Abstract
The stimulation of aldosterone production by acidosis enhances proton excretion and serves to limit disturbances in systemic acid-base equilibrium. Yet, the mechanisms by which protons stimulate aldosterone production from cells of the adrenal cortex remain largely unknown. TWIK-related acid sensitive K channels (TASK) are inhibited by extracellular protons within the physiological range and have emerged as important regulators of aldosterone production in the adrenal cortex. Here we show that congenic C57BL/6J mice with genetic deletion of TASK-1 (K2P3.1) and TASK-3 (K2P9.1) channel subunits overproduce aldosterone and display an enhanced sensitivity to steroidogenic stimuli, including a more pronounced steroidogenic response to chronic NH4Cl loading. Thus, we conclude that TASK channels are not required for the stimulation of aldosterone production by protons but their inhibition by physiological acidosis may contribute to full expression of the steroidogenic response.
Keywords: metabolic acidosis, aldosterone, renin, TASK, angiotensin II, zona glomerulosa
1. Introduction
In man, changes in aldosterone production are associated with acid-base disturbances (Jones et al., 1992, Perez et al., 1977, Schambelan et al., 1987, Sicuro et al., 1998, Yamauchi et al., 1998). For example, patients with primary hyperaldosteronism, especially those with Conn's syndrome, frequently present with hypokalemia and a mild metabolic alkalosis, whereas aldosterone deficiency disorders, whether primary (Addisons disease, selective aldosterone synthase deficiency) or secondary (hyporeninemia, mineralocorticoid blockage), are associated with hyperkalemia and metabolic acidosis (DuBose, 2000). Conversely, the regulation of aldosterone production by H ions can be considered part of a physiological feedback loop to maintain acid-base balance. Supraphysiological elevations in plasma H ion concentration imposed by acute or chronic ammonium chloride dietary loading (Perez et al., 1977, Schambelan et al., 1987), or less extreme acid-base changes produced by hemodialysis with dialysates of varied bicarbonate concentration (Jones et al., 1992), evoke increases in plasma aldosterone that are independent of changes in systemic factors known to regulate aldosterone production (e.g., Ang II, K and ACTH). Yet, although aldosterone production is regulated by blood acid-base status in vivo and in vitro, the molecular targets that mediate this action have remained largely unexplored. Twik-related acid-sensitive potassium (TASK) channels have emerged as prominent candidate molecular substrates underlying regulation of aldosterone production. Aldosterone producing zona glomerulosa (zG) cells in rodents robustly express mRNA for two members of the KCNK gene family of 2-pore domain K channels, TASK-1 and TASK-3 (Bayliss et al., 2003, Czirjak et al., 2000, Czirjak and Enyedi, 2002). These subunits can form homo- or hetero-meric channels (Czirjak and Enyedi, 2002) and by generating leak or background K currents they play a major role in setting the resting membrane voltage of cells in which they are expressed. Importantly, these channels are inhibited by extracellular protons within the physiological range (Duprat et al., 1997). Our laboratory has recently characterized TASK-1 and TASK-3 (TASK-/-) double knockout mice (Davies et al., 2008) on a mixed genetic background. Deletion of TASK subunits removes greater than 90% of recorded depolarization-elicited current from zG cells and is associated with an approximate 20 mV depolarization of the resting membrane potential. Such membrane depolarization would be expected to result in excessive aldosterone production. Indeed, TASK-/- mice phenocopy key features of nontumorous idiopathic primary hyperaldosteronism: increased urinary aldosterone, low plasma renin, high aldosterone to renin ratios, a failure to suppress excess aldosterone production on a high salt diet or to normalize it with Ang II Receptor (AT1R) blockade, and hypertension (Davies et al., 2008).
The present study was undertaken to determine if modulation of TASK channels by H ions contributes to elevated aldosterone production in response to metabolic acidosis. The results demonstrate that NH4Cl acid loading in WT mice increases urinary aldosterone excretion by a mechanism that depends principally on enhanced renin levels and Ang II activity. Notably, renin is not increased by acidosis in TASK-/- mice. Nevertheless, a smaller Ang II-independent component of the aldosterone secretory response to H ions is shared between genotypes and, surprisingly, is even augmented in TASK-/- mice. These data indicate that physiological inhibition of TASK channels by H ions is not required to evoke an increase in aldosterone production; nevertheless, TASK channel inhibition may permit development of enhanced steroidogenic responses to multiple stimuli.
