SUMMARY
Background & Aims
Lysophosphatidic acid (LPA) is a potent inducer of colon cancer and LPA receptor type 2 (LPA2) is overexpressed in colon tumors. LPA2 interacts with membrane-associated guanylate kinase with inverted orientation-3 (MAGI-3) and the Na+/H+ exchanger regulatory factor 2 (NHERF-2), but the biological effects of these interactions are unknown. We investigated the roles of MAGI-3 and NHERF-2 in LPA2-mediated signaling in human colon cancer cells.
Methods
We overexpressed or knocked down MAGI-3 in HCT116 and SW480 cells. The effects of MAGI-3 and NHERF-2 in LPA-induced cell migration, invasion, inositol phosphate generation, and NF-κB activation were determined. Expression of MAGI-3 and NHERF-2 in human colon tumor tissues was analyzed using tissue microarray analysis.
Results
NHERF-2 promoted migration and invasion of colon cancer cells, whereas MAGI-3 inhibited these processes. MAGI-3 competed with NHERF-2 for binding to LPA2 and phospholipase C (PLC)-β3. However, NHERF-2 and MAGI-3 reciprocally regulated LPA2-induced PLC activity. MAGI-3 increased the interaction of LPA2 with Gα12, whereas NHERF-2 preferentially promoted interaction between LPA2 and Gαq. MAGI-3 decreased the tumorigenic capacity of LPA2 by attenuating the activities of NF-κB and c-Jun N-terminal kinase. MAGI-3 and NHERF-2 were differentially expressed in colon adenocarcinomas, consistent with their opposing effects.
Conclusion
LPA2 is dynamically regulated by 2 distinct PDZ proteins via modulation of G protein coupling and receptor signaling. MAGI-3 is a negative regulator of LPA2 signaling.
Keywords: G-protein signaling, colorectal cancer, neoplasia, tumorigenesis
INTRODUCTION
In the gastrointestinal tract, cell migration is essential in healing of superficial epithelial injury, cell differentiation, and maintenance of barrier function 1. However, unchecked migration of cells can give rise to invasive or metastatic gastrointestinal diseases 1. Lysophosphatidic acid (LPA) is a growth factor-like phospholipid that has the potential to induce cancer progression by stimulating cell proliferation, and protecting cancer cells from chemotherapeutic treatment 2, 3. LPA mediates diverse effects through its cognate receptors that include at least 5 members of the G protein-coupled receptor (GPCR) super-family, LPA1-LPA5 4. Elevated expression of LPA2 in several types of cancer is of tremendous clinical interest given the tumor promoting activity of the aberrant LPA signaling axis 5, 6. Recently we showed that LPA2 deficiency protected mice from colitis-induced colon cancer 7.
GPCRs associate not only with G proteins, but with various other proteins that can regulate receptor activity 8. LPA2 contains a PSD-95/DlgA/ZO-1 (PDZ) binding motif at the carboxyl terminal end that enables interaction with multiple PDZ scaffold proteins, including Na+/H+ exchanger regulatory factor 2 (NHERF-2), membrane-associated guanylate kinase with inverted orientation-3 (MAGI-3), neurabin, and PDZ-RhoGEFs 6, 9–11. NHERF-2 enhances 6, 9, LPA2-dependent cell proliferation and gene expression whereas MAGI-3 or PDZ-RhoGEF interaction with LPA2 enhances receptor-mediated activation of RhoA 10, 11. However, the pathophysiological effects of these interactions have not been studied. In cells that express more than one LPA2-interacting PDZ scaffold, it is not known if LPA2 regulation by the PDZ proteins is antagonistic, additive, or synergistic. In an effort to understand the functional role of MAGI-3 and how multiple scaffold proteins in a given cell compete or coordinate to modulate the biological effects and signaling pathways elicited by LPA2, we investigated functional modulation of LPA2 by NHERF-2 and MAGI-3 in colon cancer cells.
EXPERIMENTAL PROCEDURES
Cells
HCT116 and SW480 human colon cancer cells were grown and transfected as previously described 12. pcDNA3.1 harboring MAGI-3 or NHERF-2 was previously described 6, 12. Knockdown of MAGI-3, NHERF-2, or LPA2 was performed as previously described 11. Stable expression of LPA2 was achieved by using retroviral pLPCX/VSVG-LPA2 or pLPCX (Roche, Indianapolis, IN)). Otherwise stated, cells were serum starved for 24 h followed by exposure to 1 μM LPA.
Antibodies
Animals
Mouse tissues were generated in the previously reported studies 7, 22. Mice were maintained and experiments were performed under the institutional guidelines of Emory University.
Cell invasion and migration
In vitro invasion assay was performed in BioCoat Matrigel invasion chambers (BD Bioscience, San Joe, CA). HCT116 or SW480 cell suspensions (5×105 cells/mL) were placed into the upper chamber in 0.5 mL of serum-free medium. The lower compartment was filled with serum-free medium containing 1–10μM LPA (prepared in PBS containing 0.1% BSA; Avanti Polar Lipids, Alabaster, AL) or with an inhibitor. After incubation for 24 h, cells that had migrated to the lower surface of the filters were fixed in acetone for 5 min at room temperature and visualized with H&E staining method. The staining was viewed with an Axioskop 2 plus microscope (Zeiss, Thornwood, NY). Cells were counted in several fields of triplicate membranes. For the migration assay, confluent monolayer was scraped with a pipette tip, washed with PBS, and incubated in culture medium supplemented with 10% FBS for 24 h. The cell migration was observed by a Nikon Ti-U microscope.
Inositol phosphates (IP) generation
Cells were labeled with 1 μCi of myo-[3H]-inositol (NEN Life Sciences, Boston, MA) and processed as previously described 13. See Supplementary materials for detail.
Western immunoblot and immunoprecipitation
Western blotting and immunoprecipitation was performed as previously described 11. See Supplementary materials for detail.
Cell Surface Expression Assay
The expression level of LPA2 on the plasma membrane was quantified as described 14. See Supplementary materials for detail.
Immunohistochemical analysis of colon tissue array
Human colon tissue array slides (IMH-359) were purchased from Imgenex. Immunohistochemical labeling was performed as previously described 7. The expression levels of MAGI-3 and NHERF-2 in tissue microarrays were quantified according to the published method 15. See Supplementary Materials for details.
[35S]GTP-γ-S binding assay
G protein activation was determined by measuring the binding of the non-hydrolyable analog [35S]GTP-γ-S to Gα subunits according to the method of Lazareno and Birdsall 16.
Statistical analysis
Results are presented as the mean ± standard error. Statistic significance was determined by Student’s t-test as post hoc tests after one-way analysis of variance (ANOVA) using SPSS program (Chicago, IL).
