I. Introduction
Molecular imaging aims to enable personalized medicine via imaging specific molecular and cellular targets that are relevant to the diagnosis and treatment of disease. By providing in vivo readouts of biological detail, molecular imaging complements traditional anatomical imaging modalities to allow 1) visualization of important disease-modulating molecules and cells in vivo; 2) serial investigations to image evolutionary changes in disease attributes; and 3) evaluation of the in vivo molecular effects of bio-therapeutics. The added information garnered by molecular imaging can improve risk assessment and prognosticative studies, of particular benefit in the management of cardiovascular disease (CVD).
Molecular imaging investigations of CVD continue to increase each year. Over the past year, significant gains in clinical applications and new technology have been achieved in vascular and myocardial imaging. In this update, we have the privilege to highlight recent outstanding clinical and translational molecular imaging (MOLI) studies of CVD.
II. Vascular Imaging
A. Atherosclerosis
Atherosclerosis remains a dominant focus for CVD molecular imaging studies. This field is driven by the quest to identify biologically high-risk “vulnerable” plaques (e.g. heightened plaque inflammation, neovascularization, or apoptosis). Molecular imaging therefore complements traditional anatomical imaging approaches that identify plaque structure and composition.
Clinical molecular imaging studies of atherosclerosis have grown substantially in recent years. The two leading clinical platforms remain 18F-fluorodeoxyglucose (FDG) imaging of plaque metabolic activity/inflammation by positron emission tomography (PET), and ultrasmall superparamagnetic iron oxide (USPIO) nanoparticle-enhanced MRI of plaque macrophages. These modalities primarily interrogate the carotid arteries and larger vascular beds, although recent preliminary reports suggest the potential for noninvasive coronary plaque imaging.
Clinical 18FDG PET molecular imaging studies of atherosclerosis
18FDG is a glucose analog trapped intracellularly by metabolically active cells, and therefore enables PET-based detection of metabolism. 18FDG has been used extensively in cancer and myocardial viability studies. Pioneering work from the last decade demonstrated that 18FDG could enable PET imaging of inflamed subsets of carotid atheromata of human subjects. Subsequent correlational analyses established a link between 18FDG signals and plaque macrophages, or inflammation. Consistent with the widespread ability to perform clinical 18FDG PET imaging, large vessel atherosclerosis metabolism/inflammation studies have grown substantially in the last several years.
Several recent 18FDG plaque investigations have shed new light on the clinical utility, pathophysiology, and spatial distribution of plaque metabolism/inflammation. In an observational 18FDG PET/CT study of 932 cancer patients, increased 18FDG uptake (mean large vessel plaque target-to-background ratio (TBR) ≥1.7) was the strongest predictor of a future vascular event, which occurred in 1.6% of patients at a median follow-up time of 29 months (1). Importantly, the 18FDG signal was 4-fold-more predictive of a future vascular event compared to the degree of plaque calcification noted on co-registered CT. A retrospective study of 200 patients demonstrated a correlation between cardiovascular risk factors and the number of 18FDG-positive plaques, and an inverse association between statin therapy and 18FDG-positive plaques (2).
In new pathophysiologic studies, 18FDG plaque signals rarely overlapped with plaque calcification, further suggesting that CT-detected calcification may indicate a “burnt-out” plaque phenotype. 18FDG plaque uptake was greater in patients with a history of CAD and also associated with the inflammatory serum biomarkers matrix metalloproteinase (MMP)-3 and MMP-9 (3). In multimodal 18FDG PET/CT and MRI studies of carotid plaques, one MRI study found that lipid-rich plaques had higher 18FDG signal intensities than collagen-rich or calcified plaques (4), while another study noted weak correlations between 18FDG plaque signals and plaque compositional parameters derived from CT and MRI (5). Lastly a study of ten carotid endarterectomy patients further linked 18FDG uptake to plaque inflammation (6). The inflammatory mRNA markers macrophage CD68, cathepsin K, matrix metalloproteinase (MMP)-9, and interleukin-18 positively correlated with the 18FDG TBR, with CD68 having the strongest association (r=0.71, p=0.02).
In an exciting preliminary advance, 18FDG PET/CT enabled the noninvasive detection of coronary plaque metabolism/inflammation (Figure 1) (7). Background suppression of the typically intense myocardial 18FDG signal was performed by having patients consume a low carbohydrate, high-fat meal the night before, and then imbibe a vegetable oil drink prior to the study. In subjects with good myocardial suppression, focal 18FDG signal (albeit at limited resolution) was detected in CT-demarcated coronary arterial segments, and tended to be more prevalent in patients with angiographically-confirmed CAD. Prospective studies are needed to determine whether 18FDG measures of coronary plaque inflammation will predict coronary events.
Figure 1.
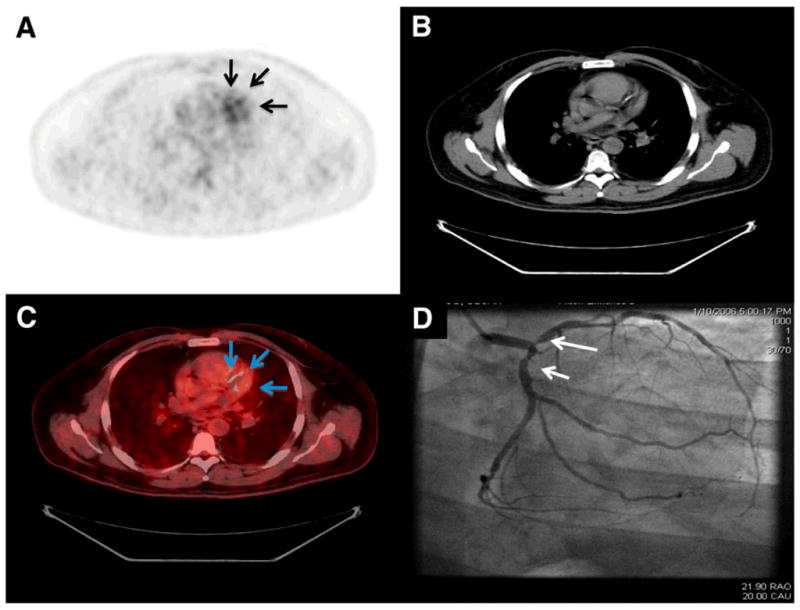
PET/CT imaging of coronary arterial inflammation/metabolic activity via 18F-fluorodeoxyglucose (FDG). Suppression of myocardial FDG signal was obtained by using a special high-fat diet prior to imaging. Representative images of (A) 18F-FDG PET (B) cardiac CT axial slice (C) Fusion PET/CT image showing elevated 18FDG signal overlying a calcified coronary artery, and (D) correlative invasive coronary angiography showing severe LAD disease. Reproduced with permission from (7).
Clinical molecular MRI studies of plaque inflammation
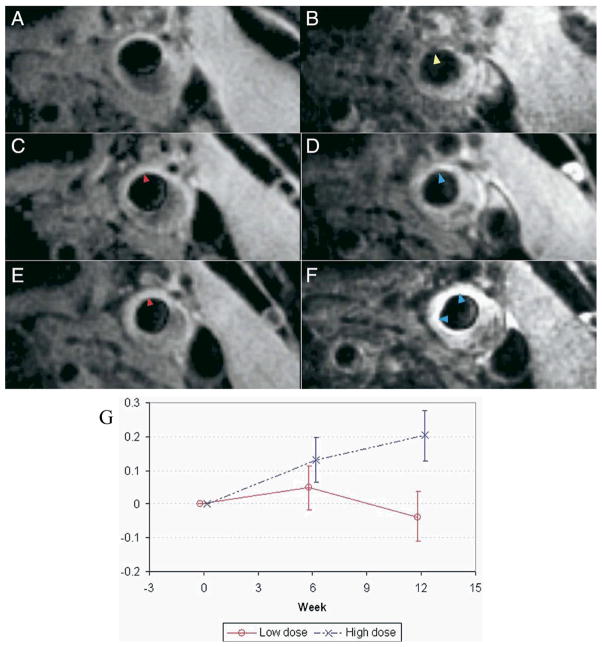
USPIO are superparamagnetic dextranated nanoparticles that are phagocytosed by plaque macrophages, and therefore can report on carotid plaque inflammation on T2-weighted MRI studies. Recent studies from the investigative group in Cambridge have added new insights into human plaque inflammation. In the ATHEROMA trial, USPIOs were utilized to report on differential changes in plaque inflammation when 47 patients were randomized to high-dose or low-dose statin pharmacotherapy (8). Compared to atorvastatin 10mg daily, atorvastatin 80mg daily significantly reduced USPIO-based plaque inflammation on carotid MRI (Figure 2). Furthermore, high-dose but not low-dose statin therapy reduced cerebral microemboli detected on concomitantly obtained transcranial Doppler studies. Remarkably, these differences were detectable after only 12 weeks, thus providing insight into the rapid clinical benefits noted in the MIRACL and PROVE-IT statin trials. This important study demonstrates the power of molecular imaging to provide a biological readout for atherosclerosis pharmacotherapies.
