Abstract
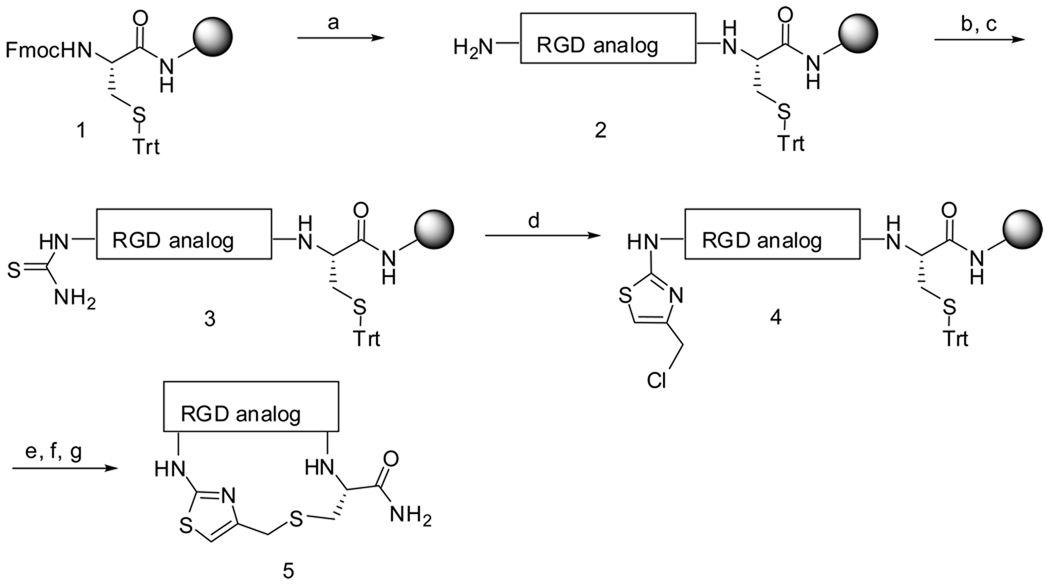
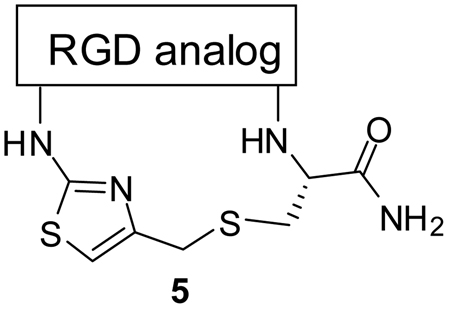
The synthesis of conformationally constrained RGD-containing integrin ligands via an efficient solid-phase intramolecular thioalkylation reaction is described. The reaction of S-nucleophiles with newly generated N-terminal 4-chloromethyl thiazoles leads to the desired cyclic RGD products 5 in high purities and good overall yields.
The RGD sequence is a well known binding motif for specific transmembrane proteins and is involved in cellular adhesion to the extracellular matrix (ECM).1 There’s a continuous interest in the development of RGD-based therapeutics that function either as agonists to promote the interaction of cells and tissues with artificial matrices, or as antagonists to control the nature of cell-cell and cell-ECM interactions. Many reviews have reported general and specific topics related to the field of integrin.2
Cyclic RGD peptides have been developed for various applications including fibrinogen receptor antagonists,3 selective αVβ3 integrin antagonists for the treatment of human tumor metastasis and tumor-induced angiogenesis,4 phagocitosis of cells undergoing apoptosis, bone remodeling and osteoporosis, diabetic retinopathy, tumor imaging and targeting2a and acute renal failure.4 RGD-containing cyclic peptides were used to target tetraphenylchlorin as novel photosensitizers for selective photodynamic activity.5 The cyclization of RGD containing peptides restricts the flexibility of peptides and, therefore, offers the possibility to present the RGD sequence in a specific conformation to provide enhanced selectivity of the ligands toward the receptors. Furthermore cyclic peptides are often more stable to peptidases, and therefore they can have improved pharmacokinetic profiles and represent promising lead compounds for further development.6
Reported approaches on the solid-phase synthesis of RGD containing cyclic peptides include intramolecular Heck reaction;7 nucleophilic substitution,8 intramolecular amide formation,9 disulfide formation,10 intramolecular Suzuki reactions,11 SNAr displacement reactions involving haloaryl electrophiles,12 intramolecular urea bond formation,13 ring closing metathesis reaction,14 and more recently click chemistry.15
Of particular interest, thioalkylation reactions offer a facile and versatile approach to the solid-phase synthesis of cyclic peptides.16,17 Examples of macrocyclizations via thioalkylation include the reaction of the thiol group of a C-terminal cysteine with an N-terminal acetyl bromide or N-terminal benzyl bromide.16,17 A conceptually different approach, wherein thioalkylation proceeds via Michael addition of a thiolate anion to an α,β-unsaturated ester, has been reported for the synthesis of cyclic thioether dipeptides,18 and more recently thioalkylation using thiol–ene click chemistry was applied for the generation of RGD analogs.19
Due to the potential clinical advantages of cyclic peptides, and the importance of the RGD motif for integrin-recognition, there remains an unmet need to develop rapid and efficient synthetic strategies for the combinatorial generation of RGD-containing cyclic peptides. Herein, we describe an efficient approach toward the generation of a new class of cyclic RGD peptides by an intramolecular thioalkylation reaction by the halogen displacement of N-terminus 4-chloro methyl thiazole peptide with the thiol group of cysteine.
