Abstract
Spiral ganglion neurons (SGNs) extend processes that interact with Schwann cells (SCs) and with oligodendrocytes (OLs) and astrocytes (ACs). We investigated the ability of these glial cells to support SGN neurite growth. In the presence of cultured ACs, OLs and SCs, SGN neurites tended to follow SCs and OLs and cross-over ACs. Most neurites initially followed the type of glial cell on which the neuronal cell body was found. To determine the influence of homogeneous populations of glia on neurite growth, SG explants were plated on cultured SCs, ACs or OLs. The number of neurites/explant extending onto SCs (463.89±16.25) was significantly greater than the number extending onto ACs (111.38±38.73) or OLs (6.75±2.21), indicating that populations of central glia inhibit SGN neurite growth. Treatment with cell-permeant cpt-cAMP or forskolin (FSK) each significantly increased the number of neurites on OLs (133.54±25.59 and 292.25±83.57, respectively). cpt-cAMP and FSK each also increased the number of neurites on ACs (213.19± 36.06 and 208.64±59.25, respectively), however the difference was not significant compared with control. The neurites on ACs and OLs failed to grow radially in a well-fasciculated pattern as on SCs. In explants plated on the borders of cultured OL-SC or AC-SC groups, more neurites extended onto SCs compared with OLs and ACs. Conditioned media from OL or AC cultures did not reduce neurite length, implying that the inhibition of neurite growth by central glia is not due to soluble factors. Taken together, these results demonstrate that homogeneous populations of central glia inhibit SGN neurite growth.
Keywords: Schwann cell, Astrocyte, Oligodendrocytes, cyclic AMP, protein kinase A, axons
1. Introduction
Spiral ganglion neurons (SGNs) are bipolar with a peripheral axon that innervates hair cells in the organ of Corti and a central axon that terminates in the cochlear nucleus. In rodents, SGNs critically depend on both peripheral and central target tissue for survival (Spoendlin, 1975, Spoendlin and Suter, 1976, Alam et al., 2007). Hence, destruction of the organ of Corti leads to degeneration of the peripheral axons and a gradual, subtotal loss of the neurons themselves (Alam et al., 2007). In humans, SGN loss following deafening occurs much more slowly suggesting ongoing neurotrophic support by cells outside of the organ of Corti and allowing for the functional stimulation of the auditory nerve with implanted electrodes (Gomaa et al., 2003, Khan et al., 2005, Linthicum and Fayad, 2009). SGNs also degenerate following transection of the central nerve trunk, with this loss occurring over an even longer time frame (Spoendlin, 1975, Spoendlin and Suter, 1976, Sugawara et al., 2005). In both instances, the gradual loss of SGNs suggests multiple sources of neurotrophic support. Glial cells represent one potential source. In rodents, Schwann cells (SCs) myelinate the peripheral axons of SGNs, the neuronal somata, and the initial portion of the central axons (Spoendlin, 1985, Toesca, 1996, Hurley et al., 2007); whereas in humans the neuronal somata remain unmyelinated (Arnold, 1987, Nadol et al., 1990). Central glia, including oligodendrocytes (OLs) and astrocytes (ACs), associate with the central axons from the peripheral-central glial transition zone (glia limitans) to the terminal synapses in the cochlear nucleus (Spoendlin, 1985, Valderrama-Canales et al., 1993, Jalenques et al., 1995, Toesca, 1996, Moore and Linthicum, 2001, Hurley et al., 2007).
The contribution of glial cells to SGN development, survival, and neurite growth remains largely unknown. In the early embryonic period, glial cells support neuronal survival, direct neuronal migration, and modify the growth of axons and dendrites. For example, ACs, OLs, and SCs secrete glial derived neurotrophic factor which promotes survival and neurite growth in a variety of neurons, including SGNs (Ylikoski et al., 1998, Altschuler et al., 1999, Rind and von Bartheld, 2002, Ernsberger, 2008, Bostrom et al., 2009). ErbB2 is required for SC development and ErbB2-null mice show a reduction in the number of afferent fibers innervating the cochlea (Morris et al., 2006). Further, those fibers that develop are disorganized demonstrated by several fibers crossing between radial bundles. These results raise the possibility that SCs are necessary for the normal development of cochlear innervation. Further, preliminary data suggest that loss of SGSCs postnatally leads to neural degeneration with preservation of the organ of Corti sensory and supporting cells, suggesting that SCs are critical for maintenance of SGNs (Akil et al., 2010).
In addition to contributing to neurogenesis, glial cells influence neural regeneration. In the peripheral nervous system, SCs typically support regenerating axons. Cultured spiral ganglion Schwann cells (SGSCs) express brain derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3), neurotrophins that support SGN survival and neurite growth, and growing SGN neurites preferentially associate with SGSCs in vitro (Hansen et al., 2001a, Bostrom et al., 2009, Whitlon et al., 2009). By contrast, inhibitory factors produced by central glia contribute to the limited ability of the adult mammalian central nervous system (CNS) axons to regenerate. (Carlstedt et al., 1989, Schnell and Schwab, 1990, Filbin, 2003, Yiu and He, 2006, Nash et al., 2009). Regeneration of SGN axons along central glial pathways to targets in the cochlear nucleus will be required for strategies aimed at repopulating the spiral ganglia in deaf patients with functional neurons (Reyes et al., 2008, Boer et al., 2009, Nishimura et al., 2009). We have begun investigating the interaction of SGN neurites with SCs, OLs, and ACs. We found that SGN neurites will readily extend along or cross over isolated ACs and OLs; however, established populations of OLs and ACs strongly inhibited SGN neurite growth. Elevation of cyclic adenosine monophosphate (cAMP) levels overcame this inhibition.
2. Experimental Procedures
2.1. Dissection of spiral ganglia
The institutional animal care and use committee at the University of Iowa approved all protocols used in this study. Spiral ganglia (SG) were isolated from postnatal day 4–5 (P4–P5) rats euthanized under cold anesthesia after placing the pups in a cardboard box on ice for 20 min (Phifer and Terry, 1986, Danneman and Mandrell, 1997). The temporal bone was harvested and the otic capsule was dissected under operating microscope in ice cold PBS. The bony cochlear capsule was removed, followed by the spiral ligament. The organ of Corti was removed, transecting the outer radial fibers, leaving the SGNs within the modiolus. Modiolar bone and surrounding connective tissue were removed. Ganglia were collected in ice cold Hanks’ balanced salt solution with calcium and magnesium (HBSS+/+, Gibco, Carlsbad, CA). When used as explants, SGs were cut into 4 pieces and placed onto the prepared culture slides.
2.2. Dissociated spiral ganglia cultures
Dissociated SG cultures were prepared as previously described (Hegarty et al., 1997, Hansen et al., 2001b). The basal culture medium included high glucose Dulbecco’s Modified Eagle’s Medium with L-glutamine (DMEM, Gibco, Carlsbad, CA) with N2 supplement (Invitrogen; Carlsbad, CA) and fresh insulin (10 μg/ml, Sigma, St. Louis, MO) supplemented with penicillin and streptomycin.
Dissociated cells were plated in 8-well culture plates (Nunc™, Rochester, NY) previously coated sequentially with polyornithine (0.1 mg/ml in 10 mM borate buffer, pH 8.4, Sigma) for 1 hr at room temperature (RT), followed by laminin (20 μg/ml in HBSS, Sigma) overnight at 4°C.
2.3. Schwann cell cultures
Bilateral sciatic nerves and brachial plexi were dissected from P4–P5 rat pups and collected in ice cold HBSS +/+. The nerves were enzymatically digested in 0.125% trypsin with EDTA (Gibco) and 0.1% collagenase (Sigma) for 30 min at 37°C. Fetal bovine serum (FBS, Gibco) at 10% final concentration was used to cease the enzymatic activity. The tissue was washed with DMEM (0%) then suspended in DMEM with 5% FBS, N2 supplement, and fresh insulin. The cells were dissociated by gentle trituration through 1 ml pipettor tip and fire-polished glass Pasteur pipettes sequentially. The cell suspension was plated on polyornithine/laminin-coated 35 mm culture dishes. After 3 to 4 days, culture medium was changed with DMEM containing 5% FBS, N2 supplement, insulin, and 20 μM of Cytosine β-D-arabinofuranoside (Sigma) to eliminate any contaminating fibroblasts. Forty-eight hr after treatment with cytosine arabinoside, we changed the medium to serum free DMEM with N2 supplement/insulin and kept cells in this condition at least 24 hr before experimental manipulation.
2.4. Astrocyte (AC) and oligodendrocyte (OL) cultures
Central glial cultures were prepared from P5 rat cerebral cortex according to a modification of the method described by McCarthy and de Vellis (McCarthy and de Vellis, 1980). Under the operating microscope, the meninges were removed and cortical tissue was minced with microforceps into 3 mm3 pieces. Enzymatic dissociation was performed with 0.125% trypsin in HBSS−/− at 37°C, 6.5% CO2. After 25 min, enzymatic activity was ceased by adding 10% FBS followed by mechanical trituration. Dissociated cortical cells in 30 ml of DMEM (10% FBS) were plated to poly-lysine coated T75 flask and maintained at 37°C, 6.5% CO2 for 9 days. On the 10th day, the cell cultures were rinsed 3 times with 10% DMEM to remove floating cells. The flask was fixed on the floor of orbital shaker and shaken at 250 rpm for 6 hr at 37°C.
