Abstract
Excessive exposure to Mn induces neurotoxicity, referred to as manganism. Exposure assessment relies on Mn blood and urine analyses, both of which show poor correlation to exposure. Accordingly, there is a critical need for better surrogate biomarkers of Mn exposure. The aim of this study was to examine the relationship between Mn exposure and early indicators of neurotoxicity, with particular emphasis on peripheral biomarkers. Male Wistar rats (180–200 g) were injected intraperitoneally with 4 or 8 doses of Mn (10 mg/kg). Mn exposure was evaluated by analysis of Mn levels in brain and blood along with biochemical end-points (see below).
Results
Brain Mn levels were significantly increased both after 4 and 8 doses of Mn compared with controls (p<0.001). Blood levels failed to reflect a dose-dependent increase in brain Mn, with only the 8-dose treated group showing significant differences (p<0.001). Brain glutathione (GSH) levels were significantly decreased in the 8-dose-treated animals (p<0.001). A significant and dose-dependent increase in prolactin levels was found for both treated groups (p<0.001) compared to controls. In addition, a decrease in motor activity was observed in the 8-dose-treated group compared to controls.
Conclusions
1) The present study demonstrates that peripheral blood level is a poor indicator of Mn brain accumulation and exposure; 2) Mn reduces GSH brain levels, likely reflecting oxidative stress; 3) Mn increases blood prolactin levels, indicating changes in the integrity of the dopaminergic system. Taken together these results suggest that peripheral prolactin levels may serve as reliable predictive biomarkers of Mn neurotoxicity.
Keywords: manganese, neurotoxicity, biomarkers, prolactin, behavior
Introduction
Manganese (Mn) is an abundant and essential metal acquired naturally through regular dietary intake (Aschner et al., 2002). Accordingly, Mn levels in organs are usually kept at optimal concentrations (Michalke et al., 2007; Au et al., 2008). Blood Mn levels are maintained by both gastrointestinal (GI) absorption and efficient biliary excretion, the latter representing the main route for Mn elimination from the body (Dorman et al., 2006; Greenberg et al., 1940). In general, humans are exposed to low levels of Mn in water, air and food. Nevertheless, Mn can accumulate in certain brain regions following elevated exposures, and Mn-induced neurotoxicity may ensue. Overexposure to Mn can also occur in occupational environments, and cases of Mn neurotoxicity (manganism) have been reported particularly in miners, smelters and workers in the alloy industry where exposures occur predominantly via the inhalation of Mn fumes or Mn-containing dusts (ATSDR, 2000; Aschner et al., 2007). The gasoline additive, methylcyclopentadyenyl manganese tricarbonyl (MMT), is another source of airborne Mn (Kaiser, 2003).
The early onset of Mn intoxication is typically subtle, but once established, usually becomes progressive and irreversible, leading to permanent neurological damage (Jiang et al., 2006; Aschner et al., 2007). Thus, early diagnosis is crucial for preventing Mn neurotoxicity in settings of occupational and environmental exposure. Once absorbed, Mn is rapidly distributed throughout the body, concentrating primarily in the bones and in metabolically active organs. However, in all studied mammalian species, including humans, the brain represents the primary target tissue in which Mn persists for the longest duration (Maeda et al., 1997; Lucchini et al., 2000; Crossgrove and Zheng, 2004; Dorman et al., 2006; Kim et al., 2007). T1-weighted magnetic resonance imaging (MRI) corroborates the accumulation of Mn predominantly in the basal ganglia, namely in the globus pallidus, striatum and substantia nigra pars reticulata (Cersosimo et al., 2006; Reaney et al., 2006; Roth, 2006).
The precise mechanisms by which Mn enters the brain are not yet fully understood. As an essential metal, it readily crosses the blood-brain-barrier (BBB) in both the adult and the developing fetus (Aschner and Aschner, 1991). Recently, Aschner et al. (2007) and Au et al. (2008) documented that facilitated diffusion, active transport, divalent metal transport 1 (DMT-1; also known as DCT-1/NRAMP2)-mediated transport and transferrin (Tf)-dependent transport mechanisms are involved in shuttling Mn across the BBB. Mn accumulates in brain regions that are normally rich in iron, most likely due to the fact that the two metals share common transporters (Tf and DMT-1). Studies in Fe-deficient rats have shown Mn accumulation in the globus pallidus, hippocampus and substantia nigra (ATSDR, 2000), corroborating a close relationship between low Fe levels and increased Mn uptake into the brain.