RESULTS
NHERF-2 and MAGI-3 reciprocally regulate LPA2-mediated cellular functions
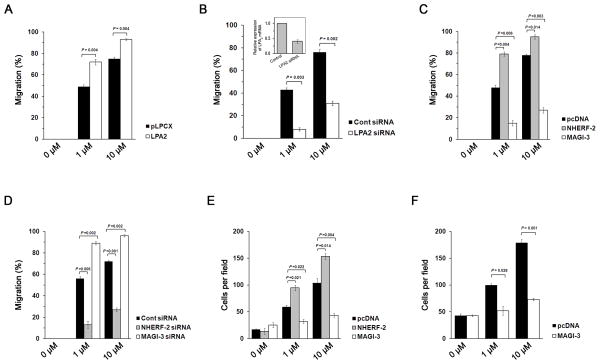
To determine the role of MAGI-3 and NHERF-2, we used human colon cancer HCT116 cells, which express NHERF-2 and MAGI-3. We have shown previously that LPA2 is the major LPA receptor in Caco-2 and other colon cancer cells 6. Consequently, silencing of LPA2 expression abrogated LPA-induced migration of HCT116 cells, whereas overexpression of LPA2 enhanced cell migration (Figure 1A–B; Supplementary Figure S1A–B). Consistent with previous reports that NHERF-2 enhances LPA2-evoked cell proliferation and gene expression, overexpression of NHERF-2 increased cell migration (Figure 1C; Supplementary Figure S1C), whereas knockdown decreased cell migration (Figure 1D; Supplementary Figure S1D) 6, 9. In contrast, overexpression of MAGI-3 in HCT116 cells suppressed LPA-induced cell migration (Figure 1C; Supplementary Figure S1C), whereas MAGI-3 knockdown resulted in an opposite effect (Figure 1D; Supplementary Figure S1D).
Figure 1. MAGI-3 negatively regulates cel l migration and invasion of HCT116 cells.
(A) Migration of HCT116 cells stably transfected with pLPCX or pLPCX/LPA2 in response to 1 μM or 10 μM of LPA was quantified. Full recovery of the wound was considered as 100%. (B) Migration of HCT116/LPA2 siRNA and control cells was determined. The inset shows LPA2 knockdown efficacy determined by quantitative RT-PCR. Migration of HCT116 cells (C) overexpressing NHERF-2 or MAGI-3, or (D) with knockdown of NHERF-2 or MAGI-3 by siRNA was determined. (E) Invasive capacity of HCT116/pcDNA, HCT116/NHERF-2 and HCT116/MAGI-3 cells was assessed. The cell numbers at the lower side of the invasion chamber per microscopic field were quantified. (F) Cell invasion of SW480 cells transfected with pcDNA or pcDNA/MAGI-3 was determined. n = 3 for each experimental set.
Next, the effects of NHERF-2 and MAGI-3 on the invasive capacity of colon cancer cells were determined by a Matrigel assay. Invasion of HCT116 cells was stimulated with increasing concentrations of LPA, which was potentiated when NHERF-2 was overexpressed (Figure 1E; Supplementary Figure S1E). In contrast, MAGI-3 inhibited LPA-mediated HCT116 cell invasion (Figure 1E; Supplementary Figure S1E). The inhibitory effect of MAGI-3 was similarly observed in SW480 cells, which endogenously express NHERF-2 and MAGI-3 (Figure 1F; Supplementary Figure S1F). These results collectively demonstrate that NHERF-2 and MAGI-3 reciprocally regulate LPA2-mediated cellular functions.
MAGI-3 negatively regulates NHERF-2 binding to LPA2 and interacts with PLC-β3
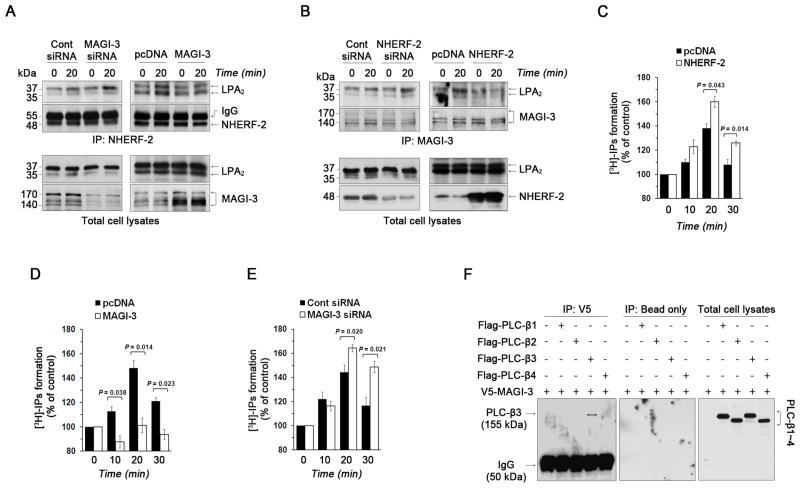
NHERF-2 and MAGI-3 bind to the same carboxyl terminal PDZ binding motif of LPA2 6, 9, 11. We explored the possibility that MAGI-3 might inhibit LPA2-mediated effects by interfering with NHERF-2 binding to LPA2. In HCT116/LPA2 cells, LPA2 co-immunoprecipitation with NHERF-2 was stimulated by LPA, indicating that LPA enhanced the LPA2-NHERF-2 interaction (Figure 2A left panel; left 2 lanes). While knockdown of MAGI-3 significantly increased the LPA2-NHERF-2 association (Figure 2A left panel; right 2 lanes), overexpression of MAGI-3 yielded an opposite effect (Figure 2A right panel). Conversely, transfection of HCT116 cells with NHERF-2 siRNA potentiated the binding of LPA2 to MAGI-3 (Figure 2B left panel), which was decreased in cells overexpressing NHERF-2 (Figure 2B right panel). Thus, these data demonstrate that MAGI-3 and NHERF-2 compete for binding to LPA2.
Figure 2. MAGI-3 competes with NHERF-2 for interaction with LPA2 to attenuate PLC activity.
(A) HCT116 cells stably expressing VSVG-LPA2 (HCT116/LPA2) were transfected with MAGI-3 siRNA or MAGI-3. Transfected cells were treated with 1 μM LPA for 20 min, NHERF-2 was immunoprecipitated, and co-immunoprecipitated VSVG-LPA2 was detected (top two panels). The bottom two panels show LPA2 and MAGI-3 in cell lysates. (B) The interaction between LPA2 and MAGI-3 in HCT116/LPA2 cells transfected with NHERF-2 siRNA or NHERF-2 was determined as described above. The top two panels show co-immunoprecipitated LPA2 and immunoprecipitated MAGI-3. The expression of LPA2 and NHERF-2 in cell lysate is shown in the bottom panels. (C) The PLC activation by LPA in HCT116/pcDNA or HCT116/NHERF-2 cells was determined as described in Methods. The data are represented as relative percent change compared with respective untreated cells. n=3. The amounts of IPs generated by LPA were determined in (D) HCT116 cells overexpressing MAGI-3 and (E) MAGI-3 knockdown cells. (F) The interaction between V5-MAGI-3 and Flag-PLCβ was determined. PLCβ expression is shown in the right panel. n ≥ 3 for each experimental set.
