Summary of recent advances
Trafficking and activation of the nucleic acid sensing TLRs is subject to unique regulatory requirements imposed by the risk of self-recognition. Like all TLRs these receptors traffick through the Golgi, however, access to the secretory pathway is controlled by a binding partner present in the ER. Receptor activation in the endolysosome is regulated through a proteolytic mechanism that requires activity of compartment-resident proteases, thereby preventing activation in other regions of the cell. Advances in our understanding of the cell biology of these receptors has been paralleled by efforts to understand their precise roles in autoimmunity. Mouse models have revealed that TLR7 and TLR9 make unique contributions to the types of self-molecules recognized in disease and possibly disease severity. Currently, methods of inhibiting TLR7 and TLR9 are being tested in clinical trials for systemic lupus erythamatosus.
Introduction to Nucleic acid sensing TLRs and autoimmunity
Innate immune sensors, including Toll-like receptors (TLRs), have evolved to recognize conserved microbial features as a strategy of pathogen detection. A subset of these receptors recognizes nucleic acids, which enables detection of microbial infection, especially of viruses. In recent years it has become apparent that this specificity for nucleic acids requires a specialized regulatory program designed to limit TLR activation to foreign rather than self nucleic acids. Nevertheless, when this regulation breaks down, TLR7 and TLR9, which respond to ssRNA and DNA, respectively, can contribute to the pathology of autoimmune diseases such as rheumatoid arthritis and (RA) and systemic lupus erythamatosus (SLE). A hallmark of such diseases is production of anti-nuclear antibodies (ANA) which form immune complexes (IC) containing nucleic acid-binding proteins, DNA, or RNA. In addition to causing damage and local inflammation when deposited in tissues, uptake of ICs can activate TLR7 and TLR9 in B cells and plasmacytoid dendritic cells (pDCs) perpetuating a cycle of autoantibody and cytokine production.
The contribution made by TLR7 and TLR9 to autoimmune disorders is now well established, and a recent focus has been to define the mechanisms that regulate these potentially self-reactive receptors. These efforts have been paralleled by clinical approaches seeking to inhibit TLR activation in patients with autoimmune disease. The aim of this review is to survey recent advances in our understanding of how these receptors are regulated, their contribution to autoimmune pathologies, and how these studies may inform therapeutic applications.
TLR9 and TLR7 ligand binding and activation
Current understanding of TLR9 and TLR7 ligand specificity is largely derived from the use of synthetic oligodeoxynucleotides (ODN) and oligoribonucleotides (ORN) respectively. TLR9 recognizes non-methylated cytosine-guanosine (CpG) motifs in DNA. However, activation can be influenced by the sequences flanking the CpG motif as well as the structure of the ODN backbone, which can protect the ODN from degradation when modified from the natural phosphodiester linkage to phosphothioate [1]. Two major classes of ODN have been defined based on their capacity to stimulate dendritic cell or B cell response [2]. Unlike TLR9, TLR7 has more flexibility in the optimal stimulatory sequence, recognizing a variety of AU rich ORN [3]. As will be discussed in later sections, sequences have been identified for TLR7 and TLR9 that inhibit signaling in the presence of competing stimulatory ODN or ORN. Interestingly, a single ODN can be used to target both receptors, and is currently in clinical trials to treat SLE [4,5].
TLR8 also recognizes ssRNA and appears to have a preference for AU rich sequences (Forsbach 2008). In human as well as mouse, TLR8 has not been associated with immunopathology. While it is not entirely clear why this is the case, it may be related to the lack of TLR8 expression in pDCs and B cells [6,7]. Of note, TLR3, which recognizes double stranded RNA, shares a similar expression profile and has also not been associated with immunopathology in human disease or mouse models.
