Abstract
Background
Active compression decompression cardiopulmonary resuscitation (ACD-CPR) plus a decrease in intrathoracic pressure during the decompression phase of CPR have been shown previously to result in improved hemodynamics when compared with standard CPR. We hypothesized that these interventions would increase survival rates with favorable neurologic function after out-of-hospital cardiac arrest (OOHCA) when compared with standard CPR.
Methods
This prospective, randomized, open, blinded, multicenter trial evaluated the safety and effectiveness of ACD-CPR plus augmentation of negative intrathoracic pressure, achieved with an impedance threshold device (intervention), compared with standard CPR (control) in patients with non-traumatic OOHCA. The primary endpoint was survival to hospital discharge with favorable neurologic function, defined as a modified Rankin Scale (mRS) ≤3. Patients meeting final selection criteria (non-traumatic arrest, presumed cardiac etiology) were included in the primary intention-to-treat analysis.
Findings
Of the 2470 provisionally enrolled patients, 817/2470 (33%) did not meet and 1653/2470 (67%) met the pre-specified final selection criteria. There were no significant differences in patient clinical profiles between the standard CPR (n=813) and the intervention (n=840) groups. Survival to hospital discharge with a mRS ≤3 was 5.8% (47/813) in the control group versus 8.9% (75/840) in the intervention group [p=0.019, OR 1.58 (CI= 1.07, 2.36)]. Overall, more patients survived to one year with intervention: 74/840 (8.8%) versus 48/813 (5.9%) (p=0.03), with similar cognitive skills, disability ratings, and quality of life indices in both groups. The overall major adverse event rate (secondary safety endpoint) was not significantly different between groups, but one category, pulmonary edema, was higher in the intervention group: 11.2% (94/840) versus 6.7% (62/813), p=0.015.
Interpretation
Compared with standard CPR, treatment with ACD-CPR with augmentation of negative intrathoracic pressure resulted in significantly increased survival to hospital discharge with favorable neurological function. One year after OOHCA, survival was significantly higher in the intervention group and there was a similar restoration of neurologic function in survivors in both groups.
Keywords: cardiopulmonary resuscitation, CPR, active compression decompression CPR, impedance threshold device, sudden death, cardiac arrest
INTRODUCTION
Greater than 800,000 Europeans and North Americans experience out-of-hospital cardiac arrest (OOHCA) annually, with overall survival averaging 5%.1,2 Poor survival rates persist, in part, because manual chest compressions and ventilation, herein termed standard cardiopulmonary resuscitation (S-CPR), is inherently inefficient, providing less than 25% of normal blood flow to the heart and brain.3 Hemodynamics are commonly compromised further by poor S-CPR technique, especially inadequate chest compressions and incomplete chest recoil.4–6
Cardiac and cerebral perfusion has been shown to increase in animals and humans during CPR by augmenting negative intrathoracic pressure during the decompression phase.7–12 Studies have demonstrated a decrease in intrathoracic pressure is linked to a simultaneous decrease in intracranial pressure: these mechanisms are thought to underlie the increase in blood flow to the heart and brain.7–14 Clinical studies have also demonstrated significant improvement in 24-hour survival rates with this approach.15,16
The potential impact of augmenting negative intrathoracic pressure during CPR on longer term survival with good neurologic function has not been previously evaluated in a clinical trial. We tested the hypothesis that when compared with S-CPR, active compression decompression CPR (ACD-CPR) plus a decrease in intrathoracic pressure during the chest recoil phase achieved with an impedance threshold device (ITD), (study intervention) would result in significantly improved survival to hospital discharge with favorable neurologic function, defined as a modified Rankin Scale (mRS) ≤ 3.17
METHODS
Study Design and Setting
This randomized, prospective, multicenter trial was performed in seven distinct geographic locations in the United States: Minneapolis, Minnesota; St. Paul, Minnesota; Whatcom County, Washington; Oshkosh, Wisconsin; Oakland and Macomb Counties, Michigan; Washtenaw and Livingston Counties, Michigan; and Indianapolis, Indiana. These sites included 46 emergency medical services (EMS) agencies in urban, suburban, and rural areas, encompassing a total population of 2.3 million.
The study was conducted after approval by the US Food and Drug Administration (FDA) under the US Code of Federal Regulations (21 CFR 50.24), Exception from Informed Consent under Emergency Circumstances, which included community consultation and public notification. Informed consent for continued participation in the trial was required from the patient or family member for neurologic assessment. The protocol was performed under an Investigational Device Exception (IDE # G050062) and approved by the FDA and 25 institutional review boards (IRBs).
Study Devices
ACD-CPR was performed with a hand-held device consisting of a suction cup attached to the chest, a handle, audible metronome set to 80 times per minute, and force gauge to guide compression depth, and chest wall recoil.15,16 The ACD-CPR requires the operator to compress to the same depth as S-CPR and then lift upward to fully decompress the chest.15,16 An ITD, with an inspiratory resistance of 16 cmH2O and < 5 cmH2O expiratory impedance, was connected to a facemask and/or advanced airway. The ITD lowered intrathoracic pressure during the decompression phase of ACD-CPR by allowing periodic positive pressure ventilation while impeding passive inspiratory gas exchange during chest recoil phase.10, 17 Both the ACD-CPR device (ResQPump,® also called CardioPump®) and the ITD (ResQPOD®) were manufactured by Advanced Circulatory Systems, Inc. (Roseville, Minnesota, USA).
Patient Population and Intention to Treat Criteria
Adults (presumed or known to be ≥18 years of age) with OOHCA were eligible for the study, based on local IRB requirements. Patients were not initially randomized if they had confirmed pre-existing Do Not Resuscitate (DNR) orders, signs of obvious clinical death, recent sternotomy, or trauma. Patients met pre-specified final enrollment criteria if the OOHCA was of presumed cardiac origin, the patient received at least one minute of CPR by EMS (to increase likelihood of receipt and potential benefit of the study intervention) and had a non-obstructed airway (managed by intubation or bag-valve-mask airway). Exclusion of non-cardiac causes for arrest, including pulmonary embolism, hemorrhage, stroke, drug overdose, and electrocution, assured meeting Utstein18 cardiac arrest criteria for the pre-specified primary study population analyses.
