INTRODUCTION
The field of tissue engineering aims to recapitulate healthy human organs and 3-D tissue structures in vitro and then transplant these constructs in vivo where they can be effectively integrated within the recipient patient and become perfused by the host circulation. To improve the design of materials for artificial tissue scaffolds, it would be ideal to have a high-throughput imaging system that allows one to directly monitor transplanted tissue constructs in live animals over an extended period of time. By combining such an assay with transgenic, cell-specific fluorescent reporters, one could monitor such parameters as tissue construct perfusion, donor cell survival, and donor-host cell interaction/integration. Here, we describe a protocol for a modified version of the classical corneal micropocket angiogenesis assay, employing it as a live imaging "window" to monitor angiogenic poly(ethylene glycol) (PEG)-based hydrogel tissue constructs.
RELATED INFORMATION
This protocol is a modified and extended version of a previously described method (Kenyon et al. 1996; Rogers et al. 2007) in which the mouse cornea is used as an in vivo test site for candidate angiogenic factors. We have customized this protocol substantially to include the use of transgenic fluorescent reporter mice as well as angiogenic factor-releasing PEG hydrogel tissue scaffolds. The PEG hydrogels in this protocol contain the Arg-Gly-Asp-Ser (RGDS) cell adhesion peptide (Gunn et al. 2005) labeled with Alexa Fluor 488, which allows live monitoring of the hydrogel within the cornea. We employ the Flk1-myr::mCherry mouse cornea as our transplantation site, thereby allowing us to monitor host blood vessel ingression within the hydrogel (Larina et al. 2009; Poché et al. 2009). The method described below is tailored specifically for the live imaging of vascularized hydrogel scaffolds, but it is important to note that this protocol can be adapted easily to a wide variety of angiogenic artificial tissues, as long as the size and dimensions of the construct are appropriate for the cornea.
MATERIALS
Reagents
 1-Vinyl-2-pyrrolidone (Sigma V3409)
1-Vinyl-2-pyrrolidone (Sigma V3409)2,2-Dimethoxy-2-phenylacetophenone (Sigma 196118)
 2,2,2-Tribromoethanol (Avertin; Fisher AC42143-0100)
2,2,2-Tribromoethanol (Avertin; Fisher AC42143-0100)Alexa Fluor 488 carboxylic acid, succinimidyl ester (Invitrogen A-20000)
Arg-Gly-Asp-Ser (RGDS; American Peptide)
Bacitracin zinc and polymyxin B sulfate ophthalmic ointment (Bausch & Lomb)
 DIPEA (N,N-diisopropylethylamine; Sigma 496219)
DIPEA (N,N-diisopropylethylamine; Sigma 496219) DMF (N,N-dimethylformamide; Sigma 648531)
DMF (N,N-dimethylformamide; Sigma 648531) DMSO (Dimethyl sulfoxide, anhydrous; Sigma D2650)
DMSO (Dimethyl sulfoxide, anhydrous; Sigma D2650)Ethanol (70%)
 Fluoromount-G (SouthernBiotech)
Fluoromount-G (SouthernBiotech)FluoSpheres polystyrene microspheres, 1.0-µm, yellow-green fluorescent (Molecular Probes/Invitrogen F-13081)
- Growth factors (see Step 15.ii):
- Fibroblast growth factor-2 (FGF-2; ProSpec Bio CYT-386)
- Platelet-derived growth factor-BB (PDGF-BB; ProSpec Bio CYT-412)
- Vascular endothelial growth factor (VEGF; Sigma V7259)
H2O (Milli-Q purified)
 HEPES-buffered saline for hydrogels (HBSH; sterile)
HEPES-buffered saline for hydrogels (HBSH; sterile)Mice (e.g., C57BL/6J, Flk1-myr::mCherry; see Discussion)
 Paraformaldehyde (4% [w/v]; Sigma)
Paraformaldehyde (4% [w/v]; Sigma) PEG polymer (e.g., PEG diacrylate or a degradable PEG-based polymer), synthesized and stored as lyophilized powder
PEG polymer (e.g., PEG diacrylate or a degradable PEG-based polymer), synthesized and stored as lyophilized powderThe PEG can be any desired molecular weight; this flexibility provides tunable mechanical properties.
