Abstract
Genetic manipulation of Plasmodium falciparum in culture through transfection has provided numerous insights into the molecular and cell biology of this parasite. The procedure is rather cumbersome, and is limited by the number of drug-resistant markers that can be used for selecting transfected parasites. Here we report a new selectable marker that could allow multiple transfections. We have taken advantage of our finding that a critical function of the mitochondrial electron transport chain (mtETC) in the erythrocytic stages of P. falciparum is the regeneration of ubiquinone as co-substrate of dihydroorotate dehydrogenase (DHODH), and that transgenic P. falciparum expressing ubiquinone-independent DHODH from yeast (yDHODH) are resistant to all mtETC inhibitors. We assessed the possibility of using yDHODH as a positive selectable marker for transfections of P. falciparum, including its use in gene disruption strategies. We constructed a transfection vector designed for gene disruption, termed pUF-1, containing the yDHODH gene as the positive selection marker in combination with a previously described fused yeast cytosine deaminase-uracil phosphoribosyl transferase gene as a negative selection marker. Transfection of the D10 strain followed by selection with atovaquone yielded positively selected parasites containing the plasmid, demonstrating that yDHODH can be used as a selective marker. Atovaquone, however, could not be used for such selection with the Dd2 strain of P. falciparum. On the other hand, we demonstrated that yDHODH transgenic parasites could be selected in both strains by Plasmodium DHODH-specific triazolopyrimidine-based inhibitors. Thus, selection with DHODH inhibitors was superior in that it successfully selected transgenic Dd2 parasites, as well as yielded transgenic parasites after a shorter period of selection. As a proof of concept, we have successfully disrupted the type II vacuolar proton-pumping pyrophosphatase gene (PfVP2) in P. falciparum by double crossover recombination, showing that this gene is not essential for the survival of blood stage parasites.
Keywords: malaria, selectable marker, atovaquone, triazolopyrimidine, gene knockout, vacuolar pyrophosphatase
1. Introduction
Molecular genetic investigations of malaria parasites hold promise for a deeper understanding of parasite biology to guide treatment and control of malaria. Although genome sequences of several parasite species have been completed [1–4], application of these advances is often hampered by difficulties in carrying out biochemical and genetic studies. Successful transfection of Plasmodium falciparum has provided an invaluable means to study a number of important biological properties of this parasite such as drug resistance and cytoadherence. The technique can also be used to gain insight into metabolic pathways, protein trafficking and parasite differentiation. Transfection is, however, a highly demanding technique in P. falciparum, with a very low efficiency. The technique has also been limited by the number of selectable markers. For genetic transformations of P. falciparum only a few positive selection markers are available—primarily human dihydrofolate reductase (DHFR), fungal blasticidin S deaminase (bsd) and bacterial neomycin phosphotransferase (neo) [5,6]. The majority of selections in P. falciparum have been carried out using human DHFR selected with WR99210, which has a large selection window (an IC50 increase of about 4000 fold was reported upon transfection with a plasmid containing human DHFR [5]). bsd plus blasticidin and neo plus G418 were reported to have much narrower selection windows, with a 10 fold or less change in IC90 (for low copy number results) [6]. In addition, it was recently reported that growth under blasticidin pressure results in the selection of resistant transport mutants in at least one strain of P. falciparum [7]. Nevertheless, reported use of bsd has been increasing, reflecting the mounting need for multi-step molecular genetic manipulations. The dearth of selectable markers limits our ability to carry out gene disruptions, complementation of mutants, and allelic replacement experiments to select specific mutants in important biological pathways. In addition, availability of additional selectable markers would be essential for the generation of double and triple gene knockouts to study the role of multiple proteins or pathways that may have redundant or alternate functions. Such markers would also be important for strain constructions requiring multiple genomic alterations.
We have previously shown that a critical role of the mitochondrial electron transport chain (mtETC) in blood-stage P. falciparum is to regenerate ubiquinone, an oxidative co-substrate of dihydroorotate dehydrogenase (DHODH). DHODH, the fourth enzyme in the pyrimidine biosynthetic pathway, is essential for malaria parasites’ survival because they cannot salvage pyrimidines [8]. Transgenic P. falciparum parasites expressing Saccharomyces cerevisiae DHODH (yDHODH), which utilizes fumarate as an electron acceptor, were completely resistant to inhibitors of the mtETC, including the antimalarial drug atovaquone, which inhibits the cytochrome bc1 complex (Complex III) [9]. In this prior work, yDHODH was expressed from an extrachromosomal plasmid that had been selected using the human DHFR marker and the inhibitor WR99210. Further investigation of these transgenic parasites revealed that they could be propagated in the presence of atovaquone during long-term culture (without any further requirement for WR99210). In this study, we report the use of yDHODH as a new selectable marker, which confers resistance to atovaquone in specific strains and, more broadly, to triazolopyrimidine-based, parasite-specific DHODH inhibitors [10]. As a proof of concept, we used the new plasmid vector, designated pUF-1, for successful double cross-over disruption of the gene encoding type II vacuolar proton-pumping pyrophosphatase (PfVP2) [11], thereby showing its non-essentiality in blood stages of the parasite.