Randomization and Intervention
Patients were assigned, using a prospective computer-generated block randomization weekly schedule (four week blocks) prepared by an independent biostatisician, to S-CPR or the study intervention protocol on a 1:1 proportional basis. Chest compressions were initiated by the first arriving basic life support (BLS) or advanced life support (ALS) EMS providers as soon as possible in both study groups. S-CPR, defibrillation, and ALS treatment were performed consistent with local policy and per the American Heart Association (AHA) guidelines.20 The compression:ventilation ratio was 30:2 during BLS for both CPR techniques. For the intervention protocol, ACD-CPR was performed at 80/minute as soon as possible. The ACD-CPR force gauge was used to help achieve the recommended compression depth and complete chest recoil. Rescuers initially attached the ITD between the ventilation bag and facemask (King Systems; Indianapolis, IN) if patients were assigned to the intervention group. It was subsequently relocated to the advanced airway. The ITD was removed if the patient had return of spontaneous circulation (ROSC) and reapplied if re-arrest occurred. The devices and facemask for the study intervention group, or a facemask alone for the S-CPR group were packaged together in a study bag and carried by rescue personnel per the randomization calendar. CPR efforts in both arms were encouraged for at least 30 minutes on scene, before terminating the resuscitation attempt. The study intervention, if ongoing, was stopped upon arrival to the hospital. In-hospital therapeutic hypothermia and cardiac revascularization for all patients was encouraged by all site investigators. 20
Study Endpoints
The pre-specified primary study endpoint was survival to hospital discharge with favorable neurologic function, defined as a modified Rankin Scale of 3 or less (mRS ≤3).18 This neurologic assessment took into account prior neurologic deficits in the evaluation and was administered by a research nurse, blinded to the study intervention, at the time of hospital discharge. A secondary safety endpoint assessed the rate of major adverse events through hospital discharge. Major adverse event categories included death, cerebral bleeding/stroke, re-arrest, pulmonary edema, chest fractures (rib/sternal), excessive bleeding, and internal thoracic and abdominal injuries.
To discern if the study intervention would result in more neurologically impaired patients, additional secondary effectiveness endpoints were assessed 90 and 365 days after OOHCA. The Cognitive Abilities Screening Instrument (CASI) assessed attention, short- and long-term memory, judgment, and spatial ability.21 The Disability Rating Index22 determined the level of functional disability, and the Beck Depression Index23, determined the level of depression and emotional stability.
Data Collection and Monitoring
Out-of-hospital data were collected according to Utstein Guidelines, from the EMS field medical record.19 In-hospital treatment, outcome, and follow-up data were collected from hospital records and neurologic assessment surveys for all consented patients or until such time as the patient/family refused consent for continued participation in the trial. All neurologic assessments were administered by trained and certified nurses who were members of the research team at each study site.
Clinical monitoring was performed throughout the study to maximize protocol adherence and quality of rescuer performance of S-CPR and the study intervention. Study sites were required to complete a run-in phase and certification process before beginning enrollment in the study.
An independent Data and Safety Monitoring Board (DSMB) monitored safety, ethical, and scientific aspects of the study. An independent Clinical Events Committee was responsible for adjudication of adverse events and for exclusion of all screened patients not meeting criteria for enrollment.
Training
A total of 4940 EMS personnel underwent didactic and hands-on training prior to starting the study and every six months thereafter. Comprehensive training in S-CPR and the study intervention emphasized: starting compressions immediately, adequate compression depth and rate, full chest recoil, maximizing “hands-on” time, appropriate ventilation rate and duration, how to ensure appropriate facemask seal for ITD use with a facemask (two-handed technique),20 how to perform ACD-CPR, 24,25 and the need to rotate personnel performing CPR every two minutes to avoid fatigue.20,24,25
Blinding
With the exception of rescuer CPR performance, all aspects of the study, including patient consent, medical record review, and neurologic evaluations, were accomplished by research staff blinded to the randomized CPR method used in each patient. This included systematic review of hospital charts for adverse events. Study coordinators provided patient information and follow-up evaluation documents that did not reveal the treatment assignment to study nurses at each site who obtained patient consent and performed the neurologic assessments. With the unavoidable exception of device failures, patient information provided in summary form to the Clinical Events Committee did not reveal the randomized treatment assignment. Hospital personnel, responsible for post-resuscitation care, were blinded to the method of CPR to the extent possible. The study sponsor remained blinded to all aggregate patient outcomes throughout the entire study, with the exception of device failures.
Sample Size and Statistical Analysis
The original sample size of the two group comparison was 1400 patients (700 per group). A single interim analysis at the 50% information point of the original study sample was prospectively planned using the Lan-DeMets alpha spending method with O’Brien-Fleming boundaries. Following the planned midpoint analysis in September 2008, the DSMB, blinded to study group assignment, recommended an upward sample size adjustment to 2696 evaluable patients (1348 per group). This was done to maintain the original design objective of 80% power to detect a group difference, without knowledge of the direction of the observed difference. In July 2009, the study was terminated early due to lack of funding, at which time 1653 patients who met final criteria had been enrolled. At the completion of the study, the original pre-specified criteria for assessing the statistical significance of study results were applied, identical to requirements that would have been applicable to full enrollment.
The study design initially included a third arm: patients randomized to S-CPR + ITD. The trial was originally designed to primarily compare S-CPR versus ACD-CPR+ ITD. The third arm was added, with one half of the proportional enrollment of the other two arms assigned initially to the third arm, to assess the relative contribution of the ITD alone to any overall treatment effect observed for the combination of devices. While blinded to outcome by study group, this arm was discontinued in November 2007 after enrolling 150 patients, due to slower than anticipated overall study enrollment and intent to focus remaining funding resources on evaluation of the primary study objective. Patients from the third arm are not included in analyses presented here.
Fisher’s Exact Test was used for analysis of the primary endpoint, with a final p-value of 0.049 required for statistical significance. It was determined a priori that all analyses would be performed on an intention-to-treat basis for all patients meeting enrollment criteria. The secondary safety endpoint was evaluated using an exact binomial test for non-inferiority with the same p-value requirement. Proportions of patients that experienced one or more major adverse events (of any kind) were compared between study groups. This comparison tested whether the patient-level major adverse event rate in the intervention group was inferior or non-inferior to that of the control arm, with a non-inferiority margin of 5%. Additional pre-specified secondary endpoints including subgroup analyses based upon age, gender, initial rhythm, time to CPR, and whether the arrests were witnessed, were evaluated using Fisher’s Exact tests and Student’s t-tests, but associated p-values are considered nominal and unadjusted without associated statistical significance levels. All tests were made using StatXact (Cytel Software, Cambridge, MA) and SPSS (SPSS, Inc., Chicago, IL) software. Continuous data are expressed as mean ± standard deviation.
Role of the funding source
Funding support was provided by a grant from the National Institutes of Health (NIH) to the sponsor (Advanced Circulatory Systems, Inc.). The protocol was approved by the NIH and a NIH representative participated on the DSMB. The sponsor participated with investigators in obtaining grant funding, study design, data interpretation, writing and the decision to submit the paper for publication. The sponsor was not involved in any patient care or assessment of patient neurologic status during the one year follow up.
