Abstract
NK cells have been shown to mediate important immunoregulatory “helper” functions in addition to their cytolytic activity. In particular, NK cells are capable of preventing maturation-related DC “exhaustion”, inducing the development of “type-1 polarized” mature DCs with an enhanced ability to produce IL-12p70, a factor essential for type-1 immunity and effective anti-cancer responses. Here we show that the NK cell-mediated type-1 polarization of DCs can be applied in the context of patients with advanced cancer to enhance the efficacy of DCs in inducing tumor-specific CTLs. NK cells isolated from late-stage (stage III and IV) melanoma patients responded with high IFNγ production and the induction of type-1-polarized DCs upon exposure to defined combinations of stimulatory agents, including IFNα plus IL-18. The resulting DCs showed strongly-enhanced IL-12p70 production upon subsequent T cell interaction, compared to immature (i)DCs (average of 19-fold enhancement) and non-polarized IL-1β/TNF-α/IL-6/PGE2-matured “standard” (s)DCs (average of 215-fold enhancement). Additional inclusion of poly-I:C during NK-DC co-cultures optimized the expression of CD80, CD86, CD40, and HLA-DR on the resulting NKDC1s, increased their CCR7-mediated migratory responsiveness to the lymph node-associated chemokine CCL21, and further enhanced their IL-12-producing capacity. When compared in vitro to iDCs and non-polarized sDCs, NKDC1s were superior in inducing functional melanoma-specific CTLs capable of recognizing multiple melanoma-associated antigens and killing melanoma cells. These results indicate that the helper function of NK cells can be utilized in clinical settings to improve the effectiveness of DC-based cancer vaccines.
Keywords: NK cells, Dendritic cells, Vaccination, CTL, Tumor immunity, Human
Introduction
Dendritic cells (DCs) play a central role in the initiation and regulation of immune responses. They act as carriers of pathogen- and damage-related information, migrating from peripheral sites of pathogen entry and tissue injury to the T cell areas of draining lymph nodes where they prime naïve T cells, providing them with the antigen-specific “signal 1” and co-stimulatory “signal 2”1. Furthermore, DCs also regulate the balance between the preferential activation of type-1, type-2, and type-17 effector mechanisms of immunity by providing naïve T cells with an additional “signal 3”2–5. The character of this DC-mediated signal 3 is influenced by the cues provided to them directly by pathogens, by factors produced by injured tissues2,6,7, or by other immune cells capable of sensing transformation or intracellular infections, including natural killer (NK) cells8,9.
The argument for the therapeutic use of DCs as cancer vaccines has been recently strengthened by the FDA approval of sipuleucel-T for the treatment of patients with castration-resistant prostate cancer10. However, in addition to their ability to deliver antigen, effective DC-based cancer vaccines also need to deliver the co-stimulatory “signal 2” and IL-12-involving “signal 3” needed for optimal T cell proliferation and differentiation, respectively11–13. Current standard protocols used for the production of “second-generation” DC vaccines emphasizing these principles yield mature DCs, but with an “exhausted” ability to produce IL-12p7014–18, a crucial factor for the development of type-1 immunity and effective anti-cancer responses19. As a result, while standard non-polarized DCs combine a fully mature status (a predictive marker of enhanced immunogenicity20,21 with high expression of CCR7, a predictive marker of their lymph node homing capability22), they display only a limited ability to produce IL-12p7023–25, ultimately restricting their capacity to induce effective anti-tumor CTL activation.
Several groups, including ours, have previously demonstrated that NK cells can regulate immune responses by activating DCs26–28 and promoting their differentiation into mature, high IL-12-producing type-1 polarized DCs (DC1s) with an enhanced capacity to induce Th1 and CTL responses29,30, the responses most desirable against cancer. These observations, together with a documented role of NK cells during the induction of anti-cancer Th1 and CTL-mediated responses in vivo31–35, suggested the possibility of using NK cells as a tool to generate more effective cancer vaccines. Previously, we reported that NK cells from healthy donors can be activated in a “two-signal” paradigm to induce DC1 polarization in a mechanism involving IFNγ29,36. The resulting NK-polarized DC1s showed a strongly-elevated capacity to produce IL-12p70 and induce Th1 and CTL responses in polyclonal superantigen-driven models of T cell activation29,36.
Here, we report for the first time that this DC1-promoting “helper” function can be effectively induced in NK cells isolated directly from patients with advanced melanoma under clinically-desirable serum-free conditions, providing a useful tool to induce high numbers of melanoma-specific CTLs capable of recognizing distinct melanoma-associated epitopes and killing melanoma cells.
Materials and Methods
Media and reagents
T cells and tumor cell lines were cultured in Iscove’s Modified Dulbecco’s Medium (IMDM) containing 10% fetal bovine serum and 1% L-glutamine and Penicillin/Streptomycin (all from Gibco, Invitrogen, Grand Island, NY). IMDM containing 5% human serum (Gemini Bio-Products, West Sacramento, CA) was used as the base medium for the outgrowth of T cell cultures. Two different serum-free medium types were used as the base medium for short-term stimulation of human NK cells as well as to generate DCs: AIM-V medium (Gibco, Invitrogen, Grand Island, NY) and CellGenix DC medium (CellGenix Technologie Transfer GmbH, Freiburg, Germany). The following factors were used throughout the study: granulocyte macrophage colony-stimulating factor (GM-CSF) and IL-4 (Schering-Plough, Kenilworth, NJ); IFNα (Intron A-IFN-α-2b; Schering-Plough); TNFα and IFNγ (both from Miltenyi Biotech, Bergisch Gladbach, Germany); IL-6 (Thermo Scientific, Waltham, MA); PGE2 (Sigma-Aldrich, St. Louis, MO); IL-18 (MBL International, Woburn, MA); IL-2 (Chiron, Emeryville, CA); IL-7 (PeproTech, Rocky Hill, NJ); and poly-I:C (Sigma-Aldrich, St. Louis, MO). The R24 anti-GD3 monoclonal antibody (mouse IgG3) used in this study was prepared at CellTech (London, UK) and provided by the National Cancer Institute (NCI) BRMP, and was stored at −80°C until use.
NK cell and CD8+ T cell isolation
Peripheral blood from patients with advanced melanoma (stage III and stage IV) and healthy donors was harvested by venipuncture under IRB-approved protocols. NK cells and CD8+ T cells (>95% pure) were isolated by negative magnetic selection using the StemSep system (StemCell Technologies Inc., Vancouver, British Columbia, Canada).
Generation of DCs
Peripheral blood mononuclear cells (PBMCs) were isolated from the peripheral blood of either healthy donors or melanoma patients (all stage III and IV donors) by density gradient separation using Lymphocyte Separation Medium (Cellgro Mediatech, Herndon, VA). Monocyte fractions were further isolated by CD14 positive selection (Miltenyi Biotech, Bergisch Gladbach, Germany). Immature DCs were generated from monocytes cultured for 6 days in 24-well plates (Falcon, Becton Dickinson Labware, Franklin Lakes, NJ) at 4×105 cells per well in GM-CSF and IL-4 (both 1,000 IU/ml). To generate “standard” mature DCs, day 6 immature DCs were cultured for an additional 48 h with IL-1β (10 ng/ml), TNFα (25 ng/ml), IL-6 (1,000 IU/ml), and PGE2 (10−6 mol/L) as previously described37.