Suspended cells were collected and filtered through sequential cell strainer clothes with 70 μm and then 22 μm sized pores (BD Bioscience, San Jose, CA ). The filtrate contained suspended OLs. The suspended cells were pelleted by centrifugation (40g, 5 min) and plated onto poly-lysine culture plates.
ACs attaching to the floor of the flask were rinsed 5 times with DMEM (0%) and then suspended by adding 2 ml of 0.25% trypsin. After 5 min, FBS was added to cease the enzymatic activity and the flask was rinsed with DMEM (0%). The ACs were collected, pelleted by centrifugation (40g, 5 min), resuspended in fresh DMEM with 10% FBS, and plated onto poly-lysine coated culture plates. Astrocytes and oligodendrocytes cultures were incubated at 37°C, 6.5% CO2 until use. Culture medium (DMEM with 10% FBS) was exchanged every 2 or 3 days.
2.5. Dissociated SG cultures on the mixture of AC & OL
One hundred μl of AC (2×104 cells/mL) and 100 μl of OL (2×105 cells/mL) were plated together onto 8-well slide in DMEM with 10% FBS. After 24 hr, 100 μl of dissociated SGN cultures were plated onto AC-OL cell layer that were attached to each well. Cultures were maintained in NT-3 (50 ng/ml, R&D systems, Minneapolis, MN) for an additional 48 hr. Here we refer to the spiral ganglion Schwann cells (SGSCs) as all S100-positive cells derived from the osseous spiral lamina and Rosenthal’s canal/modiolus, including the satellite cells.
2.6. SG explant on single cell background of AC or OL
One hundred μl of AC or OL cell suspension was plated onto 8-well culture slide with a density of 1×105cells/mL for AC and 5×105 cells/mL for OL to make single cell background. After 7 days, freshly prepared SG explants were placed on the center of the pre-plated cells and maintained in NT-3. Subsets of cultures were also treated with the cell permeant cAMP analogue, 4-chlorophenylthio-adenosine 3′:5′ cyclic monophosphate (cpt-cAMP, 1 mM, EMD Biosciences, San Diego, CA) or forskolin (5 μM, EMD Biosciences), an adenylyl cyclase activator. After 3 days, the slides were fixed and immunostained. The number of neurites/explant was scored for each explant. To facilitate scoring, any explant with >500 neurites was scored as 500.
2.7. Explant on the border between different types of glial cells
To make AC-SC or OL-SC borders, 30 μL of ACs or OLs and 30 μL of SCs were plated separately onto laminin-coated coverslips (22×22 mm2) with a diagonal arrangement making 4 borders per coverslip as closely as possible without mixing. After 3 hr to allow for cell attachment, DMEM with 10% FBS, N2 supplement, and fresh insulin was applied to cover the whole surface of coverslip. After 7 to 10 days, the cell margins were in contact with each other without overlapping of populations of cells. SG explants were then placed onto the borders between AC-SC or OL-SC and maintained for 3 days prior to fixation and immunolabeling. The number of neurites/explant growing onto each glial type was determined for each explant. For some explants, a montage of 12–21 10× fields/explant were stitched together using the scan slide feature of MetaMorph software to illustrate the overall neurite outgrowth from the explant.
2.8. Dissociated SG cultures with conditioned media (CM) from AC or OL
Dissociated SG cultures were plated in laminin coated 8-well culture slides. The cells were allowed to attach for 1 hr and the media was exchanged. Conditioned media (CM) were collected from AC or OL cultures maintained in DMEM with 10% FBS. N2 supplements and fresh insulin were added just prior to use. Control medium included DMEM with 10% FBS also maintained in the incubator for 3 days and with freshly added N2 supplements/insulin with or without NT-3. The cultures were maintained for 48 hr, fixed, and immunostained.
2.9. Characterization of neurite behavior towards different cell types
Neurite behavior in relation to glial cells was categorized into 3 types: i) Follow-neurite grows along the long axis of the glial cell processes, ii) Cross-neurite crosses over the glial cell, iii) Avoid-neurite turns to avoid the glial cell. Digital images were taken from 10 randomly chosen fields from each well and the pattern of neurite behavior was determined along the entire length of a neurite. We also analyzed the first glial cell on which each neurite extended in relation to the type of glial cell in contact with the neuronal cell body. The majority of SGNs in our cultures are monopolar. In the case of a bipolar neuron, the longer, more dominant process was analyzed.
2.10. Determination of SGN neurite length
SGN neurite length was determined as previously described from digital images of 5 randomly chosen 20× microscopic fields (Roehm et al., 2008). The length was determined by measuring longest process extending from each neurofilament 200 (NF200)-positive SGN using Image J (NIH; Bethesda, MD). The average neurite length for each condition was determined and the mean and standard deviation (SD) for each repetition was determined. Conditions were performed in duplicate or triplicate and repeated at least 3 times.
2.11. Immunofluorescent labeling
Frozen sections of rat cochleae were prepared by from P60 rats anesthetized with ketamine/xylazine (40mg/kg; 5 mg/kg, IP) according to the method of Alam et al. (Alam et al., 2007). The animals were exsanguinated by transcardial perfusion, first with 0.01 M phosphate-buffered saline, pH 7.4 (PBS), containing 0.1% sodium nitrite, then with 50 ml fixative consisting of 4% paraformaldehyde in PB. The animals were then decapitated, the cochleae exposed, the stapes removed, the round window perforated, and the scalae gently perfused with fixative. The cochleae were immersed in fixative (4% paraformaldehyde in 0.1 M PB) for 2 h at RT, washed with PBS, and decalcified by immersion in 0.12M EDTA, pH 7.0, changed daily, for 1 week at RT. The decalcified cochleae were passed through serial sucrose gradients (10%, 20% and 30%) and then embedded in OCT compound. Serial 6-μm-thick midmodiolar sections were cut on a freezing microtome and mounted on poly-L-lysine-coated glass slides. Slides were stored at −20°C until further use.
Cultures fixed with 4% paraformaldehyde at RT for 15 min and frozen sections of adult rat cochleae (6–7 μM) were permeabilized with 0.2% Triton-X in PBS for 15 min at RT. Nonspecific antibody binding was reduced with blocking buffer (5% goat serum, 2% bovine serum albumin [BSA], 0.1% Triton-X in PBS) for 30–45 min at RT. Primary antibodies, diluted in blocking buffer, were applied at 4°C overnight. The following primary antibodies were used in various combinations: anti-S100 (1:800 v/v, Sigma), anti-NF200 (1:800 v/v, Sigma), RIP (1:800 v/v, Clone NS-1, Hybridoma Core, University of Iowa, Iowa City, IA), and anti-GFAP (1:800, Abcam, Cambridge, MA). RIP is a monoclonal antibody that detects oligodendrocytes and their processes (Friedman et al., 1989). Secondary antibodies, conjugated with Alexa Fluor-488, Alexa Fluor-568, or Alexa Fluor-647 (Invitrogen), were diluted in blocking buffer (1: 800 v/v) and incubated for 1 hr at 37°C. Nuclei were labeled with 10 μg/mL of Hoechst 3342 (Sigma) for 10 min at RT.
Digital images were captured on a Leica DMR III microscope equipped with epifluorescent filters and a cooled CCD camera using Leica FW4000 software (Leica Microsystems, Bannockburn, IL) or MetaMorph software (Molecular Devices, Inc., Sunnyvale, CA ) or on a Leica SP5 laser scanning confocal microscope equipped with 405 nm, 488 nm, 543 nm, and 633 nm laser lines (Leica Microsystems).
2.12. Data Analysis
Numerical data (cell survival and neurite length) were managed with Excel software (Microsoft, Seattle, WA). Significance for differences in the number of neurites on different cell backgrounds and neurite length among treatment groups were determined by one way ANOVA followed by a post hoc Tukey’s test for parametric data or Kruskal-Wallis for non parametric data using SPSS (SPSS, Inc., Chicago, IL). Significance for differences among categorical data was determined by the Chi square test.
