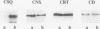Abstract
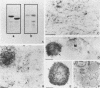
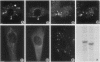
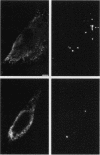
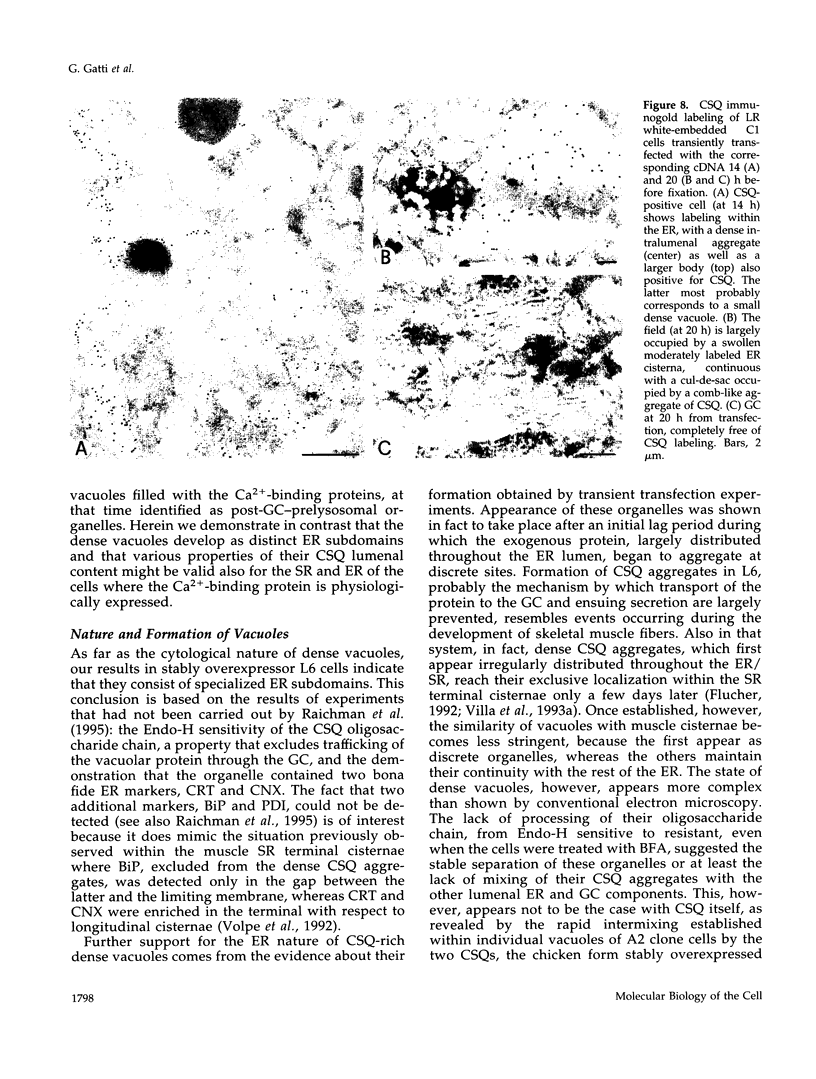
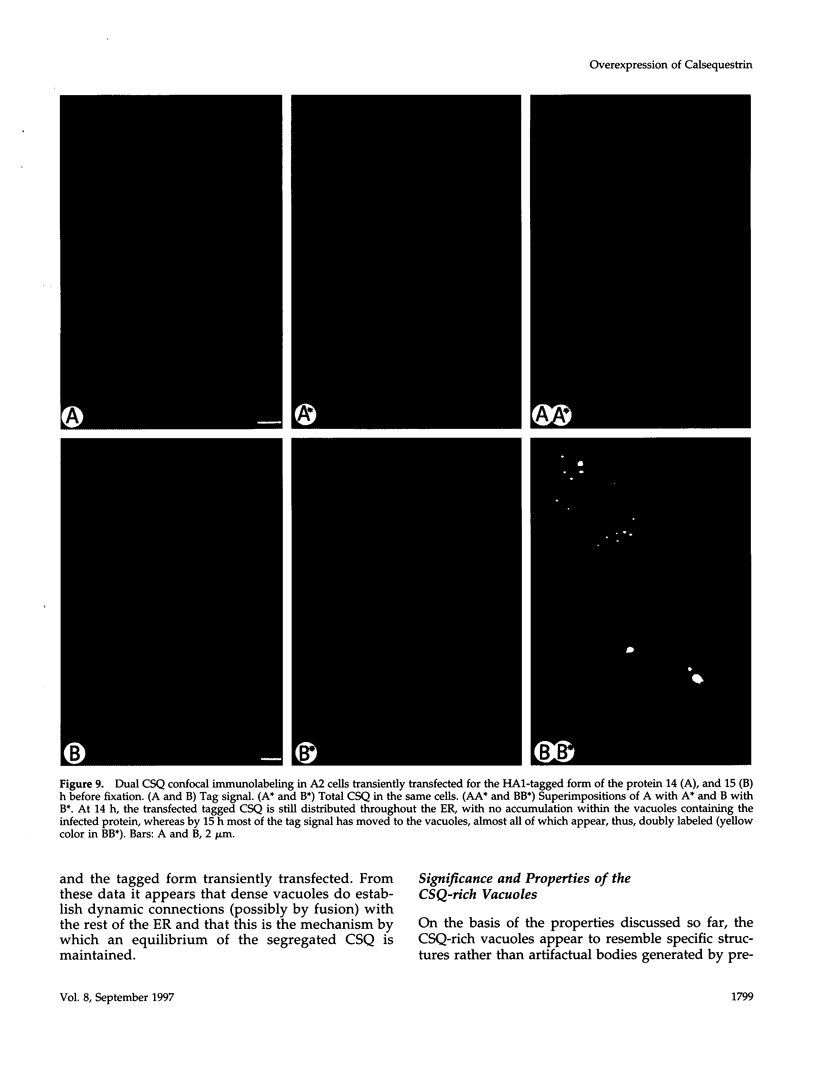
Calsequestrin (CSQ), the major low-affinity Ca(2+)-binding glycoprotein of striated muscle fibers, is concentrated to yield aggregates that occupy the lumen of the terminal cisternae of the sarcoplasmic reticulum (SR). When infected or transfected into L6 myoblast, the protein is also concentrated, however, in dense vacuoles apparently separate from the endoplasmic reticulum (ER). CSQ-rich cells appear otherwise normal; in particular, neither other proteins involved in Ca2+ homeostasis nor ER chaperones are increased. The CSQ dense vacuoles are shown herein to be specialized ER subdomains as demonstrated by 1) the endoglycosidase H sensitivity of their CSQ and 2) two markers, calreticulin and calnexin (but not others, protein disulfide isomerase and BiP), intermixed with the vacuole content. Their formation is shown to start with the aggregation of CSQ at discrete sites of the ER lumen. When cells were transfected with both CSQ and calreticulin, only the first gave rise to vacuoles; the second remained diffusely distributed within the ER lumen. The possibility that CSQ aggregation is an artifact of overexpression appears unlikely because 1) within dense vacuoles CSQ molecules are not disulfide cross-linked, 2) their turnover is relatively slow (t = 12 h), and 3) segregated CSQ is bound to large amounts of Ca2+. Transfection of a tagged CSQ into cells already overexpressing the protein revealed the continuous import of the newly synthesized protein into preassembled vacuoles. The tendency to aggregation appears, therefore, as a property contributing to the segregation of CSQ within the ER lumen and to its accumulation within specialized subdomains. The study of L6 cells expressing CSQ-rich vacuoles might thus ultimately help to unravel mechanisms by which the complexity of the sarcoplasmic reticulum is established in muscle fibers.
Full text
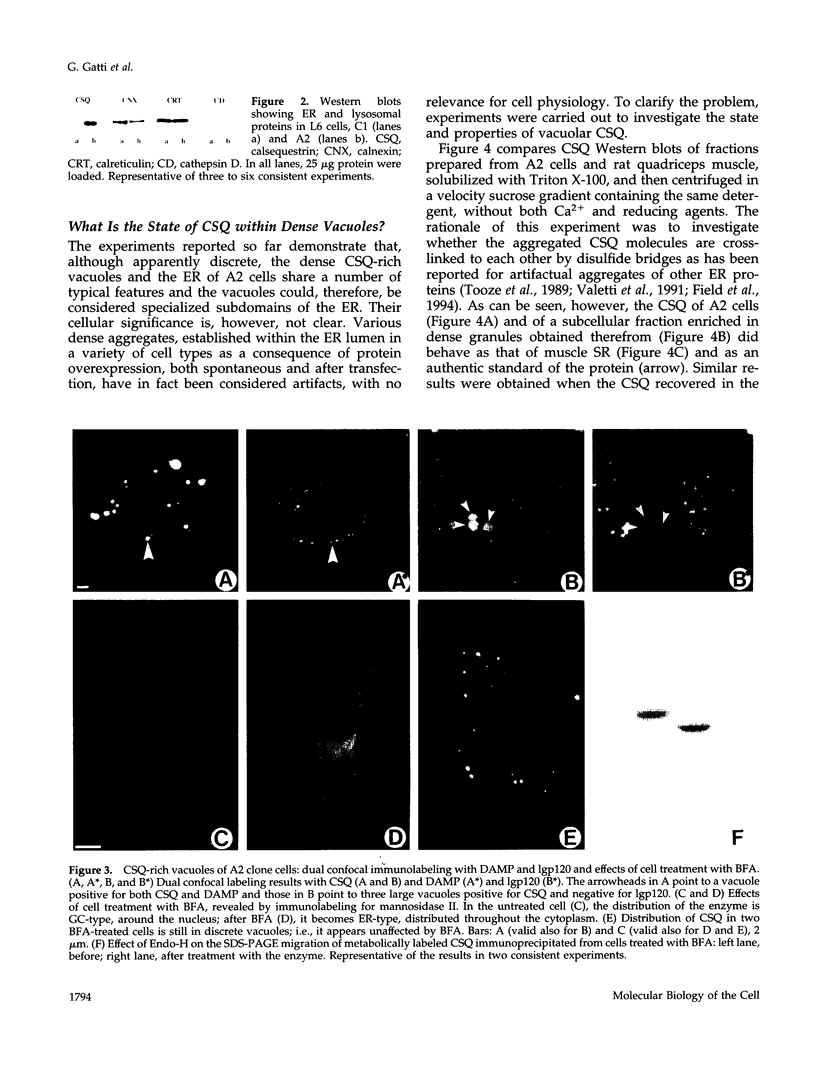
