Abstract
We investigated the color vision pattern in male and female Cebus apella monkeys by means of electroretinogram measurements and genetic analysis. Our objective was to establish a simple, fast and efficient protocol in order to determine the chromatic vision pattern in Cebus monkeys. We found five among ten possible different phenotypes, two trichromats and three dichromats. We also found that Cebus present a new allele with spectral peak near 552 nm, with the amino acid combination SFT at positions 180, 277 and 285 of the opsin gene, in addition to the previously described SYT, AFT and AFA alleles.
Keywords: New World monkeys, color vision, visual pigment, spectral tuning, primates
Introduction
The primates are the only mammals that present trichromatic vision, but not all primates are trichromatic. Humans and Old World monkeys have three different cone classes, short (S) wavelength sensitive cells with maxima near 415–430 nm, middle (M) with maxima at 530–537 nm and long (L) with maxima at 555–565 nm (Baylor et al., 1987; Jacobs, 1993). However, among the New World primates the cone phenotype is variable.
New World monkeys are divided into two families, Atelidae, with two subfamilies, and Cebidae, with four subfamilies. The family Atelidae is divided into the subfamilies Atelinae, of the howler monkey Alouatta and spider monkey Ateles, and the subfamily Pitheciinae, of titi monkey Callicebus. The family Cebidae is divided in the subfamilies Cebinae, of the capuchin monkey Cebus, Saimirinae, of the squirrel monkey Saimiri, Aotinea, of owl monkey Aotus, and Callitrichinae, of the marmoset Callithrix (Schneider et al., 1993). There are species as Aotus in which all individuals are monochromats (Jacobs et al., 1993). Other species are trichromats such as Alouatta (Jacobs et al., 1996), and in other species, such as Cebus and squirrel monkeys, the males and some females are dichromats, while other females are trichromats (Jacobs & Neitz., 1987b; Jacobs et al., 1987; Lee et al., 1996, 2000; Jacobs, 1998).
In contrast to humans and Old World monkeys that have two or more photopigment genes in the X-chromosome, most New World monkeys have only on cone pigment gene per X-chromosome. The trichromatic variation in females is based on the presence of allelic diversity at the X-chromosome opsin gene locus. Therefore, only heterozygous females have two genes that encode two different middle-to-long wavelength photopigments (Jacobs et al., 1993; Kainz, Neitz, & Neitz, 1998).
In Cebus and squirrel monkeys, three dichromatic and three trichromatic variants that arise from individual variations in cone-pigment complement have been described (Jacobs & Neitz, 1987b; Lee et al., 1996, 2000). Four cone classes were found in these species, with average spectral peak absorption of 434 (S cones), 535 (M cones), 550 (M/L cones), and 562 nm (L cones). All Cebus and squirrel monkeys contain S cones, but for the other three cone classes there are some individual variations. All males are dichromats and have any one of the three longer-wavelength cone types. Among the females there are both trichromatic and dichromatic individuals. The trichromatic females have any pair of the longer-wavelength cone types. For both dichromats and trichromats the three pigment alleles are approximately equally frequent in the population of squirrel monkeys (Jacobs & Neitz, 1987a, Jacobs & Deegan II, 2003).
In contrast to humans and Old World monkeys that have two or more photopigment genes in the X-chromosome, most New World monkeys have only one cone pigment gene per X-chromosome. The trichromatic variation in females is based on the presence of allelic diversity at the X-chromosome opsin gene locus. Therefore, only heterozygous females have two genes that encode two different middle-to-long wavelength photopigments (Jacobs et al., 1993; Kains et al., 1998).
The opsin genes from platyrrhines are very similar to those from of catarrhines primates. The amino acid sequences of the M and L pigments of humans, squirrel monkeys, and marmosets are 96% identical. Substitutions of amino acids at 3 positions (180, 277 and 285), expressed by the exons 3 and 5, are associated with shifts in the spectral peak of the pigment and the effects of these substitutions are cumulative. We can infer the photopigment phenotypes from the amino acid composition at those three sites (Neitz et al., 1991, Hunt et al., 1993, Asenjo et al., 1994). Three alleles have been described for Cebus and squirrel monkey with spectral peaks near 530–537, 545–551, and 560–564 nm. These alleles are sometimes referred to as P535, P550 and P562. The allele P535 has the combination of the amino acids Ala, Phe and Ala (AFA) in positions 180, 277 and 285, respectively. P550 has the combination Ala, Phe and Thr (AFT), and P562 has the combination Ser, Tyr and Thr (SYT) (Jacobs & Neitz, 1987b; Neitz et al. 1991; Jacobs, 1996; Shyue et al., 1998). This three-allelic set of M/L opsin has also been shown in more recent electrophysiological studies (Jacobs and Degan, 2003, Saito et al., 2005).
In this study we investigated the color vision pattern in male and female Cebus apella monkeys by means of electroretinograms (ERG) and genetic analysis. Our purpose was to establish a simple protocol, fast and efficient in order to determine the chromatic vision pattern in Cebus monkeys. The Cebus monkey is similar to the Old World monkey Macaca in many aspects such as retinal morphology (Silveira et al., 1989a,b, 1994a,b, 1998a,b, 1999; de Lima et al., 1993, 1996; Yamada et al., 1996; Andrade da Costa & Hokoç, 2000; dos Reis et al., 2002; Finlay et al., 2008) and physiology (Lee et al., 1996, 2000; Silveira et al., 1999), brain size, sulcal pattern and the relative position of homologous visual areas (Gattass et al., 1981, 1987, Rosa et al. 1988; Fiorani et al., 1989). The similarities between the Cebus and the Old World monkey brains associated with the multiplicity of color vision phenotypes makes the Cebus a valuable model in the study of various aspects of the vision, including color vision. The comparative study of color vision in the different primate species is important to understand the evolution of primate color vision. Previously to our own study, there were only few works that used electrophysiology techniques to describe the color vision phenotypes of the Cebus monkey (Jacobs & Neitz, 1987b; Lee et al., 1996, 2000). We found in the Cebus apella five different phenotypes, two trichromats and three dichromats. We also found in Cebus apella a new allele with its spectral peak near 552 nm with the amino acid combination SFT, in addition to the 3 alleles that had been described previously.