The decision to submit the paper was made collectively by all co-authors with no input from the NIH (funding source). RGH had complete access to and control of the data; all other authors could request examination of any of the data elements.
RESULTS
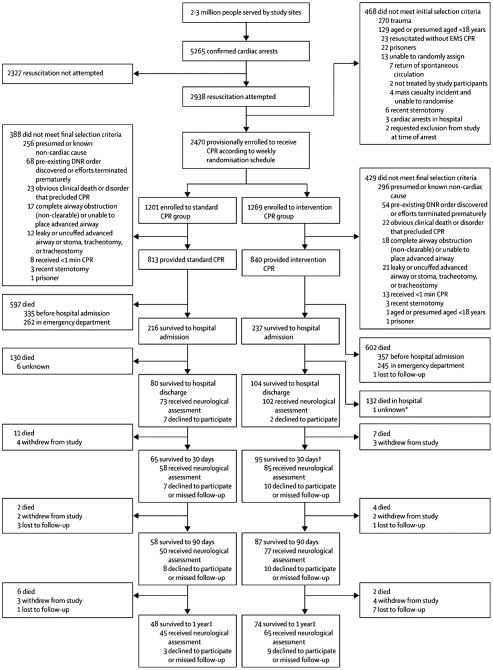
Run-in phase enrollment started in October 2005: 197 patients were enrolled and the average duration per site was 117 ± 65 days. Between March 2006 and July 2009, 2470 patients were randomized, 1653 of which met pre-specified enrollment criteria. (Figure 1) The last one-year follow-up was completed in July 2010. Distribution and exclusions in the two groups were similar. For the 1653 patients, baseline characteristics, EMS response times, and in-hospital interventions were not significantly different between groups. (Table 1) Enrollment was balanced between study groups across all sites: sites with the lowest and highest enrollment rates enrolled 4.8% and 24.8% of patients, respectively. In 80% of cases, the ITD was used first on a facemask and then switched to an advanced airway. The ITD was used only on a facemask in 5.4% of cases and only an advanced airway in 8.5% of cases.
Figure 1. Utstein18 Guidelines Patient Flow Chart.
Intervention Group received active compression decompression cardiopulmonary resuscitation with an impedance threshold device; S-CPR Group received standard cardiopulmonary resuscitation. Abbreviations: DNR= Do Not Resuscitate, ED= emergency department, EMS= emergency medical services, F/U= follow-up.
1Patients were finally excluded from the S-CPR (Control) Group for the following: presumed or known non-cardiac etiology (256); pre-existing DNR order discovered or had efforts terminated prematurely (68); signs of obvious clinical death or condition that precluded the use of CPR (23); complete airway obstruction that could not be cleared or advanced airway that could not be placed (17); leaky or uncuffed advanced airway or patient with stoma, tracheotomy, or tracheostomy (12); received <1 minute of CPR (8); recent sternotomy (3); prisoner (1).
2Patients were finally excluded from the Intervention Group for the following: presumed or known non-cardiac etiology (296); pre-existing DNR order discovered or had efforts terminated prematurely (54); signs of obvious clinical death or condition that precluded the use of CPR (22); leaky or uncuffed advanced airway or patient with stoma, tracheotomy, or tracheostomy (21); complete airway obstruction that could not be cleared or advanced airway that could not be placed (18); received <1 minute of CPR (13); recent sternotomy (3); known/presumed age <18 years (1); prisoner (1).
3Public death record search was performed at one year for all patients who withdrew or were lost to follow-up at any interval.
Table 1.
Baseline Demographics and Interventions1 [N= 1653 patients]
| Parameter | S-CPR [N=813] | Intervention2 [N=840] |
|---|---|---|
| Age, years: | 66.8 ± 14.5 | 67.0 ± 15.2 |
| 18–34 years | 12 (1.5) | 11 (1.3) |
| 35–44 years | 36 (4.4) | 47 (5.6) |
| 45–54 years | 114 (14.0) | 133 (15.8) |
| 55–64 years | 215 (26.4) | 179 (21.3) |
| 65–74 years | 172 (21.2) | 169 (20.1) |
| 75–84 years | 162 (19.9) | 192 (22.9) |
| ≥ 85 years | 102 (12.5) | 109 (13.0) |
| Gender- Male | 539 (66.3) | 558 (66.4) |
| Arrest witnessed prior to arrival of first responder | 383 (47.1) | 398 (47.4) |
| Arrest witnessed after arrival of first responder | 76 (9.3) | 80 (9.5) |
| Arrest unwitnessed | 353 (43.4) | 361 (43.0) |
| Data not available | 1 (0.1) | 1 (0.1) |
| Bystander CPR provided: | 350 (43.1) | 357(42.5) |
| Data not available | 1 (0.12) | |
| Initial cardiac arrest rhythm: | ||
| Ventricular fibrillation/pulseless ventricular tachycardia | 247 (30.4) | 292 (34.8) |
| Asystole | 379 (46.6) | 375 (44.7) |
| Pulseless electrical activity | 180 (22.1) | 170 (20.2) |
| Data not available | 7 (0.9) | 3 (0.4) |
| 911 to first response time, minutes | 6.5 ± 3.3 | 6.4 ± 3.1 |
| 911 to EMS CPR start time3 | 6.6 ± 3.4 | 6.7 ± 3.2 |
| ITD airway attachment sites: | ||
| Facemask | 717 (85.4) | |
| Endotracheal tube | 586 (69.8) | |
| Supraglottic airway (e.g., Laryngeal mask airway, Combitube™, King™) | 169 (20.2) | |
| Dose epinephrine, mg | 3.3 ± 2.1 | 3.3 ± 2.1 |
| Dose epinephrine, patients without ROSC | 3.8 ± 1.9 | 3.8 ± 1.9 |
| Duration of CPR, minutes | 27.6 ± 12.2 | 28.1 ± 11.4 |
| Duration CPR, patients without ROSC | 32.3 ± 9.5 | 32.3 ± 8.1 |
| ROSC4 during pre-hospital CPR | 324 (39.9) | 343 (40.8) |
| Enrollment by site | ||
| A | 122 (15.0) | 121 (14.4) |
| B | 155 (19.1) | 169 (20.1) |
| C | 113 (13.9) | 92 (10.9) |
| D | 189 (23.2) | 208 (24.8) |
| E | 46 (5.7) | 40 (4.8) |
| F | 149 (18.3) | 169 (20.1) |
| G | 39 (4.8) | 41 (4.9) |
| ADMITTED TO HOSPITAL | 216 (26.6) | 237 (28.2) |
| In-hospital hypothermia, % of admitted | 85 (39.4) | 92 (38.8) |
| Cardiac catheterization, % of admitted | 72 (33.3) | 100 (42.2) |
| Coronary stenting, % of admitted | 28 (13.0) | 38 (16.0) |
| Coronary bypass surgery, % of admitted | 6 (2.8) | 15 (6.3) |
| Implanted cardio-defibrillator, % of admitted | 30 (13.9) | 41 (17.3) |
Values are expressed as number of patients (percentage) unless otherwise indicated, and include all patients who met final study enrollment endpoint criteria. Plus-minus values are means ± standard deviation.