Localization & processing
It has long been suspected that the intracellular localization of the nucleic acid sensing TLRs serves to prevent recognition of nucleic acids that may be more abundant at the cells surface. Host nucleic acids gain access to the extracellular milieu through passive release from necrotic cells, and several homeostatic mechanisms exist to clear such debris. Conditions that shift this balance may lead to TLR recognition, for example, mice defective in DNAseI, the major DNAse secreted into serum, succumb to an SLE-like disease characterized by anti-nuclear antibodies and glomerulonephritis [8]. Mice lacking a functional copy of the complement protein C1q or the phagocytic receptor MER, cannot effectively clear apoptotic bodies, and an SLE-like disease develops characterized by ANA production [9,10]. Similarly, patients with a history of SLE often relapse after incidence of cellular trauma, like acute viral infection or radiotherapy [11,12].
Early evidence implicating endosomal localization as a regulatory mechanism of TLR activation came from studies with B cells expressing rheumatoid factor, a B cell receptor (BCR) specific for self-immunoglobulin. DNA-containing ICs internalized via this BCR led to TLR9 activation, in effect lowering the threshold for activation by self nucleic acids [13]. The importance of intracellular localization of TLR9 was later shown more directly through the generation of a chimeric receptor with the DNA specificity of TLR9 but the cell surface localization of TLR4 which exhibited enhanced signaling to PD DNA than TLR9 [14]. These studies indicated that subcellular localization may establish a high threshold for self-recognition, however, until recently the mechanisms controlling their expression were largely unknown.
Differential regulation of the nucleic acid sensing TLRs from those at the cell surface appears to be established in the ER. Nucleic acid sensing TLR exit from the ER is under the control of Unc93b1, a twelve pass integral membrane protein which appears to directly interact with these TLRs and escort them to the endosomal compartments for signaling [15-17]. Analysis of the glycosylation pattern of the TLR7 and TLR9 ectodomain indicates that these proteins traffic through the Golgi to access the endosome [18,19]. Whether they are routed directly to the endosomal compartments or through the cell surface is not entirely clear: studies from the Leifer group have shown that deleting a tyrosine-based motif in the cytosolic domain of TLR9 stabilizes TLR9 on the cell surface [20]. However, several groups have failed to see expression of TLR9 or other nucleic acid sensing TLRs at the cell surface [14,16].
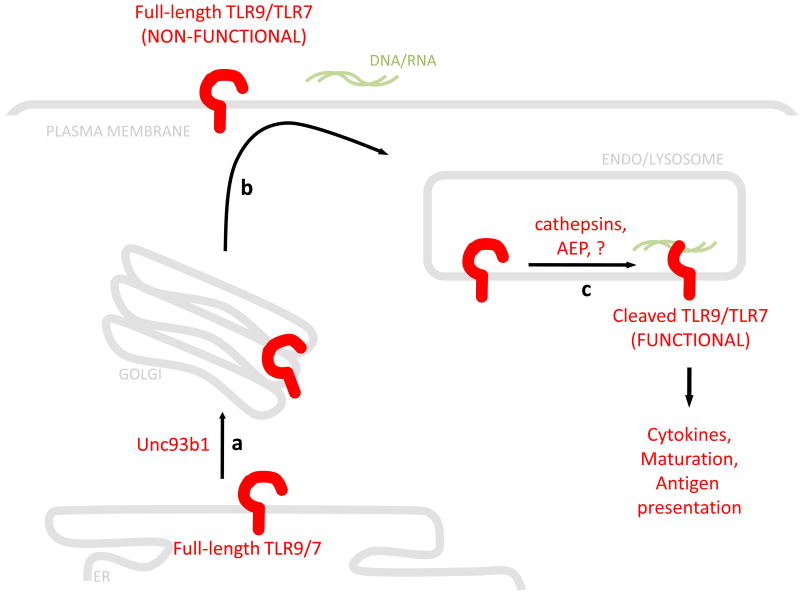
Recently, three groups have independently shown that TLR9 is proteolytically cleaved in the endolysosomal compartment by acid dependent proteases. This proteolysis is required for function, as the cleaved form alone has been shown to be sufficient to mediate signaling, can be eluted in MyD88-containing cell fractions, and has been shown to bind the signaling adaptor directly while the full length form does not [19,21,22]. In this manner, proteolysis serves to physically restricted activation of TLR9 to endosomal compartments. As suggested above, this would prevent receptors that may have accessed the cell surface, either by default or by leaking through the secretory pathway, from aberrantly recognizing self nucleic acids (Figure 1a-b). While it is not entirely clear how proteolysis mediates TLR9 activation, studies from the Golenbock group have shown that the TLR9 dimer undergoes a conformational shift in the endosomal compartments that appears that enable signaling [23]. It may be that cleavage facilitates this conformational shift, however, future studies examining this possibility directly will be required.
