following day 12 of culture. This delay is associated with the lack of activation of proMMPs during the first 12 days of culture. Analysis of MMP1, 2, 3, 7, 8, 9, 12, 13 and ADAMTS5 efficiencies at cleaving within the aggrecan IGD and CS-2 region in vitro was carried out by the digestion of bovine aggrecan with the various enzymes and Western blot analysis using aggrecan anti-G1 and anti-G3 antibodies. Of these MMPs, MMP12 was the most efficient at cleaving within the aggrecan IGD. In addition to cleavage in the IGD, MMP, 3, 7, 8 and 12 were also able to degrade the aggrecan CS-2 region. MMP3 and MMP12 were able to degrade aggrecan at the very C-terminus of the CS-2 region, cleaving the Glu2047–2048Ala bond which was previously shown to be cleaved by ADAMTS5. However, in comparison to ADAMTS5, MMP3 was about 100 times and 10 times less efficient at cleaving within the aggrecan IGD and CS-2 regions, respectively. Collectively, our results showed that the delayed activation of proMMPs and the relatively low cleavage efficiency of MMPs can explain the minor contribution of these enzymes to aggrecan catabolism in vivo. This study also uncovered a potential role for MMPs in the C-terminal truncation of aggrecan.
Keywords: Aggrecan catabolism, MMP, ADAMTS, Interglobular domain, Chondroitin-sulfate-2 region
1. Introduction
The major function of articular cartilage is to provide a smooth, lubricated interface between bones in joints and resistance to the compressive loads to which the joint is submitted during movement. The structural integrity of articular cartilage extracellular matrix (ECM) is essential for maintaining these functional properties. The ECM consists mainly of a collagen type II fibrous network, which entraps negatively charged proteoglycans. The collagen framework provides the tissue with its tension-resisting properties, while the proteoglycans are responsible for the hydration of cartilage and its ability to withstand compressive forces. The large aggregating chondroitin sulfate proteoglycan, aggrecan, is the main proteoglycan of the tissue. It consists of a~250-kDa core protein composed of three globular domains (N-terminal G1 and G2 domains and a C-terminal G3 domain) and an extended region between the G2 and G3 domains, which is heavily substituted with negatively charged glycosaminoglycan (GAG) chains (keratan sulfate (KS) and chondroitin sulfate (CS)). The GAG chains are further organized into three domains: a KS-rich region, a chondroitin sulfate-1 (CS-1) region and a chondroitin sulfate-2 (CS-2) region (Doege et al., 1991; Kiani et al., 2002). The ~15-kDa sequence between G1 and G2, the interglobular domain (IGD), is highly sensitive to proteolysis (Caterson et al., 2000). Aggrecan proteolysis in this region is extremely detrimental to the tissue function, as it results in the loss of aggrecan degradation products bearing the GAG side chains.
Aggrecan degradation occurs as an early event under pathological conditions and is mediated by pro-inflammatory cytokines, such as interleukin-1β (IL-1β) or oncostatin M (OSM), which are present within the joint (Okamoto et al., 1997; Pratta et al., 2003; Richards, 2004; van den Berg, 1999). Aggrecanases, members of the a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) family of enzymes, are known to be responsible for most of the aggrecan proteolysis occurring under these conditions (Jones and Riley, 2005; Lark et al., 1995). Both ADAMTS4 and ADAMTS5 specifically cleave the Glu373–374Ala bond located in the bovine aggrecan IGD, as well as five additional sites located in the CS-2 region (Loulakis et al., 1992; Sandy et al., 1991; Tortorella et al., 1999; Tortorella et al., 2000; Tortorella et al., 2002). Members of the matrix metalloproteinase (MMP) family, such as MMP3, can also cleave bovine aggrecan within the IGD but at a different site located between the Ser341 and Phe342 residues (Flannery et al., 1992; Fosang et al., 1991, 1992). The extent of MMP contribution to aggrecan catabolism in vivo still remains to be determined. The synthesis and secretion of these proteinases is inducible by catabolic cytokines. However, while aggrecanases may undergo activation in the Golgi prior to secretion (Wang et al., 2004a) or may be activated extracellularly upon secretion (Gao et al., 2004; Longpre et al., 2009; Malfait et al., 2008), MMPs are present in the ECM as inactive zymogens, requiring proteolytic removal of their prodomain to become active (Nagase, 1997; Springman et al., 1990). Such an activation process can be mediated in vitro by chemical agents such as sodium dodecyl sulfate (SDS) or p-amino-phenyl-mercuric acetate (APMA). However, the activation mechanism occurring under physiologic and pathologic conditions in vivo is still unclear for most MMPs.
We have previously observed no sign of MMP action in aggrecan degradation upon short-term (4–8 day) stimulation of cartilage explants with cytokines, while aggrecanases always remained the major aggrecan-cleaving activity detected in the tissue (Durigova et al., 2008a). The objective of this study was to investigate the possible mechanisms explaining the lack of detectable aggrecanolytic MMP activity in cartilage and assess the relative contribution of MMPs to aggrecan proteolysis in later stages of cytokine-stimulation. In addition, the relative efficiencies of aggrecanases and various MMPs on aggrecan IGD and CS-2 cleavage were also determined.
2. Results
In order to investigate the predominant enzymatic mechanism by which aggrecan is cleaved in long-term cultures, cartilage explants were treated with an IL-1β/OSM cytokine mixture for 19 days to promote matrix degradation. Generation of aggrecanase- or MMP-derived degradation products in the tissue resulting from cleavage within the aggrecan IGD was monitored by SDS/PAGE and immunoblotting using an anti-aggrecan G1 or anti-neoepitope antibodies recognizing the new C-terminus NITEGE373 generated by aggrecanase or DIPES341 generated by MMP cleavage (Fig. 1). Such analysis revealed that all of the free G1 fragments accumulating in the tissue during the first 9 days of cytokine stimulation are solely generated by aggrecanase action, the molecular size of these products being consistent with aggrecanase cleavage at the Glu373–374Ala site. A minor degradation product, corresponding to the MMP-derived G1-DIPES341 fragment was detected in the later stages (days 12–19) of IL-1β/OSM stimulation. A similar IGD degradation pattern and delayed MMP action relative to aggrecanases was observed after cartilage was treated with IL-1β alone (data not shown). These observations confirmed that aggrecanolytic MMP activity in response to cytokines is present in the tissue, but only after a prolonged exposure of cartilage explants to the catabolic cytokines.
Fig. 1.
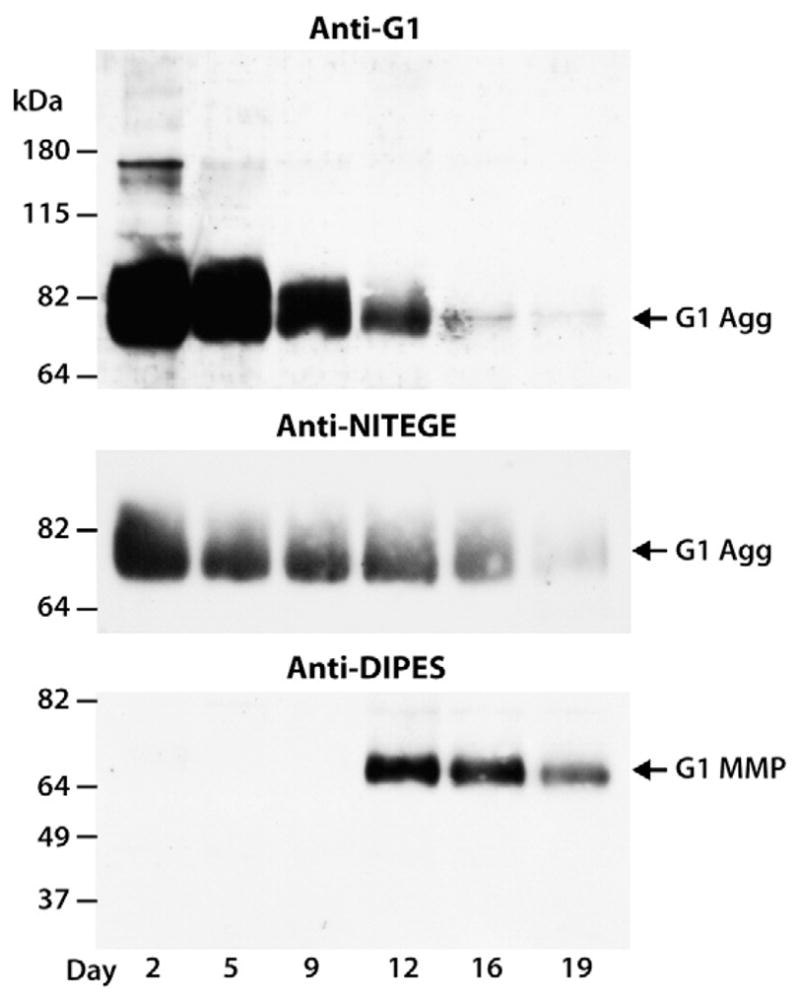
Time-course of aggrecanolysis in response to cytokine stimulation. Bovine articular cartilage explants were cultured for 19 days in the presence of IL-1β/OSM and tissue was collected at different time points and analyzed by SDS/PAGE and immunoblotting. Aggrecan degradation in the IGD was monitored using an anti-aggrecan G1 antibody (anti-G1) or anti-neoepitope antibodies directed against the new C-terminus generated by aggrecanase cleavage at the Glu373–374Ala site in the IGD (anti-NITEGE) and MMP cleavage at the Ser341–342Phe site in the IGD (anti-DIPES). Migration positions of aggrecan products generated by aggrecanase (G1 Agg) or MMP (G1 MMP) action are indicated on the right and the migration positions of molecular weight markers are indicated on the left. The days of culture are indicated below the immunoblots.