PEG-succinimidyl carbonate (Acrylate-PEG-SCM, MW 3400; Laysan Bio)
 Proparacaine hydrochloride ophthalmic solution (0.5%; Bausch & Lomb)
Proparacaine hydrochloride ophthalmic solution (0.5%; Bausch & Lomb)Reagents for gel permeation chromatography (GPC) (optional; see Step 6)
 Sigmacote (Sigma)
Sigmacote (Sigma)Sodium bicarbonate (50 mM, pH 8.5)
Viscotears solution (Novartis)
Equipment
Binder clips, small (for making hydrogel discs)
Coverslips (VWR 48393-026)
Dialysis equipment (e.g., 5-L beaker, stir bar)
Dialysis membrane, regenerated cellulose, MWCO 3500 Da (Spectrum Laboratories 132592)
Equipment for GPC (optional; see Step 6)
Forceps, blunt (Roboz RS-5130)
Forceps, curved (Fine Science Tools 11370-31)
Forceps, Dumont #5 (Fine Science Tools 11252-20)
Heating pad (Snugglesafe)
Imaris software (Bitplane)
Knife, microsurgical (Surgistar 7536)
Knife, von Graefe (Miltex 18–140)
Laboratory tissue
Lyophilizer
Microcentrifuge
Micropipettors and tips
Microscope, confocal (e.g., LSM 5 LIVE high-speed confocal microscope with a Plan-Neofluar 5×/0.15 NA objective lens; Zeiss)
Microscope, fluorescent dissection
Molding clay
Rocker (for microcentrifuge tubes)
Scalpel, #10 (Sklar Instruments)
Scissors, Vannas (Fine Science Tools 15000-08)
Sharpening stone (Fine Science Tools 29008-01) (use if von Graefe knife is new; see Step 20)
Slides, plain glass (for making hydrogel discs; Fisher 12-550B)
Slides, treated glass (for mounting flattened corneas; VWR 48311-703)
Spacer, polytetrafluoroethylene (PTFE/Teflon), 0.005-in. thick (Small Parts STE-0005-C)
Stereomicroscope, illuminated (e.g., Stemi 2000; Zeiss)
Syringe, Hamilton, 0.5-µL with cone-style needle, 50 mm, 26-gauge, 0.47-mm OD (SGE Analytical Science 000303)
Syringe, insulin (BD 328418)
Syringe with 27-gauge needle
Tubes (microcentrifuge, sterile, 1.5- and 2-mL)
 Ultraviolet (UV) lamp (e.g., Blak-Ray Model B-100AP; Entela)
Ultraviolet (UV) lamp (e.g., Blak-Ray Model B-100AP; Entela)Vortex mixer
METHOD
Hydrogel Preparation
Synthesize the PEG-RGDS
-
1
Dissolve 20 mg of RGDS in 2 mL of DMSO and add one drop of DIPEA.
-
2
Add the dissolved RGDS to acrylate-PEG-SCM at a molar ratio of 1:1.1 RGDS:acrylate-PEG-SCM (i.e., 173 mg acrylate-PEG-SCM).
-
3
Vortex the reaction for 1 min.
-
4
Incubate the reaction with gentle rocking for 24 h at room temperature.
-
5
Dialyze the completed reaction in regenerated cellulose dialysis membrane (MWCO 3500 Da) against 4 L of H2O. Change the H2O several times.
-
6
Lyophilize the resulting PEG-RGDS and store under argon at −80°C until use in Step 8.
Conjugation efficiency can be analyzed using GPC.
Add the Fluorescent Label to the PEG-RGDS
For more details about the fluorescent labeling procedure, see Hahn et al. (2006).
-
7
Dissolve the dye (Alexa Fluor 488 carboxylic acid, succinimidyl ester) in DMF at 50 µg/mL.
-
8
Dissolve the PEG-RGDS from Step 6 in sodium bicarbonate (50 mM, pH 8.5) at 3 mg/mL.
-
9
Add the dissolved dye to the PEG-RGDS solution at a 10:1 molar ratio of dye:PEG-RGDS.
-
10
Rock the mixture for 2 h at room temperature.
-
11
Lyophilize the product and store under argon at −80°C until use in Step 14.
Prepare the Hydrogels
-
12
Prepare a 300 mg/mL stock solution of the photoinitiator 2,2-dimethoxy-2-phenylacetophenone (acetophenone) in 1-vinyl-2-pyrrolidone.
-
13
Dissolve the PEG polymer (e.g., poly[ethylene glycol] diacrylate) in sterile HBSH at 200 mg/mL. Add 20 µL of 300 mg/mL acetophenone stock solution per milliliter of PEG polymer solution.
-
14
Prepare a 2× solution of fluorescently labeled PEG-RGDS by dissolving lyophilized PEG-RGDS (from Step 11) in sterile HBSH at a final concentration of 7 µmol/mL.
-
15Prepare the polymer solution:
- Combine equal volumes of the solutions prepared in Steps 13 and 14 to generate a solution with a final gel composition of 10% polymer weight (100 mg/mL), 3.5 µmol/mL labeled PEG-RGDS, and 10 µL/mL acetophenone.