2. Materials and Methods
2.1 Animals
All experiments were approved by the University of Virginia's Animal Care and Use Committee in accordance with The Guide for the Care and Use of Laboratory Animals. C57BL/6J (WT) and TASK-/- mice were 2-4 months of age and housed on a 12:12 light:dark cycle in a temperature and humidity controlled environment. Male mice were used in order to remove potential confounds of hormonal surges associated with the estrus cycle and zonation defects observed in female TASK knockout mice (Heitzmann et al., 2008). TASK-1 and TASK-3 double KO mice were generated as previously described (Lazarenko et al., 2010). For this work, mouse lines with ‘floxed’ alleles for TASK-1 (TASK-1f/f) and TASK-3 (TASK-3f/f) were backcrossed onto a C57BL/6J background by using ‘speed congenics’ (University of Virginia Transgenic Core). The floxed exon of each TASK channel gene was excised by breeding with a Cre-deleter strain, also on a C57BL/6J background (EIIa-Cre; Jackson Labs Stock #003724), and the resulting knockout lines were intercrossed to generate the double TASK-1-/-:TASK-3-/- line (TASK-/-). All knockout lines were maintained as homozygotes, with the parental C57BL/6J mouse line used as a control strain.
2.2 Metabolic cage experiments
All mice were housed in metabolic cages for a habituation period of 3-4 days and were fed a standard rodent chow and deionized drinking water before initiating an experimental protocol. After habituation, urine samples were collected daily and total urine volume and fluid intake over 24 hours were recorded. Prior to acid loading, mice had free access to sucrose water (2%) for 3 days. Acid loading was imposed over a 3 day period by delivering 0.28M NH4Cl in sucrose water. In order to assess the impact of acid loading in the absence of AT1R activity, a second group of mice received candesartan (10 mg/kg/day) in sucrose water for 2 days, prior to and then during a 3 day period of acid loading.
2.3 Urine Analysis
Urine samples were analyzed for aldosterone concentration using an aldosterone 125I radioimmunoassay kit (RIA, Diagnostic Products Corporation, Los Angeles, CA). Urinary Na and K concentrations were measured by Flame Photometry (IL943 Automatic Flame Photometer, Instrumentation Laboratory Inc., Lexington, MA). To correct for differences in urine volume, aldosterone, Na, and K concentrations were standardized to urinary creatinine concentration, measured by Jaffes' colorimetric detection with a Creatinine Assay Kit (Cayman Chemical Company, Ann Arbor, MI).
2.4 Plasma Renin Concentration and Blood Chemistry
Blood samples were taken for each mouse before the habituation period and after the 3 consecutive days of acid loading. For renin analysis, mice were put into a restraint and blood was quickly drawn from a nicked tail vein using a capillary tube, separated by centrifugation, and stored at -20 °C until analysis. Plasma Renin Concentration was measured by RIA kit (Diasorin, Stillwater, MN). After tail vein sampling, mice were anesthetized with ketamine (70 mg/kg) and dexmedetomidine (0.5 mg/kg) (ip) and blood was drawn quickly from the retro-orbital sinus in a heparinized capillary tube. Samples were analyzed immediately for blood chemistries with an iStat hand held monitor configured with an EC8+ or CG8+ cartridge (Heska, Fort Collins, CO).
2.5 Statistics
Data were analyzed using repeated measures ANOVAs and Bonferroni post hoc analysis to determine significance between groups (p<0.05).