Materials and methods
Fourteen Cebus apella monkeys, 7 males and 7 females, weighing between 2.8 and 4.0 Kg, were used. All experimental protocols were conducted following the NIH guidelines for animal research and approved by the committee for animal care and use of the Instituto de Biofísica Carlos Chagas Filho, UFRJ. The DNA for the genetic analysis was extracted from blood samples of each animal (except for animal M4) using a purification kit (PUREGENE® DNA, Gentra System). The X-linked photopigment gene was amplified through the polimerase chain reaction (PCR) as described by Mancuso et al. (2006). The PCR products were directly sequenced with the Big Dye Terminator Sequencing kit (Applied Biosystems, Foster City, CA) and analyzed on an ABI Prism 3130xl.
For the ERG record the animals were anesthetized with intramuscular administration of 0.5 ml/Kg of a 1:4 mixture of 6% ketamine hydrochloride (Ketalar, ParkeDavis) and 2% dihydrotiazine hydrochloride (Rompum, Bayer). Atropine sulfate (0.15 mg/kg) and benzodiazepine (0.8 mg/kg) were injected to prevent tracheobronchic secretion and stress, respectively. The pupil was dilated with 1% tropicamide and 10% phenylephrine hydrochloride. The ocular fundus was examined to verify that the animals were free of previous ocular pathologies. The animals were positioned at 30 cm of distance in front of the monitor and the head was stabilized with the aid of cushion pads. The right eye was occluded and the left eye ERG were obtained using a gold electrode mounted in a contact lens positioned onto the cornea of the animal.
Stimuli were presented on a color monitor (Philips-Brilliance 202-P4), using an experiment control software (CORTEX 5, NIH). The luminance of the stimuli was obtained from calibration curves measured for each monitor phosphor using the ColorCal colorimeter (Cambridge Research Systems). These stimuli consisted of the following CIE 1976 u’v’ chromaticities: white (0.290, 0.335), blue (0.151, 0.074), green (0.276, 0.617), or red (0.618, 0.344) 30 X 30 cm squares presented at the center of the monitor. First, for each animal, we measured the response to luminance at each chromaticity, presenting squares at 8.57 Hz, at luminances from 2.5 cd/m2 to the maximum available for each color with the used monitor (76 cd/m2 for white, 51.91 cd/m2 for green, 20.14 cd/m2 for red and 9.11 cd/m2 for blue). Next, the animals were submitted to counter-phase flicker photometric procedures similar to that described by Hanazawa et al. (2001) to test the animals’ sensitivity to middle and long-wavelength light. Counter-phase flickers (30 Hz) of green and red stimuli were presented in four conditions with one color intensity fixed and another varying in luminance in several steps. In the first condition, the luminance of the green stimulus was fixed at 12 cd/m2, while the luminance of the red was varied between 0 and 27 cd/m2 in 13 equidistant steps on a logarithmic scale. In the second condition, the green luminance was fixed at 8 cd/m2, while the red was varied in the same way as above. In the third condition, the red luminance was fixed at 12 cd/m2, while the green was varied between 0 and 90 cd/m2 in 19 steps. In the last condition, the luminance of the red was fixed at 8 cd/m2, while the green was varied between 0 and 27 cd/m2 in 13 steps. For all conditions, the luminance of the stimuli varied in an increasing and in a decreasing way. The luminance of the monitor during the off-period was 0 cd/m2 and the ambient luminance was maintained at 5 cd/m2 throughout the experiment.
The ERG signal was amplified, sampled and recorded by the PowerLab data acquisition unit with Chart software (ADINSTRUMENTS, ), using a low (1 Hz) and a high (100 Hz) pass filter, and an adaptive mains filter to remove the common 60 Hz frequency of electrical noise. The data were analyzed using Matlab 6.1 routines.
For each condition, 200 cycles were averaged. For the color sensitivity curves, the amplitude between the first two components of the ERG wave (N35 and P50) was computed for all luminance and color combinations. The values obtained with the increasing and decreasing luminance sensitivity curves for each color were averaged to avoid adaptive influences.
The counter-phase flicker was analyzed using two different approaches. In the first approach we computed the amplitude (valley to peak) of the averaged ERG (over 200 cycles) for each Red/Green luminance (R/G) value combination. The R/G value combination giving the smallest amplitude was considered the isoeffective intensity point (reversal point). In the second, we performed a Fast Fourier Transformation (FFT) upon the raw (non averaged) ERG data for each pair of R/G values. The power and phase of the 30 Hz component were obtained for each R/G combination. The R/G values giving the smallest power amplitude were considered the isoeffective points. We also computed the isoeffective point pairing the fixed luminance stimulus value with the average of the two variable stimulus values that show a phase inversion (Pi radians) in the FFT.
The animals were classified based on a relative sensitivity index (RSI), RSI = Ln (Ired) − Ln (Igreen), where Ired is the luminance of the red stimulus and Igreen the luminance of the green stimulus at the reversal point of the curve. This index is positive for protanopes and negative for deuteranopes. The RSIs obtained in the increasing and decreasing variable luminance curves were averaged together. Therefore, the counter-phase flicker gives 3 measurements for the RSI referred from now on as amplitude, FFT power and FFT phase RSI. These three measurements were averaged to obtain the averaged RSI.
Results
The nucleotide sequences obtained by the analysis of the opsin gene segments corresponding to exons 3 and 5 of each animal are shown in Table 1. Table 2 is a summary with the amino acid combination at positions 180, 277 and 285 and its relation to the averaged RSI. We found five dichromatic animals (three males and two females) with the combination SYT (deuteranope), two dichromats (one male and one female) with the combination AFT (protanope M/L), two male dichromats with the combination AFA (protanope), three trichromats with the combinations SYT + SFT, and one trichromat with the combinations AFT + AFA.
Table 1.