In order to discriminate whether the delayed activity of the MMPs is due to a delay in proMMP activation or a delay in the synthesis of the enzymes, the presence of both the latent and active MMPs was monitored in the cultures. Culture media from IL-1β-treated cartilage explants were collected at various time points and submitted to gelatin zymography (Fig. 2). The presence of proMMP2 and proMMP9 was detected throughout the 19 days of cytokine treatment, but active MMP2 and MMP9 was only readily detected following day 12 of culture. ProMMP9 and active MMP9 were always secreted at higher levels than their MMP2 counterparts, and showed a greater tendency to increase in abundance with time. The presence of detectable amounts of active MMP2 and MMP9 in the culture media collected on day 12 of cytokine stimulation is consistent with our previous observations on aggrecanolytic MMP activity which was also detected on day 12, assuming that all MMPs follow a similar activation profile. Thus, even though proMMPs are synthesized and present in the tissue throughout the culture period, their activation occurs only at later stages.
Fig. 2.
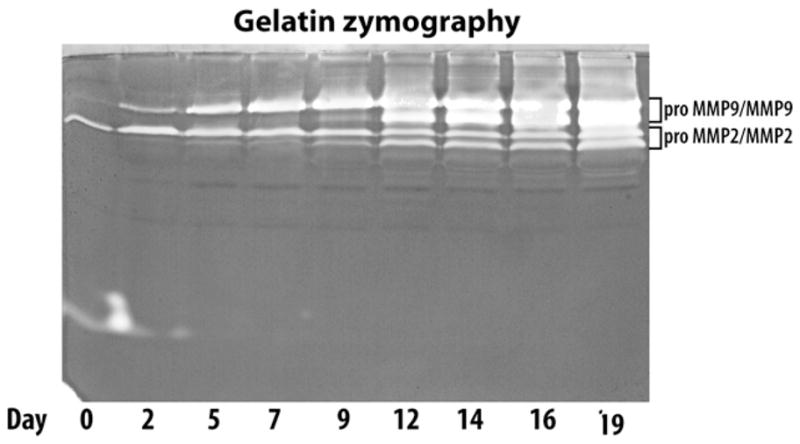
Time-course of MMP2 and MMP9 production and activation in cytokine-treated cartilage. Bovine articular cartilage explants were treated with IL-1β for 19 days. Culture media were collected at various time points, concentrated and analyzed by gelatin zymography. The migration positions of both pro- and active forms of MMP2 and MMP9 are indicated on the right. The days of culture are indicated below the zymograph.
To further investigate whether the lack of detectable MMP activity within the first 12 days of culture is due to the absence of sufficient amounts of aggrecan remaining in the tissue following aggrecanase action, cartilage explants were treated with 1-mM APMA to promote the activation of the proMMPs that are synthesized in response to IL-1β treatment. Cleavage in the IGD was monitored by SDS/PAGE and immunoblotting using an anti-aggrecan G1 antibody, which can detect both the ADAMTS- and MMP-derived aggrecan-G1 fragments (Fig. 3). This cartilage contained endogenous aggrecanase-drived G1 fragments, and analysis at early time points of IL-1β treatment revealed that only aggrecanase-derived aggrecan G1 was generated in the cultures. Addition of APMA was not sufficient to promote earlier MMP-mediated degradation of aggrecan. A G1-containing fragment corresponding in size to that generated by MMP cleavage of aggrecan at the DIPES341–342FFGVG site in the IGD was detected at day 12 and subsequent days in culture (data not shown). However, the cleavage of aggrecan IGD through MMP action was much less abundant than that mediated by aggrecanases. As APMA will presumably activate the lower levels of proMMP present prior to day 12 of culture and yet there is no evidence for MMP-mediated aggrecan cleavage at these times, there appears to be a threshold level of MMP that is necessary in order to detect MMP-mediated degradation of the IGD. Therefore, the low level of aggrecan IGD cleavage by MMPs is not due to the lack of MMP expression in cartilage cultures, but rather the delayed activation and insufficient active enzymes in the tissue.
Fig. 3.
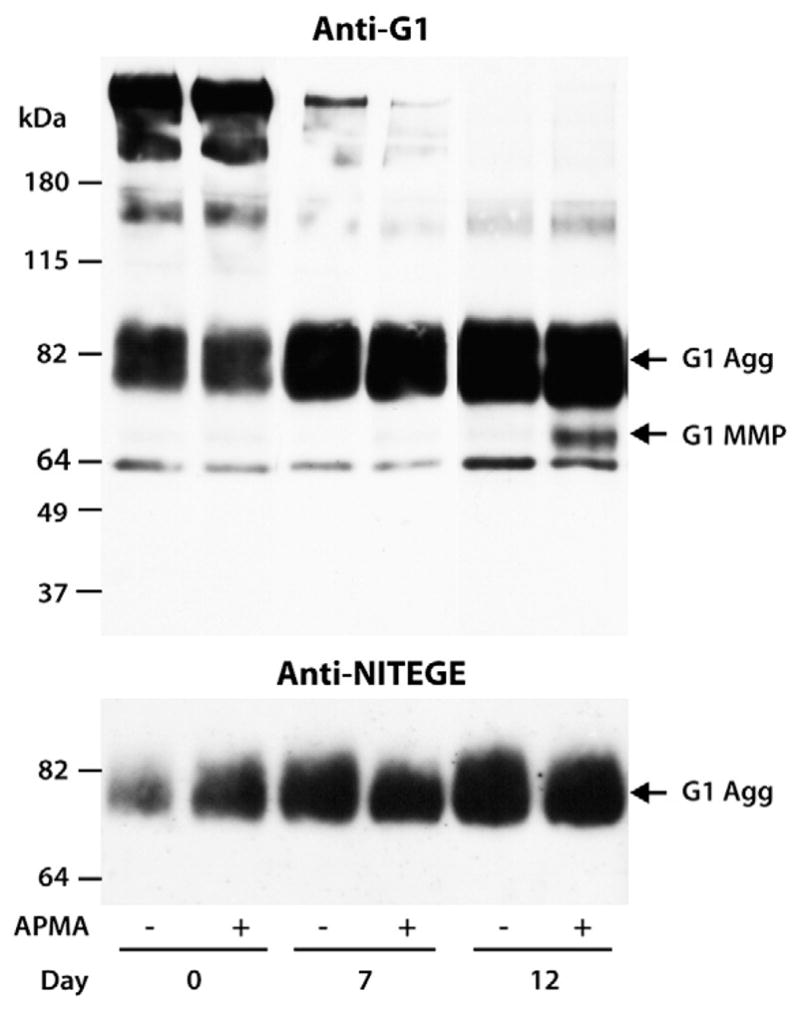
APMA-activated MMP degradation of aggrecan in cytokine-treated cartilage. Bovine articular cartilage explants were cultured for 19 days with IL-1β and treated with (+) or without (−) 1-mM APMA. Cartilage extracts were then analyzed by SDS/PAGE and immunoblotting. Cleavage within the aggrecan IGD was monitored using an anti-aggrecan G1 antibody (anti-G1) and an anti-neoepitope antibody directed against the new C-terminus generated by aggrecanase cleavage at the Glu373–374Ala site in the IGD (anti-NITEGE). Migration positions of aggrecan products generated by aggrecanase (G1 Agg) or MMP (G1 MMP) action are indicated on the right and the migration positions of molecular weight markers are indicated on the left. The days of culture are indicated below the immunoblots. The band at ~64 kDa represents non-specific staining.
However, the presence of low levels of MMP-derived G1-DIPES341 products detected in the tissue could also be explained by the reduced ability of the MMPs to further cleave within a previously aggrecanase-truncated G1-NITEGE373 aggrecan. The ability of MMPs to degrade previously cleaved aggrecan IGD was investigated by digestion of purified bovine aggrecan by ADAMTS5, followed by subsequent treatment with APMA-activated MMP3. Aggrecan G1-containing degradation products were visualized by immunoblotting using an aggrecan anti-G1 antibody (Fig. 4). No aggrecan G1-containing fragments were detectable in the preparation of bovine aggrecan. Digestion with 10-nM ADAMTS5 alone generated a free G1 whose size is characteristic of aggrecanase action at the Glu373–374Ala site in the IGD. Subsequent treatment with 1-μM MMP3 was able to completely degrade this product and generate a fragment of lower molecular size that results from MMP cleavage at the Ser341–342Phe site, indicating that the aggrecanase-generated aggrecan G1 can be further cleaved by MMP3. Similar MMP cleavage of the Asn341–342Phe bond in aggrecanase-generated porcine aggrecan G1 has been reported previously (Fosang et al., 2000). A 10-fold increase in MMP3 amount relative to ADAMTS5 was necessary to detect their respective aggrecan G1 fragments, and a similar MMP3 concentration was needed to detect IGD cleavage in intact aggrecan (see Fig. 7). Thus, the lack of abundance of MMP-derived fragments in explant cultures cannot be attributed to a difference in the ability of MMP3 to degrade aggrecanase-generated G1-Glu373 relative to intact aggrecan, but rather to the much lower affinity of this enzyme towards the aggrecan IGD compared to ADAMTS5.
Fig. 4.

