Abstract
Purpose
Magnetic resonance imaging (MRI) can provide unique information about extraocular muscle (EOM) structure and function. Prior high-resolution motility imaging studies employed T1 weighting, which provides intrinsic contrast of dark-appearing EOMs against bright orbital fat and is suitable for intravenous contrast. However, time-consuming T1 sequences are subject to motion artifacts. We evaluated an alternative T2-weighted fast spin-echo pulse sequence that emphasizes tissue-free fluid.
Methods
We prospectively used high resolution, surface coil technique for orbital MRI at 1.5T in 21 normal and 113 living strabismic subjects and 2 monkey cadavers using T2 fast spin-echo (T2FSE) weighting (long repetition time, short echo time). T2FSE was compared with T1 in 17 subjects, and with T1 in 506 different living subjects, and 12 cadavers.
Results
For 2 mm thick coronal MRIs of 312 μm resolution spanning the entire orbit, T1 acquisition required 218 seconds, whereas T2FSE required 150 seconds (31% faster). T2-defined the globe border better, and provided intrinsic contrast between EOMs and their pulleys. While both T1 and T2 demonstrated motor nerves to EOMs in living subjects, only T1 was satisfactory with injected contrast and in cadavers.
Conclusions
For motility imaging, T2FSE is faster than T1 MRI and demonstrates superior tissue details of EOMs and other orbital tissues. T2FSE of the orbits can be performed using widely available standard equipment. We suggest that T2FSE be the preferred method for clinical imaging of EOM structure, function, and innervation, although T1 may be more appropriate when intravenous contrast must be employed.
Introduction
Magnetic resonance imaging (MRI) has become increasingly employed in pediatric ophthalmology and strabismus. Technical improvements in MRI now afford the opportunity for detailed study of the functional anatomy of the extraocular muscles (EOMs), associated orbital connective tissues, and nerves in living subjects patients,1 and cranial nerves can be imaged against the surrounding cerebrospinal fluid as they exit the brainstem.2
Orbital MRI for evaluation of ocular motility employs techniques different from those appropriate to brain MRI. The positions, sizes, and shapes of the EOMs are strongly dependent on eye position, and MRI quality depends strongly on minimization of physiologic motion artifacts that do not occur in the brain. The proximity of the orbits to air-filled sinuses and their corresponding MRI signal artifacts reduces and often even reverses the imaging advantages that high-field imaging affords in brain.3 Intravenous contrast injection is often unnecessary for orbital MRI in the evaluation of strabismus. Yet, for clinical utility, orbital MRI resolution must be higher than usually employed in brain MRI. A typical whole-head MRI with 5 mm plane thickness and a 20 cm square field of view has 781 μm in-plane resolution, giving 3 μl voxel volume. A typical orbital MRI with surface coil technique, 2 mm plane thickness, and an 8 cm square field of view has 312 μm in-plane resolution, giving 0.2 μl voxel volume. The high-resolution orbital MRI thus has 15-fold higher voxel resolution than the brain MRI. A further implication of high-resolution orbital technique is that its field of view is large enough only for one orbit at a time, rather than both as is imaged using typical brain technique; twice as many sets of images are therefore required to image both orbits at high resolution. The demanding technical requirements of high-resolution orbital MRI may not be generally appreciated.
Numerous MRI parameters may be varied to image different features of tissues, including different pulse sequences.4 The authors have performed preliminary evaluations of a wide range of MRI pulse sequences and protocols, including volumetric and high field (3T) acquisitions, over the last two decades as new approaches have emerged. Despite the advantages of some of these methods for brain imaging, the techniques described here have proved superior for orbital MRI in pediatric ophthalmology and strabismus. T1 weighting was formerly recommended for high-resolution orbital MRI because with this image sequence the orbital fat is bright, against which the globe and EOMs appear with high contrast in dark signal.5 A T2 image heavily weights free fluid, such as aqueous and vitreous humors, and cerebrospinal fluid. T2-weighted fast spin-echo (T2FSE) has been developed for acquiring images rapidly3 (Clark RA, Demer JL. Differential lateral rectus [LR] compartmental contraction: A novel mechanism accounts for impaired ocular counter-rolling [OCR) in superior oblique [SO] palsy. J AAPOS 2011;15:e000 Abstract 055). This method is also known as “rapid acquisition with relaxation enhancement” (RARE).4 Such a time advantage could be of great practical importance, since motion artifacts are now the main limitation on surface coil orbital MRI resolution. It is possible, however, that T2FSE might not show the same features in the orbit as the recommended T1 MRI. While both T1 and T2FSE MRI display orbital fat as bright and extraocular muscles (EOMs) as dark, the vitreous appears dark with T1 and bright with T2FSE. Free fluid, such as in aqueous, vitreous, and cerebrospinal fluid, is bright on T2FSE. These qualitative features might have differing clinical implications in the orbit. We conducted the present study to evaluate the advantages and limitations of T2FSE for EOM imaging.