Amino acids sequences of exons 3 and 5 of the opsin gene of Cebus monkeys.
| Animal | Amino acids Exon 3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 151 | 152 | 153 | 154 | 155 | 161 | 171 | 172 | 173 | 174 | 180 | 186 | 194 | |
| M1 | A | Trp | L | V | V | Asn | V | Gly | V | V | S | P | ACG |
| M2 | A | Trp | L | V | V | Asn | V | Gly | V | V | S | P | ACG |
| M3 | A | Trp | L | V | V | Asn | V | Gly | V | V | S | P | ACG |
| M5 | A | Trp | L | V | V | Asn | V | Gly | V | V | A | P | ACG |
| M6 | A | Trp | L | V | V | Asn | V | Gly | V | V | A | P | ACA |
| M7 | A | Trp | L | V | V | Asn | V | Gly | V | V | A | P | ACA |
| F1 | A | Trp | L | V | V | Asn | V | Gly | V | V | S | P | ACG |
| F2 | A | Trp | L | V | V | Asn | V | Gly | V | V | S | P | ACG |
| F3 | A | Trp | L | V | V | Asn | V | Gly | V | V | S | P | ACG |
| F4 | A | Trp | L | V | V | Asn | V | Gly | V | V | S | P | ACG |
| F5 | A | Trp | L | V | V | Asn | V | Gly | V | V | S | P | ACG |
| F6 | A | Trp | L | V | V | Asn | V | Gly | V | V | A | P | ACG |
| F7 | A | Trp | L | V | V | Asn | V | Gly | V | V | A | P | ACA/ACG |
| Animal | Amino acids Exon 5 | ||||||
|---|---|---|---|---|---|---|---|
| 271 | 275 | 276 | 277 | 279 | 285 | 298 | |
| M1 | V | M | A | Y | V | T | A |
| M2 | V | M | A | Y | V | T | A |
| M3 | V | M | A | Y | V | T | A |
| M5 | V | L | T | F | V | T | A |
| M6 | V | V | T | F | V | A | A |
| M7 | V | V | T | F | V | A | A |
| F1 | V | M | A | Y | V | T | A |
| F2 | V | M | A | Y | V | T | A |
| F3 | V | M / L | A / T | F/Y | V | T | A |
| F4 | V | M / V | A / T | F/Y | V | T | A |
| F5 | V | M / V | A / T | F/Y | V | T | A |
| F6 | V | L | T | F | V | T | A |
| F7 | V | M / L | T | F | V | T / A | A |
Table 2.
Amino acids detected at positions 180, 277 and 285 of the opsin genes and the RSI calculated for each monkeys.
| Animal | Amino acids | RSI | ||
|---|---|---|---|---|
| 180 | 277 | 285 | ||
| M1 | S | Y | T | −0.57 |
| F1 | S | Y | T | −0.52 |
| M2 | S | Y | T | −0.43 |
| M3 | S | Y | T | −0.47 |
| M4 | S | Y | T | −0.37 |
| F2 | S | Y | T | −0.28 |
| F3 | S | Y | T | −0.12 |
| S | F | T | ||
| F4 | S | Y | T | −0.07 |
| S | F | T | ||
| F5 | S | Y | T | 0.00 |
| S | F | T | ||
| F6 | A | F | T | 0.12 |
| M5 | A | F | T | 0.15 |
| F7 | A | F | T | 0.34 |
| A | F | A | ||
| M6 | A | F | A | 0.53 |
| M7 | A | F | A | 0.58 |
All these animals and one other animal (M4), from whom we did not obtain the genetic data due to a technical problem, were studied by means of ERG. Fig. 1 shows the response to luminance flicker for each color for three animals, one deuteranopic dichromat with the amino acid combination SYT (Fig. 1A), one trichromat SYT + SFT (Fig. 1B) and one protanopic dichromat AFA (Fig. 1C). All animals presented a stronger response to the blue stimulus than to the other chromaticities at the same intensity. This result is discussed later in Section 4. As we expected, the trichromatic animals that present both L and M cones showed similar responses to the red and green stimuli. The deuteranopic animal that do not present M cones showed a smaller response to the green stimulus, while the protanopic animal that do not present L cones showed a smaller response to the red stimulus. The white intensity curve tended to follow the green curve in all cases.
Fig. 1.
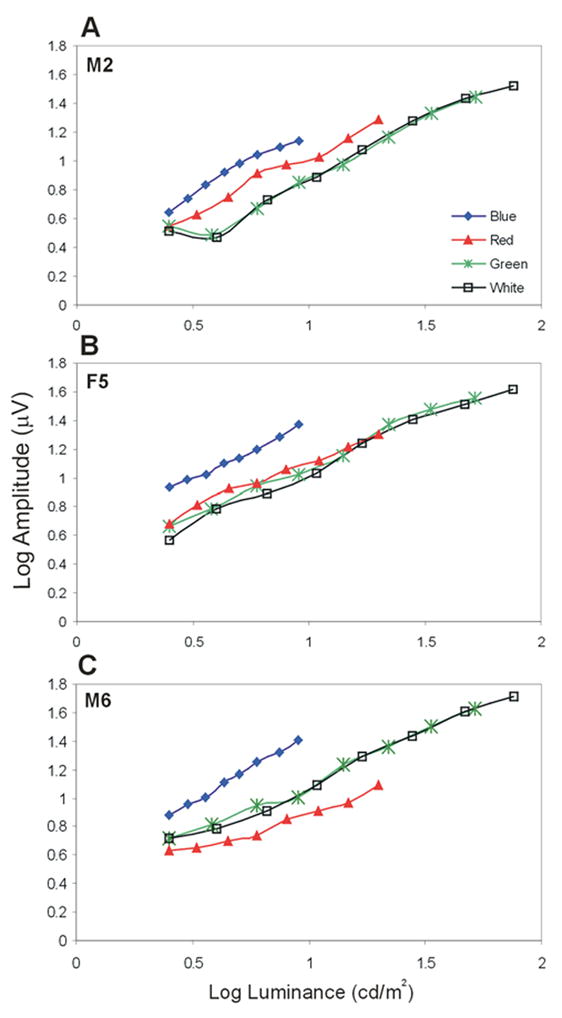
Luminance sensitivity curves for each color for 3 animals. (A) Case M2, deuteranopic dichromat with the amino acid combination SYT. (B) Case F5, trichromat SYT + SFT. (C) Case M6, protanopic dichromat AFA.