LPA2 has an intrinsic ability to activate PLC to generate diacylglycerol and inositol 1,4,5-triphosphate. The physiological significance of PLC activation is demonstrated in Supplementary Figure S2 where the LPA-induced invasion of HCT116 cells was abrogated by the presence of the PLC inhibitor U73122. Given the opposing effects of MAGI-3 and NHERF-2, we examined if NHERF-2 and MAGI-3 differentially regulated LPA2-mediated PLC signaling. Figure 2C shows that LPA stimulates total inositol phosphate (IP) accumulation, which was enhanced by overexpression of NHERF-2. In comparison, expression of MAGI-3 in HCT116 (Figure 2D) or SW480 cells (data not shown) decreased LPA2-mediated PLC activity. The negative role of MAGI-3 was corroborated by knockdown of MAGI-3 that enhanced LPA2-mediated PLC activation (Figure 2E).
To determine whether the MAGI-3-dependent decrease in PLC activity is specific for LPA2, we examined activation of PLC activity by purinergic signaling. We have shown previously that NHERF-2 enhances purinergic P2Y receptor activation 18. On the contrary, overexpression of MAGI-3 attenuated ATP-induced PLC activation, whereas a significant increase in IP accumulation resulted from MAGI-3 knockdown (Supplementary Figure S3A-B). These results suggest that the ability of MAGI-3 to be a negative regulator of PLC activity is not unique to LPA2 activation.
It was shown previously that PLC-β3 binds to NHERF-2 19, but the status of PLC interaction with MAGI-3 has not been reported. Therefore, we wondered whether the decreased PLC activity might be due to the inability to bind PLC-β by MAGI-3. We co-expressed V5-MAGI-3 and each of Flag-tagged PLC-β isoforms, PLC-β1–4, in HCT116 cells, followed by immunoprecipitation of V5-MAGI-3. Figure 2F shows that MAGI-3 specifically co-immunoprecipitated PLC-β3, but not other PLC-β isoforms, identically recapitulating the NHERF-2 interaction with PLC-β. Therefore, since both NHERF-2 and MAGI-3 specifically interact with PLC-β3, differential PLC interactions could not explain why MAGI-3 and NHERF-2 exert opposing functional effects.
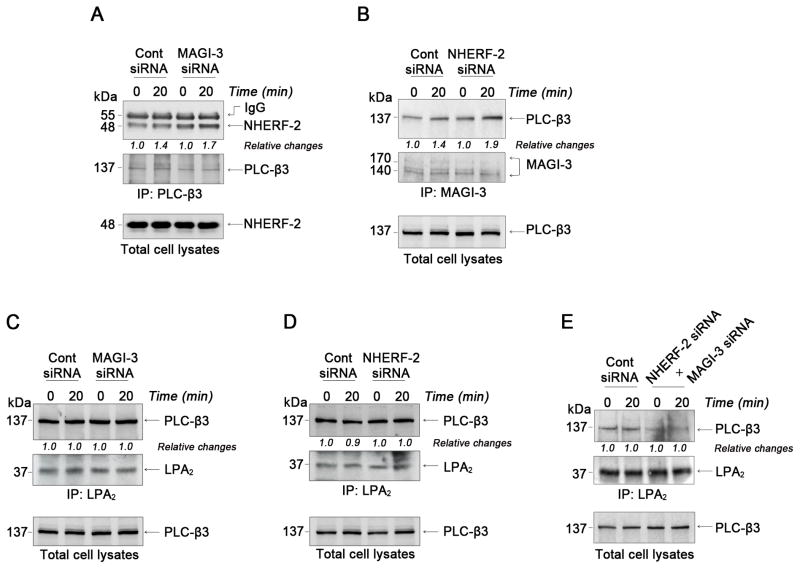
Since NHERF-2 potentiates PLC activity 19, we hypothesized that MAGI-3 might inhibit PLC activity by interfering with the association of NHERF-2 and PLC-β3. To test this possibility, we examined the effect of MAGI-3 knockdown on the NHERF-2-PLC-β3 interaction. Figure 3A shows that knockdown of MAGI-3 increased the PLC-β3-NHERF-2 association. Conversely, NHERF-2 knockdown augmented PLC-β3 interaction with MAGI-3 (Figure 3B) revealing that NHERF-2 and MAGI-3 competitively interact with PLC-β3. To address whether the LPA2-PLC-β3 association is dependent on the presence of a specific PDZ protein, we determined the amount of PLC-β3 complexed with LPA2 when either MAGI-3 or NHERF-2 was knocked down. Surprisingly, we found that the amount of PLC-β3 co-immunoprecipitated with LPA2 was not significantly modulated by knockdown of either MAGI-3 or NHERF-2 (Figure 3C–D). The possibility that PLC-β3 tethers to LPA2 by a protein other than NHERF-2 or MAGI-3 was eliminated by simultaneous knockdown of NHERF-2 and MAGI-3, which evidently decreased the amount of PLC-β3 bound to LPA2 (Figure 3E). These results imply that MAGI-3 functions in the same manner as NHERF-2 in bridging LPA2 and PLC-β3, and that the total amount of PLC-β3 associated with LPA2 is unaltered by which PDZ scaffold is bound to the receptor.
Figure 3. NHERF-2 and MAGI-3 do not alter coupling of PLC-β3 or G proteins with LPA2.
(A) Co-immunoprecipitation of NHERF-2 (top panel) with PLC-β3 (middle panel) in control siRNA or MAGI-3 siRNA transfected cells was determined. The bottom panel shows NHERF-2 expression in cell lysates. NHERF-2 co-immunoprecipitation was quantified by densitometric analysis. (B) Co-immunoprecipitation of PLC-β3 with MAGI-3 was determined in cells transfected with control siRNA or NHERF-2 siRNA. The amount of PLC-β3 associated LPA2 was determined in (C) MAGI-3 knockdown and (D) NHERF-2 knockdown cells. (E) LPA2-associated PLC-β3 was determined in cells transfected with both NHERF-2 siRNA and MAGI-3 siRNA. n = 3.
MAGI-3 and NHERF-2 differentially regulate LPA2 coupling with Gαq and Gα12, and LPA2 stability
Having shown that the amount of receptor-associated PLC-β3 is unaffected by NHERF-2 or MAGI-3, we sought to determine whether the decreased PLC activity in the presence of MAGI-3 could be explained by inefficient coupling of the Gα protein relative to NHERF-2. To this end, we measured LPA-dependent binding of the non-hydrolysable GTP analogue, GTP-γ-S, which measures the total amount of agonist-induced G protein activation. As expected, GTP-γ-S binding was increased in response to LPA (Supplementary Figure S4A). However, overexpression of neither NHERF-2 nor MAGI-3 significantly altered the GTP-γ-S binding (Supplementary Figure S4B), implying that the differential regulation of PLC activation is not due to a globally altered amount of G protein binding.