Subjects and Methods
From June 2007 through December 2010, a total of 21 normal and 106 strabismic living humans underwent high-resolution orbital MRI under a prospective protocol for study of strabismus. Retrospective comparison was made with T1-weighted MRI obtained in the same study from 1990 to 2010 in 506 living humans, 10 human cadavers, and 21 monkey cadavers. The imaging goals, use of quasicoronal imaging planes, target fixation, gaze directions, and goal of limiting scan duration on scan duration remained the same throughout the study. The aim remained to optimize the resolution possible with the existing hardware. As technical capabilities improved over the years, image plane thickness was reduced, quasisagittal imaging was added, and the total number of image planes acquired per sequence was increased, and finally, with T2FSE the acquisition time was further reduced. As a result, most of the early MRI studies in the project include views exactly comparable to the most recent studies; they differ, of course, in resolution. The direct comparisons illustrated in figures were all from the period 2007-2010, using the same scanner, the same target device, and the same surface coil array. The T1 protocol did not change during this period. A total of 15 subjects were prospectively scanned using both T1-weighted and T2FSE sequences. Orthotropic volunteers were recruited by advertising; the patients were recruited from referral strabismus practices. In all subjects, written informed consent was obtained prospectively according to a protocol approved by the institutional review board for the protection of human subjects, and in conformity with the Health Insurance Portability and Accountability Act. All subjects underwent complete ophthalmological examination, with particular attention to ocular motility.
Human cadaver specimens were obtained by anatomical donation in conformity with applicable laws. Macaque monkey specimens were obtained by tissue sharing from investigators whose protocols for the living animals had been approved by applicable animal welfare committees. After formalin fixation, 10 human and 19 monkey cadavers underwent MRI using T1, and 2 monkey cadavers using both T1 and T2FSE, employing dual 75 mm phased array surface coils (General Electric, Milwaukee, WI). Cadaver specimens were warmed to physiological temperature in a water bath immediately prior to MRI.
Imaging was performed using a 1.5 T General Electric Signa scanner (Milwaukee, WI). Orbital MRI in living subjects was performed using an array of surface coils embedded in a transparent facemask (Medical Advances, Milwaukee, WI), and fixation targets to avoid eye motion artifacts.5,6 The head was stabilized in the supine position by tightly fastening the surface coil mask to the face using headbands, and fixing the mask to the scanner gantry using foam cushions and tape. These measures avoided head rotation during scanning. An adjustable array of illuminated fixation targets was secured in front of each orbit with the center target in subjective central position for each eye, and in selected cases in secondary and tertiary gaze positions. In living humans, non-overlapping images of 2 mm thickness in a matrix of 256 × 256 were obtained over a field of view of 6–8 cm for a resolution in plane of 234–312 μm, respectively. In cadavers, some images were obtained with a field of view of 4 cm for a resolution in plane of 156 μm. Axial scout images were obtained, as well as quasicoronal images perpendicular to the long axis of the orbit, and quasisagittal images parallel to the long axis of the orbit.
All living subjects underwent MRI using a T2FSE protocol summarized in Table 1. Selected subjects also underwent additional T1 weighted imaging for comparison purposes, using published parameters.1,5 After initial T1 and T2FSE MRI was performed in some subjects, the paramagnetic MRI contrast agent gadodiamide (0.1 mmol/kg) was given intravenously before T1 MRI was repeated.7
Table 1. Comparison of MRI scan parameters.
| Parameter | T1 | T2FSE |
|---|---|---|
| Typical field of view (cm) | 8 × 8 | 8 × 8 |
| Typical in-plane resolution (μm) | 312 | 312 |
| Image plane thickness (mm) | 2 | 2 |
| Repetition time (TR, ms) | 416 | 4116 |
| Echo time (TE, ms) | 13 | 87 |
| Excitations (acquisitions) | 2 | 2 |
| Bandwidth (Hz/pixel) | 122 | 195 |
| Echo train length (echoes/TR) | 1 | 13 |
| Total imaging time (seconds)* | 218 | 150 |
| Reduction in imaging time (%) | 0 | 31 |
Imaging time is for a typical set of 18 quasicoronal image planes.