3. Results
3.1. Characterization of glial immunoreactivity in the auditory nerve
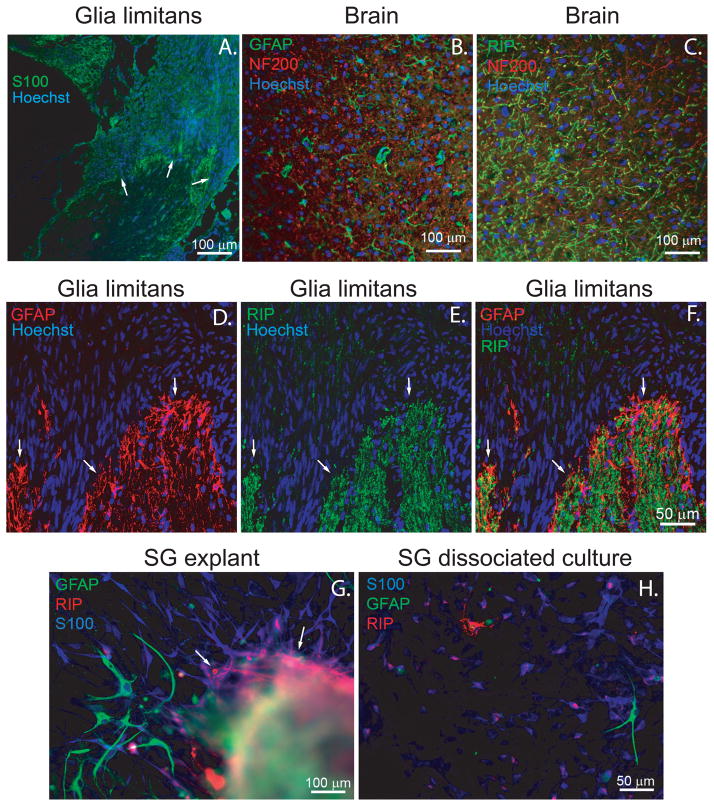
To characterize the glial cells along the central portion of the auditory nerve, we immunostained frozen sections of the adult rat cochlea (Fig. 1). We also immunostained frozen sections from the cerebral cortex with anti-GFAP and RIP antibodies to verify the specificity of these antibodies for ACs and OLs, respectively (Fig. 1). In the brain, anti-GFAP antibody labeled cells with typical astrocytic morphology and RIP antibody, which detects 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) and labels OLs (Friedman et al., 1989, Watanabe et al., 2006), labeled cells with oligodendrocyte morphological features. In the cochlea, S100-positive SCs were associated with the peripheral processes, neuronal somata in Rosenthal’s canal (RC), and the initial segment of the central axons. GFAP and RIP immuno-positive glial cells appeared at the glia limitans and extended centrally (Fig. 1).
Figure 1.
Glial cells in contact with spiral ganglion neurons and their processes express Schwann cell, astrocyte or oligodendrocyte markers. A. Frozen section of the cochlea immunostained with anti-S100 (green) and Hoechst (blue). Arrows indicate glia limitans. B&C. Frozen sections of rat cerebral cortex immunostained with anti-GFAP (green) and anti-NF200 (red) antibodies (B) or RIP (green) and anti-NF200 antibodies (C). D–F. Frozen sections of the cochlea (P60) at the glia limitans immunostained with anti-GFAP (red, D&F) and RIP (green, E&F) antibodies and Hoechst (blue). F is an overlay of GFAP and RIP immunostaining. The glia limitans is demonstrated in the intramodiolar portion of cochlear nerve and indicated by arrows. G. Area of SG explant corresponding to the intramodiolar central nerve trunk immunostained with anti-GFAP (green), anti-RIP (red), and anti-S100 (blue) antibodies. H. Dissociated SG cultures immunostained with anti-GFAP (green), RIP (red), and anti-S100 (blue) antibodies. Several GFAP-positive cells with typical astrocyte morphology migrated out from the central nerve trunk region of the SG explants (G) and composed ~2% of cells in dissociated SG cultures (H), while RIP-positive cells remained in close proximity to the central trunk region (arrows, G) and were rarely observed (<0.5% of cells) in dissociated cultures (H). Cultured SGSCs show slight RIP immunoreactivity evident as red dots on S100-positive (blue) cells (H).
In cultured explants of neonatal rat SG, GFAP-positive glial cells with astrocyte morphology extended from the portion of the explant that corresponds with the central trunk region, consistent with GFAP-positive cells at the glia limitans that are incorporated in the explant (Fig. 1). A few RIP-positive cells were also evident in the portion of the explants corresponding to the central trunk, but these cells did not migrate out of the explant. In dissociated SG cultures, ~2% of non-neuronal cells were GFAP-positive with astrocytic morphology, as previously noted (Hansen et al., 2001a). We also detected very rare (<0.5% of non-neuronal cells) RIP-positive cells in dissociated SG cultures with OL morphology. None of the cultured glial cells were simultaneously GFAP and RIP-positive. Thus in vivo the central glia associated with SGN axons express both GFAP and RIP antigens. However, in culture some of the cells manifest astrocyte characteristics including GFAP expression and astrocytic morphology while others retain RIP immunoreactivity, typical of OLs.
3.2. In mixed glial cell cultures more SGN neurites grow along SC and OL processes than along AC processes
To begin to characterize the influence of glia on SGN neurite growth, we plated ACs and OLs at 40–60% density. After 24 hr, dissociated SG cultures were then added to the cultures providing mixed cultures composed of SGNs, SGSCs, ACs, and OLs. After 48 hr, the cultures were fixed and immunostained with anti-NF200, anti-S100, and either anti-GFAP or RIP antibodies (Fig. 2).
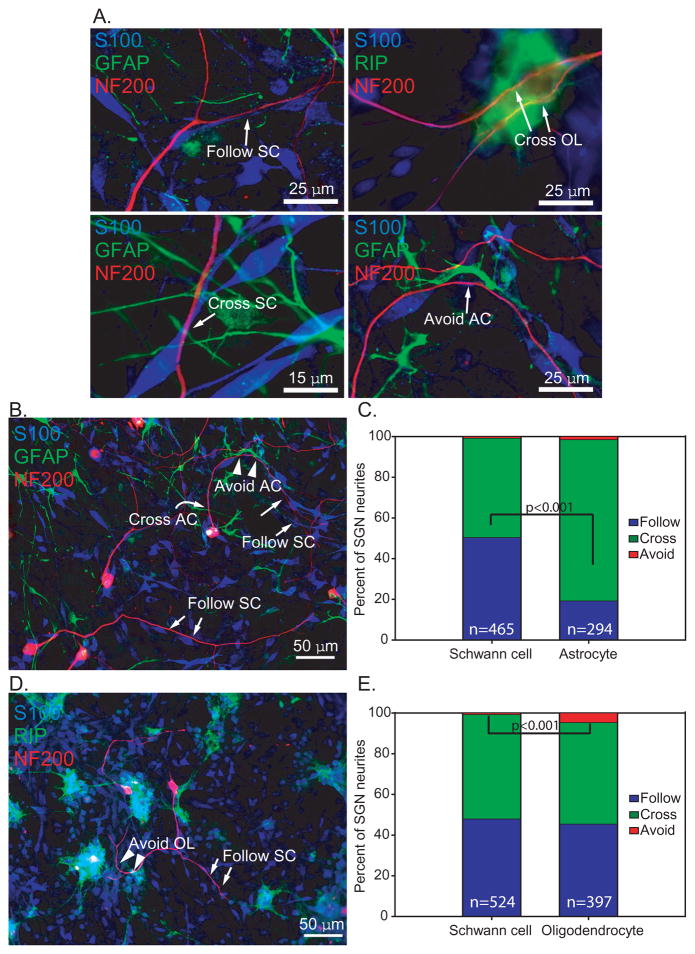
Figure 2.
Most SGN neurites extend along or cross over glial cells. Dissociated SG cultures mixed with ACs (B&C) and OLs (D&E) and immunostained with anti-NF200 (red), anti-S100 (blue), and either anti-GFAP (green) or RIP (green) antibodies. A. Neurites showed various patterns of interaction with glial cells that were classified as “follow”, “cross”, or “avoid” as illustrated here. The image showing avoidance by a nerve fiber is a higher magnification of the image in B below. B&D. Patterns of interaction with glial cells that were classified as “follow” (arrow), “cross” (curved arrow), or “avoid” (arrowhead). C. In AC-SC labeled cultures, neurites followed (50.5%) or crossed the SCs (48.8%) at nearly an equal ratio. Most neurites (79.3%) crossed ACs while 19.4% followed ACs. n=total number of processes scored. The proportion of neurites following SCs or ACs was significantly different by Chi square analysis (p<0.001) E. In OL-SC labeled cultures, 49.9% of neurites crossed OLs, 45.6% followed OLs, and a few neurites (4.5%), avoided OLs, but this was rarely observed with ACs or SCs. n=total number of processes scored. The proportion of neurites avoiding SCs or OLs was significantly different by Chi square analysis (p<0.001)
Dissociated SGNs on the mixture of SCs, ACs and OLs survived well and produced neurites that grew in association with the glial cells. The behavior of neurites towards the glial cells they encountered was categorized as either follow, cross, or avoid (Fig. 2). Due to immunostaining limitations, we analyzed neurite behavior towards SCs and ACs or towards SCs and OLs in individual wells, but not towards all 3 glial types within the same well.
In wells that were stained with SC and AC markers, neurites followed (50.5%) or crossed the SCs (48.8%) at nearly a 1:1 ratio (Fig. 2). Most neurites (79.3%) crossed ACs while 19.4% grew along the AC cellular processes. Few neurites appeared to avoid SCs (0.7% of neurites) or ACs (1.4% of neurites).