Figure 1.
Schematic of TLR localization and activation: a. TLR9 and TLR7 entry into the secretory pathway is regulated by Unc93b. b. TLRs traffic though the Golgi en route to the endolysosome, which may occur through the cell surface. c. Cleavage occurs in the endolysosome through a mechanism requiring cathepsins and/or AEP. Cleavage is required for activation of the receptor leading to cytokine induction and maturation programs. By contrast, receptors that access the cell surface are not cleaved and cannot respond to nucleic acids that are encountered.
To date cathepsins and asparagine endopeptidase have been implicated in the processing and function of TLR9 and possibly the other nucleic acid sensing TLRs [21,22,24,25]. However, it is not entirely clear if the relative contribution of these proteases is cell type specific. Additionally, the contribution of individual cathepsins to TLR9 processing and function is somewhat controversial. Cathepsin K has been identified as a target for autoimmune arthritis, due to its role in bone resorption, however, a recent report showed that cathepsin K was also required for TLR7 and TLR9 signaling [24]. Two subsequent reports could not define a TLR phenotype using cathepsin K knockouts or inhibitors, finding that multiple cathepsins or cathepsins and other acid dependent proteases may play overlapping roles in mediating nucleic acid sensing TLR function (Figure 1c) [19,21,22]. Clearly, further studies are required to define the specific proteases involved in TLR9 processing and the mechanistic details through which they operate. These data are of additional clinical importance as cathepsin inhibitors are currently in clinical trials for treatment of osteoporosis and bone metastases [26].
TLR-mediated activation of pDCs and B cells in autoimmunity
The contribution of TLR7 and TLR9 to autoimmunity is closely linked to their expression in plasmacytoid DCs (pDCs) and B cells. PDCs express a restricted repertoire of TLRs and have linked signaling through MyD88 to the potent production of Type I interferon (IFN). Activation of this pathway induces a positive feedback loop through the type I IFN receptor (IFNAR) that perpetuates these responses [27]. Over half of SLE patients have elevated levels of serum IFNα or an elevated signature of 12 Type I IFN inducible genes, the level of which correlates with disease severity [28,29].
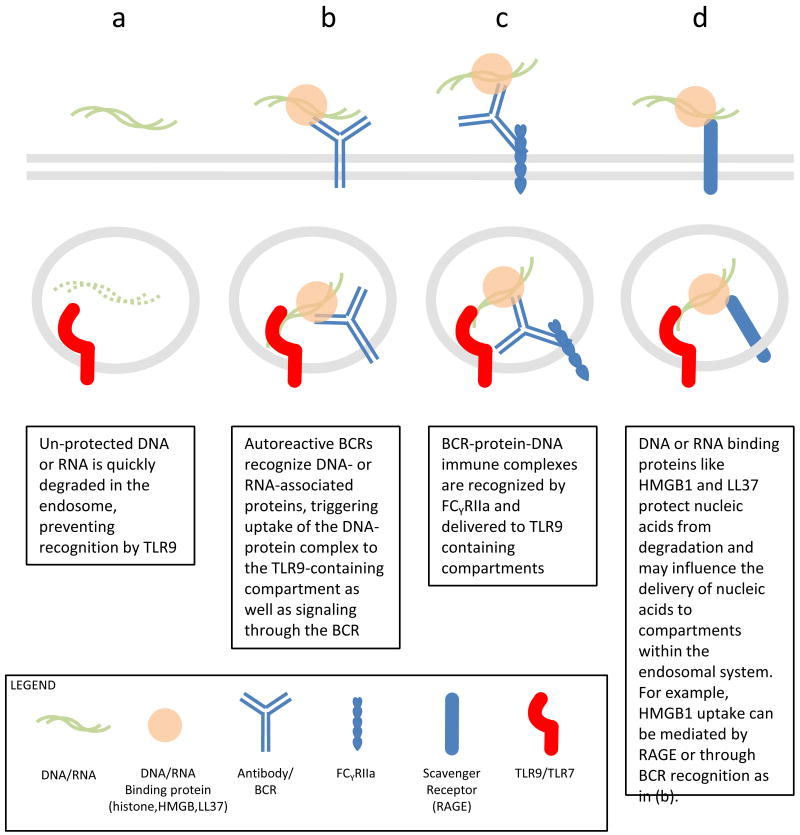
PDCs and B cells appear to have specialized cell biology to enhance delivery of potential ligands to TLR7 and TLR9 containing compartments. For example, studies using AM14 B cells, that express IgG2a BCR specific for chromatin immune complexes, have shown that BCR can deliver DNA directly to TLR9 containing compartments leading to potent B cell activation (Figure 2a-b) [13,30]. In a more recent study, internalized BCRs in complex with antigen co-localized to endosomal compartments containing TLR9 and autophagy marker phosphorylated-p38, suggesting that BCR signaling can orchestrate cellular remodeling to enhance TLR recognition of substrates [31]. An analogous system for substrate delivery appears to exist in pDCs, mediated by FcγRIIa (CD32), a low affinity receptor for IgG2a (Figure 2c). Expression of FCγRIIa enables pDCs to internalize and respond to ICs derived from SLE patients, delivering them to a TLR9 containing compartment [32].