As outlined in scheme 1, we performed the parallel synthesis of different thiazolyl containing RGD cyclic peptides. Starting from p-methylbenzhydrylamine hydrochloride (MBHA·HCl) resin-bound orthogonally protected Fmoc-Cys-(Trt)-OH 1, the thio-methyl thiazolyl macrocyclic peptidomimetics 5 were synthesized following stepwise Fmoc deprotection and standard repetitive Fmoc-amino-acid couplings20 yielding the linear RGD peptide 2. The resulting N-terminal free amine was treated with Fmoc-isothiocyanate. Following Fmoc deprotection, the thiourea was treated with 1,3-dichloroacetone (Hantzsch’s cyclocondensation21) to afford the resulting resin-bound chloromethyl thiazolyl peptide 4. The Trt group was removed with 5%TFA in DCM and the resin-bound peptide was treated with a solution of Cs2CO3 in DMF to effect an SN2 intramolecular thioalkylation reaction. The resin was treated with HF/anisole and the desired thiazolyl thioether cyclic peptides 5 were released and obtained in good yield and high purity.
We performed the parallel synthesis of 25 RGD cyclic peptides with different sizes ranging from tetrapeptide 5a, pentapeptides 5b–5g and hexapeptides 5h–5z (Table 1). All peptides were obtained in good yield and purity. The final products were purified by preparative HPLC and the identity of the products was confirmed by LC-MS and NMR spectroscopy. The NMR data show a clear singlet between 6.4 and 6.8 ppm which is specific to the proton on C-5 of the aminothiazole ring.
Table 1.
RGD-Containing Cyclic Peptides
 | ||||
|---|---|---|---|---|
| RGD analog | Observed MW | Purity (%)a | Yieldb | |
| 5a | -Arg-Gly-Asp- | 544.6 (MH+) | 81 | 45 |
| 5b | -Arg-Gly-Asp-Tyr- | 707.9 (MH+) | 83 | 42 |
| 5c | -Arg-Gly-Asp-Phe- | 691.7 (MH+) | 80 | 41 |
| 5d | -Arg-Gly-Asp-Leu- | 657.7 (MH+) | 76 | 38 |
| 5e | -Tyr-Arg-Gly-Asp- | 707.7 (MH+) | 84 | 43 |
| 5f | -Phe-Arg-Gly-Asp- | 691.7 (MH+) | 82 | 44 |
| 5g | -Leu-Arg-Gly-Asp- | 657.7 (MH+) | 84 | 42 |
| 5h | -Arg-Gly-Asp-Tyr-Tyr- | 870.9 (MH+) | 82 | 40 |
| 5i | -Arg-Gly-Asp-Phe-Tyr- | 854.9 (MH+) | 85 | 47 |
| 5j | -Arg-Gly-Asp-Leu-Tyr- | 820.9 (MH+) | 78 | 41 |
| 5k | -Arg-Gly-Asp-Tyr-Phe- | 854.9 (MH+) | 86 | 43 |
| 5l | -Arg-Gly-Asp-Phe-Phe- | 838.9 (MH+) | 88 | 45 |
| 5m | -Arg-Gly-Asp-Leu-Phe- | 804.9 (MH+) | 85 | 42 |
| 5n | -Arg-Gly-Asp-Tyr-Leu- | 820.9 (MH+) | 79 | 40 |
| 5o | -Arg-Gly-Asp-Phe-Leu- | 804.9 (MH+) | 82 | 39 |
| 5p | -Arg-Gly-Asp-Leu-Leu- | 770.9 (MH+) | 84 | 43 |
| 5q | -Tyr-Tyr-Arg-Gly-Asp- | 870.9 (MH+) | 80 | 41 |
| 5r | -Phe-Tyr-Arg-Gly-Asp- | 854.9 (MH+) | 83 | 44 |
| 5s | -Leu-Tyr-Arg-Gly-Asp- | 820.9 (MH+) | 81 | 42 |
| 5t | -Leu-Phe-Arg-Gly-Asp- | 854.9 (MH+) | 77 | 39 |
| 5u | -Phe-Phe-Arg-Gly-Asp- | 838.9 (MH+) | 79 | 41 |
| 5v | -Leu-Phe-Arg-Gly-Asp- | 804.9 (MH+) | 80 | 43 |
| 5w | -Tyr-Leu-Arg-Gly-Asp- | 820.9 (MH+) | 74 | 35 |
| 5x | -Phe-Leu-Arg-Gly-Asp- | 804.9 (MH+) | 81 | 40 |
| 5y | -Leu-Leu-Arg-Gly-Asp- | 769.9 (MH+) | 82 | 44 |
The products were run on a Vydac column, gradients 5 to 95% formic acid (0.1%) in ACN in 10 min. The purity was estimated on analytical traces at λ= 214 nm and 254 nm.