In wells that were stained with SC and OL markers, the behavior of neurites towards SCs was similar as in cultures with ACs. Nearly half of neurites (49.9%) crossed processes of OLs and 45.6% followed OL processes. Thus, fewer SGN neurites followed along ACs processes (19.4%) compared with SCs (50.5%, p<0.001) or OLs (45.6%). Few neurites (4.5%), but significantly more than towards SCs or ACs (p<0.001), appeared to avoid OLs. Taken together these results suggest that dissociated central glia do not appear to significantly inhibit or repel SGN neurite growth in vitro.
3.4. The type of glial cell contacting a SGN cell body correlates with the glial cell on which the neurite extends
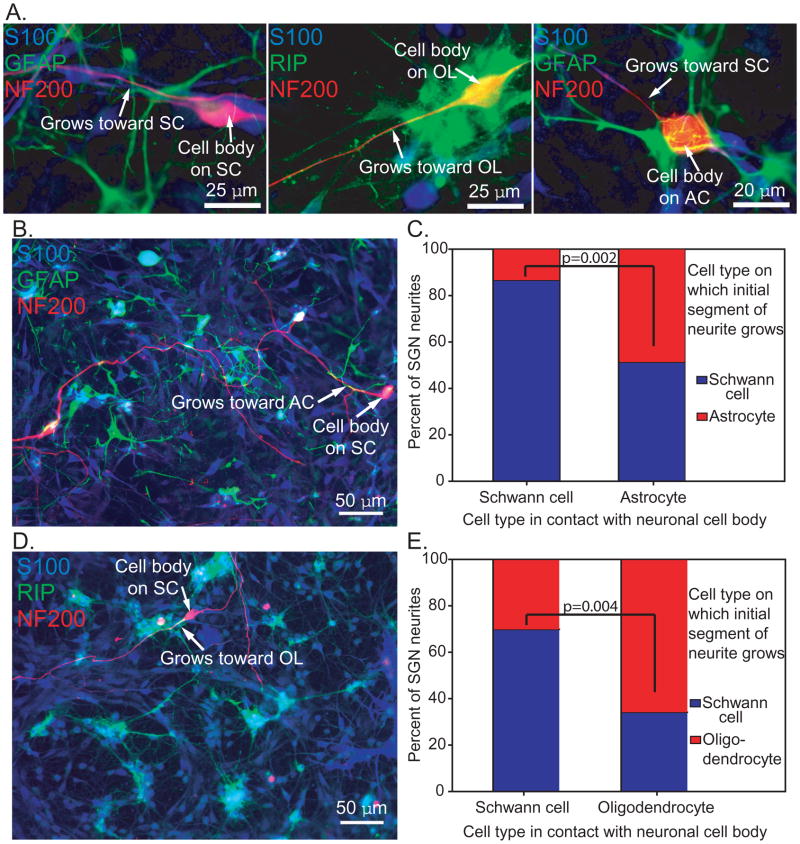
We next asked whether the type of glial cell that contacts the SGN cell body correlates with the glia on which the proximal segment of the neurite extends. Neurite growth in mixed glial cultures was assessed by (1) the type of glial cell that contacts the cell body and (2) the type of glial cell contacting the proximal segment of the neurite (Fig. 3). In most cases, SGN cell bodies contacted SCs, but some contacted central glial cells and produced neurites. The majority of SGNs with cell bodies associated with SCs initially extended neurites along SCs (86.7% in AC-SC, 70% in OL-SC). Half of SGNs (51.4%) with cell bodies associated with ACs, initially extended neurites along SCs and 48.7% initially grew on ACs. In OL-SC cultures 65.7% of neurites from SGNs with cell bodies on OLs initially extended onto OLs.
Figure 3.
SGN neurites tend to grow on the type of glial cell that contacts the cell body. Dissociated SG cultures mixed with ACs (B&C) or OLs (D&E) and immunostained with anti-NF200 (red), anti-S100 (blue), and either anti-GFAP (green) or RIP (green) antibodies. A. The type of glial cell that interacts with the initial portion of the neurite was determined in relation to the type of glial cell that was in contact with the neuron cell body. B. The neuronal soma at right side was located on a SC (arrow) and the neurite grew along the cellular processes of AC for some distance (arrow). C. Soma of the neuron was located on a SC whereas the initial segment of the neurite grew along an OL. In both AC-SC cultures (C) and OL-SC cultures (E), the majority of neurites from SGNs with cell bodies located on SCs initially extended along SCs (86.7% in AC-SC cultures and 70% in OL-SC cultures). When the SGN cell body was located on an AC, 51.4% of the neurites initially grew along SCs, and 48.7% grew along ACs. These proportions were significantly different than SGNs with cell bodies contacting SCs (p=0.002, Chi square). 65.7% of neurites from SGNs located on OLs initially extended on OLs, whereas 34.3% grew along SCs. These proportions were significantly different than SGNs with cell bodies contacting SCs (p=0.004, Chi square).
Thus, when an SGN cell body associates with a SC, the neurite usually extends along SCs. When a neuronal cell body is plated on ACs, there does not appear to be a preference for the cell type on which the neurite initially grows. However, dissociated SGNs with cell bodies associated with OLs are most likely to initially extend the neurite on OLs rather than SCs, even though SCs are abundantly available. These observations suggest that the type of glial cell that contacts a SGN cell body influences the type of cell that the neurite begins to grow on.
3.5. Astrocytes and oligodendrocytes inhibit neurite outgrowth from SG explants; increasing cAMP overcomes this inhibition
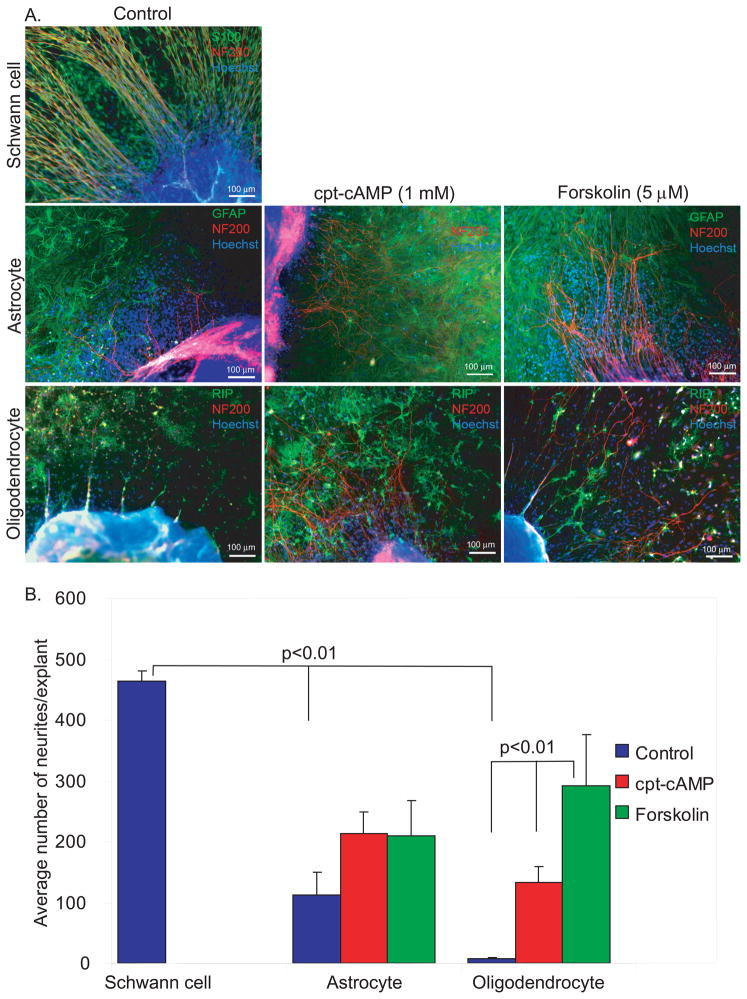
Central glia typically inhibit axon regeneration; this is attributed to a variety of factors including secreted proteins (e.g. semaphorins), the astroglial scar, extracellular matrix (ECM) material (e.g. chondroitin sulfate proteoglycans, CSPGs), and myelin-derived proteins (e.g. Nogo-A) (Schnell and Schwab, 1990, Filbin, 2003, Yiu and He, 2006, Nash et al., 2009). We found that acutely dissociated ACs and OLs did not significantly inhibit or repel SGN neurite growth. To address the influence of an established homogenous population of glia that has produced ECM proteins on SGN neurite growth, we prepared cultures of SCs, ACs, or OLs. In this case the SCs were derived from sciatic nerves and brachial plexi to facilitate generating a glial bed for neurite growth. After 7 days, SG explants were placed on a bed of established glial cultures. Three days later, the cultures were immunostained and the number of neurites extending from each explant was scored. There was extensive neurite outgrowth on the SC background with a mean of 463.89±16.25 neurites/explant (Fig. 4). To facilitate scoring, any explant with >500 neurites was scored as 500. Thus, our scoring method under-represents the actual number of neurites extending onto SCs which was typically >1,000/explant. On peripheral nerve SCs, the neurites were long and radial without significant crossing, similar to the normal peripheral cochlear nerve fascicles and similar to neurite growth in explants plated on laminin-coated substrates without pre-plating of SCs.
