Abstract
Adult risk of alcohol dependence increases the younger one first engages in intoxicating consumption. Adolescent mice drink more ethanol than do adults on a g/kg basis, an increase sometimes persisting into adulthood, and this is genotype-dependent. Most studies have used 24-hr two-bottle preference, with choice between ethanol and water. We studied the developmental onset of binge drinking using limited access ethanol drinking in the dark (DID) in male and female mice. To establish age dependence in DID magnitude, we tested HS/Npt mice of 6 ages for DID for two weeks, and, when 9 weeks old, retested them for two weeks vs naïve adult controls. Age groups drank equivalently in their first week; thus, adolescent HS/Npt mice do not show greater DID than adults. Six week old mice drank more ethanol during their second week relative to their other weeks. Ethanol DID during early adolescence (4 weeks) led to increased drinking in adulthood, as did initial DID exposure at 8 weeks. High Drinking in the Dark-1 (HDID-1) mice (4, 6, 9 weeks old), selectively bred for high blood ethanol after DID, were tested for 9 weeks. Mice beginning at 4 weeks generally drank more ethanol than those of other age groups. Comparison at the same ages showed that 9 week olds initiated at 4 weeks drank more ethanol than did naïve 9 week olds, but all three groups of age-matched mice drank equivalent amounts once 10 weeks and older. The DID test is thus sensitive to developmental age. DID intakes by young adolescent HDID-1 mice were greater than by older mice, like studies with two-bottle preference. Early DID led to increased drinking as adults only in HS/Npt mice. HDID-1 mice provide a useful animal model for exploring whether DID and continuous access preference drinking have parallel consequences when initiated in adolescence.
Keywords: adolescent, ethanol, drinking in the dark, genetics, mice, development
1. Introduction
Longitudinal epidemiological data indicate that adult propensity to be diagnosed with alcohol dependence increases the younger one first engages in intoxicating consumption (Grant and Dawson, 1997; Spear, 2002; Grant, 1998). It has been shown in rodent models that adolescents drink more ethanol on a g ethanol/kg body weight basis than do adults when given a choice between an ethanol solution and water. Using the two-bottle preference test of ethanol vs water drinking, an early study found that separate groups of juvenile BALB/cCrgl mice (3–7 weeks old) had greater preference for 10% alcohol than mice 10–20 weeks old, supporting the idea that this strain shifts developmentally from alcohol accepting to alcohol avoiding (Kakihana and McClearn, 1963). Adult mice exposed to ethanol as juveniles drink more of an ethanol solution than adults given first access during adulthood (Ho et al., 1989;Blizard et al., 2004; Tambour et al., 2008). For example, Ho and colleagues showed that C57BL/6J (B6) male mice given access to 10% ethanol beginning at 3 weeks postnatal had stable, mean licks at the ethanol bottle of about 1500/day until about 6 weeks of age, when licks increased to about 4000/day. B6 male mice given access to the same solution for the first time at 8 weeks of age had significantly fewer mean daily licks of about 3000 (Ho et al., 1989). Both groups showed stable consumption from 8 to 16 weeks of age, indicating that the increase shown by the juvenile group during the 6th week of age was not likely to be due to the duration of exposure. Gradual exposure to ascending concentrations (from 0.5% to 10%) of alcohol during adolescence and into adulthood (from 5–12 weeks) has been shown to lead to increased ethanol preference during adulthood by two substrains of BALB/c mice versus mice introduced to 10% ethanol at 12 weeks (Blizard et al., 2004). BALB/cByJ but not BALB/cJ mice exposed to either forced 10% ethanol or a choice between ethanol and water during weeks 5–12 showed greater ethanol consumption in a 2-bottle choice procedure at 12 weeks (Blizard et al., 2004). All these studies used a two-bottle preference drinking paradigm with continuous access. The amount of ethanol consumed in such a test is one of the genetically most stable traits known in adult mice (Wahlsten et al., 2006).
None of these studies has employed limited access to alcohol (i.e., for a fraction of the 24 hr day), and nearly all have employed choice between alcohol and water. We recently built upon a method where ethanol is offered to mice for 2 – 4 hr/day during their circadian dark period, leading to relatively high levels of consumption (Ryabinin et al., 2003;Sharpe et al., 2005). The Drinking in the Dark (DID) test established that C57BL/6J mice would drink enough ethanol to become intoxicated (i.e., “binge”; Rhodes et al., 2005) and tests in 12 inbred strains showed that DID is heritable, and suggested that there is substantial, but not complete, overlap of the genes influencing DID and two-bottle preference drinking (Rhodes et al., 2007). We subsequently selectively bred lines of mice for high blood ethanol concentration (BEC) after limited access to 20% ethanol beginning 3 hrs into the circadian dark (Crabbe et al., 2009). Starting with a heterogeneous control stock (HS/Npt) developed by systematic intercrossing of 8 standard inbred mouse strains, we selectively bred in two genetically independent replicates those mice that had the highest BEC after a 4 hr exposure on their second day (Crabbe et al, 2009). The HDID-1 line was recently compared with the HS/Npt control for two-bottle preference with continuous or limited access; these data also support partial overlap of genetic influences on ethanol preference and ethanol DID (Crabbe et al., submitted).
Recent work by other labs has begun to characterize the differences between adolescent and adult mice in binge models. Strong et al. (2010) showed that adolescent C57BL/6J mice (4 – 6 weeks) drank more ethanol than did adult mice using a 30 minute access period combined with scheduled water consumption. When re-exposed to ethanol in a continuous access, two-bottle choice phase, adult female mice previously exposed to the binge procedure as adolescents drank more ethanol and had greater ethanol preference than did previously naïve adult female mice. This effect was not observed when the adult mice were re-exposed to ethanol in a 2-hr limited access, two-bottle choice phase (Strong et al., 2010). In another study, 4 week old C57BL/6J and DBA/2J mice of both sexes were compared with 9 week old adults using the 2-hr DID test daily for two weeks (Moore et al., 2010a). Adolescent C57BL/6J, but not DBA/2J, mice consumed more ethanol than did adult C57BL/6J mice. A subsequent retest when the previously adolescent C57BL/6J group was 9 weeks old (and adults were then 14 weeks old) showed that the group exposed to the DID test during adolescence drank more ethanol than those previously exposed as adults (Moore et al., 2010a). As predicted by prior work (Rhodes et al., 2007), both age groups of C57BL/6J mice drank more than either age group of DBA/2J mice (Moore et al., 2010a).