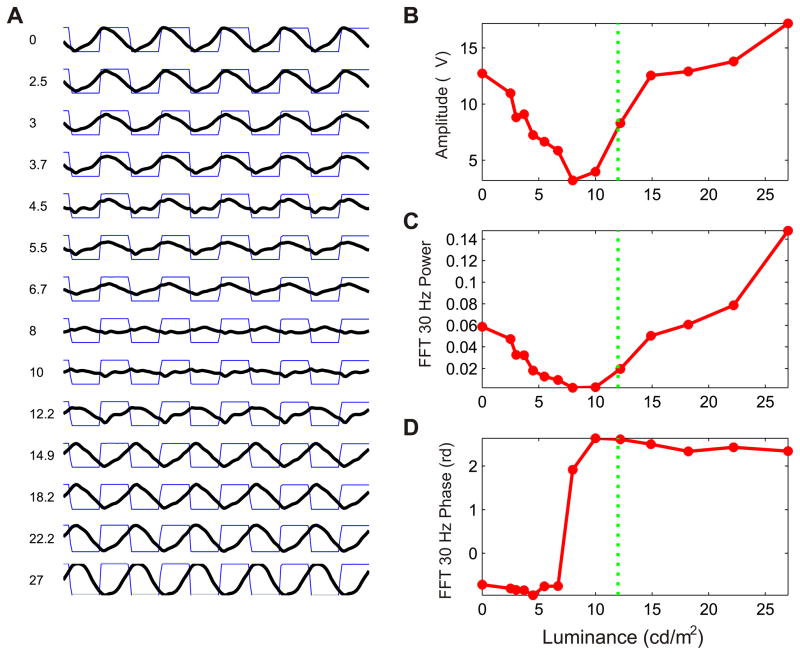
The results of the ERG recordings for the heterochromatic counter- phase flicker stimuli in the animal F2, one deuteranopic dichromat with the amino acid combination SYT, are illustrated in Fig. 2. The amplitude, FFT power and FFT phase curves on the right were constructed with the results for each condition illustrated on the left. In this case the green stimulus was fixed at 12 cd/m2 and the red stimuli varied from 0 to 27 cd/m2 in 14 steps (conditions). The strongest responses were observed in the extreme conditions where the luminance of the red stimuli was 0 or 27 cd/m2. Notice the flattening of the ERG signal at the condition of 8 cd/m2 and the increase followed by an inversion of the signal phase after that. The flattening of the signal corresponds to the point were the red and green stimuli have the same effective intensity (curve reversal point). As expected for an animal that lacks M cones, the reversal point occurred with a red stimulus luminance smaller than the green one. Fig. 3 shows the response curves for the four stimuli presentation conditions, with increasing and decreasing luminance- steps paradigms, in another deuteranopic animal. As in Fig. 2, in the conditions where the fixed stimulus was green (Fig. 3A–D), the reversal point occurred with a red stimulus luminance smaller than the green one. In the conditions where the fixed stimulus was red (Fig. 3E–H), in an opposite way, the reversal point occurred when the luminance of the green stimulus was higher than that of the red stimulus.
Fig. 2.
ERG recordings for counter-phase flicker stimuli in case F3. (A) ERG signals for the14 stimulation conditions. The numbers on the left are the luminance (cd/m2) of the red stimulus in each condition. The square waves represent the counter-phase flicker of green and red stimuli, and the continuous waves (thick lines) the ERG signal. In this case the green stimulus was fixed at 12 cd/m2 (vertical line in the curves on the right) and the red stimuli varied from 0 to 27 cd/m2 in 14 steps (conditions). Measurements obtained in the curves illustrated in A were used to construct the amplitude (B), FFT power (C) and FFT phase (D) curves.
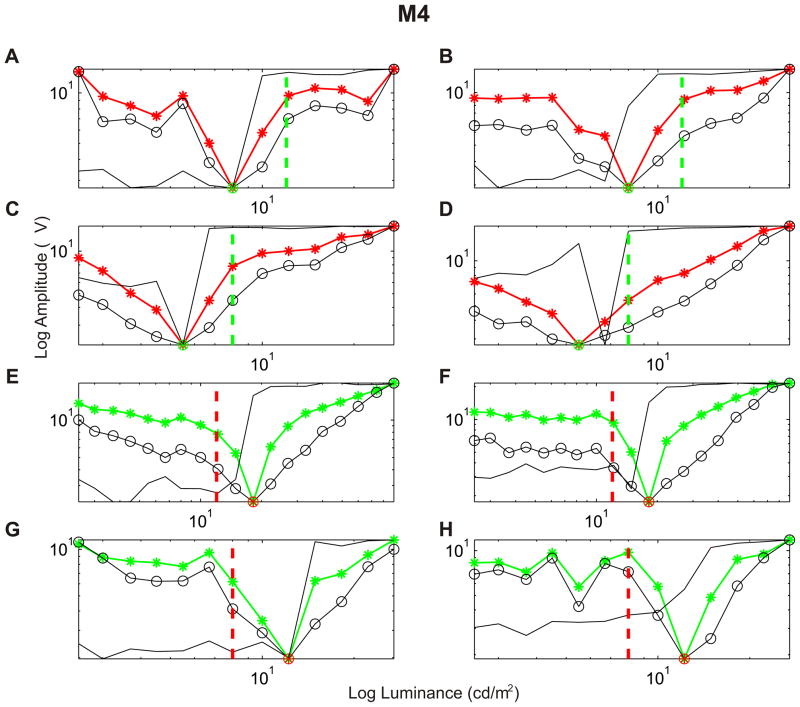
Fig. 3.
Response curves for the 4 stimuli presentation conditions in a deuteranopic animal, M4. For all conditions in each row, the luminance of the stimuli varied in an increasing (left) and decreasing (right) way. (A, B) The luminance of the green stimulus was fixed at 12 cd/m2 (vertical line), whereas the luminance of the red one was varied from 2.5 to 27 cd/m2 in 13 steps. (C, D) The luminance of the green stimulus was fixed at 8 cd/m2, whereas the luminance of the red stimulus was varied as in A. (E, F) The luminance of the red square was fixed at 12 cd/m2, whereas the luminance of the green square was varied from 2.5 to 90 cd/m2 in 19 steps. (G, H) The luminance of the red square was fixed at 8 cd/m2, whereas the luminance of the green square was varied from 2.5 to 27 cd/m2 in 13 steps. ( -*-) amplitude, (-○-) FFT power and (__) FFT phase curves.