Although Mn is a component of several critical enzymes that prevent cellular oxidative stress, such as mitochondrial superoxide dismutase (Aschner et al., 2002; Crossgrove and Zheng, 2004), it can also interact negatively with cellular dopamine, promoting dopamine autoxidation and causing dopaminergic cell death. Hypo- or hyper-activity in dopaminergic neurotransmission leads to a number of disorders, such as Parkinson’s disease and schizophrenia (Ben-Jonathan and Hnasko, 2001). Dopamine also acts by binding to D2-receptors on specific pituitary cells (lactrophs) which are responsible for the secretion of prolactin, thereby inhibiting both the release and the synthesis of this neurohormone (Ellingsen et al., 2003; Takser et al., 2004; Ellingsen et al., 2007). In addition, hypothalamic dopaminergic neurons, which provide dopaminergic efferents to the anterior pituitary gland are themselves regulated by feedback from prolactin through a short-loop feedback mechanism. A variety of other modulators of prolactin secretion (e.g., serotonin, GABA, estrogens and opioids) act at the hypothalamic level by inhibiting or reinforcing the dopaminergic tone (e.g., substance P) (Fitzgerald and Dinan, 2008). Evidence proving that dopamine regulates prolactin release in humans has also been corroborated by observations that drugs which interfere with its release affect circulating prolactin levels (Ben-Jonathan and Hnasko, 2001).
Since Mn stimulates dopamine autoxidation in dopaminergic neurons, it indirectly modulates prolactin secretion, thereby leading to an increase in circulating prolactin levels. Recently, Kim et al. (2009) studied mechanisms that govern changes in rat brain dopamine levels and prolactin production by evaluating the transacting factor of the prolactin gene (PIT-1). The results of these studies showed a increases in PIT-1 and prolactin, as well as a significant decrease in dopamine levels in rats exposed to Mn. These results are consistent with the role of PIT-1 as a regulator of both dopamine and prolactin levels.
Occupational biomonitoring in populations at risk for Mn exposure has attempted to address the utility of prolactin as a biological index of exposure (Sloot et al., 1996; Smargiassi and Mutti, 1999). Indeed, a strong relationship between Mn exposure and serum prolactin levels has been established in multiple studies (Aschner, 2006; Kim et al., 2007). Mn was positively associated with prolactin in human neonates (Takser et al., 2004) and occupationally exposed men (Mutti et al., 1996; and Smargiassi and Mutti, 1999; Ellingsen et al., 2007). However, negative relationships between Mn and prolactin have also been reported (Roels et al., 1992; Popek et al., 2006). For example, Roels et al. (1992) found that serum prolactin as a predictive measure of effect was unrelated to atmospheric Mn exposure. Such differences in outcomes may be attributable to different exposure times and differences in accumulated brain Mn levels.
In addition to its effect on the dopaminergic system, Mn also inhibits mitochondrial respiration and antioxidant systems. Mn readily induces free radical formation and oxidative stress (Chen and Liao, 2002; Hazzel and Normandin, 2002; Erikson et al. 2004; Marreilha dos Santos et al., 2010) leading to the excessive production of reactive oxygen species (ROS). ROS generation depletes GSH, perturbing the intracellular redox balance as well as the conjugation and excretion of toxic molecules (Meister, 1988, 1991; Duckande et al., 2006; Marreilha dos Santos et al., 2008). In vitro studies have shown that the inhibition of GSH synthesis and the consequent impairment of neuronal antioxidant system activity play a detrimental role in oxidative stress-mediated Mn neurotoxicity (Desole et al., 1997; Marreilha dos Santos et al., 2010). Dorman et al. (2000) detected significantly decreased striatal GSH levels following subchronic Mn sulphate (MnSO4) inhalation. A similar mechanism for Mn neurotoxicity was proposed by Erikson et al. (2004) using lipid peroxides, GSH and metallothionein as biomarkers of free radical production.
The identification and validation of biomarkers is crucial to human toxicology (Smith et al., 2007). To prevent the neurotoxic effects resulting from chronic exposure to Mn, at-risk populations must receive initial and subsequent examinations and necessary follow-up treatment using specific, sensitive and predictive biomarkers. Occupational exposure to Mn has been largely monitored by the determination of Mn levels in blood and urine. Lucchini et al. (1999) observed an association between blood Mn and exposure levels. Apostoli et al. (2000) investigated the suitability of blood and urine Mn levels for exposure assessment, concluding that the two measures could discriminate only between groups of occupationally exposed and control subjects; however, within the exposed groups, there was no association between blood and urinary Mn levels and Mn exposure. Furthermore, blood and urine Mn analyses did not provide information about previous exposures, due to Mn’s relatively short blood half-life (t1/2 < 2 h) (Li et al., 2006). Accordingly, published data question the utility of Mn blood and urine levels as biomarkers of chronic exposure, as they reveal substantial variability in Mn levels in exposed subjects. Since clinical diagnostic medicine relies on effect-related biomarkers (Liu et al., 2008), and the currently available tests (Mn in blood and urine) fail to establish this relationship, attention must be focused on alternative indicators (Smargiassi and Mutti, 1999; Apostoli et al., 2000; Montes et al., 2008).
