Abstract
Parkinson's disease (PD) is caused by oxidative stress, and erythropoietin (EPO) reduces oxidative stress in the brain. However, EPO cannot be developed as a treatment for PD, because EPO does not cross the blood-brain barrier (BBB). A brain-penetrating form of human EPO has been developed wherein EPO is fused to a chimeric monoclonal antibody (MAb) against the mouse transferrin receptor (TfR), which is designated the cTfRMAb-EPO fusion protein. The TfRMAb acts as a molecular Trojan horse to transport the fused EPO into brain via transport on the BBB TfR. Experimental PD was induced in adult mice by the intra-striatal injection of 6-hydroxydopamine, and PD mice were treated with 1 mg/kg of the cTfRMAb-EPO fusion protein intravenously (IV) every other day starting 1 hour after toxin injection. Following 3 weeks of treatment mice were euthanized for measurement of striatal tyrosine hydroxylase (TH) enzyme activity. Mice treated with the cTfRMAb-EPO fusion protein showed a 306 % increase in striatal TH enzyme activity, which correlated with improvement in 3 assays of neuro-behavior. The blood hematocrit increased 10% at 2 weeks, with no further changes at 3 weeks of treatment. A sandwich ELISA showed the immune reaction against the cTfRMAb-EPO fusion protein was variable and low titer. In conclusion, the present study demonstrates that a brain penetrating form of EPO is neuroprotective in PD following IV administration with minimal effects on erythropoiesis.
Keywords: blood-brain barrier, Parkinson's disease, erythropoietin, monoclonal antibody
1. Introduction
Erytropoietin (EPO) is neuroprotective in experimental Parkinson's disease (PD) following direct intra-cerebral injection (Xue et al, 2007). The neurotrophin must be administered directly into the brain, because EPO does not cross the blood-brain barrier (BBB) (Boado et al, 2010). EPO can be re-engineered to cross the BBB by fusion to a BBB molecular Trojan horse (MTH). The latter is a peptide or peptidomimetic monoclonal antibody (MAb) that undergoes transport across the BBB following binding to an endogenous BBB receptor such as the insulin receptor or transferrin receptor (TfR). An MTH developed for the human brain is a MAb against the human insulin receptor (HIR) (Boado et al, 2007). A HIRMAb-EPO fusion protein has been engineered that retains low nM KD of binding to both the HIR and the EPO receptor (EPOR), and rapidly penetrates the BBB in the Rhesus monkey following intravenous (IV) administration (Boado et al, 2010). However, the HIRMAb-EPO fusion protein cannot be administered IV in rodents because the HIRMAb does not recognize the rodent insulin receptor. There is no known MAb against the mouse insulin receptor that can be used as a BBB MTH. Therefore, a BBB MTH directed against the mouse TfR has been engineered, which is a chimeric MAb against the mouse TfR, designated the cTfRMAb (Boado et al, 2009). A cTfRMAb-EPO has been engineered, and this fusion protein rapidly penetrates the BBB in the mouse and the brain uptake is >2% of injected dose (ID)/gram brain following IV administration (Zhou et al, 2010). The IV administration of the cTfRMAb-EPO fusion protein in a mouse middle cerebral artery occlusion (MCAO) stroke model results in a 80% reduction in stroke volume and neural deficit, whereas IV administration of high doses of EPO has no neuroprotective effect in stroke (Fu et al, 2011).
The present study examines the neuroprotection in a mouse model of PD caused by chronic IV treatment with the cTfRMAb-EPO fusion protein. Therapeutic effects are monitored with assays of striatal tyrosine hydroxylase (TH) enzyme activity, as well as 3 models of striatal-related neurobehavior. In addition, this study examines the effect on hematocrit (Hct) of chronic dosing in the mouse with the cTfRMAb-EPO fusion protein. The effect of EPO treatment on Hct is directly related to the plasma area under the concentration curve (AUC) of the EPO analogue (Elliott et al, 2008). EPO variants with prolonged blood circulation times, and high plasma AUC, have increased effects on erythropoiesis. Conversely, an EPO analogue, such as the cTfRMAb-EPO fusion protein, that has a 14-fold lower plasma AUC as compared to EPO (Zhou et al, 2010), would be predicted to have reduced effects on erythropoiesis. Fusion of EPO to the cTfRMAb has 2 effects that both promote the use of the fusion protein for chronic treatment of neurodegeneration. First, fusion of EPO to the cTfRMAb causes binding to the BBB TfR, which results in BBB penetration of EPO from blood. Second, fusion of EPO to the cTfRMAb causes binding to the TfR in peripheral organs, which results in accelerated clearance from blood and reduced plasma AUC.