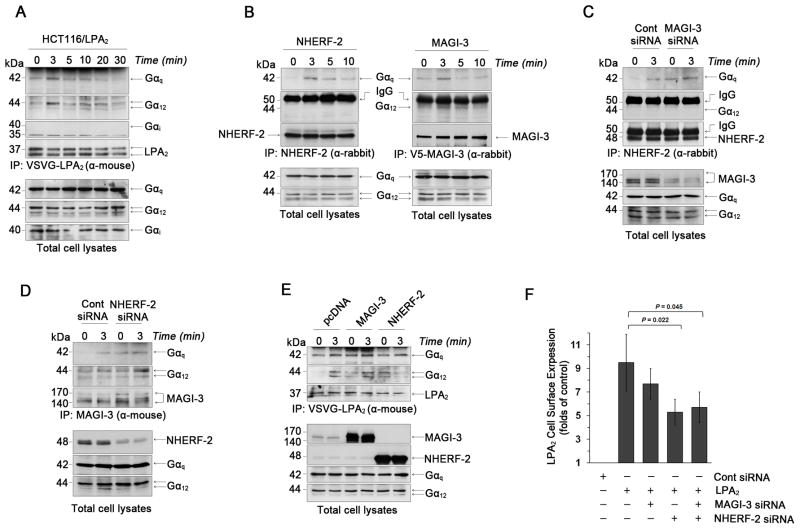
However, it is possible that different G proteins can associate with LPA2 when either NHERF-2 or MAGI-3 is co-expressed. To address this, HCT116/LPA2 cells were treated with LPA and the association of LPA2 with G proteins, Gαq, Gαi, and Gα12, that known to be activated by LPA was determined. Figure 4A shows that Gαq and Gα12, but not Gαi, co-immunoprecipitated with LPA2, and the binding of Gαq and Gα12 to LPA2 was acutely stimulated by LPA. In the next experiment, we determined whether NHERF-2 and MAGI-3 interact with same or different G proteins by immunoprecipitating NHERF-2 or MAGI-3 from HCT116/LPA2 cells. Figure 4B shows that NHERF-2 (left panel) co-immunoprecipitated Gαq, but not Gα12, whereas MAGI-3 (right panel) co-immunopreciated both Gαq and Gα12. Subsequently, knockdown of MAGI-3 potentiated Gαq association with NHERF-2 (Figure 4C). On the contrary, knockdown of NHERF-2 increased the amount of Gα12 found in the complex with MAGI-3 (Figure 4D). The association of Gαq and Gα12 with LPA2 was reproduced with overexpression of MAGI-3 (Figure 4E), whereas, in cells overexpressing NHERF-2, co-immunoprecipitation of Gαq with LPA2 was stimulated by LPA, but the LPA2-Gα12 association was decreased upon LPA treatment. The data imply that NHERF-2 enhances the PLC activity by potentiating the Gαq-PLC pathway, while MAGI-3 diverts receptor signaling between Gαq and Gα12.
Figure 4. NHERF-2 binds Gαq and stabilizes LPA2 surface expression.
(A) The association of Gα subtypes with LPA2 in HCT116/LPA2 cells treated with 1μM LPA up to 30 min was determined. Expression of Gα subtypes in total cell lysates is shown in the bottom panels. Representative figures from 3 independent experiments are shown. α-mouse; mouse monoclonal antibody. α-rabbit; rabbit polyclonal antibody. (B) Co-immunoprecipitation of Gαq or Gα12 with NHERF-2 (left panel) or V5-MAGI-3 (right panel) was determined in HCT116/LPA2 cells overexpressing NHERF-2 or V5-MAGI-3. The presence of NHERF-2 or MAGI-3, Gαq, and Gα12 in total cell lysates is shown in the bottom panels. (C) The association of Gαq or Gα12 with NHERF-2 was determined in HCT116/LPA2 cells transfected with control siRNA or MAGI-3 siRNA. (D) Co-immunoprecipitation of Gαq or Gα12 with MAGI-3 was examined in HCT116/LPA2 cells transfected with control siRNA or NHERF-2 siRNA. (E) Co-immunorpecipitation of Gαq or Gα12 with LPA2 in HCT116/LPA2 cells transfected with MAGI-3 or NHERF-2 was determined. (F) Surface expression levels of LPA2 were determined by a luminometer-based assay. n ≥ 3 for each experimental set.
Receptor internalization or expression can be altered by scaffold proteins 8. Using a luminometer-based cell surface assay, we did not see any evidence for LPA-mediated LPA2 internalization. However, the amount of LPA2 in the plasma membrane was significantly decreased with NHERF-2 knockdown (Figure 4F). On the contrary, MAGI-3 knockdown had no effect on surface expression of LPA2. Simultaneous silencing of NHERF-2 and MAGI-3 reduced LPA2 surface expression to a similar extent as that seen with NHERF-2 knockdown alone. Thus, our data reveal that NHERF-2 potentiates LPA2-elicited activities by stabilizing LPA2 surface expression together with promotion of Gαq-PLC signaling, whereas MAGI-3 attenuates LPA2 signaling by diverting between Gαq- and Gα12-dependent pathways.
MAGI-3 inhibits LPA-induced activation of NF-κB and JNK
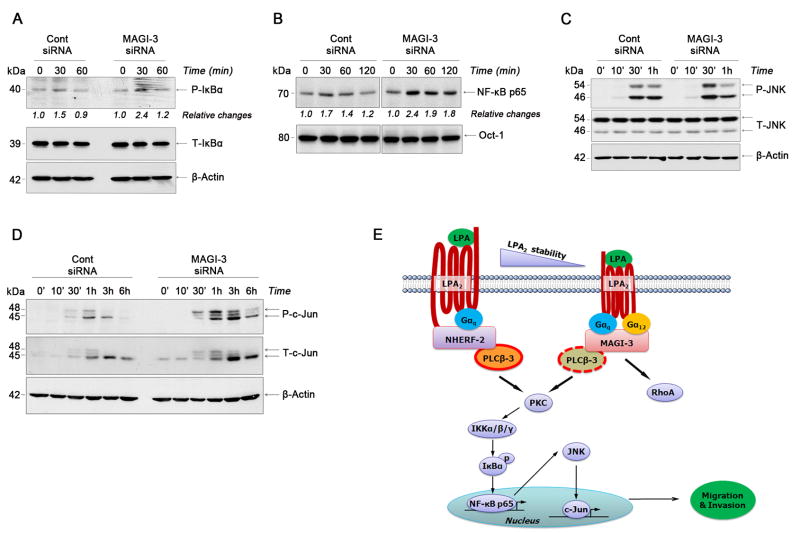
NF-κB, a pleiotropic transcription factor, plays an important role in inflammation and carcinogenesis, and its activation by LPA via PLC has been shown in other cell types 20. To further understand the mechanism whereby MAGI-3 alters signaling by LPA2, we examined the effect of LPA on NF-κB in HCT116 cells and whether MAGI-3 could modulate NF-κB activation. LPA increased the level of IκBα phosphorylation and the nuclear translocation of NF-κB p65 (Figure 5A–B, left panels). In comparison, MAGI-3 knockdown increased IκBα phosphorylation and nuclear translocation of NF-κB p65 (Figure 5A–B, right panels).