Received active compression decompression cardiopulmonary resuscitation plus an impedance threshold device
These data do not include subjects with an EMS witnessed arrest.
ROSC= return of spontaneous circulation
Compared with S-CPR, treatment with the study intervention resulted in a 53% relative increase in survival to hospital discharge with a mRS of ≤3 (the primary study endpoint): 75/840 (8.9%) vs. 47/813 (5.8%), p=0.019, OR 1.58 [CI= 1.07, 2.36]. (Table 2) In addition, there was a shift in the distribution of mRS scores in favor of improved outcomes in the intervention group, (p=0.039, Kruskal-Wallis test for ordinal responses). (Table 2)
Table 2.
Primary and Secondary Study Endpoint Outcomes1 [N= 1653 patients]
| Parameter | S-CPR [N=813] | Intervention2 [N=840] | p-value |
|---|---|---|---|
| PRIMARY COMPOSITE STUDY ENDPOINTS | |||
| Hospital Discharge-mRS3: | |||
| 0 | 3 (0.4) | 11 (1.3) | |
| 1 | 8 (1.0) | 11 (1.3) | |
| 2 | 26 (3.2) | 30 (3.6) | |
| 3 | 10 (1.2) | 23 (2.7) | 0.0394 |
| 4 | 10 (1.2) | 9 (1.1) | |
| 5 | 16 (2.0) | 18 (2.1) | |
| 6 | 727 (89.4) | 734 (87.4) | |
| Survival to hospital discharge not available | 6 (0.7) | 2 (0.2) | |
| Survived, MRS data not available | 7 (0.9) | 2 (0.2) | |
| mRS ≤ 3 (Primary Study Endpoint) | 47 (5.8%) | 75 (8.9%) | 0.019 |
| SECONDARY SURVIVAL ENDPOINTS | |||
| Survived to 24 hours post-arrest | 176 (21.6) | 197 (23.5) | 0.41 |
| Data not available | 9 (1.1) | 6 (0.7) | |
| Survived to hospital discharge | 80 (9.8) | 104 (12.4) | 0.12 |
| Data not available | 6 (0.7) | 2 (0.4) | |
| Discharge location, % of discharged: | |||
| Home | 47 (58.8) | 67 (64.4) | 0.75 |
| Other | 28 (35.0) | 35 (33.7) | |
| Data not available | 5 (6.2) | 2 (1.9) | |
| Survived to 90 Days | 58 (7.1) | 87 (10.4) | 0.029 |
| Data not available | 15 (1.8) | 8 (1.0) | |
| Survived to 1 Year | 48 (5.9) | 74 (8.8) | 0.030 |
| Data not available | 19 (2.3) | 19 (2.3) | |
| Initial arrest rhythm mRS ≤3 | |||
| VF/Pulseless VT | 40 (16.7) | 66 (22.9) | 0.06455 |
| Asystole | 3 (0.8) | 6 (1.6) | |
| PEA | 3 (1.7) | 2 (1.2) | |
| Unknown | 1 (0.1) | 1 (0.1) | |
| NEUROLOGIC ASSESSMENT | |||
| CASI6 (subjects with complete score, validity = 1) | |||
| 90 day | 93.2 ± 7.4 | 90.4 ± 13.4 | 0.251 |
| Data N/A | 19 (33) | 35 (40) | |
| 365 days | 92.9 ± 12.0 | 94.5 ± 4.5 | 0.473 |
| Data N/A | 16 (33) | 32 (43) | |
| Beck Depression Score7 | |||
| 90 day | 4.8 ± 3.9 | 6.5 ± 6.8 | 0.098 |
| Data N/A | 14 (24) | 22 (25) | |
| 365 days | 5.2 ± 6.3 | 5.5 ± 5.9 | 0.862 |
| Data N/A | 13 (27) | 17 (23) | |
Values are expressed as number of patients (percentage) unless otherwise indicated, and includes all patients who met final study enrollment endpoint criteria. P-values are based on subjects with data on status available.
Received active compression decompression cardiopulmonary resuscitation plus and impedance threshold device
mRS= modified Rankin Scale as follows: 0= asymptomatic, 1= no significant disability, 2= slight disability, 3= moderate disability, 4= moderately severe disability, 5= severe disability, 6= dead.
P-value = 0.039 for difference in distribution of mRS values.
A Mantel-Haenszel test statistic across the 3 initial arrest rhythm groups was used.
CASI = Cognitive Abilities Screening Instrument: assessment of attention, short- and long-term memory, judgment, spatial ability, concentration; based on a scale of 0–100 where 100 is a perfect score.
Beck Depression Index: evaluation of depression using a scale of 0–63 where 0–13=minimal signs of depression, 14–19=mild, 20–28=moderate, and 29–63=severe.
A subgroup analysis of survival to hospital discharge with mRS ≤ 3 based upon the first recorded rhythm demonstrated non-significantly higher survival rates in patients with ventricular fibrillation/pulseless ventricular tachycardia (VF/VT) (p=0.0645). (Table 2) For patients with a witnessed OOHCA and VF/VT initially, survival to hospital discharge was 27.6% (50/181) with S-CPR versus 34.0% (74/218) in the intervention group (p= 0.19).
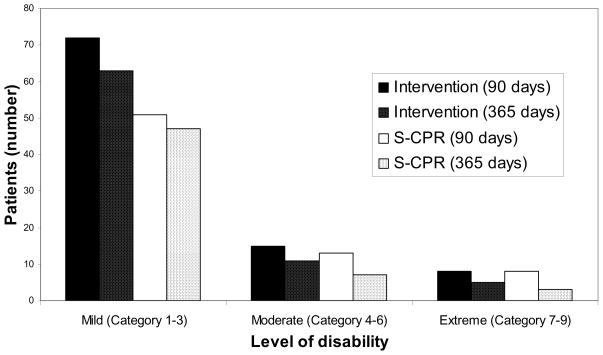
Cognitive, functional, and psychological assessments were performed in patients up to 365 days after OOHCA. The overwhelming majority of survivors had no or mild long-term neurologic deficits, with no significant difference observed between groups. (Table 2) The Disabilities Rating Scale scores, a key functional assessment of patients with severe disability, were also not significantly different between groups. (Figure 2)
Figure 2. Disabilities Rating Scores 90 and 365 days after OOHCA. There were no significant differences between study groups at 90 and 365 days after cardiac arrest.