2. Results
Experimental PD was induced in mice by the intra-striatal injection of 6-hydroxydopamine on day 1 of the study. The mice were then treated every other day with either saline or 1 mg/kg of cTfRMAb-EPO fusion protein given IV, starting 1 hour after toxin injection. The body weight (grams) at the start and end of the study was 26 ± 2 and 27 ± 1, respectively, in the saline treated mice, and was 28 ± 2 and 30 ± 2, respectively, in the fusion protein treated mice. No symptoms of immune response were observed in any of the fusion protein-treated mice during the course of the treatment.
Mice with PD were tested for apomorphine-induced and amphetamine-induced rotation at 1, 2, and 3 weeks after toxin administration, and were then euthanized for measurement of striatal TH enzyme activity. The mice treated with saline showed an increase in apomporphine-induced contralateral rotation at 1 through 3 weeks after toxin injection (Figure 1). However, the mice treated with the cTfRMAb-EPO fusion protein had a 35%, 51%, and 62% decrease in apomorphine-induced rotation at weeks 1, 2, and 3, respectively, after toxin administration (Figure 1). The mice treated with saline similarly showed an increase in amphetamine-induced ipsilateral rotation at 1 through 3 weeks after toxin injection (Figure 2). However, the mice treated with the cTfRMAb-EPO fusion protein had a 33%, 61%, and 65% decrease in amphetamine-induced rotation at weeks 1, 2, and 3, respectively, after toxin administration (Figure 2). At the end of the study, the mice were evaluated with the vibrissae-elicited forelimb placing test. The saline and fusion protein treated mice both showed maximal placing scores on the right side, which is ipsilateral to the side of toxin injection (Figure 3). The mice treated with saline showed a reduction in placing score from 167 ± 1, on the non-lesioned side, to 34 ± 2, on the lesioned side (mean ± SE, n=10 mice/group). The mice treated with the fusion protein showed a 132 % increase in placing score, relative to the saline treated mice, on the lesioned side (Figure 3).
Figure 1.
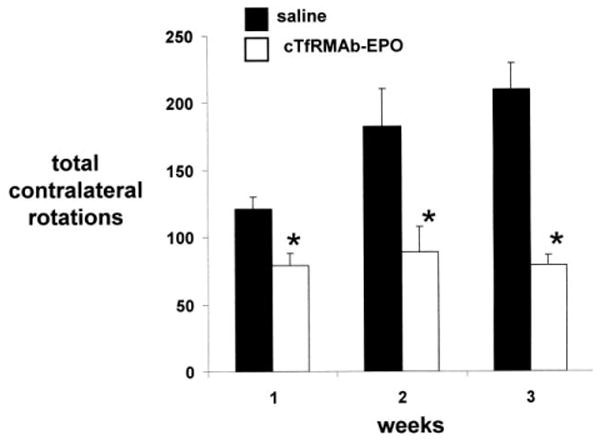
Rotation measurements following the administration of apomorphine for PD mice treated with either saline or the cTfRMAb-EPO fusion protein. Data are mean ± S.E. (n=10 mice/group). Statistical differences from the saline treated animals are p<0.005 at weeks 1 and 2 and p<0.001 at week 3 (*).
Figure 2.
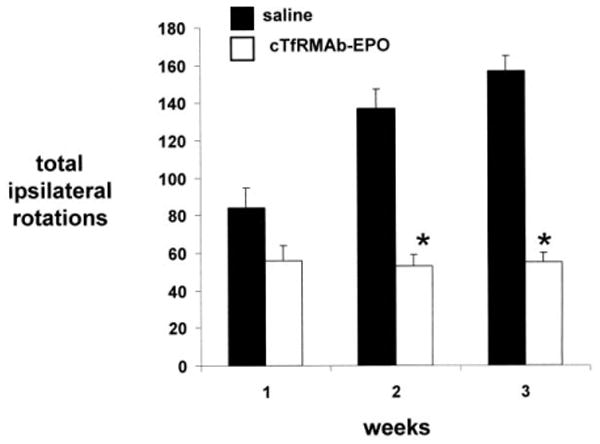
Rotation measurements following the administration of amphetamine for PD mice treated with either saline or the cTfRMAb-EPO fusion protein. Data are mean ± S.E. (n=10 mice/group). Statistical differences from the saline treated animals are p<0.001 at weeks 2 and 3 (*).