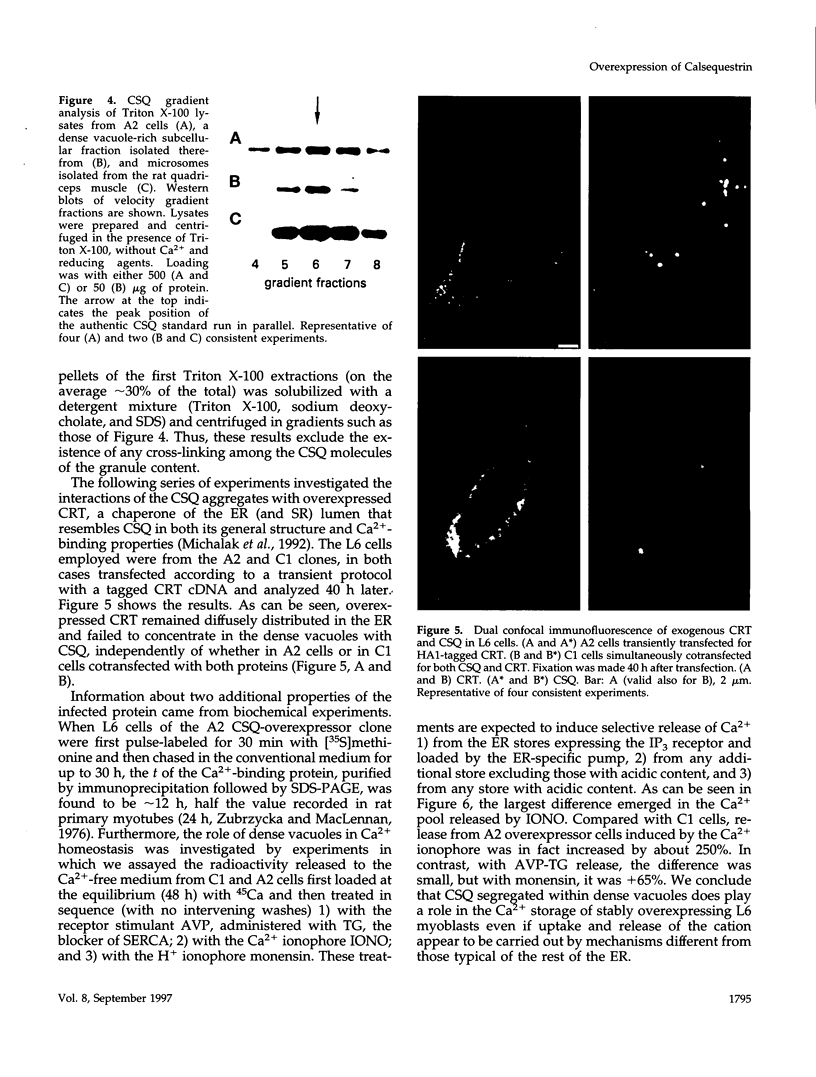
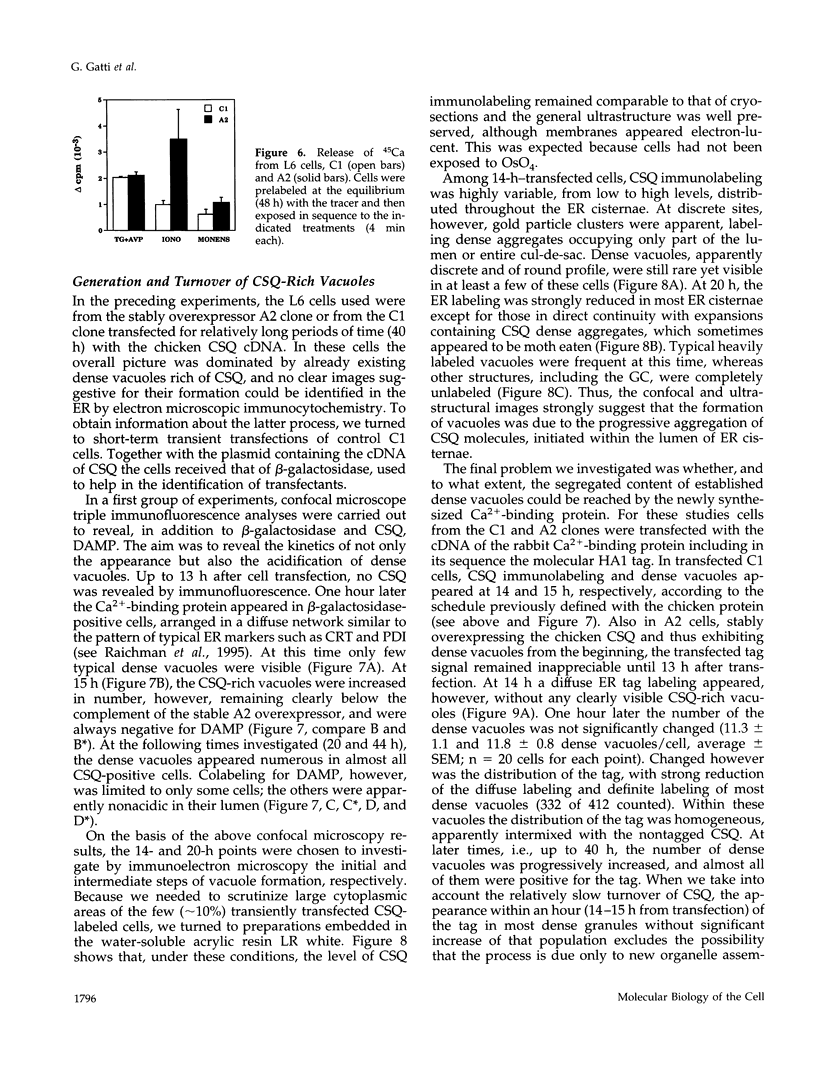
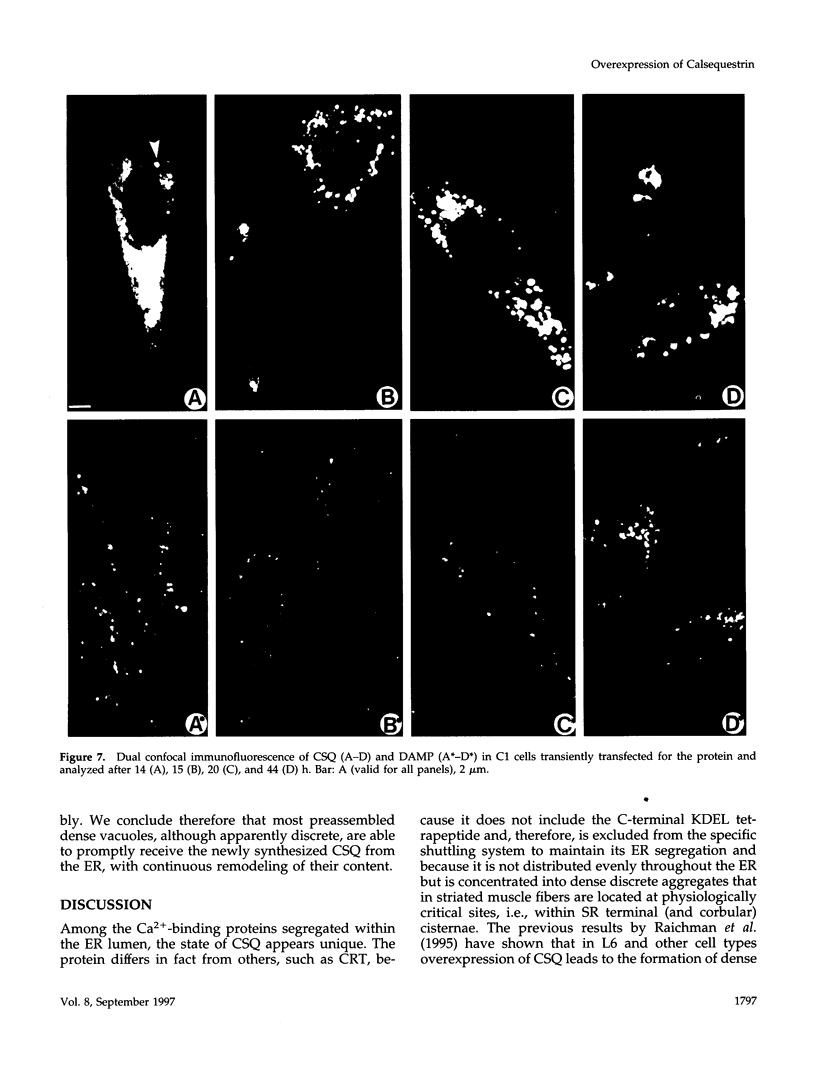
PDF



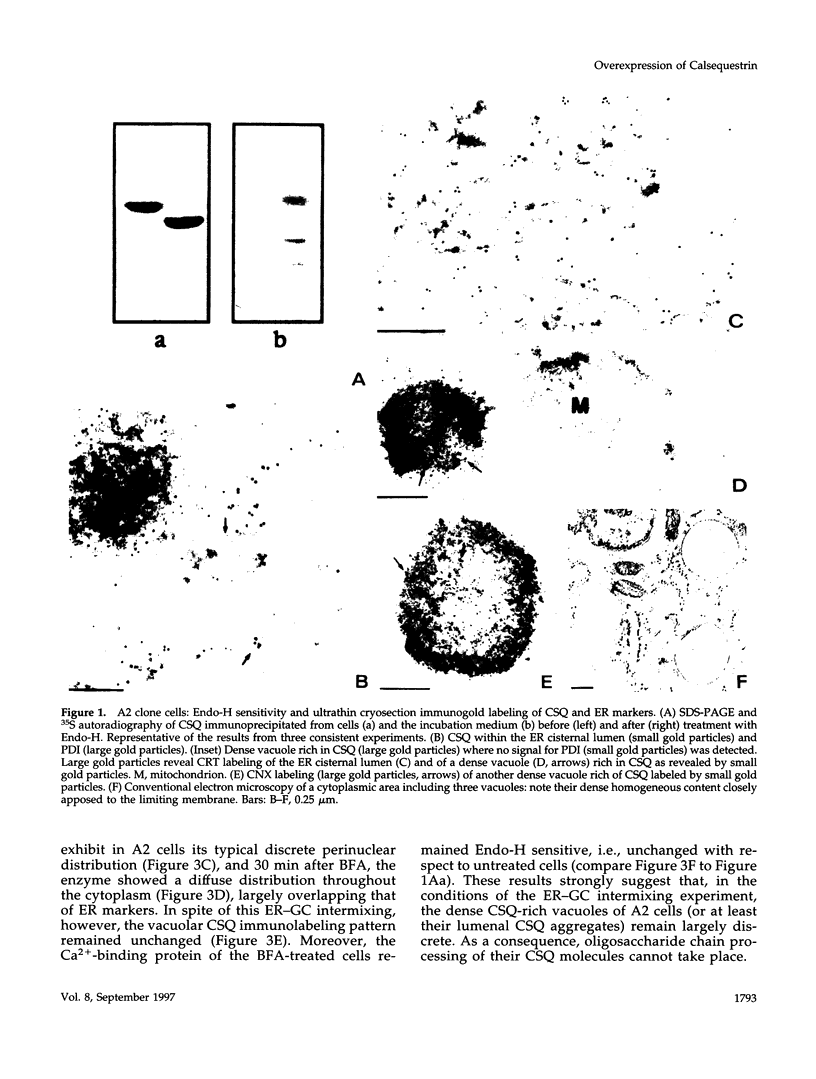




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amara J. F., Lederkremer G., Lodish H. F. Intracellular degradation of unassembled asialoglycoprotein receptor subunits: a pre-Golgi, nonlysosomal endoproteolytic cleavage. J Cell Biol. 1989 Dec;109(6 Pt 2):3315–3324. doi: 10.1083/jcb.109.6.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastianutto C., Clementi E., Codazzi F., Podini P., De Giorgi F., Rizzuto R., Meldolesi J., Pozzan T. Overexpression of calreticulin increases the Ca2+ capacity of rapidly exchanging Ca2+ stores and reveals aspects of their lumenal microenvironment and function. J Cell Biol. 1995 Aug;130(4):847–855. doi: 10.1083/jcb.130.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bole D. G., Hendershot L. M., Kearney J. F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986 May;102(5):1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J. S., Suzuki C. K., Lippincott-Schwartz J., Weissman A. M., Klausner R. D. Pre-Golgi degradation of newly synthesized T-cell antigen receptor chains: intrinsic sensitivity and the role of subunit assembly. J Cell Biol. 1989 Jul;109(1):73–83. doi: 10.1083/jcb.109.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussif O., Lezoualc'h F., Zanta M. A., Mergny M. D., Scherman D., Demeneix B., Behr J. P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci U S A. 1995 Aug 1;92(16):7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell A. H., Brandt N. R., Brunschwig J. P., Purkerson S. Localization and partial characterization of the oligomeric disulfide-linked molecular weight 95,000 protein (triadin) which binds the ryanodine and dihydropyridine receptors in skeletal muscle triadic vesicles. Biochemistry. 1991 Jul 30;30(30):7507–7513. doi: 10.1021/bi00244a020. [DOI] [PubMed] [Google Scholar]