Digital MRI images were transferred to Macintosh computers (Apple Computer, Inc, Cupertino, CA), converted into 8-bit tagged image file format (TIFF), and examined using the program NIH Image (W. Rasband, National Institutes of Health; available from zippy.nimh.nih.gov or on disk from NTIS, 5285 Port Royal Road, Springfield, VA 22161, part number PB95-500195GEI).
After MRI, cadaver orbits imaged with T1 and T2FSE were removed en bloc with periorbita intact. As previously described, the whole orbits were sectioned in the quasicoronal plane into 10 μm thick sections by a microtome (HM325, Carl-Zeiss, Thornwood, NY) after tissue processing, including formalin fixation, decalcification, dehydration, and embedding in paraffin.8-11 Selected sections were stained with Masson trichrome stain to distinguish collagen (blue), muscle (red), and nerve (purple).12 Stained sections were digitally photographed using a Nikon Eclipse microscope (Melville, NY).
The authors have extensive experience with high-resolution orbital MRI. Images were evaluated qualitatively by the authors for resolution of details of the globe, EOMs, orbital connective tissues, and nerves. This included contrast of these anatomical features against surrounding structures such as orbital fat, and pathological changes within the features. Imaging acquisition time was evaluated as total time required to acquire each image set after initiation of the imaging pulse series.
Results
Imaging Quality
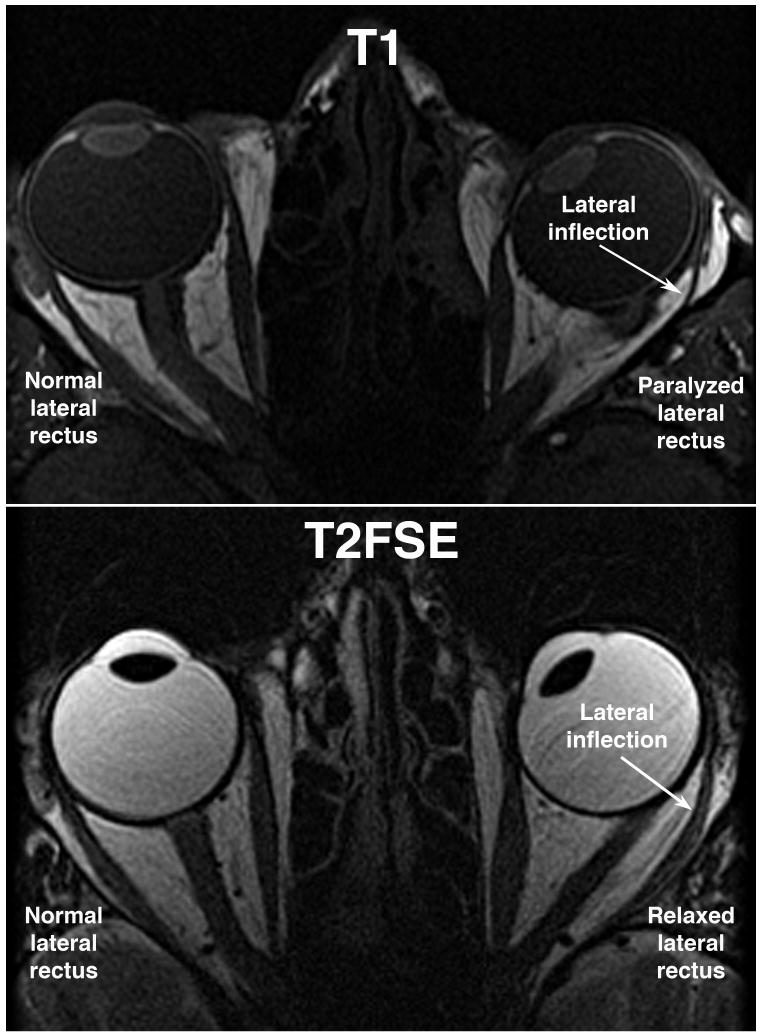
In general, T2FSE produced images of the globes and orbits that were comparable, if not better than, MRI with T1 weighting. The typical level of detail is illustrated in Figure 1, containing axial images of two different patients with left abducens paralysis. In each case, atrophy of the involved lateral rectus muscle is obvious, as well as lateral bowing and inflection of the lateral rectus path. In the T1 image, the cornea, lens, choroids, and ciliary body are clearly distinguishable against the dark vitreous humor (Figure 1, top). In the T2FSE image, the dark appearing lens is vividly contrasted against the bright vitreous humor, but the uveal tract provides no contrast against the vitreous. The optic nerve is readily visible in both T1 and T2FSE images.
FIG 1.