The Disabilities Rating Scale (DRS)21 is based on a scale of 0–29 where 0=no disability (category 1), 1=mild (2), 2–3=partial (3), 4–6=moderate (4), 7–11=moderately severe (5), 12–16=severe (6), 17–21=extremely severe (7), 22–24=vegetative state (8), and 25–29=extreme vegetative state (9).
There was no significant difference in the overall major adverse event rates between groups. (Table 3) However, pulmonary edema was more common in the intervention group (p=0.015).
TABLE 3.
Major Adverse Events1 and Device Performance [N= 1653 patients]
| Parameter | S-CPR [N=813] | Intervention2 [N=840] | p-value |
|---|---|---|---|
| Subjects had at least one adverse event reported | 766 (94.2) | 787 (93.7) | 0.681 |
| Subjects had no adverse events reported | 47 (5.8) | 53 (6.3) | <0.001 (for non-inferiority) |
| Adverse Event Category: | |||
| Death | 729 (89.7) | 734 (87.4) | 0.165 |
| Rearrest | 161 (19.8) | 184 (21.9) | 0.304 |
| Pulmonary edema | 62 (7.6) | 94 (11.2) | 0.0153 |
| Seizure following index arrest | 13 (1.6) | 11 (1.3) | 0.683 |
| Bleeding requiring transfusion or surgery | 3 (0.4) | 7 (0.8) | 0.343 |
| Chest fractures | 15 (1.8) | 12 (1.4) | 0.563 |
| Pneumothorax | 7 (0.9) | 10 (1.2) | 0.628 |
| Hemothorax | 1 (0.1) | 2 (0.2) | 1.000 |
| Cardiac tamponade | 3 (0.4) | 2 (0.2) | 0.682 |
| Cerebral bleeding | 3 (0.4) | 2 (0.2) | 0.682 |
| Aspiration | 7 (0.9) | 8 (1.0) | 1.000 |
| Internal organ injury | 2 (0.2) | 4 (0.5) | 0.687 |
| Other Adverse Event | 3 (0.4) | 1 (0.1) | 0.367 |
| Study device performance: | |||
| ITD-timing light failure | n/a | 59 (7.0)4 | |
| ACD-CPR device-inadequate attachment of suction cup to the chest | n/a | 81 (9.6)5 | |
All reported adverse events were considered to be major adverse events because of their nature and the working understanding that only serious events associated with the CPR interventions would be collected. If a patient died in the field and was not transported to the hospital, then the only major adverse event assigned to that patient was “Death.” Post-mortems were not routinely collected; a uniform assessment of other adverse events in the field was not possible. All patients who were transported to a hospital, however, had all reported adverse events prior to hospital discharge considered in the event rates, including those which occurred in the field prior to transport. Events of a like nature were combined for reporting purposes, using the categories of major adverse events outlined in the study protocol. For example, rib, sternal, and spinal fractures were all coded as “Chest fractures.” Chest organ injury and abdominal organ injury were both coded as “Internal organ injury”. Adverse events identified as “Other Adverse Event” were individually examined and included in protocol Adverse Event groups, if possible. For example, pneumomediastinum was coded into “Pneumothorax”. Adverse events described as “fluid in the ET tube or airway” were coded as evidence of “Pulmonary edema”. With only a few exceptions, subjects were reported to have only one incident of a particular AE type. In those cases (e.g., multiple chest fractures or multiple rearrests) only one event of that type was assigned to a given subject. Reported Major Adverse Events rates for individual Adverse Event types, accordingly were based on the percentage of all patients at risk who reported a given event type.
Received active compression decompression cardiopulmonary resuscitation plus and impedance threshold device.
The incidence of pulmonary edema was significantly greater in the Intervention group. The clinical relevance of this finding is unclear: the percent increase in pulmonary edema (46%) is proportional to the increase in survival in the intervention group (53%).
ITD timing light is an accessory feature to guide ventilation rate. Primary function performance of the ITD was not affected by timing light failures in any cases, as the device provided inspiratory impedance appropriately. Manufacturing changes have since been implemented to remedy timing light performance.
In 73 of 81 (90%) cases of reported difficulty maintaining suction, use of the ACD-CPR device was continued despite suction cup attachment difficulty. In 8 cases, use of the ACD-CPR device was discontinued and replaced with manual S-CPR.
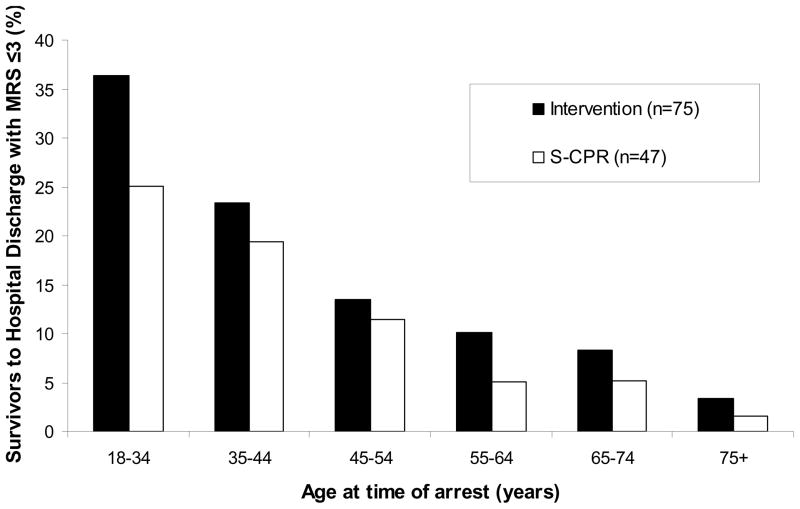
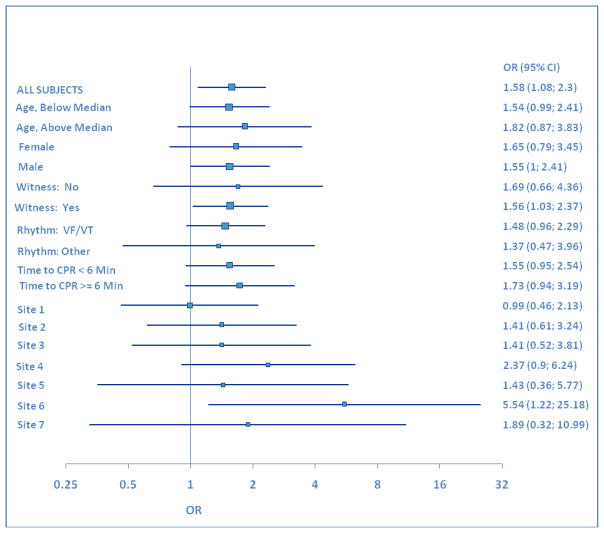
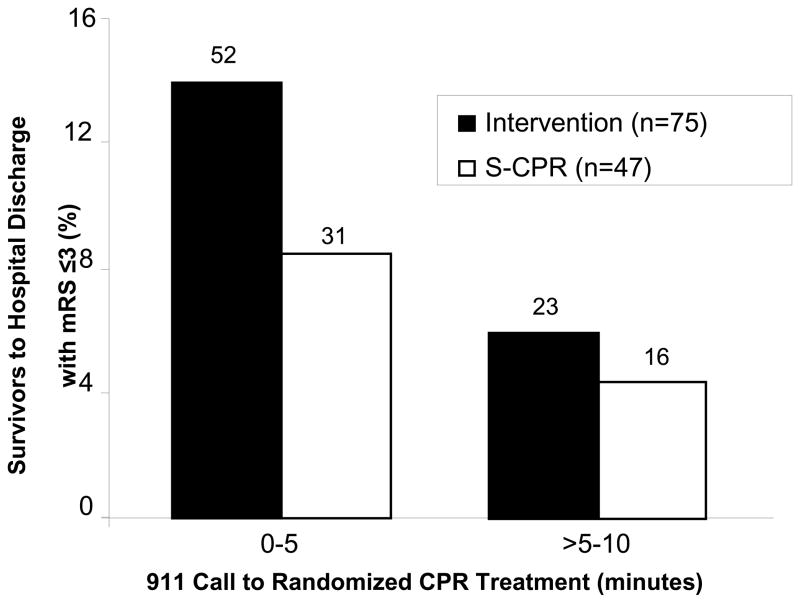