Ability of MMP3 to cleave aggrecanase-generated G1-NITEGE373. Bovine aggrecan was digested with 10-nM recombinant ADAMTS5, followed by a 16-h incubation with or without MMP3. Digested samples were then analyzed by SDS/PAGE and immunoblotting using an anti-aggrecan G1 antibody (anti-G1). Migration positions of aggrecan products generated by aggrecanase (G1 Agg) or MMP (G1 MMP) action are indicated on the right and the migration positions of molecular weight markers are indicated on the left. The concentrations of MMP3 used for aggrecan digestion are indicated below the immunoblot. The band at ~26 kDa represents biotinylated SBTI used as a loading control.
Fig. 7.
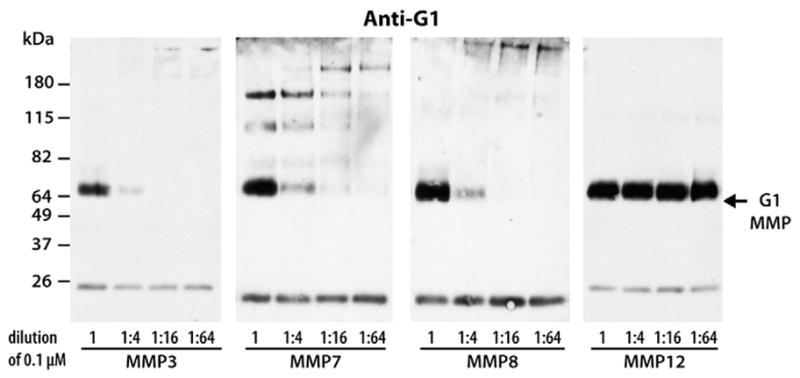
Efficiencies of MMP3, MMP7, MMP8 and MMP12 at cleaving within the aggrecan IGD. Bovine aggrecan was digested with 0.1 μM and subsequent 1:4 dilutions (1:4, 1:16, 1:64) of enzyme. Digested samples were then analyzed by SDS/PAGE and immunoblotting using an anti-aggrecan G1 antibody (anti-G1). Migration position of aggrecan products generated by MMP cleavage at the Ser341–342Phe site in the aggrecan IGD (G1 MMP) is indicated on the right and the migration positions of molecular weight markers are indicated on the left. The dilution factors for each enzyme are indicated below the immunoblot. The band at ~26 kDa represents biotinylated SBTI used as a loading control.
The previous observation showed that MMP3 has a low ability for cleaving within the previously truncated aggrecan IGD region, thereby raising a question regarding the overall ability of MMP3 to cleave within the intact aggrecan IGD. Therefore, intact aggrecan was digested with various concentration of MMP3 or ADAMTS5 and the generation of the respective degradation fragments was compared by immunoblotting using an anti-aggrecan G1 antibody (Fig. 5). This comparison showed that a concentration as low as 1 nM of ADAMTS5 was sufficient to degrade the aggrecan IGD at the aggrecanase Glu373–374Ala site, while at least 100 times more MMP3 was necessary to produce an equivalent digestion of the intact aggrecan IGD. Thus, ADAMTS5 is a much more potent IGD-degrading enzyme than MMP3, and it seems that the inefficiency of MMP cleavage observed in cytokine-stimulated cultures is due to the low affinity of MMP3 for this substrate.
Fig. 5.
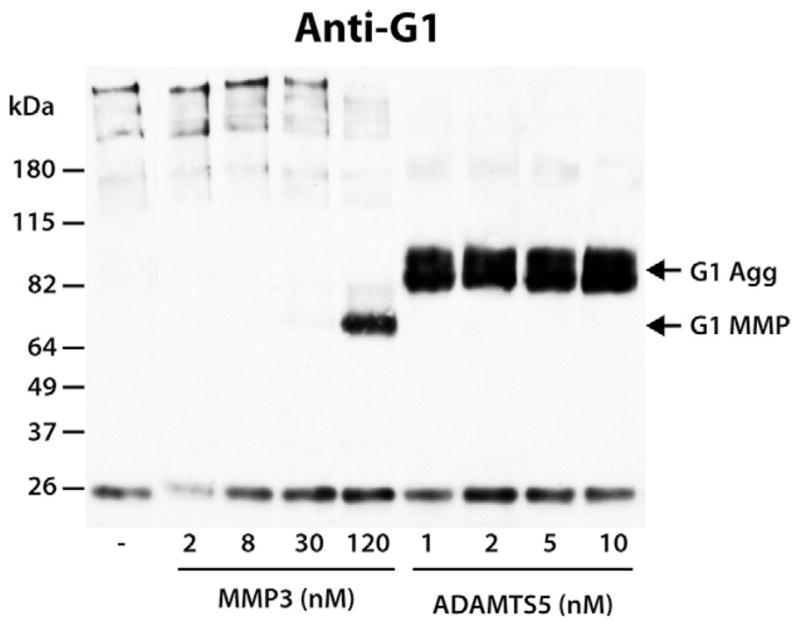
Comparison of the ability of MMP3 and ADAMTS5 to cleave within the aggrecan IGD. Bovine aggrecan was digested with increasing amounts of MMP3 or ADAMTS5. Samples were then analyzed by SDS/PAGE and immunoblotting using an anti-aggrecan G1 antibody (anti-G1). Migration positions of aggrecan products generated by aggrecanase (G1 Agg) or MMP (G1 MMP) action are indicated on the right and the migration positions of molecular weight markers are indicated on the left. The concentrations of MMP3 and ADAMTS5 used for aggrecan digestion are indicated below the immunoblot. The band at ~26 kDa represents biotinylated SBTI used as a loading control.
As MMP3 exhibited a low activity towards aggrecan IGD cleavage, it is possible that other MMPs are more efficient at cleaving this site. Collagenases (MMP1, MMP8 and MMP13), gelatinases (MMP2 and MMP9), together with the matrilysin MMP7 and metalloelastase MMP12, which are known to have broader substrate specificity, were compared with MMP3. Equimolar amounts of all MMPs were activated by 1-mM APMA and the generation of aggrecan degradation fragments was monitored by SDS/PAGE and immunoblotting using an anti-aggrecan G1 antibody (Fig. 6). A band corresponding in size to a fragment resulting from cleavage at the Ser341–342Phe site was present in the MMP3, MMP7, MMP8 and MMP12 digests and represents the major degradation product. Additional degradation fragments from cleavages at sites downstream of the Ser341–342Phe site were present in the MMP7 digests.
Fig. 6.
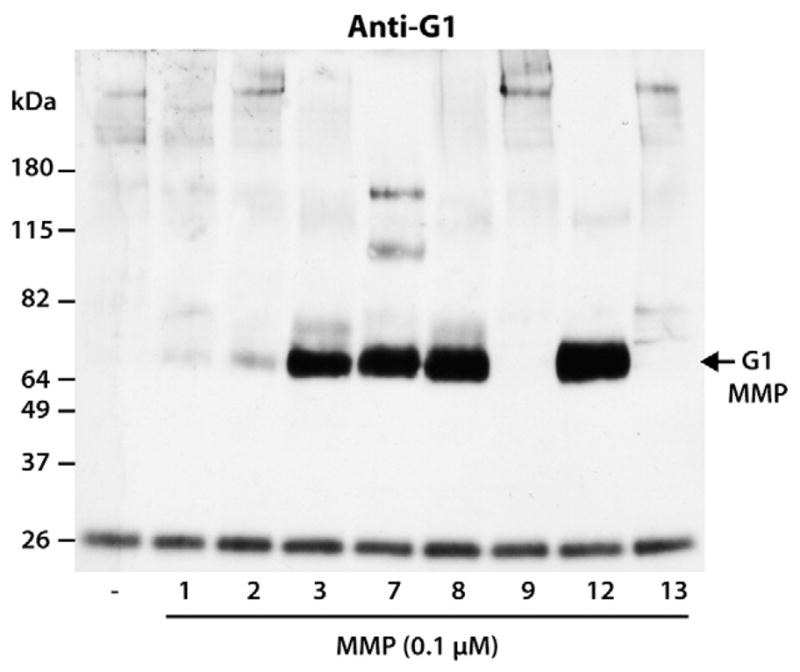
Susceptibility of aggrecan IGD to cleavage by various MMPs. Bovine aggrecan was digested with equimolar amounts of MMP1, MMP2, MMP3, MMP7, MMP8, MMP9, MMP12 and MMP13. Digested samples were then analyzed by SDS/PAGE and immunoblotting using an anti-aggrecan G1 antibody (anti-G1). Migration position of aggrecan products generated by MMP cleavage at the Ser341–342Phe site in the aggrecan IGD (G1 MMP) is indicated on the right and the migration positions of molecular weight markers are indicated on the left. The band at ~26 kDa represents biotinylated SBTI used as a loading control.
The relative efficiencies of MMP3, MMP7, MMP8 and MMP12 in generating G1-DIPES341 fragments were compared by digesting aggrecan with serial dilutions of each enzyme. Immunoblotting using anti-aggrecan G1 antibody (Fig. 7) showed that in the presence of 0.1-μM MMP3, MMP7 or MMP8 a major 70-kDa degradation product was generated by cleavage at Ser341–342Phe, while digestion with 1:4 diluted enzyme significantly reduced the amount of MMP-derived G1 degradation. Subsequent dilutions of these enzymes were not sufficient to generate such a fragment. Surprisingly, serial dilutions of MMP12 up to 1:64 (~1.5 nM of the enzyme) did not alter its ability to cleave aggrecan at this “MMP site,” and the MMP-derived fragment remained a major degradation product. This observation indicated that MMP12 is the most efficient MMP to cleave the aggrecan IGD Ser341–342Phe site in vitro.