Axial MRI in two comparable esotropic patients. T1 image above demonstrates dark signal from slower realignment of proton spin axes in the vitreous. T2FSE image below demonstrates bright signal from slower dephasing of proton spin axes in the vitreous. Horizontal rectus EOMs are well demonstrated with both methods. T1 demonstrates iris and ciliary body better than T2FSE.
Imaging Time
With two excitations, acquisition of 18 quasicoronal MRI planes 2 mm thick with an 8 cm square field of view required 218 seconds for T1, but only 150 seconds for T2FSE. This example represents a 31% time advantage for T2FSE imaging (Table 1). While TR and TE were varied slightly for each study to minimize total imaging time, this example was typical of the lower acquisition from T2FSE than T1 imaging.
Nerves and Blood Vessels
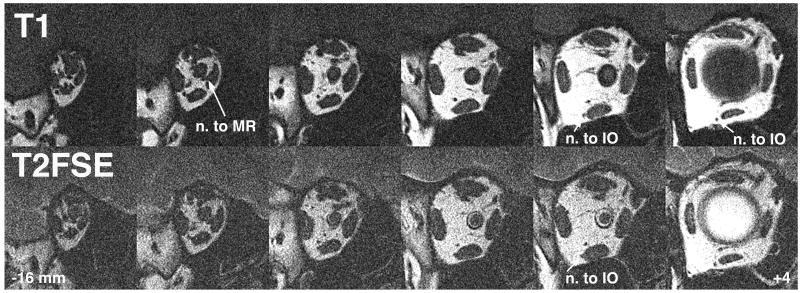
Quasicoronal images perpendicular to the long axis of the orbit are optimal for demonstration of rectus and the superior oblique EOMs in cross section. This imaging plane is also optimal to demonstrate small intraorbital motor nerves to the EOMs. As illustrated in Figure 2, posterior to the globe, T1 and T2FSE images appear almost identical. Both techniques demonstrate EOM cross sections in the deep orbit, orbital nerves, and blood vessels almost identically by their dark signals against the bright orbital fat. One feature favors T2FSE in the deep orbit: T2FSE provides vivid contrast of the bright-appearing cerebrospinal fluid against the optic nerve within its dural sheath (Figure 2, bottom row).
FIG 2.
Quasicoronal MRI in right orbit demonstrates motor nerves and blood vessels equally well for T1 and T2FSE. The ring of cerebrospinal fluid within the retrobulbar optic nerve sheath nicely defines the optic nerve with T2FSE. The vitreoretinal interface is also well defined by the bight vitreous signal with T2FSE. Lower numbers indicate anterior distance of the image plane in mm from the globe-optic nerve junction. IO, inferior oblique muscle; MR, medial rectus muscle.
Intrinsic Tissue Contrast
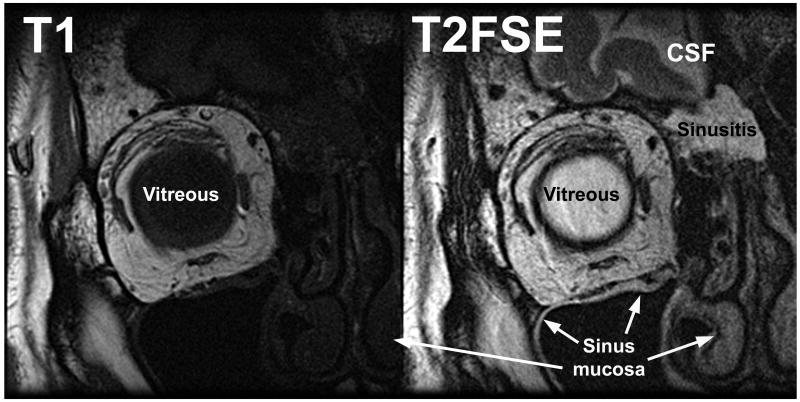
While both T1 and T2FSE provided excellent delineation of tissue boundaries demarcated by orbital fat, only T2FSE demonstrated additional boundaries delineated by variations in tissue water content. Figure 3 shows comparable quasicoronal images of the right orbit of the same patient, who has ethmoid sinusitis. The T1 image clearly demonstrates the EOMs and orbital connective tissues, but the periorbital tissues are almost uniformly dark (Figure 3 left). With T2FSE, water-containing sinus mucosa is vividly demonstrated against bones, and fluid in the sinuses and subarachnoid space appears bright (Figure 3 right). The brain is also better demonstrated with T2FSE against cerebrospinal fluid.
FIG 3.
Coronal MRI in right orbit of the same patient. Vitreous appears dark on T1 and bright on T2FSE. CSF, cerebrospinal fluid.