Figure 5. MAGI-3 suppresses LPA-induced activation of NF-κB and JNK.
(A) Phosphorylation of IκBα (P-IκBα) in cells transfected with control or MAGI-3 siRNA was determined. Total IκBα (T-IκBα) and β-actin expression is shown as controls. Relative changes in P-IκBα are indicated. All figures are representatives of 3 independent experiments. (B) Nuclear translocation of NF-κB p65 subunit was determined in control siRNA or MAGI-3 siRNA transfected cells. Oct-1 was used as a loading control for nuclear proteins. n = 3 Phosphorylation of JNK (C) and c-Jun (D) by LPA in control siRNA and MAGI-3 siRNA transfected cells are shown. n=4. (E) A putative model for LPA2-induced signaling pathways in colon cancer cells is shown. NHERF-2 and MAGI-3 competitively form macro-complexes by bridging LPA2 and PLC-β3.
In addition to the activation of NF-κB, activation of c-Jun N-terminal kinase (JNK) is often associated with cell migration-related events, such as cytoskeletal rearrangement and cell motility 21. LPA increased phosphorylation of JNK and c-Jun, which was potentiated by knockdown of MAGI-3 (Figure 5C-D). In contrast, activation of other protein kinases, such as Akt and p38 by LPA was not appreciably affected by changes in MAGI-3 expression (data not shown). The role of NF-κB, JNK, and PKC in LPA-mediated cell invasion was assessed by using NBD (nemo binding domain) peptide, SP600125, and Gö6976, an inhibitor of conventional PKCs, to inhibit NF-κB, JNK, and PKC, respectively. All the inhibitors blocked HCT116 cell invasion, indicating the involvement of PKC, NF-κB, and JNK in cell invasion (Supplementary Figure S5A).
In order to grasp the relationship between NF-κB, JNK, and PLC activation induced by LPA, we examined phosphorylation of NF-κB and JNK in the presence of inhibitors (Supplementary Figure S5B–C). Phosphorylation of IκB and JNK was blocked by U73122, but not by SP600125. Surprisingly, NBD peptide inhibited JNK phosphorylation, indicating that NF-κB stimulates JNK. Moreover, LPA-induced activation of c-Jun was inhibited by Gö6976 (Supplementary Figure S5D). The sequences of putative signaling pathways are summarized in Figure 5E.
To further correlate LPA2 with the NF-κB pathway, we examined the phosphorylation status of IκBα in intestinal tumors of LPA2-deficient (Lpar2−/−) mice that were recently reported by us 7, 22. The phosphorylation level of IκBα was elevated in adenomas of ApcMin/+ compared with normal epithelial cells of wild-type (WT) mice (Supplementary Figure S6A). In comparison, a loss of LPA2 expression in ApcMin/+ (ApcMin/+/Lpar2−/−) mice significantly decreased IκBα phosphorylation 22. Similarly, a loss of LPA2 reduced IκBα phosphorylation levels in tumors induced by azoxymethane and dextran sodium sulfate (Supplementary Figure S6A) 7.
MAGI-3 and NHERF-2 expression are altered in human colon cancer
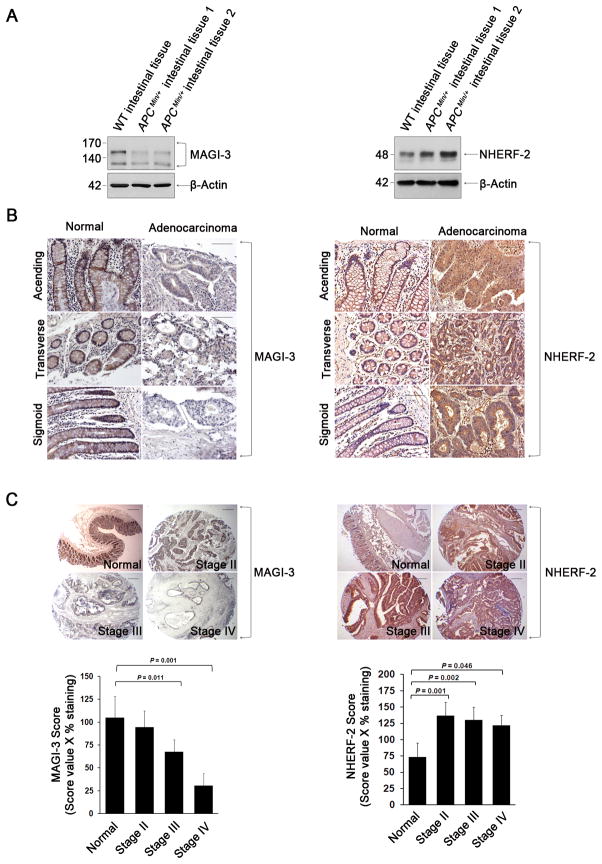
The differential roles of MAGI-3 and NHERF-2 in LPA-induced oncogenic effects prompted us to examine the expression levels of MAGI-3 and NHERF-2 in intestinal tissues. We first compared expression of these scaffolds in the intestine of WT and ApcMin/+ mice. The expression level of MAGI-3 was lower in intestinal adenomas of ApcMin/+ mice compared with normal intestinal tissue, whereas NHERF-2 showed a reverse pattern (Figure 6A). The differential levels of MAGI-3 and NHERF-2 expression were further demonstrated in human colon tissue arrays. Labeling of MAGI-3 was significantly lower in adenocarcinoma tissues in the ascending, transverse and sigmoid colon, as compared to the prominent labeling in the plasma membrane and junctional membrane of normal colonocytes (Figure 6B, left panels). The immunostaining scores of MAGI-3 based on the intensity and proportion of stained cells gradually decreased from stage II through IV (Figure 6C, left panels). In contrast, NHERF-2 expression was upregulated in human colon cancer tissues compared with healthy tissues (Figure 6B–C, right panels). Even though the biological functions of MAGI-3 and NHERF-2 probably are not limited to the LPA-induced effects, the decreased MAGI-3 expression as well as the increased NHERF-2 expression in adenocarcinomas correlate well with the opposing roles of MAGI-3 and NHERF-2 in LPA2-elicited cellular functions.
Figure 6. The expression level of MAGI-3 is down-regulated in adenocarcinomatous colon tissues.