Figure 2.
Molecular MRI of carotid plaque inflammation following high-dose statin treatment. Representative T2*-weighted left common carotid artery imaging pre- and post-USPIO administration at baseline (A and B), 6 weeks (C and D), and 12 weeks (E and F). (B) Baseline USPIO effect is present (signal loss on T2* weighted image, yellow arrowhead), indicating plaque macrophages. (C and E) Pre-USPIO signal is similar at each imaging timepoint, signifying loss of tissue retention before each round of imaging (red arrowhead). (D) Minimal USPIO signal loss is observed by 6 weeks (blue arrowhead), consistent with reduced plaque inflammation (F) At 12 weeks, even less USPIO effect is present (blue arrowheads). (G) Signal intensity change (ΔSI) compared for the 2 treatment groups at baseline, 6, and 12 weeks with 95% confidence intervals; low-dose (red line) and high-dose (dashed blue line, showing less USPIO effect and thus higher signal). Reproduced with permission from (8).
In a small cross-sectional study of subjects with carotid atherosclerosis (stenosis ε50%), Howarth et al. demonstrated that symptomatic patients had significantly more quadrants of USPIO-based macrophage inflammation compared to asymptomatic patients (75% vs. 32%, p<0.01) (9). Further studies may determine if USPIO enhancement in asymptomatic patients will portend a higher stroke risk.
New biological insights
The past year witnessed major advances in visualizing plaque inflammation, angiogenesis, apoptosis, oxidative stress and calcification. In addition, hybrid imaging strategies such as PET/CT, PET/MRI, and optical/CT imaging broadened our understanding of these processes by providing co-registered molecular and structural detail of atheromata.
Inflammation
In an integrated PET/CT dual target molecular imaging study, the macrophage-avid iodinated nanoparticle CT contrast reporter (N1177) detected plaque inflammation two hours after injection in atheroma-bearing rabbits (10). A moderately strong correlation of N1177 uptake was present in comparison to 18FDG PET signal (r=0.61) and macrophage density on immunohistochemistry (r=0.63). Worthley et al. utilized serial PET imaging of inflamed rabbit aortas to show continued 18FDG uptake when a fatty diet was sustained, and diminished 18FDG signal when animals were returned to a non-atherogenic diet (11).
Using contrast-enhanced ultrasound with P-selectin and vascular cell adhesion molecule (VCAM)-1 targeted microbubbles, Kaufmann et al. demonstrated that atheroma inflammation could be imaged prior to the development of advanced lesions (12).
Amirbekian et al. utilized a ‘positive’ contrast MRI-detectable gadolinium chelate (P947) covalently linked to an MMP peptide sequence to visualize plaque MMP expression in ApoE−/− mice (13). A two-fold greater signal enhancement was noted over the control (scrambled peptide sequence) probe P1135. Imaging of plaque MMP presence in ApoE- and LDL-receptor dual knockout mice was also demonstrated using micro-SPECT imaging of 99mTc-derivatized MMP inhibitor (14).
In an integrated PET/CT murine study, Laitinen et al. utilized the clinically-tested, integrin ανβ3-targeted tracer 18F-Galacto-RGD to identify cellular (macrophage+endothelial cell) inflammation and neoangiogenic vasculature in atheroma (15). Interestingly the 18F-Galacto-RGD enhancement associated primarily with macrophages, as neovessels were uncommon in plaques from the mouse model. Unlike 18F-FDG, the 18F-Galacto-RGD agent does not target the myocardium and thus may ultimately enable low-background, noninvasive PET imaging of coronary plaque inflammation.
New inflammation imaging agents for atherosclerosis
To image VCAM-1 expression in atheroma, Nahrendorf and colleagues developed three 18F-labeled small VCAM-1 affinity ligands for PET/CT imaging (16). The tetrameric peptide 18F-4V provided the highest specificity and affinity for VCAM-1, and enabled its noninvasive detection in murine plaques. Statin-treated animals demonstrated less in vivo plaque uptake of 18F-4V. 18F-4V also deposited in preliminary studies of myocardial infarction and cardiac transplant rejection, two diseases with augmented VCAM-1 expression.
New agents to image atherosclerotic cysteine protease activity were developed and evaluated using integrated fluorescence molecular tomography (FMT)/CT (17). Nahrendorf et al. evaluated three near-infrared fluorescence (NIRF) protease-activatable nanosensors of different sizes (5–40 nm) and pharmacokinetics, but with identical oligo-L-lysine cleavage sites. While the 5nm agent showed faster activation, the 40nm agent (PS-40, a polymeric iron oxide-containing nanoparticle) demonstrated the strongest NIR fluorescence in murine plaques. In vivo FMT, a noninvasive tomographic imaging approach, detected strong PS-40 NIRF signals in the aortic roots of ApoE−/− mice. Statin therapy resulted in diminished PS-40 signals in atheroma.
Lipinski et al. developed a new macrophage-specific molecular MRI agent using a phospolipid nanoparticle containing gadolinium, the fluorochrome NBD for target validation, and a biotinylated antibody to the human macrophage scavenger receptor B (CD36) (18). In vitro confocal fluorescence and mass spectroscopy studies demonstrated strong uptake of the agent by macrophages. Ex vivo MRI of aortic plaques from patients also demonstrated uptake of the agent by human macrophages. This probe may expand the clinical options to image plaque macrophages beyond USPIO-MRI and 18FDG-PET.
Tekabe et al. developed a new radiolabeled tracer that imaged the receptor for advanced glycation end products (RAGE), a molecule that participates in plaque inflammation. The imaging agent was created by radiolabeling a RAGE antibody fragment with technecium-99m (19). Planar gamma images revealed focal uptake in the thoracic aorta in ApoE−/− mice that was not noted when using a control antibody.
Apoptosis
Zhao et al. employed 99mTc-annexin A5, a clinically tested apoptosis imaging agent, and 18F-FDG to co-visualize plaque apoptosis and metabolism/inflammation (20). Ex vivo analyses demonstrated that both 99mTc-annexin A5 (apoptosis) and 18F-FDG (inflammation/metabolism) deposition in murine plaques increased similarly from ages 10 to 25 weeks, and was greatest in cholesterol-fed ApoE−/− mice.
Neovascularizartion
Gadofluorine-M is an ampiphilic compound with high R1 relaxivity that provides excellent T1-weighted MRI plaque contrast. To further explore its microscopic localization in atherosclerosis, Sirol et al. crafted a carbocyanine fluorescent derivative of Gadofluorine-M (21). In atheroma-bearing rabbits injected with this derivative, confocal microscopy and detailed histological analyses linked gadofluorine-M deposition and CD31+ plaque neovessels, as well as the degree of plaque macrophages.
Oxidative stress
Myeloperoxidase (MPO) activity in plaques was visualized in vivo by the positive contrast MRI agent bis-5HT-DTPA(Gd) (22). MPO catalyzes the formation of hypochlorite and other reactive oxidants that are linked to plaque progression and complication. A detailed study in rabbits established that bis-5HT-DTPA(Gd) enhanced atheromata on T1-weighted MRI, in comparison to control agents. Findings were corroborated by biochemical assays of MPO activity in the vessel wall, and by immunohistochemical studies of plaque sections. Intriguingly, MPO activity localized at plaque shoulders, further implicating this enzyme in plaque destabilization.
