Abstract
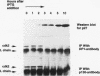
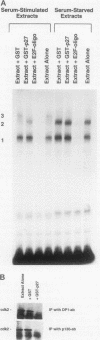
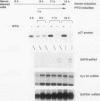
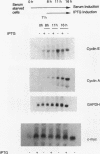
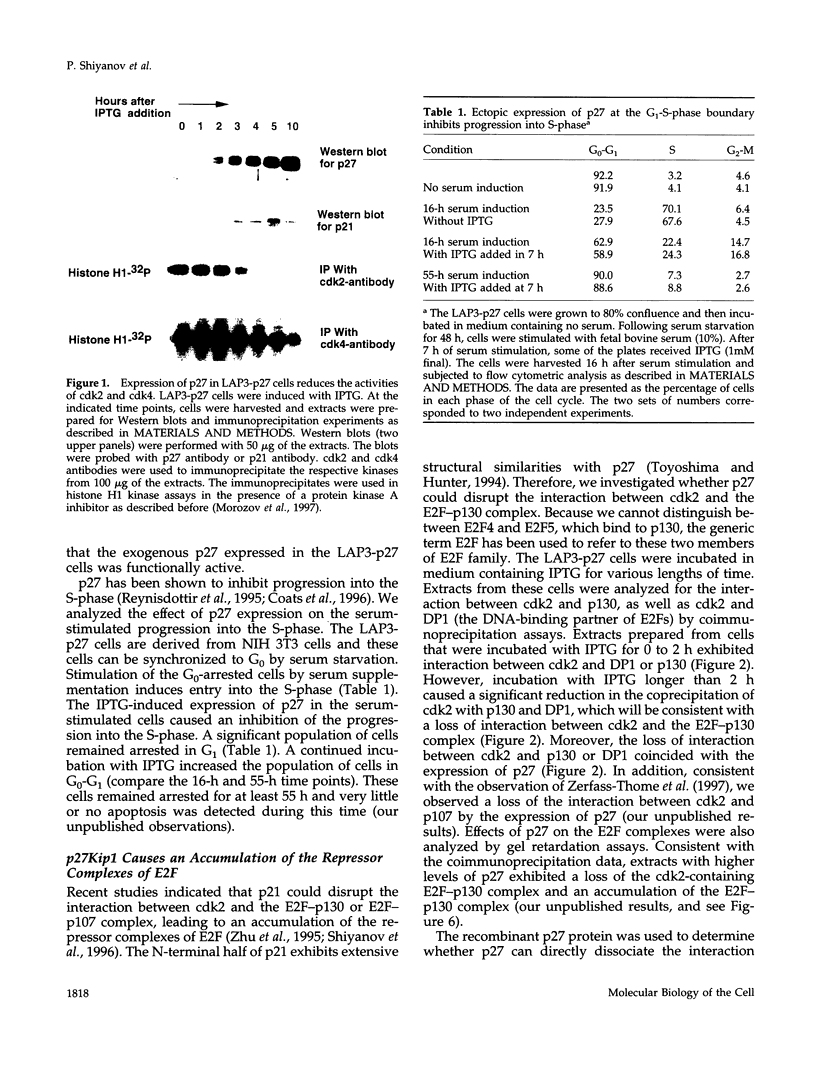
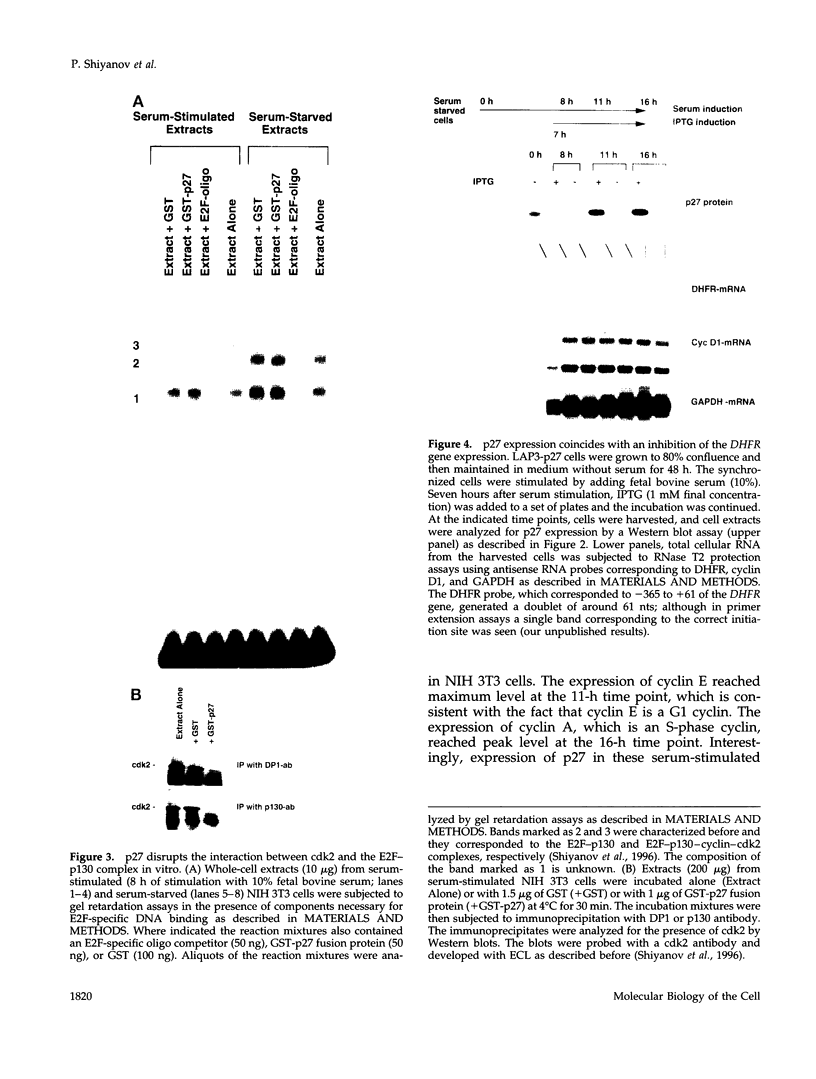
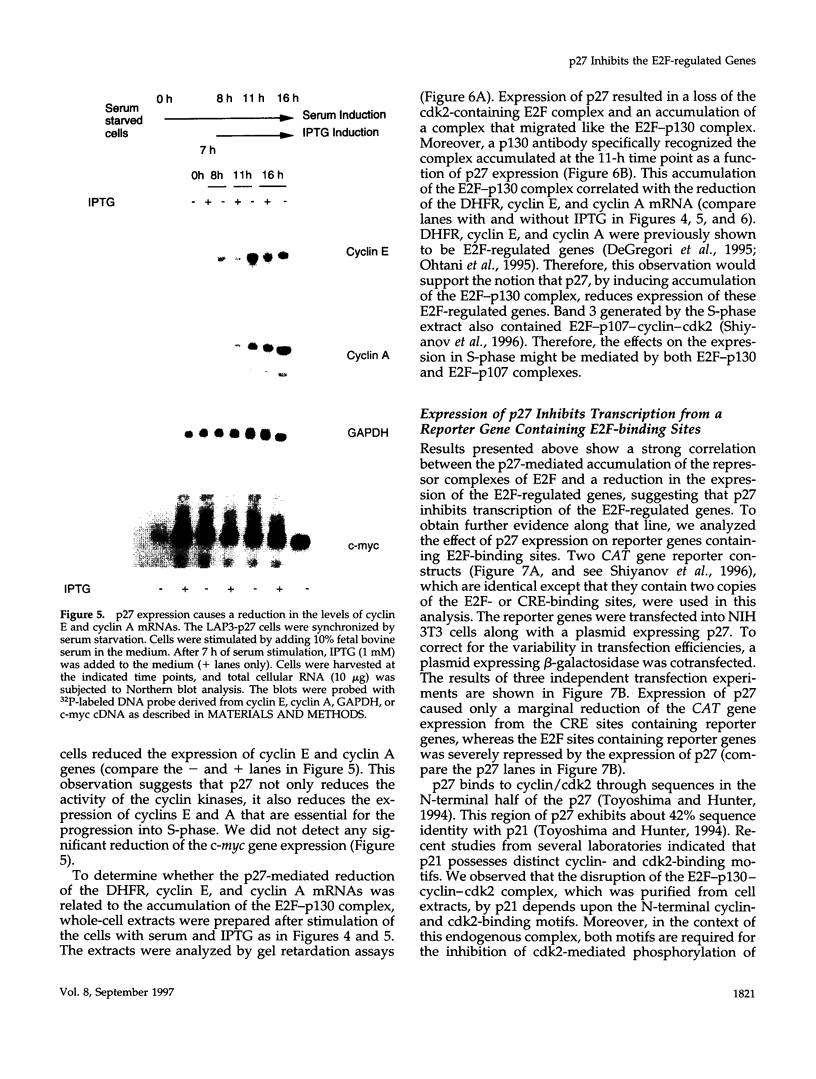
p27Kip1 is an inhibitor of the cyclin-dependent kinases and it plays an inhibitory role in the progression of cell cycle through G1 phase. To investigate the mechanism of cell cycle inhibition by p27Kip1, we constructed a cell line that inducibly expresses p27Kip1 upon addition of isopropyl-1-thio-beta-D-galactopyranoside in the culture medium. Isopropyl-1-thio-beta-D-galactopyranoside-induced expression of