Abstract
Age-dependent changes in the cellular immune response have been mainly described in CD8+ T cells, with relative sparing of CD4+ T cells. We show that in older compared to young adults, effector memory and effector CD8+ T-cell subsets responding to influenza A/H3N2 challenge have diminished cytolytic activity. In contrast, effector CD4+ T-cell subsets in older adults share similar phenotypic and functional characteristics with those from young adults. Further, we observed a diminished cytolytic T-cell response to both seasonal influenza A/H3N2 and pandemic H1N1 (pH1N1) strains in older compared to young adults who had received seasonal influenza vaccine. These results are consistent with the observed rates of serious complications from seasonal and pandemic influenza infections in different age groups, and suggest that CD4+ T cells may provide a compensatory response to influenza infection when CD8+ T cells become compromised during the aging process.
1. Introduction
Thymic involution and a decline in T cell output with advancing age, together with a lifetime of exposure to a variety of pathogens, leads to a dramatic reduction in the naïve T cell pool and a relative increase in the proportion of memory T cells. The most dramatic functional changes occur in the CD8+ T cell subset, where progressive exhaustion of this compartment leads to terminal differentiation of the memory T cells responding to a particular pathogen [1]. Influenza, particularly A/H3N2 strains, is foremost among all infectious diseases in the aged-related increase in risk for serious complications and death [2, 3]. Although influenza vaccination is the first and most important step in protecting against influenza, the underlying mechanism for the well-documented association between the age-related decline in T cell function, and increased susceptibility to serious influenza illness and loss of influenza vaccine efficacy in older adults, is poorly understood and requires further study.
The current pandemic influenza A/H1N1 virus (pH1N1) is a novel influenza virus strain which spread from Mexico to the United States in the spring of 2009 and circulated through the early winter [4]. Lower attack rates during the pH1N1 pandemic among the population age 65 years and older [5], were attributed to pre-existing antibodies from exposure to the A/H1N1 strains that circulated in the years following the 1918 influenza pandemic. However, this population remains the highest risk group for serious complications and even death from seasonal A/H3N2 and pandemic H1N1 influenza infections, particularly those with high-risk conditions [6]. These observations suggest that T-cell mediated clinical protection against complicated influenza illness is important when antibodies fail to provide sterilizing immunity against infection.
Our previous studies in vaccinated older adults have shown that measures of cell-mediated immunity are important indicators of clinical protection against illness from an influenza infection [7, 8]. These results are consistent with earlier findings showing that cytotoxic T lymphocytes (CTL) are important for recovery from influenza infection [9]. Granzyme B (GzmB) has been shown to be a key cytolytic mediator of killing of influenza-infected host cells [10-12]. Low levels of GzmB activity are associated with increased risk of influenza illness in older adults but in contrast to young adults, GzmB appears to be non-specifically expressed within a subset of CD8+ T cells in older adults [7, 8]. Thus, the identification of the phenotype of T cells that provide an influenza virus-specific cytolytic response is important for a mechanistic understanding of the correlates of clinical protection against influenza illness in the over 65 population.
As the pool of naïve and early memory T-cells declines with aging, T cells with a naïve phenotype (CD45RA+) exhibit numerous functional defects, mainly their reduced ability to expand and differentiate into virus-specific effector cells [13]. While these changes have been well documented in a number of experimental models of human PBMC, their impact on the response to live influenza virus challenge has yet to be studied. The purpose of this study was to elucidate age-related changes in the cytolytic T cell response to both influenza A/H3N2 and pH1N1 virus within the memory and effector subsets of CD4+ and CD8+ T cells. Our data show that in older adults, cytolytic activity against influenza-infected targets is severely compromised in CD8+ T cells but preserved in CD4+ T cell subsets when compared to young adults. Further, A/H3N2 strains elicit a greater cytolytic response than pH1N1 in all age groups studied and is consistent with the observed increase in serious complication rates of pH1N1 infection. These results suggest that CD4+ T cells may provide a compensatory response to influenza as a potential mechanism of protection against influenza illness when CD8+ T cells become dysfunctional in older adults.
2. Materials and Methods
2.1. Study design and participants
Fifteen young (20-25 years old) and older adults (60-70 years old or >80 years old) from the vicinity of the Greater Hartford Area of Connecticut were studied pre-vaccination and 4-weeks and 10-weeks post-vaccination in the fall and winter of 2008-2009. All subjects were recruited through written informed consent. The Institutional Review Board of the University of Connecticut Health Center approved the protocol and informed consent document. Subjects were excluded for an acute respiratory illness in the 2 weeks preceding study enrolment, insulin-requiring diabetes, any conditions or medication causing immunosuppression such as prednisone >10mg/day, or any contraindications to influenza vaccination.
2.2. Cell culture and virus stimulation
Human PBMC were isolated from venous blood samples by Histopaque gradient purification and stimulated in 0.5 ml of AIM V media (GIBCO) containing 1.0×106 PBMC and 4×106 TCID50 of influenza A/Victoria/3/75 (H3N2) virus as previously described [7] or stimulated with pandemic influenza A/TN/1-560-09 (pH1N1) virus (Generous gift from Dr. Richard J. Webby's lab, St.Jude Children's Research Hostpital, Memphis). For proliferation and/or cytotoxity assays, cells were stimulated with influenza virus 5 days or 5 μg/ml phytohemagglutinin (PHA) for 6 hr and cultured in AIM V medium containing 10% Human AB serum (Sigma) (R&D Systems).
2.3. Flow cytometry and antibodies
To determine the phenotype of T cells responding to influenza challenge, PBMC were prepared and stained as previously described [8]. Briefly, human anti-CD107a-PE (H4A3) was added to the PBMC/virus cultures, incubated for 12 hours, then the cells were washed with cold 0.2% FBS/PBS twice before adding the following conjugated monoclonal antibodies: Human anti-CD8-FITC (OKT8) and anti-CD25-APC-Cy7 (eBioscience), anti-CD127-Biotin/PE-Texas Red and anti-CCR7 PE-Cy7 (3D12) (BD Pharmingen), anti-CD4 Alex Fluor 700 (OKT4) and anti-CD45RA Pacific Blue/or eFluor 450 (HI100) (eBioscience). For intracellular staining, cells were fixed with 2% paraformaldyhyde and permeabilized with permeabilization buffer (eBioscience), then incubated with anti-GzmB Alex Fluor 647 (GB11) or anti-Perforin PE (δG9) (BD Pharmingen) antibodies. For cell sorting, human PBMC were prepared as above and sorted by using FACS Vantage SE/DIVA High Speed Sorter (BD Biosciences) running DIVA 5.0 Software. Data were acquired on the BD LSR II, and analysis using FlowJo 8.8.2 software.
2.4. Cytotoxicity Assay
Human PBMC were prepared and cultured in AIM V medium containing 10% human AB serum (Sigma) with influenza A/Victoria/3/75 or A/TN/1-560-09 (pH1N1) virus stimulation for 5-6 days. CD4+ and CD8+ T cell were selected by bead separation (Dyna Beads) and sorted into the different memory/effector subsets before the assay. PHA-activated autologous lymphoblast target cells were infected and cultured with influenza H3N2 or pH1N1 virus for 30 min and added into V-bottom 96-well plate at the dilution of 104 cells/100 μl per well in triplicate before adding sorted effector T-cell subsets at E/T (effector/target) ratios of 10:1 and 5:1; co-cultures were incubated for 4hr at 37′C [14, 15]. The lactate dehydrogenase (LDH) activity released in the cell free supernatants was determined using the CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega, Madison, WI).
2.5. Proliferation Assay
The proliferative response to influenza was assessed by the MTT Cell Proliferation Assay (ATCC), based on the reduction of MTT (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) by mitochondrial dehydrogenase of intact cells to formazan (MTT metabolic product). Briefly, human PBMC were stimulated with influenza virus for 5-6 days in AIM V medium containing 10% human AB serum. Sorted CD4+ and CD8+ T cell subsets were added to a flat bottom 96-well microtiter plate (1× 105 cells/100μl per well) with 10 μl of MTT reagent, incubated for 3-4 hr, and then 100 μl of Detergent Reagent added and incubated in the dark for 4 hr at RT. The amount of formazan produced (A570) was used to quantify the proliferative response.