-
Add angiogenic (such as VEGF) or other growth factors at desired concentrations.These factors can be incorporated as releasable and/or covalently immobilized forms.
-
16Prepare the glass slides for making hydrogel discs:
- Soak the slides in Sigmacote.
- Rinse the slides in Milli-Q H2O to remove excess Sigmacote.
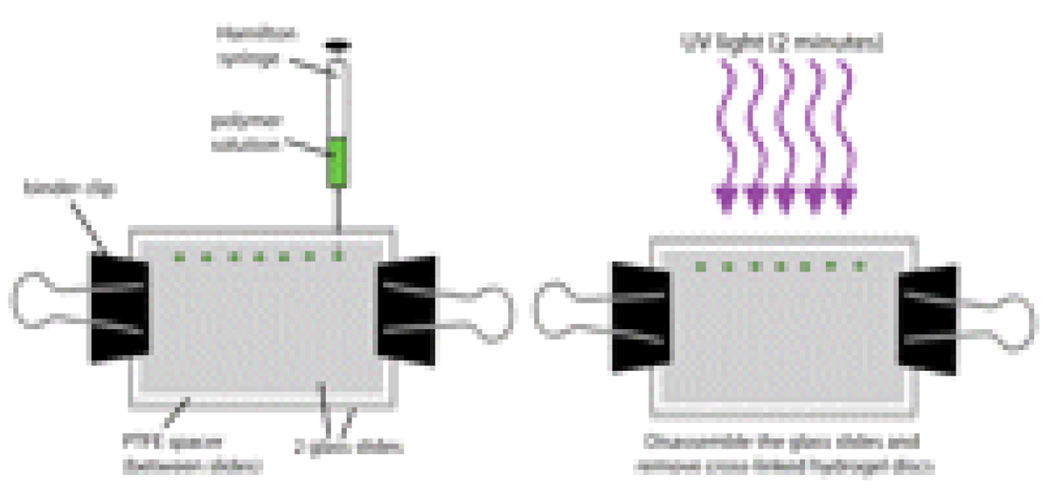
- Use binder clips to secure two glass slides separated by a 0.005-in. thick PTFE spacer.
-
17
Inject 0.12 µL of polymer solution prepared in Step 15 between the glass slides using a Hamilton syringe (see Fig. 1).
This droplet of solution will form a disc shape between the two slides.
-
18
Hold the glass slides ~5 cm from a B-100AP UV lamp (UVP, 365 nm, 10 mW/cm2) for 2 min (see Fig. 1).
The UV light will cause the liquid polymer between the glass slides to polymerize, thereby forming a hydrogel disc.
-
19
Immediately implant the gels into a cornea micropocket (see Hydrogel Implantation).
In our experience, the resulting hydrogel discs range from 500 to 700 µm in diameter and from 200 to 300 µm in height. The range in hydrogel dimensions reflects variations in swelling of the hydrogels after implantation. However, we have found that hydrogels within this size range efficiently remain in the cornea over the course of the experiment.
Figure 1.
Polymerization of hydrogel discs. See text for detailed procedure.
Hydrogel Implantation
Autoclave all surgical instruments before use. Because more than one mouse will be implanted with a hydrogel disc, clean all instruments thoroughly with 70% ethanol between each surgery.
-
20
If the von Graefe knife is new, use a sharpening stone to carefully create a more blunted tip on the knife while maintaining its sharpness.
-
21
Using a syringe with a 27-gauge needle, anesthetize one cage of mice with intraperitoneal injections of Avertin (0.4–0.75 mg/g body weight). Maintain the mice in a cage on a heating pad until ready for surgery.
-
22
Place one mouse under the stereomicroscope with illumination.
-
23
Place one drop of proparacaine hydrochloride ophthalmic solution on the mouse’s right eye and wait 30 sec.
The left eye serves as a control.
-
24
Wick the solution with a laboratory tissue and brush the eyelashes away from the eyeball.
-
25Create the micropocket:
-
Proptose the eye with blunt forceps (see Fig. 2A).Take care to contact the fur around the eye and not the eyeball directly.
-
Using the microsurgical knife, make a vertical incision (adjacent to the pupil) into the corneal stroma approximately halfway through the entire corneal thickness (see Fig. 2B).Be careful to ensure the incision is not so deep that the eye "pops" and vitreous leaks out.
- Hold the von Graefe knife perpendicular to the vertical incision and insert the tip of the knife into the incision.
- Push the knife toward the limbus and vertically in both directions so that an expanded pocket is created (see Fig. 2C).
