Abstract
BACKGROUND & AIMS
Endoplasmic reticulum (ER) stress responses (collectively known the unfolded protein response, UPR) have important roles in several human disorders, but their contribution to alcoholic pancreatitis is not known. We investigated the role of X box-binding protein 1 (XBP1), an UPR regulator, in prevention of alcohol-induced ER stress in the exocrine pancreas.
METHODS
Wild-type and Xbp1+/− mice were fed control or ethanol diets for 4 weeks. Pancreatic tissue samples were then examined by light and electron microscopy to determine pancreatic alterations; UPR regulators were analyzed biochemically.
RESULTS
In wild-type mice, ethanol activated a UPR, increasing pancreatic levels of XBP1 and XBP1 targets such as protein disulfide isomerase (PDI). In these mice, pancreatic damage was minor. In ethanol-fed Xbp1+/− mice, XBP1 and PDI levels were significantly lower than in ethanol-fed, wild-type mice. The combination of XBP1 deficiency and ethanol feeding reduced expression of regulators of ER function and the upregulation of pro-apoptotic signals. Moreover, ethanol feeding induced oxidation of PDI, which might compromise PDI-mediated disulfide bond formation during ER protein folding. In ethanol-fed Xbp1+/− mice, ER stress was associated with disorganized and dilated ER, loss of zymogen granules, accumulation of autophagic vacuoles, and increased acinar cell death.
CONCLUSIONS
Chronic ethanol feeding causes oxidative ER stress, which activates a UPR and increases XBP1 levels and activity. A defective UPR, due to XBP1 deficiency, results in ER dysfunction and acinar cell pathology.
Keywords: alcohol disease, transcription factors, ER protein folding, organelle
Introduction
Alcohol abuse is a key factor in the development of chronic pancreatitis.1 Despite the evidence supporting toxic effects for alcohol on pancreas 2–4, only a small number of alcohol abusers eventually develop pancreatitis.4 Long-term ethanol feeding to animals causes only minor pancreatic damage, but sensitizes the pancreas to develop pancreatitis at a lower threshold to stressors.5–7 These observations suggest that the pancreas mobilizes adaptive responses that restore normal function in the presence of alcohol.
The acinar cell is a chief participant in the pathobiologic responses of pancreatitis. 8 This cell specializes in the production and secretion of large amounts of digestive enzymes. To fulfill these functions, the acinar cell has an extensive endoplasmic reticulum (ER) network that regulates the folding and trafficking of proteins in the secretory pathway. Multiple chaperones and foldases assist protein folding within the ER. Quality control systems ensure the progress of correctly folded proteins into the secretory pathway or direct misfolded proteins for degradation by ER-associated degradation (ERAD) mechanisms.9 Alterations in the ER environment including oxidative stress, overloading of the ER’s protein folding capacity, or the presence of mutant proteins causes aberrant protein folding, accumulation of misfolded proteins and “ER stress”.10
ER stress plays an important role in the disease progression of several disorders including diabetes, cardiovascular diseases, neurodegenerative disorders, intestinal inflammation and alcoholic liver disease.11–14 However, whether alcohol abuse causes ER stress or alters ER stress responses in the exocrine pancreas has not been addressed.
ER stress responses are collectively known as unfolded protein response (UPR).15 The UPR has three main outputs: a transient reduction in protein translation to decrease ER protein load; upregulation of ER regulators to augment the folding and export capacity of the ER, and activation of ERAD. The UPR can also activate cell death programs under severe or prolonged ER stress, when adaptive responses are exceeded and/or a dysfunctional UPR is unable to correct ER stress.12, 16 The UPR is initiated by ER sensors that detect the presence of misfolded proteins within the ER. Activation of the ER sensor protein kinase RNA (PKR)-like ER kinase (PERK) results in phosphorylation of eukaryotic translation initiation factor 2α (eIF2α), which leads to general decrease in protein translation,17 and preferential translation of activating transcription factor 4 (ATF4) that targets genes involved in regulation of intracellular redox status and glutathione synthesis.18 While transient PERK activation alleviates ER stress, prolonged PERK activation leads to sustained blockade in protein translation, and upregulation of C/EBP homologous protein (CHOP) that triggers apoptotic responses. 19
Inositol-requiring transmembrane kinase/endonuclease 1 (IRE1) is the most evolutionarily conserved ER stress sensor. Upon ER stress, IRE1 induces splicing of the X box-binding protein 1 (XBP1) mRNA,20 resulting in translation of the transcription factor spliced-XBP1 (sXBP1). sXBP1 target genes include chaperones, oxidoreductases of the protein disulfide isomerase (PDI) family, components of the ERAD pathway, and genes required for lipid synthesis.21–23 Activation of the IRE1/XBP1 pathway acts as an adaptive response to restore the folding capacity of the ER and promote degradation of misfolded proteins and ER-localized mRNAs.15, 24 XBP1 is essential for ER development in secretory cells including pancreatic acinar cells.22 Although XBP1 actions vary between different cell types, genetic alterations of XBP1 are linked to several disorders including diabetes, IBD, atherosclerosis, and neurodegenerative disorders.13, 22, 25–27
Previous studies have demonstrated UPR activation in acinar cells and during experimental pancreatitis.28 This study sought to determine whether alcohol activates UPR signals in exocrine pancreas that are critical for adaptation to the toxic effects of ethanol and its metabolites. We have examined UPR signals in pancreas from ethanol-fed rats and mice, and observed changes in the IRE1-XBP1 pathway consistent with protective responses. Moreover, we found severe ER stress and pathological responses in acinar cells of mice with genetic inhibition of XBP1 expression during chronic ethanol feeding. Taken together, these findings establish a critical role for an adaptive UPR involving the IRE1/XBP-1 pathway in protecting the pancreas from alcohol-induced injury.
Materials and Methods
Animals and ethanol feeding
Xbp1+/− and wild-type (Xbp1+/+, BALB/c) littermate mice were obtained by breeding Xbp1+/−mice generously provided by L Glimcher. The phenotype of these mice is described elsewhere26, and in Supplementary Materials. For initial studies, we also used Wistar rats (Charles River, MA). Both rats and mice were fed control and ethanol-containing diets using the Tsukamoto-French intragastric ethanol infusion model.29 Animals were placed on diets for 6 (rats) or 4 (mice) weeks. Pancreatic tissue samples were analyzed by light and electron microscopy and biochemically by Western blotting and RT-PCR. Blood samples were analyzed for alcohol levels and markers of pancreatic (amylase and lipase) and liver (ALT) injury. For more details see Supplementary Materials.