(A) The expression levels of MAGI-3 and NHERF-2 in the intestine of WT mice and intestinal adenomatous lesions of ApcMin/+ mice are shown. (B) Representative immunohistochemical labeling of MAGI-3 (left) and NHERF-2 (right) in human colon and adenocarcinoma colon tissues are shown. Magnification; 200X. Scale bars; 10 μm. (C) Immunohistochemical staining of MAGI-3 and NHERF-2 in colon tissue sections of normal, stage II, III, and IV are shown. Magnification; 40 X. Scale bars; 10 μm. The stage-dependent histological scoring is shown in the graphs below the figures.
DISCUSSION
The role of LPA signaling in the progression of cancer is an active area of study. Since the initial demonstration of the effect of LPA on cell proliferation, the identification of LPA as the ovarian cancer activating factor in malignant ascites together with the finding of elevated levels of LPA in ovarian and other gynecological cancers have heightened the relevance of LPA to cancer 23–25. The recent report that free fatty acid generation in cancer cells produces oncogenic lipids, such as LPA and prostaglandin E2, offers provocative implication for a role of LPA in linking obesity to tumorigenesis 26. The tumorigenic effects of LPA are primarily mediated by the activation of LPA2, which is upregulated in ovarian, colon, breast, prostate, uterus, and testis cancer 5, 6, 27. Consistently, LPA2 mRNA expression was significantly elevated in adenomas of ApcMin/+ mice compared with non-dysplastic intestinal tissue 7, 22. In the present study, our data showed that the signaling and functions of LPA2 are reciprocally modulated by the dynamic and coordinated interaction of two PDZ scaffold proteins, NHERF-2 and MAGI-3.
NHERF-2 is a known positive regulator of LPA2. The interaction of NHERF2 with LPA2 enhanced LPA-induced cell proliferation, cyclooxygenase-2 expression, IL-8 secretion, and anti-apoptotic property of colon cancer cells against chemotherapy 6, 9, 17. Consistent with the earlier findings, the positive effects of NHERF-2 on LPA2 signaling are recapitulated in the present study using HCT116 and SW480 cells. On the other hand, apart from its interaction with Frizzled, β1-adrenergic receptor (β1-AR), PTEN/MMAC, and receptor tyrosine phosphatase-β, the functional role of MAGI-3 has not been widely explored 28–30. We found that overexpression of MAGI-3 inhibited LPA-induced migration and invasion of colon cancer cells, whereas knockdown of MAGI-3 recapitulated the effect of NHERF-2 overexpression. Thus, these results demonstrate that MAGI-3 is a negative regulator of LPA2-mediated cellular functions, and provide evidence that PDZ domain-containing proteins play a critical role in regulating LPA2-mediated effects.
The PLC-PKC-Ca2+ cascade is a major signaling pathway elicited by LPA2, which is potentiated by NHERF-2 9. Unlike NHERF-2, we found that MAGI-3 attenuated PLC activity despite its binding of PLC-β3. Importantly, the effect of MAGI-3 on PLC activity was not specific to LPA2 but also inhibited P2Y-mediated PLC activity, suggesting that MAGI-3 might be a negative regulator of PLC signaling by a broad range of GPCRs. The negative regulation by MAGI-3 could have risen from MAGI-3 simply displacing NHERF-2 and PLC-β3 from LPA2. Indeed, MAGI-3 competed with NHERF-2 for both LPA2 and PLC-β3. However, deletion of NHERF-2 or MAGI-3 did not alter the amount of PLC-β3 complexed with LPA2, suggesting that the change in PLC activity was not due to inefficient complexing of PLC-β3 by MAGI-3 with LPA2. Instead, the PDZ proteins facilitated LPA2 coupling of different G proteins, without a significant effect on the total GTPase activity. In colon cancer cells, LPA2 rapidly associated with Gαq and Gα12, but not with Gαi, upon activation by LPA. NHERF-2 exclusively interacted with Gαq and the presence of NHERF-2 led to a preferential enhancement of Gαq-mediated downstream signaling by LPA2. In contrast to NHERF-2, MAGI-3 facilitated the association of LPA2 with both Gαq and Gα12 to divert LPA2-mediated signaling to Gαq and Gα12-dependent pathways, thereby lessening Gαq-dependent activation of PLC. In support of MAGI-3 mediating Gα12-dependent signaling, we showed previously that MAGI-3 potentiates LPA-induced RhoA activation in colon cancer cells 11. Additionally it was shown recently that LPA2 inhibits migration of pancreatic cancer cells via the Gα12-RhoA pathway 31, implying that that the activation of Gα12-RhoA by MAGI-3 could further contribute to the inhibition of migratory response of colon cancer cells.
A number of GPCR-interacting proteins have been shown to regulate GPCR thorough multiple mechanisms involving recycling, targeting, or stability of receptor proteins 8. NHERF-1, which is highly homologous to NHERF-2, associates with β2-AR and κ-opioid receptor to promote recycling of the receptors 32, 33, and PSD-95 interacts with β1-AR to attenuate agonist-promoted receptor internalization 34. In comparison, little is known about the receptor recycling or trafficking of LPA receptors except that LPA1 is rapidly endocytosed in response to LPA in a dynamin2 and Rab5a dependent mechanism 35. We did not see any evidence for LPA-induced internalization of LPA2 based on a luminometer-based cell surface assay, although rapid LPA2 internalization and recycling might have escaped detection. Instead, our results showed that NHERF-2 enhanced the surface expression of LPA2, suggesting that LPA2 is positioned and stabilized on plasma membrane through its interaction with NHERF-2. However, MAGI- 3 showed no effect on the surface expression of LPA2. That NHERF-2, but not MAGI-3, alters LPA2 surface stability might be explained by its ability to interact with the actin cytoskeletal network through the ezrin-radixin-moesin binding motif present at the carboxyl terminal region of NHERF-2 36. Collectively, NHERF-2 facilitates migration and invasion of colon cancer cells by increasing surface expression of LPA2 and potentiating the LPA2-Gαq-PLC pathway (Figure 5E). In comparison, MAGI-3 diverts LPA2 signaling to both Gαq-PLC-3β and Gα12-RhoA pathways.
Activation of NF-κB is often linked to inflammation, but aggressive cancers and several cancer cell lines have constitutively active NF-κB, and clinical evidence linking colon cancer and NF-κB comes from epidemiological studies 37, 38. Similarly, transgenic expression of JNK in the intestine results in increased cell proliferation and migration, and JNK accelerates tumorigenesis in ApcMin/+ mice in part by cross-talk with the Wnt pathway 39. In addition, nuclear localization of β-catenin in human colon carcinoma samples was paralleled by JNK activation 40. In the present study, LPA-induced invasion of HCT116 cells was blocked by inhibition of NF-κB or JNK, demonstrating the critical role of NF-κB and JNK in the invasion of colon cancer cells. The activation of NF-κB and JNK by LPA is regulated by PLC-β as evidenced by silencing of MAGI-3 expression and inhibition by U73122. Importantly, we showed that the activation status of NF-κB pathway in intestinal tumors of ApcMin/+ mice was markedly attenuated by the loss of LPA2 expression. A similar reduction in IκBα phosphorylation was observed in colitis- associated tumors in mice. However, the molecular mechanism of LPA2-mediated NF- κB activation remains incompletely understood. Recent studies indicated that CARMA3 (caspase recruit domain and MAGUK domain containing 3) plays an essential role in LPA-induced NF-κB activation through coupling of Bcl10, an intermediate bridging factor, and Malt-1, a protein that stimulates IKK complex via interaction with Bcl10 20, 41. In addition, it was shown that JNK signaling is regulated by Bcl10-dependent NF-κB regulation in lymphocytes 20. Therefore, it is an intriguing possibility that MAGI-3 may negatively regulate LPA-induced NF-κB activation by interfering with the PKC-CARMA3- Bcl10-MALT1 cascade.
