Abstract
Hemorrhagic fevers caused by arenaviruses are among the most devastating emerging human diseases. Considering the number of individuals affected, the current lack of a licensed vaccine, and the limited therapeutic options, arenaviruses are arguable among the most neglected tropical pathogens and the development of efficacious anti-arenaviral drugs is of high priority. Over the past years significant efforts have been undertaken to identify novel potent inhibitors of arenavirus infection. High throughput screening of small molecule libraries employing pseudotype platforms led to the discovery of several potent and broadly active inhibitors of arenavirus cell entry that are effective against the major hemorrhagic arenaviruses. Mechanistic studies revealed that these novel entry inhibitors block arenavirus membrane fusion and provided novel insights into the unusual mechanism of this process. The success of these approaches highlights the power of small molecule screens in antiviral drug discovery and establishes arenavirus membrane fusion as a robust drug target. These broad screenings have been complemented by strategies targeting cellular factors involved in productive arenavirus infection. Approaches targeting the cellular protease implicated in maturation of the fusion-active viral envelope glycoprotein identified the proteolytic processing of the arenavirus glycoprotein precursor as a novel and promising target for anti-arenaviral strategies.
Keywords: arenavirus, small molecule, antiviral, inhibitors, virus entry, mechanism, Lassa, LCMV
Introduction
Arenaviruses are important emerging human pathogens
Several arenaviruses cause severe viral hemorrhagic fevers (VHF) in humans and represent a serious public health problem (Geisbert and Jahrling, 2004). Lassa virus (LASV) in Africa causes several hundred thousand infections per year resulting in significant mortality and morbidity (McCormick and Fisher-Hoch, 2002). On the South American continent, the arenaviruses Junin (JUNV), Machupo (MACV), Guanarito (GTOV), and Sabia virus (SABV) have emerged as etiological agents of severe VHF in Argentina, Venezuela, Bolivia, and Brazil, respectively (Buchmeier, de la Torre, and Peters, 2007). The worldwide-distributed prototypic arenavirus lymphocytic choriomeningitis virus (LCMV) is also a neglected human pathogen of clinical significance, especially in pediatric medicine (Barton, Mets, and Beauchamp, 2002) and represents a threat to immuno-compromised individuals, as tragically illustrated by recent fatal cases of transplant-acquired LCMV infection (Fischer et al., 2006; Palacios et al., 2008). New arenaviruses emerge on average every three years as illustrated by the recent discoveries of Chapare virus and Lujo virus that were associated with fatal hemorrhagic fever cases in Bolivia and Southern Africa, respectively (Briese et al., 2009; Delgado et al., 2008).
A hallmark of fatal arenavirus VHF is marked immunosuppression of the host and consequent uncontrolled fatal infection (Geisbert and Jahrling, 2004). Those who survive develop a vigorous anti-viral immune response during the second week of disease, control the infection, and ultimately clear the virus. Control of acute arenavirus infection appears to be primarily associated with cellular immunity rather than neutralizing antibodies (Fisher-Hoch and McCormick, 2004; McCormick and Fisher-Hoch, 2002). Neutralizing antibodies appear during convalescence and are in case of Lassa fever frequently of low titer. A highly predictive factor for disease outcome in arenavirus VHF is the viral load, indicating a close competition between viral spread and replication and the patient s immune system(McCormick and Fisher-Hoch, 2002). Anti-viral drugs that limit viral replication and spread may provide the patient s immune system a window of opportunity to develop anti-viral immune responses, to control, and ultimately clear the virus. The development of novel drugs targeting different steps in the arenavirus life cycle is therefore a promising strategy to combat arenavirus infection in humans and will be covered in the present review.
Molecular and cell biology of arenaviruses
The molecular and cell biology of arenaviruses has been covered by excellent recent reviews (Buchmeier, de la Torre, and Peters, 2007; de la Torre, 2009) and only a short summary will be given here. Arenaviruses are enveloped viruses with a bisegmented negative strand RNA genome and a life cycle restricted to the cytoplasm. Each genomic RNA segment L (ca 7.3 kb) and S (ca 3.5 kb) uses an ambisense coding strategy to direct the synthesis of two polypeptides in opposite orientation, separated by a non-coding intergenic region (IGR) with a predicted hairpin structure (Fig. 1). The S RNA encodes the viral glycoprotein precursor, GPC, and the nucleoprotein, NP, (ca 63 kDa), whereas the L RNA encodes the viral RNA dependent RNA polymerase (RdRp, or L polymerase) (ca 200 kDa), and a small RING finger protein Z (ca 11 kDa). Arenavirus GPC is synthesized as a single polypeptide chain (ca 75 kDa) and post-translationally cleaved by the cellular proprotein convertase (PC) subtilisin kexin isozyme-1 (SKI-1)/site-1 protease (S1P) to yield the mature virion glycoproteins GP1 (40-46 kDa) and GP2 (35 kDa) (Beyer et al., 2003; Lenz et al., 2001; Pinschewer et al., 2003; Rojek et al., 2008a). The GP1 part mediates virus interaction with host cell surface receptors and is located at the top of the mature GP spike present in the viral envelope. GP1 is associated via ionic interactions with the transmembrane GP2 that forms the stalk of the spike. Arenavirus GP2 resembles the fusion-active membrane-proximal parts of other enveloped viruses. The cellular receptor for LASV and most isolates of LCMV is α-dystroglycan (α-DG), a cell surface receptor for proteins of the extracellular matrix (ECM) (Cao et al., 1998). The New World arenaviruses JUNV, MACV, GTOV, and SABV can use human transferrin receptor 1 (TfR1) as a cellular receptor (Radoshitzky et al., 2007). Upon attachment to the target cell, arenavirus particles are taken up by endocytosis and delivered to acidified endosomes where low pH induces membrane fusion (Borrow and Oldstone, 1994). New World arenaviruses like JUNV that use human TfR1 enter the cell via clathrin-mediated endocytosis (Martinez, Cordo, and Candurra, 2007). In contrast, the Old World arenaviruses LASV and LCMV, which depend on α-DG, use a distinct and unusual pathway for cell entry that is independent of clathrin, caveolin, dynamin, and actin and bypasses classical routes of incoming early endosomal trafficking (Quirin et al., 2008; Rojek, Perez, and Kunz, 2008; Rojek et al., 2008b).
Figure 1. Arenavirus particle and genome organization.