Calcification
Calcified nodules and microcalcifications are now appreciated as a consequence of vascular inflammation, but the mechanism of bone mineral deposition remains unclear. In ApoE−/− mice with advanced renal failure, Aikawa et al. investigated of the role of the elastolytic protease cathepsin S (CatS) on arterial and aortic valve calcification (23). Multi-channel intravital fluorescence imaging was performed after co-injection of a specialized CatS protease-activatable NIRF probe; osteogenic activity was detected by a spectrally distinct fluorescent bisphosphanate agent (OsteoSense680). Ten weeks after nephrectomy, CatS-deficient mice (ApoE−/− CatS−/−) exhibited significantly less arterial and aortic valve calcification than control ApoE−/− CatS+/+ mice. Intriguingly, plaque size and macrophage content was similar in both nephrectomized groups, suggesting that CatS was specifically involved in osteogenesis, possibly by releasing elastin peptide degradation products that promote local mineralization. Proteolytic enzymes, such as CatS and MMP, may represent biomarkers of calcific and plaque remodeling that may provide bio-efficacy readouts for future anti-calcific therapies.
Bio-evaluation of atherosclerosis pharmacotherapies
Emerging atherosclerosis therapeutics are primarily biologics, and therefore a biological readout of drug efficacy may be advantageous compared to classical structural atheroma measurements. In addition to the above-discussed clinical ATHEROMA trial (8), several recent pre-clinical studies from the last year illustrate the utility of molecular imaging to interrogate the biological effects of anti-inflammatory therapies.
In a comprehensive report, Fujimoto et al. utilized an MMP imaging agent (99mTc-conjugated to the broad-spectrum, MMP-inhibiting macrocyclic compound RP805) to study the effects of statin therapy on plaque inflammation (24). Plaque MMP presence on in vivo micro-SPECT imaging was reduced with just one month of fluvastatin therapy. In a dual isotope SPECT protocol, Haider et al. detected both broad-spectrum MMP activity and apoptosis in inflamed rabbit plaques via combined 99mTc-RP805 (MMP) and 111In-annexin A5 imaging (macrophages/apoptosis) (25). Strong MMP and annexin A5 plaque signals on SPECT imaging were diminished by one month of fluvastatin therapy or withdrawal of the high-cholesterol diet. In addition, this study demonstrated a moderate correlation (r=0.62) between the MMP and annexin SPECT signals, as well as with plaque macrophages, MMP-9, and TUNEL+ apoptotic cells.
Intravascular coronary artery-targeted molecular imaging of inflammation
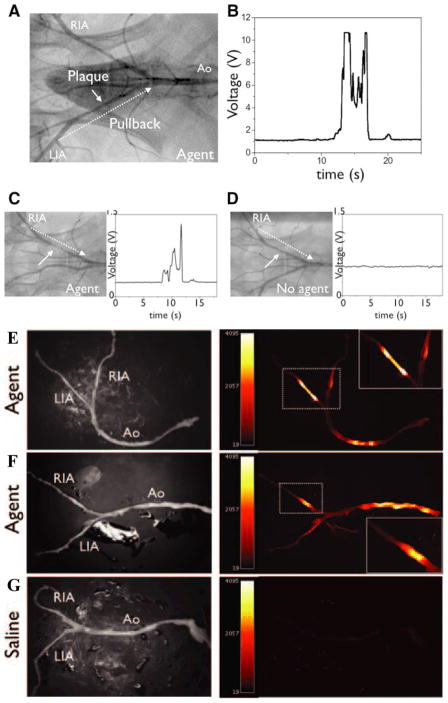
While clinical molecular imaging has made significant inroads into exploring molecular and cellular targets in larger vascular beds (carotids, aorta, iliac), strategies to interrogate the coronary arteries are limited. To address this need, a novel prototype NIRF intravascular catheter was developed and tested for real-time sensing of plaque inflammation atheroma in rabbit iliac arteries (of similar size to human coronary arteries) (26). The NIRF catheter was developed from a clinical intracoronary optical coherence tomography wire. Twenty-four hours before imaging, rabbits received a cysteine protease-activatable NIRF agent (ProSense750, 600 nmol/kg) to identify inflamed plaques. Real-time catheter pullback revealed >500% increases in the peak TBRs in ProSense750 rabbits compared to saline-injected subjects (Figure 3). Notably, intravascular NIRF imaging was performed through blood, without the need for flushing, consistent with the favorable light transmission properties of the NIR window. The availability of a clinical intravascular NIRF catheter could accelerate the detection of biologically high-risk coronary plaques.
Figure 3.
Real-time intravascular NIR fluorescence detection of atherosclerosis inflammation. (A) In vivo manual catheter pullback trajectory in the iliac arteries (dotted arrow). (B and C) Rabbits injected with a protease-activatable NIRF agent showed strong fluorescence signals in vivo, through blood, without flushing (Prosense750 group, average TBR 6.8) in angiographically-demarcated iliac plaques. (D) Saline-injected rabbits generated significantly less NIRF signal. (E, F) Paired light and NIRF signals in ex vivo arterial samples show enhanced NIRF protease activity. (G) Saline-injected control rabbits again with minimal plaque autofluorescence. RIA, right iliac artery; LIA, left iliac artery; Ao, aorta. Reproduced with permission from (26).
B. Aneurysm
Clinical 18FDG PET imaging of abdominal aortic aneurysm (AAA)
Elevated 18FDG signal occurs in subsets of aneurysms, and associates with inflammatory markers and possibly rapid expansion. Kotze et al. recently applied 18FDG PET/CT to evaluate advanced infrarenal AAAs (diameter 5.4±0.8 cm) in 14 men undergoing surveillance imaging (27). While all AAAs had reasonably high 18FDG uptake, the two aneurysms that exhibited inflammatory changes based on CT criteria (periaortic fibrosis) possessed significantly greater 18FDG signal activity. The degree of CT calcification and the initial AAA diameter did not correlate with 18FDG uptake in this small-sized study.
In an illustrative case report, Reeps et al. connected elevated 18FDG signals, inflammation, and mechanical instability in a patient with a small infrarenal AAA (4.6 cm diameter) on a staging 18FDG PET/CT scan (28). Just 6 months later, the asymptomatic AAA showed markedly increased 18FDG PET uptake in association with rapid aneurysm expansion (6.0 cm diameter). The patient underwent successful prophylactic surgical repair and biopsies were obtained from areas of high and low 18FDG activity. In areas of high 18FDG activity, the tissue showed collagen and elastin destruction, macrophage infiltration, and enhanced MMP expression. Larger investigations of 18FDG PET imaging for identifying AAAs likely to rapidly progress and/or rupture are in progress.
New experimental imaging insights
DeLeo et al. recently demonstrated the ability to image elevated MPO activity in an inflammatory intracranial aneurysmal disease model (29). Saccular inflamed aneurysms created in rabbit carotid arteries were imaged at 48 hours with a 3T whole-body clinical MRI scanner using a paramagnetic MPO contrast agent (di-5-hydroxytryptamide of gadopentetate dimeglumine). Compared to controls, MPO-specific signal was >30% enhanced in the inflamed aneurysms and exhibited delayed tissue washout. This agent may be translatable to human studies of endovascular inflammation caused by macrophage and neutrophil activity in atherosclerosis and other vascular inflammatory conditions.
Tedesco et al. investigated the role of neovascularization in murine AAA progression using a NIRF reporter targeted to the vascular endothelial growth factor (VEGF) receptor (30). At a timepoint between 21 and 28 days after angiotensin II–induced aneurysm formation, a NIRF Cy5.5-derivatized single chain VEGF homodimer was injected intravenously. Two hours later, in situ, ex vivo and fluorescence images revealed NIRF signal in aneurysmal segments. The NIRF signal reflecting AAA neovascularization correlated well with the AAA diameter measured on ultrasound (r=0.69). VEGF receptor imaging may represent a new surrogate marker for assessment of the progression and treatment of AAA.