- Clegg D. O., Helder J. C., Hann B. C., Hall D. E., Reichardt L. F. Amino acid sequence and distribution of mRNA encoding a major skeletal muscle laminin binding protein: an extracellular matrix-associated protein with an unusual COOH-terminal polyaspartate domain. J Cell Biol. 1988 Aug;107(2):699–705. doi: 10.1083/jcb.107.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colyer J., Mata A. M., Lee A. G., East J. M. Effects on ATPase activity of monoclonal antibodies raised against (Ca2+ + Mg2+)-ATPase from rabbit skeletal muscle sarcoplasmic reticulum and their correlation with epitope location. Biochem J. 1989 Sep 1;262(2):439–447. doi: 10.1042/bj2620439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello B., Chadwick C., Saito A., Chu A., Maurer A., Fleischer S. Characterization of the junctional face membrane from terminal cisternae of sarcoplasmic reticulum. J Cell Biol. 1986 Sep;103(3):741–753. doi: 10.1083/jcb.103.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani E., Margreth A. Specific protein-protein interactions of calsequestrin with junctional sarcoplasmic reticulum of skeletal muscle. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1253–1259. doi: 10.1016/0006-291x(90)91584-f. [DOI] [PubMed] [Google Scholar]
- Dunn W. A., Jr Studies on the mechanisms of autophagy: maturation of the autophagic vacuole. J Cell Biol. 1990 Jun;110(6):1935–1945. doi: 10.1083/jcb.110.6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasolato C., Zottini M., Clementi E., Zacchetti D., Meldolesi J., Pozzan T. Intracellular Ca2+ pools in PC12 cells. Three intracellular pools are distinguished by their turnover and mechanisms of Ca2+ accumulation, storage, and release. J Biol Chem. 1991 Oct 25;266(30):20159–20167. [PubMed] [Google Scholar]
- Field M. C., Moran P., Li W., Keller G. A., Caras I. W. Retention and degradation of proteins containing an uncleaved glycosylphosphatidylinositol signal. J Biol Chem. 1994 Apr 8;269(14):10830–10837. [PubMed] [Google Scholar]