3. Results
3.1 Hydration, acid-base status and urinary electrolytes
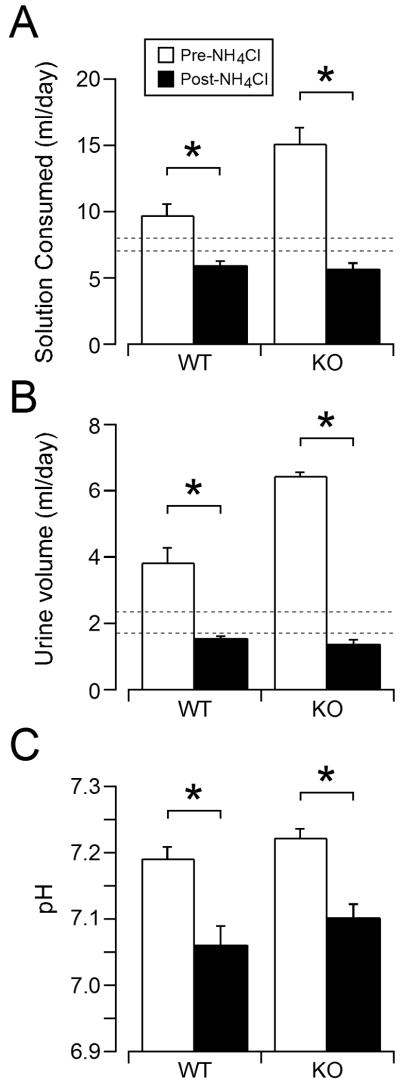
To assess whether TASK channels are required for the aldosterone secretory response to dietary acid loading, we studied age-matched WT and congenic TASK-/- male mice maintained on a diet of normal Na content (0.32%). In pilot studies, we noticed that addition of NH4Cl to the drinking water significantly decreased fluid consumption and reduced body weight. Therefore, drinking water was supplemented with 2% sucrose to achieve adequate hydration prior to and during acid loading. During a 3-day hydration period, mice of both genotypes consumed more fluid (Fig 1A, average of days 2-3) and produced more urine (Fig 1B, average of days 2-3) than a cohort of mice of either genotype drinking regular deionized water (see dashed lines on Figs. 1A & 1B; consumption: 7.4 ± 0.5 mls/day; excretion: 2.0 ± 0.1 mls/day). In both genotypes, fluid consumption and urine volume decreased over 3 days of acid loading; however these values were not different from normal consumption and excretion values of mice drinking regular water. Thus in 3-day acidotic mice, body weight was stable, plasma blood urea nitrogen remained constant, and plasma hematocrit and hemoglobin were not increased, consistent with adequate hydration (Table 1). Nonetheless, in both genotypes, hypernatremia developed after 3 days of acid loading providing a conflicting indicator of volume status and the possibility of an early onset acid-induced volume depletion (Table 1).
Figure 1.
The effect of acid loading on fluid consumption, urine volume, and blood pH of WT and KO mice. Mean ± standard error of fluid consumption (A) and urine volume (B) on the last 2 days (averaged) before and after acid-loading in WT (n = 10) and TASK-/- (n = 13) mice. Standard error (dotted lines) of a separate cohort of WT and KO mice given DH2O are also shown. Mean ± standard error of plasma pH (C) in WT and KO mice before and after acid loading. * indicates statistical significance (p<0.05).
Table 1. Effect of NH4Cl loading on blood and urine electrolyte and acid-base composition in wild-type and TASK-/- mice.