2. Materials and methods
2.1 Parasite lines, culture, transfection and selection methods
P. falciparum D10 strain parasites were used for most experiments in this study, unless mentioned otherwise. Parasites were cultured in RPMI 1640 medium supplemented with 300 mg/L L-glutamine, 10 mg/liter hypoxanthine (Sigma), 15 mM HEPES (Hyclone), 0.225% NaHCO3 (Cellgro), 0.5% Albumax II (InVitrogen), and 50 μg/ml gentamicin (Cellgro), under a gas mixture of 5% O2, 5% CO2, and 90% N2. Parasites were transfected as previously described [5]. Briefly, 0.2 cm electroporation cuvettes were loaded with 0.3 ml of 50% parasitized erythrocytes and 50 μg of plasmid DNA in incomplete cytomix solution. Electroporation conditions were 0.31 kV and 960 μFd. For stable transfections, parasites were cultured in media containing 100 nM atovaquone, or 1.5 μM compound DSM1 [10] for positive selection. For comparison of selection efficiency, the transfection was done as described above, the resulting culture was divided, and each half was selected with either atovaquone or DSM1.
2.2 Knockout vector (pUF-1) construction
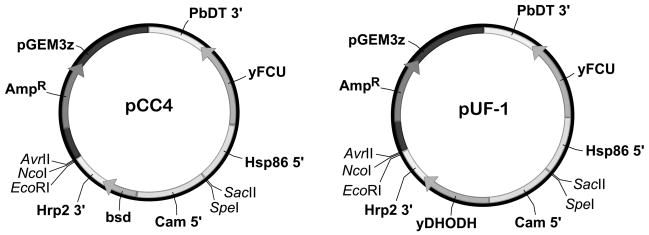
The DNA fragment containing bsd and P. falciparum calmodulin promoter (CAM 5′ UTR) was excised from pCC4 [12] with the restriction enzymes SpeI and HindIII and cloned into the pBluescript plasmid, producing pBluescript-bsd. The Saccharomyces cerevisiae DHODH gene (yDHODH) was amplified from the pHHyDHOD-GFP vector [9] by forward and reverse primers containing BamHI and HindIII restriction sites, respectively. The PCR product was digested with BamHI and HindIII and cloned into pBluescript-bsd digested with the same set of enzymes, yielding pBluescript-yDHODH. The sequence of the yDHODH gene was confirmed by automated sequencing. Since EcoRI and NcoI restriction sites were present both in the yDHODH gene and also in one of the multiple cloning sites of pCC4, we eliminated these restriction sites in the yDHODH gene by silent mutagenesis using the QuikChange protocol (Stratagene). The primer sequences used for PCR amplification and restriction site mutations are listed in Table 1. The DNA fragment of pBluescript-yDHODH* containing mutated yDHODH along with the CAM 5′ UTR was excised with SpeI and HindIII restriction enzymes and cloned into the pCC4 plasmid digested with the same restriction sites, yielding the new plasmid pUF-1 (Fig 1).
Table 1.
Primers used for PCR amplification of yDHODH and 5′ and 3′ homologous regions of the PfVP2 gene, and for silent mutagenesis of yDHODH
| PRIMER | SEQUENCEa,b |
|---|---|
| S.c.URA-1BamHIF | TACAGGATCCTTCGAAATGACAGCCAGTTTAACTACCAA |
| S.c.URA-1HindIIIR | GTACAAAGCTTTTAAATGCTGTTCAACTTCCCA |
| EcoRImutF | GGAAAATCCAAGATAGCGAATTTAACGGTATTACCGAGTTAAACTTG |
| EcoRImutR | CAAGTTTAACTCGGTAATACCGTTAAATTCGCTATCTTGGATTTTCC |
| NcoImutF | GTCCCTCTAGGCAGTATCAACTCAATGGGTTTACCAAACGAAG |
| NcoImutR | CTTCGTTTGGTAAACCCATTGAGTTGATACTGCCTAGAGGGAC |
| VP2Sense5′ | caccatgGCAAAAACCTGTAAAACATCGTATAAC |
| VP2Antisense5′ | caggatccGCAAGTGCAACATTAACGATAGC |
| VP2Sense3′ | gaactagtGCTTATATCAACAATTGGTATCTACTTAG |
| VP2Antisense3′ | aaccgcggCTAACTTCTTTCAAAACTTCTTCAGC |
Letters shown in bold italic font are the mismatched bases for mutation of the yDHODH gene.