Given these deficiencies in biomonitoring, it is prudent to perform in vivo assays to evaluate the relationship between biomarkers of exposure (blood and brain Mn levels) and effect (prolactin, GSH and behavioral assays). Accordingly, the present study was designed to address the consequences of repeated exposures to MnCl2 and to correlate its neurotoxic sequalae with reliable and predictive biomarkers of exposure (prolactin), which, in the future, could serve to validate risk in Mn-exposed human populations.
Results
Animal Weight
The rats’ weights were recorded on the first day of treatment and after the 4 and 8 doses of Mn, before the sacrifice. As shown in Table 1, on comparing the rats’ weights after the 4 and 8 doses of Mn with the respective controls, there was a reduction in the body weight gain in both treated groups but that reduction was only significant for the 8 doses group (P<0,001).
Table 1.
Growth rate measured in % of body weight gain (mean±SD), between the first and the last dose, of rats repeatedly exposed to 4 and 8 doses of MnCl2 (10 mg/kg).
| growth rate % | |
|---|---|
| Control 4 doses (saline) | 10,0±4,4 |
| Mn 4 doses | 4,7±2,1 |
| Control 8 doses (saline) | 22,3±3,7 |
| Mn 8 doses | 3,8±1,1* |
Significant different from respective control (p<0.001).
Behavioral assays
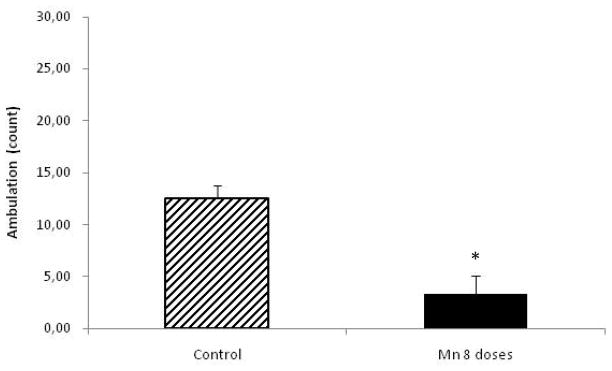
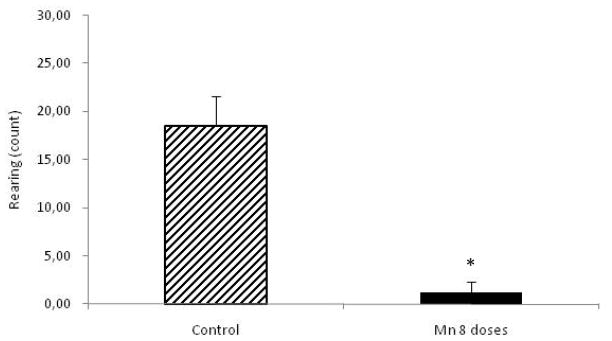
Behavioral parameters were studied in control and Mn-treated rats (8 doses) at the beginning of the experiment and 24 h after the last Mn dose, prior to sacrifice. MnCl2-treated rats showed decreased spontaneous activity compared to controls (p<0.05), both for ambulation and rearing (Fig. 1 and Fig. 2, respectively).
Figure 1.
Ambulation in rats repeatedly exposed to 8 doses of MnCl2 (10 mg/kg) vs. controls. Each bar represents the mean ± S.E.M. for n=6 (*p<0.05).
Figure 2.
Rearing in rats repeatedly exposed to 8 doses of MnCl2 (10 mg/kg) vs. controls. Each bar represents the mean ± S.E.M. for n=6 (*p<0.05).
Blood Mn Concentrations
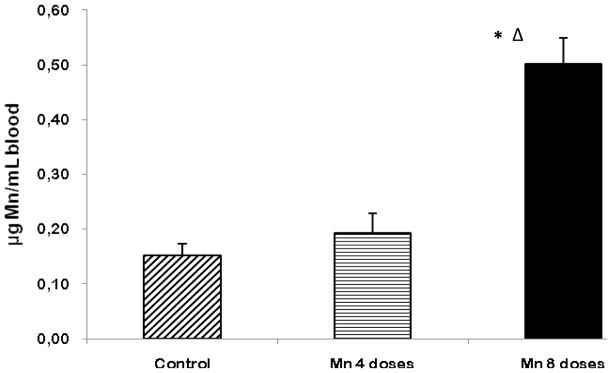
At the end of the experiment (24 h after the last dose), blood was collected, and levels of Mn were measured by means of GFAAS. Both the 4- and 8-dose Mn-treated animals had higher Mn levels in the blood compared with controls. However, the 4-dose-treated group showed no statistical differences compared with the controls. The 8-dose-treated Mn group had significantly higher (p<0.001) blood Mn concentrations compared with the controls as well as the 4-dose-treated group (p<0.01) (Fig. 3).