Figure 3.
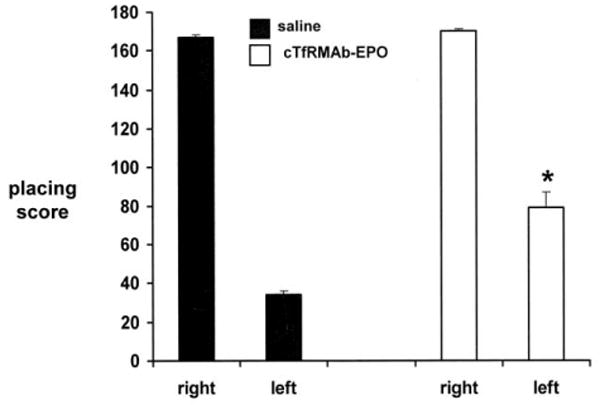
Vibrissae-elicited forelimb placing test scores for right side, which is ipsilateral to the toxin lesion, and the left side, which is contralateral to the toxin lesion, for the saline and cTfRMAb-EPO fusion protein treated mice. All scores were measured at 3 weeks following toxin injection. Data are mean ± S.E. (n=10 mice/group). Statistical differences from the saline treated animals are p<0.001 (*).
The striatal TH enzyme activity in the left, or non-lesioned striatum, 4620 ± 167 pmol/hr/mg protein, in the saline treated mice was not significantly different from the TH enzyme activity in the left striatum for the fusion protein treated mice, 4544 ± 144 pmol/hr/mg protein (mean ± SE, n=10 mice/group) (Figure 4). Similarly, there was no difference in the TH enzyme activity in the frontal cortex for the saline treated mice, 197 ± 9 pmol/hr/mg protein, vs the fusion protein treated mice, 204 ± 20 pmol/hr/mg protein (Figure 4). However, the fusion protein treatment caused a 306 % increase in striatal TH enzyme activity in the striatum on the lesioned side, from 996 ± 82 pmol/hr/mg protein, in the saline treated mice, to 3045 ± 357 pmol/hr/mg protein, in the fusion protein treated mice (Figure 4).
Figure 4.
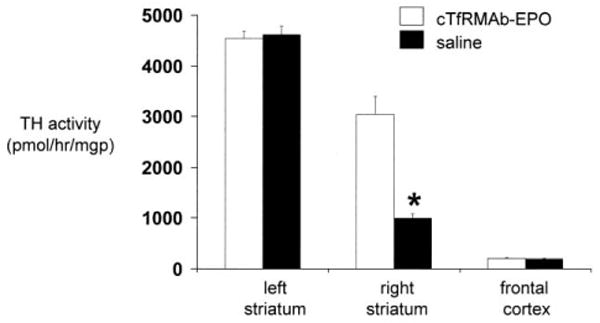
Striatal TH enzyme activity on the lesioned side and the non-lesioned side in the striatum and in the frontal cortex in mice treated with either saline or the cTfRMAb-EPO fusion protein. Brain TH activity was measured at 3 weeks after toxin administration. Data are mean ± S.E. (n=10 mice/group). Statistical differences from the saline treated animals in the right striatum are p<0.001(*).
The blood Hct in the fusion protein treated mice before treatment was 48.3 ± 1.4%, and the Hct increased 10% to 53.3 ± 2.5 % at 2 weeks of treatment with no further increase in Hct at 3 weeks of treatment (Table 1). The increase between 0 and 2 weeks was significant (p<0.01), while there were no significant differences between the Hct at 2 and 3 weeks (ANOVA).
Table 1. Hematocrit in PD mice treated with cTfRMAb-EPO fusion protein.
Data are mean ± SD (n=10 replicates/group).
p<0.01 difference from week 0; the difference between the hematocrit at weeks 2 and 3 is not significant (ANOVA).