Restriction enzyme recognition sequences are underlined.
Fig. 1. Schematic maps of pUF-1 and its parental plasmid pCC4.
In pUF-1 a modified yDHODH gene replaces the bsd gene present in the parental plasmid pCC4 (see Materials and Methods). Hsp86 5′ = 5′ UTR of PfHSP86 gene; yFCU = yeast cytosine deaminase-uracil phosphoribosyl transferase fusion gene; Cam 5′ = 5′ UTR of P. falciparum calmodulin gene; PbDT3′ = 3′UTR of P. berghei dihydrofolate reductase-thymidylate synthase gene; Hrp2 3′ = P. falciparum histidine rich protein 2 3′ UTR; bsd = blasticidin S deaminase; yDHODH = yeast dihydroorotate dehydrogenase; pGEM3z = pGEM3z plasmid (Genbank accession X65306) backbone containing an ampicillin resistance gene (AmpR)
2.3 Construction of a specific PfVP2 knockout vector
The type II vacuolar pyrophosphatase gene knockout plasmid, pUF1ΔVP2, was constructed by amplifying 5′ and 3′ regions from the PfVP2 gene, each ~1kb in size, from P. falciparum genomic DNA using specific primers listed in Table 1. Both these regions originated within the gene’s coding region and would result in a deletion of 417bp within the gene under a double crossover recombination event (Fig. 1B). The amplicons were cloned into the pBluescript SK plasmid, sequence–verified, and inserted into the pUF-1 cloning sites surrounding the yDHODH cassette. The presence of the inserts was confirmed by restriction enzyme digestion with EcoRI and NcoI for the 5′ insert and SpeI and SacII for the 3′ insert.
2.4 Southern blot
DNA obtained from the wild type and knock-out parasites was digested with the enzymes EcoRI and HindIII, and electrophoresed in a 0.8% agarose gel. Southern blot transfer and hybridization was carried out using a standard protocol [13]. Briefly, the DNA was transferred onto GeneScreen Plus membrane (Perkin Elmer) by capillary blotting. One of the homologous regions, the 3′ insert, was amplified from P. falciparum D10 genomic DNA and labeled with dATP-α32P using Prime It II random primer labeling kit (Stratagene). The probe was hybridized to the membrane overnight (42 °C) and washed with 2XSSC containing 1% SDS (60 °C). X-ray film was exposed to the blot for 24 hrs, then developed and analyzed.
2.5. Chemical synthesis
Triazolopyrimidine inhibitors of P. falciparum DHODH, 5-Methyl[1,2,4]triazolo[1,5-a]pyrimidin-7-yl)naphthalen-2-ylamine (DSM1), and 5-Methyl[1,2,4]triazolo[1,5-a]pyrimidin-7-yl)(4-trifluoromethylphenyl)amine (DSM74) were synthesized as described previously [10,14].
2.6. Growth inhibition assay
The susceptibility of wildtype and transgenic PfVP2 knockout parasites to different inhibitors used for selection in P. falciparum transfections was assessed by 3H-hypoxanthine uptake as described by Desjardins et al [15]. Briefly, the parasitemia was adjusted to 0.1% parasitemia, 2.5% hematocrit, and 200-μl aliquots were placed in wells of a microtiter plate. Serial dilutions of inhibitors (WR99210, blasticidin, atovaquone, DSM1 or artemisinin) were made in RPMI. The compounds were added to the cultures in the microtiter plate and mixed well. After incubation for 48 h under standard conditions, [2,8-3H]-hypoxanthine (12.5 Ci/mmol) in RPMI (20 μl, 0.2 μCi) was added to each well. After incubation for an additional 24 h, the cultures were lysed, and incorporated radioactivity was measured with a scintillation counter. Each measurement reported is an average of three determinations.
3. Results
3.1. Atovaquone selection of pUF-1 transfected parasites
In the elaborate protocol for gene disruption in P. falciparum, a circular plasmid first needs to be transfected into parasites and propagated as an extrachromosomal episome under positive selection pressure. Therefore we assessed the propensity of the pUF-1 vector to be used under positive selection pressure by transfecting it into the D10 strain of P. falciparum under atovaquone selection. Because atovaquone resistance due to mutation in cytochrome b is relatively common, we used 100 nM atovaquone (>100 times IC50) to minimize generation of intrinsically resistant parasites. Transgenic parasites appeared 4 weeks after transfection. Since pUF-1 vector also possesses a yeast fusion gene (cytosine deaminase-uracil phosphoribosyl transferase, FCU) that should permit pyrimidine salvage by transfected parasites, we also tried selection by atovaquone in combination with cytosine. However, selection by atovaquone plus cytosine did not reduce the time to the appearance of resistant parasites (6 weeks vs. 4 weeks were observed after splitting the transfection and selecting in parallel with and without cytosine). When we attempted selection in 3D7 and Dd2 strains of P. falciparum, we found the efficiency of selection of the pUF-1 plasmid by atovaquone to be strain dependent. Transfection of pUF-1 into 3D7 yielded transgenic parasites, but these transfectants required 15 days more than had the transfectants of the D10 strain to reach a detectable level of growth under atovaquone pressure (results of single, independent transfections). More significantly, two independent attempts to transfect pUF-1 into Dd2 failed to yield transgenic parasites, suggesting that the Dd2 line was refractory to selection with atovaquone. This observation is in agreement with the finding that the Dd2 line, while expressing the yDHODH gene, cannot grow continuously under atovaquone pressure (Ke et al., manuscript submitted).