-
-
26Insert the hydrogel disc into the micropocket:
- Using #5 forceps, gently pick up a freshly made hydrogel disc and place it on the surface of the cornea adjacent to the micropocket.
- Again, proptose the eye.
- Use the von Graefe knife to nudge the hydrogel disc into the micropocket (see Fig. 2D).
- After the disc is in place, use the von Graefe knife to seal the pocket opening by pressing down on the pocket lip where the original cut was made.
-
27
Apply bacitracin zinc and polymyxin B sulfate ointment to the eye and return the mouse to the heated cage for recovery.
-
28
Monitor the hydrogels within the corneas daily with a fluorescent dissection microscope (see Fig. 2E). If the hydrogel recipients are Flk1-myr::mCherry transgenic mice, monitor blood vessel ingression toward the angiogenic hydrogel daily (see Discussion).
See Troubleshooting.
Figure 2.
Modified corneal micropocket assay. (A–E) Shown here is an illustration detailing the steps involved in surgical implantation of fluorescently labeled hydrogel discs into the mouse cornea. By incorporating a fluorescent dye such as fluorescein isothiocyanate (FITC), the hydrogel is easily observed within the corneas of live mice (E, arrowhead). See text for details. Adapted by permission from MacMillan Publishers Ltd: [Nature Protocols] (Rogers et al. 2007), copyright 2007.
Live Imaging
Live imaging experiments will vary widely based on the particular study and microscope setup. Here we provide a specific example of a live imaging method that can be used to monitor hydrogel tissue construct perfusion by the host vasculature (see Movie 1).
-
29
Use a syringe with a 27-gauge needle to anesthetize one cage of mice by intraperitoneal injections of Avertin (0.4–0.75 mg/g body weight).
-
30
Using an insulin syringe, inject ~100 µL of 1-µm fluorescent microspheres (1.0 × 1010 beads/mL) into the tail vein.
-
31
Add one drop of 0.5% proparacaine to anesthetize the eye containing the hydrogel and wait 30 sec.
-
32
Prepare a glass coverslip through which to image the eye.
Press a ring of molding clay against the surface of the glass coverslip.
-
Fill the resulting well with Viscotears solution.
The clay ring prevents the eye from directly contacting the glass coverslip, and the Viscotears solution prevents drying of the eye.
-
33
Immediately place the animal on the stage of an inverted LSM 5 LIVE microscope equipped with an environmental chamber maintained at 37°C.
-
34
Position the eye over the glass coverslip (inside the Viscotears-filled well) parallel to the glass surface and secured to the stage.
-
35
Image the microsphere flow within the cornea, using the LSM 5 LIVE high-speed confocal microscope with a Plan-Neofluar 5×/0.15 NA objective lens (see Fig. 3).
-
36
To illustrate and quantify the movement of the microspheres, use Imaris software to track individual spheres in successive frames in an area of interest (see Fig. 3A, white box).
By doing so, one can verify circulation through induced vessels in live animals, quantify the velocity of circulating particles, and determine which vessels support continuous flow.
-
37
After completing the live imaging session, return the mouse to its heated cage for recovery and use in subsequent imaging sessions.
If the mouse is no longer needed for live imaging, it can be sacrificed and the cornea processed into flat mounted specimens (see "Corneal Flat Mounts for Immunofluorescence and Sample Archiving").
Movie 1.
High-speed confocal imaging of circulating fluorescent microspheres among an implanted corneal hydrogel. A hydrogel disc releasing PDGF-BB (320 ng) and FGF-2 (80 ng) was implanted into the cornea and fluorescent microspheres within the circulation were imaged after 11 d.
Figure 3.
High-speed confocal imaging of circulating fluorescent microspheres among an implanted corneal hydrogel. Images were acquired at 50 frames/sec using a Zeiss LSM 5 LIVE high-speed confocal microscope with a Zeiss Plan-Neofluar 5X/0.15 NA objective lens, resulting in a movie showing the circulation of microspheres through the newly induced vessels surrounding the hydrogel. (A) A single frame taken from the image series. Bright green microspheres are located within the induced vessels and surrounding the hydrogel in the cornea of a live animal. (B–D) Images from the Imaris software in which individual spheres (yellow and blue arrows) are tracked in successive frames in an area of interest (white box in A). The tracks shown represent the trajectories of 23 microspheres imaged over 15.62 sec (753 images) and are shown as colored lines. The time stamp shows that the elapsed time between each frame (B to D) is 100 msec. The velocities of fluorescent spheres that were tracked ranged from 0.65 to 1.15 mm/sec (Average = 980.58±147.89 µm/sec).