Figure 2.
Mechanisms of nucleic acid uptake and delivery to TLR containing compartments.
Accessory proteins involved in self nucleic acid recognition
Increasingly, it appears that accessory proteins may play an important role in converting mammalian nucleic acids, which have a low potential for eliciting TLR7/9 activation, into potent ligands during autoimmune inflammation [33,34]. Passive release of the DNA binding protein HMGB1 from necrotic cells has long been associated with inflammatory responses in autoimmunity and sepsis [35,36]. More recently, it has been shown that extracellular HMGB1, which can also be actively secreted from monocytes and DCs, associates with DNA released from necrotic cells or DNA-containing immune complexes to elicit TLR9 dependent responses [37,38]. The role that this DNA-binding protein plays is in part due to protection of DNA from degradation; however, HMGB1 recognition by receptor for advanced glycosylation end products (RAGE) or IgG2a may facilitate uptake and delivery of nucleic acids to intracellular compartments (Figure 2b,d)[38,39]. A recent report demonstrates that HMGB2 and HMGB3 may also contribute in innate immune sensing of nucleic acids in the cytosol, suggesting that this family of proteins may play a very general role in viral detection by both TLRs and cytosolic sensors [40].
Psoriasis, a Type I IFN driven auto-inflammatory response in the skin has recently been shown to have a TLR7 and TLR9 self-recognition component. When extracts derived from psoriatic lesions were compared with healthy skin in their ability to elicit Type I IFN responses from pDCs, LL37 was identified as the immunologically active component in psoriatic samples [41]. Previously defined as an anti-microbial peptide, LL37 was shown to bind and protect DNA and RNA from nucelases [41,42]. Unlike, HMGB1 or IC, it is not known how LL37-nucleic acid conjugates are taken up, however, LL37 enhanced DNA uptake to early endosomes. Interestingly, LL37 conjugation could induce a Type I IFN signature from ODN that do not usually favor such a responses [41]. These examples are consistent with the possibility that nucleic acids alone are not sufficient to break TLR tolerance to self and require association with other, accessory molecules to be converted to ligands for TLR recognition (Figure 1a-c). It is not unlikely that other nucleic acid binding partners will be discovered, and it is interesting to speculate that these binding partners, like LL37, play a dominant role in determining the tissue specificity of inflammatory responses that otherwise have very similar etiologies.