C. Thrombosis
Clinical molecular imaging studies of thrombosis
Vymazal et al. recently published the full phase II clinical results of EP-2104R, a fibrin-targeted, gadolinium-derivatized peptide for molecular MRI of thrombosis (31). Patients with known venous (n=14) or arterial (n=38) thrombi underwent EP-2104R-enhanced MRI. For both types of thrombi, EP-2104R increased the detection capability by enhancing thrombi not apparent on non-contrast scans (Figure 4). However, despite EP-2104R administration, 71% of venous thrombi and 16% of arterial clots remained undetected. Further investigations may optimize imaging parameters to showcase this approach beyond conventional, flow-based thrombosis diagnostic imaging methods (e.g. ultrasound, CT).
Figure 4.
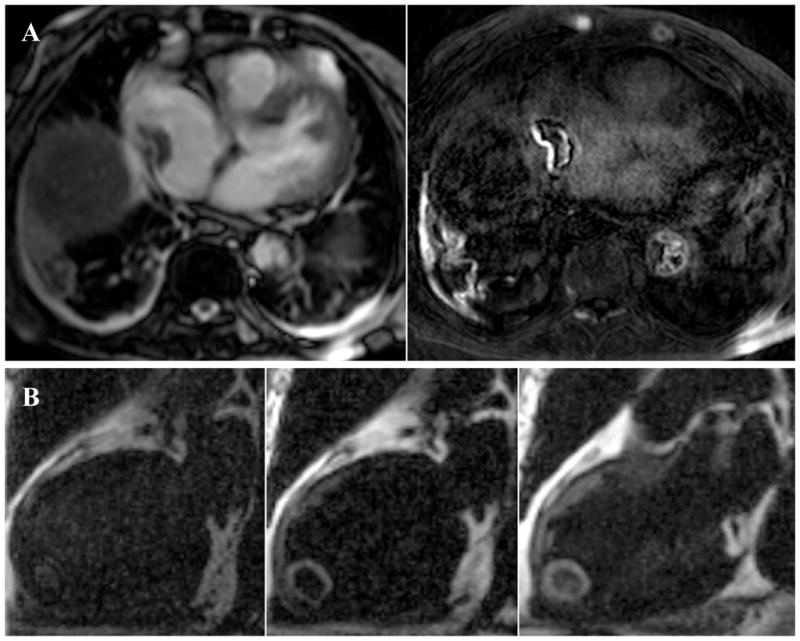
Molecular MRI of fibrin-rich thrombi using the clinical agent EP-2104R. (A) Right atrial thrombus is demonstrated in the right panel 24 hrs after EP-2104R delivery with peripheral hyperenhancement. In addition EP-2104R enhanced fibrin-bearing irregular aortic atheromata, and fibrinous pleuritis of the right lung. (B) Prior to EP-2104R injection in the left panel, an initially hidden left ventricular thrombus becomes apparent two hours after EP-2104R administration (center panel) and persists 24 hours later (right panel). Reproduced with permission from (31).
New thrombosis imaging agents
Expanding on prior optical, radionuclide, and nanoparticle agents for blood transglutaminase factor XIII (FXIIIa), Miserus et al. developed a bimodal optical-MRI agent for FXIIIa (32). FXIIIa is a hallmark of biologically acute thrombi, and is involved in fibrinolytic resistance. The authors linked a FXIIIa-targeted oligopeptide substrate to gadolinium for MRI and to the fluorochrome rhodamine for microscopic localization. In murine carotid artery thrombosis, immediate FXIIIa agent injection followed by MRI at 90 min revealed hyperintense signal at the thrombus border zones (contrast-to-noise ratio (CNR) of 2.28), and was confirmed on ex vivo two-photon rhodamine fluorescence microscopy. In contradistinction, older thrombi (24–48 hours old) demonstrated virtually no signal enhancement (CNR 0.11), consistent with the disappearance of FXIIIa in older thrombi.
D. Vascular Injury
The ability to monitor the inflammatory response following vascular injury could help elucidate the pathogenesis of many diseases including graft arteriosclerosis, in-stent restenosis and diabetic vasculopathies. In particular, MMPs play an important role in vascular remodeling following injury, To track the MMP response in experimental vascular injury, Zhang et al. utilized the previously validated SPECT radiotracer 111In-RP782 to image broad-spectrum MMP activity (33). At 2–4 weeks after guidewire mechanical injury of the carotid artery in ApoE−/− mice, RP782 was injected, and microSPECT/CT imaging was performed 2 hours later. Focal, strong signal was evident in the injured left carotid artery segment, but not the right-sided sham-treated vessel. RP782 uptake correlated directly with hyperplastic vessel-wall area expansion. Competitive inhibition experiments with excess unlabeled tracer diminished RP782 uptake. RP782 may also enable imaging MMP presence within atherosclerotic plaques as an indicator of inflammation and instability.
In a inflammatory protease imaging study, Sheth et al. utilized an intravascular NIR fluorescence endoscope to visualize cysteine protease activity in the injured aorta of rats (34). The group developed an intriguing normalization strategy to account for blood pool signal attenuation that frequently confounds quantitative real-time endovascular NIRF imaging. Capitalizing on the ratio of two NIRF signals with matched blood attenuation properties, the effects of light absorption and red blood cell scattering were effectively cancelled, providing an independent descriptor regardless of the intervening blood layer size between the emitting source and detection probe. Subsequent experiments in rats with focal aortic wall injury confirmed the ability of this technique to discriminate local vascular protease activity (NIR fluorescence).
III. Myocardial Imaging
A. Myocardial Infarction
Pharmacological studies
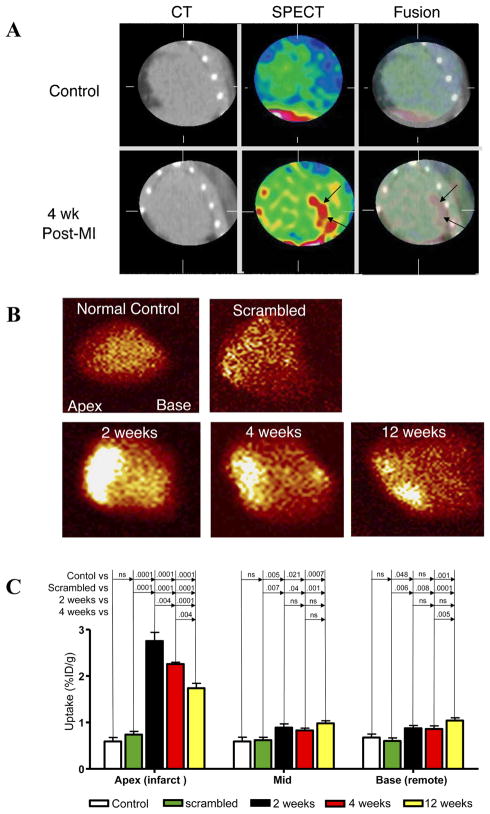
Biological imaging readouts of therapies directed against adverse myocardial remodeling remain a priority area for molecular imaging of myocardial infarction. Two recent studies by van den Borne et al. illustrate the molecular and cellular effects of key heart failure therapies such as ACE inhibitors, aldosterone antagonists, and angiotensin receptor blockers (35,36). Following induction of myocardial infarction (MI) by coronary artery occlusion, mice were treated for 4 weeks with 1, 2, or 3 drug combinations of captopril (angiotensin converting enzyme (ACE) inhibitor) or losartan (angiotensin receptor blocker) or spironolactone (aldosterone antagonist). Mice then underwent micro-SPECT/CT imaging with an integrin-specific 99mTc-labeled RGD peptide conjugated to a Cy5.5 fluorescent agent (termed CRIP) that identifies myofibroblast-related collagen deposition in the post-infarction scar (Figure 5) (35). Peak CRIP signal 2–4 weeks after infarction was significantly reduced in an additive fashion by sequential blockade of the renin-angiotensin-aldosterone axis, and corresponded with improved echocardiographic left ventricular function and diminished histologic collagen deposition. Radiolabeled CRIP noninvasively identified myocardial fibrosis following MI, and its uptake is responsive to clinically beneficial neurohumoral antagonists known to limit adverse cardiac remodeling.