Corneal Flat Mounts for Immunofluorescence and Sample Archiving
For analyzing specific cell markers, the entire cornea can be easily dissected out of the eye, processed for immunofluorescence and the entire flattened tissue mounted under a coverslip. This gives one a global assessment of the entire tissue and a means to archive the sample.
-
38
Using curved forceps, enucleate the eye.
-
39
Fix the eye in 4% paraformaldehyde overnight at 4°C.
-
40
Using the Vannas scissors, poke a hole in the sclera of the eye just behind the limbus. From this starting point, carefully cut around the entire circumference of the eye until the anterior part of the eye (containing the cornea) is separated away from the posterior eye (containing the retina).
-
41
With two #5 forceps, gently pull the lens away from the anterior eyecup.
-
42
With the #5 forceps, carefully tease out the pigmented iris, leaving behind the cornea with a thin rim of pigmented sclera (including the limbus) attached.
-
43
On a glass slide, use a #10 scalpel to make four cuts into the cornea so that the cup is now flat.
-
44
Process the cornea for flat mount immunofluorescence or mount immediately (corneal surface face-up) with Fluoromount-G (see Fig. 4).
Figure 4.
Corneal flat mounts from implanted Flk1-myr::mCherry transgenic mice. (A) Schematic depiction of a corneal flat mount showing how the tissue is separated into quadrants, with one quadrant containing the implanted hydrogel and induced host vessels. (B) Blown-up view of the boxed region in panel A. (C,D) Tiled, confocal images of an actual corneal flat mount show a green fluorescently labeled hydrogel (containing VEGF) sitting adjacent to the limbic region (C) and Flk1-myr::mCherry+ (magenta) neovessels extending toward the hydrogel (D).
TROUBLESHOOTING
Problem: Despite testing previously published growth factor types and concentrations (as positive controls), blood vessel induction is not robust.
[Step 28]
Solution: Corneal angiogenic potential varies considerably between mice of different genetic backgrounds. The C57BL/6J strain has been shown to be suitable for the micropocket assay (Kenyon et al. 1996; Rohan et al. 2000). After consistent results are achieved using established angiogenic factors (e.g., VEGF) and a suitable inbred strain, one may proceed to other candidate angiogenic factors, tissue constructs, and mouse models.
Problem: Blood vessel induction is not consistent from mouse to mouse.
[Step 28]
- Solution: Consider the following:
- Inconsistencies in angiogenesis may result from variation in the placement of the tissue construct. The researcher must achieve consistent placement of the tissue construct at a specific distance from the limbus and clock hour location. This is particularly important for characterizing a new candidate angiogenic factor or artificial tissue type for which the optimal distance from the limbus will have to be determined empirically.
- If using commercially available growth factors, keep track of the lot number of every order. If possible, reserve a particular lot number to be more certain of consistent growth factor bioactivity.
DISCUSSION
Rationale for the Cornea as a Tissue Construct Transplantation Site
The cornea is a transparent tissue covering the outer surface of the eye which, together with the lens, functions to transmit and refract light. The mature cornea consists of an outer stratified epithelial cell layer, an inner collagenous stroma with a sparse population of keratocytes, and an inner endothelial cell monolayer (for descriptions of eye anatomy and histology, see Hogan et al. [1971]; Maurice [1984], and Zieske [2004]). For efficient transmittance of light, the cornea has evolved as an avascular tissue (Ambati et al. 2006; Beebe 2008). Nevertheless, the cornea is still capable of experiencing substantial neovascularization in response to severe physical, chemical, and thermal injury, implantation of tumors and other physiological insults (Langham 1953; Gimbrone et al. 1973, 1974; Muthukkaruppan and Auerbach 1979). The mouse corneal micropocket angiogenesis assay has capitalized upon this property and is often considered the gold standard to determine whether signaling peptides, drugs, etc. function as proangiogenic or anti-angiogenic factors in vivo (Kenyon et al. 1996; Rogers et al. 2007). This assay has several advantages. For example, because the cornea is normally avascular, there are no background vessels present. Thus, the researcher does not have to make any assumptions as to whether the observed changes in vascular structure are the result of remodeling of preexisting vessels or neovascularization (Kenyon et al. 1996; Rogers et al. 2007). Another major advantage of this system is that the cornea is a relatively immune privileged site (Streilein 2003a,b). Additionally, the cornea is not essential for viability and is externally located. These properties lend themselves to minimally invasive and high-throughput transplantation of experimental tissue constructs and also allow live imaging of construct vascularization in vivo.