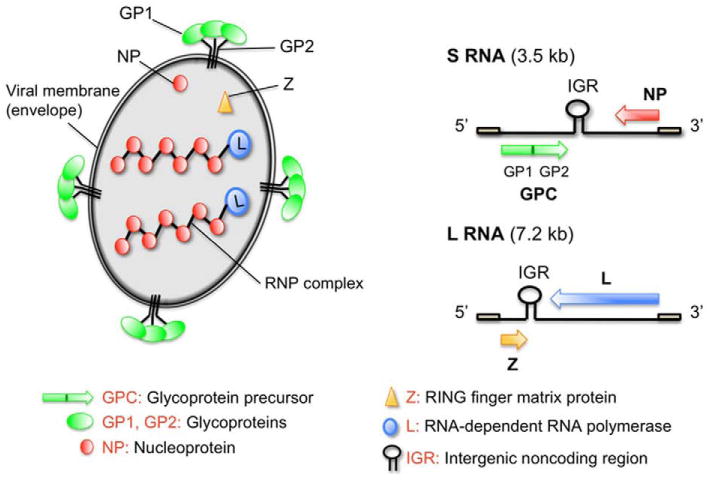
(A) Schematic representation of an arenavirus particle. The viral RNA is packaged into ribonucleoparticles (RNP) containing the viral nucleoprotein (NP). The RNA-dependent RNA polymerase (L) is associated with RNP and required for initial steps of viral transcription. The matrix protein Z associates with the inner leaflet of the viral membrane envelope and interacts with the C-terminal part of the transmembrane GP2 moiety of the mature GP decorating the virion surface. (B) The ambisense coding strategy of arenaviruses. Each of the two single-stranded RNA segments, L and S, uses an ambisense coding strategy to direct the synthesis of two polypeptides in opposite orientations and separated by an intergenic region (IGR).
Formation and release of arenavirus infectious progeny from infected cells by budding requires that assembled viral RNPs associate at the cell surface with membranes that are enriched in viral GPs. For many enveloped RNA viruses viral budding is mediated by a matrix (M) protein that acts as a bridge between the RNP and GP. In arenaviruses, the Z protein functions as the main driving force of arenavirus budding (Perez, Craven, and de la Torre, 2003; Strecker et al., 2003) and the process is mediated by the Z proline-rich late (L) domain motifs (PTAP and PPPY) known to control budding of several other viruses via interaction with specific host cell proteins (Freed, 2002).
Current drugs to combat arenaviruses
The only licensed drug for treatment of human arenavirus infection is the nucleoside analogue ribavirin (Rib) (1-β-D-ribofuranosyl-1,2,4-triazole-3-carboxamide) (Parker, 2005). In vitro and in vivo studies have documented the prophylactic and therapeutic value of Rib against several arenaviruses. Rib reduced both morbidity and mortality in humans associated with LASV infection (McCormick et al., 1986), and experimentally in MACV (Kilgore et al., 1995) and JUNV (Weissenbacher, Laguens, and Coto, 1987) infections, if given early in the course of clinical disease. The mechanism of action of Rib against arenaviruses is currently not entirely clear. Studies in the LCMV model revealed that the anti-viral effect of Rib is not associated with significant increases in virus mutation frequencies, but has a dramatic effect on viral RNA synthesis (Ruiz-Jarabo et al., 2003) Over the past two decades efforts have been made to discover novel drug candidates to combat arenaviruses and only a short and by no means comprehensive overview can be given here: Inhibitors of IMP dehydrogenase (Andrei and De Clercq, 1993), the S-adenosylhomocysteine (SAH) hydrolase (Andrei and De Clercq, 1990), phenotiazines compounds (Candurra, Maskin, and Damonte, 1996), brassinosteroids (Wachsman et al., 2000) and myristic acid (Cordo, Candurra, and Damonte, 1999) have been reported to have anti-arenaviral activity. Several zinc-finger-reactive compounds with antiretroviral potential showed activity against arenaviruses (Garcia, Candurra, and Damonte, 2000; Garcia et al., 2006) and evidence has been provided for an involvement of the viral Z protein in their mechanism of action (Garcia et al., 2010). The pyrazine derivative T-705 (6-fluoro-3-hydrody-2-pyrazinecarboxamide) showed activity against the arenaviruses JUNV, Pichinde (PICV), and Tacaribe virus (TACV) in vitro and protected against PICV infection in a hamster model (Gowen et al., 2007), with efficacy in late stage infection (Gowen et al., 2008).
Early attempts to target arenavirus entry employed sulfated polysaccharides (Andrei and De Clercq, 1990) and revealed inhibitory effects of charged polymers on arenavirus infection, however, the exact mechanism of action remained elusive. A more recent study evaluated phosphorothioate oligonucleotides in the LCMV model and revealed that amphipathic DNA polymers are potent inhibitors of arenavirus infection (Lee et al., 2008). The DNA polymers act at the level of cell entry and target the interaction between LCMVGP and its cellular receptor, α-DG, without affecting later steps in replication. While these studies are of great value as proof-of-principle, to the best of our knowledge none of these drug candidates has undergone further evaluation in pre-clinical and clinical trials.
Discovery of novel inhibitors of arenavirus entry by small molecule screening
Since entry into the host cell is the first step of every virus infection it represents a promising target for attacking the virus before it can gain control over the host cell machinery for replication. In recent years, viral entry inhibitors have emerged as a new class of anti-viral drugs, with enfuvirtide being successfully used for clinical treatment of humans infected with human immunodeficiency virus (HIV) (Este and Telenti, 2007; Qian, Morris-Natschke, and Lee, 2009) and potent small molecule inhibitors for entry of other viruses, including influenza (Vanderlinden et al., 2010) and hepatitis C virus (Baldick et al., 2010) have been reported.
Considering the limited public health infrastructure in endemic regions, highest priority is given to the development of novel synthetic drugs against human pathogenic arenaviruses that can be produced at low cost, delivered orally, and can be stored in tropical climates. To achieve this goal, several attempts at drug discovery based on screening large collections of synthetic small molecules have been undertaken. High throughput screening (HTS) of small molecular libraries has emerged as a major source of potent agonists and antagonists for a great number of molecular interaction implicated in a variety biochemical processes (Boger, Desharnais, and Capps, 2003). Despite the possible involvement of several molecular interfaces, a large number of studies on protein-protein interaction indicate that in most cases, small clusters of amino acid residues mediate the energetically most important interactions (Livnah et al., 1996; Wells, 1996; Wells and de Vos, 1996; Wrighton et al., 1996) that may be perturbed by small synthetic molecules. Libraries of small molecules can therefore be used to screen for and identify molecules that promote protein–protein interactions (agonists) or inhibit protein–protein interactions (antagonists) (Boger et al., 1998).
A first attempt using HTS of small molecules to identify inhibitors of arenavirus infection employed a virus-induced cytopathic effect (CPE)-based assay involving the non-pathogenic New World arenavirus TACV. HTS of a random library identified a potent small molecule inhibitor of TACV and several other New World arenaviruses, ST-294 (Fig. 2) (Bolken et al., 2006), providing proof-of-principle for the feasibility of this type of approach.
Figure 2. Arenavirus entry inhibitors identified in small molecule HTS.