Figure 5.
In vivo molecular imaging of myocardial remodeling via 99mtechnetium-labeled Cy5.5-RGD peptide (Tc-CRIP). (A) Micro-CT, microSPECT, and SPECT/CT fusion images in control mice (top row) or mice 4 weeks after anterior MI (bottom row) demonstrate Tc-CRIP uptake exclusively in infarcted mice. Explanted hearts from (B) controls had no significant Tc-CRIP uptake, however intense Tc-CRIP signal was present in infarcted mice 2 weeks after MI that then diminished at 4 and 12 weeks. (C) Quantitative Tc-CRIP activity was greatest in the apical infarct, followed by the mid peri-infarct zone and non-infarcted base. Reproduced with permission from (36).
New biological insights
In contrast to previous beliefs that circulating monocytes undergo end-stage differentiation upon tissue entry, Swirski and colleagues identified a large splenic reservoir of undifferentiated monocytes that are liberated after MI, hone to the site of injury, and actively engage in would healing (37). The team utilized multimodal MRI, FMT, and intravital microscopic molecular imaging methods to track monocyte migration in vivo. This new paradigm suggests that a prominent organ source of inflammatory cells can be recruited in response to local injury and participate in myocardial healing. Modulating this cascade may motivate future therapies that coerce beneficial inflammatory cell profiles to elicit favorable cardiac remodeling.
A dual-contrast molecular MRI technique has recently been employed to concurrently image acute post-MI mid-myocardial apoptosis and necrosis (38). Apoptosis was detected using a previously validated annexin-tagged cross-linked iron oxide (AnxCLIO-Cy5.5, an R2 agent similar to earlier mentioned clinical USPIOs) that identifies phosphatidylserine residues exposed on the membranes of apoptotic cells. Necrosis was detected using a delayed enhancement protocol and the fluorescent gadolinium agent Gd-NBD. In murine infarcts induced by 35 minutes of temporary coronary artery ligation, cardiomyocyte necrosis and apoptosis were discretely visualized on in vivo multicontrast MRI at an early timepoint (4–6 hrs). Capitalizing on the high spatial resolution of MRI, geographic mural differences in the infarct were noted. In the mid-myocardium of the infarct zone, large regions of T2*-mediated AnxCLIO-Cy5.5 signal (reflecting apoptosis) were visible. In contrast, the mid-myocardium was negative for delayed-gadolinium enhancement (necrosis). Necrotic zones identified by Gd-NDB enhancement localized to the subendocardium. This study identified post-reperfusion mid-myocardial areas of apoptotic but viable cardiomyocytes that might be salvageable by new, targeted therapies, offering a new focus for improvement of post-MI reperfusion injury and healing.
Sosnovik et al. additionally studied chronic myocardial apoptosis (39), a key feature of post-infarct myocardial remodeling. Utilizing a murine heart failure model characterized by chronic, low-grade (1–2%) apoptosis with scant necrosis, mice were intravenously injected with AnxCLIO-Cy5.5 for in vivo MRI and ex vivo fluorescence imaging. By MRI, myocardial pockets of AnxCLIO-Cy5.5 were observed in the subendocardium, and correlated strongly with apoptotic caspase-3 activity. This technology might be applied in various forms of chronic heart failure, including infarct-related cardiomyopathy, to better identify subjects likely to progress to end-stage heart failure.
New MOLI agents
A new high-affinity collagen-binding imaging agent for scintigraphic detection of infarct fibrosis was developed (termed collagelin, with a Kd 1.1×10−7 M for type I collagen) (40). Collagelin was discovered and synthesized from a bacterial peptide library using an antibody against anti-glycoprotein VI (GPVI), an immunoadhesin known to bind type I and type III collagens. After in vitro characterization, collagelin was labeled with 99mTc for in vivo SPECT imaging. Rats with prior healed MI (>3 weeks old) received intravenous 99mTc-collagelin. In vivo SPECT imaging revealed specific isotope uptake in fibrotic areas dense in collagen fibers and showed minimal uptake in the surrounding non-infarcted myocardium. The collagelin probe thus expands the armamentarium of imaging agents designed to assay fibrosis (35,36).
B. Cell Transplantation
Molecular imaging of transplanted cell localization, function, proliferation, durability, and cell fate/specificity is highly suited to maximize the benefit of cardiovascular regeneration. Strategies leveraging reporter gene and exogenous physical cell labeling approaches have revealed substantial new insights into transplant cell biology in the past year.
Reporter Gene Approaches
Reporter gene approaches (i.e. incorporating a gene that encodes a receptor or substrate that can localize an imaging agent) enable precise and specific tracking of cell transplants, providing accurate readouts of cell viability and differentiation. Major reporter gene strategies include bioluminescence imaging (BLI) of firefly luciferase (Fluc) gene expression and PET/SPECT imaging of herpes simplex virus thymidine kinase (HSV-tK) or the sodium-iodide symporter (NIS) gene expression.
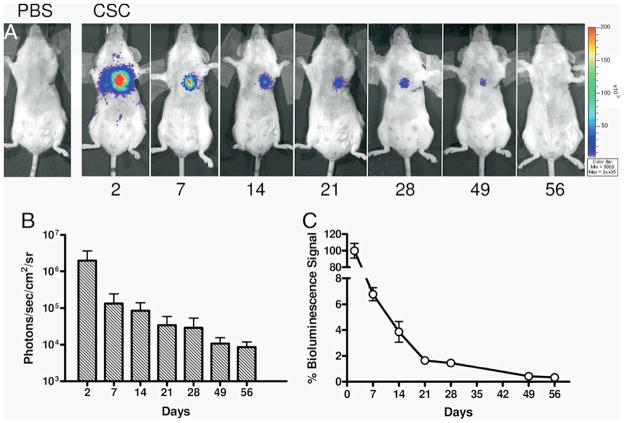
Using BLI (Fluc), Li et al. demonstrated that Sca-1 positive cardiac resident stem cells transplanted directly into mouse infarcts did not provide long-term cardiac engraftment nor benefit cardiac function (Figure 6) (41). Higuchi et al. utilized NIS-based PET reporter gene imaging to study the early survival of cardiac-transplanted human endothelial progenitor cells (EPCs). The study revealed that EPC survival was augmented by pretreatment with atorvastatin as well as co-transduction of the EPCs with VEGF (42). Swijenburg et al. utilized BLI (Fluc) to compare acute and subacute (day 7) transplants of murine bone marrow mononuclear cells into mouse infarcts, finding low engraftment in both groups. The timing of cell transplantation did not affect myocardial cell retention or impart significant benefits on cardiac function (43). Bai et al. employed BLI to demonstrate that human adipose tissue-derived cells and stem cells could survive at least 4 months in an immunopriviledged environment, and were associated with an improvement in myocardial function (44).
Figure 6.
Reporter gene optical imaging of cardiac stem cell (CSC) transplantation. (A) Firefly luciferase reporter gene labeled CSC transplantation reveals prominent bioluminescence signal at day 2 that decreased markedly over 8 weeks of longitudinal tracking. (B and C) Quantitative analyses of transplanted CSC signal changes over time and % donor cell survival. Reproduced with permission from (41).