The ability of MMP12 to cleave within the aggrecan IGD was then compared with that of ADAMTS5, using ten-fold dilutions of each enzyme. Generation of G1-containing fragments resulting from cleavage at the Glu373–374Ala site by ADAMTS5 or the Ser341–342Asn site by MMP12 were monitored by SDS/PAGE and immunoblotting with an anti-aggrecan G1 antibody (Fig. 8). Both enzymes cleaved aggrecan at their respective sites at 10 nM. However, the dilution of MMP12 to 1 nM significantly diminished its action, while degradation by ADAMTS5 was much less affected. Moreover, ADAMTS5 retained some activity at 0.1 nM, while MMP12 activity was not detectable at this concentration. Thus, even though MMP12 is the most potent IGD-degrading MMP, ADAMTS5 remains the most efficient enzyme at cleaving within this region of aggrecan.
Fig. 8.
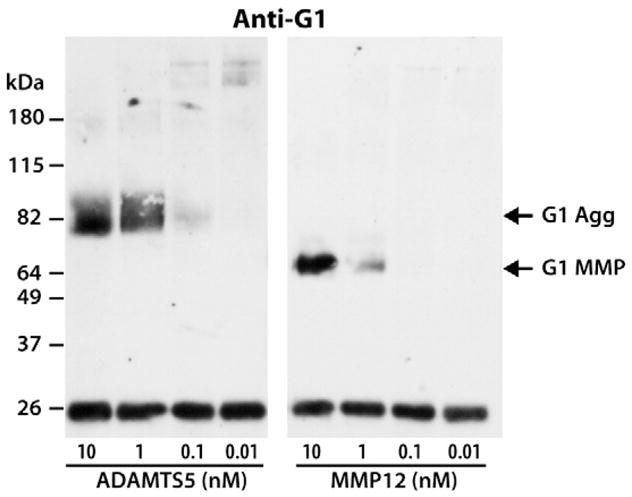
Comparison of the ability of ADAMTS5 and MMP12 to cleave within the aggrecan IGD. Bovine aggrecan was digested with ten-fold dilutions of 10-nM ADAMTS5 and 10-nM MMP12. Digested samples were then analyzed by SDS/PAGE and immunoblotting using an anti-aggrecan G1 antibody (anti-G1). Migration positions of aggrecan products generated by aggrecanase (G1 Agg) or MMP (G1 MMP) action are indicated on the right and the migration positions of molecular weight markers are indicated on the left. The concentrations of ADAMTS5 and MMP12 used for aggrecan digestion are indicated below the immunoblot. The band at ~26 kDa represents biotinylated SBTI used as a loading control.
In addition to their action in the IGD, a possible role for MMPs in the C-terminal processing of aggrecan was investigated by digesting aggrecan with equimolar amounts of various MMPs, followed by SDS/PAGE and immunoblotting with an antibody directed against the C-terminal G3 domain of aggrecan (Fig. 9, panel A). Such analysis demonstrated that MMP2, MMP9 and MMP13 had no major activity in the C-terminal truncation of aggrecan, as the majority of the detected material was identical in size to that detected in the undigested aggrecan. However, MMP1, MMP3, MMP7, MMP8 and MMP12 were able to generate G3-containing fragments in the ~180–50-kDa range, suggesting cleavage within the CS-2 region. Surprisingly, MMP3, MMP7 and MMP12 were able to cleave aggrecan near the G3 domain generating a 50-kDa fragment, reminiscent of the 50-kDa 2048ARLEI-G3 product generated in vitro by ADAMTS5 and to a lesser degree ADAMTS4 action (Durigova et al., 2008b). In order to further assess the identity of the MMP-generated 50-kDa fragment, all MMP digests as well as an ADAMTS5 digest of aggrecan (representing a positive control) were analyzed using an anti-neoepitope anti-ARLEIE antibody (Fig. 9, panel B). Samples digested with MMP2, MMP3, MMP7 and MMP12 did react with the neoepitope antibody, but the efficiencies of MMP cleavage at this site were vastly different, with MMP3 and MMP12 being the most efficient.
Fig. 9.
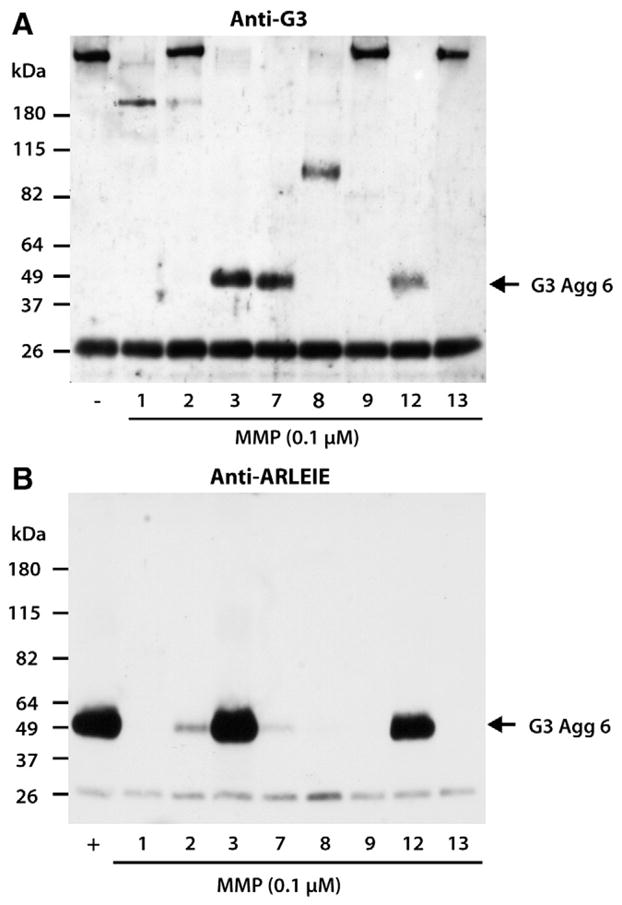
MMP-mediated cleavage within the aggrecan CS-2 region. Bovine aggrecan was digested with 10-nM ADAMTS5 (+) or equimolar amounts of MMP1, MMP2, MMP3, MMP7, MMP8, MMP9, MMP12 and MMP13. Digested samples were then analyzed by SDS/PAGE and immunoblotting using (A) an anti-aggrecan G3 (anti-G3), or (B) an anti-neoepitope antibody (anti-ARLEIE). The migration position of the C-terminal aggrecanase-mediated cleavage product 2048ARLEIE-G3 (G3 Agg 6) is indicated on the right and the migration positions of molecular weight markers are indicated on the left. The band at ~26 kDa represents biotinylated SBTI used as a loading control.
As MMP3 was the most potent of the MMPs to cleave the Glu2047–2048Ala bond in the CS-2 region, the activity of MMP3 at this site was compared to that of ADAMTS5. Purified aggrecan was digested with 10-fold dilutions of both 10-nM ADAMTS5 and 100-nM MMP3, and samples were analyzed by immunoblotting using the anti-aggrecan G3 antibody (Fig. 10). Such analysis demonstrated that 10 times more MMP3 than ADAMTS5 was required to generated the 50-kDa G3-containing fragment, thereby showing that ADAMTS5 is not only more potent than all of the tested MMPs at cleaving within the IGD but also the CS-2 region of aggrecan. In addition, it was noted that the ADAMTS5-generated 2048ARLEIE-G3 fragment is an end-cleavage product resulting from further truncation of previously cleaved aggrecan, as aggrecan digestion with lower amounts of ADAMTS5 results in multiple fragments of high molecular weight which are converted into the 50-kDa degradation product as the concentration of ADAMTS5 is increased. In contrast, the MMP3-generated 50-kDa G3-containing fragment is generated directly without prior cleavage of the aggrecan CS-2 region.
Fig. 10.
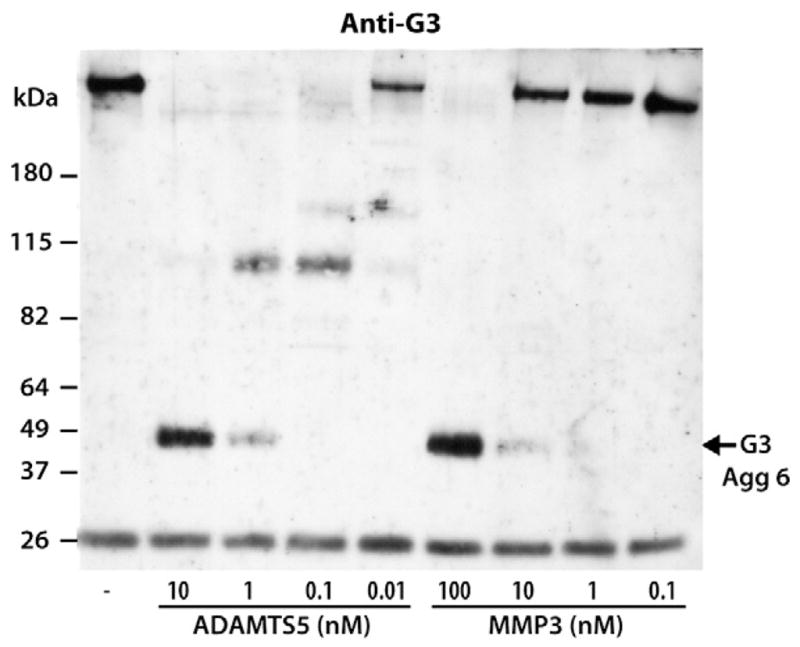
Comparison of ability of ADAMTS5 and MMP3 to cleave within the aggrecan CS-2 region. Bovine aggrecan was digested with 10-fold dilutions of 10-nM ADAMTS5 and 100-nM MMP3. Digested samples were then analyzed by SDS/PAGE and immunoblotting using an anti-aggrecan G3 antibody (anti-G3). The migration position of the C-terminal aggrecanase-mediated cleavage product 2048ARLEIE-G3 (G3 Agg 6) is indicated on the right and the migration positions of molecular weight markers are indicated on the left. The concentrations of ADAMTS5 and MMP3 used for aggrecan digestion are indicated below the immunoblot. The band at ~26 kDa represents biotinylated SBTI used as a loading control.
