Abstract
Sulforaphane, a major isothiocyanate derived from cruciferous vegetables, protects living systems against electrophile toxicity, oxidative stress, inflammation, and radiation. A major protective mechanism is the induction of a network of endogenous cytoprotective (phase 2) genes that are regulated by transcription factor Nrf2. To obtain more detailed understanding of the anti-inflammatory and immunomodulatory effects of sulforaphane, we evaluated its effect on the phagocytosis activity of RAW 264.7 murine macrophage-like cells by measuring the uptake of 2-μm diameter polystyrene beads. Sulforaphane raised the phagocytosis activity of RAW 264.7 cells but only in the absence or presence of low concentrations (1%) of fetal bovine serum. Higher serum concentration depressed phagocytosis and abolished its stimulation by sulforaphane. This stimulation did not depend on the induction of Nrf2-regulated genes since it occurred in peritoneal macrophages of nrf2−/− mice. Moreover, a potent triterpenoid inducer of Nrf2-dependent genes did not stimulate phagocytosis, whereas sulforaphane and another isothiocyanate (benzyl isothiocyanate) had comparable inducer activity to sulforaphane. It has been shown recently that sulforaphane is a potent and direct inactivator of macrophage migration inhibitory factor (MIF), an inflammatory cytokine. Moreover, addition of recombinant MIF to RAW 264.7 cells attenuated phagocytosis, but sulforaphane-inactivated MIF did not affect phagocytosis. The inactivation of MIF may therefore be involved in the phagocytosis-enhancing activity of sulforaphane.
Keywords: MIF, macrophage migration inhibitory factor; RAW 264.7 cells; Nrf2; NQO1
Introduction
Phagocytosis is a major mechanism of cellular protection and manifestation of inflammatory and immunological responsiveness. The isothiocyanate sulforaphane (SF) is derived from its glucosinolate precursor (glucoraphanin) which is abundant in cruciferous plants such as broccoli [1, 2]. There has been intense interest in the chemoprotective capabilities of SF in defending against carcinogenesis initiated by DNA-damaging electrophiles, reactive oxygen intermediates, inflammation, and radiation (UV and ionizing) damage [3-5]. These protective effects, which have been largely attributed to the upregulation of cytoprotective (phase 2) genes, have been demonstrated in systems ranging in complexity from cells in culture to humans. The potential implications of these findings for devising strategies to reduce the risk of chronic disease are widely appreciated, and SF has been identified as a potentially promising new chemoprotective agent.
Inflammation plays a key and critical role in the pathogenesis of many chronic diseases, and SF has now been shown to dampen many inflammatory responses in a variety of experimental systems. Overexpression or abnormal accumulation of inflammatory agents such as chemokines, cytokines, nitric oxide (NO), and prostaglandins (PGs) are at the root of many age-related and chronic diseases. SF has been reported to attenuate the lipopolysaccharide-induced production of tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), PGE2, and NO in murine peritoneal macrophages and murine macrophage-like RAW 264.7 cells [6, 7]. These inflammatory agents also play critical roles in TH2 (humoral) immune allergic responses such as in atopic diseases and allergic bronchitis. SF also showed protective effects against age-related decrease of TH1 (cellular) immunity [8]. The respiratory system is one of the front lines of both humoral and cellular immunities, and it has been reported that SF protects against disorders induced by xenobiotics including cigarette smoke [9] and diesel exhaust particles [10]. Phagocytic cells such as macrophages act to eliminate foreign materials that accompany inflammation. We therefore asked whether in such systems, in some of which SF had already been shown to suppress inflammation, this isothiocyanate also regulated phagocytosis. For these purposes, we used the established mouse RAW 264.7 macrophage-like cell line, as well as mouse peritoneal macrophages produced in response to an inflammatory stimulus. In both types of cells SF raised phagocytosis activity when fetal bovine serum concentrations were low or absent, probably by inactivation of macrophage migration inhibitory factor (MIF) [11] which under some conditions depresses phagocytosis of these cells [12].
Materials and Methods
Materials
Triterpenoid 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-onitrile (TP-225) [13] was a gift from M. B. Sporn (Department of Pharmacology and Toxicology, Dartmouth Medical School). Endotoxin-free recombinant MIF was from R&D Systems. L-Dopa methyl ester was from Sigma. RS-Sulforaphane was obtained from LKT Laboratories.