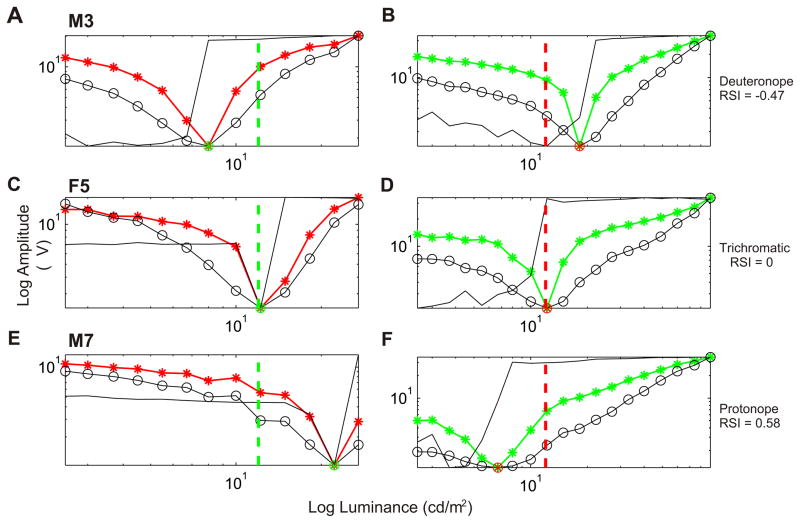
Fig. 4 illustrates the response curves of three different animals, a deuteranope with the amino acid combination SYT (M3, Fig. 4A and B), a trichromat (F5, Fig. 4C and D) and a protanope with the amino acid combination AFA (M7, Fig. 4E and F). The curves from M3 were similar to those of the two previous animals, shown in Fig. 3. In the trichromat animal the reversal point occurred when the luminances of both stimuli were equivalent. In the protanopic animal, higher red luminance as compared to green luminance is necessary to produce a reversion.
Fig. 4.
Response curves in two different conditions (right and left) for 3 animals. On the left, the luminance of the green square was fixed at 12 cd/m2, whereas the luminance of the red square was varied from 2.5 to 27 cd/m2 in 13 steps. On the right, the luminance of the red square was fixed at 12 cd/m2, whereas the luminance of the green square was varied from 2.5 to 90 cd/m2 in 19 steps. (A) Deuteranope, M3, (B) trichromat, F5, and (C) protanope, M7. For conventions see Fig. 3.
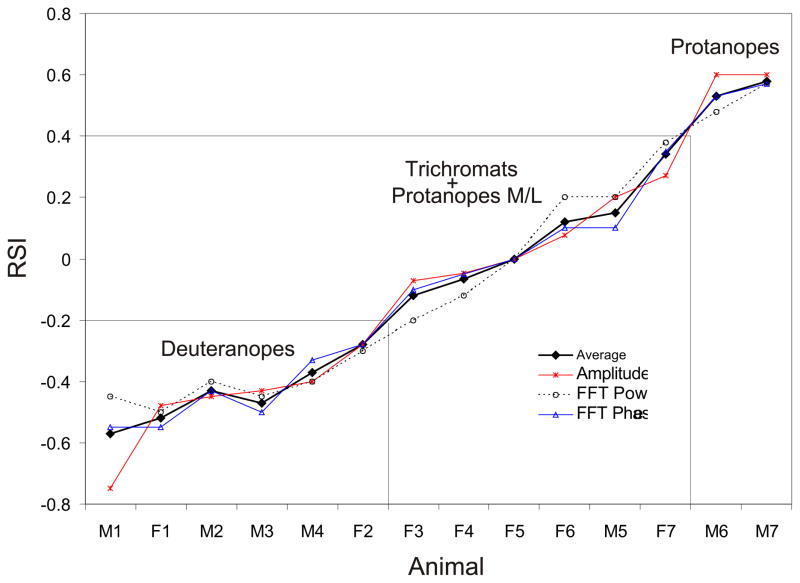
The relative sensitivity index (RSI) calculated for each animal is shown in Table 2. Based on the RSI and the amino acid combinations found for each monkey, the animals were divided in three groups (Figs. 5 and 6). The first group was classified as deuteranopic dichromats (SYT), with RSI < 0.2. In this group we found six animals, four males and two females. The second group was classified as protanopic dichromats (AFA) with RSI > 0.4. In this group two males were found. The third group, with RSI between 0.2 and 0.4, comprises the trichromat animals and the dichromats with intermediate sensitivity between green and red (M/L protanopes). We found in this group four females, three with the combinations SYT + SFT and one with AFT + AFA. The two dichromats found in this group, one male and one female, presented the amino acid combination AFT; however, dichromats with the combination SFT, not found in our sample, also would belong to this group.
Fig. 5.
Distribution of the animals based on the relative sensitivity index (RSI). The set of boxes segregate the animals in 3 groups. One group was classified as deuteranopic dichromats (SYT), with RSI < -0.2 (small box). Another group was classified as protanopic dichromats (AFA) with RSI > 0.4 (right). A third group (middle), with RSI between -0.3 and 0.4, comprises the trichromat animals and the dichromats with intermediate sensibility between green and red (protanope M/L).
Fig. 6.
Frequency histogram of the RSI for all female (dashed bar) and all male (solid bar) monkeys.
Discussion
We found in the Cebus monkey five different phenotypes, two of which are trichromat and three, dichromat. The dichromats present the amino acid combinations SYT (five animals), AFT (two animals) and AFA (two animals). The SFT combination was found only in trichromats, along with the combination SYT (three animals). In one female we found the combination AFT and AFA. Other amino acid combinations trichromats not encountered, but theoretically possible, are SYT + AFT, SYT + AFA, AFT + SFT and AFA + SFT. Table 3 shows the correlation of the predicted spectral peaks as proposed by Asenjo et al. (1994) with the alleles described.
Table 3.
Mean peak absorption spectra (λmax) predicted based on Asenjo et al. (1994) for the four alleles found in the Cebus monkeys.Values given in parentheses are the number of animals found in this study.
| λmax (nm) | Amino acid combination | |
|---|---|---|
| Dichromats | Trichromats | |
| 560–563 | SYT (6) | |
| SYT+SFT (3) | ||
| SYT+AFT | ||
| 546–553 | SFT | |
| SFT+AFT | ||
| 542–547 | AFT (2) | SYT+AFA |
| SFT+AFA | ||
| AFT+AFA (1) | ||
| 530–532 | AFA (2) | |
Jacobs and collaborators (Jacobs&Neitz, 1987b; Jacobs&Deegan, 2003) using spectral sensitivity measurements made with ERG flicker photometry show that Cebus monkeys have three possible cone pigments with maximum absorption in the middle to long wavelengths with average peak values (kmax) at about 537, 551, and 563 nm. Others studies (Lee et al., 2000; Saito et al., 2005; Shyue et al., 1998) demonstrated the correlation between genotypes and phenotypes showing that the genotype SYT corresponds to a kmax of about 560–563 nm, AFT corresponds to 545–551 nm and AFA to 530–537 nm. Animals with the SFT combination were not found in these previous genetic works, and in electrophysiology studies would be difficult to distinguish between SFT and AFT due to their similar kmax, 546–553 nm to SFT (predicted as proposed by Asenjo et al. (1994) and 545–551 nm to AFT (Saito et al., 2005; Shyue et al., 1998).