Comparison of reporter gene imaging vs. physical cell label-based imaging
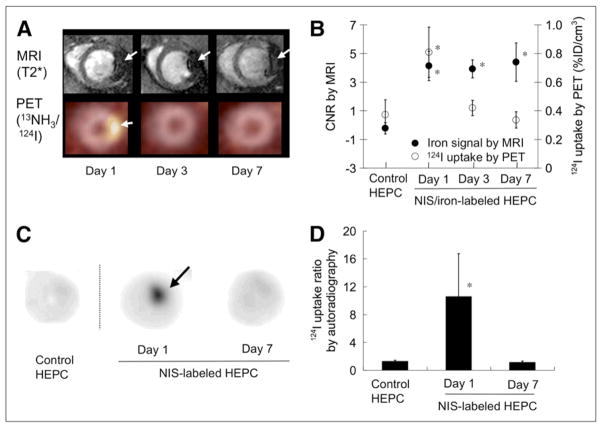
In a multimodal PET/MRI cardiac transplant study of human EPCs, Higuchi et al. demonstrated the complementary roles of reporter gene imaging (NIS, 124I) and physical cell labeling with iron oxides. PET imaging revealed a loss of cell viability by day 7 despite the persistence of iron oxide-induced signal void on MRI (Figure 7). Histological analyses demonstrated the absence of transplanted cells, corroborating the PET data, and also revealed that iron-laden macrophages were the likely source of the residual MRI signal (45). In a similar multimodal study of post-MI athymic rats transplanted with murine embryonic stem cells (ESCs) expressing the HSV-tk gene and also labeled with SPIOs, Qiao et al. demonstrated ESC survival by PET imaging, but a corresponding decrease in iron oxide MRI signal. At 4 weeks most iron oxide was histologically present within macrophages (46). Overall, while iron oxide does not appear to be a reliable reporter of long-term stem cell engraftment and survival, it can provide a marker for early transplantation and is clinically approved for use in humans.
Figure 7.
Combined reporter gene imaging (NIS-PET) and physical cell labeling (iron oxide-MRI) of cell transplantation. (A) Short-axis imaging of transplanted iron oxide (SPIOs) and NIS (sodium iodide symporter) reporter gene labeled EPCs into rat hearts at days 1, 3, and 7. (B) Mean (±SD) SPIO/MRI signals were stable over 7 days, while 124I PET uptake rapidly diminished to undetectable levels at day 7 (*P < 0.001). (C and D) Autoradiography revealed similar loss of 124I uptake over time, present as early as day 3 (*p<0.001). This study highlights the differences between reporter gene and physical cell label approaches for cell tracking. Reproduced with permission from (45).
Large animal demonstration of reporter gene imaging
In a swine study, Willmann et al. utilized a clinical PET/CT scanner to image myocardial transplants of human mesenchmyal stem cells (MSCs) incorporating the HSV-tK gene. Human MSCs (50–600 × 106) suspended in either phosphate-buffered saline or a matrigel scaffold were implanted into the myocardium of swine. Imaging of viable cells expressing the HSV-tk gene was performed following injection of 9-(4-18F-fluoro-3-[hydroxymethyl]butyl)guanine (18F-FHBG). The matrigel scaffold increased the retention of viable MSCs (47). This study provides a further step towards clinical utilization of the HSV-tk reporter gene approach for human cardiac studies, which may now be possible given a recent feasibility study in a cancer patient (48).
New reporter gene constructs
In a recent advance, Sun et al. formulated a triple fusion lentiviral construct in ESCs consisting of Fluc, HSV-tk, and the monomeric red fluorescent protein (mRFP) reporter gene to provide a three-phase imaging readout via bioluminescence, PET, and fluorescence imaging, respectively (49). The fluorescence capability enabled cell sorting/identification of ESCs expressing the triple construct. Monitoring transplanted human ESC survival over 6 months using in vivo BLI and PET imaging demonstrated long-term ESC viability with this approach. Non-invasive reporter gene monitoring is a powerful technique for stem cell tracking as only viable cells are captured, with the added benefit that daughter cells can be tracked with equal efficacy during proliferation without signal loss due to probe dilution. Kuliszewski et al. developed and validated a reporter gene imaging strategy for contrast-enhanced ultrasound (50). Bone-marrow derived rodent EPCs were stably transfected with the gene for the cell surface marker protein mouse H-2Kk. Ultrasound molecular imaging was performed by injecting microbubbles conjugated to an anti-H-2Kk antibody. Imaging demonstrated EPC growing within extracellular matrix scaffolds implanted in the subcutaneous tissue of rats. Although less sensitive than nuclear labeling approaches, this approach offers the potential for single-modality functional and molecular assessment of cardiac cell transplantation.
Physical cell labeling methods
Physical cell labeling approaches can utilize clinical imaging targets such as USPIOs for MRI, or 18F-FDG for PET. As these labels are not durable or transplant cell-specific over time, such approaches are likely to be useful in documenting acute delivery of the cell transplant payload, and to provide a highly translatable platform to optimize the clinical environment for maximal acute cell transplant retention.
Terrovitis et al. utilized the metabolic imaging agent 18F-FDG to study and manipulate acute cardiac-derived stem cell transplant retention in Wistar rats (51). 18F-FDG PET demonstrated greater cell retention at 1 hour when cell injections were sealed by fibrin glue placed at the epicardial injection site, or when cells were injected during adenosine-induced bradycardia or immediately following reversible cardiac arrest.
In addition, Adler et al. developed a new MRI-based agent, gadofluorine M-Cy3 (GdFM-Cy3, where Cy3 is a fluorochrome) for noninvasive tracking of stem cell engraftment (52). As opposed to USPIO labels for MRI, gadofluorine is primarily a T1 relaxation agent and therefore imparts positive MRI contrast, which can be particularly advantageous in low signal-to-noise ratio images. The GdFM-Cy3 paramagnetic probe forms micelles in aqueous solutions that are retained within the cell cytosol. The probe was tested by injection of GdFM-Cy3 labeled ESC-derived cardiac progenitor cells into infarcted zones of mouse hearts. Serial 9.4T MRI performed over 2 weeks revealed good agreement between the MRI-based signal and cell identification on fluorescent microscopy of excised tissues. While this probe identifies cells early after transplantation, similar to other physical agents it may suffer from dilutional and non-specific localization in other cells over time.
C. Cardiac Transplant Rejection
Current clinical diagnosis of cardiac allograft rejection requires endomyocardial biopsy. In addition to being invasive, endomyocardial biopsy-based diagnostics are limited by sampling error. Noninvasive detection of cardiac allograft rejection therefore remains an important goal for transplant medicine. Two reports from the past year highlight progress in noninvasive molecular MRI and optical imaging of the innate immune response in allograft rejection.
In a mouse model of heterotopic cardiac transplantation, Christen et al. detected allograft rejection using non-invasive MRI and optical molecular imaging readouts for macrophage phagocytosis and cathepsin protease activity (53). Macrophages were sensed with a phagocytosable clinical-type nanoparticle imaging agent for MRI, and cathepsin activity was sensed with a protease-activatable NIRF agent. Interestingly, early imaging at day 3 showed no difference between allografts and isografts, presumably due to similar levels of ischemia-reperfusion injury. By day 7, allograft rejection was the dominant inflammatory process. In vivo imaging at day 7 demonstrated increased macrophage enhancement on T2*-weighted MRI and increased NIRF cathepsin protease activity on FMT, with statistical differences in comparison to both the transplanted isograft group and the group with allografts implanted into genetically-engineered immune deficient mice.
In a second molecular and functional MRI study, USPIOs (phagocytosable clinical-type nanoparticles for MRI, 1.5–3.0 mg iron per animal) were employed in a rat abdominal heterotopic heart transplant model to detect macrophage infiltration (54). Throughout the rejection period, the USPIO signal was observed to be heterogeneous, even in advanced stages. As MRI can interrogate cardiac mechanics, the authors performed additional measurements of ventricular function by myocardial tagging and strain mapping, and found a correspondence between the extent of macrophage infiltration and the circumferential strain (Ecc). Based on these above studies, macrophage presence and altered myocardial strain may indicate early allograft rejection prior to the onset of frank LV dysfunction.
IV. Outlook
Molecular imaging studies are yielding unparalleled insight into in vivo cardiovascular biology. Experimentally, emphasis remains on developing highly sensitive imaging agents with excellent safety and pharmacokinetic profiles, and on new detection technology designed to address unmet needs. Clinical testing of promising molecular imaging agents remains a top priority for the field. For molecular imaging to gain a solid foothold in CVD clinical practice, utility beyond functional and anatomical imaging will need to be demonstrated. In the near term, molecular imaging will play a key role in assessing the biological effects of CVD therapies. In the mid-to-long term, molecular imaging should improve the risk stratification and the clinical management of many cardiovascular diseases.