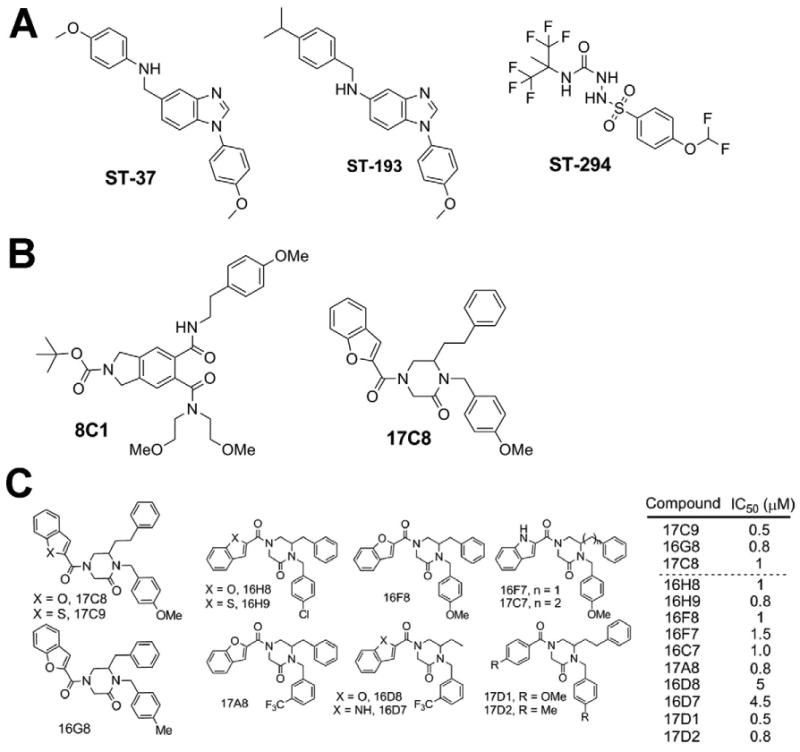
(A) Arenavirus entry inhibitors identified by HTS of a random chemical library (Bolken et al., 2006; Larson et al., 2008). (B) Inhibitors of arenavirus entry obtained from HTS of combinatorial libraries (Lee et al., 2008). (C) SAR of the candidate compounds 17C8 and 16G8 obtained from the combinatorial library HTS (Lee et al., 2008). For details please see text.
All hemorrhagic arenaviruses are classified as biosafety level (BSL)-4 pathogens requiring high security containment laboratories for work with live viruses. In order to identify specific inhibitors of cell entry of hemorrhagic arenaviruses, subsequent drug screening strategies made use of recombinant retroviruses bearing the GP of hemorrhagic arenaviruses in their envelope and a luciferase reporter in their genome (Larson et al., 2008; Lee et al., 2008). Since arenavirus cell attachment and entry are mediated exclusively by the envelope GP, these pseudotype platforms allow rapid and reliable screening of small molecule libraries in a HTS format. On one study, pre-selected combinatorial small molecule libraries were chosen instead of random libraries, allowing a significant reduction of the scope of the screen to less than 100,000 compounds. Combinatorial chemical libraries generated by solution-phase synthetic techniques (Boger et al., 1998) have proven to be powerful sources of novel inhibitors or agonists of a variety of biochemical interactions. The use of this type of library has identified erythropoieitin (EPO) mimetics (promote EPO receptor dimerization) (Berg et al., 2002), inhibitors of Myc/Max dimerization (Berg et al., 2002), inhibitors of LEF-1/β-catenin dimerization (Boger et al., 2000), inhibitors of the binding of the protease MMP-2 to αvβ3 integrin (Boger et al., 2001; Silletti et al., 2001), and inhibitors of HIV protease (Chang et al., 2010). Positive screening of circa 80,000 compounds with LASV pseudotypes yielded initially circa 1 % hits (Lee et al., 2008). Based on the combinatorial nature of the libraries, the hits tended to appear in clusters of structurally related compounds. Counter-screening employing pseudotypes of the unrelated vesicular stomatitis virus (VSV) yielded 32 single compounds and 53 mixtures of 4- to 10 compounds each that specifically blocked LASV GP-mediated infection. A series of leads were selected, including the candidate compounds 8C1 and 17C8 (Fig 2). Candidate compounds 8C1 and 17C8 efficiently blocked infection of cells with live LASV, whereas candidate 17C8, but not 8C1 was also potent against JUNV and other New World hemorrhagic arenaviruses with comparable IC50 values. Mechanistic studies revealed that both 8C1 and 17C8 blocked the very last step of viral entry, pH-dependent membrane fusion. When tested in a cell-based arenavirus GP fusion assay, these compounds efficiently blocked membrane fusion mediated by the GPs of LASV and JUNV with an IC50 between 200-350 nM. The combinatorial nature of the chemical libraries allowed the identification of clusters of structurally related compounds with differential anti-viral activity, providing the basis for a first assessment of structure-activity-relationship (SAR). Subsequent examination of selected compounds in the same series as 17C8 and the related 16G8 (Fig. 2) identified several additional candidates that exhibited potent activity against LASV GP-mediated infection (Whitby et al., 2009). These compounds showed that the benzofuran and the X-benzyl moieties (X being either methyl- or methoxy-) impart the activity of the compound against the LASV GP, as substitution at the third position around the central ring of various different-sized substituents, including methyl, benzyl, and phenethyl moieties, had no effect on the potency of the compound. Interestingly, after purification of the (R)- and (S)-isomers of 16G8, activity against the LASV pseudotypes was fully retained with (S)-16G8, whereas (R)-16G8 showed 15-fold lower activity. This indicates that the stereochemistry of the molecule is important for its function against arenavirus GP-mediated fusion. A similar effect was also observed with 17C8, with (S)-17C8 being significantly more potent than (R)-17C8 against LASV pseudotypes (Lee et. al., unpublished results). The requirement for one stereoisomer over another suggests that our compounds bind to a specific structure on the GP to exert their effects, and efforts to identify where the compounds bind the GP and block fusion are currently underway.
Another screen for LASV entry inhibitors used a chemically diverse random library of 400,000 small molecule compounds and a lentivirus pseudotype platform (Larson et al., 2008). Applying a more stringent cut-off (>75% inhibition at 5 μM), HTS resulted in a hit rate of 1.2%. However, in contrast to the combinatorial library screen, where counter-screens reduced the number of hits by less than 50% (Lee et al., 2008), 90-95% of the hits in the random library screen were found to be not specific for LASV pseudotypes and/or cytotoxic (Larson et al., 2008). The most promising hit, a unique benzimidazole derivative, ST-37 (Fig. 2), exhibited an IC50 of 16 nM against LASV pseudotypes. Subsequent SAR and lead optimization yielded the compound ST-193 (Fig. 2) that inhibited pseudotypes of LASV, JUNV, MACV, GTOV, and SABV with IC50 of 2-12 nM (Larson et al., 2008). The candidate ST-193 was also potent against live viruses, supporting the use of pseudotype platforms for this type of screens. Mechanistic studies revealed that ST-193 and the formerly identified New World arenavirus entry inhibitor ST-294 inhibit GP-mediated membrane fusion (Larson et al., 2008; York et al., 2008). Interestingly, despite potent inhibition of cell entry of hemorrhagic arenaviruses by 17C8 and ST-193, both compounds seem to be far less active against LCMV, whose GP is structurally closely related to LASV (Larson et al., 2008); Lee et al., unpublished results). The resistance of LCMV GP towards ST-193 mapped to the C-terminal portion of GP2 and involves two residues in the transmembrane domain that show divergence between LCMV on the one hand and the hemorrhagic arenaviruses, including LASV, on the other hand (Larson et al., 2008). In sum, two independent small molecule screens using pseudotype platforms in combination with different library formats identified a set of broadly active potent inhibitors of arenavirus fusion. These studies provide proof-of-principle for this type of approach and establish arenavirus membrane fusion as a robust drug target to combat a broad range of arenaviruses. A notable strength of HTS of small molecule libraries is that no information on the structure of the viral envelope protein, receptor use, and pathway of endocytosis is needed. This approach holds therefore a great potential for the rapid discovery of cell entry inhibitors for newly emerging enveloped viruses that allow pseudotyping.