Yields are based on the amount of isolated purified products and are relative to the initial loading of the resin.
Scheme 1.
Reagents and conditions: (a) Solid-phase peptide synthesis using Fmoc chemistry; (b) FmocNCS (6 equiv) in DMF (0.3 M), RT, overnight; (c) 20% piperidine/DMF; (d) 1,3-dichloroacetone (10 equiv) in DMF (0.3 M), 70 °C, overnight; (e) TFA/(But)3SiH/DCM (5:5:90), 30 min; (f) Cs2CO3 in DMF overnight; (g) HF/anisole, 0 °C, 90 min.
Acknowledgment
The authors would like to thank the State of Florida Funding, NIH (1R03DA025850-01A1, Nefzi), NIH (5P41GM081261-03, Houghten) and NIH (3P41GM079590-03S1, Houghten) for their financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES AND NOTES
- 1.a) Weide T, Modlinger A, Kessler H. Top Curr Chem. 2007;272:1–50. [Google Scholar]; (b) Ruoslahti E. Annu. Rev. Cell Dev. Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]; (c) Hynes RO. Cell. 1987;48:549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- 2.(a) Meyer A, Auernheimer AM, Kessler H. Current Pharmaceutical Design. 2006;12:2723–2724. doi: 10.2174/138161206777947740. [DOI] [PubMed] [Google Scholar]; (b) Hynes RO. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]; (c) Xiong JP, Stehle T, Goodman SL, Arnaout MAJ. Thromb Haemost. 2003;1:1642–1654. doi: 10.1046/j.1538-7836.2003.00277.x. [DOI] [PubMed] [Google Scholar]; (d) Shimaoka M, Springer TA. Nat Rev Drug Discov. 2003;2:703–716. doi: 10.1038/nrd1174. [DOI] [PubMed] [Google Scholar]
- 3.Samanen J, Ali F, Bean J, Callahan J, Huffman W, Kopple K, Peishoff C, et al. Cell. Adhes. 1994:259–290. [Google Scholar]
- 4.Schaffner P, Dard MM. CMLS, Cell. Mol. Life Sci. 2003;60:119–132. doi: 10.1007/s000180300008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frochot C, Di Stasio B, Vanderesse R, Belgy M-J, Dodeller M, Guillemin F, Viriot M-L, Barberi-Heyob M. Bioorg Chem. 2007;35:205–220. doi: 10.1016/j.bioorg.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Giannis A, Kolter T. Angew Chem. Int. Ed. Engl. 1993;32:1244–1267. [Google Scholar]; Bogdanowick-Knipps SJ, CHakrabarti S, Williams TD, Dillman RK, Siahaan TJ. J. Pept. Res. 1999;53:530–541. doi: 10.1034/j.1399-3011.1999.00052.x. [DOI] [PubMed] [Google Scholar]
- 7.Akaji K, Teruya K, Akaji M, Aimoto S. Tetrahedron. 2001;57:2293–2303. [Google Scholar]
- 8.Barker PL, et al. J. Med. Chem. 1992;35:2040–2048. doi: 10.1021/jm00089a014. [DOI] [PubMed] [Google Scholar]
- 9.a) Alcaro MC, Sabatino G, Uziel J, Chelli M, Ginanneschi M, Rovero P, Papini AM. Journal of Peptide Science. 2004;10:218–228. doi: 10.1002/psc.512. [DOI] [PubMed] [Google Scholar]; b) van Well RM, Overkleeft HS, van der Marel GA, Bruss D, Thibault G, de Groot PG, van Boom JH, Overhand M. Bioorganic & Medicinal Chemistry Letters. 2003;13:331–334. doi: 10.1016/s0960-894x(02)01022-3. [DOI] [PubMed] [Google Scholar]; c) Besong G, Billen D, Dager I, Kocienski P, Sliwinski E, Tai LR, Boyle TF. Tetrahedron. 2008;64:4700–4710. [Google Scholar]; d) Miiller A, Schumann F, Koksch M, Sewald N. Letters in Peptide Science. 1997;4:275–281. [Google Scholar]