Acknowledgments
Support Sources (FJ): Howard Hughes Medical Institute Early Career Award, American Heart Association Scientist Development Grant #0830352N, and NIH RO1 HL-108229.
Footnotes
Disclosures
We regret that additional excellent molecular imaging studies could not be included due to space limitations. FAJ was a prior consultant for VisEn Medical.
References
- 1.Rominger A, Saam T, Wolpers S, et al. 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med. 2009;50:1611–20. doi: 10.2967/jnumed.109.065151. [DOI] [PubMed] [Google Scholar]
- 2.Wassélius J, Larsson S, Sundin A, Jacobsson H. Assessment of inactive, active and mixed atherosclerotic plaques by 18F-FDG-PET; an age group-based correlation with cardiovascular risk factors. The international journal of cardiovascular imaging. 2009;25:133–40. doi: 10.1007/s10554-008-9366-5. [DOI] [PubMed] [Google Scholar]
- 3.Rudd JHF, Myers KS, Bansilal S, et al. Relationships Among Regional Arterial Inflammation, Calcification, Risk Factors, and Biomarkers: A Prospective Fluorodeoxyglucose Positron-Emission Tomography/Computed Tomography Imaging Study. Circulation: Cardiovascular Imaging. 2009;2:107–115. doi: 10.1161/CIRCIMAGING.108.811752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silvera SS, Aidi He, Rudd JHF, et al. Multimodality imaging of atherosclerotic plaque activity and composition using FDG-PET/CT and MRI in carotid and femoral arteries. Atherosclerosis. 2009;207:139–43. doi: 10.1016/j.atherosclerosis.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwee RM, Teule GJJ, van Oostenbrugge RJ, et al. Multimodality imaging of carotid artery plaques: 18F-fluoro-2-deoxyglucose positron emission tomography, computed tomography, and magnetic resonance imaging. Stroke. 2009;40:3718–24. doi: 10.1161/STROKEAHA.109.564088. [DOI] [PubMed] [Google Scholar]
- 6.Graebe M, Pedersen SF, Borgwardt L, Højgaard L, Sillesen H, Kjaer A. Molecular pathology in vulnerable carotid plaques: correlation with [18]-fluorodeoxyglucose positron emission tomography (FDG-PET) Eur J Vasc Endovasc Surg. 2009;37:714–21. doi: 10.1016/j.ejvs.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Wykrzykowska J, Lehman S, Williams G, et al. Imaging of inflamed and vulnerable plaque in coronary arteries with 18F-FDG PET/CT in patients with suppression of myocardial uptake using a low-carbohydrate, high-fat preparation. Journal of Nuclear Medicine. 2009;50:563–8. doi: 10.2967/jnumed.108.055616. [DOI] [PubMed] [Google Scholar]
- 8.Tang TY, Howarth SPS, Miller SR, et al. The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) Study. Evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. Journal of the American College of Cardiology. 2009;53:2039–50. doi: 10.1016/j.jacc.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 9.Howarth SPS, Tang TY, Trivedi R, et al. Utility of USPIO-enhanced MR imaging to identify inflammation and the fibrous cap: a comparison of symptomatic and asymptomatic individuals. European Journal of Radiology. 2009;70:555–60. doi: 10.1016/j.ejrad.2008.01.047. [DOI] [PubMed] [Google Scholar]
- 10.Hyafil F, Cornily J-C, Rudd JHF, Machac J, Feldman LJ, Fayad ZA. Quantification of inflammation within rabbit atherosclerotic plaques using the macrophage-specific CT contrast agent N1177: a comparison with 18F-FDG PET/CT and histology. Journal of Nuclear Medicine. 2009;50:959–65. doi: 10.2967/jnumed.108.060749. [DOI] [PubMed] [Google Scholar]
- 11.Worthley S, Zhang Z, Machac J, et al. In vivo non-invasive serial monitoring of FDG-PET progression and regression in a rabbit model of atherosclerosis. The international journal of cardiovascular imaging. 2009;25:251–7. doi: 10.1007/s10554-008-9377-2. [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann BA, Carr CL, Belcik JT, et al. Molecular imaging of the initial inflammatory response in atherosclerosis: implications for early detection of disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30:54–9. doi: 10.1161/ATVBAHA.109.196386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amirbekian V, Aguinaldo JGS, Amirbekian S, et al. Atherosclerosis and matrix metalloproteinases: experimental molecular MR imaging in vivo. Radiology. 2009;251:429–38. doi: 10.1148/radiol.2511080539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohshima S, Petrov A, Fujimoto S, et al. Molecular imaging of matrix metalloproteinase expression in atherosclerotic plaques of mice deficient in apolipoprotein e or low-density-lipoprotein receptor. Journal of Nuclear Medicine. 2009;50:612–7. doi: 10.2967/jnumed.108.055889. [DOI] [PubMed] [Google Scholar]
- 15.Laitinen I, Saraste A, Weidl E, et al. Evaluation of v 3 Integrin-Targeted Positron Emission Tomography Tracer 18F-Galacto-RGD for Imaging of Vascular Inflammation in Atherosclerotic Mice. Circulation: Cardiovascular Imaging. 2009;2:331–338. doi: 10.1161/CIRCIMAGING.108.846865. [DOI] [PubMed] [Google Scholar]
- 16.Nahrendorf M, Keliher E, Panizzi P, et al. 18F-4V for PET-CT imaging of VCAM-1 expression in atherosclerosis. JACC Cardiovasc Imaging. 2009;2:1213–22. doi: 10.1016/j.jcmg.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nahrendorf M, Waterman P, Thurber G, et al. Hybrid in vivo FMT-CT imaging of protease activity in atherosclerosis with customized nanosensors. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29:1444–51. doi: 10.1161/ATVBAHA.109.193086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipinski MJ, Frias JC, Amirbekian V, et al. Macrophage-specific lipid-based nanoparticles improve cardiac magnetic resonance detection and characterization of human atherosclerosis. JACC Cardiovasc Imaging. 2009;2:637–47. doi: 10.1016/j.jcmg.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tekabe Y, Li Q, Rosario R, et al. Development of Receptor for Advanced Glycation End Products-Directed Imaging of Atherosclerotic Plaque in a Murine Model of Spontaneous Atherosclerosis. Circulation: Cardiovascular Imaging. 2008;1:212–219. doi: 10.1161/CIRCIMAGING.108.788299. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y, Kuge Y, Zhao S, Strauss HW, Blankenberg FG, Tamaki N. Prolonged high-fat feeding enhances aortic 18F-FDG and 99mTc-annexin A5 uptake in apolipoprotein E-deficient and wild-type C57BL/6J mice. J Nucl Med. 2008;49:1707–14. doi: 10.2967/jnumed.108.051847. [DOI] [PubMed] [Google Scholar]
- 21.Sirol M, Moreno PR, Purushothaman K-R, et al. Increased Neovascularization in Advanced Lipid-Rich Atherosclerotic Lesions Detected by Gadofluorine-M-Enhanced MRI: Implications for Plaque Vulnerability. Circulation: Cardiovascular Imaging. 2009;2:391–396. doi: 10.1161/CIRCIMAGING.108.801712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ronald JA, Chen JW, Chen Y, et al. Enzyme-sensitive magnetic resonance imaging targeting myeloperoxidase identifies active inflammation in experimental rabbit atherosclerotic plaques. Circulation. 2009;120:592–9. doi: 10.1161/CIRCULATIONAHA.108.813998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aikawa E, Aikawa M, Libby P, et al. Arterial and aortic valve calcification abolished by elastolytic cathepsin S deficiency in chronic renal disease. Circulation. 2009;119:1785–94. doi: 10.1161/CIRCULATIONAHA.108.827972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujimoto S, Hartung D, Ohshima S, et al. Molecular imaging of matrix metalloproteinase in atherosclerotic lesions: resolution with dietary modification and statin therapy. Journal of the American College of Cardiology. 2008;52:1847–57. doi: 10.1016/j.jacc.2008.08.048. [DOI] [PubMed] [Google Scholar]