Cell Cultures
RAW 264.7 cells, a macrophage-like, Abelson leukemia virus-transformed cell line from BALB/c mice, were obtained from the American Type Culture Collection and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen) with 10% heat-inactivated fetal bovine serum (FBS, Invitrogen) at 37°C in a humidified atmosphere containing 5% CO2.
Peritoneal Macrophages
Experimental animal procedures were in compliance with the National Institutes of Health Guidelines and approved by the Johns Hopkins University Animal Care and Use Committee. C57BL/6 background- nrf2−/− mice (n = 4, female, 17 weeks old) initially established by Itoh et al. [14] and nrf2−/− hairless SKH1 mice crossed with the nrf2−/− mice in the C57BL/6 background and SKH1 mice (n = 2, female, 52 weeks old) were used. The mice were challenged by intraperitoneal injection of 4% thioglycollate solution (3 ml/mouse). After 4 days, the animals were euthanized by cervical dislocation, and the peritoneal cavity was lavaged with two 10-ml injections of cold sterile PBS. After centrifugation of the recovered fluid (5 min at 100 × g), the cell pellet was suspended in RPMI 1640 culture medium (Invitrogen). About 107 cells were obtained from each mouse.
Phagocytosis Assay
The method of Link et al. [15] was modified and used to assess the phagocytosis activity. RAW 264.7 cells were plated in 8-well glass slides (6 × 104 cells/well; Nalge Nunc International) and incubated for 24 h (37°C, 5% CO2). Cytoprotective (phase 2) inducers were added in acetonitrile (final concentration 0.1% by volume), and incubation was continued for a further 24 h, unless otherwise stated. The medium was replaced by DMEM containing polystyrene beads (2 μm diameter; Bangs Laboratories) which had been treated for 1 h at 37°C with mouse IgG (5 mg/ml; Equitech Bio). The ratio of polystyrene beads to cells was 8:1. The chamber slide was incubated at 37°C for 10 min, nonadherent beads were removed by washing with cold PBS, and cells were fixed with 3.7% formaldehyde for 12 min. Ten to fifteen randomly-selected fields were photographed in each well with a dark-field microscope (TMS; Nikon) and a digital camera (D40; Nikon). The numbers of cells and beads were confirmed with ImageJ (ver. 1.43, NIH), a software program for photo-analysis. Phagocytosis activity was expressed as a Phagocytosis Index (PI), which is the number of beads phagocytosed by 100 cells under these conditions. The agreement between the PI measurements by two independent observers who counted the same 21 randomly-selected fields was 99.7 ± 4.0%.
NQO1 Assay
NAD(P)H quinone oxidoreductase 1 (NQO1) activity in RAW 264.7 cells was measured by the Prochaska microtiter plate bioassay [16]. Cells were plated into 96-well microtiter plates (5 × 104 cells/well). After 3 h at 37°C, the medium was replaced by DMEM containing serial dilutions of phase 2 inducers in acetonitrile (final concentration 0.1% by volume) and incubated for 24 h. The cells were lysed by digitonin (0.08 % in 2 mM EDTA, pH 7.8; 75 μl/well) and aliquots (20 μl/well) were transferred to newly prepared 96-well microtiter plates for measuring cellular proteins by the bicinchoninic acid assay [17]. NQO1 activity was obtained from the initial velocity of formation of the reduced tetrazolium dye in cell lysates with an optical microtiter plate scanner (SpectraMax Plus 384; Molecular Devices). The specific enzyme activity was expressed as the ratio of reaction velocity to protein concentration, and the CD values (Concentration required to Double the NQO1 specific activity) were used to compare inducer potencies.
mRNA determination by quantitative real-time PCR
Quantitative real-time PCR analysis was performed to determine the expression of the nqo1 gene (7000 Sequence Detection System, Applied Biosystems). Total RNA was isolated from RAW 264.7 cells by a column-based RNA extraction kit (RNeasy Mini kit, Qiagen) and reverse transcribed into cDNA (iScript, BioRad). The cDNA was diluted 50-fold to measure the 18s ribosomal RNA for accurate normalization. The cDNA was subjected to quantitative real-time PCR by using the TaqMan Gene Expression Assay primer probe sets for NQO1 and 18s ribosomal RNA (assay ID Mm00500821_m1 and Hs03928990_g1, Applied Biosystems). Relative expression of the NQO1 message was normalized to the 18s RNA as an endogenous control and the expression rate was calculated using the comparative ΔΔCT method. Average relative mRNA expression and confidence intervals are indicated (number of dishes, n=3).