p27Kip1 in these cells causes a specific reduction in the expression of the E2F-regulated genes such as cyclin E, cyclin A, and dihydrofolate reductase. The reduction in the expression of these genes correlates with the p27Kip1-induced accumulation of the repressor complexes of the E2F family of factors (E2Fs). Our previous studies indicated that p21WAF1 could disrupt the interaction between cyclin/cyclin-dependent kinase 2 (cdk2) and the E2F repressor complexes E2F-p130 and E2F-p107. We show that p27Kip1, like p21WAF1, disrupts cyclin/cdk2-containing complexes of E2F-p130 leading to the accumulation of the E2F-p130 complexes, which is found in growth-arrested cells. In transient transfection assays, expression of p27Kip1 specifically inhibits transcription of a promoter containing E2F-binding sites. Mutants of p27Kip1 harboring changes in the cyclin- and cdk2-binding motifs are deficient in inhibiting transcription from the E2F sites containing reporter gene. Moreover, these mutants of p27Kip1 are also impaired in disrupting the interaction between cyclin/cdk2 and the repressor complexes of E2Fs. Taken together, these observations suggest that p27Kip1 reduces expression of the E2F-regulated genes by generating repressor complexes of E2Fs. Furthermore, the results also demonstrate that p27Kip1 inhibits expression of cyclin A and cyclin E, which are critical for progression through the G1-S phases.