In the current experiments, we asked whether adolescents exposed to alcohol using the DID test would ingest more ethanol on a gram ethanol/kilogram body weight basis than adults. We further asked whether exposure during adolescence would have lasting consequences on the amount of ethanol drunk when the DID test was readministered during (or repeatedly administered until) adulthood. In the first experiment, HS/Npt mice were exposed for two, weekly DID tests starting each week from weaning (3 weeks) until 8 weeks, and were then retested for DID during PN weeks 9 and 10. In experiment 2, we tested the hypothesis that early and/or late adolescent HDID-1 mice would escalate their drinking when exposed to weekly DID tests compared with adult mice when exposed for nine consecutive weeks.
2. Methods
2.1. Animals and Husbandry
Mice were housed in standard polycarbonate or polysulfone cages with stainless steel tops in groups of 1–4 males and 1–5 females until mating or testing. Mice were housed on Bed-o-cob® bedding (Andersons, Maumee, OH, USA) with bottles with stainless steel drinking spouts. Mice were born into a reverse light/dark cycle (12L:12D) where the room was illuminated using a red light bulb and maintained at 21 ± 2 °C. Cages were changed weekly on Mondays. Food and water were freely available (breeders: Purina 5008; offspring, from weaning on: Purina 5001; PMI Nutrition International, Brentwood, MO, USA). All mice in both experiments were naïve at the beginning of the experiments.
2.1.1. Experiment 1
HS/Npt, a genetically heterogeneous stock of mice (see Crabbe et al., 2009), were used for this experiment.
2.1.2. Experiment 2
Mice in this experiment were from the 17th selected generation of the first replicate High Drinking in the Dark (HDID-1) selected line (Crabbe et al., 2009). Colony and experimental conditions were the same as for mice in Experiment 1.
2.2. DID testing
Mice were tested using our standard 4 day Drinking in the Dark (DID) procedure (Rhodes et al., 2007). Further details are given at http://www.scripps.edu/cnad/inia/modelmousedrinkingindark.pdf. Weights were taken one hour before lights out on days 1 and 3 of each week mice were being offered ethanol. Each week, on days 1 – 3, starting three hours into the dark cycle, the water bottle was removed from the mouse cage and replaced with a 10 ml drinking tube containing 20% v/v ethanol in tap water (food was always available). Initial volumes were recorded, and 2 hrs later the final volume was recorded. The ethanol tube was removed and the water bottle was replaced. Day 4 followed the same procedure as days 1–3. However, the ethanol tubes remained in place for an additional 2 hrs, for a total of 4 hrs. As described for individual experimental designs, mice were exposed to the DID test for multiple weeks, with or without gaps in testing. In all cases, the weekly DID test comprised 4 days of DID followed by 3 days’ respite where water only was available. A difference from the published DID procedures was that no blood samples were taken for BEC assessment until the very last day of drinking (in week 10) in Experiment 1. No blood samples were collected during Experiment 2. Ethanol consumption is expressed as g ethanol/kg body weight. All procedures were approved by the Institutional Animal Care and Use Committee and adhered to NIH standards.
2.3. Experiment 1: Experimental design
Thirty-five HS/Npt mating pairs chosen from the 64th generation were set up on the same day. Twenty-six of these pairs produced litters within 2 days of each other for a total of 216 offspring, from which we selected 168 mice for testing (3 – 12 per family). Within each family, mice of each sex were randomly assigned to one of 7 age groups (see Table 1), 12 mice per sex per group. Each age group comprised mice from a minimum of 8 families. Mice were individually housed prior to the dark cycle on day 1 of their initial week of DID. Once individually housed, cages were changed every two weeks on Monday.
Table 1.
Drinking Schedule by Week.
| Age at Drinking (Week) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at Start (Group) | |||||||||||||||
| 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
| Expt. 1 (HS/Npt) | |||||||||||||||
| 3 Weeks | x | x | x | x | |||||||||||
| 4 Weeks | x | x | x | x | |||||||||||
| 5 Weeks | x | x | x | x | |||||||||||
| 6 Weeks | x | x | x | x | |||||||||||
| 7 Weeks | x | x | x | x | |||||||||||
| 8 Weeks | x | x | x | ||||||||||||
| 9 Weeks | x | x | |||||||||||||
| Expt. 2 (HDID-1) | |||||||||||||||
| 4 Weeks | x | x | x | x | x | x | x | x | x | ||||||
| 6 Weeks | x | x | x | x | x | x | x | x | x | ||||||
| 9 Weeks | x | x | x | x | x | x | x | x | x | ||||||
Mice of all age groups were randomly assigned to a drinking order (1–168) that would distribute the age groups and sexes across shelves of three drinking racks. Mice of age groups 4 – 9 wks were weaned at 21 days into same-sex cages and then these group-housed cages were randomly assigned to the rack positions of the Week 9 mice. The Week 3 mice were weaned, weighed, and then individually housed on day 1 and placed in their randomly assigned rack positions (e.g., 2, 5, 8, 9, …, 167); the other groups (Weeks 4 – 9) remained group-housed with their cages interspersed among those of the Week 3 mice. On day 8 (the first day of drinking for the Week 4 mice and the first day of drinking in the second week for the Week 3 mice), the Week 4 mice were individually housed and placed in their randomly assigned rack positions (e.g., 7, 26, 40, 46, …, 161) and so on (see Table 1). On day 15, Week 5 mice were individually housed for their first day/week of drinking, Week 4 mice began their second week of drinking, and Week 3 mice had their cages changed, remaining individually housed, and began weeks of rest from ethanol drinking. Thus, all mice experienced nearly equivalent disturbance to the rack at the same times of day for body weight measurements and cage changes, and these disturbances began at one hour prior to lights out (i.e., 4 hours prior to ethanol drinking).
The first group of mice was given its first DID test at week 3 (i.e., at weaning). An additional group was started each successive week through week 9. Each group of mice was given the DID test initially for two consecutive weeks, with three days of rest between tests (see Table 1). Mice then rested for varying amounts of time (0–4 weeks) until all mice began an additional 2 consecutive weeks of DID testing at week 9. The second week of DID testing for group 8 mice was during week 9, so these mice received 3 weeks of DID testing in a row. Similarly, the Week 7 mice received four consecutive weeks of testing. After 4 hours of drinking on day 4 of week 10, a 20 microliter blood sample was collected from the periorbital sinus using a capillary tube. This was subsequently analyzed for BEC using gas chromatography (Rustay and Crabbe, 2004).