Figure 3.
Mn levels in blood of rats repeatedly exposed to 4 and 8 doses of MnCl2 (10 mg/kg). Bars represent the mean ± S.E.M. for n=6 (*p< 0.001, vs. control; Δ p<0.01, vs. 4-dose Mn-treated group).
Brain Mn Concentrations
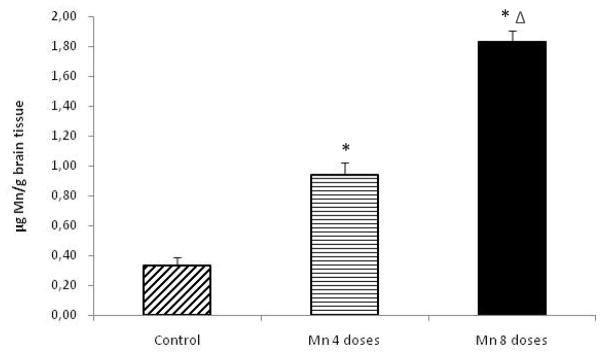
As shown in Fig. 4, rats receiving either 4 or 8 doses of MnCl2 showed a significant increase in brain Mn concentrations compared with controls (p<0.001). Moreover, brain Mn concentrations in the 8-dose-treated group were significantly increased compared to both the control and the 4-dose-treated groups (p<0.01).
Figure 4.
Mn levels in brain tissue of rats repeatedly exposed to 4 and 8 doses of MnCl2 (10 mg/kg). Bars represent the mean ± S.E.M. for n=6 (*p<0.001, vs. control; Δ p<0.01, vs. 4-dose Mn-treated group).
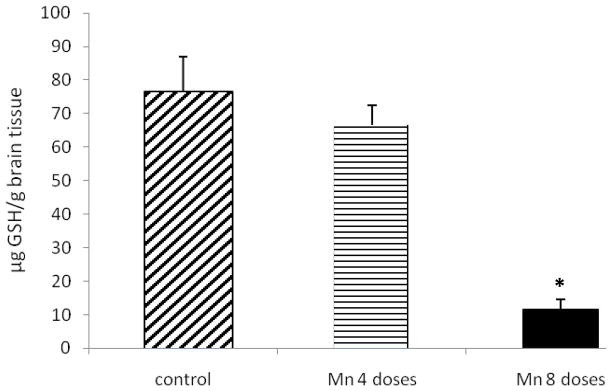
Brain GSH Levels
A decrease in brain GSH levels was observed in both Mn-treated groups; however, the decrease in GSH levels was only statistically significant in the 8-dose-treated group compared to controls (p<0.001) (Fig. 5); levels in the 4-dose-treated group remained indistinguishable from those of the controls.
Figure 5.
GSH levels in brain tissue of rats repeatedly exposed to 4 and 8 doses of MnCl2 (10 mg/kg). Bars represent the mean ± S.E.M. for n=6 (* p<0.001, vs. control).
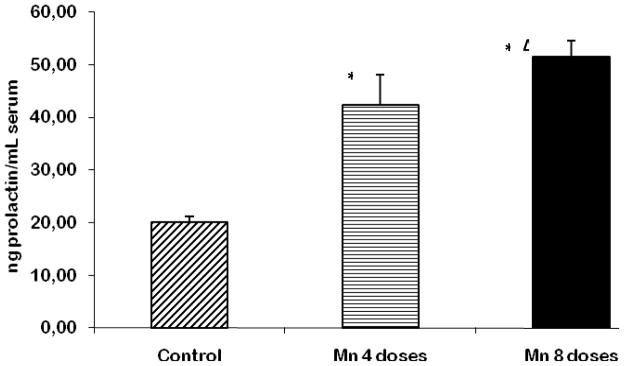
Serum Prolactin Levels
Prolactin levels were measured in rat serum, and the values were found to be significantly increased in both the 4- and 8-dose-treated Mn groups (p<0.001) compared to controls (Fig. 6). A statistical difference (p<0.05) was also observed between the two Mn-treated groups.
Figure 6.
Prolactin levels in the serum of rats repeatedly exposed to 4 and 8 doses of MnCl2 (10 mg/kg). Bars represent the mean ± S.E.M. for n=6 (* p<0.001 vs. control; Δ p<0.05, vs. 4-dose Mn-treated group).