- 10.Cheng S, Craig WS, Mullen D, Tschopp JF, Dixon D, Pierschbachert MD. J Med Chem. 1994;37:1. doi: 10.1021/jm00027a001. [DOI] [PubMed] [Google Scholar]
- 11.a) Doi T, Kamioka S, Shimazu S, Takahashi T. J. Combinatorial Chem. 2008;10:817–819. [Google Scholar]; b) Kamioka S, Shimazu S, Doi T, Takahashi T. J. Combinatorial Chem. 2008;10:681–690. doi: 10.1021/cc800089m. [DOI] [PubMed] [Google Scholar]
- 12.a) Grieco P, Cai C, Liu L, Mayorov A, Chandler K, Trivedi D, Lin G, Campiglia P, Novellino E, Hruby VJ. J Med Chem. 2008;5:2701–2707. doi: 10.1021/jm701181n. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Roedern EGv, Lohof E, Hessler G, Hoffmann M, Kessler H. J. Am. Chem. Soc. 1996;118:10156–10167. [Google Scholar]
- 13.Schmidt J, Garambois V, Rocheblave L, Martinez J, Pelegrin A, Cavelier F, Vives E. ChemBioChem. 2010;11:1083–1092. doi: 10.1002/cbic.201000062. [DOI] [PubMed] [Google Scholar]
- 14.(a) Chaleix V, Sol V, Guilloton M, Granet R, Krausz P. Tetrahedron Lett. 2004;45:5295–5299. doi: 10.1016/j.bmcl.2004.06.016. [DOI] [PubMed] [Google Scholar]; (b) Leβmann L, Waldmann H. Chem. Commun. 2006:3380–3389. doi: 10.1039/b602822e. [DOI] [PubMed] [Google Scholar]
- 15.a) Colombo M, Bianchi A. Molecules. 2010;15:178–197. doi: 10.3390/molecules15010178. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Dijkgraaf I, Rijnders AY, Soede A, Dechesne AC, van Esse GW, Brouwer AJ, Corstens FHM, Boerman OC, Rijkers DTS, Liskamp RMJ. Org. Biomol. Chem. 2007;5:935–944. doi: 10.1039/b615940k. [DOI] [PubMed] [Google Scholar]
- 16.a) Feng Y, Pattarawarapan M, Wang Z, Burgess K. Org Lett. 1999;1:121. doi: 10.1021/ol990597r. [DOI] [PubMed] [Google Scholar]; (b) Roberts KD, Lambert JN, Ede NJ, Bray AMJ. Pep. Sci. 2006;12:525–532. doi: 10.1002/psc.761. [DOI] [PubMed] [Google Scholar]; (c) Ede NJ, Poberts KDJ. Pep. Sci. 2007;13:811–821. doi: 10.1002/psc.904. [DOI] [PubMed] [Google Scholar]
- 17.a) Jung G. Angew. Chem. Int. Ed. Engl. 1991;30:1051–1068. [Google Scholar]; (b) Campiglia P, Gomez-Monterrey I, Longobardo L, Lama T, Novellino E, Grieco P. Tetrahedron Lett. 2004;45:1453–1456. [Google Scholar]; (c) Jack RW, Jung G. Curr. Opin. Chem. Biol. 2000;4:310–317. doi: 10.1016/s1367-5931(00)00094-6. [DOI] [PubMed] [Google Scholar]; (d) Kaiser D, Jack RW, Jung G. Pure Appl. Chem. 1998;70:97–104. [Google Scholar]
- 18.Crescenza A, Botta M, Corelli F, Santini A, Tafi A. J. Org. Chem. 1999;64:3019–3025. doi: 10.1021/jo981425v. [DOI] [PubMed] [Google Scholar]
- 19.Aimetti AA, Shoemaker RK, Linc C-C, Anseth KS. Chem Commun (Camb) 2010;46:4061–4063. doi: 10.1039/c001375g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fields GB, Noble RL. Int. J. Peptide Protein Res. 1990;35:161–214. doi: 10.1111/j.1399-3011.1990.tb00939.x. [DOI] [PubMed] [Google Scholar]