The design of the immunity ELISA is shown in Figure 5A. The detector reagent is biotinylated cTfRMAb-EPO fusion protein, and biotinylation of the fusion protein is verified by Western blot analysis (Figure 5B). The pre-treatment mouse plasma produced no immune reaction with cTfRMAb-EPO fusion protein, whereas there was a variable immune reaction observed at the end of the treatment study at 1:50 dilutions of plasma (Figure 5C). The plasma from 2 mice that produced maximal immune signals at 1:50 dilution, and the plasma from 2 mice that produced minimal immune signals at 1:50 dilution, were tested in the immunity ELISA at 4 dilutions of plasma (1:50, 1:300, 1:1000, and 1:3000) (Figure 5D). The absorbance was near background in all plasma samples at the 1:1000 dilution (Figure 5D).
Figure 5.
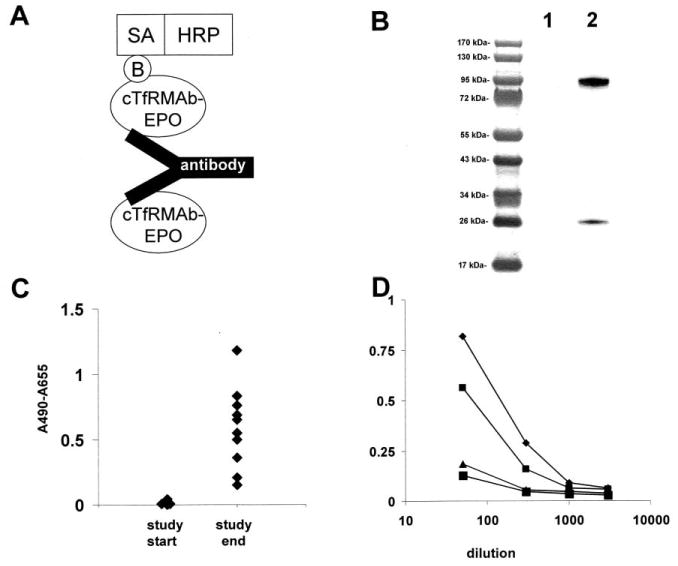
(A) Structure of the 2-site ELISA for detection of antibodies against the cTfRMAb-EPO fusion protein. The cTfRMAb-EPO fusion protein is used as the capture reagent, and the biotinylated cTfRMAb-EPO fusion protein is used as the detector reagent, along with a complex of streptavidin (SA) and horseradish peroxidase (HRP); the biotin moiety is designated, ‘B’. (B) Western blot of cTfRMAb-EPO fusion protein (lane 1) and biotinylated cTfRMAb-EPO fusion protein (lane 2) with a conjugate of avidin and biotinylated peroxidase. (C) Absorbance at 1:50 dilutions of mouse plasma taken at the beginning and the end of the 3-week treatment study of mice with the cTfRMAb-EPO fusion protein treatment groups. (D) Absorbance at dilutions (1:50, 1:300, 1:1000, and 1:3000) of plasma from 4 mice in the cTfRMAb-EPO fusion protein treatment group, including 2 mice that reacted the highest, and 2 mice that reacted the lowest, in the screen at 1:50 dilution (panel B).
3. Discussion
The results of this study are consistent with the following conclusions. First, chronic intravenous EPO therapy is neuroprotective in experimental PD, providing the EPO is first re-engineered as an IgG-EPO fusion protein, to enable receptor-mediated transport across the BBB. Second, the effect of chronic treatment with the cTfRMAb-EPO fusion protein on erythropoiesis is minimal, which is consistent with the differential pharmacokinetics of EPO vs the cTfRMAb-EPO fusion protein (Zhou et al, 2010). Third, the immune response developed in the course of the chronic fusion protein treatment was low titer and did not limit chronic drug therapy.
EPO is a large molecule drug that does not cross the BBB, in the absence of BBB disruption (Boado et al, 2010). Consequently, EPO must be administered via a trans-cranial injection route in order to observe neuroprotection in regional or global brain ischemia (Sakanaka et al, 1998; Bernaudin et al, 1999; Catania et al, 2002). In experimental PD, EPO must also be administered directly into the brain in order to produce neuroprotection, as systemic EPO has no therapeutic effect in PD (Xue et al, 2007). The intra-striatal injection of viral vectors encoding the EPO gene is also neuroprotective in experimental PD, providing the gene therapy is administered 7 days before toxin injection into the brain (Puskovic et al, 2006). The neuroprotective effect of intra-striatal EPO gene therapy in PD is comparable to the effect of intra-striatal GDNF gene therapy in PD (Puskovic et al, 2006).