The reciprocal roles of NHERF-2 and MAGI-3 on LPA2-induced effects on colon cancer cells correlate with the expression levels of NHERF-2 and MAGI-3 in human colon cancer tissues. Not only was the MAGI-3 expression lower in the tumor samples, the immunostaining scores of MAGI-3 inversely correlated with disease progression and lower scoring in late stage adenocarcinomas, whereas NHERF-2 showed opposite results. Although it is tempting to suggest the expression levels of NHERF-2 and MAGI-3 as potential biomarkers of colon cancer, we have not fully established a causal link between these scaffold proteins and colon cancer and we await additional studies.
In summary, our data demonstrate that LPA2 is dynamically regulated by two distinct PDZ proteins via modulation of G protein coupling and receptor expression. The current studies reveal the potential relevance of PDZ interactions to colon cancer cell behaviors.
Supplementary Material
Acknowledgments
Funding sources: This work was supported by grants from the National Institutes of Health R01DK071597 and R01DK071597-03S1 (CCY) and R01NS055179 (RAH).
We thank Dr. Pann-Ghill Suh for PLC-β clones. We acknowledge the Emory Digestive Disease Research Development Center (supported by DK064399) for HCT116 and SW480 cells.
Abbreviations
- LPA
lysophosphatidic acid
- PDZ
postsynaptic density 95, discs large, and zonula occludens–1
- GPCR
G protein-coupled receptors
- MAGI-3
membrane-associated guanylate kinase with inverted orientation-3
- NHERF-2
Na+/H+ exchanger regulatory factor 2
- PLC
phospholipase C
- IκBα
inhibitory kappa Bα
- NF-κB
nuclear factor-kappa κB
- JNK
c-Jun N-terminal kinase
- NBD
NEMO binding domain
- IKKγ
Inhibitor κB kinase gamma
- cPKC
conventional protein kinase C
Footnotes
No conflict of interest exists.
Author contributions: S.L.: Study concept and design, acquisition of data, analysis and interpretation of data, statistic analysis, drafting of the manuscript
S.L.R.: Study concept and design, acquisition of data, analysis and interpretation of data, statistic analysis, drafting of the manuscript
H.Z.: acquisition of data, analysis and interpretation of data
H.S.: Study concept and design, study supervision
R.A.H.: Study concept and design, analysis and interpretation of data, drafting of the manuscript, funding, study supervision
C.C.Y.: Study concept and design, analysis and interpretation of data, drafting of the manuscript, funding, study supervision
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 2.Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3:582–591. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 3.Moolenaar WH, van Meeteren LA, Giepmans BN. The ins and outs of lysophosphatidic acid signaling. Bioessays. 2004;26:870–881. doi: 10.1002/bies.20081. [DOI] [PubMed] [Google Scholar]
- 4.Choi JW, Herr DR, Noguchi K, et al. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol. 2010;50:157–186. doi: 10.1146/annurev.pharmtox.010909.105753. [DOI] [PubMed] [Google Scholar]
- 5.Goetzl EJ, Dolezalova H, Kong Y, et al. Distinctive expression and functions of the type 4 endothelial differentiation gene-encoded G protein-coupled receptor for lysophosphatidic acid in ovarian cancer. Cancer Res. 1999;59:5370–5375. [PubMed] [Google Scholar]
- 6.Yun CC, Sun H, Wang D, et al. LPA2 receptor mediates mitogenic signals in human colon cancer cells. Am J Physiol. 2005;289:C2–11. doi: 10.1152/ajpcell.00610.2004. [DOI] [PubMed] [Google Scholar]
- 7.Lin S, Wang D, Iyer S, et al. The absence of LPA2 attenuates tumor formation in an experimental model of colitis-associated cancer. Gastroenterology. 2009;136:1711–1720. doi: 10.1053/j.gastro.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ritter SL, Hall RA. Fine-tuning of GPCR activity by receptor-interacting proteins. Nat Rev Mol Cell Biol. 2009;10:819–830. doi: 10.1038/nrm2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oh YS, Jo NW, Choi JW, et al. NHERF2 specifically interacts with LPA2 receptor and defines the specificity and efficiency of receptor-mediated phospholipase C-beta3 activation. Mol Cell Biol. 2004;24:5069–5079. doi: 10.1128/MCB.24.11.5069-5079.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamada T, Ohoka Y, Kogo M, et al. Physical and functional interactions of the lysophosphatidic acid receptors with PDZ domain-containing Rho guanine nucleotide exchange factors (RhoGEFs) J Biol Chem. 2005;280:19358–19363. doi: 10.1074/jbc.M414561200. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H, Wang D, Sun H, et al. MAGI-3 regulates LPA-induced activation of Erk and RhoA. Cell Signal. 2007;19:261–268. doi: 10.1016/j.cellsig.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, Bialkowska A, Rusovici R, et al. Lysophosphatidic acid facilitates proliferation of colon cancer cells via induction of Kruppel-like factor 5. J Biol Chem. 2007;282:15541–15549. doi: 10.1074/jbc.M700702200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berridge MJ, Downes CP, Hanley MR. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982;206:587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balasubramanian S, Teissere JA, Raju DV, et al. Hetero-oligomerization between GABAA and GABAB receptors regulates GABAB receptor trafficking. J Biol Chem. 2004;279:18840–18850. doi: 10.1074/jbc.M313470200. [DOI] [PubMed] [Google Scholar]
- 15.Buck E, Eyzaguirre A, Barr S, et al. Loss of homotypic cell adhesion by epithelial-mesenchymal transition or mutation limits sensitivity to epidermal growth factor receptor inhibition. Mol Cancer Ther. 2007;6:532–541. doi: 10.1158/1535-7163.MCT-06-0462. [DOI] [PubMed] [Google Scholar]