2.4. Experiment 2: Experimental design
Twenty mating pairs of HDID-1 mice from the 16th selected generation were set up on the same day. Second through fourth litter S17 offspring were weaned at 21 days of age into same-sex cages. Three age groups of mice were formed from offspring of different litters [e.g., 9 week old mice (Week 9) were formed using second litter mice, and Week 4 mice were from fourth litters] to enable nearly simultaneous testing. Multiple families (4 – 6 per age group) were chosen from among the 20 families that had litters with birthdates within 2 days of each other (3 days for Week 9 mice). Ultimately, 12 families were represented with 2 – 9 (mode: 4) mice per family in any single age group. Mice were individually housed prior to the dark cycle on day 1 of their initial week of DID and all groups had DID testing for nine consecutive weeks (see Table 1). Once individually housed, cages were changed every two weeks on Monday.
Mice of all age groups were randomly assigned to a drinking order (1–76) that would mix the age groups and sexes across shelves on either side of the drinking rack. Upon weaning, group-housed cages were randomly assigned to the rack positions of the Week 4 mice. Random assignment and rack placements were handled analogously to the description for Experiment 1.
2.5. Statistical Analyses
Data were first examined to eliminate excessive “drinking” values we deemed spurious (http://www.scripps.edu/cnad/inia/modelmousedrinkingindark.pdf). Occasionally, this was due to a tube that leaked (and emptied), but this can also occur when a mouse plays with the tube. All 2 hr consumption values of more than 12 g/kg were deleted, a value approximately 4–5 SD greater than the means. In Experiment 1, data from 3 male mice were deleted entirely for having multiple 2-hr “consumption” values in excess of 16 g/kg (many > 20) leaving 165 mice in the study. In total, 12 animals of 165 had some data removed for reasons other than a known leaky tube (a total of 20 of the 2,950 2-hr drinking periods, or less than 1%). These data were treated as missing for statistical analyses. The range of consumption values was from 0 – 11.91 g/kg in the remaining mice. In Experiment 2, four mice had g/kg intake data greater than 12 g/kg/2 hrs or leaky tubes on multiple occasions, so their data were deleted, leaving 72 mice. Five animals of the remaining 72 had some data removed, affecting a total of 12 of the 3,240 2-hr drinking periods, or less than 0.5%. The range of 2 hr consumption values was from 0 – 11.87 g/kg in the remaining mice.
Data were analyzed by analysis of variance (ANOVA) using Systat v. 13 (Systat Software, Inc., Chicago, IL). Results were taken to be significant at p < 0.05.
3. Results
3.1. Experiment 1
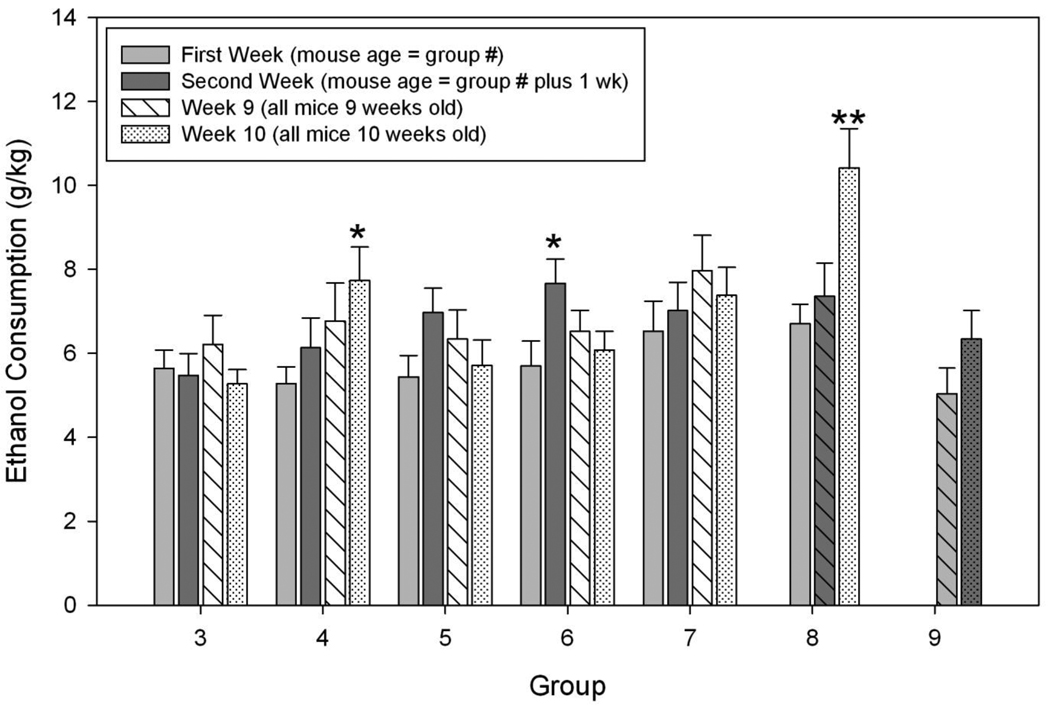
Results are shown in Figure 1, where we depict (and analyze) only the data from the 4th day of the test. For each analysis described below, we first employed the ANOVA described including sex as a factor. Female mice drank significantly more ethanol than males in nearly all analyses, but sex did not interact significantly with any other factor. For the first analysis, we asked whether age groups differed in DID on their first test. There were no significant effects (F 6,156 = 1.29, NS) indicating that the age groups drank equivalently on day 4 of their first week.
Figure 1.
Drinking in the Dark by young and adult mice of seven age groups. Male and female HS/Npt mice were given access to 20% ethanol in the Drinking in the Dark (DID) test. At the time of the initial test, mice were 3 – 9 weeks old (Group). Total ethanol consumption (g/kg) over the 4 hours on the fourth day of the DID test in each week is plotted, collapsed across sex. Each age group from 3 – 9 Weeks was tested for two consecutive weeks (First week bars are light grey; Second week bars are dark grey). Age groups 3 – 6 Weeks were then treated normally until 9 weeks of age, and then were retested for two consecutive weeks (Week 9 bars are hatched diagonally; Week 10 bars are dotted). The group 7 Week mice were tested for four consecutive weeks, while group 8 Week mice were tested for 3 consecutive weeks (see Table 1). *, significantly greater consumption than in the First week of the same age group (p < 0.05). **, significantly greater consumption than in the First or Second weeks of the same age group (p < 0.01).