2.6. Statistical analysis
Unpaired t tests were used to compare the two groups for the different CD4+ and CD8+ T-cell subsets. Analyses were performed using Origin Pro 8.0 (OriginLab, MA, www.OriginLab.com). P values <0.05 were considered statistically significant.
3. Results
3.1. A phenotype shift in T cell subsets in vaccinated older compared to young adults
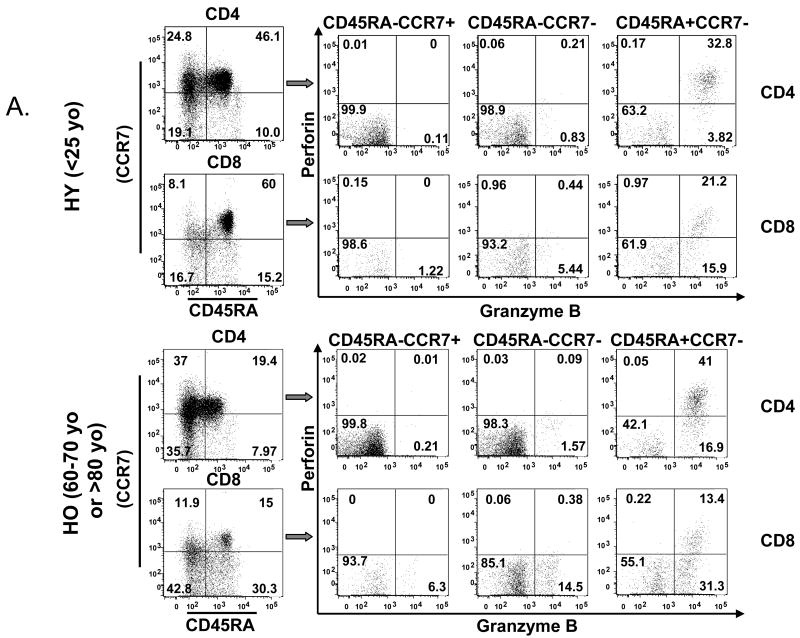
To determine the effect of age on the phenotype of naïve, memory and effector T lymphocytes responding to influenza challenge, PBMC from three age groups: 20-25 years old (yo), 60-70 yo, and >80 yo, were stimulated for 20 hours with live influenza A/H3N2 virus and analyzed by flow cytometry according to CD45RA+CCR7+ (naïve), CD45RA-CCR7+ (central memory), CD45RA-CCR7- (effector memory) and CD45RA+CCR7- (effector) subsets (Fig. 1A) [16]. Consistent with contraction of the peripheral naïve T cell pool and the effect of aging on CD4+ and CD8+ T cells [17], there was a significant decline with age in the proportion of naïve (CD45RA+CCR7+) CD4+ and CD8+ T cells (Fig. 1A, 1B, 1C, p<0.0001). The reciprocal increase in the proportion of different memory and effector subsets varied across the CD4+ and CD8+ T cells subsets.
Figure 1.
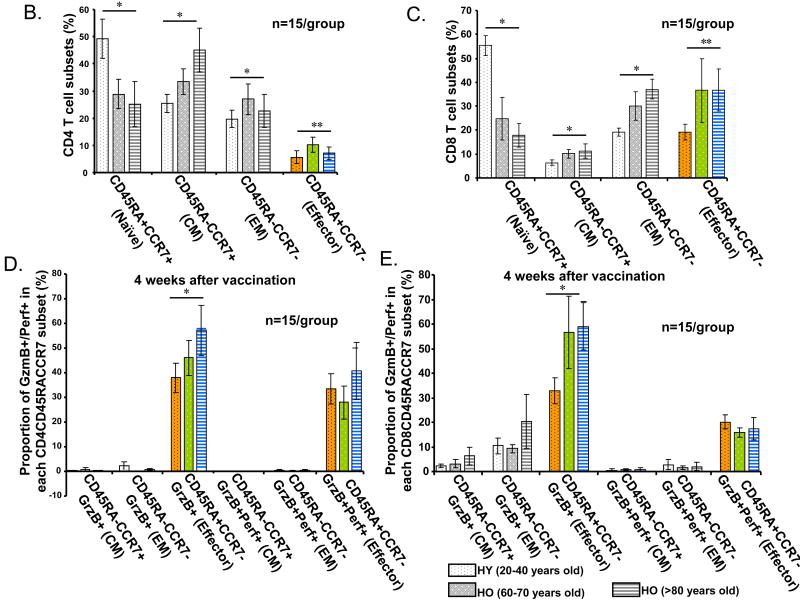
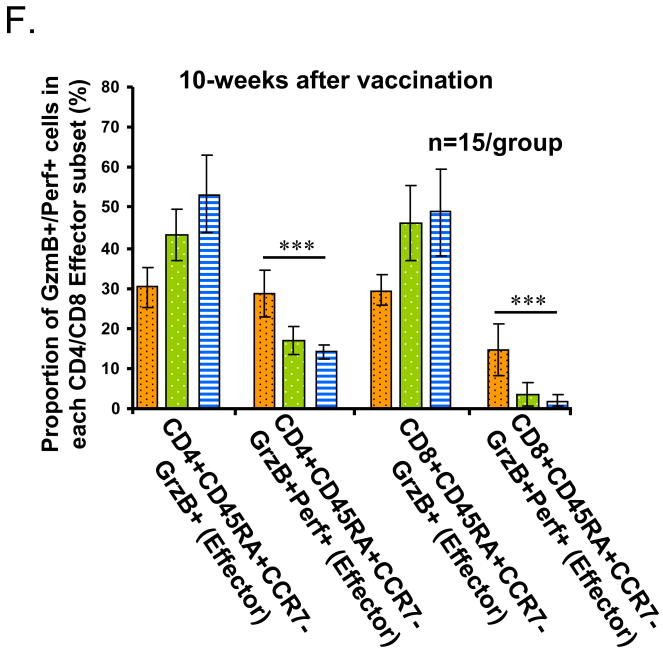
The phenotype shift and the dual expression of GzmB and Perf delineates age-related changes in different T cell subsets. PBMCs from healthy young (HY, light or yellow bars) and older (HO, 60-70 years old, dark or green bars, and HO, >80 years old, striped bars, grey or blue) adults (n=15/group) obtained at 4 or 10 weeks post vaccination were cultured with live influenza virus for 20 hr. (A) CD4+ and CD8+ subsets were defined by the expression of cell surface molecules, CD45RA and CCR7, and their intracellular cytolytic effector molecules, GzmB and Perf. The percentage of cells within each of the T-cell subsets: CD45RA+CCR7+ (Naïve), CD45RA-CCR7+ (central memory, CM), CD45RA-CCR7- (effector memory, EM), and CD45RA+CCR7- (Effector) are shown. The proportion of naïve T cells decline with age while different memory and effector subsets increase with age in (B) CD4+ and (C) CD8+ subsets. The proportion of effector T cells that are GrzB+ increase with age in both CD4+ and CD8+ subsets. In contrast, the proportion of GzmB+Perf+ effector T cells is similar between age groups in both (D) CD4+, and (E) CD8+ subsets at 4 weeks post-vaccination but declined with age by (F) 10 weeks post vaccination in both CD4+ and CD8+ T cell subsets. Error bars represent standard error of the mean. Significant differences in the means between age groups are shown as *p<0.0001, **p=0.001, and ***p<0.001.
In CD4+ T cells, the proportion of central memory T cells increased with age (Fig. 1B, p<0.0001) but in the older adult groups, there was an associated with a significant decline in the proportion of effector memory (CD45RA-CCR7-) and effector (CD45RA+CCR7-) CD4+ T cells in the >80 yo age group relative to the 60-70 yo age group (Fig. 1B). In contrast, CD8+ T cell subsets (Fig. 1C) showed an age-related increase in the proportion of central memory (p<0.0001), effector memory (p<0.0001) and effector (p=0.001) T cells. These results show that contraction of the naïve T cell pool with aging is associated with CD4+ T cell expansion mainly in the central memory subset. In contrast, CD8+ memory T cells are more differentiated to effector memory and effector T cells, which is consistent with aging having its greatest impact on the CD8+ T cell subset.