MIF tautomerase activity assay
RAW 264.7 cells were suspended in DMEM (with 0 or 10% FBS) and plated in 96-well microtiter well plates (105 cells/well). The plates were prepared in duplicate for measurement of MIF tautomerase activity and cell proliferation. After incubation (37°C, 5% CO2) for 24 h, the media were recovered and the cells were washed with PBS. Lysis buffer (40 mM HEPES, 50 mM NaCl, 1 mM EDTA, pH 7.4, 1% CHAPS) was added to each well (30 μl/well) and incubated at room temperature for 10 min. Recovered medium and cell lysates (40 μl of medium; 20 μl of cell lysate) were transferred to freshly prepared 96-well microtiter plates for measurement of MIF enzymatic activity, by a modification of the method of Rosengren et al. [18]. L-Dopachrome methyl ester was prepared just before use by mixing L-dopa methyl ester solution (13 mM, 500 μl) and sodium periodate (24 mM, 500 μl) in 19 ml of the reaction buffer (50 mM Bis-Tris, 1 mM EDTA, pH 6.2). Then 200 μl of the L-dopachrome methyl ester solution were added to each well, and the decrease in absorbance at 475 nm was monitored for 10 min after addition of the solution to be assayed. The tautomerase activity was calculated as the maximum velocity of the initial rate of decrease in absorbance, corrected for the nonenzymatic rate (usually about 0.001 per min). A molar absorption coefficient of 3,000 M−1cm−1 at 475 nm was assumed. The specific enzyme activities were normalized by dividing the tautomerase velocity by a measure of cells obtained from MTT reduction assays. Briefly, MTT was added to each well (50 μg/well) of the 96-well plate and incubated at 37°C for 2 h. Formazan products were solubilized with DMSO (150 μl/well) and diluted 10-fold. The absorbance of the solution at 570 nm was used for the normalization of MIF tautomerase activity.
MIF inactivation
Commercially available recombinant murine MIF was reconstituted in sterile PBS at 100 μg/ml. SF (5 mM, dissolved in DMSO) was added to the MIF solution to a final concentration of 100 μM and incubated at room temperature for 30 min. Excess SF was removed by gel filtration (NAP 10 Columns, GE Healthcare). The concentration of inactivated MIF in eluted fractions was determined by the bicinchoninic acid protein assay [17].
Statistical Analysis
The effects of prior treatment with SF on phagocytosis activity were evaluated by use of the Kruskal-Wallis equality of populations rank test, followed by Wilcoxon rank test, as well as by ANOVA (Analysis of Variance). Effects of incremental additions of serum were evaluated by ANOVA and Pearson’s linear regression coefficients r were calculated. ANOVA and Scheffé’s post hoc test were applied for the comparison of the PI (phagocytosis index) between the groups treated with each concentration of inducers and recombinant MIF. STATA (ver.10, StataCorp LP) was used.
Results and discussion
Effects of SF on phagocytosis activity of RAW 264.7 cells
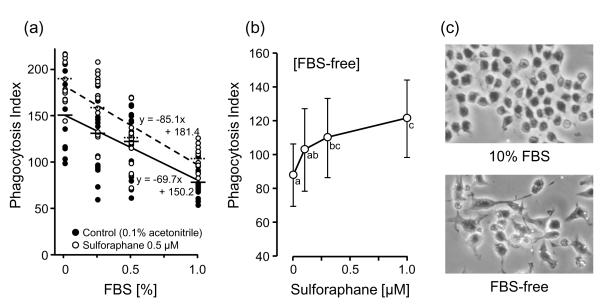
Our two initial observations were: 1) the uptake of 2-μm polystyrene beads by RAW264.7 cells was inversely related to the concentration of FBS in the culture medium (Fig. 1); and 2) SF addition stimulated this phagocytosis activity, but the degree of enhancement decreased as the FBS concentration was raised, and was not observed in cells incubated in complete medium containing 10% FBS (PI = 29.2 ± 8.5 and 29.5 ± 13.2, for 0 and 0.5 μM SF, respectively, which are not significantly different). Prior treatment of the cells for 24 h with 0.5 μM SF resulted in a highly significant increase in phagocytosis activity at low FBS concentrations (F(1, 115) = 13.98, p = 0.0003) and there was also an inverse correlation between the PI and FBS concentration. The magnitude of this stimulation was also dependent on SF concentration (Fig 1).
Fig. 1.