- Fliegel L., Ohnishi M., Carpenter M. R., Khanna V. K., Reithmeier R. A., MacLennan D. H. Amino acid sequence of rabbit fast-twitch skeletal muscle calsequestrin deduced from cDNA and peptide sequencing. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1167–1171. doi: 10.1073/pnas.84.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flucher B. E. Structural analysis of muscle development: transverse tubules, sarcoplasmic reticulum, and the triad. Dev Biol. 1992 Dec;154(2):245–260. doi: 10.1016/0012-1606(92)90065-o. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C., Kenney L. J., Varriano-Marston E. The structure of calsequestrin in triads of vertebrate skeletal muscle: a deep-etch study. J Cell Biol. 1987 Jul;105(1):49–56. doi: 10.1083/jcb.105.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Jorgensen A. O., Campbell K. P. Characterization and ultrastructural localization of a novel 90-kDa protein unique to skeletal muscle junctional sarcoplasmic reticulum. J Biol Chem. 1994 Nov 11;269(45):28359–28365. [PubMed] [Google Scholar]
- Guo W., Jorgensen A. O., Jones L. R., Campbell K. P. Biochemical characterization and molecular cloning of cardiac triadin. J Biol Chem. 1996 Jan 5;271(1):458–465. doi: 10.1074/jbc.271.1.458. [DOI] [PubMed] [Google Scholar]
- Hall D. E., Frazer K. A., Hann B. C., Reichardt L. F. Isolation and characterization of a laminin-binding protein from rat and chick muscle. J Cell Biol. 1988 Aug;107(2):687–697. doi: 10.1083/jcb.107.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S., Bruno B., Lew D. P., Pozzan T., Volpe P., Meldolesi J. Immunocytochemistry of calciosomes in liver and pancreas. J Cell Biol. 1988 Dec;107(6 Pt 2):2523–2531. doi: 10.1083/jcb.107.6.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. R., Zhang L., Sanborn K., Jorgensen A. O., Kelley J. Purification, primary structure, and immunological characterization of the 26-kDa calsequestrin binding protein (junctin) from cardiac junctional sarcoplasmic reticulum. J Biol Chem. 1995 Dec 22;270(51):30787–30796. doi: 10.1074/jbc.270.51.30787. [DOI] [PubMed] [Google Scholar]
- Jorgensen A. O., Shen A. C., Campbell K. P., MacLennan D. H. Ultrastructural localization of calsequestrin in rat skeletal muscle by immunoferritin labeling of ultrathin frozen sections. J Cell Biol. 1983 Nov;97(5 Pt 1):1573–1581. doi: 10.1083/jcb.97.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson C. M., Stang K. K., Moomaw C. R., Slaughter C. A., Campbell K. P. Primary structure and topological analysis of a skeletal muscle-specific junctional sarcoplasmic reticulum glycoprotein (triadin). J Biol Chem. 1993 Jun 15;268(17):12646–12654. [PubMed] [Google Scholar]