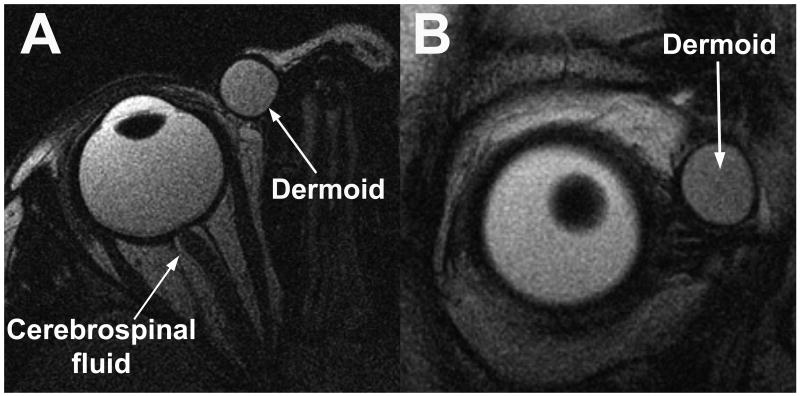
Nonorbital fluid is also well demonstrated by T2FSE. Figure 4 demonstrates a dermoid cyst in the right orbit of a child in axial and coronal views using T2FSE. The fluid-filled cyst has a bright internal signal, with a dark capsular wall demarcating the cyst from surrounding orbital fat.
FIG 4.
Axial (A) and quasicoronal (B) MRI in right orbit of child with an orbital dermoid cyst. Signal in the dermoid is bright, similar to that of the orbital fat and slightly darker than the vitreous.
Comparison with Injected Contrast
In many cases, T2FSE provided sufficient tissue contrast that injection of intravenous gadodiamide contrast was unnecessary. Quasisagittal imaging of the same dermoid cyst using T1 with intravenous gadodiamide 0.1 mmol/kg demonstrates a bright enhancing cyst wall with dark cyst contents (e-Supplement 1A, available at jaapos.org). Without necessity of contrast injection, T2FSE demonstrates bright cyst contents and a dark wall. Either image would probably suffice for surgical purposes, but T2FSE was obtained noninvasively and in less time.
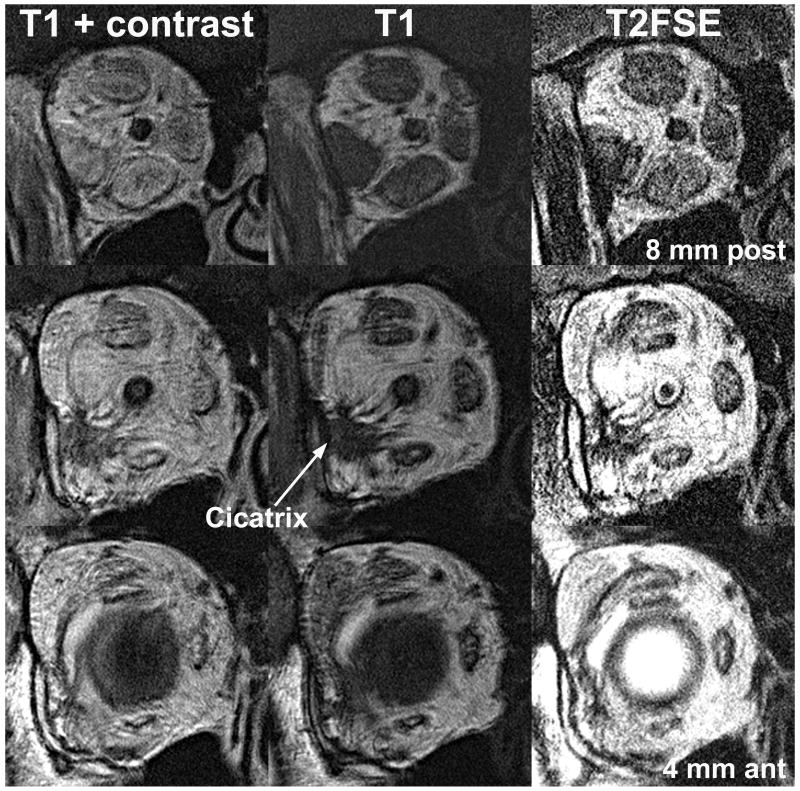
Thyroid ophthalmopathy is a good example illustrating imaging of orbital inflammation using T2FSE. Figure 5 illustrates the right orbit in a patient with active thyroid ophthalmopathy who had developed cicatrization around the lateral rectus following orbital decompression. Imaging with T1 illustrates enlarged EOMs and cicatricial bands, both appearing uniformly dark against the bright orbital fat (Figuer 5, middle column). After gadodiamide contrast injection, the enlarged EOMs enhance in a non-uniform manner suggestive of inflammation, but the poorly vascular cicatrix around the lateral rectus remains dark (Figure 5, left column). Contrast enhancement of EOMs during T1 imaging reduces their visibility against the adjacent bright orbital fat. With T2FSE in the absence of injected contrast medium, both the cicatrix and EOMs exhibit excellent contrast against the surrounding bright orbital fat (Figure 5, right column). The EOMs are more distinct against surrounding orbital fat with T2FSE than with contrast-enhanced T1, yet T2FSE demonstrates variations in signal within EOMs about as well as does the former. This signal variation within EOMs in thyroid ophthalmopathy probably indicates active myositis.