3. Discussion
The lack of detectable MMP action at early stages of cartilage catabolism in response to cytokine treatment, as observed in the presence of IL-1β or IL1β/OSM, could be explained by regulation of MMPs at different levels: the lack of MMP production in response to cytokine stimulation, the lack of proenzyme activation, or a low efficiency of the activated enzymes towards aggrecan as a substrate. With respect to MMP production, numerous cytokines have been shown to upregulate the mRNA expression of aggrecan-degrading MMPs in articular cartilage. In particular, a strong induction of MMP3 mRNA has been observed within a few hours in human chondrocytes treated with IL-1β/OSM (Barksby et al., 2006; Cawston et al., 1998; Koshy et al., 2002), and MMP protein synthesis has also been shown to be upregulated by these cytokines (Arner and Tortorella, 1995; Bonassar et al., 1996; Fuchs et al., 2004; Sanchez et al., 2004; Tetlow et al., 2001). In addition, the present data show that MMP2 and MMP9 are readily synthesized and present in the culture media throughout the 19-day treatment of bovine cartilage explants with IL-1β and that the addition of APMA to these cultures is able to promote aggrecan degradation within the IGD through MMP action. Thus, the lack of MMP synthesis in cartilage explant cultures is an unlikely explanation for the absence of detectable aggrecan-degrading MMP activity in early stages of cytokine stimulation.
Instead, the results of the present study suggest a combination of different reasons, i.e., the lack of MMP activation in the early stages of cytokine stimulation, the requirement for a threshold level of proMMP for efficient activation, and the low efficiency of MMPs relative to aggrecanases in cleaving the aggrecan core protein. In addition, this study shows that MMP3, which has been long accepted as the major aggrecan-degrading MMP in cartilage, is not the MMP that can most readily cleave within the aggrecan IGD. While MMP3, MMP7, MMP8 and MMP12 were all able to cleave the Ser341–342Phe bond in the IGD, MMP12 was by far the most active MMP at this site. MMP12, also known as a macrophage elastase, is capable of cleaving ECM components such as elastin. While it is expressed by inflammatory cells that invade the joint space in disease, it has also been shown that the overexpression of MMP12 can enhance the development of inflammatory arthritis in transgenic rabbits (Wang et al., 2004b). In addition, there is increased expression of MMP12 in the synovial tissue of patients with rheumatoid arthritis (RA) (Liu et al., 2004) and upregulation in chondrocytes by IL-1β or IL-1β/OSM (Davidson et al., 2006; Milner et al., 2006; Yang et al., 2010). Thus, these observations suggest that MMP12 can play a role in cartilage degradation in arthritis.
In the present study MMP1 and MMP13, together with the gelatinases, have little ability to cleave aggrecan compared to other MMPs. This contrasts with previous reports showing MMP13 cleavage within the IGD of aggrecan from various species (Fosang et al., 1996) and MMP1 and MMP13 cleavage within the GAG-attachment region of bovine aggrecan (Little et al., 2002; Miwa et al., 2006). The reason for this discrepancy is not immediately obvious, but it could relate to the amount of active MMP used for digestion, which is not possible to compare due to the different methods of assessing enzyme concentration and the different methods of activation used. It is certainly possible that with additional enzyme, aggrecan cleavage would be observed in the present system. In the case of work by Miwa et al. (Miwa et al., 2006), a recombinant aggrecan was used, and the severe under-glycosylation of this preparation could also influence the efficiency of cleavage by MMPs.
It is now well accepted that there is a delay between aggrecan degradation due to aggrecanases and that due to MMPs. As with our observations in organ cultures maintained in the presence of catabolic cytokines, several studies have shown that aggrecanases are responsible for the primary cleavage of aggrecan within the IGD (Lark et al., 1995; Little et al., 1999; van Meurs et al., 1999b), while aggrecan-degrading MMPs exhibit their activity only at later stages of cartilage catabolism, when proteoglycan and collagen depletion from the ECM has already occurred (Little et al., 2002; van Meurs et al., 1999a; van Meurs et al., 1999b). Thus, a difference in the temporal regulation of these enzymatic activities could account for the absence of MMP activity in the present culture system. A “delay” in aggrecanase versus MMP activities in the cartilage could be caused by different mechanisms of activation, as the aggrecanases (ADAMTS4 and ADAMTS5) may be activated intracellularly or extracellularly by proenzyme convertases (Gao et al., 2004; Longpre et al., 2009; Malfait et al., 2008; Wang et al., 2004a), while the MMPs are secreted as proenzymes that require activation in the ECM by other proteinases (Buttle et al., 1993; Ito and Nagase, 1988; Murphy et al., 1987) or components of complex proteolytic cascades whose activity can be regulated at different levels (He et al., 1989; Nagase et al., 1990; Werb et al., 1977). In addition, as MMP activity in cartilage is only detected following aggrecanase-mediated depletion of aggrecan from the ECM, it is possible that a change in the chondrocyte phenotype and metabolism caused by the matrix depletion initiates the delayed activation of the MMPs. As a result of the depletion of the surrounding matrix and alteration of the cell-matrix interactions, the chondrocytes could lose their anabolic abilities to replace the lost matrix with newly synthesized aggrecan and modify their function towards a MMP-mediated catabolism of the remaining matrix components. This is supported by the observation that MMP-derived collagenolytic activity is detected in cartilage only at later time points, after initial degradation of aggrecan (Pratta et al., 2003). Similarly, it has been reported that in the presence of catabolic stimulation, aggrecanolysis can shift to a MMP-mediated mode, but only in the later stages of aggrecan loss (Karsdal et al., 2008). This is consistent with studies carried out in OA cartilage samples that showed an accumulation of aggrecan- and collagen-degrading MMPs in the late stages of OA in the proximity of chondrocytes in the superficial zones of cartilage where signs of degenerative changes including aggrecan depletion and fibrillation were prominent (Tetlow et al., 2001).
The proteolysis of aggrecan at the Ser341–342Phe bond is a well established, preferred cleavage site attributable in vitro and in vivo to numerous MMPs (Buttner et al., 1998; Fosang et al., 1991, 1992, 1993, 1994, 1996, 1998). Additional MMP-sensitive sites have also been reported within the IGD (Fosang et al., 1992, 1998), between the G2 and the KS-rich region, and within the CS-1 region of aggrecan (Bonassar et al., 1995; Little et al., 2002), indicating that MMPs can degrade aggrecan at multiple sites. In this study, we identified an additional MMP-mediated cleavage occurring at the very C-terminus of the CS-2 region of aggrecan at the Glu2047–2048Ala bond. Surprisingly, cleavage at this site was previously observed in vivo in OSM-treated bovine cartilage, and has been shown to result from aggrecanase action (Durigova et al., 2008b). This is not the first time an MMP has been shown to cleave aggrecan at an “aggrecanase” cleavage site in vitro, as previous reports have shown that MMP8 was able to degrade within aggrecan IGD at the Glu373–374Ala bond attributed to aggrecanases (Fosang et al., 1994). However, this cleavage was only generated when high concentrations of MMP8 relative to the G1–G2 substrate were used, indicating that this is not a favored cleavage and raising the question whether such cleavage could occur in vivo. In the present study, the substrate–enzyme ratio required for MMP3 cleavage at the Glu2047–2048Ala bond was comparable to that necessary for aggrecanase cleavage in contrast to the amount of MMP3 required for the cleavage within the IGD. It is known that aggrecan molecules in the tissue are C-terminally truncated, and that such truncation resulting in the loss of the C-terminal G3 domain increases with age (Hardingham et al., 1994). It is therefore possible that MMP3 could be a candidate for C-terminal truncation of aggrecan in cartilage in vivo.
4. Experimental procedures
4.1. Tissue culture
Bovine articular cartilage from metacarpophalangeal joints of skeletally mature (18–24 months old) animals was obtained at a local abattoir. Pieces of full-depth cartilage were collected, washed twice for 15 min in Dulbecco’s modified Eagle medium (DMEM) buffered with 44-mM NaHCO3 (Sigma), 20-mM HEPES, pH 7.4, containing antibiotics (100-U/ml penicillin G sodium, 100-μg/ml streptomycin sulfate and 150-ng/ml gentamycin (medium A)), and supplemented with 0.25–μg/ml Fungizone (Gibco). Cartilage explants were left to equilibrate for 48 h at 37 °C in 12-well culture plates (Costar, 100-mg tissue (wet weight) per well per 2 ml of medium A) prior to any treatment. The medium was then changed (day 0) and the explants were cultured in the presence of catabolic cytokines for up to 19 days. Tissue and media samples were collected three times per week and fresh media containing the cytokine treatments was added to the remaining cultures. All cytokines were prepared in medium A containing 0.1-mg/ml BSA: human recombinant IL-1β (5 ng/ml) alone, or in combination with OSM (10 ng/ml) (R&D). The activation of MMPs produced in response to cytokine treatment was achieved by incubating the cartilage explants for 6 h with 1-mM APMA prior to the collection of the culture media and tissue samples. At the end of the culture period, cartilage explants were collected, snap frozen in liquid nitrogen, and stored at −80 °C.