Based on the available literature, we hypothesized that mice would increase ethanol intake over two or more successive weeks. We looked at the data in several ways to address this hypothesis. First, age groups 3 – 7 wks old drank ethanol for a total of 4 weeks (two weeks, then a break of 4 to no weeks, respectively, then two weeks), so these groups were analyzed separately by repeated measures ANOVA on week. As expected, there was a significant main effect of week (F 3,309 = 4.32, p < 0.01) and a significant interaction with group (F 12,309 = 2.13, p < 0.05). The main effects of group (F 4,103 = 1.74, p = 0.14) and sex (F 1,103 = 1.37, p = 0.06) were not significant, nor were there significant interactions of sex with week or sex X week X group (F 3–12,309 ≤ 1.04, NS). Therefore, we collapsed on sex for the remaining analyses. We next asked whether there were differences among weeks within each group. Only the 4 and 6 week old groups showed significant effects of drinking week (both Fs 3,66 > 3.69, ps < 0.05). Post hoc tests showed that the 4 week old group drank significantly more ethanol in their 10th week than they did in their 1st or 2nd week (pairwise mean differences > 2.14, ps < 0.05). The 6 week old group showed a different pattern. This group showed greater ethanol consumption in their 2nd week than any other week (pairwise mean differences > 1.15, ps < 0.05).
Next, we compared drinking by all seven age groups between their second week relative to the first. Intake increased significantly in the second week (F 1,155 = 13.7, p < 0.001) but neither the main effect of group nor its interaction with week (F 6,155 < 1.61, p > 0.15) were significant.
Two groups had more than two consecutive weeks of drinking: the 8 wk old group (3 weeks) and the 7 wk old group (4 weeks). Separate analyses showed that the 8 wk old group (F 2,42 = 10.56, p < 0.001; main effect of week) showed a pattern similar to the 4 wk old group in that their drinking during their 10th week was greater than that in their 1st or 2nd week (pairwise mean differences > 3.06, ps < 0.01). For the 7 wk old group, the main effect of week was not significant (F 3,66 = 2.21, p = 0.096).
There were no differences between sexes, among age groups, or in their interaction in BECs at the end of drinking in Week 10 (Fs 1–6,151 < 1.56, NS). BECs averaged 0.44 ± 0.04 mg/ml across the experiment, with 53 mice having BECs of zero and 62 with BECs ≥ 0.50 mg/ml (range: 0 – 2.21, data not shown).
As shown in Table 2, body weights among groups of same-sex mice were similar at the same ages (all Fs < 3.11, NS). When all mice were 9 and 10 weeks old, age group had no main or interaction effects on body weight (all Fs < 1.15, NS), indicating that prior drinking experience did not affect adult body weight.
Table 2.
Body weights by group and sex at different ages in HS/Npt mice in Experiment 1.
| Age at Drinking (Week) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Age at Start (Group) | ||||||||
| 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Female | ||||||||
| 3 Weeks | 10.7 ± 0.3 g | 14.2 ± 0.4 g | 20.3 ± 0.3 g | 20.8 ± 0.4 g | ||||
| 4 Weeks | 13.9 ± 0.3 g | 16.6 ± 0.4 g | 19.4 ± 0.3 g | 20.0 ± 0.3 g | ||||
| 5 Weeks | 17.0 ± 0.5 g | 18.9 ± 0.5 g | 21.3 ± 0.6 g | 21.4 ± 0.6 g | ||||
| 6 Weeks | 18.2 ± 0.4 g | 18.6 ± 0.4 g | 19.9 ± 0.4 g | 20.4 ± 0.4 g | ||||
| 7 Weeks | 18.5 ± 0.6 g | 19.1 ± 0.5 g | 19.9 ± 0.5 g | 20.6 ± 0.5 g | ||||
| 8 Weeks | 19.9 ± 0.3 g | 20.4 ± 0.3 g | 21.0 ± 0.3 g | |||||
| 9 Weeks | 19.4 ± 0.6 g | 19.8 ± 0.6 g | ||||||
| Male | ||||||||
| 3 Weeks | 11.2 ± 0.3 g | 16.7 ± 0.4 g | 24.2 ± 0.3 g | 24.5 ± 0.5 g | ||||
| 4 Weeks | 17.3 ± 0.6 g | 19.6 ± 0.7 g | 24.3 ± 0.6 g | 24.6 ± 0.7 g | ||||
| 5 Weeks | 21.0 ± 0.4 g | 22.2 ± 0.4 g | 25.4 ± 0.5 g | 25.9 ± 0.5 g | ||||
| 6 Weeks | 22.8 ± 0.7 g | 22.9 ± 0.8 g | 24.7 ± 0.9 g | 25.0 ± 0.8 g | ||||
| 7 Weeks | 24.1 ± 0.8 g | 24.6 ± 0.9 g | 25.5 ± 0.9 g | 25.9 ± 0.9 g | ||||
| 8 Weeks | 25.1 ± 0.8 g | 25.5 ± 0.8 g | 25.9 ± 0.7 g | |||||
| 9 Weeks | 25.1 ± 0.7 g | 25.5 ± 0.6 g | ||||||
Body weights were assessed on days 1 & 3 of each week of DID testing. The data shown represent the mean ± SEM body weight of the mice on day 1 of each week. No significant differences among groups within sex were found at any age at drinking.