Studies using pancreatic acini
Pancreatic acini were isolated from chow-fed wild-type and Xbp1+/− mice, and Wistar rats, and cultured as described.2, 30 Cells were treated for up to 24 h without or with cholecystokinin-octapeptide (CCK-8, 0.01-100 nM) or with 75 mmol/L ethanol, and then cells and conditioned media were prepared for Western blot analysis and measurements of amylase secretion. Amylase activity was measured using the Phadebas test (Pharmacia Diagnostic, NY) following manufacturer’s protocol. Culture conditions and treatments are detailed in Supplementary Materials.
Histological Analysis
Pancreatic sections of mice and rats fed ethanol or control diets were formalin fixed and H&E stained to determine parenchymal structure, inflammation, acinar cell necrosis and apoptosis (by TUNEL). Acinar ultrastructure and autophagy were examined by electron microscopy. In addition, we performed LC3B immunostaining in mouse pancreatic sections to assess accumulation of autophagic vacuoles. Details of all these procedures are provided in Supplementary Materials, and were described before. 5, 30, 31
Real time PCR and XBP1 splicing assay
RNA extracted from pancreas using TRI reagent (Molecular Research Center, OH) was subjected to RT-PCR (for CHOP, and XBP1 splicing) or qRT-PCR analysis using SyBR Green (Applied Biosystems, CA) (for ERdj4 and EDEM1) as described previously.2 XBP1 splicing was measured by specific primers flanking the splicing site. PCR products were visualized using a FluorChem-HD2 imager (Alpha Innotech, CA). Primer sequences are listed in Table S1.
Immunoblotting
Pancreatic protein extracts in RIPA buffer containing a mixture of protease and phosphatase inhibitors were resolved by SDS-PAGE for immunoblot analysis as previously described.2, 30 Antibodies and procedures are described in Supplementary materials.
PDI Redox State
PDI redox state was determined by electrophoretic migration shift in PDI after alkylation of thiol groups with AMS.32 Pancreatic protein extracts in RIPA buffer were treated with the reducing agent TCEP (10 mM) in 1% SDS for 10 min on ice, and then with 25 mM AMS for 60 min in the dark. AMS-treated proteins were separated on 8% Tris-glycine SDS-PAGE gels, and PDI was detected by Western blot using an anti-PDI antibody (Assay Designs). Since AMS increases MW by 500 daltons per thiol group, changes in electrophoretic mobility of PDI indicates different numbers of thiol-groups associated with changes in PDI redox state.
Statistical analysis
Data, presented as mean±SEM, were analyzed using SigmaStat software (SPSS Inc). Comparisons between 2 groups were performed by the Student t test. Two-way ANOVA (for normally distributed data) and post-hoc Tukey tests were used for group comparisons when two variables were present. The Kruskal–Wallis test followed by Dunn post-hoc tests were used when group data were not normally distributed. P values <0.05 were considered statistically significant.
Results
Ethanol feeding induced activation of UPR and XBP1 in rat pancreas
As reported previously, 5, 6 rats fed for 6 weeks ethanol diets using the Tsukamoto-French intragastric infusion model did not exhibit evidence of pancreatitis or acinar cell damage in H&E-stained tissue sections. Levels of blood lipase, and intrapancreatic trypsin activity were similar in ethanol-fed and control-fed rats (not shown). However, electron microscopy analysis revealed structural changes, the most prominent being an extensive distension in the endoplasmic reticulum (ER) of acinar cells (Fig. 1A). By observational analysis of several randomly selected fields, these morphological changes were estimated to affect up to 35% of the acinar cells. We also found that ethanol feeding altered ER redox status, as indicated by a 2-fold increase in the GSSG/GSH ratios in ER-enriched pancreatic fractions (Fig. S1). Taken together, these data suggested oxidative ER stress in ethanol-fed rats.
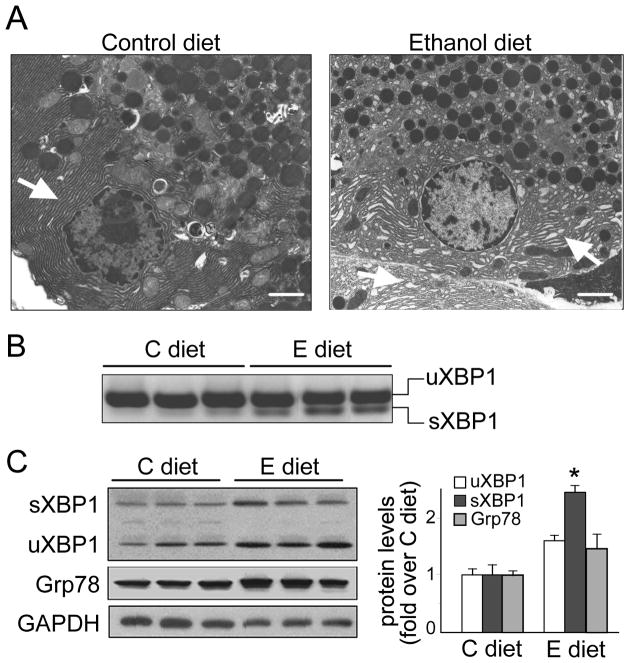
Figure 1. Ethanol feeding activates UPR and XBP1 in rat pancreas.
Rats were fed control (C) or ethanol (E) diets for 6 weeks. (A) Electron micrographs showing ultrastructure of pancreatic acinar cells. Ethanol-fed rats exhibit well preserved acinar architecture (right panel) compared to controls (left panel), but ER is dilated (closed arrows). Bars, 2 μm. (B) RT-PCR showing higher XBP1 mRNA splicing (sXBP1) in pancreas from ethanol-fed than in control-fed rats. (C) Western blot analysis of spliced (sXBP1) and unspliced (uXBP1), Grp78 and GAPDH (loading control). In B and C each lane represents an individual rat. Graph shows densitometry for XBP1 and Grp78 (means±SEM; n=5). * P<.05 vs. C diet (Student t-test).