| WT | KO | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Metabolic Parameter | ||||
| Body Wt (g) | 25.8±0.5 | 25.2±0.2 | 24.9±1.0 | 25.0±0.6 |
| NH4Cl intake (g/day) | N/A | 0.35±0.02 | N/A | 0.35±0.02 |
| UNa (mmol/mg cre) | 0.30±0.04 | 0.89±0.09* | 0.41±0.05 | 1.14±0.03* |
| UK (mmol/mg cre) | 1.01±0.14 | 2.36±0.19* | 1.35±0.16 | 3.07±0.10* |
| Blood acid-base | ||||
| pH | 7.19±0.02 | 7.06±0.03* | 7.21±0.02 | 7.10±0.02* |
| HCO3 (mmol/L) | 21.57±1.72 | 16.11±1.45* | 23.77±0.93 | 17.01±0.73* |
| PCO2 (mmHg) | 57.86±6.60 | 56.60±3.44 | 59.48±3.30 | 55.16±3.33 |
| PO2 (mmHg) | 55.80±3.12 | 51.00±2.92 | 48.80±3.18 | 56.00±3.51 |
| BE (mmol/L) | -6.86±1.34 | -14.29±1.86* | -4.78±0.62 | -12.67±0.91* |
| Blood parameters | ||||
| Cl (mmol/L) | 116.33±0.88 | 123.67±2.33 | 117.83±1.74 | 120.83±2.33 |
| Na (mmol/L) | 144.71±1.39 | 154.14±1.20* | 149.00±0.90 | 159.33±3.35* |
| K (mmol/L) | 5.8±0.39 | 4.57±0.23* | 4.10±0.17 | 3.56±0.17 |
| iCa (mmol/L) | 1.21±0.05 | 1.32±0.04 | 1.29±0.04 | 1.30±0.04 |
| Hct (%PCV) | 39.86±1.97 | 35.43±1.34* | 42.63±0.68 | 38.25±0.77* |
| Hb (g/dL) | 13.54±0.67 | 12.06±0.46* | 14.50±0.23 | 13.06±0.24* |
| BUN (mg/dL) | 28.50±2.72 | 29.33±1.86 | 28.71±1.69 | 24.00±1.86 |
Values are means ± SE of samples obtained during a 3 day pre-NH4Cl control period and during days 4-6 of NH4Cl administration. Comparisons of group means were made using a repeated measures ANOVA with Bonferroni post hoc tesing.
indicates statistical significance vs. before acid-load (p<0.05). Urinary K and Na were measured using flame photometry and renin concentration was measured with RIA. All other blood measures were determined with an iSTAT hand-held blood analyzer. PCO2, partial pressure of CO2; PO2, partial pressure of O2; BE, base excess; iCa, ionized Ca; Hct, hematocrit; Hb, hemoglobin; BUN, blood urea nitrogen.
Three days of acid loading produced a mild metabolic acidosis that was equivalent between genotypes (Fig. 1C): the pH decreased by approximately 0.12 units and was accompanied by a reduction in plasma HCO3. Neither sinus PCO2 nor PO2 changed after 3 days of acidosis (Table 1) in WT or TASK-/- mice, suggesting little to no respiratory compensation. As also shown in Table 1, acidosis was accompanied by increased urinary excretion of K in both genotypes; this produced significant hypokalemia in WT mice while the existing hypokalemia already evident in TASK-/- mice was maintained. Also, in both genotypes, acidosis increased the urinary excretion of Na while producing a hypernatremia, consistent with acid-induced impairment of water reabsorption, likely in the collecting duct.
3.2 Aldosterone secretory response to acid loading
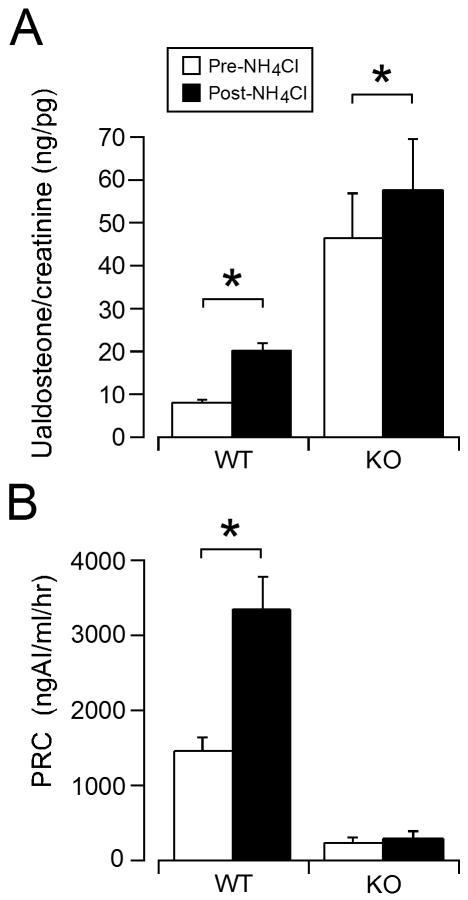
To evaluate the steroidogenic response to acidosis, we measured urinary aldosterone excretion (24 hr, normalized to creatinine), as an indicator of integrated aldosterone secretory activity in vivo. This non-invasive measure minimizes variability arising from diurnal patterns and stress-induced changes in aldosterone production. Similar to the excessive aldosterone production previously observed for TASK-/- mice on a mixed genetic background (Davies et al., 2008), we found here that urinary aldosterone excretion in this congenic line of TASK-/- mice was elevated over that of C57BL/6 mice (by ∼5-fold; Fig 2A). In WT mice, 3 days of acid loading elicited a robust increase in urinary aldosterone excretion to levels ∼2.5-fold of control; by comparison TASK-/- mice responded with a more modest increase (∼to 1.25 fold of control), although the absolute magnitude of the increase in aldosterone produced between genotypes was similar.