Full text
PDF



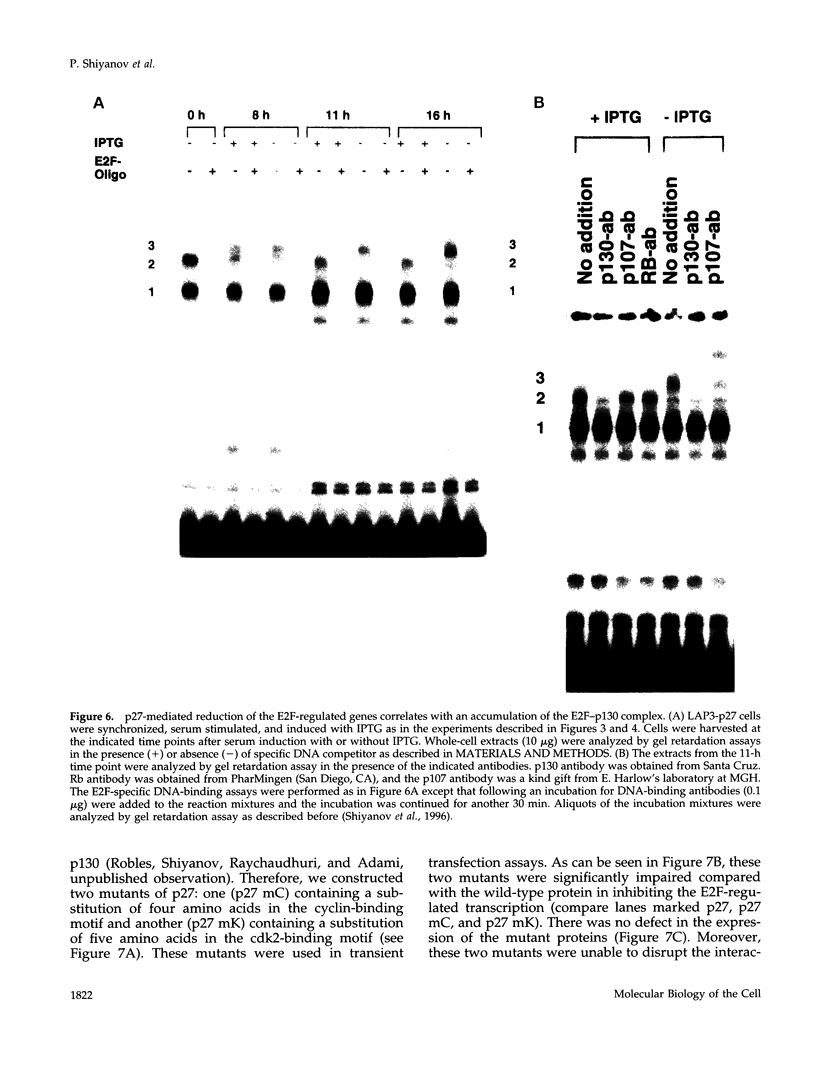
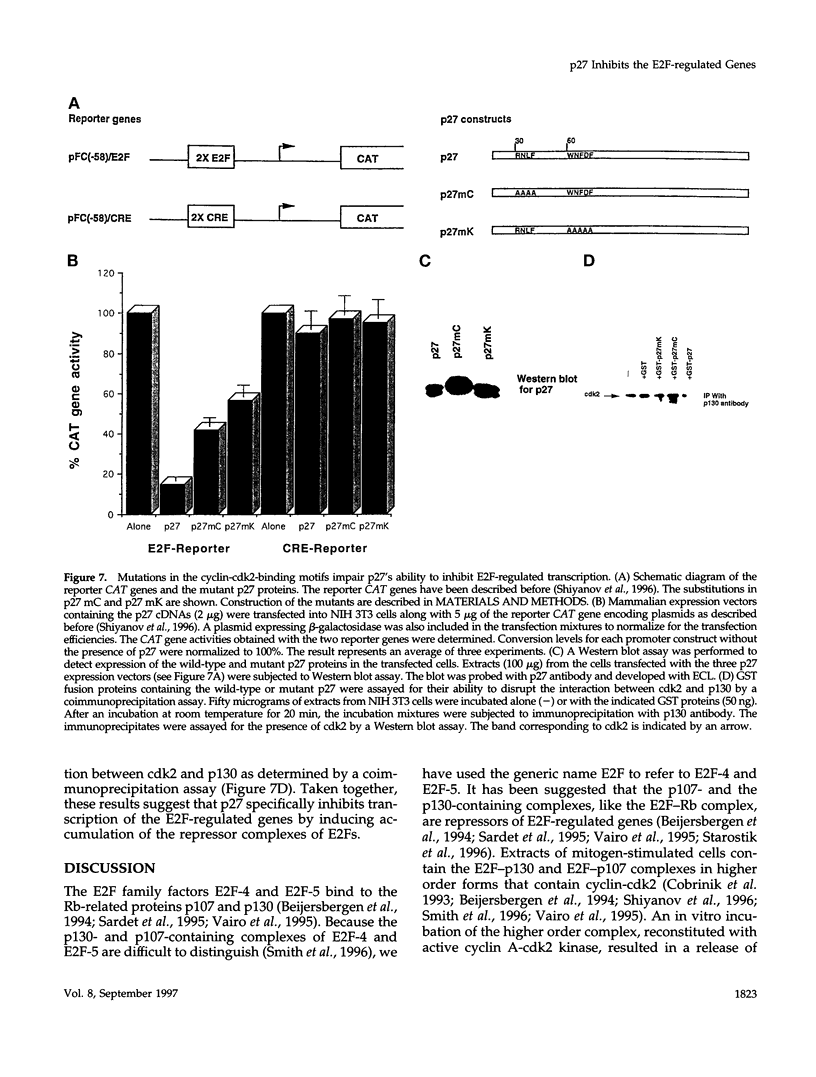




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. D., Sellers W. R., Sharma S. K., Wu A. D., Nalin C. M., Kaelin W. G., Jr Identification of a cyclin-cdk2 recognition motif present in substrates and p21-like cyclin-dependent kinase inhibitors. Mol Cell Biol. 1996 Dec;16(12):6623–6633. doi: 10.1128/mcb.16.12.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afshari C. A., Nichols M. A., Xiong Y., Mudryj M. A role for a p21-E2F interaction during senescence arrest of normal human fibroblasts. Cell Growth Differ. 1996 Aug;7(8):979–988. [PubMed] [Google Scholar]
- Arroyo M., Bagchi S., Raychaudhuri P. Association of the human papillomavirus type 16 E7 protein with the S-phase-specific E2F-cyclin A complex. Mol Cell Biol. 1993 Oct;13(10):6537–6546. doi: 10.1128/mcb.13.10.6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K. L., Lain S., Fâhraeus R., Smythe C., Lane D. P. Cell-cycle arrest and inhibition of Cdk4 activity by small peptides based on the carboxy-terminal domain of p21WAF1. Curr Biol. 1997 Jan 1;7(1):71–80. doi: 10.1016/s0960-9822(06)00029-7. [DOI] [PubMed] [Google Scholar]
- Beijersbergen R. L., Kerkhoven R. M., Zhu L., Carlée L., Voorhoeve P. M., Bernards R. E2F-4, a new member of the E2F gene family, has oncogenic activity and associates with p107 in vivo. Genes Dev. 1994 Nov 15;8(22):2680–2690. doi: 10.1101/gad.8.22.2680. [DOI] [PubMed] [Google Scholar]
- Blake M. C., Azizkhan J. C. Transcription factor E2F is required for efficient expression of the hamster dihydrofolate reductase gene in vitro and in vivo. Mol Cell Biol. 1989 Nov;9(11):4994–5002. doi: 10.1128/mcb.9.11.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Saha P., Kornbluth S., Dynlacht B. D., Dutta A. Cyclin-binding motifs are essential for the function of p21CIP1. Mol Cell Biol. 1996 Sep;16(9):4673–4682. doi: 10.1128/mcb.16.9.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats S., Flanagan W. M., Nourse J., Roberts J. M. Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science. 1996 May 10;272(5263):877–880. doi: 10.1126/science.272.5263.877. [DOI] [PubMed] [Google Scholar]
- Cobrinik D., Whyte P., Peeper D. S., Jacks T., Weinberg R. A. Cell cycle-specific association of E2F with the p130 E1A-binding protein. Genes Dev. 1993 Dec;7(12A):2392–2404. doi: 10.1101/gad.7.12a.2392. [DOI] [PubMed] [Google Scholar]
- DeGregori J., Kowalik T., Nevins J. R. Cellular targets for activation by the E2F1 transcription factor include DNA synthesis- and G1/S-regulatory genes. Mol Cell Biol. 1995 Aug;15(8):4215–4224. doi: 10.1128/mcb.15.8.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynlacht B. D., Flores O., Lees J. A., Harlow E. Differential regulation of E2F transactivation by cyclin/cdk2 complexes. Genes Dev. 1994 Aug 1;8(15):1772–1786. doi: 10.1101/gad.8.15.1772. [DOI] [PubMed] [Google Scholar]