3.2. Effect of age on effector T cells expressing GzmB and perforin
The next experiments evaluated the effect of age on the cytolytic effector function of different T-cell subsets. Previous studies have shown that perforin (Perf) and GzmB are key cytolytic effector molecules, which are stored in the granules of cytolytic T cells [18, 19]. Upon T cell receptor binding to the peptide-MHC I complex, granules containing granzymes and Perf migrate to the cell surface of and are released from the CTL. Perf facilitates the entry of GzmB into virus-infected host cells to cause apoptotic cell death, and thus is necessary for effective cytolytic activity. Thus, we analyzed the intracellular expression of effector molecules, GzmB and Perf, in different CD4+ or CD8+ T-cell subsets (Fig. 1A) to estimate their cytolytic potential in response to live influenza virus.
In CD4+ T cells, GzmB was expressed only in the effector (CD45RA+CCR7-) subset (Fig. 1D), while in CD8+ T cells, GzmB was expressed in a subset of all memory and effector cells (Fig. 1E) as has been previously shown [20]. The proportion of GzmB+ cells increased with age in these virus-stimulated CD4+ and CD8+ T cells subsets (Fig. 1D, 1E) but the proportion was greater in CD8+ T cells and there was a plateau with no further increase from the 60-70 yo to the >80 yo age group (Fig. 1D, 1E). However, our previous results showed that a large proportion of CD45RA+CD8+ T cells from older adults express GzmB at baseline and these GzmB+CD8+ T cells respond poorly to virus stimulation [8], consistent with a terminally differentiated phenotype [1]. To further distinguish the phenotype of cytolytic T cells responding to influenza virus, we evaluated these T cell subsets for the co-expression of GzmB and Perf in influenza-stimulated PBMC. A response to vaccination was detected but the increase in the proportions of GzmB+Perf+ T cells in CD4+ and CD8+ subsets was not statistically significant due to the high variability in the proportions of GzmB+Perf+ T cells in pre-vaccination samples (Supplemental Fig. 1). At 4-weeks post-vaccination, the proportion of effector CD4+ T cells that co-expressed GzmB and Perf was similar across the three age groups and to the proportion of GzmB+ effector CD4+ T cells in young adults (Fig. 1D).
In contrast, the CD8+ T cell subset showed a statistically significant reduction in the proportion of effector T cells that were GzmB+Perf+ compared to GzmB+ alone (Fig. 1E, p<0.0001). While young and older adults had similar proportions of GzmB+Perf+ effector CD8+ T cells at 4-weeks post-vaccination, there was a significant age-related decline in the proportion of GzmB+Perf+ effector T cells by 10-weeks post-vaccination (Fig. 1F), which was more marked in the CD8+ T cells compared to CD4+ T cells. These results show an age-related shift in the relative proportion of GzmB+Perf+ effector T cells responding to influenza challenge within the CD4+ and CD8+ subsets such that CD4+ T cells are the main source of effector T cells by 10-weeks post-vaccination in older adults. Since the influenza season usually occurs after 10-weeks post-vaccination, these data suggest that this decline in the effector T cell response to influenza challenge is clinically important with respect to serious outcomes of influenza infection [21].
3.3. Degranulation markers of cytolytic effector function in response to virus stimulation
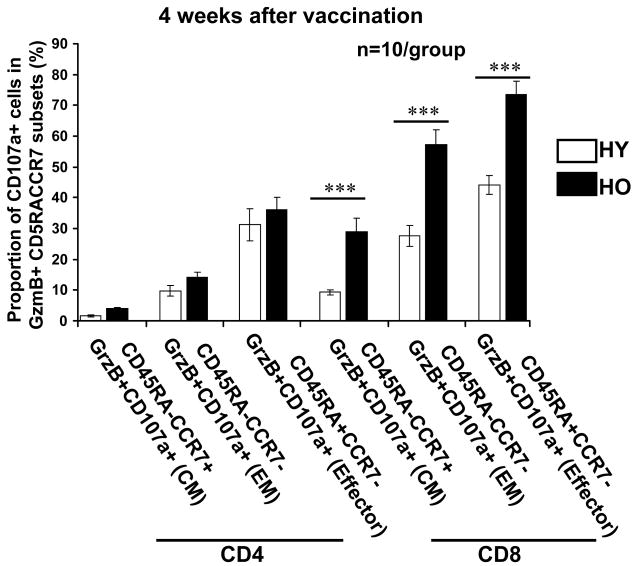
Our previous experiments showed that older adults (mainly those with congestive heart failure) who showed an increased proportion of GzmB+CD8+ T cells expressing the degranulation marker, CD107a, at baseline, mounted a poor response to influenza virus stimulation [8, 22]. In contrast, healthy older compared to young adults showed an increased proportion of GzmB+CD107a+CD8+ T cells in the response to influenza virus. Thus, we were interested in whether the relative changes in GzmB+Perf+ T cells within the CD4+ and CD8+ subsets of influenza-stimulated PBMC could be detected using CD107a. As in our previous study, healthy young and older adults showed similar proportions of CD8+ T cells (and CD4+ T cells in this study) that were CD107a+ after 12 hours of virus stimulation. Thus, we were interested in measuring degranulating activity in the response to live influenza virus (12-hour stimulation) within each of the memory and effector T-cell subsets using cell surface expression of CD107a (Fig. 2). Young and older adult PBMC showed similar proportions of degranulating cells (CD107a+) within the memory and effector GzmB+CD4+ T-cell subsets (Fig. 2). However, older compared to young adults showed a significant increase in the proportion of GzmB+CD8+ T cells expressing CD107a; the increase was >20% in the central memory and >30% in effector memory and effector subsets (p<0.001 for all comparisons) (Fig. 2). These results suggest that a larger proportion of CD8+ T cells were activated to mount a cytolytic response to influenza virus challenge. A functional assay of cytolytic activity was therefore conducted to confirm these results.
Figure 2.
Degranulating activity in GzmB+ T-cells responding to influenza virus is similar in young (HY) and older (HO, >65 years old) adults. Human PBMCs (n=10/group) obtained 10-weeks post-vaccination were cultured for 12 hr in the presence of influenza A/H3N2 live virus. Graphs show the proportion of cells expressing the degranulating marker, CD107a, was similar in the CD4+ and CD8+ T cells from young and older adults. The proportion of CD107a+GzmB+ in the different memory and effector CD4+ T cell subsets was similar in the two age groups but significantly increased in CD8+ T cells from older adults (dark bars) compared to young adults (white bars), ***p<0.001. Error bars represent standard error of the mean.