3.2. Experiment 2
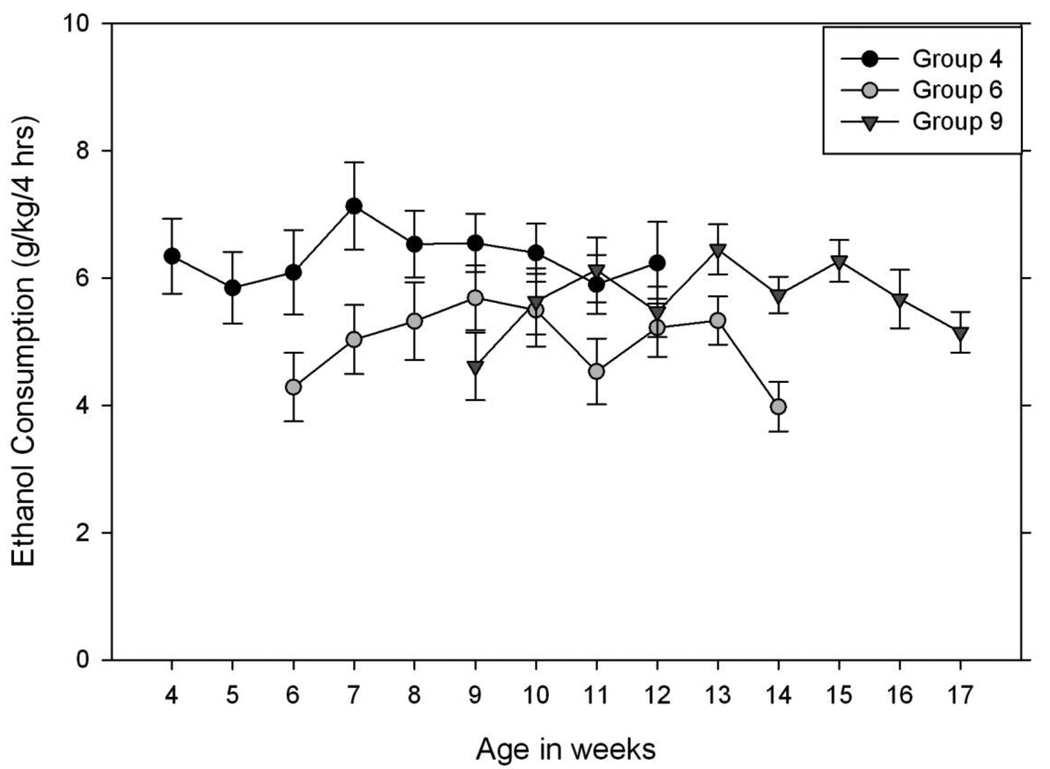
Results (again, for the 4 hr test on the 4th day of each week) are shown in Figure 2. Data were first subjected to a repeated measures ANOVA on the number of weeks of drinking, with sex and age at start as between groups factors. Significant main effects of sex (females > males; F 1,64 = 8.62, p < 0.01), age group (F 2,64 = 3.39, p < 0.05), and weeks (F 8,512 = 3.63, p < 0.001) were found. There were no significant interactions (all F <= 1.02). The group of mice beginning drinking at 4 wks of age consumed more ethanol than the other groups. The pattern of changes across weeks of testing in all groups combined was followed up with further analysis using pairwise mean difference tests on week. In all but the mice starting at 4 weeks old, consumption increased over the first 4 weeks by about 1 g/kg, before tapering back to nearly the initial value by week 9. For all subsequent analyses, we collapsed data across sex.
Figure 2.
Drinking in the Dark by early, mid-to-late adolescent and adult HDID-1 mice. Male and female mice were given access to 20% ethanol in the Drinking in the Dark (DID) test. At the time of the initial test, mice were 4, 6, or 9 weeks old. Data plotted are the mean ± SEM total ethanol consumption (g/kg) over the 4 hours on the fourth day of the DID test in each of nine weeks, collapsed across sex (see Table 1).
Next, we ignored the specific number of weeks of prior drinking experience and simply compared the groups when they reached the same ages. For the first comparison, data from the 4 week- and 6 week-initiated animals were analyzed using the data from 6 – 12 weeks old (see Figure 2). Significant main effects of group (Week 4 > Week 6; F 1,43 = 5.24, p < 0.05), and age (F 6,258 = 2.54, p < 0.05) were found. The interaction was not significant (F 6,258 = 0.87, NS). The second comparison used the data from 9 – 12 weeks old for all 3 age groups. Neither main effect was significant (Fs from 0.53 – 2.11), but there was a significant interaction of age X group (F 6,204 = 2.95, p < 0.01). This was followed up by separate ANOVAs of group within age. The effect of group was significant at 9 weeks of age (F 2,69 = 4.03, p < 0.05), but not at ages 10 – 12 weeks (Fs 0.91 – 2.84). Thus, the mice starting drinking at 4 weeks of age drank significantly more ethanol at 9 weeks of age (Tukey’s HSD = 1.94, p < 0.05) than did the naïve group (those that started drinking at 9 weeks). The group that began drinking at 6 weeks of age was not different from either of the other groups at 9 weeks of age.
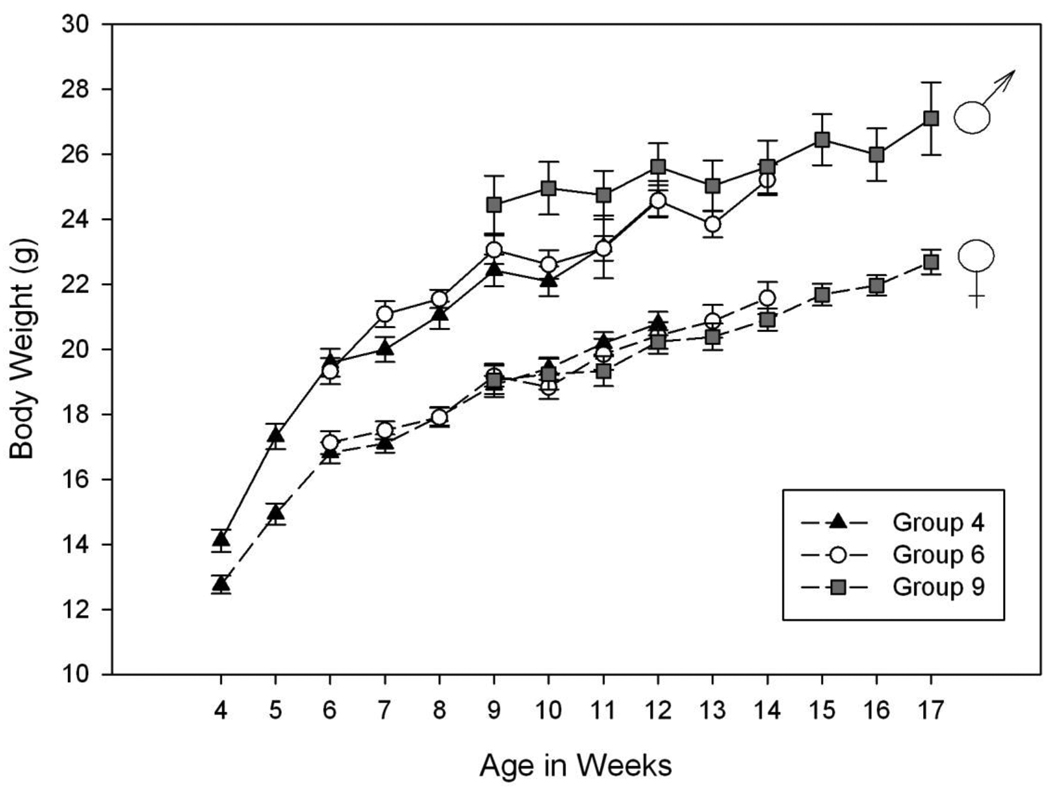
Body weights among groups of same-sex mice generally were similar at the same ages (see Figure 3); however, analyses showed that male mice in the 4 and 6 week old groups showed significantly lower body weight (22.09 ± 0.45 and 22.60 ± 0.44g, respectively) at 10 weeks of age than the male mice of the 9 week old group (24.95 ± 0.81g).