The present study shows that EPO is therapeutic in PD following intravenous administration, provided the EPO is re-engineered as a MTH-EPO fusion protein. Therapy with the MTH-EPO fusion protein need not be initiated in advance of the intra-striatal injection of the neurotoxin. Chronic IV treatment with the cTfRMAb-EPO fusion protein results in a 306 % increase in striatal TH enzyme activity (Figure 4). Prior work has shown that striatal TH enzyme activity, which is a quantitative measure of striatal recovery, correlates with immunocytochemistry of striatal TH (Zhang et al, 2004). The increase in striatal TH enzyme activity caused by systemic treatment with the cTfRMAb-EPO fusion protein is associated with an improvement in 3 assays of neurobehavior (Figures 1-3). The dose of the cTfRMAb-EPO fusion protein used in the current study was 1 mg/kg. Future work will define the dose-response relationships of IgG-EPO neuroprotection in PD. However, treatment with the IgG-EPO fusion protein in experimental stroke demonstrates a dose-response relationship of the neuroprotective properties of the fusion protein (Fu et al, 2010a). Future work will also define the extent to which intravenous IgG-EPO is neuroprotective in experimental PD following a delay in treatment relative to neurotoxin injection. Prior work shows there is recovery of the nigral-striatal tract in experimental PD when neurotrophin administration is delayed 1-2 weeks following toxin injection (Zhang and Pardridge, 2009).
The dose of the cTfRMAb-EPO fusion protein used in this study, 1 mg/kg, is equivalent to a dose of EPO of 200 ug/kg, since EPO comprises 20% of the amino acid content of the fusion protein (Zhou et al, 2010). A 200 ug/kg dose of EPO is equivalent to a dose of 20,000 units/kg, since 1 unit=10 ng of EPO. However, given the 10-fold lower plasma area under the concentration curve (AUC) of the IgG-EPO fusion protein, as compared to EPO alone (Zhou et al, 2010), the dose administered in this study is equivalent to an EPO dose of 2,000 units/kg. A systemic daily dose of 5,000 units/kg of EPO alone was not neuroprotective in experimental PD (Xue et al, 2007). The failure of systemic EPO to cause neuroprotection in PD is consistent with the lack of penetration of EPO across the BBB (Boado et al, 2010).
Chronic administration to mice of the IgG-EPO fusion protein at a dose of 1 mg/kg caused only a 10% increase in hematocrit at 2 weeks of treatment and no further increases in blood hematocrit were observed at 3 weeks (Table 1). In contrast, the systemic administration of EPO alone in the mouse at a daily dose of 4,000 units/kg results in a 52% and 75% increase in hematocrit at 2 and 4 weeks of treatment (Grignaschi et al, 2007). The differential effects of EPO and the cTfRMAb-EPO fusion protein on hematopoiesis is attributed to the different pharmacokinetic properties of the proteins. The cTfRMAb-EPO fusion protein is cleared from blood via the TfR in peripheral tissues. Owing to the TfR-mediated clearance of the fusion protein, the plasma AUC of the cTfRMAb-EPO fusion protein is at least 10-fold reduced as compared to the plasma AUC of EPO in the mouse (Zhou et al, 2010). Similarly, the plasma AUC of the HIRMAb-EPO fusion protein is 13-fold lower than the plasma AUC of EPO in the Rhesus monkey (Boado et al, 2010). The hematopoietic effect of systemic EPO is directly proportional to the plasma AUC of the EPO analogue (Elliott et al, 2008). Analogues of EPO with increased plasma AUC have enhanced effects on erythropoiesis. Conversely, analogues of EPO, such as the IgG-EPO fusion protein, with decreased plasma AUC have diminished effects on erythropoiesis.
The chronic administration of the cTfRMAb-EPO fusion protein results in an immune response directed against the protein, as demonstrated by the immunity ELISA (Figure 5C). However, this immune response is low titer and is not detected at plasma dilutions greater than 1:1000, even in the mice with the highest immune responses (Figure 5D). The re-engineering of EPO as an IgG fusion protein may promote immune tolerance. The constant region of IgG contains amino acid sequences, called Tregitopes, which induce immune tolerance (DeGroot et al, 2008).