FIG 5.
Quasicoronal MRI of right orbit with thyroid ophthalmopathy, post orbital decompression that caused cicatrization around the lateral rectus muscle. Detail with T2FSE is comparable to T1 with intravenous gadodiamide contrast, which shortens both T1 and T2. Contrast enhancement occurs in tissues in proportion to blood flow. T2FSE also gives bright signal in EOM regions that enhance with contrast in T1 imaging, which may obviate the need for contrast injection for demonstration of extraocular muscle inflammation.
Demonstration of Pulleys
In the anterior orbit, the thin rectus EOMs have been difficult to distinguish from their surrounding connective tissue pulleys using T1 MRI, unless intravenous gadodiamide contrast is injected that preferentially enhances the highly vascular EOMs within their collagenous pulleys.13,14 This is illustrated in e-Supplement 2 (available at jaapos.org), showing in coronal views with axial reformatting the horizontal rectus pulleys of the left orbit a normal subject in adduction and abduction. Without contrast injection, T2FSE can also distinguish rectus pulleys from anterior EOMs, presumably because EOMs contain more fluid than pulleys. E-Supplement 3 (available at jaapos.org) demonstrates the horizontal rectus pulleys of an esotropic patient in coronal views with axial reformatting in adduction and abduction. However, T2FSE did not provide as much contrast of EOMs against their pulleys as did gadodiamide-enhanced T1 MRI.
Cadaver Imaging
While not performed clinically, imaging of cadaveric orbits permits histological validation of MRI findings. Two formalin fixed monkey orbits were imaged by both T1 and T2FSE before en bloc embedding and serial sectioning for staining with Masson trichrome. As illustrated in e-Supplement 4 (available at jaapos.org), T1 imaging provides excellent detail of EOM and nerve borders delineated by their dark signal against the bright orbital fat, and similarly demonstrated the superior ophthalmic vein. While T2FSE also demonstrated the nerves and superior ophthalmic vein, there was poor contrast of EOMs against surrounding orbital fat. Poor EOM contrast made T2FSE unsuitable for MRI of cadaveric orbits. Cadaveric MRI using T1 weighting provided near microscopic resolution, as previously described.15
Discussion
Orbital MRI has broadened understanding of the anatomy and physiology of the EOMs and their associated connective tissues.16,17 In living humans, it is now possible to image with MRI at near microscopic resolution the physiologic changes associated with conjugate eye movements, vergence, and accommodation.18 It has been possible for some years to employ clinically available equipment to image the size, contractility, and position of each EOM. It takes time to acquire high-resolution MRI, however.
The present paper demonstrates that orbital MRI using the T2FSE sequence is about a third faster than imaging with previously recommended T1 weighting, but in living people T2FSE demonstrates as well as T1 nearly all details of EOMs, orbital connective tissues, and EOM innervation. Furthermore, T2FSE demonstrates fluid-filled features such as cysts and edematous tissues without contrast injection. While not as effective in demonstrating the rectus pulleys as gadodiamide-enhanced T1 MRI, T2FSE may still demonstrate these pulleys adequately in many cases without the invasive maneuver of intravenous contrast injection. While T2FSE is generally preferred for orbital MRI in living people, T2FSE is not suitable for cadaveric orbital imaging. This may be due to postmortem alterations in free fluid, and will not be a relevant limitation in clinical imaging.