Figure 3.
Body weights by group and sex at different ages in HDID-1 mice in Experiment 2. Body weights were assessed on days 1 & 3 of each week of DID testing. The data shown represent the mean ± SEM age of the mice (in weeks) versus the body weights (g) on day 1 of each week. Groups of male mice are shown with solid lines; female mice are shown with dashed lines. Symbols represent group age at the start of testing. No significant differences among groups within sex were found. The male mice of the 4 and 6 week groups showed significantly lower body weight at 10 weeks of age, but not at other ages, than did the male mice of the 9 week group. There were no differences in body weight among groups in female mice of the same age.
4. Discussion
Previous studies have demonstrated that C57BL/6J inbred strain mice given ethanol in binge consumption models drink more ethanol than do adults and also drink more ethanol when they become adults than do C57BL/6J mice first exposed as adults for periods of up to 14 days (Strong et al., 2010; Moore et al., 2010a). The present studies extend this finding to another genotype: early adolescent HDID-1 mice drank more ethanol in the DID test overall than did late adolescent or adult mice during a nine-week study. This was found when comparing all three age groups over the nine weeks of testing, as well as in comparisons when mice were the same ages. The group that began drinking at 4 weeks drank significantly more ethanol between 6 and 12 weeks of age than did the group that began drinking at 6 weeks. When all three age groups were compared at 9 weeks of age, the group that began drinking at 4 weeks drank significantly more than the naïve group (the 9 week group that began drinking that week). There was no statistical evidence that any individual age-initiated group of HDID-1 mice increased drinking over the course of the nine week study. The significant main effect of week appeared to be due to a gradual increase in consumption from initial values across the first 4 weeks of drinking in all but the 4 week old group, but the age groups did not differ by 10 and 12 weeks of age (Figure 2). The mice started in late adolescence (6 weeks) differed from the other two groups in that they appeared to decrease their consumption during later tests. By adulthood, they were clearly drinking less than the group that started drinking as adults. Whether this overall pattern represents an effect of early adolescent (4 weeks) exposure to enhance drinking or an effect of mid-to-late adolescent exposure (6 weeks) to reduce drinking with prolonged exposure in adulthood cannot be determined from the current data. It would be necessary to continue the DID test in all groups through ages older than 14 weeks to resolve this issue (Figure 2).
In the HS/Npt mice, the age of initiation of drinking in the DID test was not important, as all groups drank approximately the same amount of ethanol in their first week (Figure 1, light grey bars). This is consistent with another study using a heterogeneous mouse stock where greater ethanol consumption by adolescent mice relative to adults in a continuous access, two-bottle choice study took 3 weeks to develop (Tambour et al., 2008). Experiment 1 provides some support for the hypothesis that earlier drinking experience in some age groups may increase later drinking. Results showed a significant increase in consumption during the second week across groups (Figure 1). In particular, the mid-to-late adolescent 6 week group showed elevated ethanol consumption the following week that returned to initial values in their 9th & 10th weeks of age. The mice beginning drinking in their 4th or 8th week of age also showed significant effects of testing week (Figure 1). Both the groups initiated as early adolescents (4 week) and as adults (8 week) showed significantly increased ethanol consumption during their 10th week of age (a repeated test during adulthood). For the 4 week group, this reflected a steadily increasing progression of intakes across the 4 weeks of testing. The finding of elevated intake only during week 10 in the 8 week old group (and the lack of increase in the 9 week group) suggested that at least three consecutive weeks of DID testing might be necessary to see an increase over time. However, the 7 week old group, which had four consecutive weeks of DID, did not show this pattern. Furthermore, the absolute level of consumption in week 10 on day 4 by the 8 week group was clearly higher than that of any group in any week (Figure 1). We also examined data from days 1 – 3 of each week by this group and found that week 10 intakes on these days were similar to those of weeks 8 and 9., Had the 9 week group been tested for another week and shown an increase similar to the 8 week group, it might have been possible to suggest that there was a developmental difference between the 7 week and 8 week group. Unfortunately, the 9 week group was only tested for 2 weeks. Thus, we cannot exclude the possibility that the dramatic increase of about 3 g/kg in the 8 week group in week 10 was stochastic. In Experiment 2, the 9 week group of HDID-1 mice also showed an increase of consumption of about 1.5 g/kg over the first three weeks, which appeared to stabilize later (Figure 2).
Tambour et al. (2008) found that adult (10 weeks) and adolescent mice (4 weeks) that had equal durations of exposure (8 subsequent weeks) eventually did not differ in consumption (i.e., when the mice were all adult); however, this was because the adult group’s drinking eventually increased to match the adolescent consumption rather than because the adolescents decreased their consumption. Thus, the return to first week values of ethanol consumption of the 6 week HS/Npt group when they were 9 – 10 weeks old, relative to their increased consumption when they were 7 weeks old, was unexpected. The finding in Experiment 2 that the 6 week HDID-1 group also appeared to drink more ethanol during the middle of their test and return to approximately initial levels during their last week of drinking (Figure 2) suggests that there may be some developmental changes during this time that may be influencing DID. Alternatively, the low intake of the 6 week-initiated group during week 14 may be a stochastic event.
The 4 week old HS/Npt group appeared to increase their consumption gradually over the four weeks of testing (Figure 1), while the 4 week old HDID-1 group remained fairly stable at a higher level than the groups started at older ages (Figure 2). Aside from genotype, the primary difference between these two studies is that the HS/Npt mice were tested for DID for two weeks and then had 3 weeks off before drinking again, while the HDID-1 drank for 9 consecutive weeks. Although this wasn’t tested directly, it suggests that there are developmental factors active near or before 4 weeks of age in the HDID-1 line that may develop later in the HS/Npt mice. Further work would obviously be needed to test this hypothesis.
The 3 – 6 week age groups of HS/Npt mice all experienced two weeks of drinking and then a period from 1 (6 week group) to 4 (3 week group) weeks without ethanol access. This hiatus could have produced an increase in drinking when mice began drinking again at 9 weeks of age, a phenomenon known as the alcohol deprivation effect (Sinclair & Senter, 1968; Melendez et al., 2006; Füllgrabe et al., 2007). However, Figure 1 shows no evidence for such an escalation in drinking. It is possible that continuous access to ethanol access to a choice between ethanol and water, and/or a longer period of DID prior to deprivation are required to elicit the alcohol deprivation effect. Further work would be necessary to clarify these issues.