Effect of sulforaphane and fetal bovine serum (FBS) on phagocytosis activity of RAW 264.7 cells. (a) Each circle represents the phagocytosis index (PI) of each photo-field treated with 0.1% (v/v) acetonitrile as a control (●) and 0.5 μM SF (○) for 24 h. Horizontal bars show the averages of each group (control: —, 0.5 μM SF: - - -). Regression lines of PI values in control (—) and the SF-treated condition (- - -) are shown, as well as the regression equations. The linear correlation coefficient r are −0.620 and −0.736 for control and SF-treated cells, respectively. (b) Cells were incubated with 0.1% (v/v) acetonitrile as control and with (0.1, 0.3, and 1.0 μM SF) for 24 h in the absence of FBS. PI values are shown as means ± SD (number of fields counted n = 28 and 29 for the control and the treated cells). Points not sharing a common letter are significantly different (p < 0.05, ANOVA with Scheffé’s test). (c) Photographs of RAW 264.7 cells incubated in the presence (10%) or absence of FBS. The bright white circles are the 0.2 μm polystyrene beads.
Lowering of serum in the culture medium affects cell cycle dynamics and induces apoptosis in RAW 264.7 cells [19, 20]. In the absence of FBS, RAW 264.7 cell proliferation was depressed, and the cells showed significant changes in their morphology, including increased size and greatly elongated spindle shapes (Fig. 1). How these morphological changes relate to phagocytosis is unclear.
Participation of the Keap1/Nrf2 pathway in stimulation of phagocytosis by SF
SF is a potent inducer of the Keap1/Nrf2 pathway [21-23]. Nrf2 has been also reported to interact with other cell-signaling pathways involved in cell proliferation and cell death such as the aryl hydrocarbon receptor (AhR) [24], nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) [25], p53 [26], and Notch [27]. We assessed the involvement of the Keap1/Nrf2 pathway in the enhancement of phagocytosis activity by SF treatment in two ways: 1) by examining the ability of Nrf2 inducers other than SF to stimulate phagocytosis, and 2) by determining whether stimulation of phagocytosis occurred in nrf2−/− macrophages.
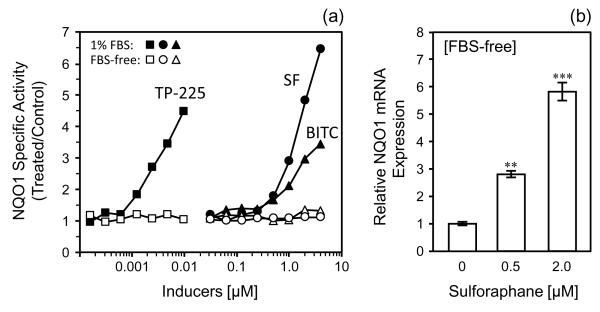
The inducer potencies of SF, benzyl isothiocyanate (BITC) and the triterpenoid TP-225 on the NQO1 activity of RAW 264.7 cells were measured in the presence of FBS (Fig. 2). The CD (Concentration required to Double) values for NQO1 induction for SF, BITC and TP-225 in DMEM containing 1% FBS were 0.57, 0.86, and 0.0014 μM, respectively. In contrast, no induction was observed with the same concentrations of these agents in the absence of FBS. On the other hand, treatment with both 0.5 and 2.0 μM SF induced NQO1 mRNA expression 2.8- and 5.8-fold even in the absence of FBS (Fig. 2). This suggests that lack of serum may disrupt posttranscriptional synthesis of Nrf2-dependent proteins. Treatment with SF or BITC (1.0 μM, 24 h) significantly stimulated the phagocytosis activity of RAW 264.7 cells in serum-free DMEM, but TP-225 (a powerful inducer of Nrf2) [13] showed no effect on phagocytosis in the range of 0.005 to 0.02 μM (Table 1). These results suggest that stimulation of phagocytosis is not a common property of all inducers of the phase 2 response, but that it may be related to the specific chemistry of isothiocyanates, and be independent of the Keap1/Nrf2 pathway.
Fig. 2.
Induction of NQO1 in RAW 264.7 cells by three phase 2 response inducers: sulforaphane (SF), benzyl isothiocyanate (BITC), and triterpenoid TP-225. (a) NQO1 enzyme specific activity (treated/control) after 24 h induction in presence of 1% FBS (●, ▲, ■) or FBS-free medium (○, △, □). (b) Induction of NQO1 mRNA expression after treatment with SF for 4 h in absence of FBS. Expression of mRNA as a function of SF concentration was determined by real-time PCR and expressed as relative values and 95% confidence intervals. 18s ribosomal RNA was used as the internal control for normalization. **p < 0.01, ***p < 0.001, compared to 0 μM SF control (ANOVA with Scheffé’s test).