- Fero M. L., Rivkin M., Tasch M., Porter P., Carow C. E., Firpo E., Polyak K., Tsai L. H., Broudy V., Perlmutter R. M. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell. 1996 May 31;85(5):733–744. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- Firpo E. J., Koff A., Solomon M. J., Roberts J. M. Inactivation of a Cdk2 inhibitor during interleukin 2-induced proliferation of human T lymphocytes. Mol Cell Biol. 1994 Jul;14(7):4889–4901. doi: 10.1128/mcb.14.7.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotedar R., Fitzgerald P., Rousselle T., Cannella D., Dorée M., Messier H., Fotedar A. p21 contains independent binding sites for cyclin and cdk2: both sites are required to inhibit cdk2 kinase activity. Oncogene. 1996 May 16;12(10):2155–2164. [PubMed] [Google Scholar]
- Ikeda M. A., Jakoi L., Nevins J. R. A unique role for the Rb protein in controlling E2F accumulation during cell growth and differentiation. Proc Natl Acad Sci U S A. 1996 Apr 16;93(8):3215–3220. doi: 10.1073/pnas.93.8.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. G., Schwarz J. K., Cress W. D., Nevins J. R. Expression of transcription factor E2F1 induces quiescent cells to enter S phase. Nature. 1993 Sep 23;365(6444):349–352. doi: 10.1038/365349a0. [DOI] [PubMed] [Google Scholar]
- Kato J. Y., Matsuoka M., Polyak K., Massagué J., Sherr C. J. Cyclic AMP-induced G1 phase arrest mediated by an inhibitor (p27Kip1) of cyclin-dependent kinase 4 activation. Cell. 1994 Nov 4;79(3):487–496. doi: 10.1016/0092-8674(94)90257-7. [DOI] [PubMed] [Google Scholar]
- Kiyokawa H., Kineman R. D., Manova-Todorova K. O., Soares V. C., Hoffman E. S., Ono M., Khanam D., Hayday A. C., Frohman L. A., Koff A. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1). Cell. 1996 May 31;85(5):721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- Koff A., Ohtsuki M., Polyak K., Roberts J. M., Massagué J. Negative regulation of G1 in mammalian cells: inhibition of cyclin E-dependent kinase by TGF-beta. Science. 1993 Apr 23;260(5107):536–539. doi: 10.1126/science.8475385. [DOI] [PubMed] [Google Scholar]
- Krek W., Ewen M. E., Shirodkar S., Arany Z., Kaelin W. G., Jr, Livingston D. M. Negative regulation of the growth-promoting transcription factor E2F-1 by a stably bound cyclin A-dependent protein kinase. Cell. 1994 Jul 15;78(1):161–172. doi: 10.1016/0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- LaBaer J., Garrett M. D., Stevenson L. F., Slingerland J. M., Sandhu C., Chou H. S., Fattaey A., Harlow E. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997 Apr 1;11(7):847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- Labow M. A., Baim S. B., Shenk T., Levine A. J. Conversion of the lac repressor into an allosterically regulated transcriptional activator for mammalian cells. Mol Cell Biol. 1990 Jul;10(7):3343–3356. doi: 10.1128/mcb.10.7.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. H., Mori S., Raychaudhuri P. trans-Activation by the hnRNP K protein involves an increase in RNA synthesis from the reporter genes. J Biol Chem. 1996 Feb 16;271(7):3420–3427. doi: 10.1074/jbc.271.7.3420. [DOI] [PubMed] [Google Scholar]
- Means A. L., Slansky J. E., McMahon S. L., Knuth M. W., Farnham P. J. The HIP1 binding site is required for growth regulation of the dihydrofolate reductase gene promoter. Mol Cell Biol. 1992 Mar;12(3):1054–1063. doi: 10.1128/mcb.12.3.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg K., Starz M. A., Lees J. A. E2F-4 switches from p130 to p107 and pRB in response to cell cycle reentry. Mol Cell Biol. 1996 Apr;16(4):1436–1449. doi: 10.1128/mcb.16.4.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morosov A., Phelps W. C., Raychaudhuri P. Activation of the c-fos gene by the HPV16 oncoproteins depends upon the cAMP-response element at -60. J Biol Chem. 1994 Jul 15;269(28):18434–18440. [PubMed] [Google Scholar]
- Morozov A., Shiyanov P., Barr E., Leiden J. M., Raychaudhuri P. Accumulation of human papillomavirus type 16 E7 protein bypasses G1 arrest induced by serum deprivation and by the cell cycle inhibitor p21. J Virol. 1997 May;71(5):3451–3457. doi: 10.1128/jvi.71.5.3451-3457.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozov A., Subjeck J., Raychaudhuri P. HPV16 E7 oncoprotein induces expression of a 110 kDa heat shock protein. FEBS Lett. 1995 Sep 11;371(3):214–218. doi: 10.1016/0014-5793(95)00884-c. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992 Oct 16;258(5081):424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- Nourse J., Firpo E., Flanagan W. M., Coats S., Polyak K., Lee M. H., Massague J., Crabtree G. R., Roberts J. M. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 1994 Dec 8;372(6506):570–573. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- Ohtani K., DeGregori J., Nevins J. R. Regulation of the cyclin E gene by transcription factor E2F1. Proc Natl Acad Sci U S A. 1995 Dec 19;92(26):12146–12150. doi: 10.1073/pnas.92.26.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestov D. G., Lau L. F. Genetic selection of growth-inhibitory sequences in mammalian cells. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12549–12553. doi: 10.1073/pnas.91.26.12549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K., Kato J. Y., Solomon M. J., Sherr C. J., Massague J., Roberts J. M., Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994 Jan;8(1):9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- Polyak K., Lee M. H., Erdjument-Bromage H., Koff A., Roberts J. M., Tempst P., Massagué J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994 Jul 15;78(1):59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- Poon R. Y., Toyoshima H., Hunter T. Redistribution of the CDK inhibitor p27 between different cyclin.CDK complexes in the mouse fibroblast cell cycle and in cells arrested with lovastatin or ultraviolet irradiation. Mol Biol Cell. 1995 Sep;6(9):1197–1213. doi: 10.1091/mbc.6.9.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynisdóttir I., Polyak K., Iavarone A., Massagué J. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev. 1995 Aug 1;9(15):1831–1845. doi: 10.1101/gad.9.15.1831. [DOI] [PubMed] [Google Scholar]
- Roberts J. M., Koff A., Polyak K., Firpo E., Collins S., Ohtsubo M., Massagué J. Cyclins, Cdks, and cyclin kinase inhibitors. Cold Spring Harb Symp Quant Biol. 1994;59:31–38. doi: 10.1101/sqb.1994.059.01.006. [DOI] [PubMed] [Google Scholar]
- Sardet C., Vidal M., Cobrinik D., Geng Y., Onufryk C., Chen A., Weinberg R. A. E2F-4 and E2F-5, two members of the E2F family, are expressed in the early phases of the cell cycle. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):2403–2407. doi: 10.1073/pnas.92.6.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers W. R., Rodgers J. W., Kaelin W. G., Jr A potent transrepression domain in the retinoblastoma protein induces a cell cycle arrest when bound to E2F sites. Proc Natl Acad Sci U S A. 1995 Dec 5;92(25):11544–11548. doi: 10.1073/pnas.92.25.11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Roberts J. M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995 May 15;9(10):1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- Shiyanov P., Bagchi S., Adami G., Kokontis J., Hay N., Arroyo M., Morozov A., Raychaudhuri P. p21 Disrupts the interaction between cdk2 and the E2F-p130 complex. Mol Cell Biol. 1996 Mar;16(3):737–744. doi: 10.1128/mcb.16.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slansky J. E., Li Y., Kaelin W. G., Farnham P. J. A protein synthesis-dependent increase in E2F1 mRNA correlates with growth regulation of the dihydrofolate reductase promoter. Mol Cell Biol. 1993 Mar;13(3):1610–1618. doi: 10.1128/mcb.13.3.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slingerland J. M., Hengst L., Pan C. H., Alexander D., Stampfer M. R., Reed S. I. A novel inhibitor of cyclin-Cdk activity detected in transforming growth factor beta-arrested epithelial cells. Mol Cell Biol. 1994 Jun;14(6):3683–3694. doi: 10.1128/mcb.14.6.