Induction of IFNγ production by NK cells
NK cells were isolated and plated in 96 well plates at 1×105 cells/well. NK cells were stimulated with IFNα (1,000 IU/ml) together with either IL-18 (1 µg/ml), K562 cells (2×104 cells/well), or melanoma (FEM-X) cells (1×104 cells/well) in a final adjusted volume of 200 µl. When stated, anti-GD3 antibody (R24) was used to opsonize FEM-X cells. To accomplish this, 1×106 FEM-X cells were placed in 1 ml of tumor culture media and exposed to the R24 antibody at 1 µg/ml for 30 min at room temperature. Cells were then washed three times to remove excess antibody before use.
DC and NK cell co-cultures
Previously isolated and cryopreserved autologous NK cells were thawed and added to DC cultures either directly or separated by Transwell culture inserts (Costar-3413; 0.4µm pore size) at 1.5×105 cells/well to day 6 DC cultures in the presence of IFNα (1,000 IU/ml) and IL-18 (1 µg/ml). When stated, poly-I:C(20 µg/ml) was also added 20 h after co-culture initiation, which was previously determined to be optimal for enhancing its effects.
Flow cytometry
Two and three-colored cell surface immunostaining analyses were performed using a Beckman Coulter Epics XL Flow Cytometer. FITC-labeled anti-human CD86, CD40, and CD3 monoclonal antibodies and the corresponding FITC-isotype (mouse IgG1) control antibodies were purchased from BD Biosciences (San Jose, CA). PE-labeled anti-human CD83 and the corresponding PE-isotype (mouse IgG2b) control monoclonal antibodies were purchased from BD Biosciences (San Jose, CA). PE-Cy5-labeled anti-human HLA-DR and the corresponding PE-Cy5-isotype (mouse IgG1) control monoclonal antibodies were purchased from Beckman Coulter (Brea, CA). PE-labeled anti-human CCR7 monoclonal antibody was purchased from R&D Systems (Minneapolis, MN) and the corresponding PE-isotype (mouse IgG2a) control antibody was purchased from BD Biosciences (San Jose, CA). PE-labeled MART-1 tetramer (ELAGIGILTV) and the control influenza virus tetramer (GILGFVFTL) were purchased from Beckman Coulter (Brea, CA). Before staining, the cells were treated for 20 min at 4°C in PBS buffer containing 0.1% NaN3, 2% human serum, 0.5% BSA, and 1 µg/ml of mouse IgG (Sigma-Aldrich, St. Louis, MO) to block non-specific Fc receptor binding sites. Cells were stained for 40 min at 4°C followed by washing with PBS buffer containing 0.1% NaN3 and 0.5% BSA, then fixed and stored in 2% paraformaldehyde until analysis.
DC production of IL-12p70
Dendritic cells were harvested, washed, and plated in 96-well plates at 2×104 cells/well. To mimic the interaction with CD40L-expressing Th cells, CD40L-transfected J558 cells (a gift from Dr. P. Lane, University of Birmingham, United Kingdom), which in previous studies proved equivalent to activated CD4+ T cells and soluble CD40L15,38, were added at 5×104 cells/well. Supernatants were collected after 24 h and analyzed by IL-12p70 ELISA (Endogen, Woburn, MA).
Chemotaxis
Dendritic cell migration was induced by CCL21 (6C-Kine-Biosource, Camarillo, CA) and measured using a 96-well 8um pore ChemoTx system (Neuro Probe, Gaithersburgh, MD). 25×103 DC in AIM-V medium were placed on the top of the membrane and permitted to migrate for 90 min at °C. Enumeration of migrated DC was determined by counting four random areas in the bottom chamber. Results are expressed as mean DC numbers ± SD of four random areas in duplicate wells.
CTL induction
HLA-A2+ melanoma patient-derived CD8+ T cells (5×105 cells) were plated in 48-well plates and sensitized by autologous DCs (5×104 cells) that were pulsed with the HLA-A2-restricted peptides MART-1 (26–35), gp100 (209–217), and tyrosinase (368–376). Added to the mix were γ-irradiated (3,000 rad) CD40L-transfected J558 cells (5×104), which acted as a surrogate for CD40L-expressing CD4+ Th cells. At day 4, T cell cultures were supplemented with IL-2 (50 IU/ml) and IL-7 (10 ng/ml). The CD8+ T cells were expanded following an additional in vitro stimulation (day 12) with irradiated peptide-pulsed autologous PBMCs (1:1 T cell:PBMC ratio). At day 24, the differentially-induced CD8+ T cell lines were stimulated with target cells to determine the generated frequency of melanoma-specific CD8+ T cells by IFNγ enzyme-linked immunospot (ELISPOT), using either T2 cells (pulsed with the relevant individual antigenic melanoma peptides or the irrelevant HPV-E7 peptide (43–62), or left unpulsed as an additional nonspecific control) or the HLA-A2+ and HLA-A2− melanoma cell line targets FEM-X and MEL-397, respectively. The pan-MHC class I blocking antibody (W6/32) was used to determine MHC class I restriction. CTL activity was further assessed by standard 4 h 51Cr-release cytotoxicity assays using the antigen relevant HLA-A2+ and irrelevant HLA-A2− melanoma cell lines FEM-X and MEL-397, respectively.
Statistical analysis
Data was analyzed using unpaired and paired t tests (two-tailed) and one-way and two-way ANOVA, where appropriate. Significance was judged at an α of 0.05.
Results
Intact “helper” activity of NK cells from melanoma patients: Two-signal activation requirement
We previously reported that type-I IFNs synergize with IL-18 or exposure to the NK-sensitive K562 leukemic cell line to induce IFNγ production and DC-activating “helper” function by healthy donor-derived NK cells29,36. Such two-signal-activated NK cells from healthy donors were shown to significantly enhance the CTL-inducing properties of DCs, as measured by superantigen-based polyclonal assays29,36.
In order to test whether NK cells from patients with advanced cancer are similarly functional and whether they respond to the above stimuli in standardized, clinically-desirable serum-free conditions, we first analyzed the cytokine-producing capacity of NK cells derived from late-stage (stage III and IV) melanoma patients. NK cells were exposed to various activating combinations under serum-free conditions, including IFNα with IL-18, IFNα with the NK cell-sensitive K562 leukemic cell line, or IFNα with the nominally NK cell-insensitive FEM-X melanoma cell line. In accordance with their undisturbed ability to perform helper functions, melanoma patient-derived NK cells produced high levels of IFNγ when stimulated with the combination of IFNα and IL-18, although not when stimulated with either of these factors alone (Fig. 1A, top). Similarly, the combination of IFNα with NK-sensitive K562 cells or with opsonized NK-insensitive FEM-X melanoma tumor cells, but not with any of these individual stimuli, effectively induced freshly-isolated NK cells from melanoma patients to secrete IFNγ (Fig. 1A, middle and bottom).