Table 1.
Effects of phase 2 inducers on phagocytosis activity in RAW 264.7 cells in absence of Fetal Bovine Serum
| Compound | [μM] | Phagocytosis Index | |
|---|---|---|---|
| SF | 0 | 87.9 ± 18.7 | (28)* |
| 0.1 | 102.8 ± 24.5 | (29) | |
| 0.3 | 110.1 ± 23.6** | (29) | |
| 1.0 | 121.4 ± 23.1*** | (29) | |
| BITC | 0 | 90.4 ± 17.3 | (22) |
| 0.1 | 88.1 ± 20.0 | (22) | |
| 0.3 | 95.5 ± 29.1 | (22) | |
| 1.0 | 122.1 ± 21.1*** | (22) | |
| TP-225 | 0 | 108.9 ± 25.0 | (18) |
| 0.005 | 109.6 ± 20.5 | (18) | |
| 0.01 | 113.2 ± 20.0 | (17) | |
| 0.02 | 102.4 ± 12.8 | (17) |
number of fields counted. PI values are expressed as means ± SD.
p < 0.01,
p < 0.001, compared to inducer-free control (ANOVA with Scheffé’s test).n = number of fields counted. PI values are expressed as means ± SD.
This possibility was strengthened by the finding that the effects of SF on phagocytosis were also observed after only a very short (4 h) treatment. The phagocytosis index for untreated (number of fields counted n=29), 0.3 μM SF (n=30), and 1.0 μM SF (n=27) treated cells were 70.8 ± 35.0, 123.2 ± 43.7, and 132.7 ± 39.3, respectively; (p < 0.001 for increases compared to control).
In further support that the Keap1/Nrf2 pathway is not involved, the effect of SF was assessed on peritoneal macrophages from two strains of nrf2−/− mice which had either the genetic background of C57BL/6 mice or were 50% hybrids of C57BL/6 and SKH1 hairless mice. Peritoneal cells obtained from these mice were plated at 105 cells/well in 8-well chamber slides and incubated for 3 h (37°C, 5% CO2). After removal of nonadherent cells with PBS, SF (0.1, 0.3, and 1 μM) or 0.1% (v/v) acetonitrile were added to the medium (RPMI 1640) in the absence of FBS and incubated for 24 h. Exposure to SF of peritoneal macrophages obtained from SKH1 wild-type female mice resulted in a dose-dependent stimulation of phagocytosis. The PIs for these macrophages were increased 15-22% (p < 0.001) at SF concentrations of 0.3-1.0 μM (data not shown). However, macrophages from nrf2−/− mice, both from C57BL/6 and SKH1/C57BL/6 hybrids showed lower PI, but greater sensitivity to stimulation by SF (Table 2). This strongly suggests that induction of Nrf2-dependent genes is not required for stimulation of phagocytosis by SF.
Table 2.
Effects of sulforaphane on phagocytosis activity in primary peritoneal macrophages of nrf2−/− mice
| Sulforaphane [μM] |
Phagocytosis Index in nrf2−/− mice |
|||
|---|---|---|---|---|
| C57BL/6 | Hairless | |||
| 0 | 39.8 ± 19.0 | (120)* | 105.1 ± 30.9 | (57) |
| 0.1 | 62.6 ± 25.5*** | (120) | 127.1 ± 26.9** | (56) |
| 0.3 | 62.2 ± 31.0*** | (120) | 131.4 ± 36.4*** | (56) |
| 1.0 | 60.7 ± 21.4*** | (120) | 139.4 ± 26.9*** | (53) |
number of fields counted. PI values are expressed as means ± SD.
p < 0.01,
p < 0.001, compared to 0 μM SF control (ANOVA with Scheffé’s test).