- 21.(a) Hantzsch AR, Weber JH. Ber. 1887;20:3118–3132. [Google Scholar]; (b) Garcia-Egido E, Wong SYF, Warrington BH. Lab Chip. 2002;2:31–33. doi: 10.1039/b109360f. [DOI] [PubMed] [Google Scholar]; (c) Lin PY, Hou RS, Wang HM, Kang IJ, Chen LC. Journal of the Chinese Chemical Society. 2009;56:455–458. [Google Scholar]; (d) Kearney PC, Fernandez M, Flygare JA. J. Org. Chem. 1998;63:196–200. doi: 10.1021/jo971542a. [DOI] [PubMed] [Google Scholar]; (e) Arutyunyan S, Nefzi A. J. Combinatorial Chem. 2010;12:315–317. doi: 10.1021/cc9001907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.General procedure for the solid-phase synthesis of cyclic peptide 5a: One bag of resin 1 (100 mg, 0.115 mmol) was put into a small polyethylene bottle and the Fmoc group was deprotected with 15 mL of a solution of 20% piperidine in DMF (2×10min). The resin was then washed with 15 ml DMF (3×) and 15 mL DCM (3×). L-Fmoc-Asp(Bzl)-OH (6 equiv, 0.307 g, 0.69 mmol) was coupled in the presence of hydroxybenzotriazole (HOBt, 6 equiv, 0.094 g, 0.69 mmol) and diisopropylcarbodiimide (DIC, 6 equiv, 0.101 ml, 0.69 mmol) in 15 mL anhydrous DMF for 2h at room temperature. The resin-bound dipeptide was washed with DMF (3×) and DCM (3×). Completion of the coupling was monitored by the ninhydrin test. The Fmoc group was deprotected with 15 mL 20% piperidine in DMF (2×10min) and followed by the coupling of L-Fmoc-Gly-OH (6 equiv, 0.205 g, 0.69 mmol) using the same reaction conditions. The Fmoc group was deprotected and the resin-bound tripeptide was coupled to L-Fmoc-Arg(Pmc)-OH (6 equiv, 0.457 g, 0.69 mmol) in the same conditions to yield following Fmoc deprotection the corresponding resin-bound protected linear peptide 2a. The resulting N-terminal free amine of resin-bound linear peptide 2a was treated with Fmoc-isothiocyanate (6 equiv, 0.193 g, 11.04 mmol) in 15 mL DMF anhydrous overnight at room temperature. Following Fmoc deprotection with a solution of 20% piperidine in DMF, the resin-bound N-terminal thiourea was treated with 1,3-dichloroacetone (10 equiv, 0.145 g, 18.4 mmol) in DMF anhydrous overnight at 70 °C to afford following Hantzsch’s cyclocondensation the resulting resin-bound chloro methyl thiazolyl peptide 4a. The Trt group was removed in the presence of TFA/(But)3SiH/DCM (5:5:90) for 30 min. The resin was washed with DCM (5×) and DIEA/DCM (5:95) and was treated overnight with a solution of Cs2CO3 (10 eq, 0.325 g) in 15 ml DMF at room temperature to undergo an SN2 intramolecular thialkylation. The resin was treated with HF/anisole for 90 min at 0 °C, and the desired thiazolyl thioether cyclic peptides 5a was obtained following extraction with 95% acetic acid in water and lyophilization as a white powder (61.9 mg). The cyclic peptide 5a was purified by preparative reverse-phase HPLC. 5a: 1H NMR (500 MHz, DMSO-d6). δppm 8.78 (bs, 1H), 8.37 (bs, 1H), 8.30 (bs, 1H), 7.42 (bs, 1H), 7.32 (s, 1H), 7.09 (s, 1H), 6.41 (s, 1H), 4.47 (dd, J= 13.3 Hz, J= 5.5 Hz, 1H), 4.12 (dd, J= 12.7 Hz, J= 7.6 Hz, 1H), 3.93 (m, 2H), 3.64 (d, J=13.3 Hz, 1H), 3.53 (d, J=13.5 Hz, 1H), 3.14 (m, 1H), 3.05 (dd, J= 13.6 Hz, J= 8.6 Hz, 1H), 2.97 (m, 1H), 2.84 (dd, J= 13.6 Hz, J= 4.7 Hz 1H), 2.38 (m, 2H), 2.02 (m, 1H), 1.88 (m, 1H), 1.51 (m, 2H).