3.2. Selection with PfDHODH inhibitors
As shown previously, a critical step blocked by atovaquone is the DHODH reaction in the pyrimidine biosynthesis pathway [9]. Although all strains expressing the yDHODH gene are resistant to atovaquone (and other mtETC-inhibitors) when examined in the standard 48 hr growth inhibition assay, long-term growth of transgenic parasites under atovaquone pressure varies greatly among different parasite strains (Ke et al., unpublished results). This variation could be explained by the fact that, while atovaquone inhibits the ubiquinone regenerating system, some, but not all, parasite lines may require the function of ubiquinone-dependent enzymes in addition to DHODH for their long-term growth. In that case, it should also be possible to select for transfectants expressing yDHODH using specific inhibitors of PfDHODH, such as the triazolopyrimidine compound DSM1 [10,14]. We tested the ability of these compounds to select pUF-1 transfectants of P. falciparum D10 parasites, using 1.5 μM DSM1 for positive selection. In parallel, we also used 100 nM atovaquone for comparison. Several Plasmodium gene knockout constructs made with pUF-1, including one for the likely blood-stage nonessential gene PfCSP (PFC0210c) [16], were used to compare the selection efficiency. We observed that transfections with pUF-1 constructs yielded resistant parasites earlier under DSM1 selection compared to selection under atovaquone (~2-4 weeks versus ~4–6 weeks) (Table 2), suggesting that DSM1 is a more efficient selective agent than atovaquone, at least under the conditions employed. We also used another PfDHODH inhibitor, DSM74 [13], as a selection agent, and observed similar results. In contrast to atovaquone selection, we found in two independent experiments that pUF-1 transfected Dd2 lines could be selected successfully with DSM1, but not with atovaquone. The Dd2 transgenic parasites selected with DSM1 appeared in approximately 3–4 weeks. The responses of D10 and Dd2 in 2 independent transfections are compared in Table 3. These results underscore the superiority of using a direct inhibitor of PfDHODH for positive selection of pUF-1 transfected parasites in these P. falciparum strains.
Table 2.
Time to detection of transfected parasites under selection with atovaquone or DSM1
| Construct | Atovaquone selection (in weeksa) | DSM1 selection (in weeksa) |
|---|---|---|
| pUF-1/ΔAMT1 | 5 | 4 |
| pUF-1/ΔCSP | 6 | 4 |
| pUF-1/ΔRPL2 | 6 | 4 |
| pUF-1/ΔMQO | 8 | 3 |
| pUF-1/ΔRNAPOL* | 4 | 2 |
| pUF-1/ΔNDH2* | 5 | 3 |
| pUF-1/ΔDHODH* | 5 | 2 |
| pUF-1/ΔG3PDH* | 5 | 3 |
| Average difference | −2.4 | |
Weeks following transfection when the transgenic parasites were observed in Giemsa stained smears.
Indicates experiments in which a single transfection was split into two flasks, each of which was placed under selection by a different one of the inhibitors.
Table 3.
Comparison of atovaquone and DSM1 selection in P. falciparum D10 and Dd2 strains
| Transfection construct | Strain | Time until appearance of parasite growth (weeks) under selection by: |
|
|---|---|---|---|
| Atovaquone | DSM1 | ||
| pUF-1/ΔMQO | D10 | 8 | 3 |
| Dd2* | no growth | 4 | |
| pUF-1/ΔNDH2 | D10 | 5 | 3 |
| Dd2* | no growth | 3 | |
Selection was carried out in parallel with atovaquone and DSM1. Under atovaquone selection no growth of transfected Dd2 parasites occurred during the length of the experiment (8 weeks).