- 16.Lazareno S, Birdsall NJ. Pharmacological characterization of acetylcholine-stimulated [35S]-GTP gamma S binding mediated by human muscarinic m1-m4 receptors: antagonist studies. Br J Pharmacol. 1993;109:1120–1127. doi: 10.1111/j.1476-5381.1993.tb13738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rusovici R, Ghaleb A, Shim H, et al. Lysophosphatidic acid prevents apoptosis of Caco-2 colon cancer cells via activation of mitogen-activated protein kinase and phosphorylation of Bad. Biochim Biophys Acta. 2007;1770:1194–1203. doi: 10.1016/j.bbagen.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fam SR, Paquet M, Castleberry AM, et al. P2Y1 receptor signaling is controlled by interaction with the PDZ scaffold NHERF-2. Proc Natl Acad Sci USA. 2005;102:8042–8047. doi: 10.1073/pnas.0408818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang JI, Heo K, Shin KJ, et al. Regulation of phospholipase C-beta 3 activity by Na+/H+ exchanger regulatory factor 2. J Biol Chem. 2000;275:16632–16637. doi: 10.1074/jbc.M001410200. [DOI] [PubMed] [Google Scholar]
- 20.Grabiner BC, Blonska M, Lin PC, et al. CARMA3 deficiency abrogates G protein-coupled receptor-induced NF-κB activation. Genes Dev. 2007;21:984–996. doi: 10.1101/gad.1502507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia Y, Karin M. The control of cell motility and epithelial morphogenesis by Jun kinases. Trends Cell Biol. 2004;14:94–101. doi: 10.1016/j.tcb.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Lin S, Lee SJ, Shim H, et al. The Absence of LPA receptor 2 Reduces the Tumorigenesis by ApcMin Mutation in the Intestine. Am J Physiol. 2010 doi: 10.1152/ajpgi.00321.2010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Corven EJ, Groenink A, Jalink K, et al. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell. 1989;59:45–54. doi: 10.1016/0092-8674(89)90868-4. [DOI] [PubMed] [Google Scholar]
- 24.Xu Y, Gaudette DC, Boynton JD, et al. Characterization of an ovarian cancer activating factor in ascites from ovarian cancer patients. Clin Cancer Res. 1995;1:1223–1232. [PubMed] [Google Scholar]
- 25.Xu Y, Shen Z, Wiper DW, et al. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA. 1998;280:719–723. doi: 10.1001/jama.280.8.719. [DOI] [PubMed] [Google Scholar]
- 26.Nomura DK, Long JZ, Niessen S, et al. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. 2010;140:49–61. doi: 10.1016/j.cell.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitayama J, Shida D, Sako A, et al. Over-expression of lysophosphatidic acid receptor-2 in human invasive ductal carcinoma. Breast Cancer Res. 2004;6:R640–646. doi: 10.1186/bcr935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adamsky K, Arnold K, Sabanay H, et al. Junctional protein MAGI-3 interacts with receptor tyrosine phosphatase β (RPTPβ) and tyrosine-phosphorylated proteins. J Cell Sci. 2003;116:1279–1289. doi: 10.1242/jcs.00302. [DOI] [PubMed] [Google Scholar]
- 29.Wu Y, Dowbenko D, Spencer S, et al. Interaction of the tumor suppressor PTEN/MMAC with a PDZ domain of MAGI3, a novel membrane-associated guanylate kinase. J Biol Chem. 2000;275:21477–21485. doi: 10.1074/jbc.M909741199. [DOI] [PubMed] [Google Scholar]
- 30.Yao R, Natsume Y, Noda T. MAGI-3 is involved in the regulation of the JNK signaling pathway as a scaffold protein for frizzled and Ltap. Oncogene. 2004;23:6023–6030. doi: 10.1038/sj.onc.1207817. [DOI] [PubMed] [Google Scholar]
- 31.Komachi M, Tomura H, Malchinkhuu E, et al. LPA1 receptors mediate stimulation, whereas LPA2 receptors mediate inhibition, of migration of pancreatic cancer cells in response to lysophosphatidic acid and malignant ascites. Carcinogenesis. 2009;30:457–465. doi: 10.1093/carcin/bgp011. [DOI] [PubMed] [Google Scholar]
- 32.Hall RA, Premont RT, Chow CW, et al. The beta2-adrenergic receptor interacts with the Na+/H+-exchanger regulatory factor to control Na+/H+ exchange. Nature. 1998;392:626–630. doi: 10.1038/33458. [DOI] [PubMed] [Google Scholar]
- 33.Huang P, Steplock D, Weinman EJ, et al. kappa Opioid receptor interacts with Na+/H+-exchanger regulatory factor-1/Ezrin-radixin-moesin-binding phosphoprotein-50 (NHERF-1/EBP50) to stimulate Na+/H+ exchange independent of Gi/Go proteins. J Biol Chem. 2004;279:25002–25009. doi: 10.1074/jbc.M313366200. [DOI] [PubMed] [Google Scholar]
- 34.Hu LA, Tang Y, Miller WE, et al. beta 1-adrenergic receptor association with PSD-95. Inhibition of receptor internalization and facilitation of beta 1-adrenergic receptor interaction with N-methyl-D-aspartate receptors. J Biol Chem. 2000;275:38659–38666. doi: 10.1074/jbc.M005938200. [DOI] [PubMed] [Google Scholar]
- 35.Murph MM, Scaccia LA, Volpicelli LA, et al. Agonist-induced endocytosis of lysophosphatidic acid-coupled LPA1/EDG-2 receptors via a dynamin2- and Rab5-dependent pathway. J Cell Sci. 2003;116:1969–1980. doi: 10.1242/jcs.00397. [DOI] [PubMed] [Google Scholar]
- 36.Yun CH, Lamprecht G, Forster DV, et al. NHE3 kinase A regulatory protein E3KARP binds the epithelial brush border Na+/H+ exchanger NHE3 and the cytoskeletal protein ezrin. J Biol Chem. 1998;273:25856–25863. doi: 10.1074/jbc.273.40.25856. [DOI] [PubMed] [Google Scholar]
- 37.Nakshatri H, Bhat-Nakshatri P, Martin DA, et al. Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol Cell Biol. 1997;17:3629–3639. doi: 10.1128/mcb.17.7.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charalambous MP, Lightfoot T, Speirs V, et al. Expression of COX-2, NF-κB-p65, NF-κB-p50 and IKKα in malignant and adjacent normal human colorectal tissue. Br J Cancer. 2009;101:106–115. doi: 10.1038/sj.bjc.6605120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sancho R, Nateri AS, de Vinuesa AG, et al. JNK signalling modulates intestinal homeostasis and tumourigenesis in mice. EMBO J. 2009;28:1843–1854. doi: 10.1038/emboj.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phelps RA, Chidester S, Dehghanizadeh S, et al. A two-step model for colon adenoma initiation and progression caused by APC loss. Cell. 2009;137:623–634. doi: 10.1016/j.cell.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klemm S, Zimmermann S, Peschel C, et al. Bcl10 and Malt1 control lysophosphatidic acid-induced NF-κB activation and cytokine production. Proc Natl Acad Sci USA. 2007;104:134–138. doi: 10.1073/pnas.0608388103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.