Figure 2.
Urinary aldosterone and plasma renin concentration of WT and KO mice before and after acid loading. A. Mean ± standard error of urinary aldosterone concentration per creatinine on the last 2 days (averaged) before and during acid-loading in WT (n = 10) and TASK-/- KO (n = 13) mice. B. Mean ± standard error of plasma renin concentration before and during acid-loading in WT and TASK-/- KO mice. * indicates statistical significance (p<0.05).
To assess the participation of the renin-angiotensin system in the secretory response to NH4Cl loading, we measured plasma renin concentration before and after inducing acidosis in WT and TASK-/- mice. In WT mice, acidosis was associated with a substantial increase in renin levels (∼3-fold over control; Fig 2B). In TASK-/- mice, as previously reported, renin concentration was lower than in WT mice (Fig. 2B, (Davies et al., 2008)); in response to acid loading renin levels were unaltered in TASK-/- mice despite systemic responses to acid that were equivalent to those obtained in WT mice (degree of acidosis and hypernatremia, Table 1).
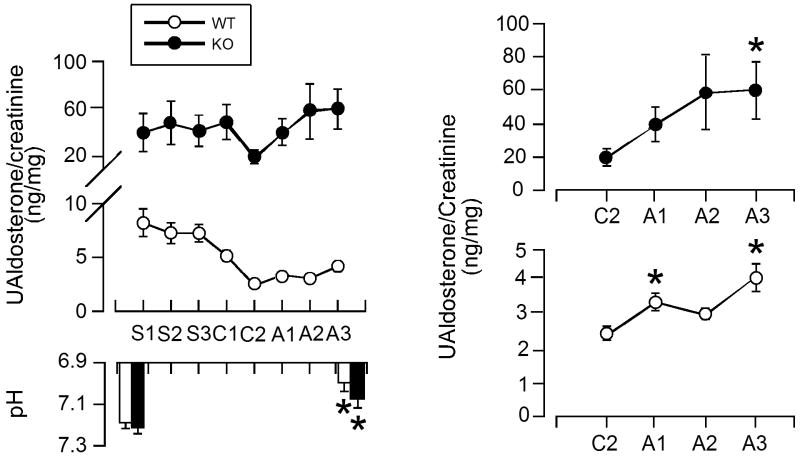
We used candesartan, an insurmountable AT1R blocker, to assess the Ang II-dependent component of the aldosterone secretory response to H ions. An oral dose of candesartan was administered in the drinking water (10 mg/kg/day; candesartan cilexetil [AstraZeneca]) 2 days prior to and for 3 days during acid loading (Fig. 3A). In WT mice, aldosterone production before and during acid loading was principally Ang II-dependent; candesartan decreased urinary aldosterone secretion by 70% and although subsequent acid loading significantly increased production, the increase induced by acidosis amounted to only 14% of that observed in the absence of AT1R blockade (Δ Ualdo:creatinine = 1.7 vs 12.1 ng/mg respectively). This change in responsiveness to H ion in WT mice was not attributable to a reduction in the degree of acidosis induced by NH4Cl loading, as the decrease in pH was equivalent in the presence or absence of candesartan (Fig. 1C, 3B).
Figure 3.
Daily urinary aldosterone concentration of WT and KO mice with acid loading and AT1R blockade. A (top). Daily means ± standard error of urinary aldosterone concentration per creatinine during control (S1-3, sucrose), AT1R blockade (C1-2, 2% sucrose + candesartan), and acid loading (A1-3, sucrose + candesartan + NH4Cl) in WT (n = 12) and TASK-/- KO (n = 6) mice. A (bottom). pH of blood samples taken before experimental protocol and after acid-loading with candesartan. B. The last 4 days of AT1R blockade for KO (top) and WT (bottom) are shown on a magnified scale. * indicates statistical significance (p<0.05).