Ethanol feeding induced UPR activation in rat pancreas, as indicated by significant increases in mRNA and protein levels of sXBP1 and small increase of the ER chaperone Grp78 (Figs. 1B-1C). Because XBP1 is essential for ER development in the acinar cell,22 we hypothesized that XBP1 activation (sXBP) promotes adaptive signals in response to ER stress induced by ethanol or other stressors. In support of this, we found that sXBP1 levels increased in rat pancreatic acini in response to toxic concentrations of CCK-8 and during culture conditions (Fig. S2).
XBP1 deficiency enhanced ER stress in pancreatic acinar cells
Our data obtained from rat pancreas prompted us to investigate the role of sXBP1 in protecting the pancreas against ethanol-induced damage. We reasoned that, if sXBP1 indeed protects the pancreas, genetic deletion should unmask the toxic effects of ethanol. For subsequent studies, we used Xbp1+/− mice because Xbp1−/− mice are not viable. 33 Xbp1+/− mice exhibit a 30% decrease in pancreatic levels of XBP1 (spliced + unspliced XBP1) in steady state conditions, and a partial decrease in PDI, a XBP1 target required for disulfide bond formation during protein folding (Fig S4). These data substantiate that these mice are indeed deficient in XBP1. Xbp1+/− mice on standard chow diet are fertile and healthy, with growth and pancreatic function similar to wild-type littermates (Table S2 and Fig S4), but display hepatic ER stress when placed on long-term high fat diets.26
We first examined whether Xbp1+/− acinar cells are prone to acute ER stress induced by toxic concentrations of CCK (100 nM). Pancreatic acini isolated from wild-type and Xbp1+/− mice were treated for 30 min with CCK-8 (0.01–100 nM). In wild-type cells, 100 nM CCK-8 elicited UPR activation, as indicated by a 2-fold increase in sXBP1 over that in unstimulated cells, and marked phosphorylation of PERK and eIF2α (Fig. 2A). CCK-stimulated Xbp1+/− cells had up to 50% less sXBP1 and displayed greater susceptibility to ER stress, as demonstrated by activation of PERK in response to concentrations of CCK-8 (0.1 nM) that did not induce this activation in wild-type cells (Fig. 2A). XBP1 deficiency also led to reduced amylase secretion in response to CCK or after 24 h culture (Figs 2B-C). Moreover, after 24 h in culture, Xbp1+/− cells exhibited lower levels of sXBP1 and PDI as compared to control cells (Figs. 2C-2D).
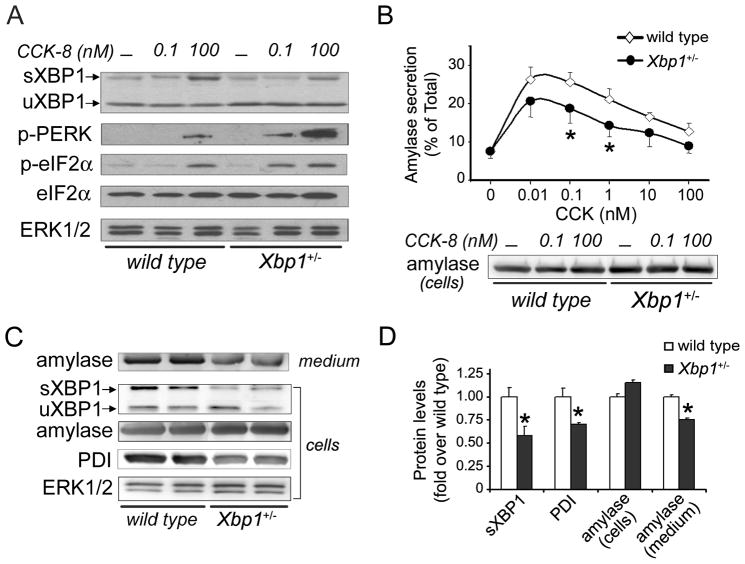
Figure 2. Pancreatic acini isolated from Xbp1+/− mice are prone to ER stress.
(A) and (B) Pancreatic acini isolated from wild-type and Xbp1+/− mice were incubated with and without CCK-8 for 30 min. (A) Immunoblot shows protein levels of sXBP1 and uXBP1, phospho-PERK, phospho- and total eIF2α after stimulation with 0.1 nM or 100 nM CCK-8. (B) Graph shows amylase secretion by acini stimulated with CCK-8 (mean±SEM, 4 independent studies). Immunoblot shows cellular amylase content. * P<.05 vs. wild-type (two-way ANOVA and Tukey post-tests: CCK, P<.001; genotype, P=0.002, interaction, P=0.624). (C) Immunoblots show amylase content in media, and cellular levels of sXBP1, amylase, PDI and ERK1/2 (loading control) in unstimulated acini cultured for 24 h (n=2 independent experiments). (D) Graph shows densitometry for immunoblots depicted in panel C (mean±SEM, n=3). * P<.05 vs. wild-type (Student t-test).
XBP1 deficiency and ethanol feeding led to ER stress and pancreas pathology
We next examined whether long-term ethanol feeding induces ER stress and UPR activation in mouse pancreas. Of note, short-term ethanol treatment (24 h) did not induced substantial activation of UPR pathways in cultured mouse pancreatic acini (not shown), although markedly increased mRNA levels of unspliced XBP1 (Fig S3).
Xbp1+/− and littermate wild-type mice were placed on the Tsukamoto-French intragastric ethanol infusion diets for 4 weeks, and then sacrificed (see Supplementary materials for details). Blood ethanol levels were comparable in ethanol-fed wild-type and Xbp1+/− mice (Fig. S5). No significant differences in body weight gain were observed in control-fed mice, irrespective of genotype, or ethanol-fed wild-type mice (Fig. S5). In contrast, body weight gain in ethanol-fed Xbp1+/− mice declined after the third week, and was significantly reduced at sacrifice. Blood ALT and amylase levels were elevated in these mice (Fig. S5), although only ALT levels reached statistical significance compared to wild-type controls.