Figure 4.
Increasing cAMP overcomes the inhibition of SGN neurite growth by astrocytes or oligodendrocytes. A. SGN neurite growth from SG explants plated on SCs, ACs, or OLs. Cultures were immunostained with anti-NF200 (red) and either anti-S100, anti-GFAP, or RIP (green) antibodies as indicated. Nuclei were labeled with Hoechst (blue). Neurite outgrowth on SCs was robust, radially oriented, and fasciculated. B. Mean B number of neurites/explant on different cell backgrounds. There was significantly reduced neurite outgrowth on ACs or OLs (p<0.01). Application of cpt-cAMP or forskolin significantly increased the number of neurites on OLs (p<0.01). The increase in neurite outgrowth on ACs by cpt-cAMP (1 mM) or forskolin (5 μM) was not statistically significant different from untreated cultures (p=0.30).
Neurite outgrowth was significantly reduced on ACs (111.38±38.73 neurites/explant) and, especially, OLs (6.75±2.21) compared with outgrowth on SCs (p<0.01, each). Treatment of explants on OLs with cell-permeant cpt-cAMP (133.54±25.59) or forskolin (FSK) (292.25±83.57), an adenylyl cyclase activator, significantly increased neurite outgrowth compared with control cultures (p<0.001). Similarly, treatment of explants on ACs with cpt-cAMP (213.19±36.06) or FSK (208.64±59.25) increased neurite outgrowth although the difference was not statistically significant compared with control cultures (111.38±38.73, p=0.30). In explants plated on ACs or OLs, elevation of cAMP did not restore outgrowth to the extent observed on SCs.
3.6. SG explants placed on glial borders extend more neurites onto SCs compared with ACs and OLs
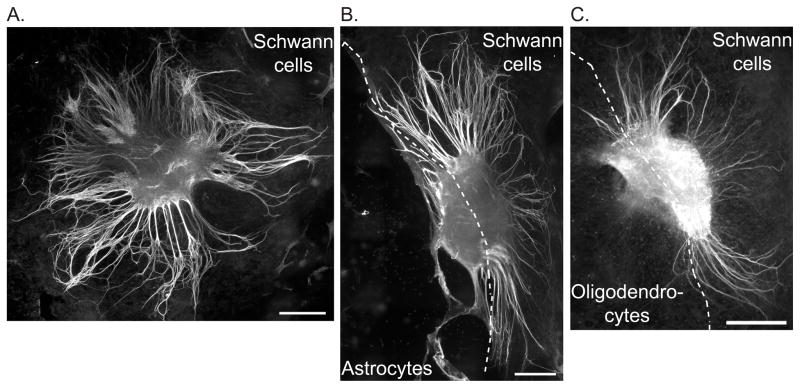
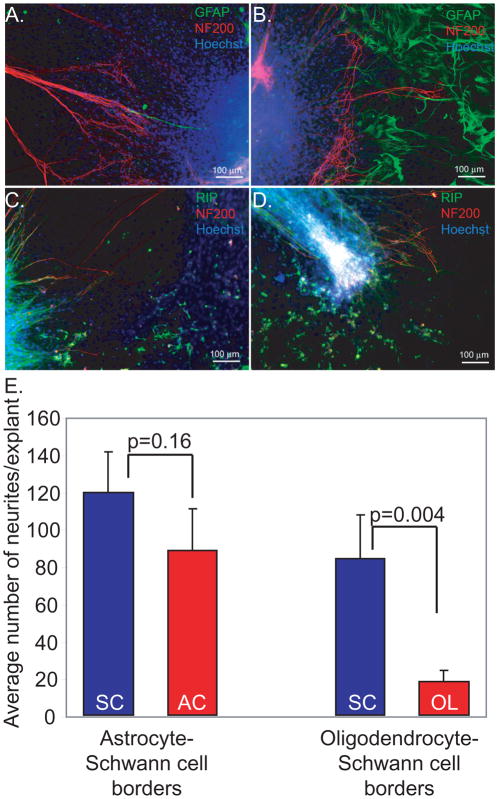
To further explore the influence of central glia on SG neurite growth we established SC cultures with borders of either OLs or ACs. After 7 days, explants were placed at the edge of the cell borders and the number of neurites that extended onto each glial type was determined. Figure 5 presents representative montages derived from 12–21 10× fields/explant stitched together using the scan slide feature of MetaMorph software. The dashed line represents the glial border. At the border of AC-SC, more neurites grew toward the SC area (119.29±21.42) than the AC area (88.24±22.08), but the difference was not statistically significant (p=0.16) (Fig. 6). Although some neurites developed from explants toward the AC area, the neurites did not extend deeply into the AC area as on SC background; rather they curved back toward the explant when they faced the AC area.
Figure 5.
Astrocytes and oligodendrocytes limit neurite growth from SG explants. SG explants were placed on SCs (A) or the border between AC-SC (B) or OL-SC (C). Cultures were immunostained with anti-NF200 antibody. They were also labeled with either anti-GFAP or RIP antibodies (not shown) to identify the glial borders. The images represent a montage of 12–21 10× fields stitched together using the scan slide feature of MetaMorph software. Scale bars=400 μm.
Figure 6.
More SGN neurites extend onto Schwann cells compared with astrocytes or oligodendrocytes. Cultures were immunostained with anti-NF200 (red) and either anti-GFAP or RIP (green) antibodies as indicated. Nuclei were labeled with Hoechst (blue). Regions without green colored-cells represent SCs. (A, B) Explant on the border between SCs (A) and ACs (B). (C, D) Explant on border between OLs and SCs. E. Mean number of neurites per explant plated on glial borders. More neurites grew toward SCs (119.29±21.42) than ASs (88.24±22.08) from explants placed on the border of AS-SC, but the difference was not statistically significant (p=0.16). At the border of OL-SC, significantly fewer neurites grew towards the OLs (17.70±6.18) than SCs (84.04±23.12) (p=0.004).
Fewer neurites extended towards the OL area (17.70±6.18) compared with than SC area (84.04±23.12) (p=0.004). These results confirm that established populations of ACs or OLs inhibit SGN neurite growth.
3.7. Conditioned media from ACs enhances neurite growth
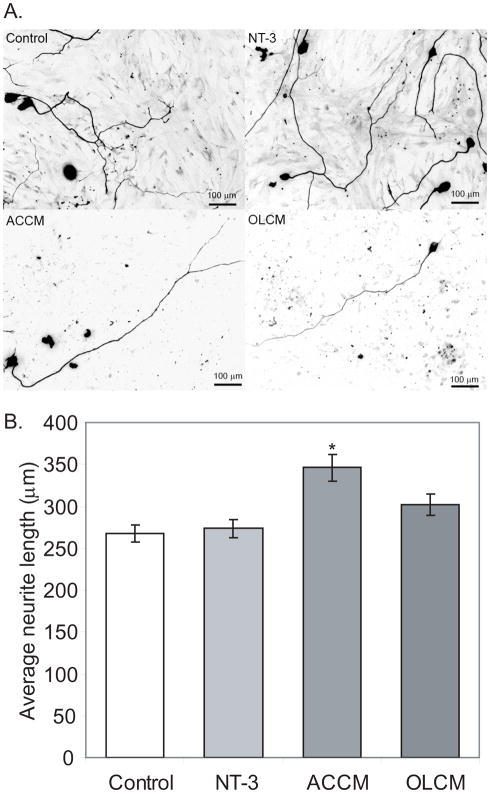
We next asked whether secreted factors from ACs or OLs would inhibit SGN neurite growth. We collected 3-day conditioned media (CM) from AC and OL cultures. Control medium consisted of 10% FBS DMEM maintained in the incubator for 3 days with or without NT-3. ACCM significantly increased SGN neurite length (346.21±16.19 μm) compared with control medium (267.36±9.97 μm) and NT-3 (273.55±10.65 μm) (p<0.01) (Fig. 7). Similarly, the SGN neurites in cultures maintained in OLCM were longer (301.83±12.76) than neurites maintained in control medium, though the difference was not statistically significant. These results suggest that the inhibitory effect of ACs and OLs on SGN neurite growth is unlikely to be due to secreted substances, rather it likely results from ECM materials.
Figure 7.
Conditioned media from astrocytes promotes neurite growth. A. Dissociated SG cultures grown in NT-3 (50 ng/ml), astrocyte conditioned medium (ACCM), oligodendrocyte conditioned medium (OLCM) or control medium and immunostained with anti-NF200 antibodies. B. Mean neurite length was significantly longer in cultures treated with ACCM compared with other groups (* p<0.01).