In TASK-/- mice, the characteristic excessive production of aldosterone persisted during AT1R blockade; indeed despite a maximal oral dose of candesartan, aldosterone production remained greatly elevated over WT mice (Fig. 3). Nevertheless, AT1R blockade in TASK-/- mice decreased urinary aldosterone levels by 54%, which is remarkable given the extremely low plasma renin concentrations. These data suggest that TASK-/- mice, like patients with primary hyperaldosteronism, are exquisitely sensitive to Ang II. Surprisingly, in the presence of AT1R blockade, acid loading in TASK-/- mice induced a marked increase in aldosterone production that was of greater magnitude than in WT mice. Urinary aldosterone concentration increased 3-fold in TASK-/- mice (from 20 to 60 ng/mg), compared to only ∼1.6-fold increase (from 2.5 to 4.1 ng/mg) in WT mice. Notably, unlike WT mice the absolute level of aldosterone production elicited by acid loading in TASK-/- mice was similar in the presence or absence of AT1R blockade (Fig 2A, 3A). This raises the possibility that when the restraining activity of TASK channels is removed, H ions can drive aldosterone production to maximal values and function as a strong regulator of steroidogenesis independent of Ang II. Taken together these data indicate that TASK-/- deletion mice are more sensitive than WT mice to multiple steroidogenic stimuli, such as Ang II and H ions. They also indicate that inhibition of TASK channels by H ions is not required to mount a steroidogenic response to acid.
4. Discussion
Our data refutes the hypothesis that the physiological inhibition of TASK channel activity by H ions is required for stimulation of aldosterone production by mild metabolic acidosis. We draw this conclusion based on two key observations. First, although metabolic acidosis elicited a robust increase in aldosterone production in WT mice, most (but not all) of the steroidogenic mechanism can be attributed to activation of the renin-angiotensin system; acidosis invoked a large rise in plasma renin and only a modest increase in aldosterone production with AT1R blockade. Secondly, aldosterone production in TASK-/- mice was increased by acid-loading, and a particularly robust acid-sensitivity was unmasked by AT1R blockade that was even greater than seen in WT mice. Therefore, since the absence of TASK channels enhances acid-induced aldosterone production, it is possible that physiological inhibition of TASK channels by protons in WT mice reduces their restraining action on aldosterone production to augment steroidogenesis.
It is important to note that the acidosis induced in the current study by in vivo acid loading is mild, producing a decrement of 0.12 pH units. Based on responses of cloned TASK channels to H ions (Berg et al., 2004), such a change in pH from 7.2 to 7.08 would be expected to decrease the activity of homomeric TASK-1 channels (pKa of 7.5) from 19 to 11% of maximal, homomeric TASK-3 channels (pKa of 6.8) from 91 to 81% of maximal and that of heteromeric channels (pKa of 7.3) from 41 to 31% of maximal. Thus, an 8-10% reduction in TASK channel conductance would be predicted by this small change in pH and itself may be insufficient to permit a sizeable physiological steroidogenic response to acid loading that is independent of renin-angiotensin system activation. The relative subunit composition of TASK channels in mouse zG cells is not established. However, in rat zG cells the pharmacological characteristics of recorded macroscopic currents and relative mRNA TASK subunit abundance indicates that TASK-3 homomeric channels are likely the dominant channel type underlying the high resting K conductance (Czirjak and Enyedi, 2002). Moreover, the measured increase in aldosterone production elicited by H ions may be minimized by systemic physiological changes induced by metabolic acidosis known to reduce aldosterone production, such as hypokalemia and hypernatremia. Indeed, in our studies acidosis was accompanied by a decrease in the concentration of plasma K and an increase in the concentration of plasma Na. Thus compensatory acid- induced electrolyte changes could contribute to the smaller measured increase in aldosterone production elicited by acid under AT1R blockade.