MIF inactivation and phagocytosis
Synthesis and secretion of the cytokine MIF are early and prominent inflammatory responses of macrophages and other cells. MIF is a homotrimer (114 amino acids in humans) that promotes the tautomerization of dopachrome, an enzymatic activity that can be quantified spectroscopically [11]. Recently three laboratories [28-30] independently reported that a number of isothiocyanates, including SF, potently and irreversibly inactivated the enzymatic function of MIF by modifying its highly nucleophilic N-terminal proline residues. Indeed, MIF was identified as the most prominent target of phenethyl isothiocyanate in Jurkat T lymphocytes [28]. Furthermore, Onodera and colleagues [12] reported earlier that treatment of RAW 267.4 cells with either 1 or 10 μg/ml MIF for 3 h increased their phagocytosis index for latex beads. These observations are in apparent conflict with the above findings that two isothiocyanates (SF and BITC) that inactivate MIF both enhance phagocytosis in these cells by mechanisms that may involve the participation of MIF. This paradox led us to undertake the following experiments.
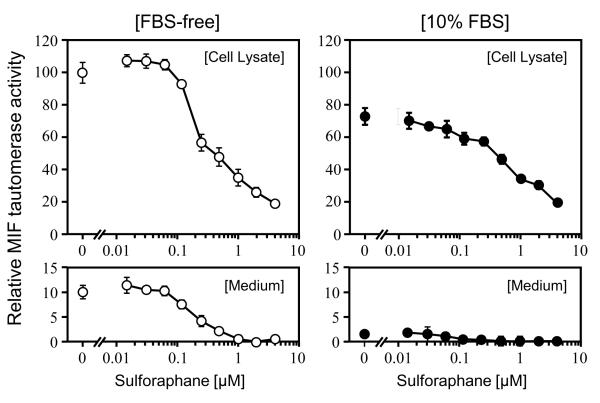
RAW 264.7 cells were treated for 24 h with a range of concentrations of SF (0 - 4 μM) in the medium containing either 0 or 10% FBS, and the dopachrome tautomerase activity was measured in the lysates of washed cells and in the culture medium. These activities were both normalized to the proliferation ability of the cells under similar treatment (Fig. 3). The presence of 10% FBS reduced MIF tautomerase activity levels in both compartments. Furthermore, addition of SF also lowered the MIF tautomerase activity in a concentration-dependent manner in these compartments. Thus, the presence of either SF or FBS suppresses MIF enzymatic activity levels. In contrast, as shown above, FBS depresses and SF enhances phagocytosis in RAW 264.7 cells.
Fig. 3.
Inactivation of MIF by sulforaphane treatment in RAW 264.7 cells. Relative MIF tautomerase activity of cell lysates (upper boxes) and medium (lower boxes) incubated with serial dilutions of SF for 24 h in the medium in the absence (○) and presence (●) of 10% FBS are expressed as means ± SD (n = 6).
We therefore examined the effect on phagocytosis of the direct treatment of RAW 264.7 cells with recombinant mouse MIF (2, 10, 50 ng/ml) for 24 h in serum-free medium (Fig. 4). This treatment dramatically suppressed the phagocytosis index in proportion to MIF concentration. However, if recombinant MIF was first treated with SF to abolish its tautomerase activity completely, and the excess SF was removed, these preparations no longer stimulated phagocytosis (Fig.4). This phenomenon was abolished in the presence of 10% FBS. We therefore conclude that at least part of the stimulating activity of SF on phagocytosis is mediated by its modification of MIF (abolition of tautomerase activity; conformational changes).
Fig. 4.
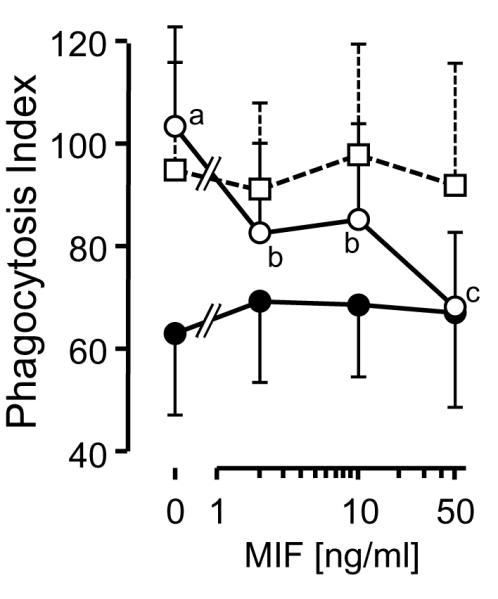
Effect of addition of recombinant mouse MIF on phagocytosis activity of RAW 264.7 cells. PI values of cells treated with intact MIF (○) and sulforaphane-inactivated MIF (□) in serum-free medium and intact MIF in the medium containing 10% FBS (●) for 24 h are shown as means ± SD (number of fields counted n = 28-30). Points not sharing common letters are significantly different from each other (p < 0.05, ANOVA with Scheffé’s test).