3.3. PfVP2 gene is not essential in P. falciparum blood stages
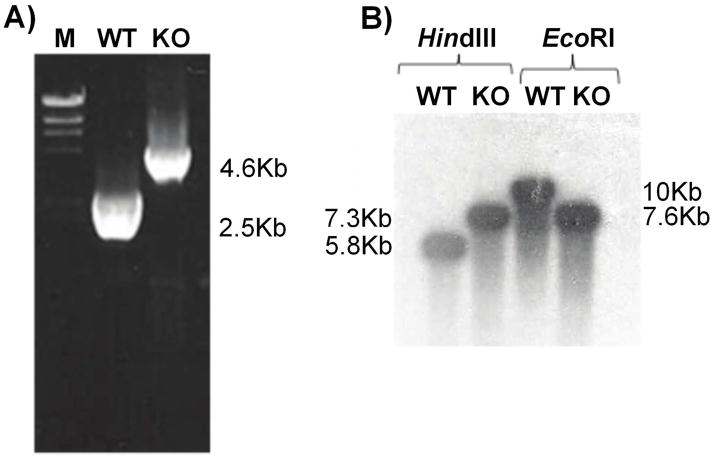
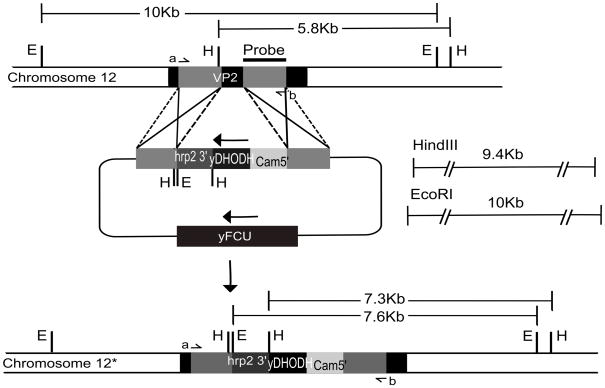
We have previously shown that P. falciparum encodes two distinct proton-pumping vacuolar pyrophosphatases, the functions of which are not clear at this point [11,17]. We tested the usefulness of the pUF-1 vector for gene disruption by targeting the K+-insensitive type II pyrophosphatase gene. The knock out plasmid pUF1ΔVP2, containing PfVP2 gene 5′ and 3′ fragments flanking the yDHODH cassette in pUF-1, was successfully transfected into the D10 strain of P. falciparum. After 5 weeks of positive selection with atovaquone, transgenic parasites containing pUF1ΔVP2 were obtained. As atovaquone resistance can arise due to mutations in cytochrome b, the gene encoding cytochrome b was amplified from positively selected parasites and sequenced. No mutations were found, indicating that the resistance to atovaquone was due to the expression of the yDHODH gene product. An initial negative selection of these positively selected parasites with 2 μM 5-fluorocytosine, which would reveal deletion of the FCU gene under double crossover recombination, did not yield parasites after 28 days of culture. We then used a drug cycling protocol to increase the chances of a double crossover event. Each cycle consisted of 21 days of culture in the absence of atovaquone, followed by growth in the presence of atovaquone to select resistant parasites. After two drug-off and drug-on cycles, followed by negative selection by 5-fluorocytosine treatment, doubly resistant parasites were obtained, suggesting a successful double crossover disruption of the PfVP2 gene. Diagnostic PCR yielded the expected 2.5 kb and 4.1 kb amplicons from wild type and knock-out DNAs, respectively (Fig 3A). To further confirm the VP2 gene knockout, a Southern blot was carried out. DNA from wild type and transgenic parasites produced the expected band sizes, 5.7 kb and 7.2 kb, respectively, when digested with HindIII and 10 kb and 7.5 kb bands, respectively, after EcoRI digestion (Fig 3B).
Fig. 3. Confirmation of VP2 gene knockout.
A) Diagnostic PCR. Genomic DNA isolated from wild type and transgenic parasites served as template for PCR reactions using primers VP2 Sense5′ and VP2 Antisense3′ (Table 2; positions indicated in Fig 2). The PCR produced 4.6 kb and 2.5 kb products with knock out and wild type genomic DNA, respectively. M- Marker DNA fragments (1kb, Promega), WT- Wild type, KO- VP2 knock out.
B) Southern blot analysis of genomic DNA from D10 wild type and D10 VP2 knock out parasites digested with HindIII or EcoRI. The probe, generated from a 3′ segment of the VP2 gene (position shown in Fig. 2), hybridized to 5.8 kb (HindIII) and 10 kb (EcoRI) fragments of wild type DNA, and to 7.3 kb (HindIII) and 7.6 kb (EcoRI) fragments of transgenic DNA.