- Kuznetsov G., Bush K. T., Zhang P. L., Nigam S. K. Perturbations in maturation of secretory proteins and their association with endoplasmic reticulum chaperones in a cell culture model for epithelial ischemia. Proc Natl Acad Sci U S A. 1996 Aug 6;93(16):8584–8589. doi: 10.1073/pnas.93.16.8584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis M. J., Pelham H. R. Ligand-induced redistribution of a human KDEL receptor from the Golgi complex to the endoplasmic reticulum. Cell. 1992 Jan 24;68(2):353–364. doi: 10.1016/0092-8674(92)90476-s. [DOI] [PubMed] [Google Scholar]
- Lewis V., Green S. A., Marsh M., Vihko P., Helenius A., Mellman I. Glycoproteins of the lysosomal membrane. J Cell Biol. 1985 Jun;100(6):1839–1847. doi: 10.1083/jcb.100.6.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak M., Milner R. E., Burns K., Opas M. Calreticulin. Biochem J. 1992 Aug 1;285(Pt 3):681–692. doi: 10.1042/bj2850681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell R. D., Simmerman H. K., Jones L. R. Ca2+ binding effects on protein conformation and protein interactions of canine cardiac calsequestrin. J Biol Chem. 1988 Jan 25;263(3):1376–1381. [PubMed] [Google Scholar]
- Montero M., Brini M., Marsault R., Alvarez J., Sitia R., Pozzan T., Rizzuto R. Monitoring dynamic changes in free Ca2+ concentration in the endoplasmic reticulum of intact cells. EMBO J. 1995 Nov 15;14(22):5467–5475. doi: 10.1002/j.1460-2075.1995.tb00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller S. R., Sullivan P. D., Clegg D. O., Feinstein S. C. Efficient transfection and expression of heterologous genes in PC12 cells. DNA Cell Biol. 1990 Apr;9(3):221–229. doi: 10.1089/dna.1990.9.221. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Nilsson T., Warren G. Retention and retrieval in the endoplasmic reticulum and the Golgi apparatus. Curr Opin Cell Biol. 1994 Aug;6(4):517–521. doi: 10.1016/0955-0674(94)90070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa S., Hirata A., Nakano A. Inhibition of endoplasmic reticulum (ER)-to-Golgi transport induces relocalization of binding protein (BiP) within the ER to form the BiP bodies. Mol Biol Cell. 1994 Oct;5(10):1129–1143. doi: 10.1091/mbc.5.10.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda T., Farquhar M. G. A non-autophagic pathway for diversion of ER secretory proteins to lysosomes. J Cell Biol. 1992 Oct;119(1):85–97. doi: 10.1083/jcb.119.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y. W., Sharp A. H., Snyder S. H., Yau K. W. Localization of the inositol 1,4,5-trisphosphate receptor in synaptic terminals in the vertebrate retina. Neuron. 1991 Apr;6(4):525–531. doi: 10.1016/0896-6273(91)90055-5. [DOI] [PubMed] [Google Scholar]
- Perrin D., Sönnichsen B., Söling H. D., Phuc N. V. Purkinje cells of rat and chicken cerebellum contain calreticulin (CaBP3). FEBS Lett. 1991 Dec 2;294(1-2):47–50. doi: 10.1016/0014-5793(91)81340-e. [DOI] [PubMed] [Google Scholar]
- Pizzo P., Fasolato C., Pozzan T. Dynamic properties of an inositol 1,4,5-trisphosphate- and thapsigargin-insensitive calcium pool in mammalian cell lines. J Cell Biol. 1997 Jan 27;136(2):355–366. doi: 10.1083/jcb.136.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T., Rizzuto R., Volpe P., Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994 Jul;74(3):595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- Raichman M., Panzeri M. C., Clementi E., Papazafiri P., Eckley M., Clegg D. O., Villa A., Meldolesi J. Differential localization and functional role of calsequestrin in growing and differentiated myoblasts. J Cell Biol. 1995 Feb;128(3):341–354. doi: 10.1083/jcb.128.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott B. T., Simmerman H. K., Collins J. H., Nadal-Ginard B., Jones L. R. Complete amino acid sequence of canine cardiac calsequestrin deduced by cDNA cloning. J Biol Chem. 1988 Jun 25;263(18):8958–8964. [PubMed] [Google Scholar]