TLR7 and TLR9 in autoimmune models and therapeutics
In the last decade, several mouse models have been developed to examine the contribution of TLR7 and TLR9 to autoimmune diseases. There has been some suggestion that TLR7 may play a more prevalent role in human disease, while the underlying reasons for this are unclear, it is known that over expression of TLR7 but not TLR9 can accelerate disease onset in mice. Autoimmunity in the BXSB strain is linked to the Yaa locus (Y-linked autoimmune accelerator), a translocation of the telomeric end of the X chromosome, containing TLR7, to the Y chromosome [43]. The resulting duplication of TLR7 appears to be responsible for production of anti-RNP and induction of lupus nephritis, as crossing TLR7 knockouts to a BXSB background ameliorates these elements of disease [44]
Recent work from the Schlomchik group suggests that TLR7 and TLR9 may have opposing contributions to autoimmune pathology. MRL/lpr mice have a recessive mutation in the FAS gene lpr (lymphoaddenopathy), leading to a general defect in FAS mediated apoptosis, and lymphocyte expansion as well as production of ANA, elevated serum IgG and glomerulonephrtis. Crossing TLR9 or TLR7 knockouts onto the MLR/lpr background revealed these TLRs are required for production of anti-RNP antibodies and DNA associated ANA, respectively [45,46]. Interestingly, TLR7 knockouts were protected from diseases, displaying reduced lymphocyte activation and serum IgG, whereas TLR9 knockouts exhibited accelerated disease progression including pDC and lymphocyte expansion, production of anti-RNP and serum titers of IFNα [46,47]. Whether this reciprocal relationship holds true in other SLE models and human disease remains to be tested, as discussed below, inhibitory ODN currently in clinical trials are designed to target both TLR7 and TLR9.
Until recently, SLE and RA have predominantly been treated with chloroquine, anti-TNF therapy and administration of glucocorticoids. These treatments are largely non-specific and carry the burden of severe side affects, particularly in the case of sustained steroid treatment. The use of inhibitory oligos have been shown to block disease progression in terms of ANA production, glomerulonephritis and delay mortality in (NZBxNZW)F1 mice, which develop a lupus-like disease very similar to human progression [5]. Glucocorticoids are often used in patients, based on their potent anti-inflammatory effects which stem, at least in part, from inhibition of the transcription factor NFκB. However a recent study suggests that chronic signaling through TLRs during SLE renders pDCs resistant to glucocorticoid-mediated NFκB inhibition [48]. These data suggest that inhibitory oligos may have a dual effect of blocking TLR-mediated inflammation directly as well as removing a barrier to glucocorticoid induced NFκB inhibition, effectively reducing the threshold of glucocorticoid treatment required to achieve the same effect in the context of intact TLR signaling.