Pancreas from all control-fed mice and ethanol-fed wild-type mice appeared normal by H&E staining, and lacked evidence of significant acinar cell necrosis or inflammation (Figs. 3A and 3C). In contrast, ethanol-fed Xbp1+/− mice displayed patchy areas of acinar cell necrosis and areas where acinar cells were replaced by stromal cells and small tubular complexes (Fig. 3A, panel c). In these mice, acinar cell necrosis represented 9% of the total parenchyma area (Fig 3C). Ethanol-fed Xbp1+/− mice also exhibited patchy areas with marked reduction in zymogen granules (Fig. 3A, panel d), suggesting defects in protein processing in the secretory pathway. These morphological changes correlated with a 25% reduction in the number of zymogen granules per cell, assessed by EM analysis (Fig. 3D), and a 30% reduction in amylase levels, as determined by Western blotting (Fig. 3E). In ethanol-fed Xbp1+/− mice, few neutrophils could be observed mostly in necrotic areas. Inflammation, scored as indicated in Supplementary materials, was: control-fed wild-type and Xbp1+/− mice, 0; ethanol-fed wild-type mice, 0.2±0.2; ethanol-fed Xbp1+/− mice, 0.7±0.2 (mean±SEM; n=5–7 mice).
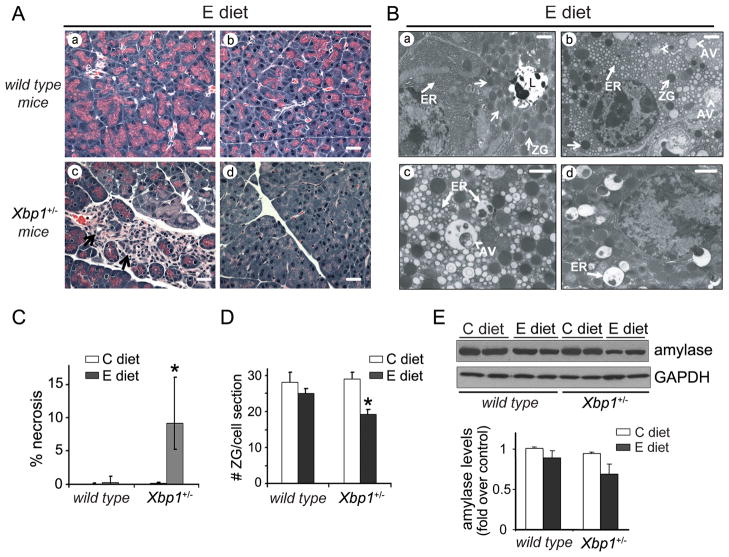
Figure 3. Ethanol feeding causes significant pancreatic damage in Xbp1+/− mice.
Wild-type and Xbp1+/− mice were fed either control or ethanol diet. (A) Representative pancreatic H&E staining from ethanol-fed wild-type (panels a-b) and Xbp1+/− mice (panels c-d) after 4-weeks on diets. Pancreas of ethanol-fed wild-type mice appeared normal. Pancreas of ethanol-fed Xbp1+/− mice displayed patchy areas of acinar cell necrosis (c, white arrow), and areas with abundant stroma cells and tubular complexes (c, black arrows). In panel d, the typical eosin staining in apical areas of acini is decreased, suggesting loss of zymogen granules. Pancreatic sections from all control-fed mice appeared normal (not shown). Bars, 20 μm. (B) Electron micrographs from ethanol-fed wild-type (panel a) and Xbp1+/− mice (panels b-d) demonstrate features of ER stress in Xbp1+/− mice. Acinar cells in wild-type mice show regular ultrastructure, with a slightly dilated ER (a, closed arrows). In Xbp1+/− mice, acinar cells often displayed extensively dilated ER (b; higher magnification in c) with occasional accumulation of dense materials (d). ZG, zymogen granules (open arrows); AV, autophagic vacuoles (arrowheads); L, acinar lumen; Bars, 1 μm. (C) Graph shows percentage of acinar cell necrosis per total pancreatic area evaluated in H&E tissue sections (median±25th and 75th percentiles; n=4–6 mice). * P<.05 vs. wild-type mice (Kruskal–Wallis test and Dunn posthoc test). (D). Graph shows number of zymogen granules per cell section measured in EM pancreatic sections (mean±SEM, n=4–6 mice). * P<.05 vs. wild-type mice (two-way ANOVA and Tukey post-tests). Criteria for quantification in C and D are explained in Supplementary Materials. (E) Immunoblots showing pancreatic levels of amylase and GAPDH (loading control). Each lane represents an individual mouse. Graph shows amylase quantification relative to control-fed wild-type mice (mean±SEM, n=5).
Electron microscopy revealed morphologic evidence of ER stress in approximately 25-38% of acinar cells from ethanol-fed Xbp1+/− mice (Fig. 3B). As illustrated in Fig. 3B, the predominant findings were a disorganized ultrastructure with extensively dilated ER, occasionally with dense lumenal inclusions, a hallmark of ER stress, a reduction in the number of zymogen granules (Fig. 3D), and accumulated autophagic vacuoles (Fig. 3, panels b-d). Consistent with this, pancreatic levels of the autophagosomal marker LC3B were significantly higher in ethanol-fed Xbp1+/− mice than in wild-type controls (Fig. S6).
XBP1 deficiency and ethanol feeding increased UPR in mouse pancreas
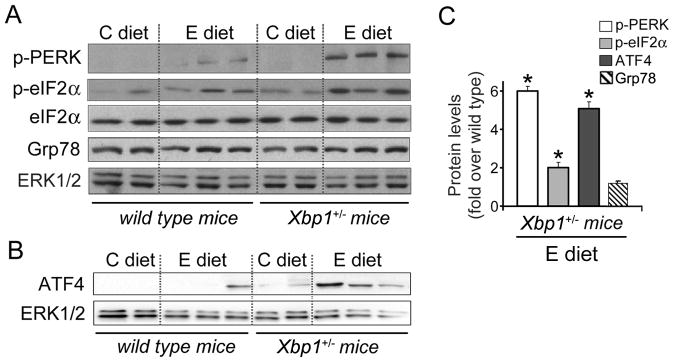
We next assessed activation of the IRE1/XBP1 and PERK pathways, including expression of ATF4, a key transcription factor regulating antioxidant programs and amino acid import.18 In addition, we examined protein levels of Grp78, a critical regulator of ER function. Of note, although protein levels of Grp78 increase during acute stress and are a good indicator of UPR activation, steady levels usually are achieved after chronic or mild stress. 34
As we observed in rats, ethanol feeding induced significant XBP1 mRNA splicing, and upregulated sXBP1 (2.5-fold) as well as IRE1 (1.4-fold) protein levels in wild-type mice (Fig. 4). We also observed a modest increase in PERK and eIF2α phosphorylation and some induction of ATF4, without significant changes in protein levels of the chaperone Grp78 (Fig. 5).