- Tooze J., Hollinshead M., Ludwig T., Howell K., Hoflack B., Kern H. In exocrine pancreas, the basolateral endocytic pathway converges with the autophagic pathway immediately after the early endosome. J Cell Biol. 1990 Aug;111(2):329–345. doi: 10.1083/jcb.111.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J., Kern H. F., Fuller S. D., Howell K. E. Condensation-sorting events in the rough endoplasmic reticulum of exocrine pancreatic cells. J Cell Biol. 1989 Jul;109(1):35–50. doi: 10.1083/jcb.109.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valetti C., Grossi C. E., Milstein C., Sitia R. Russell bodies: a general response of secretory cells to synthesis of a mutant immunoglobulin which can neither exit from, nor be degraded in, the endoplasmic reticulum. J Cell Biol. 1991 Nov;115(4):983–994. doi: 10.1083/jcb.115.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux D., Tooze J., Fuller S. Identification by anti-idiotype antibodies of an intracellular membrane protein that recognizes a mammalian endoplasmic reticulum retention signal. Nature. 1990 Jun 7;345(6275):495–502. doi: 10.1038/345495a0. [DOI] [PubMed] [Google Scholar]
- Velasco A., Hendricks L., Moremen K. W., Tulsiani D. R., Touster O., Farquhar M. G. Cell type-dependent variations in the subcellular distribution of alpha-mannosidase I and II. J Cell Biol. 1993 Jul;122(1):39–51. doi: 10.1083/jcb.122.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa A., Podini P., Clegg D. O., Pozzan T., Meldolesi J. Intracellular Ca2+ stores in chicken Purkinje neurons: differential distribution of the low affinity-high capacity Ca2+ binding protein, calsequestrin, of Ca2+ ATPase and of the ER lumenal protein, Bip. J Cell Biol. 1991 May;113(4):779–791. doi: 10.1083/jcb.113.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa A., Podini P., Nori A., Panzeri M. C., Martini A., Meldolesi J., Volpe P. The endoplasmic reticulum-sarcoplasmic reticulum connection. II. Postnatal differentiation of the sarcoplasmic reticulum in skeletal muscle fibers. Exp Cell Res. 1993 Nov;209(1):140–148. doi: 10.1006/excr.1993.1294. [DOI] [PubMed] [Google Scholar]