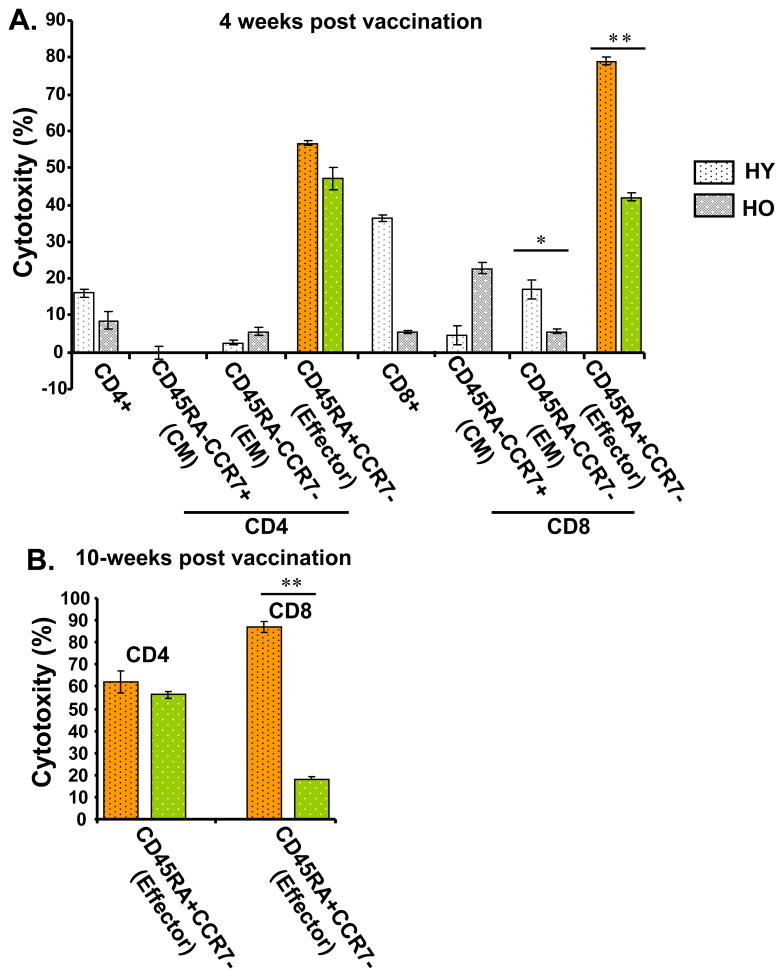
3.4. Cytolytic activity in virus-specific CD4+and CD8+ effector T-cell subsets
To further investigate the cytolytic function of different T-cell subsets responding to influenza virus, cytotoxicity assays were performed. CD4+ or CD8+ T cells were stimulated with H3N2, sorted into the different memory and effector T cells subsets, and then used as effector cells (E) in cytotoxicity assays with PHA-stimulated, autologous PBMCs infected with influenza virus as target cells (T) (Fig. 3). Results are reported as % specific lysis at an E:T ratio = 10:1. There was no cytolytic activity detected in unstimulated PBMC. In virus-stimulated CD4+ T cells from young and older (60-75 years old) adults, we found similar levels of cytolytic activity in the effector (CD45RA+CCR7-) and effector memory (CD45RA-CCR7-) subsets at 4 weeks (Fig. 3A) and 10-weeks (Fig. 3B) post-vaccination. In contrast, young compared to older adults exhibited significantly higher cytolytic activity in CD8+ T cells within the effector memory (p<0.01) and effector (p<0.005) subsets at 4-weeks post-vaccination (Fig. 3A). By 10 weeks post-vaccination, there was a significant further decline in influenza virus-specific cytolytic activity of effector CD8+ T cells in older compared to young adults (Fig. 3B, p<0.005). A corresponding diminished proliferative response in older compared to young adults after five days of stimulation with the A/H3N2 strain was demonstrated in the CD8+ effector T cell subset (Supplemental Fig. 2).
Figure 3.
Effector CD4+ T cells in older adults (HO, >65 years old, dark or green bars) responding to influenza virus demonstrate comparable cytolytic activity compared to young adults (HY, light or yellow bars), but diminished cytolytic activity in the CD8+ T-cell subset (n=6/group) at (A) 4-weeks and (B) 10-weeks post-vaccination. PBMCs from young and older adults were prepared as effector or target cells for assays of cytotoxicity. Effector (E) CD4+ and CD8+ T cells (prepared by bead selection) were stimulated with influenza A/H3N2 live virus for 5-6 days, then sorted into central memory (CM), effector memory (EM), and Effector T-cell subsets. Autologous target (T) cells were prepared by PHA stimulation of PBMC and then infected with influenza A/H3N2 virus. Cytotoxicity was calculated as mean % specific lysis for E:T ratios of 10:1. Results are shown for each of the CD4+ and CD8+ subsets, a differences between age groups shown as *p<0.01, **p<0.005. Error bars represent standard error of the mean.
Taken together, these results suggest that a split (killed) virus vaccine stimulates a more sustained memory T cell response in the CD4+ relative to the CD8+ subset. While similar proportions of GzmB+Perf+ effector (CD45RA+CCR7-) T cells can be stimulated in both CD4+ and CD8+ T cell subsets in young and older adults at 4-weeks post-vaccination, there is a decline in this effector CD8+ T cell subset by 10-weeks post-vaccination in older adults. In addition, significantly lower levels of cytolytic activity in effector CD8+ T cells in older compared to young adults at 4-weeks post-vaccination, and a further reduction in older adults by 10 weeks post-vaccination are evidence of functional defects in the CD8+ T cell response. In contrast, cytolytic activity in CD4+ effector T cells was similar in young and older adults and sustained from 4-weeks to 10-weeks post-vaccination in effector CD4+ T cells; although the frequency of this T cell subset declined by 10-weeks post-vaccination in older adults but was not as marked as in the CD8+ T cell subset. Overall, a more sustained memory CD4+ T cell response was elicited by influenza vaccination in older adults while the response to vaccination was sustained in both CD4+ and CD8+ subsets in young adults.
3.4. Phenotype of virus-specific cytotoxic T cells in vaccinated young and older adults
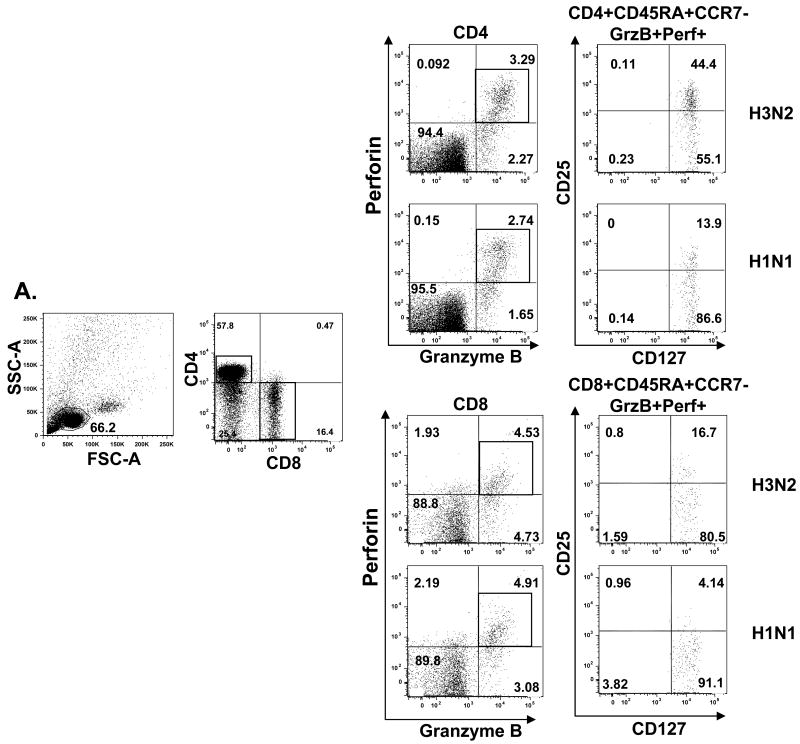
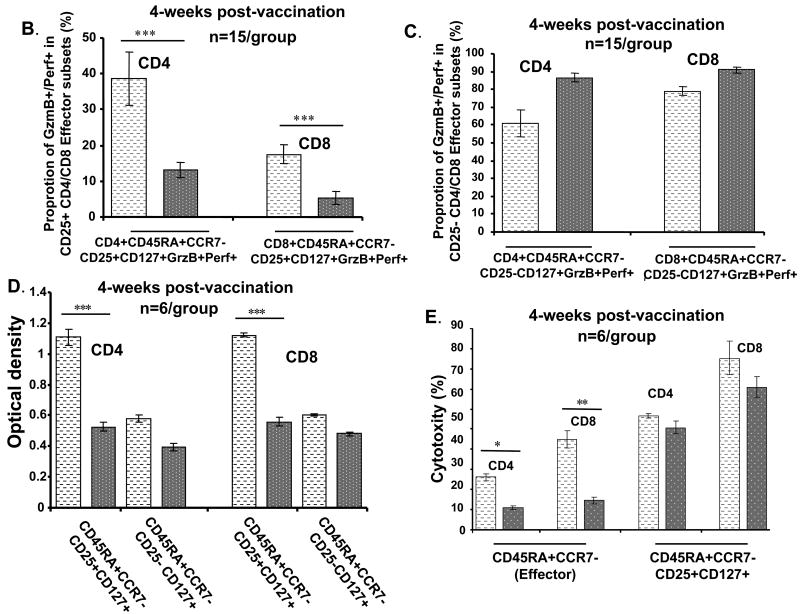
In this study, we distinguished the T-cells subsets and evaluated their effector function based on T-cell surface markers CD45RA and CCR7 as well as effector molecules, GzmB and Perf, and related cytotolytic activity. We found that effector T cells that become GzmB+Perf+ in response to influenza challenge, are the T cells with high cytolytic activity against influenza-infected targets. We were next interested in further characterizing the phenotype of the GzmB+Perf+ T cells responding to influenza stimulation. Since the expression of IL-7 receptor alpha (IL-7Rα) can distinguish functional subsets of CD8+ T cells specific for different respiratory viruses in humans [23], we used antibody to CD127 (IL-7Rα) in this analysis. In our study, most of the effector T cells (GzmB+Perf+) expressed CD127 and could be further separated into two groups based on expression of the surface marker, CD25 (IL-2 receptor) (Fig. 4A). The CD25+ effector T-cell subset we identified has the phenotype, CD45RA+CCR7-CD25+CD127+, which is consistent with high proliferative capacity in response to A/H3N2 compared to pH1N1 in the CD25+ subset, and to both strains in the CD25-subset (Fig. 4D). Comparable levels of cytolytic activity on a per cell basis were generated in the CD25+ subset in response to H3N2 and pH1N1 strains (Fig. 4E), whereas the CD25- subset had ∼50% of the cytolytic activity in the CD25+ subset (data not shown).