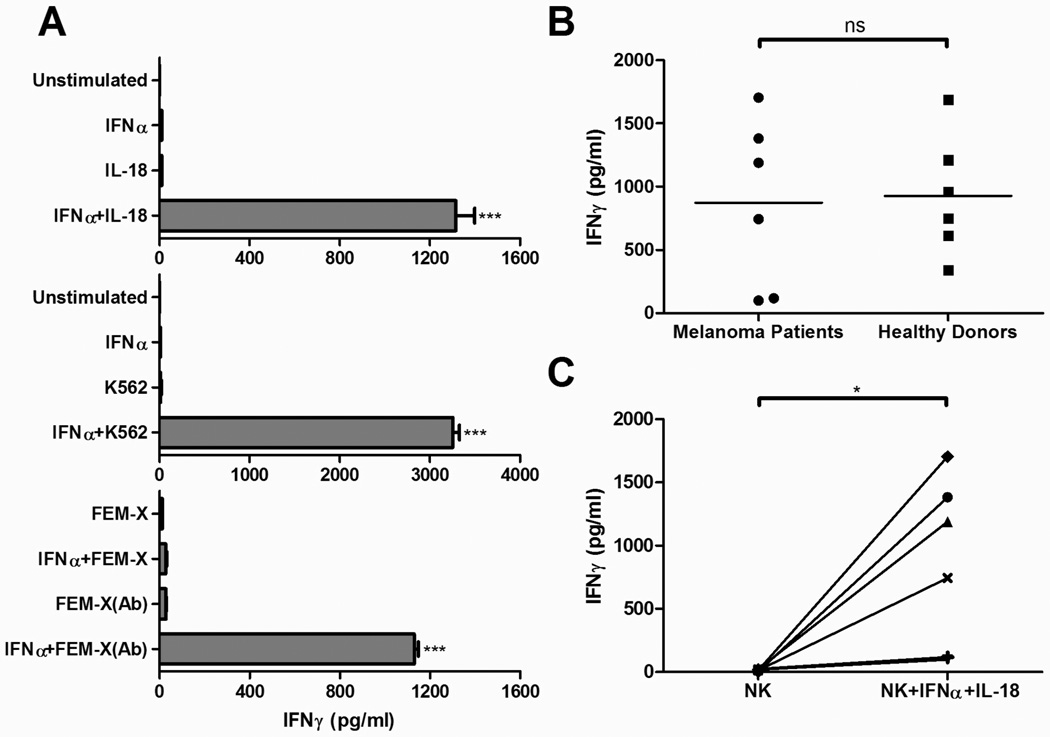
Figure 1. Two-signal activation requirement for IFNγ production by NK cells isolated from late-stage melanoma patients.
Negatively-isolated NK cells (1×105 cells/well) were incubated for 24 h in the presence of the indicated combinations of activating factors. Supernatants were subsequently assayed by ELISA for the presence of IFNγ. A, NK cell production of IFNγ in response to stimulation with IFNα (1000 IU/ml) and/or IL-18 (1 µg/ml) (top); IFNα and/or exposure to NK-sensitive K562 leukemia tumor cells (middle); or IFNα and/or exposure to antibody (R24)-opsonized, nominally NK-resistant FEM-X melanoma cells (bottom). The data shown represents one of six independent experiments, which all yielded similar results. Data recorded as the mean (± SD) of triplicate cultures. ***p<0.001 compared to all groups. B, Comparison of IFNγ production by NK cells derived from six melanoma patients or six healthy donors in response to stimulation with IFNα and IL-18. Data recorded as the mean of triplicate cultures for each patient or healthy donor. ns: p>0.05. C, Comparison of IFNγ production by unstimulated or IFNα/IL-18-stimulated NK cells isolated from individual melanoma patients. Data is presented as the mean of triplicate cultures for each patient (total of 6 patients). *p<0.05.
While immune cells from tumor-bearing individuals are known to display multiple functional defects39, the ability of NK cells to respond to two-signal stimulation was similar when comparing healthy donors and melanoma patients, although a significant variation in the absolute levels of IFNγ production was observed in both groups of donors (Fig. 1B). Despite this variability, all patients demonstrated strong increases in IFNγ secretion following activation (Fig. 1C), suggesting intact NK helper function even in patients with late-stage cancer.
NK cells from melanoma patients prime DCs for an enhanced ability to produce IL-12p70
Having established that melanoma patients’ NK cells are competent in their ability to respond to two-signal stimulation with high IFNγ production, we tested if these two-signal-activated NK cells could also promote the development of autologous type-1-polarized DCs (DC1s) with an elevated, rather than “exhausted”14,15, ability to produce IL-12p70. To accomplish this, cryopreserved autologous NK cells from late-stage melanoma patients were thawed and added to day 6 immature DCs for 48 h in the presence of IL-18 and IFNα
As shown in Figure 2, while DCs matured with the “standard” cytokine cocktail of IL-1β/TNF-α/IL-6/PGE2 (sDCs), a vaccine protocol used extensively in recent clinical trials37,40, showed a diminished capacity to produce IL-12p70 (compared to immature (i)DCs from the same donors), the DCs induced by two-signal-activated NK cells produced greatly enhanced levels of IL-12p70 (Fig. 2A). Control DCs exposed to the mix of NK cell-activating factors (IL-18 and IFNα) in the absence of NK cells failed to produce elevated levels of IL-12p70, demonstrating that NK cells are themselves critical, rather than solely IL-18 and IFNα, for this enhancement of IL-12-production by DCs.
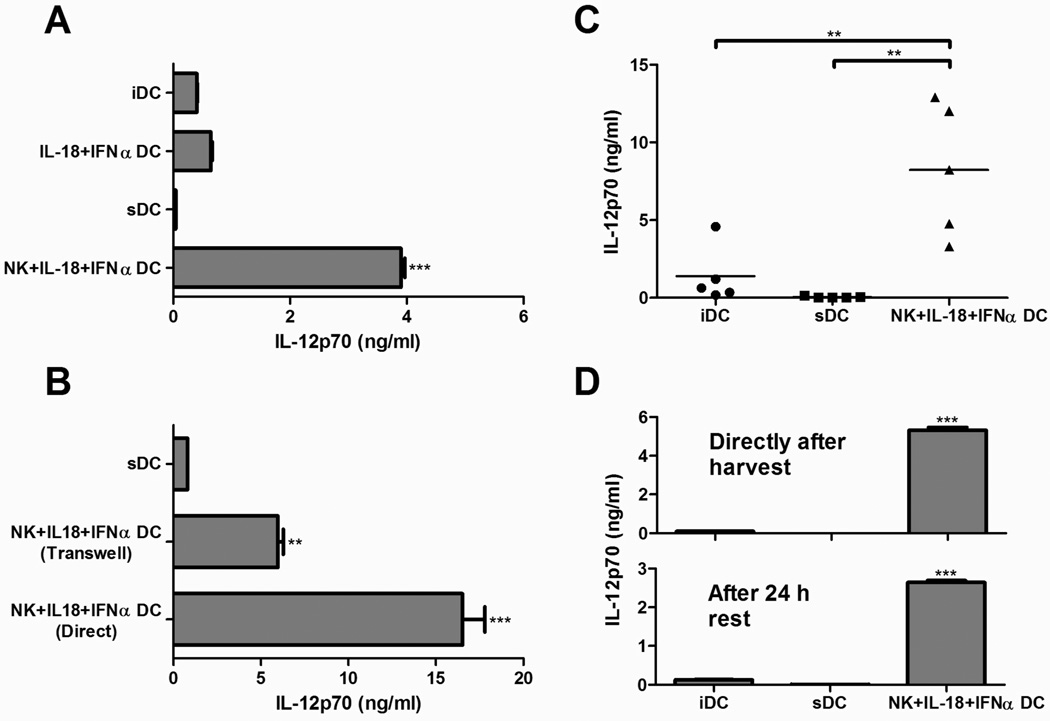
Figure 2. Two-signal-activated NK cells from late-stage melanoma patients stably induce DCs with an enhanced capacity to produce IL-12p70.