Although the same types of RAW 267.4 macrophage-like cells were used in our and Onodera’s experiments, contradictory effects of MIF on phagocytosis were observed in the two systems: inhibition in our, and stimulation in Onodera’s system. However, the experimental systems were quite different with respect to the FBS concentration of the medium (0% and 10%); the types and sizes of beads used for quantifying phagocytosis (2.0 μm polystyrene and 0.75 μm latex beads); antibody (polystyrene beads were coated with IgG, latex beads were uncoated); time of exposure to MIF (24 h and 48 h) and to beads (10 min and 120 min); and ratio of bead number to phagocytic cells.
Conclusion
The present study demonstrates that SF stimulates the phagocytosis of RAW 264.7 macrophages in culture in the absence, or at low concentrations of fetal bovine serum. This effect does not require Nrf2-dependent induction of phase 2 cytoprotective genes and may involve inactivation of MIF.
Acknowledgments
This work was carried out while H.S. was a Kagome Visiting Scholar at the Johns Hopkins University School of Medicine. These studies were supported by grants from: NIH (R01 CA094076), the Lewis B. and Dorothy Cullman Foundation, the W. Patrick McMullan III Family Fund, and the American Institute for Cancer Research, Washington DC. We thank Thomas W. Kensler for the nrf2−/− mice, and Michael J. Caterina and Tiffany M. Link for instruction on quantifying phagocytosis, and Albena Dinkova-Kostova for helpful and stimulating discussion during the preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc. Natl. Acad. Sci. U S A. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56:5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- [3].Talalay P, Fahey JW, Holtzclaw WD, Prestera T, Zhang Y. Chemoprotection against cancer by phase 2 enzyme induction. Toxicol. Lett. 1995;82:173–179. doi: 10.1016/0378-4274(95)03553-2. [DOI] [PubMed] [Google Scholar]
- [4].Juge N, Mithen RF, Traka M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell. Mol. Life Sci. 2007;64:1105–1127. doi: 10.1007/s00018-007-6484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang Y, Tang L. Discovery and development of sulforaphane as a cancer chemopreventive phytochemical. Acta. Pharmacol. Sin. 2007;28:1343–1354. doi: 10.1111/j.1745-7254.2007.00679.x. [DOI] [PubMed] [Google Scholar]
- [6].Lin W, Wu RT, Wu T, Khor TO, Wang H, Kong AN. Sulforaphane suppressed LPS-induced inflammation in mouse peritoneal macrophages through Nrf2 dependent pathway. Biochem. Pharmacol. 2008;76:967–973. doi: 10.1016/j.bcp.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Liu H, Dinkova-Kostova AT, Talalay P. Coordinate regulation of enzyme markers for inflammation and for protection against oxidants and electrophiles. Proc. Natl. Acad. Sci. U S A. 2008;105:15926–15931. doi: 10.1073/pnas.0808346105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kim HJ, Barajas B, Wang M, Nel AE. Nrf2 activation by sulforaphane restores the age-related decrease of TH1 immunity: Role of dendritic cells. J. Allergy Clin. Immunol. 2008;121:1255–1261. doi: 10.1016/j.jaci.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Malhotra D, Thimmulappa R, Acien A. Navas, Sandford A, Elliott M, Singh A, Chen L, Zhuang X, Hogg J, Pare P, Tuder RM, Biswal S. Decline in Nrf2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am. J. Respir. Crit. Care Med. 2008;178:592–604. doi: 10.1164/rccm.200803-380OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [10].Ritz SA, Wan J, Diaz-Sanchez D. Sulforaphane-stimulated phase II enzyme induction inhibits cytokine production by airway epithelial cells stimulated with diesel extract. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;292:33–39. doi: 10.1152/ajplung.00170.2006. [DOI] [PubMed] [Google Scholar]
- [11].Cooke G, Armstrong ME, Donnelly SC. Macrophage migration inhibitory factor (MIF), enzymatic activity and the inflammatory response. Bio Factors. 2009;35:165–168. doi: 10.1002/biof.27. [DOI] [PubMed] [Google Scholar]