Transgenic Reporter Mice
Given the characterization of a multitude of DNA regulatory sequences corresponding to many specific cell types as well as the ever-expanding repertoire of fluorescent proteins covering the entire color spectrum, we are in a position to specifically label several cell types within a given system and visually differentiate these cells from one another (Dickinson et al. 2001; Larina et al. 2009). Through the development of fluorescent fusion proteins such as those tethered to the cell membrane (e.g., the endothelial expressed Flk1-myr::mCherry) or the cell nucleus (e.g., the endothelial expressed Flk1-H2B::YFP) (Fraser et al. 2005; Larina et al. 2009; Nowotschin et al. 2009a,b; Poché et al. 2009), we are now achieving even greater spatial resolution of cellular relationships which can be monitored in vivo over many weeks or even months.
PEG Hydrogels in Tissue Engineering
Biomaterials provide a scaffold for delivery of growth factors and cell seeding, and both natural and synthetic materials have been used. Although natural materials, such as collagen and its gelatin derivatives, closely resemble the natural extracellular matrix and are commonly used in tissue engineering (Drury and Mooney 2003), synthetic polymers offer more flexibility in terms of tissue scaffold customization. Based on alteration of molecular weights, chemical structures, and cross-linking modes, synthetic scaffolds enable precise modification and reproduction of various parameters, such as mechanical properties, degradation rates, and drug delivery rates (Patel and Mikos 2004). Three commonly used synthetic polymers are poly(lactide-co-glycolide) (PLGA), polyurethane, and PEG, which all exhibit a large degree of versatility and biocompatibility (Patel and Mikos 2004). The synthetic, biocompatible polymer PEG diacrylate (PEGDA) resists protein absorption and subsequent nonspecific cell adhesion, thus providing a "blank slate" which can be modified with bioactive ligands to promote desired responses, such as cell adhesion, migration, proliferation, and possibly differentiation (DeLong et al. 2005). Additionally, PEGDA hydrogels are hydrophilic materials with mechanical properties that can be tuned to match that of soft tissue (West and Hubbell 1999; Hahn et al. 2006). The polymer can be photopolymerized via light by incorporating a photosensitive chemical into the polymer solution, and mild cross-linking conditions enable cellular encapsulation into the material while maintaining cell viability. Bioactivity is commonly incorporated into PEG materials by attaching the free amine or carboxyl group of a peptide, such as the cell adhesive peptide RGDS (Gunn et al. 2005), or protein (DeLong et al. 2005; Hahn et al. 2006; Leslie-Barbick et al. 2009) to a bifunctional PEG molecule, leaving the other end free to cross-link into the hydrogel. These hydrogels can also be rendered biodegradable by incorporating a matrix metalloproteinase-sensitive sequence into the polymer backbone (West and Hubbell 1999). Finally, as compared to other artificial scaffolds, PEGDA hydrogels are transparent, thereby allowing one to easily visualize encapsulated cells and ingression of host vasculature. Growth factor-releasing PEG-based hydrogels have been implanted into the cornea micropocket and have been shown to lead to the development of functional vessels contiguous with the circulation (Poché et al. 2009).
Advantages and Disadvantages of the Corneal Micropocket Assay
Due to its existence as a nonessential tissue, external localization, immune privilege, and transparency, the mouse cornea micropocket offers an accessible, high-throughput system in which to rapidly test a variety of tissue constructs in vivo. In our hands, we can implant 70 hydrogel constructs per day. This way, one can quickly screen many candidate tissue constructs and assess specific properties, such as host vessel ingression, perfusion, donor cell survival and integration, and scaffold degradation. Another obvious advantage of the cornea model is that the mouse has two eyes. Thus, one eye can be used as an internal control, thereby reducing the number of mice needed for characterization of test and control constructs. Finally, because the mouse eye is externally localized and the cornea is a transparent tissue, live imaging studies are easily performed. There are two main drawbacks of the cornea as a transplantation site. First, due to the size of the eye, there is an inherent limitation on the size of the implanted tissue construct. Also, the cornea is a highly specialized tissue consisting of a unique microenvironment, and one has to take this into consideration when making comparisons to other transplantation sites. It is important to note that cornea is not the only mouse transplantation site that is amenable to live imaging of tissue constructs. The cranial window (Yuan et al. 1994) and dorsal skin fold (Leunig et al. 1992) have been successfully employed in live imaging studies. Although these methods are better suited for the implantation of larger tissue constructs and constitute more "typical" cellular environments as compared to the highly specialized, avascular cornea, the surgical procedures are fairly laborious for the researcher and invasive to the animal. We propose a combined approach, taking advantage of the relative ease of the corneal micropocket surgery to analyze many candidate tissue construct designs first in the cornea. Once constructs with the desired properties are identified, larger versions of these constructs can then be generated and validated in other in vivo settings, such as the cranial window and dorsal skin fold.