Discussion
The control and prevention of chronic Mn-induced neurotoxicity has particular clinical relevance in occupational, clinical and environmental toxicology as overexposure to this metal can lead to progressive and permanent neurodegenerative damage, resulting in a syndrome analogous to idiopathic Parkinson’s disease (Aschner et al., 2002, Crossgrove and Zheng, 2004; Aschner et al., 2007). The need to biomonitor populations at risk has become increasingly important in preventing early and irreversible neurological damage. Thus, it is imperative that reliable, sensitive and predictive biomarkers are available for identifying populations most susceptible to the debilitating health consequences which can result from Mn exposure. As this objective cannot be met with observational epidemiological studies alone, the present study was designed to correlate Mn exposure with its neurotoxic effects, and, at the same time, to monitor adequate biomarkers of exposure and effect. In vivo assays were performed in 2 groups of rats exposed to 4 and 8 doses of MnCl2 over 8 consecutive days. The effects of Mn exposure were followed by behavioral assessments, as well as the analysis of biomarkers of exposure (Mn concentrations in brain and in blood) and effect (brain GSH and serum prolactin). Changes in behavior were used as indicators of neurotoxic effects.
A significant and progressive decrease in the rats’ weights was noted in response to Mn treatments (Table 1). These results are consistent with other published data (Torrente et al., 2005; Finkelstein et al., 2007). Motor activity measured at the end of the experiment (after 8 doses) revealed a significant decrease (P<0.05) in spontaneous activity for both ambulation and rearing in Mn-treated animals compared to controls (Fig. 1 and Fig. 2, respectively). The observed decrease in spontaneous activity in the open field due to MnCl2 treatment is consistent with earlier observations (Roels et al., 1997; Zheng et al., 2003; Ávila et al., 2010). This Mn-induced decrease in motor activity most likely reflects the vulnerability of the dopaminergic system (Verity, 1999; Hazell and Normandin, 2002).
Brain and blood Mn levels were selected as biomarkers of exposure. Mn has a short blood half-life (t1/2 < 2 h) (Maynard and Cotzias, 1955) and following exposure, it is quickly eliminated from the circulation. Currently, there are no predictive, reliable or consistent biomarkers that exhibit a correlation between Mn exposure and its neurotoxic effects. Recently, Apostoli et al. (2000) investigated the suitability of blood and urinary Mn levels for exposure assessment, concluding that while both blood and urine Mn can discriminate between exposed and unexposed workers, the variability of these indicators was exceedingly and unacceptably high (with only 13% of the variance explained), thereby failing to provide adequate and accurate usage for the biological monitoring of at-risk populations.
As shown in Fig. 3, blood Mn levels in both the Mn-treated groups were higher than those of the controls. However, the blood Mn level was only significantly higher in the 8-dose-treated group. These results corroborate those reported by others, suggesting that blood Mn concentration is a poor biomarker for Mn exposure (Garcia-Aranda et al., 1983), especially since even a 4-dose exposure, in which Mn blood levels were indistinguishable from controls, led to significant increases in brain Mn (Fig. 4) and serum prolactin concentrations (Fig. 6). Additionally, the Mn concentration in the brain was shown to be dose-dependently higher in the 4- and 8-dose Mn-treated groups (~3 and ~5 fold, respectively) compared to controls (Fig. 4).
Based on previous proposed mechanisms of Mn neurotoxicity (Erikson et al., 2008; Kim et al., 2009), we also analyzed two other biomarkers of effect (brain GSH and serum prolactin levels). Our results (Fig. 5) show a significant decrease in GSH levels in rats exposed to 8 doses of MnCl2 and an insignificant decrease in the 4-dose Mn-treated group. A decrease in GSH has already been observed in other studies with rats exposed to Mn (7.5, 15.0, 30.0 mg/kg body weight) (Yin et al., 2008), thus corroborating the presence of oxidative stress. In a recent in vitro study performed in our lab, we also confirmed a decrease in GSH levels in rat brain endothelial cells (RBE4 cells) exposed to several concentrations of MnCl2 (Marreilha dos Santos et al., 2008).
It is well known that Mn produces ROS with the depletion of antioxidant defense mechanisms (Stredrick et al., 2004). Nevertheless, alterations in antioxidant defenses do not represent the only known consequences of Mn-induced toxicity. In fact, Mn stimulates dopamine autoxidation in the dopaminergic neurons and modulates prolactin secretion, leading to an increase in circulating prolactin levels. Given that prolactin levels are regulated by dopamine, Mn-induced dopamine depletion has been posited to increase prolactin release and prolactin serum levels. This hypothesis was confirmed by our data (Fig. 6), which show increased levels of serum prolactin in male rats exposed to either 4 or 8 doses of Mn. Prolactin levels in both groups were significantly higher than in controls, as well as different from each other, demonstrating a dose-dependent effect (Fig. 6).