In addition to serving as promising leads for drug development against human pathogenic arenaviruses, the prototypic fusion inhibitors identified by these recent studies can also be used as molecular probes to dissect the mechanism of arenavirus membrane fusion. This is of particular interest in the context of arenaviruses as studies over the past years revealed unusual and unique features of arenavirus fusion that are not shared by other enveloped viruses. The GPs of arenaviruses show overall similarity to fusion-active class I envelope glycoproteins of other enveloped viruses (Eschli et al., 2006), but is unusual in that it contains a stable signal peptide (SSP) of remarkable length that is retained as an essential component of the fusion-active mature GP complex with the composition SSP/GP1/GP2 (Eichler et al., 2003; York et al., 2004). The arenavirus SSP contains 58 amino acids with two putative transmembrane domains and both the N- and the C-terminus in the cytosol and plays an important role in transport, maturation, and fusion-activity of the GP (Agnihothram, York, and Nunberg, 2006; Agnihothram et al., 2007; Saunders et al., 2007; York and Nunberg, 2006; York and Nunberg, 2007). In particular amino acid substitutions of the conserved residue K33 in the short ectodomain loop of SSP modulate the fusion pH of arenavirus GP, indicating an interaction between SSP and the fusion machinery of GP2. The New World arenavirus entry inhibitor ST-294 has been used in mechanistic studies to illuminate molecular details of JUNV fusion (York et al., 2008). Evidence was provided that ST-294 acts as a fusion inhibitor, targeting the interaction of the fusion-active GP2 subunit with SSP, and that amino acid substitutions at the residue K33 of SSP confer resistance to ST-294. Both ST-294 and the broadly active arenavirus fusion inhibitor ST-193 inhibit dissociation of GP1 from the SSP/GP1/GP2 complex induced by low pH, which occur concomitant with conformational changes in the fusion-active GP2 part (York et al., 2008).
The proteolytic processing of arenavirus glycoprotein precursor as a novel target for anti-viral therapy
A crucial step in the life cycle of arenaviruses is the proteolytic processing of the arenavirus GP precursor (GPC) by the cellular protease SKI-1/S1P, yielding fusion-active, mature GP. Processing of arenavirus GPC by SKI-1/S1P is essential for productive infection and viral spread (Beyer et al., 2003; Kunz et al., 2003; Lenz et al., 2001; Rojek et al., 2008a). In the host cell, SKI-1/S1P is involved in proteolytic processing of a defined set of cellular proteins, including the sterol regulatory element-binding proteins (SREBP-1 and SREBP-2), involved in lipid metabolism (Brown and Goldstein, 1997; Sakai et al., 1998), and the activating transcription factor 6 (ATF6), involved in the regulation of the cellular unfolded protein response (Schroder and Kaufman, 2005; Ye et al., 2000). Interestingly, apart from the arenaviruses, SKI-1/S1P is also implicated in the proteolytic processing of the envelope GPs of the highly pathogenic Bunyavirus Crimean Congo HF virus (Vincent et al., 2003).
Efforts to develop specific inhibitors of SKI-1/S1P resulted in the design of recombinant serpins able to block SKI-1/S1P activity (Pullikotil et al., 2004). Expression of SKI-1/S1P-adapted α1-antitrypsin variants efficiently inhibited the processing of LASV GPC and had a pronounced impact on production of infectious LASV from infected cells (Maisa et al., 2009). Another study evaluated a cell-permeable, peptide-based inhibitor that combines a reactive chloromethylketone (CMK) moiety with peptides derived from the LASV GPC recognition motif of SKI-1/S1P, decanoyl (dec)-RRLL-CMK (Pasquato et al., 2006; Rojek et al., 2010). Dec-RRLL-CMK functions as a fast-acting suicide inhibitor of SKI-1/S1P (Pasquato et al., 2006) and showed significant anti-viral effects against the prototypic LCMV (Rojek et al., 2010). Off-target effects unrelated to SKI-1/S1P-mediated GPC processing were excluded employing a recombinant LCMV variant bearing a canonical furin recognition site (RRRR) in its GPC. Combination of the protease inhibitor with Rib resulted in additive drug effects. Remarkably, in cells deficient in SKI-1/S1P, the furin-dependent LCMV variant established persistent infection, whereas wild-type LCMV underwent extinction without emergence of SKI-1/S1P -independent escape variants. Together, these studies strongly suggested that inhibitors of SKI-1/S1P protease represent promising and powerful anti-arenaviral drug candidates.
The SKI-1/S1P-adapted serpins and the peptide inhibitor dec-RRLL-CMK used in these proof-of-principle studies both have limitations in their potential use as an anti-viral in vivo. Recently small molecule HTS performed by Pfizer Inc. identified a novel SKI-1/S1P inhibitor, the amino-pyrrolidine amide compound PF-429242 (Hawkins et al., 2008; Hay et al., 2007). PF-429242 blocked SKI-1/S1P-mediated processing of the transcription factor SREBP in cell culture and treatment of mice with PF-429242 reduced expression levels of hepatic SREBP target genes and lowered rates of cholesterol and fatty acid synthesis (Hawkins et al., 2008). PF-429242 showed high stability, low toxicity and pharmacokinetic properties that made it an interesting drug candidate in the context of arenavirus infection. Indeed, PF-429242 efficiently prevented processing of GPC of LCMV and LASV, which correlated with the compound's potent anti-viral activity against LCMV and LASV in cultured cells (Urata et al., 2010). In contrast, a recombinant LCMV expressing a GPC whose processing is mediated by furin was highly resistant to PF-429242 treatment. Mechanistically, PF-429242 did not affect virus RNA replication or budding, and had an only modest effect on virus cell entry, indicating that the anti-arenaviral activity of PF-429242 was mostly related to its ability to inhibit SKI-1/S1P-mediated GPC processing. These findings support the feasibility of using the small molecule SKI-1/S1P inhibitor PF-429242 as a novel anti-arenaviral drug.