4.2. Extraction of aggrecan
Aggrecan was extracted from adult bovine nasal cartilage with 4-M guanidinium chloride (GuCl) and then purified by CsCl density centrifugation, as described previously (Bayliss and Roughley, 1985). Density-gradient centrifugation was performed at 100 000 g at 10 °C for 48 h in order to separate proteoglycans from other proteins. Resulting gradients were fractionated and assayed for density. Fractions of density >1.55 g/ml were pooled and extensively dialyzed against water, followed by dialysis against 100 volumes of 100-mM potassium acetate. Finally the samples were dialyzed at 4 °C against water, freeze-dried and stored at 4 °C until subsequent use in enzymatic digestion experiments. Nasal cartilage rather than articular cartilage was used for in vitro digestion as it possesses a higher G3 content, and while aggrecan from the two tissues may have differences in their pattern of GAG substitution there is no evidence that this influences the sites of MMP cleavage.
4.3. Enzymatic digestions
All aggrecan digestions by ADAMTS5 and/or MMPs were performed at 37 °C for 16 h in 50-μl 25-mM Tris–HCl, pH 7.4, containing 250-mM NaCl, 5-mM CaCl2, 0.025% Brij 35 and 0.01% NaN3 (MMP buffer) using 100 μg of purified aggrecan. The concentration of active ADAMTS5 was determined by titration with the N-terminal domain of tissue inhibitor of metalloproteinase-3 (TIMP-3) (Gendron et al., 2007), while titration with TIMP-2 (a kind gift from Dr. Gillian Murphy) was used to determine the concentration of active MMP. For this purpose, each MMP was incubated with known molar concentrations of TIMP2 in the presence of a fluorogenic peptide substrate (Mca-PLGL-Dpa-AR-NH2, from R&D) and activity recorded by excitation at 320 nm and emission at 405 nm. The molar concentration of active enzyme was then calculated from the TIMP2 inhibition profile. For digestions by recombinant aggrecanase, an isoform of ADAMTS5 lacking the C-terminal thrombospondin domain was prepared as described previously (Gendron et al., 2007). This isoform was shown to be the most active of all isoforms of ADAMTS5. For MMP digestions, purified MMP1, MMP2, MMP7, MMP8, MMP12 (all from R&D), MMP9, MMP3 and MMP13 (all from Abcam) were used. All MMPs were activated by addition of 1-mM APMA (Sigma) to the reaction mixture. At the end of the digestion period the enzymatic reactions were stopped by incubation with 5-mM EDTA for 30 min at 37 °C. For all samples, 100-ng biotinylated soybean trypsin inhibitor (SBTI) was added to each tube to monitor sample recovery, and 9 volumes of ethanol were added to precipitate the samples overnight at −20 °C. Precipitated material was recovered by centrifugation at 4 °C, washed twice with 5 volumes of cold 75% ethanol, and dried under vacuum. Samples were then resuspended in keratanase buffer (10-mM sodium acetate, pH 6.0) for subsequent keratanase and chondroitinase treatment and sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS/PAGE) and immunoblotting analysis.
4.4. Keratanase/Chondroitinase treatment
All samples were incubated overnight at 37 °C in the presence of 20-mU/ml keratanase II (Seikagaku). The buffer was then adjusted to 0.1-M Tris/HCl, 0.1-M sodium acetate, pH 7.3 (chondroitinase buffer) for subsequent treatment with 200-mU/ml chondroitinase ABC (MP Biomedicals) for 6 h at 37 °C. Both enzymatic reactions were inactivated by boiling the samples for 5 min.
4.5. SDS/PAGE and immunoblotting
Cartilage explants were extracted with 10 volumes (v/w) 4-M GuCl, 100-mM sodium acetate, pH 6.0, containing proteinase inhibitors for 48 h at 4 °C. An aliquot of 100 μl, used for Western blot analysis, was precipitated with 9 volumes of ethanol and treated with keratanase as described above. Cartilage matrix components in the tissue and culture media samples were resolved by SDS/PAGE on Novex 4–12% gradient NuPAGE Bis-Tris gels (Invitrogen) under reducing conditions, and transferred to nitrocellulose membranes for immunoblotting as recommended by the manufacturer. Aggrecan and its degradation products were detected using rabbit polyclonal antibodies recognizing the amino-terminal G1 (anti-G1) (Sztrolovics et al., 2002) or the carboxy-terminal G3 (anti-G3) domains (Roughley et al., 2003) of bovine aggrecan, or anti-neoepitope antibodies raised against the 2048ARLEIE-G3 aggrecan fragment (anti-ARLEIE) (Durigova et al., 2008b). Antibodies recognizing the new C-terminus resulting from the MMP cleavage at the Ser341–342Phe site in the IGD (anti-DIPES) or that from aggrecanase cleavage at the Glu373–374Ala site in the IGD were generated by the method described previously (Mort and Roughley, 2004). An anti-rabbit Ig-biotinylated secondary antibody (Amersham) was used to detect the binding of the primary antibody, followed by incubation with a streptavidin-biotinylated horseradish peroxidase (HRP) complex (Amersham), and bands were visualized by ECL.
4.6. Gelatin zymography
Aliquots (300 μl) of media collected over a 19-day culture period of cartilage explants in the presence of IL-1 were precipitated overnight at −20 °C by the addition of 3 volumes of cold acetone, followed by two washes with 2 volumes of cold acetone. The concentrated material was recovered by centrifugation at 4 °C, dried under a vacuum and resuspended in 30-μl MMP buffer, mixed with 4 X sample buffer (10% SDS, 25% glycerol, 0.1% bromophenol blue and 0.25-M Tris, pH 6.8) and loaded without boiling onto a 10% acrylamide (Bio-Rad) gel copolymerized with 1-mg/ml gelatin (Sigma). Human recombinant MMP2 (50 ng, R&D) and MMP9 (6 ng, Abcam) were used as positive controls. The samples were run for 1 h 30 min at 120 V at 4 °C under non-reducing conditions. After electrophoresis, the gel was washed twice for 30 min in 2.5% Triton X-100 at room temperature, to allow enzyme renaturation, then rinsed and incubated overnight at 37 °C in 50-mM Tris-HCl, pH 8, 2-mM CaCl2, 0.02% NaN3. During enzyme renaturation, the autoprocessing of the proenzyme occurs to generate active enzyme, so allowing both active and proenzyme in the original sample to be identified at migration positions corresponding to their original sizes (Birkedal-Hansen and Taylor, 1982). The next day, the gel was stained for 1 h with Coomassie solution (0.5% CBB R250) and destained in 20% methanol–10% acetic acid solution until proteolytic activity appeared as clear bands against the blue background. Clear bands, representing both the pro- and a smaller active form of the enzyme were visible.
Acknowledgments
This work was supported by the Shriners of North America, the Canadian Institutes of Health Research (JSM and PJR Grant MOP 49458), the Wellcome Trust (HN grant 075473) and the National Institutes of Health (HN Grant AR40994). We would like to thank Dr. J. C. Krupa for determining the concentrations of active MMPs by titration with TIMP-2 and Guylaine Bédard for preparing the figures.