- 25.Haider N, Hartung D, Fujimoto S, et al. Dual molecular imaging for targeting metalloproteinase activity and apoptosis in atherosclerosis: molecular imaging facilitates understanding of pathogenesis. J Nucl Cardiol. 2009;16:753–62. doi: 10.1007/s12350-009-9107-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaffer FA, Vinegoni C, John MC, et al. Real-time catheter molecular sensing of inflammation in proteolytically active atherosclerosis. Circulation. 2008;118:1802–9. doi: 10.1161/CIRCULATIONAHA.108.785881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kotze CW, Menezes LJ, Endozo R, Groves AM, Ell PJ, Yusuf SW. Increased metabolic activity in abdominal aortic aneurysm detected by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) Eur J Vasc Endovasc Surg. 2009;38:93–9. doi: 10.1016/j.ejvs.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Reeps C, Gee MW, Maier A, et al. Glucose metabolism in the vessel wall correlates with mechanical instability and inflammatory changes in a patient with a growing aneurysm of the abdominal aorta. Circulation: Cardiovascular Imaging. 2009;2:507–9. doi: 10.1161/CIRCIMAGING.109.858712. [DOI] [PubMed] [Google Scholar]
- 29.DeLeo MJ, Gounis MJ, Hong B, Ford JC, Wakhloo AK, Bogdanov AA. Carotid artery brain aneurysm model: in vivo molecular enzyme-specific MR imaging of active inflammation in a pilot study. Radiology. 2009;252:696–703. doi: 10.1148/radiol.2523081426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tedesco MM, Terashima M, Blankenberg FG, et al. Analysis of in situ and ex vivo vascular endothelial growth factor receptor expression during experimental aortic aneurysm progression. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29:1452–7. doi: 10.1161/ATVBAHA.109.187757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vymazal J, Spuentrup E, Cardenas-Molina G, et al. Thrombus imaging with fibrin-specific gadolinium-based MR contrast agent EP-2104R: results of a phase II clinical study of feasibility. Investigative radiology. 2009;44:697–704. doi: 10.1097/RLI.0b013e3181b092a7. [DOI] [PubMed] [Google Scholar]
- 32.Miserus R-JJHM, Herías MV, Prinzen L, et al. Molecular MRI of early thrombus formation using a bimodal alpha2-antiplasmin-based contrast agent. JACC Cardiovasc Imaging. 2009;2:987–96. doi: 10.1016/j.jcmg.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, Nie L, Razavian M, et al. Molecular imaging of activated matrix metalloproteinases in vascular remodeling. Circulation. 2008;118:1953–60. doi: 10.1161/CIRCULATIONAHA.108.789743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheth RA, Tam JM, Maricevich MA, Josephson L, Mahmood U. Quantitative endovascular fluorescence-based molecular imaging through blood of arterial wall inflammation. Radiology. 2009;251:813–21. doi: 10.1148/radiol.2513081450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van den Borne SWM, Isobe S, Verjans JW, et al. Molecular imaging of interstitial alterations in remodeling myocardium after myocardial infarction. Journal of the American College of Cardiology. 2008;52:2017–28. doi: 10.1016/j.jacc.2008.07.067. [DOI] [PubMed] [Google Scholar]
- 36.van den Borne SWM, Isobe S, Zandbergen HR, et al. Molecular imaging for efficacy of pharmacologic intervention in myocardial remodeling. JACC Cardiovasc Imaging. 2009;2:187–98. doi: 10.1016/j.jcmg.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 37.Swirski FK, Nahrendorf M, Etzrodt M, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–6. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sosnovik DE, Garanger E, Aikawa E, et al. Molecular MRI of cardiomyocyte apoptosis with simultaneous delayed-enhancement MRI distinguishes apoptotic and necrotic myocytes in vivo: potential for midmyocardial salvage in acute ischemia. Circulation: Cardiovascular Imaging. 2009;2:460–7. doi: 10.1161/CIRCIMAGING.109.859678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sosnovik DE, Nahrendorf M, Panizzi P, et al. Molecular MRI detects low levels of cardiomyocyte apoptosis in a transgenic model of chronic heart failure. Circulation: Cardiovascular Imaging. 2009;2:468–75. doi: 10.1161/CIRCIMAGING.109.863779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muzard J, Sarda-Mantel L, Loyau S, et al. Non-invasive molecular imaging of fibrosis using a collagen-targeted peptidomimetic of the platelet collagen receptor glycoprotein VI. PLoS ONE. 2009;4:e5585. doi: 10.1371/journal.pone.0005585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Z, Lee A, Huang M, et al. Imaging survival and function of transplanted cardiac resident stem cells. Journal of the American College of Cardiology. 2009;53:1229–40. doi: 10.1016/j.jacc.2008.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Higuchi T, Anton M, Saraste A, et al. Reporter gene PET for monitoring survival of transplanted endothelial progenitor cells in the rat heart after pretreatment with VEGF and atorvastatin. J Nucl Med. 2009;50:1881–6. doi: 10.2967/jnumed.109.067801. [DOI] [PubMed] [Google Scholar]
- 43.Swijnenburg R-J, Govaert JA, van der Bogt KEA, et al. Timing of bone marrow cell delivery has minimal effects on cell viability and cardiac recovery after myocardial infarction. Circulation: Cardiovascular Imaging. 2010;3:77–85. doi: 10.1161/CIRCIMAGING.109.872085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bai X, Yan Y, Song Y-H, et al. Both cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarction. European Heart Journal. 2010;31:489–501. doi: 10.1093/eurheartj/ehp568. [DOI] [PubMed] [Google Scholar]
- 45.Higuchi T, Anton M, Dumler K, et al. Combined reporter gene PET and iron oxide MRI for monitoring survival and localization of transplanted cells in the rat heart. Journal of Nuclear Medicine. 2009;50:1088–94. doi: 10.2967/jnumed.108.060665. [DOI] [PubMed] [Google Scholar]
- 46.Qiao H, Zhang H, Zheng Y, et al. Embryonic stem cell grafting in normal and infarcted myocardium: serial assessment with MR imaging and PET dual detection. Radiology. 2009;250:821–9. doi: 10.1148/radiol.2503080205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Willmann JK, Paulmurugan R, Rodriguez-Porcel M, et al. Imaging gene expression in human mesenchymal stem cells: from small to large animals. Radiology. 2009;252:117–27. doi: 10.1148/radiol.2513081616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yaghoubi SS, Jensen MC, Satyamurthy N, et al. Noninvasive detection of therapeutic cytolytic T cells with 18F-FHBG PET in a patient with glioma. Nature clinical practice Oncology. 2009;6:53–8. doi: 10.1038/ncponc1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun N, Lee A, Wu JC. Long term non-invasive imaging of embryonic stem cells using reporter genes. Nat Protoc. 2009;4:1192–201. doi: 10.1038/nprot.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuliszewski MA, Fujii H, Liao C, et al. Molecular imaging of endothelial progenitor cell engraftment using contrast-enhanced ultrasound and targeted microbubbles. Cardiovasc Res. 2009;83:653–62. doi: 10.1093/cvr/cvp218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Terrovitis J, Lautamäki R, Bonios M, et al. Noninvasive quantification and optimization of acute cell retention by in vivo positron emission tomography after intramyocardial cardiac-derived stem cell delivery. Journal of the American College of Cardiology. 2009;54:1619–26. doi: 10.1016/j.jacc.2009.04.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adler ED, Bystrup A, Briley-Saebo KC, et al. In vivo detection of embryonic stem cell-derived cardiovascular progenitor cells using Cy3-labeled Gadofluorine M in murine myocardium. JACC Cardiovasc Imaging. 2009;2:1114–22. doi: 10.1016/j.jcmg.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Christen T, Nahrendorf M, Wildgruber M, et al. Molecular imaging of innate immune cell function in transplant rejection. Circulation. 2009;119:1925–32. doi: 10.1161/CIRCULATIONAHA.108.796888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu YL, Ye Q, Sato K, Foley LM, Hitchens TK, Ho C. Noninvasive evaluation of cardiac allograft rejection by cellular and functional cardiac magnetic resonance. JACC Cardiovasc Imaging. 2009;2:731–41. doi: 10.1016/j.jcmg.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]