Analyses were performed to determine the effects of age, study site, gender, and date of treatment, on the primary study endpoint. The average age of survivors to hospital discharge with a mRS ≤3 was 56.0 ± 15.0 years in the S-CPR group and 56.4 ± 15.4 years in the study intervention group (p=0.87). Age distribution of patients surviving to hospital discharge with favorable neurologic function is shown in Figure 3. Consistent differences between study groups were observed throughout the entire study, independent of age, study site, gender, and date of treatment. (Figures 4 and 5) In the control group, 12/274 females (4.4%) and 35/539 males (6.5%) survived to hospital discharge with a mRS ≤3, versus 20/282 (7.1%) females and 55/558 (9.9%) in the intervention group, respectively. In addition, the therapeutic benefit of CPR in both groups was highly dependent upon time to the start of CPR. (Figure 6) There were no survivors with favorable neurologic function in either group when professional rescuer CPR was initiated more than 10 minutes after the 911 call.
Figure 3.
Age Distribution of Patients Surviving to Hospital Discharge with a Favorable Neurologic Function. Results are shown as percent of patients/age group. Favorable Neurologic function was defined as MRS ≤ 3.
Figure 4.
Analyses were performed to determine the effects of age, study site, gender, and date of treatment, on the primary study endpoint. Consistent differences between study groups were observed throughout the entire study, independent of age, study site, gender, and date of treatment.
Figure 5.
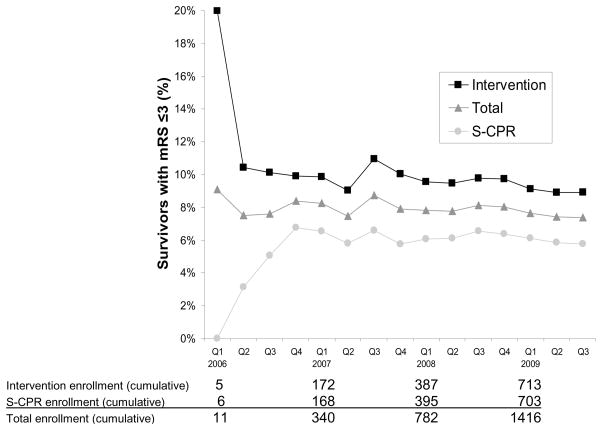
Cumulative Rates of Achieving the Primary Endpoint (mRS ≤3 at Hospital Discharge). Results are shown for pivotal phase enrollment (N=1653) by quarter. Consistent results in both groups were demonstrated throughout the entire duration of the study. Enrollment in Site 6 was initiated in the 4th Quarter (Q) of 2007 and in Site 7 in the 1st Quarter of 2009.
Figure 6. Survival to Hospital Discharge with Favorable Neurologic Function by Time to CPR Treatment. Survival to hospital discharge with MRS ≤ 3 was significantly increased in the Intervention Group [p = 0.019, OR 1.58 (CI = 1.07, 2.36)]. There no survivors in either group if CPR was initiated > 10 min after the 911 call. Values above the bars are number of patients that survived to hospital discharge with favorable neurologic function, defined as mRS ≤3.
Abbreviations: mRS = Modified Rankin Scale, Intervention= impedance threshold device plus active compression decompression cardiopulmonary resuscitation, S-CPR= standard cardiopulmonary resuscitation.
A total of 817 patients were excluded from the primary analysis who did not meet pre-specified enrollment criteria (e.g. non-cardiac causes or inability to ventilate). When data from these patients were combined with those meeting pre-specified enrollment criteria, 71/1201 (5.9%) treated with S-CPR survived to hospital discharge with a mRS ≤3 versus 101/1269 (8.0%) in the intervention group (p=0.057, OR=1.37, CI=0.99, 1.90).
Primary endpoint data were missing on 17 patients known to survive to hospital admission; informed consent for study participation was refused by 14 patients or family members. A sensitivity analysis was performed to determine the potential impact of the missing cases, assuming patients known to be dead within one year from public death records or those known to be discharged to a nursing home most likely had a mRS >3 at hospital discharge. The difference between the S-CPR and intervention groups remained significantly different (p = 0.018).
DISCUSSION
These results demonstrated that treatment with ACD-CPR and enhancement of negative intrathoracic pressure during the decompression phase of CPR significantly increased survival to hospital discharge rates with favorable neurologic function by 53% relative to S-CPR after OOHCA of presumed cardiac etiology (P=0.019). Furthermore, a greater than 50% increase in overall survival rates was observed to one year in the intervention group compared with controls. Consistency of the intervention benefit was observed independent of gender, age, date of enrollment, or study site. Neurologic function, assessed by a number of cognitive, functional, and psychological indices, was similar between groups at 90 and 365 days after the OOHCA. Importantly, there was no increase in the number of patients with severe neurologic impairment. There were no differences in overall major adverse event rates between groups, though the incidence of pulmonary edema was increased by 50% in the device group, coexistent with the increase in survival with favorable neurologic function. In addition, the current findings strongly support the need for rapid deployment of all CPR interventions to maximize the benefits of CPR.