Since SKI-1/S1P plays an important role in a number of physiological processes, including lipid metabolism and ER stress, the development of inhibitors that specifically target processing of arenavirus GPC while having minimal effects on the processing SKI-1/S1P's cellular substrates has of course highest priority. Nevertheless, SKI-1/S1P inhibitors like PF-429242 are promising since hemorrhagic fevers caused by arenaviruses in humans are acute diseases with a relatively rapid course of infection (Geisbert and Jahrling, 2004; McCormick and Fisher-Hoch, 2002). Consequently, drug intervention to treat hemorrhagic arenaviruses would be restricted to short period of times. Dietary supplementation of cholesterol and other lipids to patients in intensive care appears feasible and would limit unwanted side-effects of PF-429242 on lipid metabolism. Moreover, cells deficient in SKI-1/S1P are still able mount a partial ER stress response (Ye et al., 2000), suggesting that short term inhibition of the SKI-1/S1P activity may not result in unacceptable levels of toxicity. Therefore, candidate drugs that lack absolute specificity and to some degree affect the processing of cellular targets of S1P may still represent important candidates to be evaluated as antiviral drugs.
Conclusions and Outlook
Considering the significance of arenaviruses as important public health problems, the development of novel and potent anti-arenaviral drugs that can be manufactured at low costs remains an urgent need. Small molecule HTS performed over the past years identified novel classes of anti-viral drug candidates and provided proof-of-concept for the feasibility of this strategy for anti-arenaviral drug discovery. The successful identification of potent and specific inhibitors for arenavirus cell entry illustrate that small molecule HTS approaches for anti-viral drug discovery require neither structural information on the virus, nor knowledge on cellular factors involved. This type of approach is therefore highly suitable for rapid drug development against newly emerging human pathogenic viruses, provided their envelope proteins are amenable for pseudotyping. In addition to viral entry, virtually every step of the arenavirus replication cycle could be targeted by this approach. The development of replicon systems for LASV (Hass et al., 2004), as well as a novel and powerful cell-based luciferase assay suitable for HTS of inhibitors of arenavirus budding (Capul and de la Torre, 2008) open the possibility to screen for compounds that act at additional steps in the viral life cycle. The availability of inhibitors against distinct steps of viral multiplication would open the possibility of combinatorial drug use, reducing the risk of emergence of drug-resistance.
The successful use of newly discovered arenavirus entry inhibitors to study mechanisms of arenavirus fusion (York et al., 2008) highlights the potential of small molecule screens to provide novel small molecule agonists and antagonists that can be used as molecular probes to dissect biomolecular interactions. In particular the use of reversible small molecule inhibitors with mid- to low nanomolar binding affinities and defined drug targets allows the specific perturbation of molecular interactions in a dynamic manner due to micro-reversibility of inhibition. Target-specific small molecule compounds identified in HTS may thus complement other existing techniques such as genomic knock-out, RNA interference, and perturbation by antibodies in studies of the molecular interactions underlying arenavirus infection.
Acknowledgments
This is manuscript number 21013 from the Department of Immunology & Microbial Science at The Scripps Research Institute. We would like to thank Dr. Michael B. A. Oldstone (Scripps Research Institute, La Jolla) for his generous support and Dr. Dale L. Boger and Dr. Juan Carlos de la Torre for helpful discussions. Andrew M. Lee was supported by US PHS grant AI55540 and a Ruth Kirschstein NRSA award (AI072994). Stefan Kunz was supported by a Swiss National Science Foundation grant Nr. 3100A0-120250/1 and the Marie Curie International Reintegration Grant Nr. 224780 of the European Community. Antonella Pasquato received support from research funds of the University of Lausanne to S. Kunz.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agnihothram SS, York J, Nunberg JH. Role of the stable signal peptide and cytoplasmic domain of G2 in regulating intracellular transport of the Junin virus envelope glycoprotein complex. J Virol. 2006;80(11):5189–98. doi: 10.1128/JVI.00208-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnihothram SS, York J, Trahey M, Nunberg JH. Bitopic membrane topology of the stable signal peptide in the tripartite Junin virus GP-C envelope glycoprotein complex. J Virol. 2007;81(8):4331–7. doi: 10.1128/JVI.02779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrei G, De Clercq E. Inhibitory effect of selected antiviral compounds on arenavirus replication in vitro. Antiviral Res. 1990;14(4-5):287–99. doi: 10.1016/0166-3542(90)90009-v. [DOI] [PubMed] [Google Scholar]
- Andrei G, De Clercq E. Molecular approaches for the treatment of hemorrhagic fever virus infections. Antiviral Res. 1993;22(1):45–75. doi: 10.1016/0166-3542(93)90085-w. [DOI] [PubMed] [Google Scholar]
- Baldick CJ, Wichroski MJ, Pendri A, Walsh AW, Fang J, Mazzucco CE, Pokornowski KA, Rose RE, Eggers BJ, Hsu M, Zhai W, Zhai G, Gerritz SW, Poss MA, Meanwell NA, Cockett MI, Tenney DJ. A novel small molecule inhibitor of hepatitis C virus entry. PLoS Path. 2010;6(9) doi: 10.1371/journal.ppat.1001086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton LL, Mets MB, Beauchamp CL. Lymphocytic choriomeningitis virus: emerging fetal teratogen. Am J Obstet Gynecol. 2002;187(6):1715–6. doi: 10.1067/mob.2002.126297. [DOI] [PubMed] [Google Scholar]
- Berg T, Cohen SB, Desharnais J, Sonderegger C, Maslyar DJ, Goldberg J, Boger DL, Vogt PK. Small-molecule antagonists of Myc/Max dimerization inhibit Myc-induced transformation of chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 2002;99(6):3830–5. doi: 10.1073/pnas.062036999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer WR, Popplau D, Garten W, von Laer D, Lenz O. Endoproteolytic processing of the lymphocytic choriomeningitis virus glycoprotein by the subtilase SKI-1/S1P. J Virol. 2003;77(5):2866–72. doi: 10.1128/JVI.77.5.2866-2872.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boger DL, Desharnais J, Capps K. Solution-phase combinatorial libraries: modulating cellular signaling by targeting protein-protein or protein-DNA interactions. Angew Chem Int Ed Engl. 2003;42(35):4138–76. doi: 10.1002/anie.200300574. [DOI] [PubMed] [Google Scholar]