Previously isolated and cyropreserved NK cells (1.5×105 cells) were added to autologous day 6 DCs (2–3×105 cells/well) in the presence of IFNα (1000 IU/ml) and IL-18 (1 µg/ml). After 48 h, the DCs were harvested, plated (2×104 cells/well), and exposed to J558-CD40L to induce IL-12p70 production. IL-12p70 concentrations in 24 h supernatants were determined by ELISA. A, IL-12p70 production by untreated immature DCs (iDCs), DCs treated with the standard cytokine maturation cocktail of TNFα/IL-1β/IL-6/PGE2 (sDCs), or DCs treated with IL-18/IFNα with or without autologous NK cells. Data recorded as the mean (± SD) of triplicate cultures. Data shown was obtained from one representative experiment of five performed, all yielding similar results. ***p<0.001 compared to all groups. B, IL-12p70 production by DCs treated with the standard cytokine cocktail (sDCs) or autologous NK cells with IL-18/IFNα in direct or transwell-separated co-cultures. Data recorded as the mean (± SD) of triplicate cultures. Data from one representative experiment of two performed, both of which yielded similar results. ***p<0.001 compared to all groups, **p<0.01 compared to sDC group. C, IL-12p70 production by untreated immature DCs (iDCs), DCs treated with the standard cytokine maturation cocktail (sDCs), or DCs treated with autologous NK cells and IL-18/IFNα. Data recorded as the mean of triplicate cultures for each patient (total of 5 patients). **p<0.01. D, IL-12p70 production by differentially-matured DCs stimulated with CD40L directly after harvesting (top) or after an additional 24 h of culture in the absence of maturation factors (bottom). Data presented as the mean (± SD) of triplicate cultures for each patient. Data from one representative experiment of three performed, all of which yielded similar results. ***p<0.001 compared to all groups.
We performed transwell experiments to address whether cell-to-cell contact played a role in this NK cell-induced enhancement of IL-12p70 expression. In accordance with the previously-demonstrated key role of the soluble factor IFNγ in NK cell-mediated DC polarization29, two-signal-activated NK cells could enhance the IL-12p70-producing capacity of bystander DCs independent of cell-to-cell contact, although the IL-12-enhancing effects were maximal in the presence of cell contact (Fig. 2B).
Consistent with the notion that the ability of NK cells to perform “helper” functions is preserved even in patients with advanced cancer, similar results could be consistently obtained with blood from different patients with stage III–IV melanoma (Fig. 2C). On average, the NK cell-induced DC1s demonstrated over 200-fold greater capacity to produce IL-12p70 compared to sDCs generated from the same individual patient, and over 19-fold greater capacity compared to immature DCs from the same patient. This degree of enhancement was comparable to our observations from healthy donors29,36. Such enhanced ability to produce IL-12 was preserved for at least 24 h after harvesting of the DCs (Fig. 2D), suggesting that the function of these NK cell-induced DC1s will remain intact following their therapeutic application and migration to draining lymph nodes in clinical settings.
NKDC1s express high levels of maturation-associated co-stimulatory, antigen presentation, and lymph node migratory molecules: Stability of the NKDC1 phenotype
The effective induction of primary T cell responses during vaccination requires the action of fully mature DCs that express high levels of co-stimulatory and antigen presentation molecules, and that are capable of migrating to lymph nodes in response to CCR7 ligands22. While the production of IL-12p70 is indeed critical to their ability to induce tumor-specific Th1 cells and CTLs23–25, the IL-12-producing capacity of DCs often inversely correlates with the maturation status of the DC15,38. Therefore, we examined the maturation status of the DCs in our NK-DC co-culture system by surface flow cytometric analysis. As shown in Figure 3, the DCs co-cultured with autologous NK cells in the presence of IL-18 and IFNα demonstrated a partially-activated phenotype, manifested by enhanced expression of the co-stimulatory molecules CD80, CD86, and CD40, compared to immature DCs. However, additional co-stimulation with a TLR3/RIG-I/MDA5-ligand, polyinosinic:polycytidylic acid (poly-I:C), was needed to optimize expression of these molecules to levels comparable to the sDC maturation cocktail.
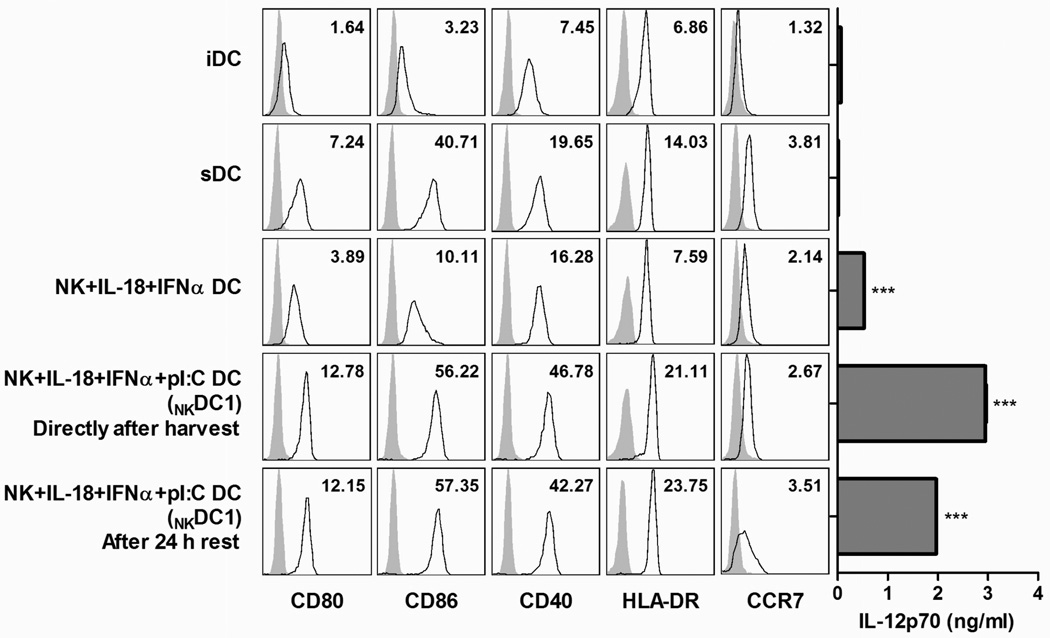
Figure 3. Inclusion of poly-I:C in NK-DC co-cultures results in NKDC1s with optimal surface expression of T cell-activating molecules and CCR7 and optimal ability to produce IL-12p70.
Surface expression (open histograms) of CD80, CD86, CD40, HLA-DR, and CCR7 on untreated immature DCs (iDCs), DCs treated with the standard cytokine maturation cocktail (sDCs), or DCs treated with autologous NK cells and IL-18/IFNα with or without poly-I:C. Surface expression was analyzed directly after DC harvesting or after an additional 24 h of culture in the absence of maturation factors. Gray histograms represent isotype controls. Inset numbers represent fold MFI increase over isotype controls. Right: The corresponding IL-12p70 production after J558-CD40L-stimulation. Data from one representative experiment of three performed, all of which yielded similar results. ***p<0.001 for NKDC1s (NK/IL-18/IFNα/poly-I:C DCs) compared to iDCs, sDCs, and NK/IL-18/IFNα DCs, or for NK/IL-18/IFNα DCs compared to iDCs and sDCs.
Similar to their IL-12-producing capacity, the mature surface phenotype of NKDC1s was maintained after 24 h of additional culture in the absence of maturation factors (Fig. 3, bottom). In all cases, the presence of NK cells in the maturation cultures was critical in inducing optimal DC maturation, compared to the IL-18/IFNα-exposed- or IL-18/IFNα/poly-I:C-exposed DCs (without NK cells) from the same patients (data not shown).