This investigation builds upon prior studies demonstrating that ACD-CPR and a means to lower intrathoracic pressure during the chest recoil phase transforms the chest into an active bellows to more effectively circulate blood during CPR to the heart and the brain and increase short term survival rates.7–12,15,16,26–31 The current study further demonstrated that it is practicable to teach and implement ACD-CPR and ITD skills in urban, suburban, and rural EMS environments. Given that the US study sites are similar in practice to most EMS systems in the US and that study devices have already been successfully integrated into emergency services at locations in the two largest European countries, this approach should be generalizable to any EMS system that follows current European Resuscitation Council or American Heart Association Guidelines.
In this study, the first statistically significant difference in clinical outcomes was observed at the time of hospital discharge; differences in ROSC and hospital admission rates were not statistically significant. Based upon preclinical and clinical studies demonstrating greater blood flow to the heart and brain with ACD CPR and augmentation of lower intrathoracic pressure (7, 10–12, 14, 26, 27,29), we speculate that improved cerebral perfusion during CPR in the intervention group resulted in reduced cerebral ischemia but that recovery and restoration of brain function may take more time than the recovery of cardiac function. These findings also support the hypothesis that better perfusion outside the hospital could result in better candidates for cardiac catheterization (more stable patients) in the intervention group, resulting in a trend towards higher cardiac catheterization rates.
This study has several limitations. First, EMS rescuers were not blinded to the CPR method. However, the primary outcome and subsequent neurologic assessments were blinded to intervention status, thus limiting potential bias to the extent feasible. Second, it was not possible to determine the relative contribution of ACD-CPR alone, the ITD alone, or the rescuer feedback elements including the timing lights, metronome, and force gauge to the positive study outcome. Animal and human data suggest that each component is essential to observe benefits with this combined approach.5,6,11,14,16,30 A potential limitation is that study enrollment was terminated early due to lack of funding and subsequent data could have either strengthened or weakened the primary findings. Finally, despite best efforts by the research team, some surviving patients refused to provide consent for further participation or release of data. Given the unique circumstances and limitations associated with obtaining informed consent under emergency circumstances, collection of follow-up data on 100% of patients remains a challenge for all such studies.
In conclusion, compared with standard CPR, ACD-CPR with augmentation of negative intrathoracic pressure resulted in significantly increased survival to hospital discharge with favorable neurological function. One year after OOHCA, survival rates with similar neurologic functionality were also significantly higher in the intervention group.
Research in Context: Four clinical trials that included a total of 644 patients, and also a single meta-analysis performed prior to the current clinical investigation provide strong support for the concept that ACD CPR with augmentation of negative intrathoracic pressure improves hemodynamics, short-term survival rates, and the potential for longer-term survival rates with favorable neurologic function (15–17, 29,30). These trials confirmed what had been observed in animal studies (7, 10–12, 14, 26, 27), that circulation is significantly enhanced with ACD CPR and augmentation of negative intrathoracic pressure. It has been previously observed that ACD CPR transforms the human chest into an active bellows: its use increased minute ventilation to 13.5 ± 5.5 L/min compared with 7.8±5.3 L/min observed with standard CPR (9). One important study on the mechanism of the combination of ACD CPR and the ITD demonstrated that ACD CPR by itself did not significantly reduce airway pressures during the decompression phase of CPR, as respiratory gases rushed into the lungs with each chest decompression (17). However, that same study showed that when used in combination with an ITD to impede inspiratory gases selectively during the recoil phase, ACD CPR significantly lowered intrathoracic pressures during chest decompression (17). Building upon this clinical foundation, the current investigation in 1653 patients treated with either standard CPR or the study intervention provides definitive evidence that ACD CPR with augmentation of negative intrathoracic pressure improves survival to hospital discharge with favorable neurologic function. Importantly, at one year following cardiac arrest, there was a continued 50% relative increase in survival in patients in the study intervention group. The neurologic function in survivors in both groups was similar one year after cardiac arrest. In addition, ACD CPR with augmentation of negative intrathoracic pressure was safe: with the exception of an increased incidence of pulmonary edema, which paralleled the 50% survival increase observed in the study intervention group, there were no other significant adverse events or adverse device events. There was no evidence that use of ACD CPR with augmentation of negative intrathoracic pressure increased the number of patients with significant neurological impairment. The current study also provides further support for the feasibility to teach and implement the use of ACD CPR with an ITD in variety of EMS environments.
Supplementary Material
Acknowledgments
Funding:
The study was supported by a grant from the United States National Institutes of Health (R44-HL065851-03).
Footnotes
Clinical Trials registration number: NCT00189423
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nichol G, Thomas E, Callaway CW, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300(12):1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hollenberg J, Herlitz J, Lindqvist J, et al. Improved survival after out-of-hospital cardiac arrest is associated with an increase in proportion of emergency crew--witnessed cases and bystander cardiopulmonary resuscitation. Circulation. 2008 Jul 22;118(4):389–96. doi: 10.1161/CIRCULATIONAHA.107.734137. [DOI] [PubMed] [Google Scholar]
- 3.Andreka P, Frenneaux MP. Haemodynamics of cardiac arrest and resuscitation. Curr Opin Crit Care. 2006;12:198–203. doi: 10.1097/01.ccx.0000224861.70958.59. [DOI] [PubMed] [Google Scholar]
- 4.Wik L, Kramer-Johansen J, Myklebust H, et al. Quality of cardiopulmonary resuscitation during out-of-hospital cardiac arrest. JAMA. 2005;293(3):299–304. doi: 10.1001/jama.293.3.299. [DOI] [PubMed] [Google Scholar]