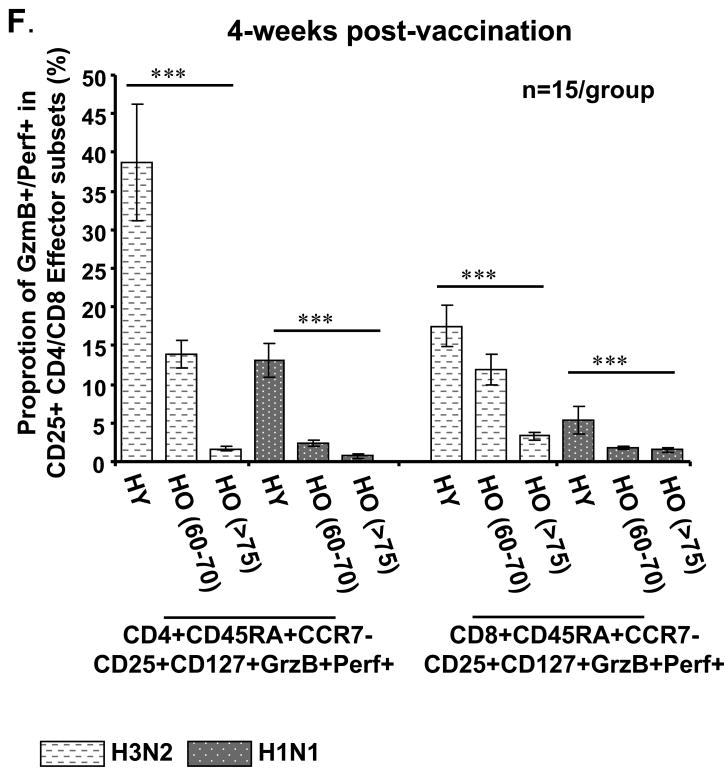
Figure 4.
Comparison of the cytotoxic effector T-cell response to seasonal A/H3N2 and pandemic influenza H1N1 (pH1N1) virus in young and older adults who received seasonal influenza split-virus vaccine. Human PBMCs from healthy young (HY) and healthy old (HO, 60-70 yo or >80 yo) (n=15/group) obtained at 4 weeks post vaccination were cultured with live influenza virus A/H3N2 (light bars) or pH1N1 (dark bars) for 5-6 days. (A) In HY, CD4+ and CD8+ subsets that were GzmB+Perf+ were defined by the expression of cell surface molecules, CD45RA, CCR7, CD25, CD127. Differences in the response to pH1N1 vs. A/H3N2 are shown as ***p<0.001, *p<0.01, and **p<0.005 in (B) through (F). The effector (CD45RA+CCR7+CD127+) subsets of CD4+ and CD8+ for (B) CD25+ and (C) CD25- T cells are shown for HY. The proportion of effector T cells in CD25+ subset was significantly lower in pH1N1 compared to A/H3N2-stimulated PMBC for both CD4+ and CD8+ T cells. (D) PBMCs from HY were stimulated with influenza A/H3N2 or pH1N1 live virus for 5-6 days, then CD4+ or CD8+ CD45RA+CCR7-CD25+CD127+ and CD45RA+CCR7-CD25-CD127+ T-cell subsets were sorted, and proliferation assays were performed. The proliferative response was significantly higher in the CD25+ compared to the CD25- in the CD4+ and CD8+ effector T cells subsets. (E) PBMCs from young adults were stimulated with live virus for 5 days, sorted and prepared as effectors (E), or stimulated with PHA for autologous targets (T) for assays of cytotoxicity as in Fig. 3 (n=6/group). Cytotoxicity was calculated as mean % specific lysis for E:T ratios of 5:1. (F) PBMCs from HY and HO, 60-70 yo and >75 yo, were stimulated for 5-6 days with A/H3N2 or pH1N1 and the effector (CD45RA+CCR7+CD25+CD127+) T cell response compared across the different aged groups and stimulating viruses. An age-related decline in both CD4+ and CD8+ effector T cell responses and diminished response to pH1N1 vs. A/H3N2 was observed. Error bars represent standard error of the mean.
3.5. Effector T cell response to A/H3N2 vs. pH1N1 after seasonal influenza vaccination
In this study, we were also interested in comparing the proliferative and cytolytic T cell response to A/H3N2 and pH1N1 within the effector T-cell subset (CD45RA+CCR7-). Since A/H3N2, A/H1N1, and pH1N1 strains share some T cell epitopes within the internal proteins derived from the virus [20], seasonal vaccine could stimulate a T cell response to A/H3N2 and pH1N1 strains. These experiments specifically focused on the phenotype of the effector T-cell subset after 20 hours of virus stimulation, and the proliferative and CTL response after in vitro stimulation (5-6 days) with live influenza virus. No changes in the T cell phenotypes were observed from 20 hours to 5-6 days of in vitro stimulation. All CD45RA+CCR7- GzmB+Perf+ T cells were CD127+ but there was a significantly lower proportion of effector T cells that were CD25+ in pH1N1 vs. A/H3N2-stimulated PBMC (p<0.001) (Fig. 4A and 4B, p<0.001), and a corresponding higher proportion of CD25- effector T cells in both CD4+ and CD8+ subsets (Fig. 4C). The lower proliferative response stimulated by the pH1N1 compared to the A/H3N2 strains is demonstrated in the CD25+ subsets of CD4+ and CD8+ effector T cells from young adults (Fig. 4D), and is associated with lower cytolytic activity within the effector (CD45RA+CCR7-) subsets of CD4+ (p<0.01) and CD8+ (p<0.005) T cells (Fig. 4E). The CD25+CD127+ effector subsets of CD4+ or CD8+ T cells from young adults exhibited similar levels of cytolytic activity against A/H3N2 and pH1N1-infected targets following stimulation with live virus (Fig. 4E). The proportion of CD25+CD127+ effector T cells responding to A/H3N2 and pH1N1 strains significantly declined with advancing age group in both the CD4+ (p<0.001) and CD8+ (p<0.001) T-cell subsets (Fig. 4F). Compared to the influenza A/H3N2 strain, there was a very limited CD25+ effector CD8+ T cell response to pH1N1 stimulation in both older age groups with no statistical difference observed between the two subtypes (less than 3% of total effector GzmB+Perf+ T cells) (Fig. 4F).