All modern clinical MRI installations are capable of performing T2FSE imaging without hardware additions or modifications. Faster orbital imaging using T2FSE not only saves costly scanner time and reduces the burden on patient cooperation, but shorter imaging time reduces motion artifacts and thus generally improves image quality. Orbital MRI in strabismus is frequently employed to demonstrate functional effects of gaze direction on EOMs and associated connective tissues. This functional application of MRI necessitates repetition of MRI acquisition in up to three separate planes for each orbit separately in each of several controlled gaze directions, so that it is common to perform 10 to 20 image acquisitions in the same study. Even accounting for overall patient setup time, a 68-second reduction in time for each of these 10 to 20 acquisitions adds up to a significant practical benefit. Patient motion during scanning is the resolution-limiting factor in modern surface coil MRI of the orbit. Use of surface coils for orbital MRI offers improvements in signal and resolution that are additive with use of T2FSE sequences.
Wider use of T2FSE for orbital imaging would improve the availability and convenience of orbital MRI. Most clinical MRI facilities do not perform T2FSE imaging of the orbits unless the physician ordering the scan specifies this technique. We suggest that T2FSE be the preferred method for clinical imaging of EOM structure, function, and innervation, although T1 is more appropriate when intravenous contrast must be employed. Strabismologists may wish to promote the use of T2FSE when they refer their patients for high-resolution orbital MRI.
Supplementary Material
e-Supplement 1. Sagittal MRI of dermoid cyst of right orbit of child shown in Figure 4 imaged using T1 with gadodiamide contrast (A) and by T2FSE without contrast (B). Note that T1 with contrast demonstrates a bright enhancing cyst wall with dark cyst contents, while T2FSE demonstrates bright cyst contents and a dark wall.
e-Supplement 2. Coronal T1 MRI in normal subject after intravenous gadodiamide 0.1 mmol/kg does not enhance horizontal rectus pulleys, but demonstrates pulleys because it enhances EOM fibers within the pulleys. Axial views are resliced from coronal image sets. LR, lateral rectus muscle; MR, medial rectus muscle; ON, optic nerve.
e-Supplement 3. Coronal T2FSE MRI in an esotropic patient without contrast demonstrates pulleys because EOM fibers contain more free fluid than avascular pulley tissues. Pulleys are not as well demonstrated as by T1 contrast imaging. Axial views are resliced from coronal image sets. LR, lateral rectus muscle; MR, medial rectus muscle; ON, optic nerve.
e-Supplement 4. Quasicoronal images of right orbit of formalin-fixed rhesus monkey. T1 provides better contrast of EOMs against orbital fat than does T2FSE. The poorer contrast of EOMs on T2FSE is because of profound post-mortem T2 lengthening due to vascular stasis, cellular edema in EOM fibers that increases their T2 signal, and subnormal tissue temperature that decreases the normally high fat signal. The superior ophthalmic vein (SOV) and optic nerve are well demonstrated by both sequences. NFVB, neurofibrovascular bundle.
Acknowledgments
Grant Support: Supported by grants from the National Eye Institute: EY08313 and EY00331. Also supported by a Research to Prevent Blindness Walt and Lilly Disney Award.
Footnotes
Presented as a poster at the 35th Annual Meeting of the American Association for Pediatric Ophthalmology and Strabismus, San Francisco, California, April 17-21, 2009.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Demer JL. A 12 year, prospective study of extraocular muscle imaging in complex strabismus. J AAPOS. 2003;6:337–347. doi: 10.1067/mpa.2002.129040. [DOI] [PubMed] [Google Scholar]
- 2.Seitz J, Held P, Strotzer M, et al. MR imaging of cranial nerve lesions using six different high-resolution T1 and T2(*)-weighted 3D and 2D sequences. Acta Radiologica. 2002;43:349–353. doi: 10.1080/j.1600-0455.2002.430401.x. [DOI] [PubMed] [Google Scholar]
- 3.Kuhl CK, Traber F, Schild HH. Whole-body high-field-strength (3.0-T) MR Imaging in Clinical Practice. Part I. Technical considerations and clinical applications. Radiology. 2008;246:675–96. doi: 10.1148/radiol.2463060881. [DOI] [PubMed] [Google Scholar]