- Boger DL, Ducray P, Chai W, Jiang W, Goldberg J. Higher order iminodiacetic acid libraries for probing protein-protein interactions. Bioorg Med Chem Lett. 1998;8(17):2339–44. doi: 10.1016/s0960-894x(98)00423-5. [DOI] [PubMed] [Google Scholar]
- Boger DL, Goldberg J, Satho S, Ambroise Y, Cohen SB, Vogt PK. Non-amide based combinatorial libraries derived from N-Boc-iminodiacetic acid: solution-pahse synthesis of a 150-membered piperazinone library with activity against LEF-1/beta-catenin mediated transcription. Helv Chim Acta. 2000;83:1825–1845. [Google Scholar]
- Boger DL, Goldberg J, Silletti S, Kessler T, Cheresh DA. Identification of a novel class of small-molecule antiangiogenic agents through the screening of combinatorial libraries which function by inhibiting the binding and localization of proteinase MMP2 to integrin alpha(V)beta(3) J Am Chem Soc. 2001;123(7):1280–8. doi: 10.1021/ja003579+. [DOI] [PubMed] [Google Scholar]
- Bolken TC, Laquerre S, Zhang Y, Bailey TR, Pevear DC, Kickner SS, Sperzel LE, Jones KF, Warren TK, Amanda Lund S, Kirkwood-Watts DL, King DS, Shurtleff AC, Guttieri MC, Deng Y, Bleam M, Hruby DE. Identification and characterization of potent small molecule inhibitor of hemorrhagic fever New World arenaviruses. Antiviral Res. 2006;69(2):86–97. doi: 10.1016/j.antiviral.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow P, Oldstone MB. Mechanism of lymphocytic choriomeningitis virus entry into cells. Virology. 1994;198(1):1–9. doi: 10.1006/viro.1994.1001. [DOI] [PubMed] [Google Scholar]
- Briese T, Paweska JT, McMullan LK, Hutchison SK, Street C, Palacios G, Khristova ML, Weyer J, Swanepoel R, Egholm M, Nichol ST, Lipkin WI. Genetic detection and characterization of Lujo virus, a new hemorrhagic fever-associated arenavirus from southern Africa. PLoS Pathog. 2009;5(5):e1000455. doi: 10.1371/journal.ppat.1000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89(3):331–40. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- Buchmeier MJ, de la Torre JC, Peters CJ. Arenaviridae: the viruses and their replication. In: Knipe DL, Howley PM, editors. Fields Virology. 4th. Lippincott-Raven; Philadelphia: 2007. pp. 1791–1828. [Google Scholar]
- Candurra NA, Maskin L, Damonte EB. Inhibition of arenavirus multiplication in vitro by phenotiazines. Antiviral Res. 1996;31(3):149–58. doi: 10.1016/0166-3542(96)06956-2. [DOI] [PubMed] [Google Scholar]
- Cao W, Henry MD, Borrow P, Yamada H, Elder JH, Ravkov EV, Nichol ST, Compans RW, Campbell KP, Oldstone MB. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science. 1998;282(5396):2079–81. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- Capul AA, de la Torre JC. A cell-based luciferase assay amenable to high-throughput screening of inhibitors of arenavirus budding. Virology. 2008;382(1):107–14. doi: 10.1016/j.virol.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MW, Giffin MJ, Muller R, Savage J, Lin YC, Hong S, Jin W, Whitby LR, Elder JH, Boger DL, Torbett BE. Identification of broad-based HIV-1 protease inhibitors from combinatorial libraries. Biochem J. 2010;429(3):527–32. doi: 10.1042/BJ20091645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordo SM, Candurra NA, Damonte EB. Myristic acid analogs are inhibitors of Junin virus replication. Microbes Infect. 1999;1(8):609–14. doi: 10.1016/s1286-4579(99)80060-4. [DOI] [PubMed] [Google Scholar]
- de la Torre JC. Molecular and cell biology of the prototypic arenavirus LCMV: implications for understanding and combating hemorrhagic fever arenaviruses. Ann N Y Acad Sci. 2009;1171 1:E57–64. doi: 10.1111/j.1749-6632.2009.05048.x. [DOI] [PubMed] [Google Scholar]
- Delgado S, Erickson BR, Agudo R, Blair PJ, Vallejo E, Albarino CG, Vargas J, Comer JA, Rollin PE, Ksiazek TG, Olson JG, Nichol ST. Chapare virus, a newly discovered arenavirus isolated from a fatal hemorrhagic fever case in Bolivia. PLoS Pathog. 2008;4(4):e1000047. doi: 10.1371/journal.ppat.1000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler R, Lenz O, Strecker T, Eickmann M, Klenk HD, Garten W. Identification of Lassa virus glycoprotein signal peptide as a trans-acting maturation factor. EMBO Rep. 2003;4(11):1084–8. doi: 10.1038/sj.embor.7400002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschli B, Quirin K, Wepf A, Weber J, Zinkernagel R, Hengartner H. Identification of an N-terminal trimeric coiled-coil core within arenavirus glycoprotein 2 permits assignment to class I viral fusion proteins. J Virol. 2006;80(12):5897–907. doi: 10.1128/JVI.00008-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Este JA, Telenti A. HIV entry inhibitors. Lancet. 2007;370(9581):81–8. doi: 10.1016/S0140-6736(07)61052-6. [DOI] [PubMed] [Google Scholar]
- Fischer SA, Graham MB, Kuehnert MJ, Kotton CN, Srinivasan A, Marty FM, Comer JA, Guarner J, Paddock CD, DeMeo DL, Shieh WJ, Erickson BR, Bandy U, DeMaria A, Jr, Davis JP, Delmonico FL, Pavlin B, Likos A, Vincent MJ, Sealy TK, Goldsmith CS, Jernigan DB, Rollin PE, Packard MM, Patel M, Rowland C, Helfand RF, Nichol ST, Fishman JA, Ksiazek T, Zaki SR. Transmission of lymphocytic choriomeningitis virus by organ transplantation. N Engl J Med. 2006;354(21):2235–49. doi: 10.1056/NEJMoa053240. [DOI] [PubMed] [Google Scholar]
- Fisher-Hoch SP, McCormick JB. Lassa fever vaccine. Expert Rev Vaccines. 2004;3(2):189–97. doi: 10.1586/14760584.3.2.189. [DOI] [PubMed] [Google Scholar]
- Freed EO. Viral late domains. J Virol. 2002;76(10):4679–87. doi: 10.1128/JVI.76.10.4679-4687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia CC, Candurra NA, Damonte EB. Antiviral and virucidal activities against arenaviruses of zinc-finger active compounds. Antivir Chem Chemother. 2000;11(3):231–7. doi: 10.1177/095632020001100306. [DOI] [PubMed] [Google Scholar]
- Garcia CC, Djavani M, Topisirovic I, Borden KL, Salvato MS, Damonte EB. Arenavirus Z protein as an antiviral target: virus inactivation and protein oligomerization by zinc finger-reactive compounds. J Gen Virol. 2006;87(Pt 5):1217–28. doi: 10.1099/vir.0.81667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia CC, Topisirovic I, Djavani M, Borden KL, Damonte EB, Salvato MS. An antiviral disulfide compound blocks interaction between arenavirus Z protein and cellular promyelocytic leukemia protein. Biochem Biophys Res Commun. 2010;393(4):625–30. doi: 10.1016/j.bbrc.2010.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisbert TW, Jahrling PB. Exotic emerging viral diseases: progress and challenges. Nat Med. 2004;10(12 Suppl):S110–21. doi: 10.1038/nm1142. [DOI] [PubMed] [Google Scholar]