Besides enhanced expression of co-stimulatory factors and the ability to produce high levels of IL-12p70, the capacity of DCs to induce immune responses and serve as effective cancer vaccines is also influenced by their ability to migrate in response to lymph node-produced chemokines, dependent on DC expression of CCR713,41. Similar to maturation-associated co-stimulatory molecules, CCR7 surface expression was enhanced by DC exposure to two-signal-activated NK cells, especially with the additional presence of poly-I:C (Fig. 3, bottom). In agreement with prior reports22, this enhanced expression of CCR7 was found to be functional in terms of migratory responsiveness to CCL21, a lymph node-associated chemokine ligand for CCR7, and was greatly augmented by direct NK-DC cell contact in transwell experiments (Supplemental Fig. 1, Supplemental Digital Content 1, demonstrating transwell CCR7 staining and in vitro CCL21 chemotaxis). Moreover, CCR7 expression on NKDC1s was further modestly increased after 24 h of additional culture in fresh media (Fig. 3, bottom), consistent with our recent report describing the CCR7 regulation in type-1-polarized DCs induced by soluble NK cell-related factors42.
Similar to the enhanced expression of surface molecules involved in T cell stimulation and lymph node-homing, the presence of poly-I:C in the IL-18/IFNα-activated NK-DC co-cultures further augmented the IL-12p70-producing capacity of DCs (Fig. 3, right), making such conditions preferable for our prospective applications. While poly-I:C stimulation alone can result in the augmentation of IL-12-production by maturing DCs43, the high capacity for IL-12-production observed in NKDC1s could not be stably imprinted by the combination of IL-18, IFNα, and poly-I:C in the absence of NK cells (Supplemental Fig. 2, Supplemental Digital Content 2, demonstrating IL-12p70 production by DCs exposed to cytokine combinations in the absence or presence of NK cells), demonstrating the key role for NK cells.
NKDC1s induce high numbers of tumor-specific CTLs capable of recognizing multiple melanoma antigens and killing melanoma cells
To determine the relative ability of NKDC1s to induce tumor-specific CTL responses, NKDC1s generated from HLA-A2+ melanoma patients (stage III and IV) were loaded with HLA-A2-restricted melanoma-associated antigenic peptides and used to sensitize autologous blood-isolated CD8+ T cells in vitro. Parallel control cultures included iDCs or sDCs, in order to compare NKDC1s to, respectively, immature/partially-mature DCs used in the FDA-approved prostate vaccine44, or to fully-mature DCs extensively tested in past clinical trials37,40. DCs exposed to IL-18/IFNα/poly-I:C in the absence of NK cells were also used as additional controls. Following two rounds of in vitro sensitization, the expanded CD8+ T cells were harvested and used as responders against HLA-A2+ T2 cells pulsed with individual peptides, or against HLA-A2+ melanoma cells (FEM-X) or control HLA-A2− melanoma cells (MEL-397).
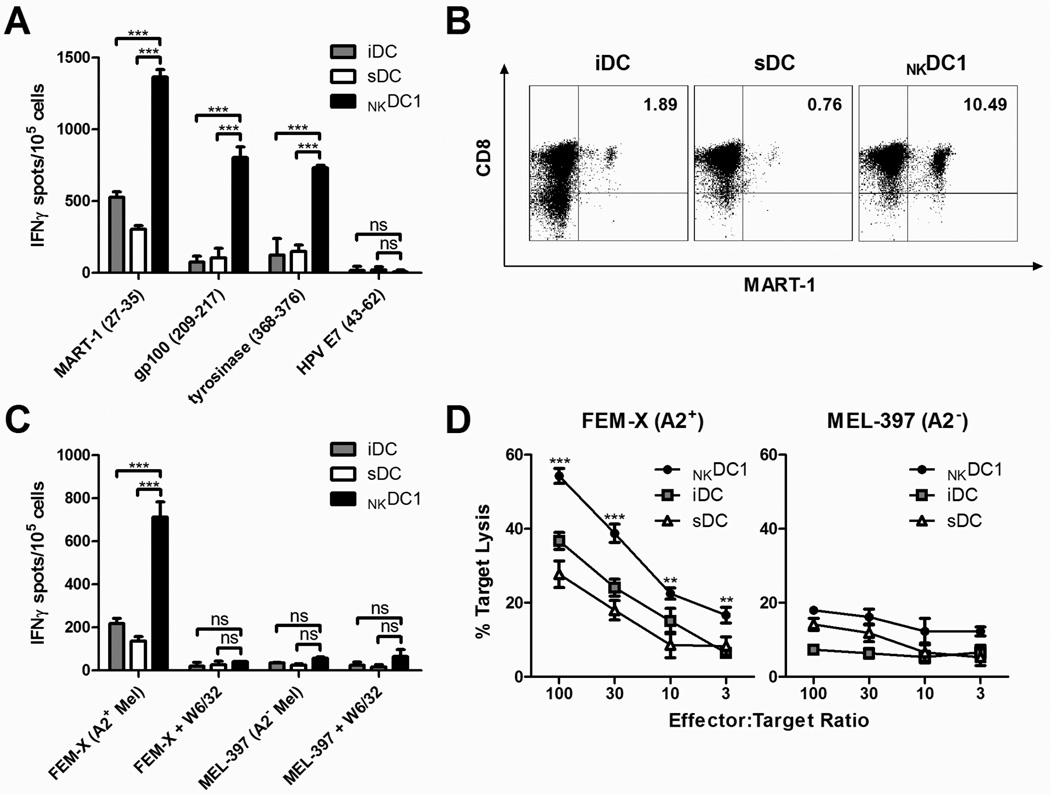
As shown in Figures 4A and 4B, NKDC1s proved to be superior in generating high numbers of functional melanoma-specific CTLs, as determined by IFNγ ELISPOT against distinct MART-1, gp100, and tyrosinase epitopes, and by tetramer staining of MART-1-specific T cell receptors. This enhanced CTL-inducing activity of NKDC1s was strictly dependent on the presence of NK cells, and was not observed in the DCs activated by IL-18/IFNα/poly-I:C alone (Supplemental Fig. 3, Supplemental Digital Content 3, demonstrating melanoma-antigen-specific CTL induction by IL-18/IFNα/poly-I:C-treated DCs in the absence or presence of NK cells). Importantly, NKDC1-sensitized CD8+ T cell cultures contained a larger percentage of CTLs not only capable of specifically recognizing peptide-loaded T2 cells, but also capable of specifically detecting and killing HLA-A2+ melanoma cells (Figs. 4C,D). This demonstrates that NKDC1-sensitized CD8+ T cells are able to detect physiologic amounts of tumor-associated antigens and are capable of killing actual tumor cells, which often show enhanced resistance to immune elimination45–47.
Figure 4. NKDC1s are efficient inducers of melanoma-specific CTLs.