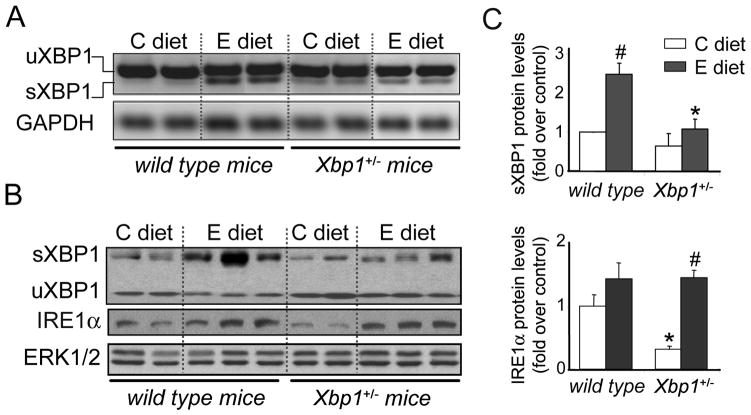
Figure 4. Ethanol-induced XBP1 activation is blunted in pancreas of Xbp1+/− mice.
Wild-type and Xbp1+/- mice were fed either control or ethanol diet for 4 weeks. (A) Pancreatic XBP1 splicing was assessed by RT-PCR. GAPDH was examined as a housekeeping gene. (B) Immunoblot analyses of spliced and unspliced XBP1, IRE1α, and ERK1/2 (loading control) in pancreas from wild-type and XBP+/− mice (panels A, B, each lane represents an individual mouse). (C) Graphs show quantification of sXBP1 and IRE1α protein expression relative to control-fed wild-type mice (mean±SEM, n=5 mice). * P<.05 vs. wild-type mice; # P<.05 vs. C diet (two-way ANOVA and Tukey post-tests).
Figure 5. XBP1 deficiency augments pancreatic UPR in ethanol-fed mice.
Immunoblot analyses of key UPR regulators in pancreas from wild-type and Xbp1+/− mice fed control or ethanol diet for 4 weeks. Shown are expression levels of phospho-Thr980-PERK, total and phospho-Ser51-eIF2α, Grp78 (A), and ATF4 (B). ERK1/2 was used as loading control. Each lane represents an individual mouse. (C) Densitometric quantification of phospho-PERK, phospho-eIF2α and ATF4 levels. Graphs show increased protein content in ethanol-fed Xbp1+/− mice, relative to ethanol-fed wild-type mice. * P<.05 vs. wild-type mice (Student t-test).
In spite of ethanol-induced IRE1 upregulation, both XBP1 mRNA splicing and protein levels were basal in ethanol-fed Xbp1+/− mice (Fig. 4). In these mice, ethanol-feeding significantly increased PERK and eIF2α phosphorylation, and ATF4 expression (Fig. 5), an effect consistent with more severe and prolonged ER stress. As we observed in wild-type mice, Grp78 protein levels were not elevated in ethanol-fed Xbp1+/− mice.
XBP1 deficiency and ethanol feeding altered expression of ER regulators in mouse pancreas
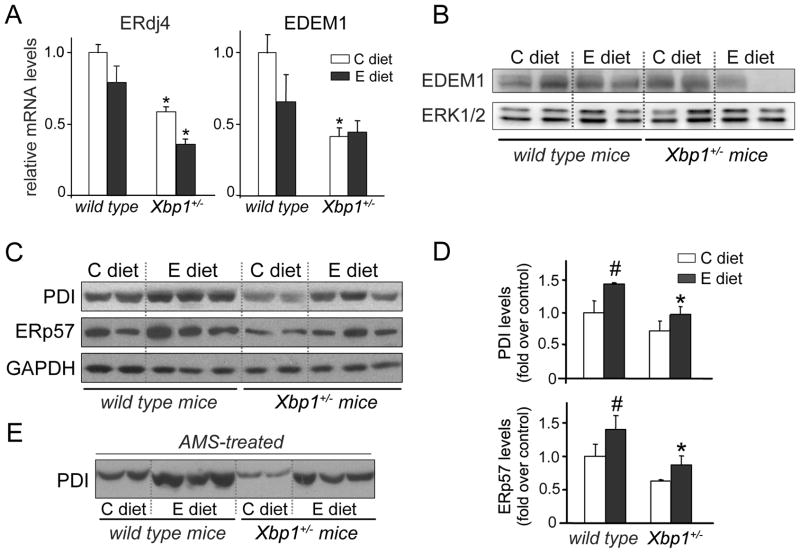
We next analyzed the expression of sXBP1 targets involved in ER protein folding and degradation. We selected the chaperones ER-localized DnaJ homologue (ERdj4) and ER degradation-enhancing alpha-mannosidase-like1 (EDEM1), and two members of the PDI family, PDI and ERp57. ERdj4 stimulates the ATPase activity of Grp78,35 and EDEM1 is an ERAD component required for degradation of misfolded glycoproteins.36
ERdj4 and EDEM1 expression was measured by qRT-PCR. EDEM1 protein levels were also assessed by Western blotting. Ethanol slightly reduced mRNA of both ERdj4 and EDEM1 in wild-type mice, suggesting that ethanol may affect the transcription and/or stability of these mRNAs. However, XBP1 deficiency reduced further mRNA levels of both genes (Fig. 6A). Similarly, EDEM1 protein levels were markedly lower in ethanol-fed Xbp1+/− mice than in wild-type mice (Fig. 6B), indicating that sXBP1 levels were insufficient to sustain levels of these proteins.
Figure 6. XBP1 deficiency diminishes ER components involved in protein folding and ERAD.
Pancreatic expression of XBP1 targets were determined in wild-type and Xbp1+/− mice fed either ethanol or control diet. (A) qRT-PCR analyses of the chaperones ERdj4 and EDEM1 (mean±SEM; n=3). mRNA values were standardized to those of mouse ARP and expressed relative to control-fed wild-type mice. *P<.05 vs. wild-type mice (two-way ANOVA). Immunoblots show pancreatic protein content of EDEM1 (B) and the ER oxidoreductases PDI and ERp57 (C). ERK1/2 and GAPDH were used as loading controls. (D) Quantitation of PDI and ERp57 expression in immunoblots relative to C diet, normalized to those of GAPDH. * P<.05 vs. wild-type mice; # P<.05 vs. C diet (two-way ANOVA and Tukey post-tests). (E) Immunoblot shows selective changes in PDI electrophoretic mobility associated with reduction/alkylation of thiol groups by TCEP and AMS treatment. AMS increases MW about 500 daltons per thiol group. As judged by the relative mobility of the bands, alkylation was less in samples from ethanol-fed mice, suggesting oxidation of fewer PDI free thiol groups. In B, C and E, each lane represents an individual mouse.