In our study, metabolic acidosis in WT mice produced a large rise in aldosterone production that was largely secondary to activation of the renin-angiotensin system. At present, there are no published data supporting a direct effect of H ions on renin secretion, which is considered to be principally controlled by perfusion pressure, Na delivery to the macula densa and sympathetic nerve activity (Koeppen and Stanton, 2001). Based on our data, the volume status of 3-day acidotic mice is uncertain. Therefore, we cannot rule out a decrease in effective circulating volume as one cause of the measured increase in renin concentration in acidotic WT mice. In acidosis, an increase in sympathetic nerve activity also may provide a stimulus for the secretion of renin, as acidosis stimulates chemosensitive cells of the carotid body and brainstem. Interestingly in this regard, we saw no acid-induced change in renin levels in TASK-/- mice, consistent with the implication of TASK channel activity in carotid body chemosensory responses (Tan et al., 2009). Taken together, the measured increase in renin concentration in acidotic WT mice is likely the integrated consequence of multiple controlling mechanisms. It is noteworthy therefore, that the renin concentration of TASK-/- mice remained relatively refractory to change, despite similar acid-induced changes in blood chemistries, suggesting that TASK channel activity may be important in the control of renin secretion.
Our current findings that congenic TASK-/- mice have strikingly low renin attendant with elevated urinary aldosterone excretion confirm results of previous studies using TASK-/- mice of mixed genetic background. Yet, a large Ang II-sensitive component to the aldosterone secretory response was revealed with the administration of candesartan; urinary aldosterone fell to ∼50% of basal. Similarly, under AT1R blockade, acidosis elicited an aldosterone secretory response in TASK-/- mice that was greatly enhanced over that of WT mice. Taken together, these data show that TASK-/- mice display increased sensitivity to two steroidogenic stimuli. The mechanisms underlying these enhanced responses remain largely unexplored but could include the more depolarized resting membrane voltage of TASK-/- zG cells which, via voltage-gated calcium channels, would increase calcium entry and result in a calcium-dependent increase in the capacity and/or flux through the mineralocorticoid synthetic pathway.
5. Conclusions
In WT mice, stimulation of aldosterone production by NH4Cl loading is largely secondary to activation of the renin-angiotensin system; AT1R blockade reveals only a small residual component that is Ang II-independent. By contrast, mice with a genetic deletion of both TASK-1 and TASK-3 subunits responded to acid loading with a robust increase in aldosterone production in the presence of AT1R blockade. Therefore, we conclude that TASK-/- mice demonstrate an enhanced sensitivity to steroidogenic stimuli but that pH-mediated inhibition of TASK channels is not primarily responsible for a steroidogenic response to mild acidosis.
Acknowledgments
This research was supported by NIH grant R01 HL089717.
List of Abbreviations
- Ang II
Angiotensin II
- ACTH
Adrenocorticotropic hormone
- TASK
Twik-related acid-sensitive potassium channels
- zG
zona glomerulosa
- AT1R
Type 1 Angiontensin II receptor
- WT
wild-type
- RIA
radioimmunoassay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nick A. Guagliardo, Email: Nag4g@virginia.edu.
Junlan Yao, Email: Jy9n@virginia.edu.
Douglas A. Bayliss, Email: Dab3y@virginia.edu.