- Gowen BB, Smee DF, Wong MH, Hall JO, Jung KH, Bailey KW, Stevens JR, Furuta Y, Morrey JD. Treatment of late stage disease in a model of arenaviral hemorrhagic fever: T-705 efficacy and reduced toxicity suggests an alternative to ribavirin. PLoS One. 2008;3(11):e3725. doi: 10.1371/journal.pone.0003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen BB, Wong MH, Jung KH, Sanders AB, Mendenhall M, Bailey KW, Furuta Y, Sidwell RW. In vitro and in vivo activities of T-705 against arenavirus and bunyavirus infections. Antimicrob Agents Chemother. 2007;51(9):3168–76. doi: 10.1128/AAC.00356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass M, Golnitz U, Muller S, Becker-Ziaja B, Gunther S. Replicon system for Lassa virus. J Virol. 2004;78(24):13793–803. doi: 10.1128/JVI.78.24.13793-13803.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins JL, Robbins MD, Warren LC, Xia D, Petras SF, Valentine JJ, Varghese AH, Wang IK, Subashi TA, Shelly LD, Hay BA, Landschulz KT, Geoghegan KF, Harwood HJ., Jr Pharmacologic inhibition of site 1 protease activity inhibits sterol regulatory element-binding protein processing and reduces lipogenic enzyme gene expression and lipid synthesis in cultured cells and experimental animals. J Pharmacol Exp Ther. 2008;326(3):801–8. doi: 10.1124/jpet.108.139626. [DOI] [PubMed] [Google Scholar]
- Hay BA, Abrams B, Zumbrunn AY, Valentine JJ, Warren LC, Petras SF, Shelly LD, Xia A, Varghese AH, Hawkins JL, Van Camp JA, Robbins MD, Landschulz K, Harwood HJ., Jr Aminopyrrolidineamide inhibitors of site-1 protease. Bioorg Med Chem Lett. 2007;17(16):4411–4. doi: 10.1016/j.bmcl.2007.06.031. [DOI] [PubMed] [Google Scholar]
- Kilgore PE, Peters CJ, Mills JN, Rollin PE, Armstrong L, Khan AS, Ksiazek TG. Prospects for the control of Bolivian hemorrhagic fever. Emerg Infect Dis. 1995;1(3):97–100. doi: 10.3201/eid0103.950308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz S, Edelmann KH, de la Torre JC, Gorney R, Oldstone MBA. Mechanisms for lymphocytic choriomeningitis virus glycoprotein cleavage, transport, and incorporation into virions. Virology. 2003;314:168–178. doi: 10.1016/s0042-6822(03)00421-5. [DOI] [PubMed] [Google Scholar]
- Larson RA, Dai D, Hosack VT, Tan Y, Bolken TC, Hruby DE, Amberg SM. Identification of a broad-spectrum arenavirus entry inhibitor. J Virol. 2008;82(21):10768–75. doi: 10.1128/JVI.00941-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AM, Rojek JM, Gundersen A, Stroher U, Juteau JM, Vaillant A, Kunz S. Inhibition of cellular entry of lymphocytic choriomeningitis virus by amphipathic DNA polymers. Virology. 2008;372(1):107–17. doi: 10.1016/j.virol.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz O, ter Meulen J, Klenk HD, Seidah NG, Garten W. The Lassa virus glycoprotein precursor GP-C is proteolytically processed by subtilase SKI-1/S1P. Proc Natl Acad Sci U S A. 2001;98(22):12701–5. doi: 10.1073/pnas.221447598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livnah O, Stura EA, Johnson DL, Middleton SA, Mulcahy LS, Wrighton NC, Dower WJ, Jolliffe LK, Wilson IA. Functional mimicry of a protein hormone by a peptide agonist: the EPO receptor complex at 2.8 A. Science. 1996;273(5274):464–71. doi: 10.1126/science.273.5274.464. [DOI] [PubMed] [Google Scholar]
- Maisa A, Stroher U, Klenk HD, Garten W, Strecker T. Inhibition of Lassa Virus Glycoprotein Cleavage and Multicycle Replication by Site 1 Protease-Adapted alpha(1)-Antitrypsin Variants. PLoS Negl Trop Dis. 2009;3(6):e446. doi: 10.1371/journal.pntd.0000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez MG, Cordo SM, Candurra NA. Characterization of Junin arenavirus cell entry. J Gen Virol. 2007;88(Pt 6):1776–84. doi: 10.1099/vir.0.82808-0. [DOI] [PubMed] [Google Scholar]
- McCormick JB, Fisher-Hoch SP. Lassa fever. Curr Top Microbiol Immunol. 2002;262:75–109. doi: 10.1007/978-3-642-56029-3_4. [DOI] [PubMed] [Google Scholar]
- McCormick JB, King IJ, Webb PA, Scribner CL, Craven RB, Johnson KM, Elliott LH, Belmont-Williams R. Lassa fever. Effective therapy with ribavirin. N Engl J Med. 1986;314(1):20–6. doi: 10.1056/NEJM198601023140104. [DOI] [PubMed] [Google Scholar]
- Palacios G, Druce J, Du L, Tran T, Birch C, Briese T, Conlan S, Quan PL, Hui J, Marshall J, Simons JF, Egholm M, Paddock CD, Shieh WJ, Goldsmith CS, Zaki SR, Catton M, Lipkin WI. A new arenavirus in a cluster of fatal transplant-associated diseases. N Engl J Med. 2008;358(10):991–8. doi: 10.1056/NEJMoa073785. [DOI] [PubMed] [Google Scholar]
- Parker WB. Metabolism and antiviral activity of ribavirin. Virus Res. 2005;107(2):165–71. doi: 10.1016/j.virusres.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Pasquato A, Pullikotil P, Asselin MC, Vacatello M, Paolillo L, Ghezzo F, Basso F, Di Bello C, Dettin M, Seidah NG. The proprotein convertase SKI-1/S1P. In vitro analysis of Lassa virus glycoprotein-derived substrates and ex vivo validation of irreversible peptide inhibitors. J Biol Chem. 2006;281(33):23471–81. doi: 10.1074/jbc.M513675200. [DOI] [PubMed] [Google Scholar]
- Perez M, Craven RC, de la Torre JC. The small RING finger protein Z drives arenavirus budding: implications for antiviral strategies. Proc Natl Acad Sci U S A. 2003;100(22):12978–83. doi: 10.1073/pnas.2133782100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinschewer DD, Perez M, Sanchez AB, de la Torre JC. Recombinant lymphocytic choriomeningitis virus expressing vesicular stomatitis virus glycoprotein. Proc Natl Acad Sci U S A. 2003;100(13):7895–900. doi: 10.1073/pnas.1332709100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullikotil P, Vincent M, Nichol ST, Seidah NG. Development of protein-based inhibitors of the proprotein of convertase SKI-1/S1P: processing of SREBP-2, ATF6, and a viral glycoprotein. J Biol Chem. 2004;279(17):17338–47. doi: 10.1074/jbc.M313764200. [DOI] [PubMed] [Google Scholar]
- Qian K, Morris-Natschke SL, Lee KH. HIV entry inhibitors and their potential in HIV therapy. Med Res Rev. 2009;29(2):369–93. doi: 10.1002/med.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirin K, Eschli B, Scheu I, Poort L, Kartenbeck J, Helenius A. Lymphocytic choriomeningitis virus uses a novel endocytic pathway for infectious entry via late endosomes. Virology. 2008;378(1):21–33. doi: 10.1016/j.virol.2008.04.046. [DOI] [PubMed] [Google Scholar]