We have reported data only from the 4th day each week. On this day, animals are offered 4 hr access to ethanol, and the DID assay is based on intakes (and blood ethanol levels) achieved by drinking on that day. We also analyzed data for Days 1–3 each week for each experiment, following the same ANOVA approach described in the Results. Experiment 2's outcome looked essentially the same during the first 3 days as during the 4th day; only the details of the statistical outcomes differed. For Experiment 1, the picture was a bit more complicated. Some of the week-specific differences revealed during the 4 hr DID test were not seen on the earlier days when drinking was limited to 2 hr each day. For example, the very high intakes of the 8 week old group tested at 10 weeks seen on Day 4 were not apparent during Days 1–3. For any reader interested in access to all the data from either experiment, we will happily supply data files.
There is considerable evidence that adolescent rats drink more ethanol on a g/kg basis than do adult rats (e.g., McBride et al., 2005; Maldonado-Devincci et al., 2010; Hargreaves et al., 2009; Walker et al., 2008); although there are also several studies that have found the opposite results (e.g., Siegmund et al., 2005; Bell et al., 2006; Füllgrabe et al., 2007). Some studies in rats have found that adolescent exposure to ethanol increases later drinking during adulthood (e.g., Maldonado-Devincci et al., 2010; Hargreaves et al., 2009), although others have seen lower ethanol consumption in adulthood (e.g., Siegmund et al., 2005). It is difficult to say what factors might explain the differences in results. Aside from a great number of procedural variations, whether males or females or both were studied, the ages of the rats at onset and at adulthood, and rat strain (e.g., Wistar, Sprague-Dawley, the alcohol preferring P rat, among others) also vary widely in this literature. The facilitating effects of early exposure on adult drinking may be expressed to a greater degree in high-drinking genotypes (Bell & Rodd in Barron et al., 2005; Tolliver & Samson, 1991), and may not be seen when ethanol vapor exposure is substituted for early drinking exposure (Slawecki and Betancourt, 2002).
The mechanisms underlying these effects are not known. Adolescent rodents are less sensitive than adults for many (although not all) measures of sensitivity to acute ethanol and also less likely to display withdrawal symptoms, and this differential sensitivity shows genotype dependence (Hefner and Holmes, 2007; Linsenbardt et al., 2009; Moore et al., 2010b). The differences cannot generally be explained by pharmacokinetic differences (Spear and Varlinskaya, 2005), although at least in mice, pharmacokinetic differences have explained some specific, strain-dependent behavioral sensitivity differences between adolescents and adults (Linsenbardt et al., 2009). BECs in Experiment 1 did not differ from those we have seen in HS/Npt mice after only two days of DID, suggesting that there may not have been pharmacokinetic adaptation in response to longer term DID (cf. Crabbe et al., 2009). Some difficulties in interpretation of studies with adolescents include whether the test apparatus are appropriately scaled for each age group and whether the test measure is age-appropriate at the time of the test (for example, see Moore et al., 2010b). Furthermore, equilibrating the dosages of ethanol between adolescents and adults is difficult. For example, comparison of g/kg intake across animals of different ages may be problematic if there are developmental changes in pharmacokinetics or drug distribution across body tissues (Barron et al., 2005). In one continuous access two-bottle preference study, adolescent C57BL/6J mice that started drinking at 3 weeks accelerated their drinking between the ages of 4 and 6 weeks as their body weight increased, and drinking plateaued when body weight stabilized (Ho et al., 1989). In our HS/Npt study, the group beginning drinking at 6 weeks showed their greatest drinking in their 7th week of age, which is similar to the age at which their rate of weight gain began to slow; however, the 4 week group showed their greatest drinking in their 10th week; presumably well after their rate of weight gain slowed (see Figure 1 and Table 2). While the 6 week group HDID-1 mice did increase their drinking over their first few weeks in a pattern similar to their change in body weight, the drinking of the 4 week group was already higher than those of the other groups when the mice were only 13–14 g (see Figure 3). Thus, the hypothesis that drinking changes mirror body weight changes warrants further study.
Together these studies show that the DID test can be sensitive to age-related differences. They show that the HDID-1 line, selectively bred for high blood ethanol concentration after drinking in the dark, is another useful genetic animal model in which to examine development of high ethanol consumption. The relationship between age of onset of testing and alcohol intake is similar to that seen in most two-bottle preference drinking studies with continuous access. This relationship, however, was genotype dependent and was not seen in the HS/Npt stock. Similarly, the effect of early exposure to elevate adult drinking was partially supported in the current work, but only in the HS/Npt stock when drinking started at 4 weeks of age. Thus, either adolescence per se, or perhaps experience, can elevate ethanol drinking under certain conditions, and the genotype of the mice appears to be one important factor.
Acknowledgments
The authors thank Jason P. Schlumbohm, Andy Jade Cameron, Stephanie E. Spence, Larry C. Huang, Chia-Hua Yu, and Kate L. Mordarski for help with the experiments and data curation and presentation. Supported by the Dept of Veterans Affairs, AA107860 and AA13519 from the NIH, and an NIH LRP award (PM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lauren Lyon Brown, Email: brolaure@ohsu.edu.
John C. Crabbe, Email: crabbe@ohsu.edu.