The amino acid composition SFT was described only once before in the spider monkey, Ateles geoffroyi (Hiramatsu et al., 2005) and was considered novel among all vertebrates examined to that date. As suggested by these authors, the P552 allele may have arisen by a combination of P545 and P560 resulting in the exchange of exon 3, or by a replacement of Tyr at site 277 by Phe in P560. Two alleles were found in the spider monkeys, one of which was the common type P560 (SYT), and the other, new one, was the allele SFT. These two alleles would form a sister group with the capuchin-marmoset clade in the phylogenetic tree, separately from human genes.
Previous works have been shown, through analyses of nucleotide differences in exons 3, 4 and 5 of genes from different genera of New World monkeys, that most have a triallelic arrangement (Boissinot et al., 1998; Hunt et al., 1998). This polymorphism might have been maintained by the selective advantage of heterozygous females. One exception to the triallelic rule was offered recently by Jacobs and Deegan (2005), who provided evidence for the presence of a total of five spectrally discrete pigments in the Callicebus moloch monkey. This finding is in line with our results showing four alleles in Cebus apella. It also suggests that the M/L opsin genes can present variation wider than presently known.
Cebus monkey has been chosen as a New World monkey model for studies of the visual cortical organization. As it is a diurnal monkey comparable in many aspects to the most studied Old World monkey macaque, it is an important link between more common platyrrhine models, such as marmoset and owl monkeys, and Old World monkey visual cortex (Fiorani et al., 1989; Gattass et al., 1981, 1987; Lee et al., 1996; Rosa, Soares, Fiorani, & Gattass, 1993). Lee et al. (2000) performed retinal ganglion cell single unit measurements in dichromatic and trichromatic Cebus monkeys and compared with macaque data. Results in trichromatic animals strongly resembled those from the macaque. Cells of parvocellular pathway showed characteristic frequency-dependent changes in responsivity to luminance and chromatic modulation, cells of the magnocellular pathway showed frequency-doubled responses to chromatic modulation, and their surrounds received a chromatic input revealed on changing the phase of heterochromatically modulated lights. Ganglion cells of dichromats, however, showed no trace of cone opponency except in those cells which received S-cone input. One purpose of this work was to develop an easy and fast protocol to distinguish among the different patterns of color vision found in the Cebus monkeys that would be used in subsequent studies concerned with color analysis in cortical areas. Here we adapted the method described by Hanazawa et al. (2001), by replacing the lights from the LEDs and diffusers used as stimuli to the light emitted by the phosphors of a CRT monitor, and creating a relative sensitivity index (RSI) to classify the animals.
The classical technique flicker photometry use monochromatic lights with fixed wavelength to stimulate the cones; however, with the development of image-processing technology we have observed a growing use of monitor systems for color vision research (Horwitz, Chichilnisky, & Albrigth, 2007; Mancuso et al., 2006; Miyahara et al., 2004; Smith & Pokorny, 1995). The color specification on a monitor is determined by combinations of the three phosphors. The trichromatic stimulus can be easily generated and modulated using a color CRT monitor. Brainard, Calderone, Nugent, and Jacobs (1999) studying responses to stimuli parametrically modulated in color space concluded that it is straightforward to record signals to color modulations presented on a CRT by using the flicker photometric ERG.
The traditional ERG flicker photometry adjust the intensity of a flickering test stimulus until it produces an ERG comparable with that produced by a similarly flickering reference light. Equations for a range of monochromatic test light that cover the spectrum permits the determination of full spectral sensitivity curves for the cones. This method is more informative, however it requires longer experiments than the ERG protocol developed in this work that measure the relative sensitivity to red and green lights and classify the animals based on a relative sensitivity index (RSI). Short experiments are important in our case in order to decrease the anesthetic time and to preserve the health of the monkeys that will be used in subsequent more extensive studies.
In this work we first measured the response to luminance at each chromaticity and constructed luminance response curves for each animal. All animals presented a stronger response to the blue stimulus than to the other chromaticities, at the same intensity. This large response could be due to the addition of the response of the rods that are strongly stimulated at lower frequencies with small wavelength stimuli. In humans, tested at mesopic light levels, rod signals preferentially enhance the percept of blue relative to yellow (Buck, Thomas, Connor, Green, & Quintana, 2008). The other color functions are probably not influenced by the rods inasmuch as the trichromatic animal showed similar responses to the white, red and green stimuli. As we expected, deuteranopes presented a smaller response to the green stimulus, while protanopes presented a smaller response to the red stimulus. Although these curves are quite informative, the classification of the animals can be done based only on the RSI, in the case of the males, and with the genetic analyze in the case of the some females. The RSI values used as reference to classify the animals, RSI smaller than 0.2 to deuteranopes and bigger than 0.4 to protanopes, are not rigid values. These values were chosen between the largest value found for a genetically confirmed deuteranope (SYT) and the smaller value found to a genetically confirmed trichromat, and between the largest value found to a genetically confirmed trichromat and the smaller value found to a genetically confirmed protanope (AFA). Based on our ERG measurements and comparisons with the genetic analysis, we found that the RSI consistently differs among the three types of dichromatic males, with M, M/L or L cones. Thus, in the case of the animal M4, we can say that it is a deuteranope and must have a SYT amino acid combination in spite of not having the genetic confirmation since its RSI is smaller than 0.2. However, among the females, we can easily distinguish only between deuteranopes that present L cones and protanopes that present M cones. The discrimination among dichromats presenting M/L cones and the various types of trichromats are more difficult due to the proximity of the RSI presented by these groups. In these cases the genetic analysis or more complex ERG flicker photometry with full spectral sensitivity measurements is necessary to confirm the classification of the animal.
Acknowledgments
The authors are grateful to Rafael Linden for his generous contribution with the ERG recording. We also thank Edil Saturato, Liliane Heringer, Paulo Coutinho, Gervásio Coutinho and Thereza Monteiro for technical support. We are also in debt to Elizabeth S. Machado for helping in the DNA extraction procedure. This research was supported by grants from the following institutions: CNPq, FAPERJ, PRONEX, FINEP, CAPES, FAPESP, Research to Prevent Blindness.