The mechanism of action of EPO on the nigral-striatal tract may be related to the property of EPO to reduce neuronal apoptosis (Wei et al, 2006). In addition, EPO has an anti-oxidant effect in brain (Sifringer et al, 2010), which may ameliorate the oxidative stress in the nigra-striatal tract that is caused by the injection of the 6-hydroxydopamine neurotoxin (Aluf et al, 2010). EPO in the brain may serve to protect neurons against oxidative stress, which may explain the increased incidence of PD in humans with chronic low EPO levels (Ferruccci et al, 2007). Analogues of EPO, such as the cTfRMAb-EPO fusion protein for the mouse, or the HIRMAb-EPO fusion protein for humans, which have enhanced BBB penetration and diminished effects on erythropoiesis, are new potential treatments for neurodegeneration.
4. Experimental procedures
4.1 6-hydroxydopamine model and treatment
All procedures were approved by the UCLA Animal Research Committee. Adult male C57BL/6J mice (Jackson Labs, Bar Harbor, ME) weighing 26-34 g were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) intraperitoneally. Animals received a unilateral intra-cerebral injection of a total of 12 μg of 6-hydroxydopamine·HBr (Sigma Chemical Co.) dissolved in 0.02% ascorbic acid in 0.9% saline. The 6-hydroxydopamine (6 μg in 2 μL) was injected into the right striatum at 2 locations as described previously (Fu et al, 2010b). The toxin was injected into the striatum at sites with the following stereotaxic coordinates: +1.0 mm relative to bregma, 2.1 mm relative to midline, 2.9 mm below the skull surface (site 1); +0.3 mm relative to bregma, 2.3 mm relative to midline, 2.9 mm below the skull surface (site 2). Mice were treated intravenously with either saline (n=10 mice), or the cTfRMAb-EPO fusion protein, 1.0 mg/kg (n=10 mice), every 2 days over the following 3 weeks, with the first dose given 1 hour after toxin injection into the brain. Drug was injected IV via the tail vein in a volume of 50 uL/mouse. The mice were euthanized at 3 weeks following toxin administration for measurement of striatal TH enzyme activity.
The genetic engineering of the cTfRMAb-EPO fusion protein has been described previously (Zhou et al, 2010). The 166 amino acid mature human EPO, minus the signal peptide, was fused to the carboxyl terminus of the heavy chain of the cTfRMAb. The cTfRMAb-EPO fusion protein was expressed by stably transfected Chinese hamster ovary cells cultured in serum free medium, and was purified by protein G affinity chromatography, as described previously (Zhou et al, 2010). The fusion protein was formulated in 0.01 M Tris buffered saline, pH=5.5, and was stored either sterile filtered at 4°C or at -70°C.
4.2 Behavioral testing
Beginning 1 week after the toxin administration, mice were tested weekly for apomorphine- and amphetamine-induced rotation, which was performed on separate days, as described previously (Fu et al, 2010b). A vibrissae-elicited forelimb placing trial was carried out as described previously for rats (Anstrom et al, 2007), as adapted for the mouse (Fu et al, 2010b).
4.3 Tyrosine hydroxylase enzyme activity and hematocrit
TH enzyme activity in mouse brain striatum (left and right side) and in frontal cortex was measured with [3,5-3H]-L-tyrosine (Perkin Elmer, Boston, MA) as substrate. The labeled [3H]-water and L-DOPA was separated with a charcoal separation technique, as described previously (Fu et al, 2010b). TH enzyme activity was measured at 37°C for 30 min, and expressed as pmol/hour/mg protein. Hematocrit was measured on whole blood, removed from the retro-orbital sinus, with a CritSpin 3 microhematocrit centrifuge (Iris Sample Processing, Inc., Westwood, MA) at 14,000 g for 2 minutes.