References
- Barron S, White A, Swartzwelder HS, Bell RL, Rodd ZA, Slawecki CJ, Ehlers CL, Levin ED, Rezvani AH, Spear LP. Adolescent vulnerabilities to chronic alcohol or nicotine exposure: findings from rodent models. Alcohol Clin Exp Res. 2005;29:1720–1725. doi: 10.1097/01.alc.0000179220.79356.e5. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Sable HJK, Schultz JA, Hsu CC, Lumeng L, Murphy JM, McBride WJ. Daily patterns of ethanol drinking in peri-adolescent and adult alcohol-preferring (P) rats. Pharmacol Biochem Behav. 2006;83:35–46. doi: 10.1016/j.pbb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Blizard DA, Vandenbergh DJ, Jefferson AL, Chatlos CD, Vogler GP, McClearn GE. Effects of periadolescent ethanol exposure on alcohol preference in two BALB substrains. Alcohol. 2004;34:177–185. doi: 10.1016/j.alcohol.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Rhodes JS, Yu C-H, Brown LL, Finn DA. A line of mice selected for high blood ethanol concentrations shows drinking in the dark to intoxication. Biological Psychiatry. 2009;65:662–670. doi: 10.1016/j.biopsych.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Spence SE, Brown LL, Metten P. Alcohol preference drinking in a mouse line selectively bred for High Drinking in the Dark. doi: 10.1016/j.alcohol.2010.12.001. (submitted; under revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Füllgrabe MW, Vengeliene V, Spanagel R. Influence of age at drinking onset on the alcohol deprivation effect and stress-induced drinking in female rats. Pharmacol Biochem Behav. 2007;86:320–326. doi: 10.1016/j.pbb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Grant BF. The impact of a family history of alcoholism on the relationship between age at onset of alcohol use and DSM-IV alcohol dependence. Alcohol Health & Research World. 1998;22:144–148. [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Hargreaves GA, Monds L, Gunasekaran N, Dawson B, McGregor IS. Intermittent access to beer promotes binge-like drinking in adolescent but not adult Wistar rats. Alcohol. 2009;43:305–314. doi: 10.1016/j.alcohol.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Hefner K, Holmes A. An investigation of the behavioral actions of ethanol across adolescence in mice. Psychopharmacology. 2007;191:311–322. doi: 10.1007/s00213-006-0646-2. [DOI] [PubMed] [Google Scholar]
- Ho A, Chin AJ, Dole VP. Early experience and the consumption of alcohol by adult C57BL/6J mice. Alcohol. 1989;6:511–515. doi: 10.1016/0741-8329(89)90060-8. [DOI] [PubMed] [Google Scholar]
- Kakihana R, McClearn GE. Development of alcohol preference in BALB/c mice. Nature. 1963;199:511–512. doi: 10.1038/199511a0. [DOI] [PubMed] [Google Scholar]
- Linsenbardt DN, Moore EM, Gross CD, Goldfarb KJ, Blackman LC, Boehm SL., II Sensitivity and tolerance to the hypnotic and ataxic effects of ethanol in adolescent and adult C57BL/6J and DBA/2J mice. Alcohol Clin Exp Res. 2009;33:464–476. doi: 10.1111/j.1530-0277.2008.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Devincci AM, Alipour KK, Michael LA, Kirstein CL. Repeated binge ethanol administration during adolescence enhances voluntary sweetened ethanol intake in adulthood in male and female rats. Pharmacol Biochem Behav. 2010;96:476–487. doi: 10.1016/j.pbb.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Bell RL, Rodd ZA, Strother WN, Murphy JM. Adolescent alcohol drinking and its long-range consequences: studies with animal models. Recent Dev Alcohol. 2005;17:123–142. doi: 10.1007/0-306-48626-1_6. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Middaugh LD, Kalivas PW. Development of an alcohol deprivation and escalation effect in C57BL/6J mice. Alcohol Clin Exp Res. 2006;30:2017–2025. doi: 10.1111/j.1530-0277.2006.00248.x. [DOI] [PubMed] [Google Scholar]
- Moore EM, Mariani JN, Linsenbardt DN, Melón LC, Boehm SL., II Adolescent C57BL/6J (but not DBA/2J) mice consume greater amounts of limited-access ethanol compared to adults and display continued elevated ethanol intake into adulthood. Alcohol Clin Exp Res. 2010a;34:737–742. doi: 10.1111/j.1530-0277.2009.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EM, Linsenbardt DN, Melón LC, Boehm SL., II Ontogenetic differences in adolescent and adult C57BL/6J and DBA/2J mice: anxiety-like, locomotor, and consummatory behaviors. Dev Psychobiol. 2010b Sept 30; doi: 10.1002/dev.20501. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiology and Behavior. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu C-H, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain and Behavior. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Rustay NR, Crabbe JC. Genetic analysis of rapid tolerance to ethanol's incoordinating effects in mice: Inbred strains and artificial selection. Behav Genet. 2004;34:441–451. doi: 10.1023/B:BEGE.0000023649.60539.dd. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Sharpe AL, Tsivkovskaia NO, Weitemier AZ. Ethanol self-administration during the circadian dark phase. Alcohol Clin Exp Res. 2003;27 S122,183A. [Google Scholar]
- Sharpe AL, Tsivkovskaia NO, Ryabinin AE. Ataxia and c-Fos expression in mice drinking ethanol in a limited access session. Alcohol Clin Exp Res. 2005;29:1419–1426. doi: 10.1097/01.alc.0000174746.64499.83. [DOI] [PubMed] [Google Scholar]
- Siegmund S, Vengeliene V, Singer MV, Spanagel R. Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcohol Clin Exp Res. 2005;29:1139–1145. doi: 10.1097/01.alc.0000171928.40418.46. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Senter RJ. Development of an alcohol-deprivation effect in rats. Q J Stud Alcohol. 1968;29:863–867. [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M. Effects of adolescent ethanol exposure on ethanol consumption in adult rats. Alcohol. 2002;26:23–30. doi: 10.1016/s0741-8329(01)00192-6. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and the college drinker: biological basis of propensity to use and misuse alcohol. J Stud Alcohol. 2002 Suppl:71–81. doi: 10.15288/jsas.2002.s14.71. [DOI] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence: Alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol. 2005;17:143–159. [PubMed] [Google Scholar]
- Strong MN, Yoneyama N, Fretwell AM, Snelling C, Tanchuck MA, Finn DA. “Binge” drinking experience in adolescent mice shows sex differences and elevated ethanol intake in adulthood. Horm Behav. 2010;58:82–90. doi: 10.1016/j.yhbeh.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambour S, Brown LL, Crabbe JC. Gender and age at drinking onset affect voluntary ethanol consumption but neither the alcohol deprivation effect nor the response to stress in mice. Alcohol Clin Exp Res. 2008;32:1–7. doi: 10.1111/j.1530-0277.2008.00798.x. [DOI] [PubMed] [Google Scholar]
- Tolliver GA, Samson HH. The influence of early postweaning ethanol exposure on oral self-administration behavior in the rat. Pharmacol Biochem Behav. 1991;38:575–580. doi: 10.1016/0091-3057(91)90016-u. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Bachmanov A, Finn DA, Crabbe JC. Stability of inbred mouse strain differences in behavior and brain size between laboratories and across decades. Proceedings of the National Academy of Sciences USA. 2006;103:16364–16369. doi: 10.1073/pnas.0605342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Walker JL, Ehlers CL. Dissociable effects of ethanol consumption during the light and dark phase in adolescent and adult Wistar rats. Alcohol. 2008;42:83–89. doi: 10.1016/j.alcohol.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]