We are cognizant that the studies were carried out in males and the possibility exists that the result would differ were they conducted in females, reflecting gender-specific prolactin levels. Although the tuberoinfundibular dopaminergic system can be considered as the final common pathway of all neural mechanisms controlling prolactin secretion, other neurotransmitters, neuropeptides and hormones modulate the sensitivity of lactotrope cells to dopaminergic tonic inhibition (Gudelsky, 1981). Among the various neuromodulators, estrogens play an important role, since they increase the amount of prolactin excreted. It has been previously shown that estrogen exerts a variety of actions on hypophyseal function, including a direct stimulatory effect on the pituitary to increase prolactin synthesis and/or release (Nicoll and Meites, 1962; Chen et al., 1970). The increasing blood concentrations of estrogen during late pregnancy and during some stages of estrous cycle appear responsible for the elevated levels of prolactin in females. According with Ferroni et al. (1992), in female workers exposed to low perchloroethylene concentrations in dry-cleaning shops, a shift in the distribution of prolactin levels rather than an increased prevalence of abnormally high values were observed, as compared to controls. While most of the workers exposed to metals are men, for women exposed to Mn the need for proper female control group should be considered. Furthermore, baseline serum prolactin levels for women should be measured. Finally, consideration should be directed at the interaction between prolactin levels during pregnancy and risk to Mn.
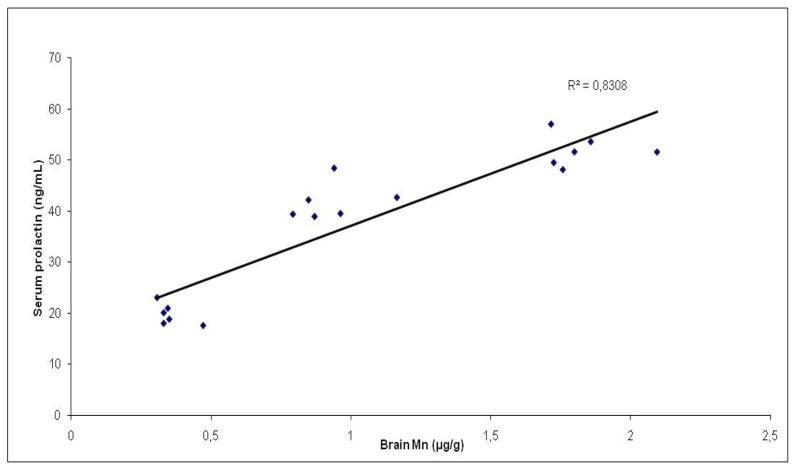
The best correlations for the exposure effects of Mn were noted for brain Mn content and prolactin serum levels (Fig. 7), as well as prolactin and neurobehavioral assays suggesting that prolactin may serve as a sensitive biomarker of cumulative exposure to Mn and as a predictive biomarker of neurotoxic effects (Table 2). To confirm this data, further research is currently being conducted, specifically directed at correlating serum prolactin levels with neurobehavioral changes in rats repetitively and simultaneously exposed to Mn and other metals, thus mimicking various occupational exposures.
Figure 7.
Correlation between Mn levels in the brain and prolactin levels in the serum of rats repeatedly exposed to 4 and 8 doses of MnCl2 (10 mg/kg; n=6).
Table 2.
Correlation between biomarkers/behavioral parameters with serum prolactin and brain Mn in rats exposed to 4 and 8 doses of Mn Cl2 (10 mg/Kg).
| Correlation | R2 |
|---|---|
| Brain Mn/Serum prolactin | 0,8306 |
| Blood Mn/Serum prolactin | 0,4870 |
| Brain GSH/Brain Mn | 0,7850 |
| Brain GSH/Serum prolactin | 0,4108 |
| Blood Mn/Brain Mn | 0,7524 |
| Rearing/Prolactin | 0,8623 |
| Ambulation/Prolactin | 0,8715 |
Materials and Methods
Chemicals
Manganese chloride tetrahydrate (MnCl2·4H2O, 99.99%: Sigma-Aldrich), nitric acid (HNO3, 65% suprapure: Merck), hydrogen peroxide (H2O2, 30%: Aldrich), sterile saline solution.