As of yet, it is unclear how inhibitory ODN exert their effect on TLR signaling. Binding assays reveal that stimulatory and inhibitory ODN bind the receptor with the similar affinity, and it is likely that inhibitory ODN antagonize TLR7 and TLR9 directly [49]. Interestingly, when compared to treatment with stimulatory ODN, inhibitory ODN treatment does not result in a conformational shift of the TLR dimer. Whether receptor processing is affected by treatment with inhibitory ODN is not known [4]. Clearly, future studies will be informative towards understand how TLR9 processing, dimer formation and ligand binding control the activation or inhibition of these receptors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 2.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annual review of immunology. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 3.Forsbach A, Nemorin JG, Montino C, Müller C, Samulowitz U, Vicari AP, Jurk M, Mutwiri GK, Krieg AM, Lipford GB, et al. Identification of RNA sequence motifs stimulating sequence-specific TLR8-dependent immune responses. Journal of immunology (Baltimore, Md : 1950) 2008;180:3729–3738. doi: 10.4049/jimmunol.180.6.3729. [DOI] [PubMed] [Google Scholar]
- 4.Barrat FJ, Coffman RL. Development of TLR inhibitors for the treatment of autoimmune diseases. Immunological reviews. 2008;223:271–283. doi: 10.1111/j.1600-065X.2008.00630.x. [DOI] [PubMed] [Google Scholar]
- *5.Barrat FJ, Meeker T, Chan JH, Guiducci C, Coffman RL. Treatment of lupus-prone mice with a dual inhibitor of TLR7 and TLR9 leads to reduction of autoantibody production and amelioration of disease symptoms. European journal of immunology. 2007;37:3582–3586. doi: 10.1002/eji.200737815. [DOI] [PubMed] [Google Scholar]; This work describes a single ODN that blocks TLR7 and TLR9 activation and disease severity in autoimmune prone mice. This immuno-regulatory ODN is currently under clinical trials for the treatment of SLE.
- 6.Rothenfusser S, Hornung V, Krug A, Towarowski A, Krieg AM, Endres S, Hartmann G. Distinct CpG oligonucleotide sequences activate human gamma delta T cells via interferon-alpha/-beta. European journal of immunology. 2001;31:3525–3534. doi: 10.1002/1521-4141(200112)31:12<3525::aid-immu3525>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, Liu YJ. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. The Journal of experimental medicine. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Napirei M, Karsunky H, Zevnik B, Stephan H, Mannherz HG, Möröy T. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nature genetics. 2000;25:177–181. doi: 10.1038/76032. [DOI] [PubMed] [Google Scholar]
- 9.Cohen PL, Caricchio R, Abraham V, Camenisch TD, Jennette JC, Roubey RA, Earp HS, Matsushima G, Reap EA. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J Exp Med. 2002;196:135–140. doi: 10.1084/jem.20012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Botto M, Dell'Agnola C, Bygrave AE, Thompson EM, Cook HT, Petry F, Loos M, Pandolfi PP, Walport MJ. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 11.Pinn ME, Gold DG, Petersen IA, Osborn TG, Brown PD, Miller RC. Systemic lupus erythematosus, radiotherapy, and the risk of acute and chronic toxicity: the Mayo Clinic Experience. Int J Radiat Oncol Biol Phys. 2008;71:498–506. doi: 10.1016/j.ijrobp.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Ramos-Casals M. Viruses and lupus: the viral hypothesis. Lupus. 2008;17:163–165. doi: 10.1177/0961203307086268. [DOI] [PubMed] [Google Scholar]
- 13.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 14.Barton GM, Kagan JC, Medzhitov R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nature immunology. 2006;7:49–56. doi: 10.1038/ni1280. [DOI] [PubMed] [Google Scholar]
- 15.Brinkmann MM, Spooner E, Hoebe K, Beutler B, Ploegh HL, Kim YM. The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. The Journal of cell biology. 2007;177:265–275. doi: 10.1083/jcb.200612056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature. 2008;452:234–238. doi: 10.1038/nature06726. [DOI] [PubMed] [Google Scholar]
- 17.Tabeta K, Hoebe K, Janssen EM, Du X, Georgel P, Crozat K, Mudd S, Mann N, Sovath S, Goode J, et al. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nature immunology. 2006;7:156–164. doi: 10.1038/ni1297. [DOI] [PubMed] [Google Scholar]
- *18.Chockalingam A, Brooks JC, Cameron JL, Blum LK, Leifer CA. TLR9 traffics through the Golgi complex to localize to endolysosomes and respond to CpG DNA. Immunology and cell biology. 2009;87:209–217. doi: 10.1038/icb.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]; Through analysis of glycosylation, this study showed that a fraction of full length TLR9 trafficks through the Golgi en route to the endosome.