3.4. yDHODH transgenic parasites remain susceptible to compounds used for the selection of other markers
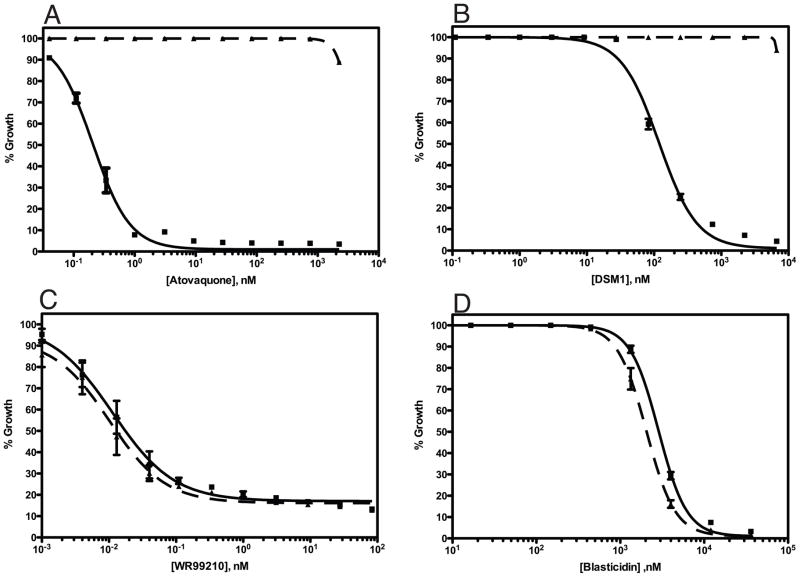
To test whether the yDHODH marker could be used in combination with other selectable markers, we analyzed the susceptibility of the ΔVP2 transgenic parasites to other compounds commonly used for selection in P. falciparum, including WR99210 and blasticidin. Using the standard 3H-hypoxanthine incorporation assay, we found that transgenic parasites expressing yDHODH were fully susceptible to the other selection compounds (Fig. 4). The IC50 values found for the transgenic and wild type parasites were 0.010 nM and 0.011 nM, respectively, for WR99210; and 2840 nM and 2041 nM for blasticidin. The transgenic parasites are completely resistant to atovaquone and DSM1 with IC50 values of >2250 nM atovaquone versus 0.2073 nM atovaquone for wild type parasites and >6667 nM versus 118 nM DSM1 for the wild type parasites.
Fig. 4. yDHODH transgenic PfVP2 knockout parasites are susceptible to the compounds used for other selectable markers.
Growth inhibition of wild type D10 and ΔVP2::yDHODH transgenic parasites was assessed by standard 3H-hypoxanthine incorporation assay for atovaquone (A), DSM1 (B), WR99210 (C), and blasticidin (D). The calculated IC50 values for these compounds are listed in the Results section. Solid lines, wild type D10 parasites; dashed lines, ΔVP2::yDHODH parasites.
4. Discussion
One of the major limiting factors in the genetic transformations of malaria parasites is the small number of selectable markers available. Marker genes that have been successfully used in Plasmodium include methotrexate-resistant human DHFR, bsd and neo [5,6]. Here we report the use of the yeast DHODH gene as a new selectable marker for transfections in the human malaria parasite P. falciparum, where it confers resistance to the antimalarial antimitochondrial drug atovaquone and to PfDHODH inhibitors. In our earlier study of the role of the mitochondrial electron transport chain (mtETC), we used yDHODH as a ubiquinone-independent substitute for the parasites’ endogenous DHODH, allowing us to observe the effects of inhibition of the mtETC independently of effects on pyrimidine biosynthesis. We found that the yDHODH transgenic parasites are resistant not only to atovaquone but also to all other mtETC inhibitors [9]. In these experiments, we corroborated the essential role of parasite DHODH and established the major role of the parasite mtETC in serving this enzyme.
To test the selectability of yDHODH, we constructed the plasmid pUF-1, containing the yDHODH gene under the control of a Plasmodium calmodulin promoter. Parasites transfected with this plasmid can be selected with atovaquone, as well as with the PfDHODH-specific inhibitor compounds DSM1 and DSM74. In the present study, the transfections were primarily carried out in the D10 strain of P. falciparum. Selection was also successful in P. falciparum 3D7 strain, albeit with a somewhat longer outgrowth period in the case of atovaquone selection. We were unsuccessful in selecting P. falciparum Dd2 parasites transfected with pUF-1 using atovaquone. Yet selection of pUF-1 transfected parasites with DHODH-specific inhibitors was possible in Dd2, as well as the other strains. These results appear to imply that in the Dd2 strain the mtETC has additional critical function(s) that are less important in other strains of P. falciparum. Comparison of the selection of transgenic parasites in the presence of atovaquone or DSM1 indicated that DSM1 is the superior selection agent, yielding transfectants about 2 weeks earlier than selection with atovaquone after transfection of D10.