4. Discussion
SGNs gradually die following loss of hair cells and supporting cells from the organ of Corti or sectioning of the central trunk of the auditory nerve (Spoendlin, 1975, Sugawara et al., 2005, Alam et al., 2007). In humans the loss of neurons is significantly delayed. Consequently, cochlear implantation with stimulating electrodes provides auditory perception to patients deafened by hair cell loss; however, neural degeneration following hair cell loss likely limits the effectiveness of cochlear implants (Gillespie and Shepherd, 2005, Roehm and Hansen, 2005, Pettingill et al., 2007, Xu and Pfingst, 2008). Further, regrowth of the peripheral axons to more closely approximate the stimulating electrodes would enhance the spatial resolution and overall performance (Rubinstein, 2004, Xu and Pfingst, 2008). Thus, there is lively interest in identifying factors and therapeutic strategies that promote SGN survival and axonal regeneration (Gillespie and Shepherd, 2005, Roehm and Hansen, 2005, Pettingill et al., 2007, Boer et al., 2009).
In contrast to many central neural tracts and peripheral nerves, the influence of glia on SGN health and regeneration remains largely unexplored. To begin to understand the interaction of glia with SGNs, we first sought to further characterize the glial composition of the cochlear nerve. The glia limitans represents the transition zone from peripheral myelin to central myelin (Toesca, 1996). As previously shown, S100-positive SCs provide peripheral myelin and surround the peripheral axons, the SGN cell bodies, and the initial portion of the central axons (Spoendlin, 1985, Toesca, 1996, Hurley et al., 2007). In contrast to rodents, SGN cell bodies are not typically myelinated in humans (Arnold, 1987, Nadol et al., 1990). Based on immunostaining of cochlear sections, explants, and dissociated cultures, we identified glia with astrocytic and oligodendrocytic features in the cochlear nerve. The glia at the glia limitans retained some characteristics of ACs including GFAP immunoreactivity with some of these cells demonstrating an astrocytic morphology in culture. Nevertheless, these glia produce central myelin and are RIP-positive typical of OLs (Hurley et al., 2003, Hurley et al., 2007). Thus, in cochlear sections the central glia demonstrate both GFAP and RIP immunoreactivity; however, in our cultures, these central glia appear to adopt either an AC or OL morphology with corresponding GFAP or RIP-immunoreactivity, respectively. This difference between the in vivo and in vitro staining with anti-GFAP and RIP antibodies may reflect changes that the cells adopt in culture. Alternatively, it may reflect development differences since the cultures are prepared from neonatal animals whereas the cochlear sections were derived from mature animals.
Interestingly, when dissociated SGNs are plated on mixed glial cells, neurites tend to grow along the type of glial cells that contact the neuronal somata. In our preparations, it was not possible to establish the sequence of neurite/glia interactions, thus our descriptions represent a static picture of these interactions. The tendency of neurites to grow in contact with the same type of glial cell that contacts neuron cell body is most apparent in neurons contacting SCs or OLs. A similar finding was observed by Grimpe et al. They seeded dorsal root ganglion (DRG) neurons on confrontation SC-AC cultures. The majority of axons (81.8%) that began growing on a SC continued their growth only on SCs, while 3.7% of axons grew only on AC (Grimpe et al., 2005). On the other hand, when the DRG neurons were plated on ACs, about half (53.3%) of the axons grew on ACs only. Taken together, these observations raise the possibility that glia provide instructive cues that favor neurite growth on similar cells. Alternatively, neurons whose cell bodies contact a particular type of glia may express a subset of receptors that favor neurite growth on similar types of cells.
Following injury, adult mammalian CNS axons do not spontaneously regenerate. Cell autonomous factors, the astroglial scar and associated ECM proteins, and CNS myelin each contribute to the lack of CNS axonal regeneration (Bomze et al., 2001, Liu et al., 2002, Yiu and He, 2003, Kretz et al., 2004, Jiao et al., 2005, Liu et al., 2006, Busch and Silver, 2007, Park et al., 2008). Several of the proteins produced by ACs and OLs that inhibit axon growth have been identified including myelin-associated glycoprotein (MAG); nogo-A, oligodendrocyte-myelin protein (OMgp); netrin-1; semaphorin 4D; ephrinB3; and chondroitin sulfate proteoglycans (CSPGs) (McKerracher et al., 1994, Chen et al., 2000, Wang et al., 2002b, Moreau-Fauvarque et al., 2003, Benson et al., 2005, Carulli et al., 2005, Low et al., 2008). Here we show that ACs and OLs strongly inhibit SGN neurite growth, compared to peripheral nerve SCs. However, the inhibition of SGN neurite growth by central glia does not appear to be mediated by secreted factors. Rather, it requires a population of cells and is not observed in neurite behavior towards isolated ACs or OLs; nor does CM from ACs or OLs inhibit SGN neurite growth. In fact, we found that ACCM provided a modest, but significant, increase in SGN neurite length. These observations suggest that the inhibitory effect of central glia in our culture conditions is likely due to ECM proteins such as CSPGs rather than glial cell surface proteins (e.g. ephrins) or secreted factors (e.g. semaphorins) (Nash et al., 2009).
Many of the ligands produced by central glia engage receptor networks such as the Nogo receptor, p75NTR, and LINGO-1 complex, and activate overlapping signaling pathways including protein kinase C and RhoA/rho-associated kinase (ROCK) to inhibit axon regeneration (Lehmann et al., 1999, Niederost et al., 2002, Wang et al., 2002a, Fournier et al., 2003, Yiu and He, 2003, Nash et al., 2009). Thus, elimination or blocking of receptors and inhibition of downstream signals (e.g. ROCK) represent therapeutic strategies to overcome the inhibitory effect of central glia on axon growth (Lehmann et al., 1999, Fournier et al., 2002, GrandPre et al., 2002, Fournier et al., 2003). Significantly, H-1152, a ROCK inhibitor, has recently been shown to enhance SGN neurite growth indicating that ROCK inhibits SGN neurite growth (Lie et al., 2010).
An alternative strategy is to manipulate the neuron to render it unresponsive to inhibitory ligands. Elevation of cAMP overcomes the inhibitory effect of central myelin on axon regeneration in vitro and in vivo; an effect that is dependent, at least in part, on cAMP activation of Ca2+/cAMP responsive element binding protein-mediated transcription (Cai et al., 2001b, Cai et al., 2002, Qiu et al., 2002, Gao et al., 2004, Hannila and Filbin, 2008). Elevation of cAMP also appears to directly alter the response of growth cones to repulsive cues (e.g. semaphorins) converting the response to attraction (Song et al., 1998, Henley et al., 2004). Similar to other neurons, we found that elevation of cAMP levels enhances SGN neurite growth in the presence of inhibitory central glia. In addition to promoting SGN neurite growth on central glia, elevation of cAMP enhances SGN survival by activating protein kinase A signaling in the cytoplasm (Hegarty et al., 1997, Hansen et al., 2001a, Bok et al., 2003).
In contrast to central glia, we found that SCs support and direct neurite growth. Whitlon, et al. reported that SGN neurites preferentially associate with SCs and suggested that SCs promote the regrowth of damaged SGN peripheral axons in the cochlea (Whitlon et al., 2009). We observed that SCs support radial neurite outgrowth from explants and likely facilitate fasciculation. By contrast, neurites growing on central glia, including those from cultures with elevated cAMP, tend to wander randomly and do not organize into bundles. In addition to supporting and directing neurite growth, SGSCs likely represent an important source of trophic factors for SGNs. SGSCs express neurotrophins that promote SGN survival including BDNF and NT-3 and the loss of SCs is associated with SG neural degeneration (Hansen et al., 2001a, Morris et al., 2006, Akil et al., 2010), further supporting the notion that SGSCs contribute to the trophic support of the SGNs.
Repopulation of SGNs with stem cell precursors recently emerged as a potential therapeutic strategy for improving cochlear implant performance in patients with profound sensorineural hearing loss or for providing reinnervation of regenerated hair cells (Reyes et al., 2008, Boer et al., 2009, Nishimura et al., 2009). Such newly generated SGNs will need to complete a functional neural circuit from the hair cells to the cochlear nucleus to provide functional recovery. If axonal regeneration along central glia is necessary as in case of SG regeneration using stem cell transplantation, increasing cAMP levels, either pharmacologically or by manipulating the developmental stage of the neurons, may be one way to overcome the inhibitory effect of central glia (Cai et al., 2001a, Qiu et al., 2002).