Concomitant with increased sXBP1 protein levels (Fig. 4B), PDI and ERp57 were increased by 40% in ethanol-fed wild-type mice compared to controls (Figs. 6C-6D). However, for ethanol fed animals levels of these oxidoreductases were reduced in Xbp1+/− mice compared to wild-type mice. Overall, these data indicate that sXBP1 is an important regulator of key ER oxidoreductases and chaperones in mouse pancreas.
Ethanol feeding promoted oxidation of PDI in mouse pancreas
PDI is a ER thiol-rich enzyme that catalyzes the formation and isomerization of disulfide bonds in folding proteins in the secretory pathway.37 PDI activity depends on the redox state of catalytic cysteine residues. PDI reduced during oxidative protein folding requires re-oxidization for further catalytic cycles. These cycles are maintained by electron transfers between PDI, ER oxidases, molecular oxygen and glutathione.38
In view of our data showing that ethanol feeding changed GSSG/GSH ratios in pancreas (Fig. S1), we asked whether ethanol altered PDI redox state. To assess this, pancreatic samples were subjected to alkylation reactions with AMS as described in Materials and Methods. We found that, in response to alkylation, PDI from control-fed mice was shifted in MW, whereas PDI from ethanol-fed mice was less shifted, suggesting fewer free thiol groups (Fig. 6E). These data provide evidence that PDI is oxidized in ethanol-fed mice, a state that likely compromises its ability to assist disulfide bond formation resulting in misfolding. On the basis of these findings, we speculate that both decreased PDI expression due to XBP1 deficiency and ethanol-induced oxidation of PDI contribute to ER stress severity in ethanol-fed Xbp1+/− mice.
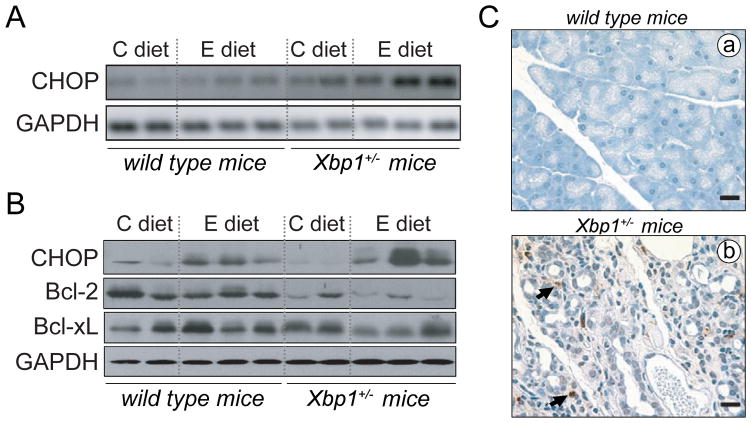
XBP1 deficiency and ethanol feeding led to CHOP upregulation and apoptosis in mouse pancreas
Upon ER stress, the UPR activates both survival and pro-apoptotic signals.15 Pro-apoptotic signals may prevail under circumstances of prolonged ER stress not alleviated by an adaptive UPR. In particular, CHOP upregulation downstream of ATF4 signaling triggers an apoptotic response that includes reduced transcription of the anti-apoptotic Bcl-2.39, 40
Compared to wild-type, CHOP mRNA and protein expression were significantly increased in ethanol-fed Xbp1+/− mice (Fig. 7), consistent with the high levels of ATF4 found in these mice (Fig. 5). Ethanol also modestly increased protein levels of CHOP in wild-type although to a lesser extent than in Xbp1+/− mice (Fig 7B), an effect that may be associated with increased CHOP translation under sustained eIF2α phosphorylation (Fig 5A).27 Protein levels of Bcl-2 were significantly lower in Xbp1+/− mice than in wild-type controls, and were reduced further in ethanol-fed Xbp1+/− mice (Fig. 7B). In these mice, CHOP upregulation and reduced Bcl-2 levels were associated with the appearance of apoptotic acinar cells, as determined by TUNEL staining. TUNEL-positive nuclei were found mainly in areas with extensive loss of acinar cells (Fig. 7C), where represent up to 4% of total nuclei. In contrast, apoptotic nuclei were absent in pancreas from control-fed mice, irrespective of the genotype (not shown), and in ethanol-fed wild-type mice (Fig. 7C).
Figure 7. XBP1 deficiency and ethanol feeding induce upregulation of CHOP and apoptosis in mouse pancreas.
(A) CHOP mRNA expression was assessed in pancreas from control- and ethanol-fed wild-type and XBP+/− mice by RT-PCR. GAPDH was used as a housekeeping gene. (B) Immunoblots show pancreatic levels of CHOP and the antiapoptotic proteins, Bcl-2 and Bcl-xL. In A, B, each lane represents an individual mouse. (C) Apoptotic cells were assessed by TUNEL staining of pancreatic sections. Ethanol-fed wild-type mice lacked apoptotic nuclei (panel a). Similar results were found for all mice fed control diet (not shown). In Xbp1+/− mice fed ethanol, TUNEL positive nuclei (brown staining) were found mainly in damaged areas of parenchyma displaying extensive loss of acinar cells (panel b). Apoptotic cells comprise acinar and stroma cells. Bars, 20 μm.
Discussion
Our results demonstrate that alcohol feeding in rodents induces UPR activation in pancreas, as indicated by increased expression of IRE1 and sXBP1. The results further demonstrate that when sXBP1 levels are diminished, as in Xbp1+/− mice, ethanol feeding induces ER stress and pancreas pathology. Based on these findings, we conclude that an adaptive UPR involving IRE1/XBP1 protects against alcohol-induced pathology in the exocrine pancreas.