References
- Andrade-da-Costa BLS, Hokoc JN. Photoreceptor topography of the retina in the New World monkey Cebus apella. Vision Research. 2000;40:2395–2409. doi: 10.1016/s0042-6989(00)00104-8. [DOI] [PubMed] [Google Scholar]
- Asenjo AB, Rim J, Oprian DD. Molecular determinants of human red/ green color discrimination. Neuron. 1994;12:1131–1138. doi: 10.1016/0896-6273(94)90320-4. [DOI] [PubMed] [Google Scholar]
- Baylor DA, Nunn BJ, Schnapf JL. Spectral sensitivity of cones of the monkey Macaca fascicularis. Journal of Physiology. 1987;357:145–160. doi: 10.1113/jphysiol.1987.sp016691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissinot S, Tan Y, Shyue SK, Schneider H, Sampaio I, Neiswanger K, et al. Origins and antiquity of X-linked triallelic color vision systems in New World monkeys. Proceedings of the National Academy of Sciences USA. 1998;95:13749–13754. doi: 10.1073/pnas.95.23.13749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH, Calderone JB, Nugent AK, Jacobs GH. Flicker ERG response to stimuli parametrically modulated in color space. Investigative Ophthalmology & Visual Science. 1999;40:2840–2847. [PubMed] [Google Scholar]
- Buck SL, Thomas LP, Connor CR, Green KB, Quintana T. Time course of rod influences on hue perception. Visual Neuroscience. 2008;25:517–520. doi: 10.1017/S0952523808080279. [DOI] [PubMed] [Google Scholar]
- de Lima SMA, Silveira LCL, Perry VH. The M-ganglion cell density gradient in New-World monkeys. Brazilian Journal of Medical and Biological Research. 1993;26:961–964. [PubMed] [Google Scholar]
- de Lima SMA, Silveira LCL, Perry VH. Distribution of M retinal ganglion cells in diurnal and nocturnal New-World monkeys. Journal of Comparative Neurology. 1996;368:538–552. doi: 10.1002/(SICI)1096-9861(19960513)368:4<538::AID-CNE6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- dos Reis JWL, de Carvalho WA, Saito CA, Silveira LCL. The morphology of horizontal cells in the retina of the capuchin monkey, Cebus apella: How many horizontal cell classes are found in dichromatic primates? Journal of Comparative Neurology. 2002;443:105–123. [PubMed] [Google Scholar]
- Finlay BL, Franco ECS, Yamada ES, Crowley JC, Parsons MP, Muniz JAPC, et al. Number and topography of cones, rods and optic nerve axons in New and Old World primates. Visual Neuroscience. 2008;25:289–299. doi: 10.1017/S0952523808080371. [DOI] [PubMed] [Google Scholar]
- Fiorani M, Jr, Gattass R, Rosa MGP, Sousa APB. Visual area MT in the Cebus monkey: Location, visuotopic organization, and variability. Journal of Comparative Neurology. 1989;287:98–118. doi: 10.1002/cne.902870108. [DOI] [PubMed] [Google Scholar]
- Gattass R, Gross CG, Sandell JH. Visual topography of V2 in the macaque. Journal of Comparative Neurology. 1981;201:519–539. doi: 10.1002/cne.902010405. [DOI] [PubMed] [Google Scholar]
- Gattass R, Sousa APB, Rosa MGP. Visual topography of V1 in the Cebus monkey. Journal of Comparative Neurology. 1987;259:529–548. doi: 10.1002/cne.902590404. [DOI] [PubMed] [Google Scholar]
- Hanazawa A, Mikami A, Angelika PS, Takenaka O, Goto S, Onishii A, et al. Electroretinogram analysis of relative spectral sensitivity in genetically identified dichromatic macaques. PNAS. 2001;98:8124–8127. doi: 10.1073/pnas.141236598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu C, Tsutsui T, Matsumoto Y, Aureli L, Fedigan LM, Kawamura S. Color vision polymorphism in wild capuchins (Cebus capucinus) and spider monkeys (Ateles geoffroyi) in Costa Rica. American Journal of Primatology. 2005;67:447–461. doi: 10.1002/ajp.20199. [DOI] [PubMed] [Google Scholar]
- Horwitz GD, Chichilnisky EJ, Albrigth TD. Cone inputs to simple and complex cells in VI of awake macaque. Journal of Neurophysiology. 2007;97:3070–3081. doi: 10.1152/jn.00965.2006. [DOI] [PubMed] [Google Scholar]
- Hunt DM, Dulai KS, Cowing JA, Juillot C, Mollon JD, Bowmaker JK, et al. Molecular evolution of trichromacy in primates. Vision Research. 1998;38:3299–3306. doi: 10.1016/s0042-6989(97)00443-4. [DOI] [PubMed] [Google Scholar]
- Hunt DM, Williams AJ, Bowmaker JK, Mollons JD. Structure and evolution of the polymorphic photopigment gene of the marmoset. Vision Research. 1993;33:147–154. doi: 10.1016/0042-6989(93)90153-n. [DOI] [PubMed] [Google Scholar]
- Jacobs GH. The distribution and nature of colour vision among the mammals. Biological Reviews of Cambridge Philosophical Society. 1993;68:413–417. doi: 10.1111/j.1469-185x.1993.tb00738.x. [DOI] [PubMed] [Google Scholar]
- Jacobs GH. Primate photopigments and primate color vision. Proceedings of the National Academy of Sciences USA. 1996;93:577–581. doi: 10.1073/pnas.93.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs GH. A perspective on color vision in platyrrhine monkeys. Vision Research. 1998;38:3307–3313. doi: 10.1016/s0042-6989(97)00405-7. [DOI] [PubMed] [Google Scholar]
- Jacobs GH, Deegan JF., II Cone pigment variations in four genera of New World monkeys. Vision Research. 2003;43:227–236. doi: 10.1016/s0042-6989(02)00565-5. [DOI] [PubMed] [Google Scholar]
- Jacobs GH, Deegan JF., II Polymorphic New World monkeys with more than three M/L cone types. Journal of the Optical Society of America A: Optics, Image, Science and Vision. 2005;22:2072–2080. doi: 10.1364/josaa.22.002072. [DOI] [PubMed] [Google Scholar]
- Jacobs GH, Deegan JF, II, Neitz JA, Crognale MA, Neitz M. Photopigments and color vision in the nocturnal monkey, Aotus. Vision Research. 1993;33:1773–1783. doi: 10.1016/0042-6989(93)90168-v. [DOI] [PubMed] [Google Scholar]