Immature (i)DCs, sDCs, and NKDC1s from HLA-A2+ stage III and stage IV melanoma patients were pulsed with MHC Class I-restricted melanoma-associated peptides and used to sensitize autologous CD8+ T cells. CTLs were assayed on day 24 of culture. A, Frequencies of IFNγ-producing CD8+ T cells responsive to T2 cells loaded with individual peptides, as determined by ELISPOT assay. Data recorded as the mean (± SD) of triplicate cultures. Data shown is from one representative experiment of three performed. ***p<0.001, ns: p>0.05. B, Flow cytometric analysis showing percentage of tetramer-positive MART-1-specific CD8+ T cells generated through in vitro stimulation with melanoma peptide-pulsed, differentially-activated DCs. Inset numbers represent percentages of CD8+ MART-1+ cells. Results from one representative experiment of three performed. C, Frequencies of IFNγ-producing CD8+ T cells responsive to the relevant (HLA-A2+) and irrelevant (HLA-A2−) target melanoma cell lines FEM-X and MEL-397, respectively, as determined by ELISPOT assay. Blockade with the W6/32 pan-MHC Class I-neutralizing antibody was used to demonstrate the MHC Class I-dependence of the T cell recognition. Data recorded as the mean (± SD) of triplicate cultures. Data shown is from one representative experiment of three performed. ***p<0.001, ns: p>0.05. D, Antigen-specific cytotoxic activity of CTLs induced by NKDC1s, iDCs, or sDCs against FEM-X (HLA-A2+) and MEL-397 (HLA-A2−) melanoma cell lines, as determined by standard 4 h 51Cr-release assay. Data recorded as the mean (± SD) of triplicate cultures. Similar data were obtained in two additional experiments. ***p<0.001, **p<0.01 compared to all groups.
Discussion
Numerous preclinical studies and clinical trials have individually employed either NK cells or DCs as tools in the immunotherapy of cancer. While the results of animal studies, as well as observations of clinical responses in individual cancer patients, have shown the potential benefits of such NK- and DC-based cancer therapies, their overall clinical efficacy has been disappointing48–50. The current results and recent demonstrations that NK cells can play a critical immunoregulatory “helper” role to support the induction of Th1 and CTL-mediated responses in mouse models31–35 and human in vitro studies29,36 suggest the potential for improving the effectiveness of DC-based anti-tumor vaccination strategies by exploiting the interactions between NK cells and antigen-carrying DCs.
We previously showed that type-I IFNs and tumor-associated activation ligands expressed on NK-sensitive K562 cells can synergistically induce the NK-mediated polarization of DC1s in healthy donors, in a mechanism dependent on IFNγ production. The current study, showing consistent generation in serum-free conditions of functional NKDC1s from the blood of different patients with advanced (stage III and IV) melanoma, demonstrates the potential for translating these findings into clinically-relevant settings using NK cells and DCs isolated directly from cancer patients. The ability of IL-18 to act in synergy with IFNα as a substitute for tumor lines provides a user-friendly, highly-reproducible, and potentially safer method of harnessing the DC1-polarizing activity of NK cells. The added positive effect of poly-I:C on the IL-12-producing function of NKDC1s and their expression of maturation-associated co-stimulatory and lymph node-homing molecules is consistent with its ability to enhance the cross-talk between NK cells and DCs recently observed in human in vitro51,52 and mouse in vivo53–55 settings.
In addition to promoting effective NK-DC interactions (and the resulting type-1 polarization of DCs) ex vivo during the generation of cell-based vaccines, the two-signal activation paradigm required for NK cell helper activity provides a rationale for in vivo approaches involving co-delivery of such cytokines as IL-18 and IFNα, or the combination of IFNα and tumor-specific opsonizing antibodies. Such therapies are likely to be particularly effective when further combined with adoptive transfer of ex vivo expanded/activated NK cells. In the case of antibody-utilizing therapies, in addition to the IgG1- or IgG3-antibody-triggered activation of CD16 on NK cells and resulting cytokine production56,57 and DC1-polarization29, antibody-directed NK-mediated lysis of nominally NK cell-resistant tumors may also provide potential antigen for cross-presentation by bystander DC, further enhancing active immunization.
A number of questions still remain concerning the potential differential impact of distinct combinations of NK cell-activating factors on NK cells and their ability to modulate DC function. It has been shown that NK cells, in analogy to Th cell differentiation, can also differentiate into polarized subsets displaying different cytokine patterns, producing a wide variety of factors including IFNγ, TNFα, IL-4, IL-5, IL-13, and IL-10 with both immune-stimulatory and immune-suppressive functions58. It is therefore conceivable that depending on the mode of their activation, instead of promoting DC-mediated type-1 immunity, NK cells activated by a particular stimulus may instead drive a type-2 response59,60, or even suppress DC function altogether61, thus highlighting the need for the careful selection of NK cell-activating signals to be used in clinical settings.
The current data demonstrate the feasibility and rationale for the clinical application of immunotherapies of melanoma and other cancers utilizing the positive feedback between NK cells and DCs. The high activity of antigen-loaded NKDC1s in inducing tumor-specific CTLs makes them interesting candidates for clinical evaluation as cancer vaccines as an alternative to standard DCs or type-1-polarized DCs induced in less physiologic conditions, using the combination of NK cell-related soluble factors17. While NK-derived IFNγ appears to be the obligatory polarizing component in the development of NKDC1s18,29, additional factors may also likely be involved, as indicated in our transwell experiments demonstrating enhanced DC function when direct contact between the two cell types was permitted. This latter effect may indicate the involvement of additional membrane-bound molecules, as observed in related systems26–28,62, but may also reflect the close proximity of the two cell types and higher concentrations of soluble factors. The potential contribution of additional NK cell-related factors to the helper activity of NK cells and the phenomena of DC activation is a subject of our current analyses.
Supplementary Material
Supplemental Digital Content 1. Supplemental Figure 1 demonstrating transwell CCR7 staining and in vitro CCL21 chemotaxis.
NKDC1s have enhanced lymph node migratory capacity. Surface expression of CCR7 (left; shaded histograms) and in vitro migration toward CCL21 (right) of untreated immature DCs (iDCs) or DCs treated with autologous NK cells and IL-18/IFNα/poly-I:C (NKDC1s) in direct or transwell-separated co-cultures.
Supplemental Digital Content 2. Supplemental Figure 2 demonstrating IL-12p70 production by DCs exposed to cytokine combinations in the absence or presence of NK cells.
NK cell requirement for the optimal induction of IL-12p70-producing capacity of DCs co-stimulated with poly-I:C. IL-12p70 production by J558-CD40L-stimulated immature DCs (iDCs), DCs treated with the standard cytokine cocktail (sDCs), or DCs treated with IL-18/IFNa or IL-18/IFNα/poly-I:C with or without autologous NK cells. Data recorded as the mean (± SD) of triplicate cultures. Data shown was obtained from one representative experiment of three performed, all yielding similar results.
Supplemental Digital Content 3. Supplemental Figure 3 demonstrating melanoma-antigen-specific CTL induction by IL-18/IFNα/poly-I:C-treated DCs in the absence or presence of NK cells.
NK cell requirement for the optimal induction of DC1s with a high capacity to induce melanoma-antigen-specific CTLs. IL-18/IFNα/poly-I:C-stimulated DCs in the absence or presence (NKDC1s) of autologous NK cells from HLA-A2+ stage III and stage IV melanoma patients were pulsed with HLA-A2-restricted melanoma-associated peptides and used to sensitize autologous CD8+ T cells. CTLs were assayed on day 24 of culture. A, Frequencies of IFNγ-producing CD8+ T cells responsive to T2 cells loaded with individual peptides, as determined by ELISPOT assay. Data recorded as the mean (± SD) of triplicate cultures. Data shown is from one representative experiment of three performed. ***p<0.001, ns: p>0.05. B, Flow cytometric analysis showing percentage of tetramer-positive MART-1-specific CD8+ T cells generated through in vitro stimulation with melanoma peptide-pulsed, differentially-activated DCs. Inset numbers represent percent CD8+MART-1+ cells. Results from one representative experiment of three performed.