- Villa A., Podini P., Panzeri M. C., Söling H. D., Volpe P., Meldolesi J. The endoplasmic-sarcoplasmic reticulum of smooth muscle: immunocytochemistry of vas deferens fibers reveals specialized subcompartments differently equipped for the control of Ca2+ homeostasis. J Cell Biol. 1993 Jun;121(5):1041–1051. doi: 10.1083/jcb.121.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe P., Villa A., Damiani E., Sharp A. H., Podini P., Snyder S. H., Meldolesi J. Heterogeneity of microsomal Ca2+ stores in chicken Purkinje neurons. EMBO J. 1991 Nov;10(11):3183–3189. doi: 10.1002/j.1460-2075.1991.tb04880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe P., Villa A., Podini P., Martini A., Nori A., Panzeri M. C., Meldolesi J. The endoplasmic reticulum-sarcoplasmic reticulum connection: distribution of endoplasmic reticulum markers in the sarcoplasmic reticulum of skeletal muscle fibers. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6142–6146. doi: 10.1073/pnas.89.13.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada I., Ou W. J., Liu M. C., Scheele G. Chaperone function of calnexin for the folding intermediate of gp80, the major secretory protein in MDCK cells. Regulation by redox state and ATP. J Biol Chem. 1994 Mar 11;269(10):7464–7472. [PubMed] [Google Scholar]
- Waldron R. T., Short A. D., Gill D. L. Thapsigargin-resistant intracellular calcium pumps. Role in calcium pool function and growth of thapsigargin-resistant cells. J Biol Chem. 1995 May 19;270(20):11955–11961. doi: 10.1074/jbc.270.20.11955. [DOI] [PubMed] [Google Scholar]
- Willnow T. E., Rohlmann A., Horton J., Otani H., Braun J. R., Hammer R. E., Herz J. RAP, a specialized chaperone, prevents ligand-induced ER retention and degradation of LDL receptor-related endocytic receptors. EMBO J. 1996 Jun 3;15(11):2632–2639. [PMC free article] [PubMed] [Google Scholar]
- Wuytack F., Raeymaekers L., Verbist J., Jones L. R., Casteels R. Smooth-muscle endoplasmic reticulum contains a cardiac-like form of calsequestrin. Biochim Biophys Acta. 1987 May 29;899(2):151–158. doi: 10.1016/0005-2736(87)90395-6. [DOI] [PubMed] [Google Scholar]
- Zubrzycka E., MacLennan D. H. Assembly of the sarcoplasmic reticulum. Biosynthesis of calsequestrin in rat skeletal muscle cell cultures. J Biol Chem. 1976 Dec 25;251(24):7733–7738. [PubMed] [Google Scholar]