- **19.Ewald SE, Lee BL, Lau L, Wickliffe KE, Shi GP, Chapman HA, Barton GM. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature. 2008;456:658–662. doi: 10.1038/nature07405. [DOI] [PMC free article] [PubMed] [Google Scholar]; Along with [**21] these studies were the first to identify proteolysis of TLR9 in the endolysosome and that processing required for TLR9 activation. This work showed that TLR7 is processed similarly and processing prevents TLR7/9 activation at the cell surface.
- 20.Leifer CA, Brooks JC, Hoelzer K, Lopez J, Kennedy MN, Mazzoni A, Segal DM. Cytoplasmic targeting motifs control localization of toll-like receptor 9. The Journal of biological chemistry. 2006;281:35585–35592. doi: 10.1074/jbc.M607511200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **21.Park B, Brinkmann MM, Spooner E, Lee CC, Kim YM, Ploegh HL. Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll-like receptor 9. Nature immunology. 2008;9:1407–1414. doi: 10.1038/ni.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]; Along with (**19) these were the first reports of TLR9 proteolysis. This paper also attributed receptor processing in macrophage to cathepsin activity.
- *22.Sepulveda F, Maschalidi S, Colisson R, Heslop L, Ghirelli C, Sakka E, Lennon-Duménil A, Amigorena S, Cabanie L, Manoury B. Critical Role for Asparagine Endopeptidase in Endocytic Toll-like Receptor Signaling in Dendritic Cells. Immunity. 2009;31:737–748. doi: 10.1016/j.immuni.2009.09.013. [DOI] [PubMed] [Google Scholar]; This work confirmed TLR9 processing and described a unique requirement for asparagine endopeptidase in TLR9 processing in dendritic cells.
- 23.Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, Golenbock DT. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nature immunology. 2004;5:190–198. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 24.Asagiri M, Hirai T, Kunigami T, Kamano S, Gober HJ, Okamoto K, Nishikawa K, Latz E, Golenbock DT, Aoki K, et al. Cathepsin K-dependent toll-like receptor 9 signaling revealed in experimental arthritis. Science (New York, NY) 2008;319:624–627. doi: 10.1126/science.1150110. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto F, Saitoh S, Fukui R, Kobayashi T, Tanimura N, Konno K, Kusumoto Y, Akashi-Takamura S, Miyake K. Cathepsins are required for Toll-like receptor 9 responses. Biochemical and biophysical research communications. 2008;367:693–699. doi: 10.1016/j.bbrc.2007.12.130. [DOI] [PubMed] [Google Scholar]
- 26.Lewiecki EM. Odanacatib, a cathepsin K inhibitor for the treatment of osteoporosis and other skeletal disorders associated with excessive bone remodeling. IDrugs. 2009;12:799–809. [PubMed] [Google Scholar]
- 27.Hall JC, Rosen A. Type I interferons: crucial participants in disease amplification in autoimmunity. Nat Rev Rheumatol. 2009;6:40–49. doi: 10.1038/nrrheum.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 29.Hooks JJ, Moutsopoulos HM, Geis SA, Stahl NI, Decker JL, Notkins AL. Immune interferon in the circulation of patients with autoimmune disease. N Engl J Med. 1979;301:5–8. doi: 10.1056/NEJM197907053010102. [DOI] [PubMed] [Google Scholar]
- 30.Viglianti GA, Lau CM, Hanley TM, Miko BA, Shlomchik MJ, Marshak-Rothstein A. Activation of autoreactive B cells by CpG dsDNA. Immunity. 2003;19:837–847. doi: 10.1016/s1074-7613(03)00323-6. [DOI] [PubMed] [Google Scholar]
- 31.Chaturvedi A, Dorward D, Pierce SK. The B cell receptor governs the subcellular location of Toll-like receptor 9 leading to hyperresponses to DNA-containing antigens. Immunity. 2008;28:799–809. doi: 10.1016/j.immuni.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Means TK, Latz E, Hayashi F, Murali MR, Golenbock DT, Luster AD. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. The Journal of clinical investigation. 2005;115:407–417. doi: 10.1172/JCI23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krieg AM. An innate immune defense mechanism based on the recognition of CpG motifs in microbial DNA. J Lab Clin Med. 1996;128:128–133. doi: 10.1016/s0022-2143(96)90004-9. [DOI] [PubMed] [Google Scholar]