Acknowledgements
This work was supported by the NIH grants CA095128, CA101944, and CA114931, and the Clinical and Translational Science Institute Multidisciplinary Predoctoral Fellowship Program (5TL1RR024155-04). The authors thank Drs. Julie Urban and Eva Wieckowski for stimulating discussions and critically reading the manuscript, and Tina Kilgore and Sharon Coutch for administrative assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: All authors have declared there are no financial conflicts of interest in regards to this work.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–567. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 3.Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 4.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 5.van Beelen AJ, Zelinkova Z, Taanman-Kueter EW, et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27:660–669. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 7.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 8.Kalinski P, Giermasz A, Nakamura Y, et al. Helper role of NK cells during the induction of anticancer responses by dendritic cells. Mol Immunol. 2005;42:535–539. doi: 10.1016/j.molimm.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 9.Kalinski P, Moser M. Consensual immunity: success-driven development of T-helper-1 and T-helper-2 responses. Nat Rev Immunol. 2005;5:251–260. doi: 10.1038/nri1569. [DOI] [PubMed] [Google Scholar]
- 10.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 11.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 12.Steinman RM. Dendritic cells in vivo: a key target for a new vaccine science. Immunity. 2008;29:319–324. doi: 10.1016/j.immuni.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 13.de Vries IJ, Lesterhuis WJ, Scharenborg NM, et al. Maturation of dendritic cells is a prerequisite for inducing immune responses in advanced melanoma patients. Clin Cancer Res. 2003;9:5091–5100. [PubMed] [Google Scholar]
- 14.Kalinski P, Schuitemaker JH, Hilkens CM, Wierenga EA, Kapsenberg ML. Final maturation of dendritic cells is associated with impaired responsiveness to IFN-gamma and to bacterial IL-12 inducers: decreased ability of mature dendritic cells to produce IL-12 during the interaction with Th cells. J Immunol. 1999;162:3231–3236. [PubMed] [Google Scholar]
- 15.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000;1:311–316. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 16.Kalinski P, Vieira PL, Schuitemaker JH, de Jong EC, Kapsenberg ML. Prostaglandin E(2) is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood. 2001;97:3466–3469. doi: 10.1182/blood.v97.11.3466. [DOI] [PubMed] [Google Scholar]
- 17.Mailliard RB, Wankowicz-Kalinska A, Cai Q, et al. alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64:5934–5937. doi: 10.1158/0008-5472.CAN-04-1261. [DOI] [PubMed] [Google Scholar]
- 18.Vieira PL, de Jong EC, Wierenga EA, Kapsenberg ML, Kalinski P. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J Immunol. 2000;164:4507–4512. doi: 10.4049/jimmunol.164.9.4507. [DOI] [PubMed] [Google Scholar]
- 19.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 20.Albert ML, Jegathesan M, Darnell RB. Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nat Immunol. 2001;2:1010–1017. doi: 10.1038/ni722. [DOI] [PubMed] [Google Scholar]
- 21.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dieu MC, Vanbervliet B, Vicari A, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zitvogel L, Robbins PD, Storkus WJ, et al. Interleukin-12 and B7.1 co-stimulation cooperate in the induction of effective antitumor immunity and therapy of established tumors. Eur J Immunol. 1996;26:1335–1341. doi: 10.1002/eji.1830260624. [DOI] [PubMed] [Google Scholar]
- 24.Nishioka Y, Hirao M, Robbins PD, Lotze MT, Tahara H. Induction of systemic and therapeutic antitumor immunity using intratumoral injection of dendritic cells genetically modified to express interleukin 12. Cancer Res. 1999;59:4035–4041. [PubMed] [Google Scholar]
- 25.Xu S, Koski GK, Faries M, et al. Rapid high efficiency sensitization of CD8+ T cells to tumor antigens by dendritic cells leads to enhanced functional avidity and direct tumor recognition through an IL-12-dependent mechanism. J Immunol. 2003;171:2251–2261. doi: 10.4049/jimmunol.171.5.2251. [DOI] [PubMed] [Google Scholar]
- 26.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Munz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343–351. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195:335–341. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–333. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mailliard RB, Son YI, Redlinger R, et al. Dendritic cells mediate NK cell help for Th1 and CTL responses: two-signal requirement for the induction of NK cell helper function. J Immunol. 2003;171:2366–2373. doi: 10.4049/jimmunol.171.5.2366. [DOI] [PubMed] [Google Scholar]
- 30.Agaugue S, Marcenaro E, Ferranti B, Moretta L, Moretta A. Human natural killer cells exposed to IL-2, IL-12, IL-18, or IL-4 differently modulate priming of naive T cells by monocyte-derived dendritic cells. Blood. 2008;112:1776–1783. doi: 10.1182/blood-2008-02-135871. [DOI] [PubMed] [Google Scholar]
- 31.Martin-Fontecha A, Thomsen LL, Brett S, et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5:1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 32.Kelly JM, Darcy PK, Markby JL, et al. Induction of tumor-specific T cell memory by NK cell-mediated tumor rejection. Nat Immunol. 2002;3:83–90. doi: 10.1038/ni746. [DOI] [PubMed] [Google Scholar]
- 33.Strbo N, Oizumi S, Sotosek-Tokmadzic V, Podack ER. Perforin is required for innate and adaptive immunity induced by heat shock protein gp96. Immunity. 2003;18:381–390. doi: 10.1016/s1074-7613(03)00056-6. [DOI] [PubMed] [Google Scholar]
- 34.Mocikat R, Braumuller H, Gumy A, et al. Natural killer cells activated by MHC class I(low) targets prime dendritic cells to induce protective CD8 T cell responses. Immunity. 2003;19:561–569. doi: 10.1016/s1074-7613(03)00264-4. [DOI] [PubMed] [Google Scholar]
- 35.Westwood JA, Kelly JM, Tanner JE, Kershaw MH, Smyth MJ, Hayakawa Y. Cutting edge: novel priming of tumor-specific immunity by NKG2D-triggered NK cell-mediated tumor rejection and Th1-independent CD4+ T cell pathway. J Immunol. 2004;172:757–761. doi: 10.4049/jimmunol.172.2.757. [DOI] [PubMed] [Google Scholar]
- 36.Mailliard RB, Alber SM, Shen H, et al. IL-18-induced CD83+CCR7+ NK helper cells. J Exp Med. 2005;202:941–953. doi: 10.1084/jem.20050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jonuleit H, Kuhn U, Muller G, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 38.Kapsenberg ML, Kalinski P. The concept of type 1 and type 2 antigen-presenting cells. Immunol Lett. 1999;69:5–6. doi: 10.1016/s0165-2478(99)00096-6. [DOI] [PubMed] [Google Scholar]
- 39.Chen IH, Lai YL, Wu CL, et al. Immune impairment in patients with terminal cancers: influence of cancer treatments and cytomegalovirus infection. Cancer Immunol Immunother. 2009 doi: 10.1007/s00262-009-0753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Engell-Noerregaard L, Hansen TH, Andersen MH, Thor Straten P, Svane IM. Review of clinical studies on dendritic cell-based vaccination of patients with malignant melanoma: assessment of correlation between clinical response and vaccine parameters. Cancer Immunol Immunother. 2009;58:1–14. doi: 10.1007/s00262-008-0568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Vries IJ, Krooshoop DJ, Scharenborg NM, et al. Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Cancer Res. 2003;63:12–17. [PubMed] [Google Scholar]