Animals
Experiments were performed according to the Guidelines for Animal Experimentation as set forth by the NIH. Male Wistar rats (Charles River Laboratories ®, Barcelona, Spain) weighing 150–171g were used for the experiments. Animals were housed in an isolated room and adapted to controlled environmental conditions for 10 days prior to experimentation. Room temperature was maintained at 24°C, with a relative humidity control of 50–70% and a 12-h light/dark cycle. Animals had free access to tap water and to a daily dose of food (Letica1 Ref. IPM-R20). General husbandry conditions were checked daily and the rats’ weights recorded.
Mn Exposure
Rats were randomly assigned to 3 separate groups. Two groups of six rats received a daily i.p. injection of Mn. One Mn-exposed group received a total of 4 doses of MnCl2 (10 mg/Kg), and the other Mn-exposed group received 8 doses of MnCl2 (10mg/kg). Eight untreated rats were used as controls (control animals received daily injections of sterile saline during 4 or 8 consecutive days). The animals were sacrificed 24 h after the last dose was administered.
Behavioral Assays
Spontaneous Locomotor Activity
Motor activity was assessed in the control and Mn-treated groups receiving a total of 8 doses of Mn. Motor activity was first assessed at the beginning of the experiment and after the last treatment, just prior to sacrifice. The order of testing was randomized, and the observer was blinded to the animals’ treatments.
Two behavioral parameters were studied: ambulation (number of crossings within 5 min in an open field) and rearing (number of times within 5 min where both forelegs were elevated from the floor in the same open field). The floor of the open field measured 60 cm × 90 cm and was divided into six equal squares. The floor was surrounded by an opaque wall 30 cm high. The rats were tested individually, and after each session, the open field was swiped with alcohol. Because a large number of environmental conditions can affect motor activity (e.g., sound level, cage design, lighting, temperature, humidity, and various odors), recordings were carried out in an isolated room at a fixed hour of the day with controlled light and sound, thus eliminating any confounding variables. At the beginning of each session, the animals were placed at the center of the open field, and the behavior of each rat was observed for 5 min (Ladefoged et al., 1994).
Tissue Collection and Analytical Procedures
Before sacrifice (24 h after the last dose), all rats were anesthetized with pentobarbital (20 mg/kg, i.p.), and blood was collected by cardiac puncture. Immediately after sacrifice, the brain was dissected out, weighed and stored at −80°C.
Several parameters were determined by analytical procedures (see below). Mn was measured in the brain and blood by atomic absorption spectrometry; brain Fe levels were measured by atomic absorption spectrometry; GSH was analyzed by high performance liquid chromatography (HPLC); and prolactin levels were determined by an enzyme immunoassay method.
Analytical Methods
Determination of Mn in biological samples
Microwave-Assisted Acid Digestion
Brain and blood samples were digested with an oxidizing acid mixture of 4:1 (v/v) 65% suprapure nitric acid: hydrogen peroxide, prior to graphite furnace atomic absorption spectrometry (GFAAS) analysis. Eighty mg of brain homogenate were weighed, and 2.9 mL of the oxidizing mixture were added to a cup. The cups were sealed, placed inside the respective bombs (Parr Microwave Acid Digestion Bombs ®) and subjected to microwave irradiation (30″, 900 W). Solutions were kept at 4°C until analysis.
Graphite Furnace Atomic Absorption Spectrometry
Mn concentrations were determined by GFAAS with a Perkin Elmer AAnalyst™ 700 atomic absorption spectrometer equipped with an HGA Graphite Furnace, a programmable sample dispenser (AS 800 Auto Sampler, and WinLab 32 for AA software). Mg (NO3)2·6 H2O (0.84 mol/L) was used as a chemical modifier. Each measurement consisted of two injections into the graphite furnace. Results were expressed as micrograms of Mn per g of brain tissue and micrograms of Mn per milliliter of blood. Calibration curves were obtained with standard solutions of 5, 10, 15 and 25 μg/L of MnCl2. The limit of detection was 0.05 μg Mn/L.
Brain GSH Analysis
Sample Preparation
Brain tissue was homogenized, and samples were weighed (approximately 20 mg). PBS solution was added (10 times the weight in volume), and samples were macerated. Samples were then sonicated (30 cycles; Conditions: Cycle 0.5, Amplitude 100) and centrifuged for 10 minutes at 10,000 rpm. The supernatant was collected in 1 ml Eppendorf tubes, and samples were analyzed by HPLC. Samples were handled on ice, and only non-metallic utensils were used.
HPLC analyses
GSH levels in brain tissue were measured by HPLC. The equipment was supplied with a Supelcosil LC-18-S reverse phase column, a Waters 2475 fluorimeter detector and a Waters 2695 separation module. The data were analyzed with Water Empower II Software.