- 34.Messina JP, Gilkeson GS, Pisetsky DS. Stimulation of in vitro murine lymphocyte proliferation by bacterial DNA. J Immunol. 1991;147:1759–1764. [PubMed] [Google Scholar]
- 35.Scaffidi P, Misteli T, Bianchi M. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 36.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 37.Ivanov S, Dragoi AM, Wang X, Dallacosta C, Louten J, Musco G, Sitia G, Yap GS, Wan Y, Biron CA, et al. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood. 2007;110:1970–1981. doi: 10.1182/blood-2006-09-044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian J, Avalos A, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nature immunology. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 39.Avalos AM, Kiefer K, Tian J, Christensen S, Shlomchik M, Coyle AJ, Marshak-Rothstein A. RAGE-independent autoreactive B cell activation in response to chromatin and HMGB1/DNA immune complexes. Autoimmunity. 43:103–110. doi: 10.3109/08916930903384591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yanai H, Ban T, Wang Z, Choi M, Kawamura T, Negishi H, Nakasato M, Lu Y, Hangai S, Koshiba R, et al. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature. 2009;462:99–103. doi: 10.1038/nature08512. [DOI] [PubMed] [Google Scholar]
- **41.Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, Cao W, Wang YH, Su B, Nestle FO, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]; This report identified LL37 as a host protein, upregulated in psoriatic lesions, that is required to elicit autoimmune inflamation in skin. LL37 was shown to bind DNA, retain ligand in early endosomes, and elicit TLR9 dependent type I interferon from dendritic cells.
- 42.Ganguly D, Chamilos G, Lande R, Gregorio J, Meller S, Facchinetti V, Homey B, Barrat FJ, Zal T, Gilliet M. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. The Journal of experimental medicine. 2009;206:1983–1994. doi: 10.1084/jem.20090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J, Liang C, Bartov G, McDaniel LD, Zhou XJ, et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9970–9975. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santiago-Raber ML, Baudino L, Izui S. Emerging roles of TLR7 and TLR9 in murine SLE. Journal of autoimmunity. 2008;33:231–238. doi: 10.1016/j.jaut.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Christensen SR, Kashgarian M, Alexopoulou L, Flavell RA, Akira S, Shlomchik MJ. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. The Journal of experimental medicine. 2005;202:321–331. doi: 10.1084/jem.20050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *46.Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]; This work describes the contribution of TLR7 and TLR9 to the generation of antibodies directed towards RNA binding proteins and DNA binding proteins, respectively. Further, it demonstrated that TLR9 may regulate TLR7 induced pathologies in the MLR/lpr model of autoimmunity.
- 47.Nickerson KM, Christensen SR, Shupe J, Kashgarian M, Kim D, Elkon K, Shlomchik MJ. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. Journal of immunology (Baltimore, Md : 1950) 2010;184:1840–1848. doi: 10.4049/jimmunol.0902592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **48.Guiducci C, Gong M, Xu Z, Gill M, Chaussabel D, Meeker T, Chan JH, Wright T, Punaro M, Bolland S, et al. TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature. 2010;465:937–941. doi: 10.1038/nature09102. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work shows that TLR7 and TLR9 signaling in pDCs renders them resisitant to glucocorticoid induced apoptosis. Treatment with immuno-regulatory ODN reversed this resistance, a process that likely hinges on NFκB induction.
- 49.Rutz M, Metzger J, Gellert T, Luppa P, Lipford GB, Wagner H, Bauer S. Toll-like receptor 9 binds single-stranded CpG-DNA in a sequence- and pH-dependent manner. European journal of immunology. 2004;34:2541–2550. doi: 10.1002/eji.200425218. [DOI] [PubMed] [Google Scholar]