Acknowledgments
Support: NIDCD KO8 DC006211
Abbreviations
- AC
astrocyte
- ACCM
astrocyte conditioned medium
- BDNF
brain derived neurotrophic factor
- cAMP
cyclic adenosine monophosphate
- CM
conditioned media
- CNS
central nervous system
- cpt-cAMP
4-chlorophenylthio-adenosine 3′:5′ cyclic monophosphate
- CSPG
chondroitin sulfate proteoglycan
- DRG
dorsal root ganglion
- ECM
extracellular matrix
- FSK
forskolin
- NT-3
neurotrophin-3
- OL
oligodendrocyte
- OLCM
oligodendrocyte conditioned medium
- RIP
monoclonal antibody clone NS-1 that detects oligodendrocytes
- ROCK
RhoA/rho-associated kinase
- SC
Schwann cell
- SG
spiral ganglia
- SGN
spiral ganglion neuron
- SGSC
spiral ganglion Schwann cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akil O, Sun Y, Grabowski G, Lustig LR. Cochlea Spiral Ganglion Cells Degeneration and Hearing Loss as a Consequence of Schwann Cells Death in the Saposin B KO Mice ARO Abstract. 2010:891. doi: 10.1523/JNEUROSCI.3920-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam SA, Robinson BK, Huang J, Green SH. Prosurvival and proapoptotic intracellular signaling in rat spiral ganglion neurons in vivo after the loss of hair cells. J Comp Neurol. 2007;503:832–852. doi: 10.1002/cne.21430. [DOI] [PubMed] [Google Scholar]
- Altschuler RA, Cho Y, Ylikoski J, Pirvola U, Magal E, Miller JM. Rescue and regrowth of sensory nerves following deafferentation by neurotrophic factors. Ann N Y Acad Sci. 1999;884:305–311. doi: 10.1111/j.1749-6632.1999.tb08650.x. [DOI] [PubMed] [Google Scholar]
- Arnold W. Myelination of the human spiral ganglion. Acta Otolaryngol Suppl. 1987;436:76–84. doi: 10.3109/00016488709124979. [DOI] [PubMed] [Google Scholar]
- Benson MD, Romero MI, Lush ME, Lu QR, Henkemeyer M, Parada LF. Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc Natl Acad Sci U S A. 2005;102:10694–10699. doi: 10.1073/pnas.0504021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer JC, Carney KE, van der Zee S. Differentiation of mouse embryonic stem cells into spiral ganglion neurons: a therapeutic approach to deafness. J Neurosci. 2009;29:5711–5712. doi: 10.1523/JNEUROSCI.0433-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok J, Zha XM, Cho YS, Green SH. An extranuclear locus of cAMP-dependent protein kinase action is necessary and sufficient for promotion of spiral ganglion neuronal survival by cAMP. J Neurosci. 2003;23:777–787. doi: 10.1523/JNEUROSCI.23-03-00777.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomze HM, Bulsara KR, Iskandar BJ, Caroni P, Skene JH. Spinal axon regeneration evoked by replacing two growth cone proteins in adult neurons. Nat Neurosci. 2001;4:38–43. doi: 10.1038/82881. [DOI] [PubMed] [Google Scholar]
- Bostrom M, Khalifa S, Bostrom H, Liu W, Friberg U, Rask-Andersen H. Effects of neurotrophic factors on growth and glial cell alignment of cultured adult spiral ganglion cells. Audiol Neurootol. 2009;15:175–186. doi: 10.1159/000251915. [DOI] [PubMed] [Google Scholar]
- Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17:120–127. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Cai D, Deng K, Mellado W, Lee J, Ratan RR, Filbin MT. Arginase I and polyamines act downstream from cyclic AMP in overcoming inhibition of axonal growth MAG and myelin in vitro. Neuron. 2002;35:711–719. doi: 10.1016/s0896-6273(02)00826-7. [DOI] [PubMed] [Google Scholar]
- Cai D, Qiu J, Cao Z, McAtee M, Bregman BS, Filbin MT. Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J Neurosci. 2001a;21:4731–4739. doi: 10.1523/JNEUROSCI.21-13-04731.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Qiu J, Cao Z, McAtee M, Bregman BS, Filbin MT. Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J Neurosci. 2001b;21:4731–4739. doi: 10.1523/JNEUROSCI.21-13-04731.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlstedt T, Cullheim S, Risling M, Ulfhake B. Nerve fibre regeneration across the PNS-CNS interface at the root-spinal cord junction. Brain Res Bull. 1989;22:93–102. doi: 10.1016/0361-9230(89)90133-0. [DOI] [PubMed] [Google Scholar]
- Carulli D, Laabs T, Geller HM, Fawcett JW. Chondroitin sulfate proteoglycans in neural development and regeneration. Curr Opin Neurobiol. 2005;15:116–120. doi: 10.1016/j.conb.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- Danneman PJ, Mandrell TD. Evaluation of five agents/methods for anesthesia of neonatal rats. Lab Anim Sci. 1997;47:386–395. [PubMed] [Google Scholar]
- Ernsberger U. The role of GDNF family ligand signalling in the differentiation of sympathetic and dorsal root ganglion neurons. Cell Tissue Res. 2008;333:353–371. doi: 10.1007/s00441-008-0634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- Fournier AE, Gould GC, Liu BP, Strittmatter SM. Truncated soluble Nogo receptor binds Nogo-66 and blocks inhibition of axon growth by myelin. J Neurosci. 2002;22:8876–8883. doi: 10.1523/JNEUROSCI.22-20-08876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier AE, Takizawa BT, Strittmatter SM. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci. 2003;23:1416–1423. doi: 10.1523/JNEUROSCI.23-04-01416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman B, Hockfield S, Black JA, Woodruff KA, Waxman SG. In situ demonstration of mature oligodendrocytes and their processes: an immunocytochemical study with a new monoclonal antibody, rip. Glia. 1989;2:380–390. doi: 10.1002/glia.440020510. [DOI] [PubMed] [Google Scholar]
- Gao Y, Deng K, Hou J, Bryson JB, Barco A, Nikulina E, Spencer T, Mellado W, Kandel ER, Filbin MT. Activated CREB is sufficient to overcome inhibitors in myelin and promote spinal axon regeneration in vivo. Neuron. 2004;44:609–621. doi: 10.1016/j.neuron.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Gillespie LN, Shepherd RK. Clinical application of neurotrophic factors: the potential for primary auditory neuron protection. Eur J Neurosci. 2005;22:2123–2133. doi: 10.1111/j.1460-9568.2005.04430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomaa NA, Rubinstein JT, Lowder MW, Tyler RS, Gantz BJ. Residual speech perception and cochlear implant performance in postlingually deafened adults. Ear Hear. 2003;24:539–544. doi: 10.1097/01.AUD.0000100208.26628.2D. [DOI] [PubMed] [Google Scholar]
- GrandPre T, Li S, Strittmatter SM. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002;417:547–551. doi: 10.1038/417547a. [DOI] [PubMed] [Google Scholar]
- Grimpe B, Pressman Y, Lupa MD, Horn KP, Bunge MB, Silver J. The role of proteoglycans in Schwann cell/astrocyte interactions and in regeneration failure at PNS/CNS interfaces. Mol Cell Neurosci. 2005;28:18–29. doi: 10.1016/j.mcn.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Hannila SS, Filbin MT. The role of cyclic AMP signaling in promoting axonal regeneration after spinal cord injury. Exp Neurol. 2008;209:321–332. doi: 10.1016/j.expneurol.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen MR, Vijapurkar U, Koland JG, Green SH. Reciprocal signaling between spiral ganglion neurons and Schwann cells involves neuregulin and neurotrophins. Hear Res. 2001a;161:87–98. doi: 10.1016/s0378-5955(01)00360-4. [DOI] [PubMed] [Google Scholar]
- Hansen MR, Zha XM, Bok J, Green SH. Multiple distinct signal pathways, including an autocrine neurotrophic mechanism, contribute to the survival-promoting effect of depolarization on spiral ganglion neurons in vitro. J Neurosci. 2001b;21:2256–2267. doi: 10.1523/JNEUROSCI.21-07-02256.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty JL, Kay AR, Green SH. Trophic support of cultured spiral ganglion neurons by depolarization exceeds and is additive with that by neurotrophins or cyclic AMP, and requires elevation of [Ca2+]i within a set range. J Neurosci. 1997;17:1959–1970. doi: 10.1523/JNEUROSCI.17-06-01959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley JR, Huang KH, Wang D, Poo MM. Calcium mediates bidirectional growth cone turning induced by myelin-associated glycoprotein. Neuron. 2004;44:909–916. doi: 10.1016/j.neuron.2004.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley PA, Clarke M, Crook JM, Wise AK, Shepherd RK. Cochlear immunochemistry--a new technique based on gelatin embedding. J Neurosci Methods. 2003;129:81–86. doi: 10.1016/s0165-0270(03)00211-5. [DOI] [PubMed] [Google Scholar]
- Hurley PA, Crook JM, Shepherd RK. Schwann cells revert to non-myelinating phenotypes in the deafened rat cochlea. Eur J Neurosci. 2007;26:1813–1821. doi: 10.1111/j.1460-9568.2007.05811.x. [DOI] [PubMed] [Google Scholar]
- Jalenques I, Albuisson E, Despres G, Romand R. Distribution of glial fibrillary acidic protein (GFAP) in the cochlear nucleus of adult and aged rats. Brain Res. 1995;686:223–232. doi: 10.1016/0006-8993(95)00463-z. [DOI] [PubMed] [Google Scholar]