- Radoshitzky SR, Abraham J, Spiropoulou CF, Kuhn JH, Nguyen D, Li W, Nagel J, Schmidt PJ, Nunberg JH, Andrews NC, Farzan M, Choe H. Transferrin receptor 1 is a cellular receptor for New World haemorrhagic fever arenaviruses. Nature. 2007;446(7131):92–6. doi: 10.1038/nature05539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojek JM, Lee AM, Nguyen N, Spiropoulou CF, Kunz S. Site 1 protease is required for proteolytic processing of the glycoproteins of the South American hemorrhagic fever viruses Junin, Machupo, and Guanarito. J Virol. 2008a;82(12):6045–51. doi: 10.1128/JVI.02392-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojek JM, Pasqual G, Sanchez AB, Nguyen NT, de la Torre JC, Kunz S. Targeting the proteolytic processing of the viral glycoprotein precursor is a promising novel antiviral strategy against arenaviruses. J Virol. 2010;84(1):573–84. doi: 10.1128/JVI.01697-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojek JM, Perez M, Kunz S. Cellular entry of lymphocytic choriomeningitis virus. J Virol. 2008;82(3):1505–17. doi: 10.1128/JVI.01331-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojek JM, Sanchez AB, Nguyen NT, de la Torre JC, Kunz S. Different mechanisms of cell entry by human-pathogenic Old World and New World arenaviruses. J Virol. 2008b;82(15):7677–87. doi: 10.1128/JVI.00560-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Jarabo CM, Ly C, Domingo E, de la Torre JC. Lethal mutagenesis of the prototypic arenavirus lymphocytic choriomeningitis virus (LCMV) Virology. 2003;308(1):37–47. doi: 10.1016/s0042-6822(02)00046-6. [DOI] [PubMed] [Google Scholar]
- Sakai J, Nohturfft A, Goldstein JL, Brown MS. Cleavage of sterol regulatory element-binding proteins (SREBPs) at site- 1 requires interaction with SREBP cleavage-activating protein. Evidence from in vivo competition studies. J Biol Chem. 1998;273(10):5785–93. doi: 10.1074/jbc.273.10.5785. [DOI] [PubMed] [Google Scholar]
- Saunders AA, Ting JP, Meisner J, Neuman BW, Perez M, de la Torre JC, Buchmeier MJ. Mapping the landscape of the lymphocytic choriomeningitis virus stable signal peptide reveals novel functional domains. J Virol. 2007;81(11):5649–57. doi: 10.1128/JVI.02759-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–89. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- Silletti S, Kessler T, Goldberg J, Boger DL, Cheresh DA. Disruption of matrix metalloproteinase 2 binding to integrin alpha vbeta 3 by an organic molecule inhibits angiogenesis and tumor growth in vivo. Proc Natl Acad Sci U S A. 2001;98(1):119–24. doi: 10.1073/pnas.011343298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecker T, Eichler R, Meulen J, Weissenhorn W, Dieter Klenk H, Garten W, Lenz O. Lassa virus Z protein is a matrix protein and sufficient for the release of virus-like particles [corrected] J Virol. 2003;77(19):10700–5. doi: 10.1128/JVI.77.19.10700-10705.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urata S, Yun N, Pasquato A, Paessler S, Kunz S, de la Torre JC. Antiviral Activity of a Small Molecule Inhibitor of Arenavirus Glycoprotein Processing by the Cellular Site 1 Protease. J Virol. 2010 doi: 10.1128/JVI.02019-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderlinden E, Goktas F, Cesur Z, Froeyen M, Reed ML, Russell CJ, Cesur N, Naesens L. Novel inhibitors of influenza virus fusion: structure-activity relationship and interaction with the viral hemagglutinin. J Virol. 2010;84(9):4277–88. doi: 10.1128/JVI.02325-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent MJ, Sanchez AJ, Erickson BR, Basak A, Chretien M, Seidah NG, Nichol ST. Crimean-Congo hemorrhagic fever virus glycoprotein proteolytic processing by subtilase SKI-1. J Virol. 2003;77(16):8640–9. doi: 10.1128/JVI.77.16.8640-8649.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachsman MB, Lopez EM, Ramirez JA, Galagovsky LR, Coto CE. Antiviral effect of brassinosteroids against herpes virus and arenaviruses. Antivir Chem Chemother. 2000;11(1):71–7. doi: 10.1177/095632020001100107. [DOI] [PubMed] [Google Scholar]
- Weissenbacher MC, Laguens RP, Coto CE. Argentine hemorrhagic fever. Curr Top Microbiol Immunol. 1987;134:79–116. doi: 10.1007/978-3-642-71726-0_4. [DOI] [PubMed] [Google Scholar]
- Wells JA. Hormone mimicry. Science. 1996;273(5274):449–50. doi: 10.1126/science.273.5274.449. [DOI] [PubMed] [Google Scholar]
- Wells JA, de Vos AM. Hematopoietic receptor complexes. Annu Rev Biochem. 1996;65:609–34. doi: 10.1146/annurev.bi.65.070196.003141. [DOI] [PubMed] [Google Scholar]
- Whitby LR, Lee AM, Kunz S, Oldstone MB, Boger DL. Characterization of lassa virus cell entry inhibitors: determination of the active enantiomer by asymmetric synthesis. Bioorg Med Chem Lett. 2009;19(14):3771–4. doi: 10.1016/j.bmcl.2009.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrighton NC, Farrell FX, Chang R, Kashyap AK, Barbone FP, Mulcahy LS, Johnson DL, Barrett RW, Jolliffe LK, Dower WJ. Small peptides as potent mimetics of the protein hormone erythropoietin. Science. 1996;273(5274):458–64. doi: 10.1126/science.273.5274.458. [DOI] [PubMed] [Google Scholar]
- Ye J, Rawson RB, Komuro R, Chen X, Dave UP, Prywes R, Brown MS, Goldstein JL. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell. 2000;6(6):1355–64. doi: 10.1016/s1097-2765(00)00133-7. [DOI] [PubMed] [Google Scholar]
- York J, Dai D, Amberg SM, Nunberg JH. pH-induced activation of arenavirus membrane fusion is antagonized by small-molecule inhibitors. J Virol. 2008;82(21):10932–9. doi: 10.1128/JVI.01140-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York J, Nunberg JH. Role of the stable signal peptide of Junin arenavirus envelope glycoprotein in pH-dependent membrane fusion. J Virol. 2006;80(15):7775–80. doi: 10.1128/JVI.00642-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York J, Nunberg JH. Distinct requirements for signal peptidase processing and function in the stable signal peptide subunit of the Junin virus envelope glycoprotein. Virology. 2007;359(1):72–81. doi: 10.1016/j.virol.2006.08.048. [DOI] [PubMed] [Google Scholar]
- York J, Romanowski V, Lu M, Nunberg JH. The signal peptide of the Junin arenavirus envelope glycoprotein is myristoylated and forms an essential subunit of the mature G1-G2 complex. J Virol. 2004;78(19):10783–92. doi: 10.1128/JVI.78.19.10783-10792.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