- Jacobs GH, Neitz J. Inheritance of color vision in a New World monkey (Saimiri sciureus) Proceedings of the National Academy of Sciences USA. 1987a;84:2545–2549. doi: 10.1073/pnas.84.8.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs GH, Neitz J. Polymorphism of the middle wavelength cone in two species of South American monkey: Cebus apella and Callicebus molloch. Vision Research. 1987b;27:1263–1268. doi: 10.1016/0042-6989(87)90202-1. [DOI] [PubMed] [Google Scholar]
- Jacobs GH, Neitz J, Crognale M. Color vision polymorphism and its photopigment basis in a callitrichid monkey (Saguinus fuscicollis) Vision Research. 1987;27:2089–2100. doi: 10.1016/0042-6989(87)90123-4. [DOI] [PubMed] [Google Scholar]
- Jacobs GH, Neitz M, Deegan JF, II, Neitz J. Trichromatic colour vision in New World monkeys. Nature. 1996;382:156–158. doi: 10.1038/382156a0. [DOI] [PubMed] [Google Scholar]
- Kainz PM, Neitz J, Neitz M. Recent evolution of uniform trichromacy in a New World monkey. Vision Research. 1998;38:3315–3320. doi: 10.1016/s0042-6989(98)00078-9. [DOI] [PubMed] [Google Scholar]
- Lee BB, Silveira LCL, Yamada ES, Hunt DM, Kremers J, Martin PR, et al. Visual response of ganglion cells of a New-World primate, the capuchin monkey, Cebus apella. Journal of Physiology. 2000;528:573–590. doi: 10.1111/j.1469-7793.2000.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BB, Silveira LCL, Yamada ES, Kremers J. Parallel pathways in the retina of Old and New World primates. Revista Brasileira de Biologia. 1996;56(Suppl 1):323–338. [PubMed] [Google Scholar]
- Mancuso K, Neitz M, Neitz J. An adaptation of the Cambridge Colour Test for use with animals. Visual Neuroscience. 2006;23:695–701. doi: 10.1017/S0952523806233364. [DOI] [PubMed] [Google Scholar]
- Miyahara R, Pokorny J, Smith VC, Szewczyk E, McCartin J, Cadwell K, et al. Computerized color vision test based upon postreceptoral channel sensitivities. Vision Neuroscience. 2004;21:465–469. doi: 10.1017/s0952523804213177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neitz M, Neitz J, Jacobs GH. Spectral tuning of pigments underlying red–green color vision. Science. 1991;252:971–974. doi: 10.1126/science.1903559. [DOI] [PubMed] [Google Scholar]
- Rosa MGP, Soares JGM, Fiorani M, Gattass R. Cortical afferents of visual area MT in the Cebus monkey: Possible homologies between New and Old World monkeys. Visual Neuroscience. 1993;10:827–855. doi: 10.1017/s0952523800006064. [DOI] [PubMed] [Google Scholar]
- Rosa MGP, Sousa APB, Gattass R. Representation of the visual field in the second visual area in the Cebus monkey. Journal of Comparative Neurology. 1988;275:326–345. doi: 10.1002/cne.902750303. [DOI] [PubMed] [Google Scholar]
- Saito A, Kawamura S, Mikami A, Ueno Y, Hiramatsu C, Koida K, et al. Demonstration of a genotype–phenotype correlation in the polymorphic color vision of a non-callitrichine New World Monkey, capuchin (Cebus apella) American Journal of Primatology. 2005;67:471–485. doi: 10.1002/ajp.20201. [DOI] [PubMed] [Google Scholar]
- Schneider H, Schneider MPC, Sampaio I, Harada ML, Stanhope M, Czelusniak J, et al. Molecular phylogeny of New World monkeys (platyrrhini, primates) Molecular Phylogenetics and Evolution. 1993;2:225–242. doi: 10.1006/mpev.1993.1022. [DOI] [PubMed] [Google Scholar]
- Shyue SK, Boissinot S, Schneider H, Sampaio I, Schneider MP, Abee CR, et al. Molecular genetics of spectral tuning in New World monkey color vision. Journal of Molecular Evolution. 1998;46:697–702. doi: 10.1007/pl00006350. [DOI] [PubMed] [Google Scholar]
- Silveira LCL, Lee BB, Yamada ES, Kremers J, Hunt DM. Postreceptoral mechanisms of colour vision in New World primates. Vision Research. 1998;38:3329–3337. doi: 10.1016/s0042-6989(97)00335-0. [DOI] [PubMed] [Google Scholar]
- Silveira LCL, Lee BB, Yamada ES, Kremers J, Hunt DM, Martin PR, et al. Ganglion cells of a short wavelength sensitive cone pathway in New World monkeys: Morphology and physiology. Visual Neuroscience. 1999;16:333–343. doi: 10.1017/s0952523899162138. [DOI] [PubMed] [Google Scholar]
- Silveira LCL, Picanço-Diniz CW, Oswaldo-Cruz E. The distribution and size of ganglion cells in the retina of large Amazon rodents. Visual Neuroscience. 1989;2:221–235. doi: 10.1017/s0952523800001140. [DOI] [PubMed] [Google Scholar]
- Silveira LC, Picanço-Diniz CW, Sampaio LF, Oswaldo-Cruz E. Retinal ganglion cell distribution in the Cebus monkey: A comparison with cortical magnification factors. Vision Research. 1989;29:1471–1483. doi: 10.1016/0042-6989(89)90131-4. [DOI] [PubMed] [Google Scholar]
- Silveira LCL, Yamada ES, Perry VH, Picanço-Diniz CW. M and P retinal ganglion cells of diurnal and nocturnal New-World monkeys. NeuroReport. 1994;5:2077–2081. doi: 10.1097/00001756-199410270-00022. [DOI] [PubMed] [Google Scholar]
- Smith VC, Pokorny J. Chromatic-discrimination axes, CRT phosphor spectra, and individual variation in color vision. Journal of Optical Society of America A. 1995;12:27–35. doi: 10.1364/josaa.12.000027. [DOI] [PubMed] [Google Scholar]
- Yamada E, Silveira LCL, Gomes FL, Lee BB. The retinal ganglion cell classes of New World primates. Brazilian Journal of Biology. 1996;56:381–396. [PubMed] [Google Scholar]
- Yamada ES, Silveira LCL, Perry VH. Morphology, dendritic field size, somal size, density and coverage of M and P retinal ganglion cells of dichromatic Cebus monkeys. Visual Neuroscience. 1996;13:1011–1029. doi: 10.1017/s0952523800007677. [DOI] [PubMed] [Google Scholar]