As a proof of concept, we have used pUF-1 vector for double crossover disruption of the P. falciparum PfVP2 gene. Proton-pumping vacuolar-type pyrophosphatases (V-PPases) are widely distributed in plants, protozoa, bacteria and archaea. Plasmodium has two different vacuolar pyrophosphatases, PfVP1 (product of PF14_0541) and PfVP2 (product of PFL1700c). PfVP1 encodes a type I, K+-dependent V-PPase and PfVP2 encodes a type II, K+-independent V-PPase [11,17]. The successful disruption of the PfVP2 gene after 2 rounds of drug on and off cycling suggests that this gene is not essential for parasite growth in erythrocytic stages. We are currently assessing subtle effects of this gene disruption on pH homeostasis in malaria parasites. Our results also demonstrate that pUF-1 is suitable for use in gene knockout experiments. As a further demonstration of the utility of this plasmid, we examined pUF-1/ CSP transfected parasites (Table 2). After 2 drug off and on cycles, followed by negative selection, we obtained a knockout of the PfCSP gene (See Fig. S1), verifying that this gene is nonessential in P. falciparum.
This transfection vector should be very useful in studies where other selectable markers cannot be employed. For example, investigation of genes encoding enzymes of the folate pathway in P. falciparum requires a selectable marker that does not depend upon resistance to the antifolate drugs pyrimethamine or WR99210. In such a scenario, pUF-1 plasmid constructs may prove useful. Clearly, the transgenic parasites remain susceptible to other compounds commonly used for selection; hence this vector can be used together with vectors utilizing one or more of the other available markers. The use of triazolopyrimidine inhibitors of parasite DHODH as selective agents should facilitate the use of the plasmid in additional P. falciparum strains. The transfection vector described here should be of particular use in multiple gene knockout studies, in which two or more genes are disrupted in the parasites to study the role of possibly redundant genes or of interacting pathways. We have, in fact, successfully carried out 2 sets of double knockouts using pUF-1 in combination with another plasmid (pCC1, containing the hDHFR marker; data not shown). Clearly, additional vectors can be constructed with the yDHODH marker for use in additional types of experiments, such as knock-in or mutant complementation. Such studies are currently in progress in our laboratory.
Supplementary Material
A) Schematic diagram of the CSP locus on chromosome 3, the pUF-1 derived knock out plasmid (pUF1ΔCSP) used to disrupt the endogenous CSP gene and the predicted CSP locus after double cross over integration in P. falciparum. The location of the probe and the expected sizes of the fragments detected by Southern blot analysis of genomic DNA digested with EcoRV and SpeI are shown.
B) Southern blot analysis of genomic DNA from wildtype D10 and CSP knock out parasites. EcoRV and SpeI digests of genomic DNA of wildtype D10 parasites produce 8.7 kb and 10 kb fragments respectively. However, the knock out allele produces 3 kb and 7 kb fragments, respectively, indicating that the CSP gene is disrupted. The probe used for hybridization is indicated in panel A
Fig. 2. Schematic diagram of the knockout strategy for the VP2 gene.
The VP2 locus on P. falciparum chromosome 12, the pUF-1 derived knock out plasmid (pUF1ΔVP2) used to disrupt the endogenous VP2 gene and the predicted VP2 locus after double cross over integration are shown. The binding sites of primers used in PCR reactions to confirm the knock out are indicated by small arrows labeled a, b. The location of the probe and the expected sizes of the fragments detected by Southern blot analysis of genomic DNA digested with EcoRI (E) and HindIII (H) are shown.
Acknowledgments
We thank Dr. Alan Cowman for providing the plasmid pCC4. This work was supported by the United States National Institutes of Health grants AI028398 (to ABV), U01AI075594 (to MAP and PKR), and AI053680 (to MAP and PKR). We also acknowledge support from Drexel University College of Medicine. MAP acknowledges the support of the Welch Foundation (I-1257). MAP holds the Carolyn R. Bacon Professorship in Medical Science and Education.