References
- Bayliss DA, Sirois JE, Talley EM. The TASK family: two-pore domain background K+ channels. Mol Interv. 2003;3:205–219. doi: 10.1124/mi.3.4.205. erratum appears in Mol Interv. 2003 Aug;3(5):252. [DOI] [PubMed] [Google Scholar]
- Berg AP, Talley EM, Manger JP, Bayliss DA. Motoneurons express heteromeric TWIK-related acid-sensitive K+ (TASK) channels containing TASK-1 (KCNK3) and TASK-3 (KCNK9) subunits. J Neurosci. 2004;24:6693–6702. doi: 10.1523/JNEUROSCI.1408-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czirjak G, Fischer T, Spat A, Lesage F, Enyedi P. TASK (TWIK-related acid-sensitive K+ channel) is expressed in glomerulosa cells of rat adrenal cortex and inhibited by angiotensin II. Mol Endocrinol. 2000;14:863–874. doi: 10.1210/mend.14.6.0466. [DOI] [PubMed] [Google Scholar]
- Czirjak G, Enyedi P. TASK-3 dominates the background potassium conductance in rat adrenal glomerulosa cells. Mol Endocrinol. 2002;16:621–629. doi: 10.1210/mend.16.3.0788. [DOI] [PubMed] [Google Scholar]
- Czirjak G, Enyedi P. Formation of functional heterodimers between the TASK-1 and TASK-3 two-pore domain potassium channel subunits. J Biol Chem. 2002;277:5426–5432. doi: 10.1074/jbc.M107138200. [DOI] [PubMed] [Google Scholar]
- Davies LA, Hu C, Guagliardo NA, Sen N, Chen X, Talley EM, Carey RM, Bayliss DA, Barrett PQ. TASK channel deletion in mice causes primary hyperaldosteronism. Proc Natl Acad Sci U S A. 2008;105:2203–2208. doi: 10.1073/pnas.0712000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBose TD., Jr Molecular and pathophysiologic mechanisms of hyperkalemic metabolic acidosis. Trans Am Clin Climatol Assoc. 2000;111:122–33. discussion 133-134. [PMC free article] [PubMed] [Google Scholar]
- Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J. 1997;16:5464–5471. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzmann D, Derand R, Jungbauer S, Bandulik S, Sterner C, Schweda F, El Wakil A, Lalli E, Guy N, Mengual R, Reichold M, Tegtmeier I, Bendahhou S, Gomez-Sanchez CE, Aller MI, Wisden W, Weber A, Lesage F, Warth R, Barhanin J. Invalidation of TASK1 potassium channels disrupts adrenal gland zonation and mineralocorticoid homeostasis. EMBO J. 2008;27:179–187. doi: 10.1038/sj.emboj.7601934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GV, Wall BM, Williams HH, Presley DN, Sapir DG, Cooke CR. Modulation of plasma aldosterone by physiological changes in hydrogen ion concentration. Am J Physiol. 1992;262:R269–275. doi: 10.1152/ajpregu.1992.262.2.R269. [DOI] [PubMed] [Google Scholar]
- Koeppen BM, Stanton BA. Renal Physiol. Mosby; 2001. Regulation of Effective Circulating Volume and NaCl Balance; pp. 93–116. [Google Scholar]
- Lazarenko RM, Willcox SC, Shu S, Berg AP, Jevtovic-Todorovic V, Talley EM, Chen X, Bayliss DA. Motoneuronal TASK channels contribute to immobilizing effects of inhalational general anesthetics. J Neurosci. 2010;30:7691–7704. doi: 10.1523/JNEUROSCI.1655-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez GO, Oster JR, Vaamonde CA, Katz FH. Effect of NH4Cl on plasma aldosterone, cortisol and renin activity in supine man. J Clin Endocrinol Metab. 1977;45:762–767. doi: 10.1210/jcem-45-4-762. [DOI] [PubMed] [Google Scholar]
- Schambelan M, Sebastian A, Katuna BA, Arteaga E. Adrenocortical hormone secretory response to chronic NH4Cl-induced metabolic acidosis. Am J Physiol. 1987;252:E454–460. doi: 10.1152/ajpendo.1987.252.4.E454. [DOI] [PubMed] [Google Scholar]
- Sicuro A, Mahlbacher K, Hulter HN, Krapf R. Effect of growth hormone on renal and systemic acid-base homeostasis in humans. Am J Physiol. 1998;274:F650–657. doi: 10.1152/ajprenal.1998.274.4.F650. [DOI] [PubMed] [Google Scholar]
- Tan ZY, Lu Y, Whiteis CA, Simms AE, Paton JF, Chapleau MW, Abboud FM. Chemoreceptor hypersensitivity, sympathetic excitation, and overexpression of ASIC and TASK channels before the onset of hypertension in SHR. Circ Res. 2009;106:536–545. doi: 10.1161/CIRCRESAHA.109.206946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Harada T, Kurono M, Matsui N. Effect of exercise-induced acidosis on aldosterone secretion in men. Eur J Appl Physiol Occup Physiol. 1998;77:409–412. doi: 10.1007/s004210050352. [DOI] [PubMed] [Google Scholar]