Abbreviations
- ADAMTS
a disintegrin and metalloproteinase with thrombospondin motifs
- APMA
p-amino-phenyl-mercuric acetate
- anti-ARLEIE
an antibody directed against the ARLEIE sequence within aggrecan
- CS-2
chondroitin sulfate-2 region
- ECM
extracellular matrix
- G1
globular domain 1
- G3
globular domain 3
- GAG
glycosaminoglycan
- IGD
interglobular domain
- IL-1β
interleukin-1beta
- MMP
matrix metalloproteinase
- OSM
oncostatin M
- OA
osteoarthritis
- RA
rheumatoid arthritis
- SDS/PAGE
sodium dodecyl sulfate/polyacrylamide gel electrophoresis
References
- Arner EC, Tortorella MD. Signal transduction through chondrocyte integrin receptors induces matrix metalloproteinase synthesis and synergizes with interleukin-1. Arthritis Rheum. 1995;38:1304–1314. doi: 10.1002/art.1780380919. [DOI] [PubMed] [Google Scholar]
- Barksby HE, Hui W, Wappler I, Peters HH, Milner JM, Richards CD, Cawston TE, Rowan AD. Interleukin-1 in combination with oncostatin M up-regulates multiple genes in chondrocytes: Implications for cartilage destruction and repair. Arthritis Rheum. 2006;54:540–550. doi: 10.1002/art.21574. [DOI] [PubMed] [Google Scholar]
- Bayliss MT, Roughley PJ. The properties of proteoglycan prepared from human articular cartilage by using associative caesium chloride gradients of high and low starting densities. Biochem J. 1985;232:111–117. doi: 10.1042/bj2320111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkedal-Hansen H, Taylor RE. Detergent-activation of latent collagenase and resolution of its component molecules. Biochem Biophys Res Commun. 1982;107:1173–1178. doi: 10.1016/s0006-291x(82)80120-4. [DOI] [PubMed] [Google Scholar]
- Bonassar LJ, Frank EH, Murray JC, Paguio CG, Moore VL, Lark MW, Sandy JD, Wu JJ, Eyre DR, Grodzinsky AJ. Changes in cartilage composition and physical properties due to stromelysin degradation. Arthritis Rheum. 1995;38:173–183. doi: 10.1002/art.1780380205. [DOI] [PubMed] [Google Scholar]
- Bonassar LJ, Stinn JL, Paguio CG, Frank EH, Moore VL, Lark MW, Sandy JD, Hollander AP, Poole AR, Grodzinsky AJ. Activation and inhibition of endogenous matrix metalloproteinases in articular cartilage: Effects on composition and biophysical properties. Arch Biochem Biophys. 1996;333:359–367. doi: 10.1006/abbi.1996.0402. [DOI] [PubMed] [Google Scholar]
- Buttle DJ, Handley CJ, Ilic MZ, Saklatvala J, Murata M, Barrett AJ. Inhibition of cartilage proteoglycan release by a specific inactivator of cathepsin B and an inhibitor of matrix metalloproteinases: Evidence for two converging pathways of chondrocyte-mediated proteoglycan degradation. Arthritis Rheum. 1993;36:1709–1717. doi: 10.1002/art.1780361210. [DOI] [PubMed] [Google Scholar]
- Buttner FH, Hughes CE, Margerie D, Lichte A, Tschesche H, Caterson B, Bartnik E. Membrane type 1 matrix metalloproteinase (MT1-MMP) cleaves the recombinant aggrecan substrate rAgg1mut at the ‘aggrecanase’ and the MMP sites. Characterization of MT1-MMP catabolic activities on the interglobular domain of aggrecan. Biochem J. 1998;333:159–165. doi: 10.1042/bj3330159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterson B, Flannery CR, Hughes CE, Little CB. Mechanisms involved in cartilage proteoglycan catabolism. Matrix Biol. 2000;19:333–344. doi: 10.1016/s0945-053x(00)00078-0. [DOI] [PubMed] [Google Scholar]
- Cawston TE, Curry VA, Summers CA, Clark IM, Riley GP, Life PF, Spaull JR, Goldring MB, Koshy PJ, Rowan AD, Shingleton WD. The role of oncostatin M in animal and human connective tissue collagen turnover and its localization within the rheumatoid joint. Arthritis Rheum. 1998;41:1760–1771. doi: 10.1002/1529-0131(199810)41:10<1760::AID-ART8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Davidson RK, Waters JG, Kevorkian L, Darrah C, Cooper A, Donell ST, Clark IM. Expression profiling of metalloproteinases and their inhibitors in synovium and cartilage. Arthritis Res Ther. 2006;8:R124. doi: 10.1186/ar2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doege KJ, Sasaki M, Kimura T, Yamada Y. Complete coding sequence and deduced primary structure of the human cartilage large aggregating proteoglycan, aggrecan: Human-specific repeats, and additional alternatively spliced forms. J Biol Chem. 1991;266:894–902. [PubMed] [Google Scholar]
- Durigova M, Roughley PJ, Mort JS. Mechanism of proteoglycan aggregate degradation in cartilage stimulated with oncostatin M. Osteoarthritis Cart. 2008a;16:98–104. doi: 10.1016/j.joca.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Durigova M, Soucy P, Fushimi K, Nagase H, Mort JS, Roughley PJ. Characterization of an ADAMTS-5-mediated cleavage site in aggrecan in OSM-stimulated bovine cartilage. Osteoarthritis Cart. 2008b;16:1245–1252. doi: 10.1016/j.joca.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Flannery CR, Lark MW, Sandy JD. Identification of a stromelysin cleavage site within the interglobular domain of human aggrecan: Evidence for proteolysis at this site in vivo in human articular cartilage. J Biol Chem. 1992;267:1008–1014. [PubMed] [Google Scholar]
- Fosang AJ, Neame PJ, Hardingham TE, Murphy G, Hamilton JA. Cleavage of cartilage proteoglycan between G1 and G2 domains by stromelysins. J Biol Chem. 1991;266:15579–15582. [PubMed] [Google Scholar]
- Fosang AJ, Neame PJ, Last K, Hardingham TE, Murphy G, Hamilton JA. The interglobular domain of cartilage aggrecan is cleaved by PUMP, gelatinases, and cathepsin B. J Biol Chem. 1992;267:19470–19474. [PubMed] [Google Scholar]
- Fosang AJ, Last K, Knauper V, Neame PJ, Murphy G, Hardingham TE, Tschesche H, Hamilton JA. Fibroblast and neutrophil collagenases cleave at two sites in the cartilage aggrecan interglobular domain. Biochem J. 1993;295:273–276. doi: 10.1042/bj2950273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosang AJ, Last K, Neame PJ, Murphy G, Knauper V, Tschesche H, Hughes CE, Caterson B, Hardingham TE. Neutrophil collagenase (MMP-8) cleaves at the aggrecanase site E373-A374 in the interglobular domain of cartilage aggrecan. Biochem J. 1994;304:347–351. doi: 10.1042/bj3040347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosang AJ, Last K, Knauper V, Murphy G, Neame PJ. Degradation of cartilage aggrecan by collagenase-3 (MMP-13) FEBS Lett. 1996;380:17–20. doi: 10.1016/0014-5793(95)01539-6. [DOI] [PubMed] [Google Scholar]
- Fosang AJ, Last K, Fujii Y, Seiki M, Okada Y. Membrane-type 1 MMP (MMP-14) cleaves at three sites in the aggrecan interglobular domain. FEBS Lett. 1998;430:186–190. doi: 10.1016/s0014-5793(98)00667-x. [DOI] [PubMed] [Google Scholar]
- Fosang AJ, Last K, Stanton H, Weeks DB, Campbell IK, Hardingham TE, Hembry RM. Generation and novel distribution of matrix metalloproteinase-derived aggrecan fragments in porcine cartilage explants. J Biol Chem. 2000;275:33027–33037. doi: 10.1074/jbc.M910207199. [DOI] [PubMed] [Google Scholar]
- Fuchs S, Skwara A, Bloch M, Dankbar B. Differential induction and regulation of matrix metalloproteinases in osteoarthritic tissue and fluid synovial fibroblasts. Osteoarthritis Cart. 2004;12:409–418. doi: 10.1016/j.joca.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Gao G, Plaas A, Thompson VP, Jin S, Zuo F, Sandy JD. ADAMTS4 (aggrecanase-1) activation on the cell surface involves C-terminal cleavage by glycosylphosphatidyl inositol-anchored membrane type 4-matrix metalloproteinase and binding of the activated proteinase to chondroitin sulfate and heparan sulfate on syndecan-1. J Biol Chem. 2004;279:10042–10051. doi: 10.1074/jbc.M312100200. [DOI] [PubMed] [Google Scholar]
- Gendron C, Kashiwagi M, Lim NH, Enghild JJ, Thogersen IB, Hughes C, Caterson B, Nagase H. Proteolytic activities of human ADAMTS-5: Comparative studies with ADAMTS-4. J Biol Chem. 2007;282:18294–18306. doi: 10.1074/jbc.M701523200. [DOI] [PubMed] [Google Scholar]
- Hardingham TE, Fosang AJ, Dudhia J. The structure, function and turnover of aggrecan, the large aggregating proteoglycan from cartilage. Eur J Clin Chem Clin Biochem. 1994;32:249–257. [PubMed] [Google Scholar]
- He CS, Wilhelm SM, Pentland AP, Marmer BL, Grant GA, Eisen AZ, Goldberg GI. Tissue cooperation in a proteolytic cascade activating human interstitial collagenase. Proc Natl Acad Sci USA. 1989;86:2632–2636. doi: 10.1073/pnas.86.8.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A, Nagase H. Evidence that human rheumatoid synovial matrix metalloproteinase 3 is an endogenous activator of procollagenase. Arch Biochem Biophys. 1988;267:211–216. doi: 10.1016/0003-9861(88)90025-2. [DOI] [PubMed] [Google Scholar]
- Jones GC, Riley GP. ADAMTS proteinases: A multi-domain, multi-functional family with roles in extracellular matrix turnover and arthritis. Arthritis Res Ther. 2005;7:160–169. doi: 10.1186/ar1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsdal MA, Madsen SH, Christiansen C, Henriksen K, Fosang AJ, Sondergaard BC. Cartilage degradation is fully reversible in the presence of aggrecanase but not matrix metalloproteinase activity. Arthritis Res Ther. 2008;10:R63. doi: 10.1186/ar2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiani C, Chen L, Wu YJ, Yee AJ, Yang BB. Structure and function of aggrecan. Cell Res. 2002;12:19–32. doi: 10.1038/sj.cr.7290106. [DOI] [PubMed] [Google Scholar]