Oxidative stress is a likely factor causing pancreatic ER stress in ethanol-fed animals, as shown previously in other organs.14, 41 In support of this, antioxidants reduce ER stress and improve protein secretion.18, 42 We found evidence of oxidative stress in ethanol-fed animals, including increased ER GSSG/GSH ratio, and PDI oxidation. PDI catalyzes disulfide bond formation in folding proteins, a key step for their maturation and stabilization in the secretory pathway.37 Since PDI requires GSH for catalytic redox cycles, 32 we conclude that ethanol depletes GSH required to modulate the redox state of PDI and folding proteins, and subsequently elicits aberrant protein folding, accumulation of misfolded proteins, and, ER stress. PDI oxidation can be especially critical in the acinar cell, as many digestive enzymes have multiple disulfide bonds.
Several studies using genetically engineered cells and mice support a protective role for sXBP1 against ER stress. sXBP1 induces the transcription of genes needed to expand the ER/Golgi networks and augment the folding capacity of the ER.21, 43 In addition, XBP1 regulates genes involved in oxidative stress responses including glutathione peroxidases.25 Our work identifies genes downstream of XBP1 that likely contribute to the protective effects of XBP1 against ethanol-induced ER stress. Ethanol-induced sXBP1 upregulation in pancreas of wild-type mice was associated with increased levels of PDI and ERp57. Conversely, PDI and ERp57 were reduced in the absence of sXBP1 upregulation (Xbp1+/− mice). Similarly, Xbp1+/− pancreatic acini had lower levels of PDI compared to wild-type cells, an effect associated with reduced amylase secretion, supporting the concept that sXBP1 is necessary for the secretory machinery of the acinar cell.22 In particular, adequate levels of PDI may be critical in ethanol-fed animals to compensate for impaired PDI activity due to ethanol-induced PDI oxidation.
Levels of the XBP1 targets ERdj4 and EDEM1 were reduced in Xbp1+/− mice, an effect that was more pronounced in ethanol-fed compared to control-fed mice. Since ERdj4 modulates the ATPase activity of Grp78,35 a ER chaperone critical for folding and export of proteins, reduced ERdj4 levels can result in ER stress. Similarly, a decrease in EDEM1 leads to impaired ERAD,36 and has been linked to increased autophagy.44 In this respect, the increase in autophagic vacuoles found in ethanol-fed Xbp1+/− mice may reflect compensatory responses to assist the defective ERAD in removing aberrant proteins.
We speculate that impaired chaperone-encoding gene expression due to XBP1 deficiency and dysfunction of PDIs due to oxidative stress favor illegitimate disulfide bond formation. The need to re-shuffle such bonds for correct protein folding aggravate ER oxidative stress by further depletion of reducing equivalents and ROS generation associated with oxidative re-folding.38 Furthermore, impaired ERAD may compromise degradation of aberrant proteins leading to accumulation of misfolded proteins, autophagy and dysregulation of the protein flux through the secretory pathway.
Severe ER stress in ethanol-fed Xbp1+/− mice is supported by the robust activation of PERK found in these mice and the induction of apoptosis. Our data suggest that activation of the PERK pathway can be counteradaptive in the context of XBP1 deficiency and ethanol treatment because triggers CHOP upregulation. Associated with increased CHOP expression, we found increased cell death, as was shown in other systems. 19, 45 UPR-induced apoptosis has been also linked to activation of c-jun N-terminal kinase (JNK) signaling by IRE1. 46 However, we did not find differences in JNK activation between ethanol-fed wild-type or Xbp1+/− mice (not shown), suggesting that the IRE1 pathway does not contribute to apoptosis in our experimental model.
In conclusion, our results elucidate adaptive signals of the UPR as key components protecting the pancreatic acinar cell against ethanol-induced injury. Alterations in the balance between UPR-induced adaptive and apoptotic signals by environmental or genetic factors likely lead to alcoholic pancreatitis in humans.
Supplementary Material
Acknowledgments
We gratefully thank L Glimcher and AH Lee for providing founder breeding pairs of Xbp1+/− mice. We acknowledge the support of the Department of Veterans Affairs, and the Animal and Morphology Cores of the Research Center for ALPD & Cirrhosis (P50-A11999) for their assistance with intragastric ethanol feeding and morphological analyses of pancreatic tissues. We thank R Waldron for his contribution to manuscript revision. This work was supported by NIAAA (R21 AA016010) and NCCAM (1P01AT003960).
Abbreviations used in this paper
- ARP
acidic ribosomal phosphoprotein P0
- AMS
acetamido-maleimidylstilbene-disulfonic acid
- ATF4
activating transcription factor 4
- C diet
control diet
- CHOP
C/EBP homoologous protein
- E diet
ethanol-containing diet
- EDEM1
ER degradation-enhancing alpha-mannosidase-like 1
- ER
endoplasmic reticulum
- ERAD
endoplasmic reticulum associated degradation
- ERdj4
ER localized DnaJ homologue
- eIF2α
eukaryotic translation initiation factor 2α
- PDI
protein disulfide isomerase
- PERK
protein kinase RNA (PKR)-like ER kinase
- TCEP
Tris-2-carboxyethyl-phosphine
- sXBP1
spliced X-box-binding protein-1
- uXBP1
unspliced X-box-binding protein-1
- UPR
unfolded protein response
Footnotes
Disclosures: no conflicts of interest exist.