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. J., Leone G., DeGregori J., Jakoi L., Nevins J. R. The accumulation of an E2F-p130 transcriptional repressor distinguishes a G0 cell state from a G1 cell state. Mol Cell Biol. 1996 Dec;16(12):6965–6976. doi: 10.1128/mcb.16.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starostik P., Chow K. N., Dean D. C. Transcriptional repression and growth suppression by the p107 pocket protein. Mol Cell Biol. 1996 Jul;16(7):3606–3614. doi: 10.1128/mcb.16.7.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden B., Marsh K., Yates J. A vector that replicates as a plasmid and can be efficiently selected in B-lymphoblasts transformed by Epstein-Barr virus. Mol Cell Biol. 1985 Feb;5(2):410–413. doi: 10.1128/mcb.5.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima H., Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994 Jul 15;78(1):67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- Vairo G., Livingston D. M., Ginsberg D. Functional interaction between E2F-4 and p130: evidence for distinct mechanisms underlying growth suppression by different retinoblastoma protein family members. Genes Dev. 1995 Apr 1;9(7):869–881. doi: 10.1101/gad.9.7.869. [DOI] [PubMed] [Google Scholar]
- Vindeløv L. L., Christensen I. J., Nissen N. I. A detergent-trypsin method for the preparation of nuclei for flow cytometric DNA analysis. Cytometry. 1983 Mar;3(5):323–327. doi: 10.1002/cyto.990030503. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. The retinoblastoma protein and cell cycle control. Cell. 1995 May 5;81(3):323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- Weintraub S. J., Chow K. N., Luo R. X., Zhang S. H., He S., Dean D. C. Mechanism of active transcriptional repression by the retinoblastoma protein. Nature. 1995 Jun 29;375(6534):812–815. doi: 10.1038/375812a0. [DOI] [PubMed] [Google Scholar]
- Zerfass-Thome K., Schulze A., Zwerschke W., Vogt B., Helin K., Bartek J., Henglein B., Jansen-Dürr P. p27KIP1 blocks cyclin E-dependent transactivation of cyclin A gene expression. Mol Cell Biol. 1997 Jan;17(1):407–415. doi: 10.1128/mcb.17.1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Hannon G. J., Beach D. p21-containing cyclin kinases exist in both active and inactive states. Genes Dev. 1994 Aug 1;8(15):1750–1758. doi: 10.1101/gad.8.15.1750. [DOI] [PubMed] [Google Scholar]
- Zhu L., Harlow E., Dynlacht B. D. p107 uses a p21CIP1-related domain to bind cyclin/cdk2 and regulate interactions with E2F. Genes Dev. 1995 Jul 15;9(14):1740–1752. doi: 10.1101/gad.9.14.1740. [DOI] [PubMed] [Google Scholar]
- Zhu L., van den Heuvel S., Helin K., Fattaey A., Ewen M., Livingston D., Dyson N., Harlow E. Inhibition of cell proliferation by p107, a relative of the retinoblastoma protein. Genes Dev. 1993 Jul;7(7A):1111–1125. doi: 10.1101/gad.7.7a.1111. [DOI] [PubMed] [Google Scholar]