- Koshy PJ, Lundy CJ, Rowan AD, Porter S, Edwards DR, Hogan A, Clark IM, Cawston TE. The modulation of matrix metalloproteinase and ADAM gene expression in human chondrocytes by interleukin-1 and oncostatin M: A time-course study using real-time quantitative reverse transcription-polymerase chain reaction. Arthritis Rheum. 2002;46:961–967. doi: 10.1002/art.10212. [DOI] [PubMed] [Google Scholar]
- Lark MW, Gordy JT, Weidner JR, Ayala J, Kimura JH, Williams HR, Mumford RA, Flannery CR, Carlson SS, Iwata M. Cell-mediated catabolism of aggrecan: Evidence that cleavage at the “aggrecanase” site (Glu373-Ala374) is a primary event in proteolysis of the interglobular domain. J Biol Chem. 1995;270:2550–2556. doi: 10.1074/jbc.270.6.2550. [DOI] [PubMed] [Google Scholar]
- Little CB, Flannery CR, Hughes CE, Mort JS, Roughley PJ, Dent C, Caterson B. Aggrecanase versus matrix metalloproteinases in the catabolism of the interglobular domain of aggrecan in vitro. Biochem J. 1999;344:61–68. [PMC free article] [PubMed] [Google Scholar]
- Little CB, Hughes CE, Curtis CL, Janusz MJ, Bohne R, Wang-Weigand S, Taiwo YO, Mitchell PG, Otterness IG, Flannery CR, Caterson B. Matrix metalloproteinases are involved in C-terminal and interglobular domain processing of cartilage aggrecan in late stage cartilage degradation. Matrix Biol. 2002;21:271–288. doi: 10.1016/s0945-053x(02)00004-5. [DOI] [PubMed] [Google Scholar]
- Liu M, Sun H, Wang X, Koike T, Mishima H, Ikeda K, Watanabe T, Ochiai N, Fan J. Association of increased expression of macrophage elastase (matrix metalloproteinase 12) with rheumatoid arthritis. Arthritis Rheum. 2004;50:3112–3117. doi: 10.1002/art.20567. [DOI] [PubMed] [Google Scholar]
- Longpre JM, McCulloch DR, Koo BH, Alexander JP, Apte SS, Leduc R. Characterization of proADAMTS5 processing by proprotein convertases. Int J Biochem Cell Biol. 2009;41:1116–1126. doi: 10.1016/j.biocel.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Loulakis P, Shrikhande A, Davis G, Maniglia CA. N-terminal sequence of proteoglycan fragments isolated from medium of interleukin-1-treated articular-cartilage cultures. Putative site(s) of enzymic cleavage. Biochem J. 1992;284:589–593. doi: 10.1042/bj2840589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfait AM, Arner EC, Song RH, Alston JT, Markosyan S, Staten N, Yang Z, Griggs DW, Tortorella MD. Proprotein convertase activation of aggrecanases in cartilage in situ. Arch Biochem Biophys. 2008;478:43–51. doi: 10.1016/j.abb.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Milner JM, Rowan AD, Cawston TE, Young DA. Metalloproteinase and inhibitor expression profiling of resorbing cartilage reveals pro-collagenase activation as a critical step for collagenolysis. Arthritis Res Ther. 2006;8:R142. doi: 10.1186/ar2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa HE, Gerken TA, Huynh TD, Flory DM, Hering TM. Mammalian expression of full-length bovine aggrecan and link protein: formation of recombinant proteoglycan aggregates and analysis of proteolytic cleavage by ADAMTS-4 and MMP-13. Biochim Biophys Acta. 2006;1760:472–486. doi: 10.1016/j.bbagen.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Mort JS, Roughley PJ. Production of antibodies against degradative neoepitopes in aggrecan. Meth Mol Med. 2004;100:237–250. doi: 10.1385/1-59259-810-2:237. [DOI] [PubMed] [Google Scholar]
- Murphy G, Cockett MI, Stephens PE, Smith BJ, Docherty AJ. Stromelysin is an activator of procollagenase: A study with natural and recombinant enzymes. Biochem J. 1987;248:265–268. doi: 10.1042/bj2480265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem. 1997;378:151–160. [PubMed] [Google Scholar]
- Nagase H, Enghild JJ, Suzuki K, Salvesen G. Stepwise activation mechanisms of the precursor of matrix metalloproteinase 3 (stromelysin) by proteinases and (4-aminophenyl)mercuric acetate. Biochemistry. 1990;29:5783–5789. doi: 10.1021/bi00476a020. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Yamamura M, Morita Y, Harada S, Makino H, Ota Z. The synovial expression and serum levels of interleukin-6, interleukin-11, leukemia inhibitory factor, and oncostatin M in rheumatoid arthritis. Arthritis Rheum. 1997;40:1096–1105. doi: 10.1002/art.1780400614. [DOI] [PubMed] [Google Scholar]
- Pratta MA, Yao W, Decicco C, Tortorella MD, Liu RQ, Copeland RA, Magolda R, Newton RC, Trzaskos JM, Arner EC. Aggrecan protects cartilage collagen from proteolytic cleavage. J Biol Chem. 2003;278:45539–45545. doi: 10.1074/jbc.M303737200. [DOI] [PubMed] [Google Scholar]
- Richards CD. Matrix catabolism in arthritis: Priming the guns with oncostatin M. J Rheumatol. 2004;31:2326–2328. [PubMed] [Google Scholar]
- Roughley PJ, Barnett J, Zuo F, Mort JS. Variations in aggrecan structure modulate its susceptibility to aggrecanases. Biochem J. 2003;375:183–189. doi: 10.1042/BJ20030609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C, Deberg MA, Burton S, Devel P, Reginster JY, Henrotin YE. Differential regulation of chondrocyte metabolism by oncostatin M and interleukin-6. Osteoarthritis Cart. 2004;12:801–810. doi: 10.1016/j.joca.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Sandy JD, Neame PJ, Boynton RE, Flannery CR. Catabolism of aggrecan in cartilage explants: Identification of a major cleavage site within the interglobular domain. J Biol Chem. 1991;266:8683–8685. [PubMed] [Google Scholar]
- Springman EB, Angleton EL, Birkedal-Hansen H, Van Wart HE. Multiple modes of activation of latent human fibroblast collagenase: Evidence for the role of a Cys73 active-site zinc complex in latency and a “cysteine switch” mechanism for activation. Proc Natl Acad Sci USA. 1990;87:364–368. doi: 10.1073/pnas.87.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztrolovics R, White RJ, Roughley PJ, Mort JS. The mechanism of aggrecan release from cartilage differs with tissue origin and the agent used to stimulate catabolism. Biochem J. 2002;362:465–472. doi: 10.1042/0264-6021:3620465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001;44:585–594. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Tortorella MD, Burn TC, Pratta MA, Abbaszade I, Hollis JM, Liu R, Rosenfeld SA, Copeland RA, Decicco CP, Wynn R, Rockwell A, Yang F, Duke JL, Solomon K, George H, Bruckner R, Nagase H, Itoh Y, Ellis DM, Ross H, Wiswall BH, Murphy K, Hillman MC, Jr, Hollis GF, Newton RC, Magolda RL, Trzaskos JM, Arner EC. Purification and cloning of aggrecanase-1: A member of the ADAMTS family of proteins. Science. 1999;284:1664–1666. doi: 10.1126/science.284.5420.1664. [DOI] [PubMed] [Google Scholar]
- Tortorella MD, Liu RQ, Burn T, Newton RC, Arner E. Characterization of human aggrecanase 2 (ADAM-TS5): Substrate specificity studies and comparison with aggrecanase 1 (ADAM-TS4) Matrix Biol. 2002;21:499–511. doi: 10.1016/s0945-053x(02)00069-0. [DOI] [PubMed] [Google Scholar]
- Tortorella MD, Pratta M, Liu RQ, Austin J, Ross OH, Abbaszade I, Burn T, Arner E. Sites of aggrecan cleavage by recombinant human aggrecanase-1 (ADAMTS-4) J Biol Chem. 2000;275:18566–18573. doi: 10.1074/jbc.M909383199. [DOI] [PubMed] [Google Scholar]
- van den Berg WB. The role of cytokines and growth factors in cartilage destruction in osteoarthritis and rheumatoid arthritis. Z Rheumatol. 1999;58:136–141. doi: 10.1007/s003930050163. [DOI] [PubMed] [Google Scholar]
- van Meurs J, van Lent P, Stoop R, Holthuysen A, Singer I, Bayne E, Mudgett J, Poole R, Billinghurst C, van der Kraan P, Buma P, van den Berg W. Cleavage of aggrecan at the Asn341-Phe342 site coincides with the initiation of collagen damage in murine antigen-induced arthritis: A pivotal role for stromelysin 1 in matrix metalloproteinase activity. Arthritis Rheum. 1999a;42:2074–2084. doi: 10.1002/1529-0131(199910)42:10<2074::AID-ANR7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- van Meurs JB, van Lent PL, Holthuysen AE, Singer II, Bayne EK, van den Berg WB. Kinetics of aggrecanase- and metalloproteinase-induced neoepitopes in various stages of cartilage destruction in murine arthritis. Arthritis Rheum. 1999b;42:1128–1139. doi: 10.1002/1529-0131(199906)42:6<1128::AID-ANR9>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Wang P, Tortorella M, England K, Malfait AM, Thomas G, Arner EC, Pei D. Proprotein convertase furin interacts with and cleaves pro-ADAMTS4 (Aggrecanase-1) in the trans-Golgi network. J Biol Chem. 2004a;279:15434–15440. doi: 10.1074/jbc.M312797200. [DOI] [PubMed] [Google Scholar]
- Wang X, Liang J, Koike T, Sun H, Ichikawa T, Kitajima S, Morimoto M, Shikama H, Watanabe T, Sasaguri Y, Fan J. Overexpression of human matrix metalloproteinase-12 enhances the development of inflammatory arthritis in transgenic rabbits. Am J Pathol. 2004b;165:1375–1383. doi: 10.1016/S0002-9440(10)63395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z, Mainardi CL, Vater CA, Harris ED., Jr Endogenous activiation of latent collagenase by rheumatoid synovial cells: Evidence for a role of plasminogen activator. N Engl J Med. 1977;296:1017–1023. doi: 10.1056/NEJM197705052961801. [DOI] [PubMed] [Google Scholar]
- Yang S, Kim J, Ryu JH, Oh H, Chun CH, Kim BJ, Min BH, Chun JS. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med. 2010;16:687–693. doi: 10.1038/nm.2153. [DOI] [PubMed] [Google Scholar]