Author contributions: DT, JN and JG, data acquisition; SWF, technical support and analysis of histological tissue samples; IG, qRT-PCR analysis; FSG, study concept and critical study supervision; SJP, study concept and design, analysis and interpretation of data, obtaining funding and general study supervision; AL, all the aspects of the study including study concept and design, obtaining funding, acquisition, analysis and interpretation of data, statistical analysis, drafting of the manuscript and study supervision.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sarles H. Alcoholic Pancreatitis. New York: McGraw Hill; 1992. [Google Scholar]
- 2.Lugea A, Gukovsky I, Gukovskaya AS, et al. Nonoxidative ethanol metabolites alter extracellular matrix protein content in rat pancreas. Gastroenterology. 2003;125:1845–1859. doi: 10.1053/j.gastro.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 3.Gukovskaya AS, Mouria M, Gukovsky I, et al. Ethanol metabolism and transcription factor activation in pancreatic acinar cells in rats. Gastroenterology. 2002;122:106–118. doi: 10.1053/gast.2002.30302. [DOI] [PubMed] [Google Scholar]
- 4.Pandol SJ, Saluja AK, Imrie CW, et al. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132:1127–1151. doi: 10.1053/j.gastro.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 5.Gukovsky I, Lugea A, Shahsahebi M, et al. A rat model reproducing key pathological responses of alcoholic chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2008;294:G68–G79. doi: 10.1152/ajpgi.00006.2007. [DOI] [PubMed] [Google Scholar]
- 6.Pandol SJ, Periskic S, Gukovsky I, et al. Ethanol diet increases the sensitivity of rats to pancreatitis induced by cholecystokinin octapeptide. Gastroenterology. 1999;117:706–716. doi: 10.1016/s0016-5085(99)70465-8. [DOI] [PubMed] [Google Scholar]
- 7.Vonlaufen A, Xu Z, Daniel B, et al. Bacterial endotoxin: a trigger factor for alcoholic pancreatitis? Evidence from a novel, physiologically relevant animal model. Gastroenterology. 2007;133:1293–1303. doi: 10.1053/j.gastro.2007.06.062. [DOI] [PubMed] [Google Scholar]
- 8.Gorelick FS, Thrower E. The acinar cell and early pancreatitis responses. Clin Gastroenterol Hepatol. 2009;7:S10–S14. doi: 10.1016/j.cgh.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harding HP, Calfon M, Urano F, et al. Transcriptional and translational control in the Mammalian unfolded protein response. Annu Rev Cell Dev Biol. 2002;18:575–599. doi: 10.1146/annurev.cellbio.18.011402.160624. [DOI] [PubMed] [Google Scholar]
- 10.Pahl HL. Signal transduction from the endoplasmic reticulum to the cell nucleus. Physiol Rev. 1999;79:683–701. doi: 10.1152/physrev.1999.79.3.683. [DOI] [PubMed] [Google Scholar]
- 11.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 12.Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev. 2006;86:1133–1149. doi: 10.1152/physrev.00015.2006. [DOI] [PubMed] [Google Scholar]
- 13.Kaser A, Lee AH, Franke A, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124:1488–1499. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- 15.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 16.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 18.Harding HP, Zhang Y, Zeng H, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 19.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida H, Matsui T, Yamamoto A, et al. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 21.Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee AH, Chu GC, Iwakoshi NN, et al. XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. EMBO J. 2005;24:4368–4380. doi: 10.1038/sj.emboj.7600903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sriburi R, Jackowski S, Mori K, et al. XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J Cell Biol. 2004;167:35–41. doi: 10.1083/jcb.200406136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313:104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Adachi M, Zhao S, et al. Preventing oxidative stress: a new role for XBP1. Cell Death Differ. 2009;16:847–857. doi: 10.1038/cdd.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 27.Wek RC, Cavener DR. Translational control and the unfolded protein response. Antioxid Redox Signal. 2007;9:2357–2371. doi: 10.1089/ars.2007.1764. [DOI] [PubMed] [Google Scholar]
- 28.Kubisch CH, Sans MD, Arumugam T, et al. Early activation of endoplasmic reticulum stress is associated with arginine-induced acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2006;291:G238–G245. doi: 10.1152/ajpgi.00471.2005. [DOI] [PubMed] [Google Scholar]
- 29.Tsukamoto H, Mkrtchyan H, Dynnyk A. Intragastric ethanol infusion model in rodents. Methods Mol Biol. 2008;447:33–48. doi: 10.1007/978-1-59745-242-7_3. [DOI] [PubMed] [Google Scholar]
- 30.Lugea A, Nan L, French SW, et al. Pancreas recovery following cerulein-induced pancreatitis is impaired in plasminogen-deficient mice. Gastroenterology. 2006;131:885–899. doi: 10.1053/j.gastro.2006.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lugea A, Gong J, Nguyen J, et al. Cholinergic Mediation of Alcohol-Induced Experimental Pancreatitis. Alcohol Clin Exp Res. 2010 doi: 10.1111/j.1530-0277.2010.01264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chakravarthi S, Bulleid NJ. Glutathione is required to regulate the formation of native disulfide bonds within proteins entering the secretory pathway. J Biol Chem. 2004;279:39872–39879. doi: 10.1074/jbc.M406912200. [DOI] [PubMed] [Google Scholar]
- 33.Reimold AM, Etkin A, Clauss I, et al. An essential role in liver development for transcription factor XBP-1. Genes Dev. 2000;14:152–157. [PMC free article] [PubMed] [Google Scholar]
- 34.Rutkowski DT, Arnold SM, Miller CN, et al. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006;4:e374. doi: 10.1371/journal.pbio.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen Y, Meunier L, Hendershot LM. Identification and characterization of a novel endoplasmic reticulum (ER) DnaJ homologue, which stimulates ATPase activity of BiP in vitro and is induced by ER stress. J Biol Chem. 2002;277:15947–15956. doi: 10.1074/jbc.M112214200. [DOI] [PubMed] [Google Scholar]
- 36.Oda Y, Hosokawa N, Wada I, et al. EDEM as an acceptor of terminally misfolded glycoproteins released from calnexin. Science. 2003;299:1394–1397. doi: 10.1126/science.1079181. [DOI] [PubMed] [Google Scholar]
- 37.Ellgaard L, Ruddock LW. The human protein disulphide isomerase family: substrate interactions and functional properties. EMBO Rep. 2005;6:28–32. doi: 10.1038/sj.embor.7400311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimizu Y, Hendershot LM. Oxidative folding: cellular strategies for dealing with the resultant equimolar production of reactive oxygen species. Antioxid Redox Signal. 2009 doi: 10.1089/ars.2009.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song B, Scheuner D, Ron D, et al. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118:3378–3389. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCullough KD, Martindale JL, Klotz LO, et al. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen G, Ma C, Bower KA, et al. Ethanol promotes endoplasmic reticulum stress-induced neuronal death: involvement of oxidative stress. J Neurosci Res. 2008;86:937–946. doi: 10.1002/jnr.21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malhotra JD, Miao H, Zhang K, et al. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci U S A. 2008;105:18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Acosta-Alvear D, Zhou Y, Blais A, et al. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell. 2007;27:53–66. doi: 10.1016/j.molcel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 44.Hetz C, Thielen P, Matus S, et al. XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev. 2009;23:2294–2306. doi: 10.1101/gad.1830709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ji C, Mehrian-Shai R, Chan C, et al. Role of CHOP in hepatic apoptosis in the murine model of intragastric ethanol feeding. Alcohol Clin Exp Res. 2005;29:1496–1503. doi: 10.1097/01.alc.0000174691.03751.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Urano F, Wang X, Bertolotti A, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.