- Jiao J, Huang X, Feit-Leithman RA, Neve RL, Snider W, Dartt DA, Chen DF. Bcl-2 enhances Ca(2+) signaling to support the intrinsic regenerative capacity of CNS axons. Embo J. 2005;24:1068–1078. doi: 10.1038/sj.emboj.7600589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AM, Handzel O, Burgess BJ, Damian D, Eddington DK, Nadol JB., Jr Is word recognition correlated with the number of surviving spiral ganglion cells and electrode insertion depth in human subjects with cochlear implants? Laryngoscope. 2005;115:672–677. doi: 10.1097/01.mlg.0000161335.62139.80. [DOI] [PubMed] [Google Scholar]
- Kretz A, Kugler S, Happold C, Bahr M, Isenmann S. Excess Bcl-XL increases the intrinsic growth potential of adult CNS neurons in vitro. Mol Cell Neurosci. 2004;26:63–74. doi: 10.1016/j.mcn.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Lehmann M, Fournier A, Selles-Navarro I, Dergham P, Sebok A, Leclerc N, Tigyi G, McKerracher L. Inactivation of Rho signaling pathway promotes CNS axon regeneration. J Neurosci. 1999;19:7537–7547. doi: 10.1523/JNEUROSCI.19-17-07537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie M, Grover M, Whitlon DS. Accelerated neurite growth from spiral ganglion neurons exposed to the Rho kinase inhibitor H-1152. Neuroscience. 2010;169:855–862. doi: 10.1016/j.neuroscience.2010.05.020. [DOI] [PubMed] [Google Scholar]
- Linthicum FH, Jr, Fayad JN. Spiral ganglion cell loss is unrelated to segmental cochlear sensory system degeneration in humans. Otol Neurotol. 2009;30:418–422. doi: 10.1097/mao.0b013e31819a8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BP, Cafferty WB, Budel SO, Strittmatter SM. Extracellular regulators of axonal growth in the adult central nervous system. Philos Trans R Soc Lond B Biol Sci. 2006;361:1593–1610. doi: 10.1098/rstb.2006.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BP, Fournier A, GrandPre T, Strittmatter SM. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- Low K, Culbertson M, Bradke F, Tessier-Lavigne M, Tuszynski MH. Netrin-1 is a novel myelin-associated inhibitor to axon growth. J Neurosci. 2008;28:1099–1108. doi: 10.1523/JNEUROSCI.4906-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- Moore JK, Linthicum FH., Jr Myelination of the human auditory nerve: different time courses for Schwann cell and glial myelin. Ann Otol Rhinol Laryngol. 2001;110:655–661. doi: 10.1177/000348940111000711. [DOI] [PubMed] [Google Scholar]
- Moreau-Fauvarque C, Kumanogoh A, Camand E, Jaillard C, Barbin G, Boquet I, Love C, Jones EY, Kikutani H, Lubetzki C, Dusart I, Chedotal A. The transmembrane semaphorin Sema4D/CD100, an inhibitor of axonal growth, is expressed on oligodendrocytes and upregulated after CNS lesion. J Neurosci. 2003;23:9229–9239. doi: 10.1523/JNEUROSCI.23-27-09229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JK, Maklad A, Hansen LA, Feng F, Sorensen C, Lee KF, Macklin WB, Fritzsch B. A disorganized innervation of the inner ear persists in the absence of ErbB2. Brain Res. 2006;1091:186–199. doi: 10.1016/j.brainres.2006.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadol JB, Jr, Burgess BJ, Reisser C. Morphometric analysis of normal human spiral ganglion cells. Ann Otol Rhinol Laryngol. 1990;99:340–348. doi: 10.1177/000348949009900505. [DOI] [PubMed] [Google Scholar]
- Nash M, Pribiag H, Fournier AE, Jacobson C. Central nervous system regeneration inhibitors and their intracellular substrates. Mol Neurobiol. 2009;40:224–235. doi: 10.1007/s12035-009-8083-y. [DOI] [PubMed] [Google Scholar]
- Niederost B, Oertle T, Fritsche J, McKinney RA, Bandtlow CE. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J Neurosci. 2002;22:10368–10376. doi: 10.1523/JNEUROSCI.22-23-10368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura K, Nakagawa T, Ono K, Ogita H, Sakamoto T, Yamamoto N, Okita K, Yamanaka S, Ito J. Transplantation of mouse induced pluripotent stem cells into the cochlea. Neuroreport. 2009;20:1250–1254. doi: 10.1097/WNR.0b013e32832ff287. [DOI] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettingill LN, Richardson RT, Wise AK, O’Leary SJ, Shepherd RK. Neurotrophic factors and neural prostheses: potential clinical applications based upon findings in the auditory system. IEEE Trans Biomed Eng. 2007;54:1138–1148. doi: 10.1109/TBME.2007.895375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phifer CB, Terry LM. Use of hypothermia for general anesthesia in preweanling rodents. Physiol Behav. 1986;38:887–890. doi: 10.1016/0031-9384(86)90058-2. [DOI] [PubMed] [Google Scholar]
- Qiu J, Cai D, Dai H, McAtee M, Hoffman PN, Bregman BS, Filbin MT. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34:895–903. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- Reyes JH, O’Shea KS, Wys NL, Velkey JM, Prieskorn DM, Wesolowski K, Miller JM, Altschuler RA. Glutamatergic neuronal differentiation of mouse embryonic stem cells after transient expression of neurogenin 1 and treatment with BDNF and GDNF: in vitro and in vivo studies. J Neurosci. 2008;28:12622–12631. doi: 10.1523/JNEUROSCI.0563-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rind HB, von Bartheld CS. Anterograde axonal transport of internalized GDNF in sensory and motor neurons. Neuroreport. 2002;13:659–664. doi: 10.1097/00001756-200204160-00025. [DOI] [PubMed] [Google Scholar]
- Roehm PC, Hansen MR. Strategies to preserve or regenerate spiral ganglion neurons. Curr Opin Otolaryngol Head Neck Surg. 2005;13:294–300. doi: 10.1097/01.moo.0000180919.68812.b9. [DOI] [PubMed] [Google Scholar]
- Roehm PC, Xu N, Woodson EA, Green SH, Hansen MR. Membrane depolarization inhibits spiral ganglion neurite growth via activation of multiple types of voltage sensitive calcium channels and calpain. Mol Cell Neurosci. 2008;37:376–387. doi: 10.1016/j.mcn.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein JT. How cochlear implants encode speech. Curr Opin Otolaryngol Head Neck Surg. 2004;12:444–448. doi: 10.1097/01.moo.0000134452.24819.c0. [DOI] [PubMed] [Google Scholar]
- Schnell L, Schwab ME. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990;343:269–272. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, Poo M. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Retrograde degeneration of the cochlear nerve. Acta Otolaryngol. 1975;79:266–275. doi: 10.3109/00016487509124683. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Anatomy of cochlear innervation. Am J Otolaryngol. 1985;6:453–467. doi: 10.1016/s0196-0709(85)80026-0. [DOI] [PubMed] [Google Scholar]
- Spoendlin H, Suter R. Regeneration in the VIII nerve. Acta Otolaryngol. 1976;81:228–236. doi: 10.3109/00016487609119954. [DOI] [PubMed] [Google Scholar]
- Sugawara M, Corfas G, Liberman MC. Influence of supporting cells on neuronal degeneration after hair cell loss. J Assoc Res Otolaryngol. 2005;6:136–147. doi: 10.1007/s10162-004-5050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toesca A. Central and peripheral myelin in the rat cochlear and vestibular nerves. Neurosci Lett. 1996;221:21–24. doi: 10.1016/s0304-3940(96)13273-0. [DOI] [PubMed] [Google Scholar]
- Valderrama-Canales FJ, Gil-Loyzaga P, Merchan-Perez A, Sanchez JG. Astrocyte cytoarchitecture in cochlear nuclei of the rat: an immunocytochemical study. ORL J Otorhinolaryngol Relat Spec. 1993;55:313–316. doi: 10.1159/000276446. [DOI] [PubMed] [Google Scholar]
- Wang KC, Kim JA, Sivasankaran R, Segal R, He Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002a;420:74–78. doi: 10.1038/nature01176. [DOI] [PubMed] [Google Scholar]
- Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, He Z. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002b;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Sakurai Y, Ichinose T, Aikawa Y, Kotani M, Itoh K. Monoclonal antibody Rip specifically recognizes 2′,3′-cyclic nucleotide 3′-phosphodiesterase in oligodendrocytes. J Neurosci Res. 2006;84:525–533. doi: 10.1002/jnr.20950. [DOI] [PubMed] [Google Scholar]
- Whitlon DS, Tieu D, Grover M, Reilly B, Coulson MT. Spontaneous association of glial cells with regrowing neurites in mixed cultures of dissociated spiral ganglia. Neuroscience. 2009;161:227–235. doi: 10.1016/j.neuroscience.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Pfingst BE. Spectral and temporal cues for speech recognition: implications for auditory prostheses. Hear Res. 2008;242:132–140. doi: 10.1016/j.heares.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu G, He Z. Signaling mechanisms of the myelin inhibitors of axon regeneration. Curr Opin Neurobiol. 2003;13:545–551. doi: 10.1016/j.conb.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylikoski J, Pirvola U, Virkkala J, Suvanto P, Liang XQ, Magal E, Altschuler R, Miller JM, Saarma M. Guinea pig auditory neurons are protected by glial cell line-derived growth factor from degeneration after noise trauma. Hear Res. 1998;124:17–26. doi: 10.1016/s0378-5955(98)00095-1. [DOI] [PubMed] [Google Scholar]