ACKNOWLEDGMENTS
We thank Melissa Scott for assistance and members of the Dickinson lab for critical reading of the manuscript. R.A.P. acknowledges support from National Institutes of Health (NIH) F32 EY019436. J.E.S. acknowledges support from a National Science Foundation Graduate Research Fellowship (NSF GRF). J.L.W. acknowledges support from NIH P20 EB007076. M.E.D. acknowledges support from NIH P20 EB007076, R01 EB005173, and R01 HL077187.
Footnotes
 Caution
Caution
1-Vinyl-2-pyrrolidone
1-Vinyl-2-pyrrolidone is toxic and a carcinogen. It is harmful by inhalation, ingestion, or skin absorption. Wear appropriate gloves, safety glasses, and use a respirator.
 Caution
Caution
DIPEA (Diisopropylethylamine)
DIPEA (diisopropylethylamine) is extremely destructive to the mucous membranes, upper respiratory tract, skin, and eyes. It may be harmful by ingestion or skin absorption. Inhalation may be fatal. Wear appropriate gloves and safety glasses and always use in a chemical fume hood. Keep away from heat, sparks, and open flame.
 Caution
Caution
Poly(ethylene glycol) diacrylate
Poly(ethylene glycol) diacrylate is an irritant and may be harmful by inhalation, ingestion, or skin absorption. Wear appropriate gloves and safety goggles. Do not breathe the vapor, mist, or gas.
 Recipe
Recipe
HEPES-buffered saline for hydrogels (HBSH)
100 mM NaCl
10 mM HEPES (pH 7.4)
Copyright © 2010 by Cold Spring Harbor Laboratory Press. Terms of Service. All rights reserved. Anyone using the procedures outlined in these protocols does so at their own risk. Cold Spring Harbor Laboratory makes no representations or warranties with respect to the material set forth in these protocols and has no liability in connection with their use. All materials used in these protocols, but not limited to those highlighted with the Warning icon, may be considered hazardous and should be used with caution. For a full listing of cautions. For a full listing of cautions, click here.
All rights reserved. No part of these pages, either text or images, may be used for any reason other than personal use. Reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means-electronic, mechanical, or otherwise-for reasons other than personal use is strictly prohibited without prior written permission.
REFERENCES
- 1.Ambati BK, Nozaki M, Singh N, Takeda A, Jani PD, Suthar T, Albuquerque RJ, Richter E, Sakurai E, Newcomb MT, et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beebe DC. Maintaining transparency: A review of the developmental physiology and pathophysiology of two avascular tissues. Semin Cell Dev Biol. 2008;19:125–133. doi: 10.1016/j.semcdb.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeLong SA, Moon JJ, West JL. Covalently immobilized gradients of bFGF on hydrogel scaffolds for directed cell migration. Biomaterials. 2005;26:3227–3234. doi: 10.1016/j.biomaterials.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 4.Dickinson ME, Bearman G, Tille S, Lansford R, Fraser SE. Multi-spectral imaging and linear unmixing add a whole new dimension to laser scanning fluorescence microscopy. Biotechniques. 2001;31:1272–1278. doi: 10.2144/01316bt01. [DOI] [PubMed] [Google Scholar]
- 5.Drury JL, Mooney DJ. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 6.Fraser ST, Hadjantonakis AK, Sahr KE, Willey S, Kelly OG, Jones EA, Dickinson ME, Baron MH. Using a histone yellow fluorescent protein fusion for tagging and tracking endothelial cells in ES cells and mice. Genesis. 2005;42:162–171. doi: 10.1002/gene.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gimbrone MA, Jr, Leapman SB, Cotran RS, Folkman J. Tumor angiogenesis: Iris neovascularization at a distance from experimental intraocular tumors. J Natl Cancer Inst. 1973;50:219–228. doi: 10.1093/jnci/50.1.219. [DOI] [PubMed] [Google Scholar]
- 8.Gimbrone MA, Jr, Cotran RS, Leapman SB, Folkman J. Tumor growth and neovascularization: An experimental model using the rabbit cornea. J Natl Cancer Inst. 1974;52:413–427. doi: 10.1093/jnci/52.2.413. [DOI] [PubMed] [Google Scholar]
- 9.Gunn JW, Turner SD, Mann BK. Adhesive and mechanical properties of hydrogels influence neurite extension. J Biomed Mater Res A. 2005;72:91–97. doi: 10.1002/jbm.a.30203. [DOI] [PubMed] [Google Scholar]