- 5.Yannopoulos D, McKnite S, Aufderheide TP, et al. Effects of incomplete chest wall decompression during cardiopulmonary resuscitation on coronary and cerebral perfusion pressures in a porcine model of cardiac arrest. Resuscitation. 2005;64(3):363–372. doi: 10.1016/j.resuscitation.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Aufderheide TP, Pirrallo RG, Yannopoulos D, et al. Incomplete chest wall decompression: a clinical evaluation of CPR performance by EMS personnel and assessment of alternative manual chest compression-decompression techniques. Resuscitation. 2005;64(3):353–362. doi: 10.1016/j.resuscitation.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Voelckel WG, Lurie KG, Sweeney M, et al. Effects of active compression-decompression cardiopulmonary resuscitation with the inspiratory threshold valve in a young porcine model of cardiac arrest. Pediatr Res. 2002;51(4):523–527. doi: 10.1203/00006450-200204000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Langhelle A, Strømme T, Sunde K, Wik L, Nicolaysen G, Steen PA. Inspiratory impedance threshold valve during CPR. Resuscitation. 2002;52(1):39–48. doi: 10.1016/s0300-9572(01)00442-7. [DOI] [PubMed] [Google Scholar]
- 9.Shultz JJ, Coffeen P, Sweeney M, et al. Evaluation of standard and active compression-decompression CPR in an acute human model of ventricular fibrillation. Circulation. 1994;89(2):684–693. doi: 10.1161/01.cir.89.2.684. [DOI] [PubMed] [Google Scholar]
- 10.Lurie KG, Voelckel WG, Plaisance P, et al. Use of an inspiratory impedance threshold valve during cardiopulmonary resuscitation: a progress report. Resuscitation. 2000;44:219–230. doi: 10.1016/s0300-9572(00)00160-x. [DOI] [PubMed] [Google Scholar]
- 11.Lurie KG, Coffeen P, Shultz J, McKnite S, Detloff B, Mulligan K. Improving active compression-decompression cardiopulmonary resuscitation with an inspiratory impedance valve. Circulation. 1995;91(6):1629–1632. doi: 10.1161/01.cir.91.6.1629. [DOI] [PubMed] [Google Scholar]
- 12.Aufderheide TP, Alexander C, Lick C, et al. From laboratory science to six emergency medical services systems: new understanding of the physiology of cardiopulmonary resuscitation increases survival rates after cardiac arrest. Crit Care Med. 2008;36(11):S397–S404. doi: 10.1097/ccm.0b013e31818a7e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yannopoulos D, McKnite SH, Metzger A, Lurie KG. Intrathoracic pressure regulation for intracranial pressure management in normovolemic and hypovolemic pigs. Crit Care Med. 2006;34(Suppl):S495–S500. doi: 10.1097/01.CCM.0000246082.10422.7E. [DOI] [PubMed] [Google Scholar]
- 14.Lurie KG, Lindner K. Recent advances in CPR. J Card Electro. 1997;8(5):584–600. doi: 10.1111/j.1540-8167.1997.tb00827.x. [DOI] [PubMed] [Google Scholar]
- 15.Wolcke BB, Mauer DK, Schoefmann MF, et al. Comparison of standard cardiopulmonary resuscitation versus the combination of active compression-decompression cardiopulmonary resuscitation and an inspiratory impedance threshold device for out-of-hospital cardiac arrest. Circulation. 2003;108(18):2201–2205. doi: 10.1161/01.CIR.0000095787.99180.B5. [DOI] [PubMed] [Google Scholar]
- 16.Plaisance P, Lurie KG, Vicaut E, et al. Evaluation of an impedance threshold device in patients receiving active compression-decompression cardiopulmonary resuscitation for out of hospital cardiac arrest. Resuscitation. 2004;61(3):265–271. doi: 10.1016/j.resuscitation.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 17.Plaisance P, Soleil C, Lurie KG, Vicaut E, Ducros L, Payen D. Use of an inspiratory impedance threshold device on a facemask and endotracheal tube to reduce intrathoracic pressures during the decompression phase of active compression decompression cardiopulmonary resuscitation. Crit Care Med. 2005;33(5):990–994. doi: 10.1097/01.ccm.0000163235.18990.f6. [DOI] [PubMed] [Google Scholar]
- 18.Lai SM, Duncan PW. Stroke recovery profile and the Modified Rankin assessment. Neuroepidemiology. 2001;20(1):26–30. doi: 10.1159/000054754. [DOI] [PubMed] [Google Scholar]
- 19.Cummins RO, Chamberlain DA, Abramson NS, et al. Recommended guidelines for uniform reporting of data from out-of-hospital cardiac arrest: the Utstein Style. Ann Emerg Med. 1991;20:861–874. [PubMed] [Google Scholar]
- 20.American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiac care. Circulation. 2005;112:IV-1–IV-203. doi: 10.1161/CIRCULATIONAHA.105.166550. [DOI] [PubMed] [Google Scholar]
- 21.McCurry SM, Edland SD, Teri L, Kukull WA, Bowen JD, McCormick WC, Larson EB. The cognitive abilities screening instrument (CASI): data from a cohort of 2524 cognitively intact elderly. Int J Geriatr Psychiatry. 1999 Oct;14(10):882–888. [PubMed] [Google Scholar]
- 22.Rappaport M, Hall KM, et al. Disability Rating scale for severe head trauma: Coma to community. Archives of Physical Medicine and Rehabilitation. 1982;63(3):118–123. [PubMed] [Google Scholar]
- 23.Craven JL, Rodin GM, Littlefield C. The Beck Depression Inventory as a screening device for major depression in renal dialysis patients. Int J Psychiatry Med. 1988;18(4):365–374. doi: 10.2190/m1tx-v1ej-e43l-rklf. [DOI] [PubMed] [Google Scholar]
- 24.Schneider T, Wik L, Baubin M, et al. ACD CPR-instructor and student manual for teaching and training. Part I: the workshop. Resuscitation. 1996;32(3):203–206. doi: 10.1016/0300-9572(96)00946-x. [DOI] [PubMed] [Google Scholar]
- 25.Wik L, Schneider T, Baubin M, et al. ACD CPR-instructor and student manual for teaching and training. Part II: a student and instructor manual. Resuscitation. 1996;32(3):206–212. doi: 10.1016/0300-9572(96)82051-x. [DOI] [PubMed] [Google Scholar]
- 26.Bahlmann L, Klaus S, Baumeier W, et al. Brain metabolism during CPR assessed with microdialysis. Resuscitation. 2003;59(2):255–260. doi: 10.1016/s0300-9572(03)00211-9. [DOI] [PubMed] [Google Scholar]
- 27.Raedler C, Voelckel WG, Wenzel v, et al. Vasopressor response in a porcine model of hypothermic cardiac arrest is improved with active compression decompression cardiopulmonary resuscitation using the inspiratory impedance threshold valve. Anesth Analg. 2002;95(6):1496–1502. doi: 10.1097/00000539-200212000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Srinivasan V, Nadkarni VM, Yannopoulos D, et al. Rapid induction of cerebral hypothermia is enhanced with active compression decompression plus inspiratory impedance threshold device cardiopulmonary resuscitation in a porcine model of cardiac arrest. J Am Coll Cardiol. 2006;47(4):835–841. doi: 10.1016/j.jacc.2005.09.062. [DOI] [PubMed] [Google Scholar]
- 29.Plaisance P, Lurie KG, Payen D. Inspiratory impedance during active compression decompression cardiopulmonary resuscitation: a randomized evaluation in patients in cardiac arrest. Circulation. 2000;101(9):989–994. doi: 10.1161/01.cir.101.9.989. [DOI] [PubMed] [Google Scholar]
- 30.Cabrini L, Beccaria P, Landoni G, et al. Impact of impedance threshold devices on cardiopulmonary resuscitation: a systematic review and meta-analysis of randomized controlled studies. Crit Care Med. 2008;36(5):1625–1632. doi: 10.1097/CCM.0b013e318170ba80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.