These results suggest that the main phenotype of CD4+ and CD8+ T cells responding to influenza virus are within the CD45RA+CCR7- CD25+CD127+ subset. In other words, these are the cells that express IL-2R (CD25) and thus have recently undergone proliferation and have the highest level of cytolytic activity. Once these T cells become CD25-, their cytolytic activity declines to approximately 50% of the CD25+ effector T cells suggesting that they are becoming exhausted. The expression of IL-7R (CD127) is consistent with the phenotype of influenza-specific CD8+ T cells in PBMC [24] and presumably the phenotype of T cells responding to dendritic cells or macrophages presenting influenza-derived peptides in PBMC cultures. Activation of these CD8+ T cells in the lungs has been characterized by a reduction in the expression of IL-7R [24]. Similar data on the expression of IL-7R in lung-residing CD4+ T cells has not to our knowledge been published. Our results suggest that the phenotype of cytolytic effectors responding in vitro to influenza is CD45RA+CCR7- CD25+CD127+ in both CD4+ and CD8+ T cell subsets.
4. Discussion
The need for more effective influenza vaccines in the older population is well recognized but there have been significant challenges. The limitations of antibody responses as a sole predictor of vaccine efficacy are increasingly recognized [25]. Our work has shown that serum antibody titers against different influenza strains (measured by the hemagglutination inhibition assay) do not distinguish those older individuals who subsequently develop influenza illness from those who do not [7, 8]. Of particular relevance, the phenomenon of original antigenic sin has been the postulated mechanism for lower attack rates for both pandemic and seasonal H1N1 influenza compared to A/H3N2 strains in older adults. However, the serious complication rates of influenza infection in older adults are similar across the different subtypes of influenza A and thus at a population level, influenza A/H3N2 has had a greater impact in older adults relative to seasonal H1N1 [3] and pandemic H1N1 [26]. Understanding the age-related defects in the cell-mediated immune response, which is cross-reactive between the subtypes of influenza, is of paramount importance to the development of influenza vaccines with enhanced efficacy in the over 65 population.
A multitude of changes in the immune system occur with aging but the specific mechanisms that increase risk for influenza illness and limit the protective effects of vaccination in older adults are poorly understood. The importance of T-cell mediated clinical protection against influenza in older adults is increasingly recognized and has underscored the importance of including cellular immune measures in the assessment of vaccine efficacy in the over 65 population [25, 26]. Age-related changes in T cell responses are associated with a decline in the antibody response to influenza vaccination [27, 28] but mechanistic links have not been made nor have these changes been correlated with protection against influenza. These observations suggest that the traditional role of vaccines in providing antibody-mediated protection against infection or “sterilizing immunity” in young adults, is replaced by T-cell mediated clearance of the virus once infection occurs, thus providing “clinical protection” against disease in older adults.
We have previously shown that GzmB activity in influenza-activated PBMC correlates with protection against influenza illness in older adults and potentially could be used to predict influenza vaccine efficacy in older adults [7, 8]. However, we have also shown that a subset of the CD8+ T cells express GzmB and CD107a in association with increased levels of GzmB activity in resting PBMC from older adults, a subset of T cells and increased GzmB activity that was not seen in young adults. Thus, we were concerned that this age-related change was related to terminal differentiation of T cells that are CD28- and no longer cytolytic [29]. The GzmB+ T cells that we have observed in the absence of virus stimulation may represent CMV-specific CTL that have been identified within both CD4+ [30] and CD8+ subsets [31] of older adults and are sustained at high frequencies in human PBMC. We have identified influenza-specific T cells that are activated in response to virus stimulation, as GzmB+Perf+. Since both CD4+ and CD8+ cytolytic effector T cells specific for influenza have been found in lung tissue [24, 32], we studied these GzmB+Perf+ T cell subsets to determine age-related changes that could impact on the cytolytic defense mechanisms against influenza-infected host cells.
Our results suggest that with aging, there is relative preservation of cytolytic function in CD4+ T cells responding to influenza, while the initial CD8+ T cell response to vaccination rapidly declines by 10-weeks post-vaccination. Previous studies of lung-residing CD4+ and CD8+ T cells suggest that both effector T cell subsets participate in influenza anti-viral defense mechanisms [23, 33] and thus, CD4+ CTL may provide an increasingly important role in clinical protection against influenza with aging. Previous studies of the CD4+ T cell response to influenza vaccination in older adults have used the vaccine (killed virus) for in vitro stimulation [34] and thus would not be applicable to our study of CD4+ cytolytic effectors. Since CD4+ effector T-cell subsets in older adults share phenotypic and functional characteristics with CD4+ and CD8+ effector T-cell subsets in young adults, they might play an important role on controlling influenza infection especially in the relative absence of functional cytotoxic CD8+ T cells in older adults. A diminished proliferative response to influenza virus stimulation in older compared to young adults was also demonstrated in these effector memory and effector CD8+ T cell subsets, while similar proliferative responses were found in these CD4+ T cells subsets (data not shown). The cytolytic activity of the central memory (CD45RA-CCR7+) CD8+ T-cell subset in older adults suggests that this subset contains CD8+ T cells that do not behave as true central memory T cells and represent a phenotype shift with aging. Future experiments will further delineate the phenotype of these central memory CD8+ T cells and determine age-related changes in their function.
The identification of influenza virus-specific CTL (GzmB+Perf+) using the degranulating marker, CD107a, unexpectedly yielded a large proportion of GzmB+CD8+ T cells that expressed CD107a in older adults. Functional assays confirmed the reduction in cytolytic activity in CD8+ T cells responding to influenza challenge. These results suggest that the cytolytic potential on a per cell basis is reduced in influenza-specific CD8+ T cells of healthy older adults. In addition, a significant fraction of these CD8+ T cells may be approaching a terminally differentiated state, as we had previously shown that CD45RA+GzmB+ CD8+ T cells mount a poor response to influenza virus [9]. Recent studies suggest that a significant portion of the CD45RA+CD8+ T cells that accumulate with aging are due to the T cell response to chronic viral infections such as CMV [1]. With continuous exposure to the virus, these CMV-specific T cells are driven to become terminally differentiated T cells, preferentially exhausting the CD8+ T cell compartment and leading to immune compromise in older adults. It has been previously shown that IL-7Rα is expressed on T cells responding to acute viral infections such as influenza, but is not expressed on most CMV-specific T cells [23, 33].
We showed that most of the GzmB+Perf+ effector T cells responding to influenza virus, expressed IL-7Rα (CD127) suggesting that the dual expression of these cytolytic mediators could distinguish between terminally differentiated T cells and those T cells that could mount an effective influenza-specific cytolytic response to virus challenge. It should be noted that CD127+CD45RA+GzmB+Perf+ T cells are the phenotype of effector T cells in PBMC [24], which is maintained over the five days of in vitro stimulation and is the phenotype of T cell subset with demonstrated cytolytic activity in our experiments. This phenotype contrasts with the phenotype of CTL in the lungs, which have been activated by influenza-infected lung epithelial cells [23, 24].
To determine a mechanism for increased susceptibility to serious complications of influenza infection, we identified the phenotype of cytolytic T cells. The effector T-cell subset (GzmB+Perf+CD45RA+CCR7-CD127+) that expressed IL-2R (CD25) had high proliferative capacity and effective virus-specific cytolytic function in healthy young adults. In contrast, the CD25- effector subset exhibited limited proliferation and diminished cytolytic function in response to influenza virus. The proliferative response that yielded IL-2R+ effector T cells following in vitro virus stimulation was dramatically reduced in both CD4+ and CD8+ T cells in older compared to young adults. This result is consistent with the previously identified age-related decline in the in vitro proliferative response, which is IL-2 mediated [35]. Differences in the serious complication rates of pH1N1 relative to A/H3N2 infection in young adults, and A/H3N2 and pH1N1 infection in older relative to young adults, may be explained by a reduction in both the proliferative and cytolytic response to these influenza subtypes. These results may be helpful in understanding the limitations of split-virus vaccines for stimulating cross-reactive CTL responses, which could provide heterologous protection against different influenza subtypes and strains if effectively stimulated through vaccination.
Lower pH1N1 attack rates in older compared to young adults can be attributed to sterilizing immunity provided by pre-existing antibody titers. These antibodies may also contribute to a reduction in illness severity when infection occurs in older adults. In contrast, young adults would have lower antibody titers to pH1N1 but demonstrate a higher CD4+ and CD8+ effector T cell response to the virus following vaccination with the seasonal H1N1 strain (Fig. 4). Thus, the combined immunity provided by humoral and cellular mechanisms would explain the consistent case:fatality rates across the age spectrum and increases in these rates related to underlying high risk conditions in all age groups [36, 37]. Protective antibody titers against pH1N1 were mainly observed in those adults over age 80 years old [38] and this was consistent with very low attack rates in this subset of older adults.