- 10.Hahn MS, Taite LJ, Moon JJ, Rowland MC, Ruffino KA, West JL. Photolithographic patterning of polyethylene glycol hydrogels. Biomaterials. 2006;27:2519–2524. doi: 10.1016/j.biomaterials.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 11.Hogan MJ, Alvarado JA, Wedell JE. Histology of the human eye. Philadelphia, PA: Saunders; 1971. [Google Scholar]
- 12.Kenyon BM, Voest EE, Chen CC, Flynn E, Folkman J, D'Amato RJ. A model of angiogenesis in the mouse cornea. Invest Ophthalmol Vis Sci. 1996;37:1625–1632. [PubMed] [Google Scholar]
- 13.Langham M. Observations on the growth of blood vessels into the cornea; application of a new experimental technique. Br J Ophthalmol. 1953;37:210–222. doi: 10.1136/bjo.37.4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larina IV, Shen W, Kelly OG, Hadjantonakis AK, Baron MH, Dickinson ME. A membrane associated mCherry fluorescent reporter line for studying vascular remodeling and cardiac function during murine embryonic development. Anat Rec (Hoboken) 2009;292:333–341. doi: 10.1002/ar.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leslie-Barbick JE, Moon JJ, West JL. Covalently-immobilized vascular endothelial growth factor promotes endothelial cell tubulogenesis in poly(ethylene glycol) diacrylate hydrogels. J Biomater Sci Polym Ed. 2009;20:1763–1779. doi: 10.1163/156856208X386381. [DOI] [PubMed] [Google Scholar]
- 16.Leunig M, Yuan F, Menger MD, Boucher Y, Goetz AE, Messmer K, Jain RK. Angiogenesis, microvascular architecture, microhemodynamics, and interstitial fluid pressure during early growth of human adenocarcinoma LS174T in SCID mice. Cancer Res. 1992;52:6553–6560. [PubMed] [Google Scholar]
- 17.Maurice D. The cornea and sclera. In: Davson H, editor. The eye. Vol. 1B. New York: Academic Press; 1984. pp. 1–158. [Google Scholar]
- 18.Muthukkaruppan V, Auerbach R. Angiogenesis in the mouse cornea. Science. 1979;205:1416–1418. doi: 10.1126/science.472760. [DOI] [PubMed] [Google Scholar]
- 19.Nowotschin S, Eakin GS, Hadjantonakis AK. Dual transgene strategy for live visualization of chromatin and plasma membrane dynamics in murine embryonic stem cells and embryonic tissues. Genesis. 2009a;47:330–336. doi: 10.1002/dvg.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nowotschin S, Eakin GS, Hadjantonakis AK. Live-imaging fluorescent proteins in mouse embryos: Multi-dimensional, multi-spectral perspectives. Trends Biotechnol. 2009b;27:266–276. doi: 10.1016/j.tibtech.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel ZS, Mikos AG. Angiogenesis with biomaterial-based drug- and cell-delivery systems. J Biomater Sci Polym Ed. 2004;15:701–726. doi: 10.1163/156856204774196117. [DOI] [PubMed] [Google Scholar]
- 22.Poché RA, Larina IV, Scott ML, Saik JE, West JL, Dickinson ME. The Flk1-myr::mCherry mouse as a useful reporter to characterize multiple aspects of ocular blood vessel development and disease. Dev Dyn. 2009;238:2318–2326. doi: 10.1002/dvdy.21886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers MS, Birsner AE, D'Amato RJ. The mouse cornea micropocket angiogenesis assay. Nat Protoc. 2007;2:2545–2550. doi: 10.1038/nprot.2007.368. [DOI] [PubMed] [Google Scholar]
- 24.Rohan RM, Fernandez A, Udagawa T, Yuan J, D'Amato RJ. Genetic heterogeneity of angiogenesis in mice. FASEB J. 2000;14:871–876. doi: 10.1096/fasebj.14.7.871. [DOI] [PubMed] [Google Scholar]
- 25.Streilein JW. Ocular immune privilege: The eye takes a dim but practical view of immunity and inflammation. J Leukoc Biol. 2003a;74:179–185. doi: 10.1189/jlb.1102574. [DOI] [PubMed] [Google Scholar]
- 26.Streilein JW. Ocular immune privilege: Therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003b;3:879–889. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- 27.West JL, Hubbell JA. Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules. 1999;32:241–244. [Google Scholar]
- 28.Yuan F, Salehi HA, Boucher Y, Vasthare US, Tuma RF, Jain RK. Vascular permeability and microcirculation of gliomas and mammary carcinomas transplanted in rat and mouse cranial windows. Cancer Res. 1994;54:4564–4568. [PubMed] [Google Scholar]
- 29.Zieske JD. Corneal development associated with eyelid opening. Int J Dev Biol. 2004;48:903–911. doi: 10.1387/ijdb.041860jz. [DOI] [PubMed] [Google Scholar]