Abbreviations
- bsd
blasticidin S deaminase
- DHFR
dihydrofolate reductase
- DHODH
dihydroorotate dehydrogenase
- DSM1
5-Methyl[1,2,4]triazolo[1,5-a]pyrimidin-7-yl)naphthalen-2-ylamine
- DSM74
5-Methyl[1,2,4]triazolo[1,5-a]pyrimidin-7-yl)(4-trifluoromethylphenyl)amine
- FCU
yeast (Saccharomyces cerevisiae) cytosine deaminase-uracil phosphoribosyl transferase fusion gene
- mtETC
mitochondrial electron transport chain
- PfDHODH
Plasmodium falciparum DHODH
- UTR
untranslated region (of DNA)
- V-PPase
proton-pumping vacuolar-type pyrophosphatase
- yDHODH
yeast DHODH (Saccharomyces Genome Database: Ura1p)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlton JM, Adams JH, Silva JC, Bidwell SL, Lorenzi H, Caler E, et al. Comparative genomics of the neglected human malaria parasite Plasmodium vivax. Nature. 2008;455:757–63. doi: 10.1038/nature07327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlton JM, Angiuoli SV, Suh BB, Kooij TW, Pertea M, Silva JC, et al. Genome sequence and comparative analysis of the model rodent malaria parasite Plasmodium yoelii yoelii. Nature. 2002;419:512–9. doi: 10.1038/nature01099. [DOI] [PubMed] [Google Scholar]
- 4.Pain A, Böhme U, Berry AE, Mungall K, Finn RD, Jackson AP, et al. The genome of the simian and human malaria parasite Plasmodium knowlesi. Nature. 2008;455:799–803. doi: 10.1038/nature07306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fidock DA, Wellems TE. Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil. Proc Natl Acad Sci USA. 1997;94:10931–6. doi: 10.1073/pnas.94.20.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mamoun CB, Gluzman IY, Goyard S, Beverley SM, Goldberg DE. A set of independent selectable markers for transfection of the human malaria parasite Plasmodium falciparum. Proc Natl Acad Sci U S A. 1999;96:8716–20. doi: 10.1073/pnas.96.15.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill DA, Pillai AD, Nawaz F, Hayton K, Doan L, Lisk G, Desai SA. A blasticidin S-resistant Plasmodium falciparum mutant with a defective plasmodial surface anion channel. Proc Natl Acad Sci USA. 2007;104:1063–1068. doi: 10.1073/pnas.0610353104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutteridge WE, Dave D, Richards WH. Conversion of dihydroorotate to orotate in parasitic protozoa. Biochim Biophys Acta. 1979;582:390–401. doi: 10.1016/0304-4165(79)90131-4. [DOI] [PubMed] [Google Scholar]
- 9.Painter HJ, Morrisey JM, Mather MW, Vaidya AB. Specific role of mitochondrial electron transport in blood-stage Plasmodium falciparum. Nature. 2007;446:88–91. doi: 10.1038/nature05572. [DOI] [PubMed] [Google Scholar]
- 10.Phillips MA, Gujjar R, Malmquist NA, White J, El Mazouni F, Baldwin J, Rathod PK. Triazolopyrimidine-based dihydroorotate dehydrogenase inhibitors with potent and selective activity against the malaria parasite Plasmodium falciparum. J Med Chem. 2008;51:3649–53. doi: 10.1021/jm8001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McIntosh MT, Drozdowicz YM, Laroiya K, Rea PA, Vaidya AB. Two classes of plant-like vacuolar-type H(+)-pyrophosphatases in malaria parasites. Mol Biochem Parasitol. 2001;114:183–95. doi: 10.1016/s0166-6851(01)00251-1. [DOI] [PubMed] [Google Scholar]
- 12.Maier AG, Braks JA, Waters AP, Cowman AF. Negative selection using yeast cytosine deaminase/uracil phosphoribosyl transferase in Plasmodium falciparum for targeted gene deletion by double crossover recombination. Mol Biochem Parasitol. 2006;150:118–21. doi: 10.1016/j.molbiopara.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 14.Gujjar R, Marwaha A, EI Mazouni E, White J, White KL, Creason S, et al. Identification of a metabolically stable triazolopyrimidine-based dihydroorotate dehydrogenase inhibitor with antimalarial activity in mice. J Med Chem. 2009;9;52:1864–72. doi: 10.1021/jm801343r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desjardins RE, Canfield CJ, Haynes JD, Chulay JD. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979;16(6):710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ménard R, Sultan AA, Cortes C, Altszuler R, van Dijk MR, Janse CJ, et al. Circumsporozoite protein is required for development of malaria sporozoites in mosquitoes. Nature. 1997;385:336–40. doi: 10.1038/385336a0. [DOI] [PubMed] [Google Scholar]
- 17.McIntosh MT, Vaidya AB. Vacuolar type H+ pumping pyrophosphatases of parasitic protozoa. Int J Parasitol. 2002;32:1–14. doi: 10.1016/s0020-7519(01)00325-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) Schematic diagram of the CSP locus on chromosome 3, the pUF-1 derived knock out plasmid (pUF1ΔCSP) used to disrupt the endogenous CSP gene and the predicted CSP locus after double cross over integration in P. falciparum. The location of the probe and the expected sizes of the fragments detected by Southern blot analysis of genomic DNA digested with EcoRV and SpeI are shown.
B) Southern blot analysis of genomic DNA from wildtype D10 and CSP knock out parasites. EcoRV and SpeI digests of genomic DNA of wildtype D10 parasites produce 8.7 kb and 10 kb fragments respectively. However, the knock out allele produces 3 kb and 7 kb fragments, respectively, indicating that the CSP gene is disrupted. The probe used for hybridization is indicated in panel A