- 42.Muthuswamy R, Mueller-Berghaus J, Haberkorn U, Reinhart TA, Schadendorf D, Kalinski P. PGE(2) transiently enhances DC expression of CCR7 but inhibits the ability of DCs to produce CCL19 and attract naive T cells. Blood. 2010;116:1454–1459. doi: 10.1182/blood-2009-12-258038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verdijk RM, Mutis T, Esendam B, et al. Polyriboinosinic polyribocytidylic acid (poly(I:C)) induces stable maturation of functionally active human dendritic cells. J Immunol. 1999;163:57–61. [PubMed] [Google Scholar]
- 44.Small EJ, Fratesi P, Reese DM, et al. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol. 2000;18:3894–3903. doi: 10.1200/JCO.2000.18.23.3894. [DOI] [PubMed] [Google Scholar]
- 45.Huang B, Zhao J, Li H, et al. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 2005;65:5009–5014. doi: 10.1158/0008-5472.CAN-05-0784. [DOI] [PubMed] [Google Scholar]
- 46.Ravi R, Fuchs EJ, Jain A, et al. Resistance of cancers to immunologic cytotoxicity and adoptive immunotherapy via X-linked inhibitor of apoptosis protein expression and coexisting defects in mitochondrial death signaling. Cancer Res. 2006;66:1730–1739. doi: 10.1158/0008-5472.CAN-05-3377. [DOI] [PubMed] [Google Scholar]
- 47.Su Z, Kuball J, Barreiros AP, et al. Nitric oxide promotes resistance to tumor suppression by CTLs. J Immunol. 2006;176:3923–3930. doi: 10.4049/jimmunol.176.7.3923. [DOI] [PubMed] [Google Scholar]
- 48.Basse PH, Whiteside TL, Chambers W, Herberman RB. Therapeutic activity of NK cells against tumors. Int Rev Immunol. 2001;20:439–501. doi: 10.3109/08830180109054416. [DOI] [PubMed] [Google Scholar]
- 49.Engleman EG. Dendritic cell-based cancer immunotherapy. Semin Oncol. 2003;30:23–29. doi: 10.1016/s0093-7754(03)00229-x. [DOI] [PubMed] [Google Scholar]
- 50.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perrot I, Deauvieau F, Massacrier C, et al. TLR3 and Rig-like receptor on myeloid dendritic cells and Rig-like receptor on human NK cells are both mandatory for production of IFN-gamma in response to double-stranded RNA. J Immunol. 2010;185:2080–2088. doi: 10.4049/jimmunol.1000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gerosa F, Gobbi A, Zorzi P, et al. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J Immunol. 2005;174:727–734. doi: 10.4049/jimmunol.174.2.727. [DOI] [PubMed] [Google Scholar]
- 53.Miyake T, Kumagai Y, Kato H, et al. Poly I:C-induced activation of NK cells by CD8 alpha+ dendritic cells via the IPS-1 and TRIF-dependent pathways. J Immunol. 2009;183:2522–2528. doi: 10.4049/jimmunol.0901500. [DOI] [PubMed] [Google Scholar]
- 54.Akazawa T, Ebihara T, Okuno M, et al. Antitumor NK activation induced by the Toll-like receptor 3-TICAM-1 (TRIF) pathway in myeloid dendritic cells. Proc Natl Acad Sci U S A. 2007;104:252–257. doi: 10.1073/pnas.0605978104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kamath AT, Sheasby CE, Tough DF. Dendritic cells and NK cells stimulate bystander T cell activation in response to TLR agonists through secretion of IFN-alpha beta and IFN-gamma. J Immunol. 2005;174:767–776. doi: 10.4049/jimmunol.174.2.767. [DOI] [PubMed] [Google Scholar]
- 56.Carson WE, Parihar R, Lindemann MJ, et al. Interleukin-2 enhances the natural killer cell response to Herceptin-coated Her2/neu-positive breast cancer cells. Eur J Immunol. 2001;31:3016–3025. doi: 10.1002/1521-4141(2001010)31:10<3016::aid-immu3016>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 57.Parihar R, Dierksheide J, Hu Y, Carson WE. IL-12 enhances the natural killer cell cytokine response to Ab-coated tumor cells. J Clin Invest. 2002;110:983–992. doi: 10.1172/JCI15950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peritt D, Robertson S, Gri G, Showe L, Aste-Amezaga M, Trinchieri G. Differentiation of human NK cells into NK1 and NK2 subsets. J Immunol. 1998;161:5821–5824. [PubMed] [Google Scholar]
- 59.Aktas E, Akdis M, Bilgic S, et al. Different natural killer (NK) receptor expression and immunoglobulin E (IgE) regulation by NK1 and NK2 cells. Clin Exp Immunol. 2005;140:301–309. doi: 10.1111/j.1365-2249.2005.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marcenaro E, Della Chiesa M, Bellora F, et al. IL-12 or IL-4 prime human NK cells to mediate functionally divergent interactions with dendritic cells or tumors. J Immunol. 2005;174:3992–3998. doi: 10.4049/jimmunol.174.7.3992. [DOI] [PubMed] [Google Scholar]
- 61.Moretta A, Marcenaro E, Sivori S, Della Chiesa M, Vitale M, Moretta L. Early liaisons between cells of the innate immune system in inflamed peripheral tissues. Trends Immunol. 2005;26:668–675. doi: 10.1016/j.it.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 62.Vitale M, Della Chiesa M, Carlomagno S, et al. NK-dependent DC maturation is mediated by TNFalpha and IFNgamma released upon engagement of the NKp30 triggering receptor. Blood. 2005;106:566–571. doi: 10.1182/blood-2004-10-4035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Supplemental Figure 1 demonstrating transwell CCR7 staining and in vitro CCL21 chemotaxis.
NKDC1s have enhanced lymph node migratory capacity. Surface expression of CCR7 (left; shaded histograms) and in vitro migration toward CCL21 (right) of untreated immature DCs (iDCs) or DCs treated with autologous NK cells and IL-18/IFNα/poly-I:C (NKDC1s) in direct or transwell-separated co-cultures.
Supplemental Digital Content 2. Supplemental Figure 2 demonstrating IL-12p70 production by DCs exposed to cytokine combinations in the absence or presence of NK cells.
NK cell requirement for the optimal induction of IL-12p70-producing capacity of DCs co-stimulated with poly-I:C. IL-12p70 production by J558-CD40L-stimulated immature DCs (iDCs), DCs treated with the standard cytokine cocktail (sDCs), or DCs treated with IL-18/IFNa or IL-18/IFNα/poly-I:C with or without autologous NK cells. Data recorded as the mean (± SD) of triplicate cultures. Data shown was obtained from one representative experiment of three performed, all yielding similar results.
Supplemental Digital Content 3. Supplemental Figure 3 demonstrating melanoma-antigen-specific CTL induction by IL-18/IFNα/poly-I:C-treated DCs in the absence or presence of NK cells.
NK cell requirement for the optimal induction of DC1s with a high capacity to induce melanoma-antigen-specific CTLs. IL-18/IFNα/poly-I:C-stimulated DCs in the absence or presence (NKDC1s) of autologous NK cells from HLA-A2+ stage III and stage IV melanoma patients were pulsed with HLA-A2-restricted melanoma-associated peptides and used to sensitize autologous CD8+ T cells. CTLs were assayed on day 24 of culture. A, Frequencies of IFNγ-producing CD8+ T cells responsive to T2 cells loaded with individual peptides, as determined by ELISPOT assay. Data recorded as the mean (± SD) of triplicate cultures. Data shown is from one representative experiment of three performed. ***p<0.001, ns: p>0.05. B, Flow cytometric analysis showing percentage of tetramer-positive MART-1-specific CD8+ T cells generated through in vitro stimulation with melanoma peptide-pulsed, differentially-activated DCs. Inset numbers represent percent CD8+MART-1+ cells. Results from one representative experiment of three performed.