- 4.Mayr R, Gottschall J, Gruber H, Neuhuber W. Internal structure of cat extraocular muscle. Anat Embryol. 1975;148:25–34. doi: 10.1007/BF00315560. [DOI] [PubMed] [Google Scholar]
- 5.Demer JL, Miller JM. Orbital imaging in strabismus surgery. In: Rosenbaum AL, Santiago AP, editors. Clinical Strabismus Management: Principles and Techniques. Philadelphia: WB Saunders; 1999. pp. 84–98. [Google Scholar]
- 6.Clark RA, Miller JM, Demer JL. Three-dimensional location of human rectus pulleys by path inflections in secondary gaze positions. Invest Ophthalmol Vis Sci. 2000;41:3787–97. [PubMed] [Google Scholar]
- 7.Oh SY, Poukens V, Cohen MS, Demer JL. Structure-function correlation of laminar vascularity in human rectus extraocular muscles. Invest Ophthalmol Vis Sci. 2001;42:17–22. [PubMed] [Google Scholar]
- 8.Demer JL, Oh SY, Poukens V. Evidence for active control of rectus extraocular muscle pulleys. Invest Ophthalmol Vis Sci. 2000;41:1280–90. [PubMed] [Google Scholar]
- 9.Kono R, Poukens V, Demer JL. Quantitative analysis of the structure of the human extraocular muscle pulley system. Invest Ophthalmol Vis Sci. 2002;43:2923–32. [PubMed] [Google Scholar]
- 10.Kono R, Poukens V, Demer JL. Superior oblique muscle layers in monkeys and humans. Invest Ophthalmol Vis Sci. 2005;46:2790–29. doi: 10.1167/iovs.04-1147. [DOI] [PubMed] [Google Scholar]
- 11.Miller JM, Demer JL, Poukens V, Pavlowski DS, Nguyen HN, Rossi EA. Extraocular connective tissue architecture. J Vis. 2003;3:240–51. doi: 10.1167/3.3.5. [DOI] [PubMed] [Google Scholar]
- 12.Sheehan DC, Hrapchak BB. Theory and Practice of Histotechnology. St. Louis: Mosby; 1973. pp. 95–116. [Google Scholar]
- 13.Demer JL, Clark RA. Magnetic resonance imaging of human extraocular muscles during static ocular counter-rolling. J Neurophysiol. 2005;94:3292–3302. doi: 10.1152/jn.01157.2004. [DOI] [PubMed] [Google Scholar]
- 14.Kono R, Clark RA, Demer JL. Active pulleys: Magnetic resonance imaging of rectus muscle paths in tertiary gazes. Invest Ophthalmol Vis Sci. 2002;43:2179–88. [PubMed] [Google Scholar]
- 15.Karim S, Clark RA, Poukens V, Demer JL. Quantitative magnetic resonance imaging and histology demonstrates systematic variation in human intraorbital optic nerve size. Invest Ophthalmol Vis Sci. 2004;45:1047–51. doi: 10.1167/iovs.03-1246. [DOI] [PubMed] [Google Scholar]
- 16.Demer JL. Pivotal role of orbital connective tissues in binocular alignment and strabismus. The Friedenwald lecture. Invest Ophthalmol Vis Sci. 2004;45:729–38. doi: 10.1167/iovs.03-0464. [DOI] [PubMed] [Google Scholar]
- 17.Demer JL. Anatomy of Strabismus. In: Taylor D, Hoyt C, editors. Pediatric Ophthalmology and Strabismus. London: Elsevier; 2005. pp. 849–61. [Google Scholar]
- 18.Demer JL, Kono R, Wright W. Magnetic resonance imaging of human extraocular muscles in convergence. J Neurophysiol. 2003;89:2072–85. doi: 10.1152/jn.00636.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
e-Supplement 1. Sagittal MRI of dermoid cyst of right orbit of child shown in Figure 4 imaged using T1 with gadodiamide contrast (A) and by T2FSE without contrast (B). Note that T1 with contrast demonstrates a bright enhancing cyst wall with dark cyst contents, while T2FSE demonstrates bright cyst contents and a dark wall.
e-Supplement 2. Coronal T1 MRI in normal subject after intravenous gadodiamide 0.1 mmol/kg does not enhance horizontal rectus pulleys, but demonstrates pulleys because it enhances EOM fibers within the pulleys. Axial views are resliced from coronal image sets. LR, lateral rectus muscle; MR, medial rectus muscle; ON, optic nerve.
e-Supplement 3. Coronal T2FSE MRI in an esotropic patient without contrast demonstrates pulleys because EOM fibers contain more free fluid than avascular pulley tissues. Pulleys are not as well demonstrated as by T1 contrast imaging. Axial views are resliced from coronal image sets. LR, lateral rectus muscle; MR, medial rectus muscle; ON, optic nerve.
e-Supplement 4. Quasicoronal images of right orbit of formalin-fixed rhesus monkey. T1 provides better contrast of EOMs against orbital fat than does T2FSE. The poorer contrast of EOMs on T2FSE is because of profound post-mortem T2 lengthening due to vascular stasis, cellular edema in EOM fibers that increases their T2 signal, and subnormal tissue temperature that decreases the normally high fat signal. The superior ophthalmic vein (SOV) and optic nerve are well demonstrated by both sequences. NFVB, neurofibrovascular bundle.