4.4 Immunity ELISA
The presence of anti-cTfRMAb-EPO fusion protein antibodies in mouse plasma was measured with a 2-site sandwich ELISA described previously (Pardridge et al, 2009). The cTfRMAb-EPO fusion protein is used as the capture reagent and biotinylated cTfRMAb-EPO fusion protein as the detector reagent. The mouse plasma was diluted in PBS. The capture reagent was plated overnight at 4C in 96-wells in 100 uL (250 ng)/well in 0.05 M NaHCO3/8.3. The wells were blocked with PBS containing 1% bovine serum albumin (PBSB), followed by the addition of 100 uL/well of the diluted mouse plasma. After a 60 min incubation at 37C, the wells were washed with PBSB, and the wells were incubated with biotinylated cTfRMAb-EPO fusion protein (50 ng/well) for 60 min. The wells were washed with PBSB, followed by incubation with 100 uL (500 ng/well) of a streptavidin-peroxidase conjugate (#SA-5004, Vector Labs) for 30 min at room temperature (RT). The wells were washed with PBSB, and 100 uL/well of o-phenylenediamine/H2O2 developing solution (#P5412, Sigma Chemical Co, St. Louis, MO) was added for a 15 min incubation in the dark at RT. The reaction was stopped by the addition of 100 uL/well of 1 M HCl, followed by the measurement of absorbance at 492 nm and 650 nm. The A650 was subtracted from the A492. The (A492-A650) for the PBSB blank was then subtracted from the (A492-A650) for the sample. Mouse plasma samples were screened with the immunity ELISA at 1:50 dilutions in PBS. For subsequent studies, mouse plasma samples were diluted 1:50, 1:300, 1:1000, or 1:3000 in PBS to assess the titer of the anti-cTfRMAb-EPO antibody response.
The cTfRMAb-EPO fusion protein was biotinylated with sulfo-biotin-LC-LC-N-hydroxysuccinimide, where LC=long chain (#21338, Pierce Chemical Co.). The biotinylation of the cTfRMAb-EPO fusion protein was confirmed by SDS-PAGE and Western blotting, where the blot was probed with avidin and biotinylated peroxidase. The non-biotinylated cTfRMAb-EPO fusion protein was tested as a negative control.
4.5.1 Statistical analysis
Statistical differences between saline and fusion protein treated mice were determined with Student's t-test, and by analysis of variance (ANOVA) with Bonferronni correction.
Acknowledgments
This research was supported by NIH grant NS065917.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aluf Y, Vaya J, Khatib S, Loboda Y, Kizhner S, Finberg JPM. Specific oxidative stress profile associated with partial striatal dopaminergic depletion by 6-hydroxydopamine as assessed by a novel multifunctional marker molecule. Free Radic Res. 2010;44:635–644. doi: 10.3109/10715761003692529. [DOI] [PubMed] [Google Scholar]
- Anstrom KK, Schallert T, Woodlee MT, Shattuck A, Roberts DC. Repetitive vibrissae-elicited forelimb placing before and immediately after unilateral 6-hydroxydopamine improves outcome in a model of Parkinson's disease. Behav Brain Res. 2007;179:183–191. doi: 10.1016/j.bbr.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Bernaudin M, Marti HH, Roussel S, Divoux D, Nouvelot A, MacKenzie ET, Petit E. A Potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab. 1999;19:643–651. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- Boado RJ, Hui EK, Lu JZ, Pardridge WM. Drug targeting of erythropoietin across the primate blood-brain barrier with an IgG molecular Trojan horse. J Pharmacol Exp Ther. 2010;333:961–969. doi: 10.1124/jpet.109.165092. [DOI] [PubMed] [Google Scholar]
- Boado RJ, Zhang Y, Wang Y, Pardridge WM. Engineering and expression of a chimeric transferrin receptor monoclonal antibody for blood-brain barrier delivery in the mouse. Biotechnol Bioeng. 2009;102:1251–1258. doi: 10.1002/bit.22135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boado RJ, Zhang YF, Zhang Y, Pardridge WM. Humanization of anti-human insulin receptor antibody for drug targeting across the human blood-brain barrier. Biotechnol Bioeng. 2007;96:381–391. doi: 10.1002/bit.21120. [DOI] [PubMed] [Google Scholar]
- Catania MA, Marciano MC, Parisi A, Sturiale A, Buemi M, Grasso G. Erythropoietin prevents cognition impairment induced by transient brain ischemia in gerbils. Eur J Pharmacol. 2002;437:147–150. doi: 10.1016/s0014-2999(02)01292-x. [DOI] [PubMed] [Google Scholar]