Current split-virus influenza vaccines stimulate a poor CTL response in both CD4+ and CD8+ subsets. For older adults in whom antibodies may not prevent influenza infection, the age-related decline in the memory and effector CTL response to influenza infection corresponds to the increased risk of complicated influenza illness in older adults. The marked decline in the CD8+ relative to the CD4+ T cell response to influenza vaccination is consistent with previous observations in vaccinia-challenged PBMC following smallpox vaccination [39-41]. Similar to our previous results showing no correlation between antibody titers and measures of cellular immunity, these earlier results showed no correlation between antibody titers and levels of vaccinia-specific CD4+ and CD8+ memory T cells [39]. In contrast, the decline in CD8+ memory T cells following smallpox vaccination appeared to be independent of age. Our results are consistent with an age-dependent effect on the duration of the response to influenza vaccination.
In light of the rapid loss of CD8+ T cell memory, we have identified CD4+ T cell subsets as a potential alternate target for designing new influenza vaccines. However, it remains to be determined whether novel influenza vaccines targeting the cell-mediated immune response could more fully realize the cytolytic potential of CD8+ T cells and improve protection in older adults. High dose influenza vaccines that would deliver more CD4 epitopes for antigen presentation may be one strategy. Alternatively, adjuvants that could more effectively present both CD4 and CD8 epitopes may offer an enhanced response in both T cell subsets. We have shown that older adults mount a very good GzmB response to infection [42]and this response can be re-stimulated upon influenza vaccination 8-10 months later [8]. However, it has yet to determine whether CD4+ and/or CD8+ T cells are the T cell subset responding to influenza infection in the lungs in humans. Importantly, the relevance of a CD4+ cytolytic T cell response to an enhanced response to influenza is dependent on the expression of MHC II and presentation of influenza epitopes on infected lung epithelial cells. It remains to be demonstrated whether these CD4+ T cells are functional cytolytic effectors that can clear influenza virus from the lungs in humans.
In summary, our results showed that age-related changes in CD8+ T cells are associated with severely compromised influenza virus-specific cytolytic activity. Current split-virus influenza vaccines provide a weak stimulus to the CTL response to influenza virus. In older compared to young adults, there is reduced cytolytic activity stimulated by influenza virus and the response to vaccination in CD8+ T cells appears short-lived. Effector CD4+ T cells in older adults appear to have preserved cytolytic function relative to CD8+ T cells, and may provide an alternate target for the development of more protective influenza vaccines in older people.
Supplementary Material
Supplemental Figure 1. Human PBMCs from healthy young (HY, light bars) and older (HO, 60-70 years old, dark bars, and HO, >80 years old, striped bars) adults (n=15/group) obtained at the time before vaccination were cultured with live influenza virus for 20 hr. CD4+ and CD8+ subsets were defined by the expression of cell surface molecules, CD45RA and CCR7, and their intracellular cytolytic effector molecules, GzmB and Perf. The percentage of cells within effector T-cell subset CD45RA+CCR7- are shown. Error bars represent standard error of the mean.
Supplemental Figure 2. Human PBMCs from healthy young (HY, light bars) and older (HO, 75-85 years old, dark bars) adults (n=6/group) were stimulated with influenza seasonal A/H3N2 live virus for 5-6 days, CD4+ or CD8+ and their effector CD45RA+CCR7- subsets were sorted, and proliferation assays were performed. A consistent proliferative capacity in effector CD4+ T cells across the different age groups was observed; in contrast there was an age-related decline in CD8+ T cells.
Acknowledgments
The authors thank the volunteers for their participation in the study, and the staff of the Lowell P. Weicker, Jr. General Clinical Research Center, UConn Center on Aging and the technicians in the McElhaney laboratory for their assistance with subject recruitment, study co-ordination, and blood sample processing.
This work was sponsored by the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases, R01 AI68265 (J. E. M., Principal Investigator). The study was conducted through the Lowell P. Weicker, Jr. General Clinical Research Center funded by the NIH, National Center for Research Resources (Grant Number MO1 RR06192) at the University of Connecticut Health Center (UCHC), and in collaboration with the UConn Center on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pawelec G, Akbar A, Caruso C, Solana R, Grubeck-Loebenstein B, Wikby A. Human immunosenescence: is it infectious? Immunol Rev. 2005 Jun;205:257–68. doi: 10.1111/j.0105-2896.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 2.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. Jama. 2003 Jan 8;289(2):179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 3.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, et al. Influenza-associated hospitalizations in the United States. Jama. 2004 Sep 15;292(11):1333–40. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 4.Update: influenza activity---United States, August 30, 2009- January 9, 2010. MMWR Morb Mortal Wkly Rep. 59:38–43. [PubMed] [Google Scholar]
- 5.Outbreaks of 2009 Pandemic Influenza A (H1N1) Among Long-Term--Care Facility Residents---Three States. MMWR Morb Mortall Wkly Rep. 2009;59:74–7. [PubMed] [Google Scholar]
- 6.Nichol KL, Margolis KL, Wuorenma J, Von Sternberg T. The efficacy and cost effectiveness of vaccination against influenza among elderly persons living in the community. N Engl J Med. 1994 Sep 22;331(12):778–84. doi: 10.1056/NEJM199409223311206. [DOI] [PubMed] [Google Scholar]
- 7.McElhaney JE, Xie D, Hager WD, Barry MB, Wang Y, Kleppinger A, et al. T cell responses are better correlates of vaccine protection in the elderly. J Immunol. 2006 May 15;176(10):6333–9. doi: 10.4049/jimmunol.176.10.6333. [DOI] [PubMed] [Google Scholar]
- 8.McElhaney JE, Ewen C, Zhou X, Kane KP, Xie D, Hager WD, et al. Granzyme B: Correlates with protection and enhanced CTL response to influenza vaccination in older adults. Vaccine. 2009 Apr 21;27(18):2418–25. doi: 10.1016/j.vaccine.2009.01.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMichael AJ, Gotch FM, Noble GR, Beare PA. Cytotoxic T-cell immunity to influenza. N Engl J Med. 1983 Jul 7;309(1):13–7. doi: 10.1056/NEJM198307073090103. [DOI] [PubMed] [Google Scholar]
- 10.Johnson BJ, Costelloe EO, Fitzpatrick DR, Haanen JB, Schumacher TN, Brown LE, et al. Single-cell perforin and granzyme expression reveals the anatomical localization of effector CD8+ T cells in influenza virus-infected mice. Proc Natl Acad Sci U S A. 2003 Mar 4;100(5):2657–62. doi: 10.1073/pnas.0538056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lord SJ, Rajotte RV, Korbutt GS, Bleackley RC. Granzyme B: a natural born killer. Immunol Rev. 2003 Jun;193:31–8. doi: 10.1034/j.1600-065x.2003.00044.x. [DOI] [PubMed] [Google Scholar]
- 12.Lawrence CW, Ream RM, Braciale TJ. Frequency, specificity, and sites of expansion of CD8+ T cells during primary pulmonary influenza virus infection. J Immunol. 2005 May 1;174(9):5332–40. doi: 10.4049/jimmunol.174.9.5332. [DOI] [PubMed] [Google Scholar]
- 13.Pfister G, Weiskopf D, Lazuardi L, Kovaiou RD, Cioca DP, Keller M, et al. Naive T cells in the elderly: are they still there? Ann N Y Acad Sci. 2006 May;1067:152–7. doi: 10.1196/annals.1354.018. [DOI] [PubMed] [Google Scholar]