- [12].Onodera S, Suzuki K, Matsuno T, Kaneda K, Takagi M, Nishihira J. Macrophage migration inhibitory factor induces phagocytosis of foreign particles by macrophages in autocrine and paracrine fashion. Immunol. 1997;92:131–137. doi: 10.1046/j.1365-2567.1997.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dinkova-Kostova AT, Liby KT, Stephenson KK, Holtzclaw WD, Gao X, Suh N, Williams C, Risingsong R, Honda T, Gribble GW, Sporn MB, Talalay P. Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc. Natl. Acad. Sci. U S A. 2005;102:4584–4589. doi: 10.1073/pnas.0500815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- [15].Link TM, Park U, Vonakis BM, Raben DM, Soloski MJ, Caterina MJ. TRPV has a pivotal role in macrophage particle binding and phagocytosis. Nat. Immunol. 2010;11:232–239. doi: 10.1038/ni.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fahey JW, Dinkova-Kostova AT, Stephenson KK, Talalay P. The “Prochaska” microtiter plate bioassay for inducers of NQO1. Methods Enzymol. 2004;382:243–258. doi: 10.1016/S0076-6879(04)82014-7. [DOI] [PubMed] [Google Scholar]
- [17].Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- [18].Rosengren E, Bucala R, Aman P, Jacobsson L, Odh G, Metz CN, Rorsman H. The immunoregulatory mediator macrophage migration inhibitory factor (MIF) catalyzes a tautomerization reaction. Mol. Med. 1996;2:143–149. [PMC free article] [PubMed] [Google Scholar]
- [19].Wei J, Sun Z, Chen Q, Gu J. Serum deprivation induced apoptosis in macrophage is mediated by autocrine secretion of type I IFNs. Apoptosis. 2006;11:545–554. doi: 10.1007/s10495-006-5146-7. [DOI] [PubMed] [Google Scholar]
- [20].Chin BY, Petrache I, Choi AMK, Choi ME. Transforming growth factor β1 rescues serum deprivation-induced apoptosis via the mitogen-activated protein kinase (MAPK) pathway in macrophage. J. Biol. Chem. 1999;274:11362–11368. doi: 10.1074/jbc.274.16.11362. [DOI] [PubMed] [Google Scholar]
- [21].Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. U S A. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- [23].Dinkova-Kostova AT, Talalay P. NAD(P)H: quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch. Biochem. Biophys. 2010;501:116–123. doi: 10.1016/j.abb.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Shin S, Wakabayashi N, Misra V, Biswal S, Lee GH, Agoston ES, Yamamoto M, Kensler TW. NRF2 modulates aryl hydrocarbon receptor signaling: Influence on adipogenesis. Mol. Cell. Biol. 2007;27:7188–7197. doi: 10.1128/MCB.00915-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhäuser C. Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J. Biol. Chem. 2001;276:32008–32015. doi: 10.1074/jbc.M104794200. [DOI] [PubMed] [Google Scholar]
- [26].Faraonio R, Vergara P, Di Marzo D, Pierantoni MG, Napolitano M, Russo T, Cimino F. p53 suppresses the Nrf2-dependent transcription of antioxidant response genes. J. Biol. Chem. 2006;281:39776–39784. doi: 10.1074/jbc.M605707200. [DOI] [PubMed] [Google Scholar]
- [27].Wakabayashi N, Shin S, Slocum SL, Agoston ES, Wakabayashi J, Kwak M-K, Misra V, Biswal S, Yamamoto M, Kensler TW. Regulation of Notch1 signaling by Nrf2: Implications for tissue regeneration. Science Signal. 2010;3:ra52. doi: 10.1126/scisignal.2000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Brown KK, Blaikie FH, Smith RAJ, Tyndall JDA, Lue H, Bernhagen J, Winterbourn CC, Hampton MB. Direct modification of the proinflammatory cytokine macrophage migration inhibitory factor by dietary isothiocyanates. J. Biol. Chem. 2009;284:32423–32433. doi: 10.1074/jbc.M109.047092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ouertatani-Sakouhi H, El-Turk F, Fauvet B, Roger T, Le Roy D, Karpinar DP, Leng L, Bucala R, Zweckstetter M, Calandra T, Lashuel HA. A new class of isothiocyanate-based irreversible inhibitors of macrophage migration inhibitory factor. Biochemistry. 2009;48:9858–9870. doi: 10.1021/bi900957e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cross JV, Rady JM, Foss FW, Lyons CE, Macdonald TL, Templeton DJ. Nutrient isothiocyanates covalently modify and inhibit the inflammatory cytokine macrophage migration inhibitory factor (MIF) Biochem. J. 2009;423:315–321. doi: 10.1042/BJ20091170. [DOI] [PMC free article] [PubMed] [Google Scholar]