- De Groot AS, Moise L, McMurry JA, Wambre E, Van Overtvelt L, Moingeon P, Scott DW, Martin W. Activation of natural regulatory T cells by IgG Fc-derived peptide “Tregitopes”. Blood. 2008;112:3303–3311. doi: 10.1182/blood-2008-02-138073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott S, Pham E, Macdougall IC. Erythropoietins: a common mechanism of action. Exp Hematol. 2008;36:1573–1584. doi: 10.1016/j.exphem.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Guralnik JM, Bandinelli S, Semba RD, Lauretani F, Corsi A, Ruggiero C, Ershler WB, Longo DL. Unexplained anaemia in older persons is characterised by low erythropoietin and low levels of pro-inflammatory markers. Brit J Hemat. 2007;136:849–855. doi: 10.1111/j.1365-2141.2007.06502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu A, Zhou QH, Hui EK, Lu JZ, Boado RJ, Pardridge WM. Neuroprotection in experimental stroke in the rat with an IgG-erythropoietin fusion protein. Brain Res. 2010a;1360:193–197. doi: 10.1016/j.brainres.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Fu A, Zhou QH, Hui EK, Lu JZ, Boado RJ, Pardridge WM. Intravenous treatment of experimental Parkinson's disease in the mouse with an IgG-GDNF fusion protein that penetrates the blood-brain barrier. Brain Res. 2010b;1352:208–213. doi: 10.1016/j.brainres.2010.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu A, Hui EK, Lu JZ, Boado RJ, Pardridge WM. Neuroprotection in stroke in the mouse with intravenous erythropoietin-Trojan horse fusion protein. Brain Res. 2011;1369:203–207. doi: 10.1016/j.brainres.2010.10.097. [DOI] [PubMed] [Google Scholar]
- Grignaschi G, Zennaro E, Tortarolo M, Calvaresi N, Bendotti C. Erythropoietin does not preserve motor neurons in a mouse model of familial ALS. Amyotroph Lateral Scler. 2007;8:31–35. doi: 10.1080/17482960600783456. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Boado RJ. Pharmacokinetics and safety in rhesus monkeys of a monoclonal antibody-GDNF fusion protein for targeted blood-brain barrier delivery. Pharm Res. 2009;26:2227–2236. doi: 10.1007/s11095-009-9939-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puskovic V, Wolfe D, Wechuck J, Krisky D, Collins J, Glorioso JC, Fink DJ, Mata M. HSV-Mediated Delivery of Erythropoietin Restores Dopaminergic Function in MPTP-Treated Mice. Mol Ther. 2006;14:710–715. doi: 10.1016/j.ymthe.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Wen TC, Matsuda S, Masuda S, Morishita E, Nagao M, Sasaki R. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci USA. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifringer M, Brait D, Weichelt U, Zimmerman G, Endesfelder S, Brehmer F, von Haefen C, Friedman A, Soreq H, Bendix I, Gerstner B, Felderhoff-Mueser U. Erythropoietin attenuates hyperoxia-induced oxidative stress in the developing rat brain. Brain Behav Immun. 2010;24:792–799. doi: 10.1016/j.bbi.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Wei L, Han BH, Li Ying, Keogh CL, Holtzman DM, Yu SP. Cell Death Mechanism and Protective Effect of Erythropoietin after Focal Ischemia in the Whisker-Barrel Cortex of Neonatal Rats. J Pharmacol Exp Ther. 2006;317:109–116. doi: 10.1124/jpet.105.094391. [DOI] [PubMed] [Google Scholar]
- Xue YQ, Zhao LR, Guo WP, Duan WM. Intrastriatal adminstration of erythropoietin protects dopaminergic neurons and improves neurobehavioral outcome in a rat model of Parkinson's disease. Neuroscience. 2007;146:1245–1258. doi: 10.1016/j.neuroscience.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Schlachetzki F, Zhang Y, Boado RJ, Pardridge WM. Normalization of striatal tyrosine hydroxylase and reversal of motor impairment in experimental Parkinsonism with intravenous nonviral gene therapy and a brain-specific promoter. Human Gene Ther. 2004;15:339–350. doi: 10.1089/104303404322959498. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Pardridge WM. Near complete rescue of experimental Parkinson's disease with intravenous, non-viral GDNF gene therapy. Pharm Res. 2009;26:1059–1063. doi: 10.1007/s11095-008-9815-9. [DOI] [PubMed] [Google Scholar]
- Zhou QH, Boado RJ, Lu JZ, Hui EK, Pardridge WM. Re-engineering erythropoietin as an IgG fusion protein that penetrates the blood-brain barrier in the mouse. Mol Pharm. 2010;7:2148–2155. doi: 10.1021/mp1001763. [DOI] [PubMed] [Google Scholar]