- 14.McElhaney JE, Pinkoski MJ, Upshaw CM, Bleackley RC. The cell-mediated cytotoxic response to influenza vaccination using an assay for granzyme B activity. J Immunol Methods. 1996 Mar 28;190(1):11–20. doi: 10.1016/0022-1759(95)00235-9. [DOI] [PubMed] [Google Scholar]
- 15.Powers DC, McElhaney JE, Florendo OA, Jr, Manning MC, Upshaw CM, Bentley DW, et al. Humoral and cellular immune responses following vaccination with purified recombinant hemagglutinin from influenza A (H3N2) virus. J Infect Dis. 1997 Feb;175(2):342–51. doi: 10.1093/infdis/175.2.342. [DOI] [PubMed] [Google Scholar]
- 16.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999 Oct 14;401(6754):708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 17.Olsson J, Wikby A, Johansson B, Lofgren S, Nilsson BO, Ferguson FG. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech Ageing Dev. 2000 Dec 20;121(1-3):187–201. doi: 10.1016/s0047-6374(00)00210-4. [DOI] [PubMed] [Google Scholar]
- 18.Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 2002;20:323–70. doi: 10.1146/annurev.immunol.20.100201.131730. [DOI] [PubMed] [Google Scholar]
- 19.Barry M, Bleackley RC. Cytotoxic T lymphocytes: all roads lead to death. Nat Rev Immunol. 2002 Jun;2(6):401–9. doi: 10.1038/nri819. [DOI] [PubMed] [Google Scholar]
- 20.Touvrey C, Derre L, Devevre E, Corthesy P, Romero P, Rufer N, et al. Dominant human CD8 T cell clonotypes persist simultaneously as memory and effector cells in memory phase. J Immunol. 2009 Jun 1;182(11):6718–26. doi: 10.4049/jimmunol.0803095. [DOI] [PubMed] [Google Scholar]
- 21.Shahid Z, Kleppinger A, Gentleman B, Falsey AR, McElhaney JE. Clinical and immunologic predictors of influenza illness among vaccinated older adults. Vaccine. doi: 10.1016/j.vaccine.2010.07.036. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, et al. Sensitive and viable identification of antigen-specific CD8+T cells by a flow cytometric assay for degranulation. Journal of Immunological Methods. 2003 Oct 1;281(1-2):65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 23.van Leeuwen EM, de Bree GJ, Remmerswaal EB, Yong SL, Tesselaar K, ten Berge IJ, et al. IL-7 receptor alpha chain expression distinguishes functional subsets of virus-specific human CD8+ T cells. Blood. 2005 Sep 15;106(6):2091–8. doi: 10.1182/blood-2005-02-0449. [DOI] [PubMed] [Google Scholar]
- 24.de Bree GJ, van Leeuwen EM, Out TA, Jansen HM, Jonkers RE, van Lier RA. Selective accumulation of differentiated CD8+ T cells specific for respiratory viruses in the human lung. J Exp Med. 2005 Nov 21;202(10):1433–42. doi: 10.1084/jem.20051365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Effros RB. Role of T lymphocyte replicative senescence in vaccine efficacy. Vaccine. 2007 Jan 8;25(4):599–604. doi: 10.1016/j.vaccine.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 26.http://www.cdc.gov/h1n1flu/vaccination/accine_seniros.htm. 2009.
- 27.Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O'Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol. 2001 Dec;75(24):12182–7. doi: 10.1128/JVI.75.24.12182-12187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saurwein-Teissl M, Lung TL, Marx F, Gschosser C, Asch E, Blasko I, et al. Lack of antibody production following immunization in old age: association with CD8(+)CD28(-) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002 Jun 1;168(11):5893–9. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- 29.Effros RB, Dagarag M, Spaulding C, Man J. The role of CD8+ T-cell replicative senescence in human aging. Immunol Rev. 2005 Jun;205:147–57. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 30.Casazza JP, Betts MR, Price DA, Precopio ML, Ruff LE, Brenchley JM, et al. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J Exp Med. 2006 Dec 25;203(13):2865–77. doi: 10.1084/jem.20052246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005 Sep 5;202(5):673–85. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Bree GJ, Daniels H, Schilfgaarde M, Jansen HM, Out TA, van Lier RA, et al. Characterization of CD4+ memory T cell responses directed against common respiratory pathogens in peripheral blood and lung. J Infect Dis. 2007 Jun 1;195(11):1718–25. doi: 10.1086/517612. [DOI] [PubMed] [Google Scholar]
- 33.de Bree GJ, Heidema J, van Leeuwen EM, van Bleek GM, Jonkers RE, Jansen HM, et al. Respiratory syncytial virus-specific CD8+ memory T cell responses in elderly persons. J Infect Dis. 2005 May 15;191(10):1710–8. doi: 10.1086/429695. [DOI] [PubMed] [Google Scholar]
- 34.Kang I, Hong MS, Nolasco H, Park SH, Dan JM, Choi JY, et al. Age-associated change in the frequency of memory CD4+ T cells impairs long term CD4+ T cell responses to influenza vaccine. J Immunol. 2004 Jul 1;173(1):673–81. doi: 10.4049/jimmunol.173.1.673. [DOI] [PubMed] [Google Scholar]
- 35.Gardner EM, Gonzalez EW, Nogusa S, Murasko DM. Age-related changes in the immune response to influenza vaccination in a racially diverse, healthy elderly population. Vaccine. 2006 Mar 6;24(10):1609–14. doi: 10.1016/j.vaccine.2005.09.058. [DOI] [PubMed] [Google Scholar]
- 36.Pebody RG, McLean E, Zhao H, Cleary P, Bracebridge S, Foster K, et al. Pandemic Influenza A (H1N1) 2009 and mortality in the United Kingdom: risk factors for death, April 2009 to March 2010. Euro Surveill . 2010 May 20;15(20) [PubMed] [Google Scholar]
- 37.Hadler JL, Konty K, McVeigh KH, Fine A, Eisenhower D, Kerker B, et al. Case fatality rates based on population estimates of influenza-like illness due to novel H1N1 influenza: New York City, May-June 2009. PloS one. 2010;5(7):e11677. doi: 10.1371/journal.pone.0011677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skowronski DM, Hottes TS, Janjua NZ, Purych D, Sabaiduc S, Chan T, et al. Prevalence of seroprotection against the pandemic (H1N1) virus after the 2009 pandemic. Cmaj. 2010 Nov 23;182(17):1851–6. doi: 10.1503/cmaj.100910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, et al. Duration of antiviral immunity after smallpox vaccination. Nature medicine. 2003 Sep;9(9):1131–7. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 40.Amara RR, Nigam P, Sharma S, Liu J, Bostik V. Long-lived poxvirus immunity, robust CD4 help, and better persistence of CD4 than CD8 T cells. Journal of virology. 2004 Apr;78(8):3811–6. doi: 10.1128/JVI.78.8.3811-3816.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Combadiere B, Boissonnas A, Carcelain G, Lefranc E, Samri A, Bricaire F, et al. Distinct time effects of vaccination on long-term proliferative and IFN-gamma-producing T cell memory to smallpox in humans. The Journal of experimental medicine. 2004 Jun 7;199(11):1585–93. doi: 10.1084/jem.20032083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shahid Z, Kleppinger A, Gentleman B, Falsey AR, McElhaney JE. Clinical and immunologic predictors of influenza illness among vaccinated older adults. Vaccine. 2010 Aug 31;28(38):6145–51. doi: 10.1016/j.vaccine.2010.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Human PBMCs from healthy young (HY, light bars) and older (HO, 60-70 years old, dark bars, and HO, >80 years old, striped bars) adults (n=15/group) obtained at the time before vaccination were cultured with live influenza virus for 20 hr. CD4+ and CD8+ subsets were defined by the expression of cell surface molecules, CD45RA and CCR7, and their intracellular cytolytic effector molecules, GzmB and Perf. The percentage of cells within effector T-cell subset CD45RA+CCR7- are shown. Error bars represent standard error of the mean.
Supplemental Figure 2. Human PBMCs from healthy young (HY, light bars) and older (HO, 75-85 years old, dark bars) adults (n=6/group) were stimulated with influenza seasonal A/H3N2 live virus for 5-6 days, CD4+ or CD8+ and their effector CD45RA+CCR7- subsets were sorted, and proliferation assays were performed. A consistent proliferative capacity in effector CD4+ T cells across the different age groups was observed; in contrast there was an age-related decline in CD8+ T cells.