Determination of Prolactin in Serum
Serum prolactin was quantified by an enzyme immunoassay (Citomed ®). Plates were read in an Anthos Zenyth 3100 microplate detector. The limit of quantification was 0.6 ng/mL.
Statistical Analysis
All analyses were conducted with Microsoft Office Excel® 2003 and SPSS Statistics® version 16.0, 2007. All results were analyzed for normal distribution with the Kolmogorov-Smirnov test. Variables were compared by tests for homogeneity of variance (Levene’s test) and one-way analysis of variance (ANOVA). For multiple comparisons, ANOVA complemented with post hoc Tukey’s HSD (all pairwise), and the Dunnett’s test (pairwise versus control) was performed. Data are presented as mean values ± standard error of the mean (S.E.M.). The limit for statistical significance was set at p<0.05 (significance level of 95%).
Acknowledgments
The authors wish to thank Doctor Rita Castro for her support with the HPLC analysis and Dr. Virginia Carvalho for assisting in the GFAAS analysis. Support for MA was provided by funds from the National Institutes of Health (NIEHS ES10563) and by the I-MED, Research Institute for Medecines and Pharmaceutical Sciences, University of Lisbon.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apostoli P, Lucchini R, Alessio L. Are current biomarkers suitable for the assessment of manganese exposure in individual workers? Am J Ind Med. 2000;37(3):283–290. doi: 10.1002/(sici)1097-0274(200003)37:3<283::aid-ajim6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Aschner M, Aschner JL. Manganese neurotoxicity: Cellular effects and blood-brain barrier transport. Neurosci Biobehav Rev. 1991;15(3):333–340. doi: 10.1016/s0149-7634(05)80026-0. [DOI] [PubMed] [Google Scholar]
- Aschner M, Connor JR, Dorman DC, Malecki EA, Vrana KE. Manganese in Health and Disease. In: Massaro EJ, editor. Handbook of Neurotoxicology. Humana Press Inc; Totowa: 2002. pp. 195–209. [Google Scholar]
- Aschner M. Manganese as a potential confounder of serum prolactin. Environ Health Perspect. 2006;114(8):A458–A458. doi: 10.1289/ehp.114-a458a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Guilarte T, Schneider J, Zheng W. Manganese: Recent advances in understanding its transport and neurotoxicity Toxicol. Appl Pharmacol. 2007;21(2):131–147. doi: 10.1016/j.taap.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Diseases) Toxicological Profile for Manganese. U.S. Department of Health and Human Services, Public Health Service; 2000. [Google Scholar]
- Au C, Benedetto A, Aschner M. Manganese transport in eukaryotes: The role of DMT1. NeuroToxicology. 2008;29:569–576. doi: 10.1016/j.neuro.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ávila DS, Colle D, Gubert P, Palma AS, Puntel G, Manarin F, Noremberg S, Nascimento PC, Aschner M, Rocha JBT, Félix AAS. A possible neuroprotective action of a vinylic telluride against Mn-induced neurotoxicity. Toxicol Sci. 2010;115(1):194–201. doi: 10.1093/toxsci/kfq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Jonathan N, Hnasko R. Dopamine as a Prolactin (PRL) Inhibitor. Endocr Rev. 2001;22(6):724–763. doi: 10.1210/edrv.22.6.0451. [DOI] [PubMed] [Google Scholar]
- Cersosimo MG, Koller WC. The diagnosis of manganese-induced parkinsonism. NeuroToxicology. 2006;27(3):340–346. doi: 10.1016/j.neuro.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Chen CL, Meites J. Effects of estrogen and progesterone on serum and pituitary prolactin levels in ovariectomized rats. Endocrinology. 1970;86:503–505. doi: 10.1210/endo-86-3-503. [DOI] [PubMed] [Google Scholar]
- Chen C-J, Liao SL. Oxidative Stress Involves in Astrocytic Alterations Induced by Manganese. Exp Neurol. 2002;175(1):216–225. doi: 10.1006/exnr.2002.7894. [DOI] [PubMed] [Google Scholar]
- Crossgrove J, Zheng W. Manganese toxicity upon overexposure. NMR Biomed. 2004;17(8):544–553. doi: 10.1002/nbm.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desole MS, Espolio G, Migheli R, Sircana S, Delogu MR, Fresu L, Miele M, de Natale G, Miele E. Glutathione deficiency potentiates manganese toxicity in rat striatum and brainstem and in PC12 cells. Pharmacol Res. 1997;36(4):285–292. doi: 10.1006/phrs.1997.0197. [DOI] [PubMed] [Google Scholar]
- Dorman DC, Struve MF, Vitarella D, Byerly FL, Goetz J, Miller R. Neurotoxicity of manganese chloride in neonatal and adult CD rats following Subchronic (21 days) high-dose oral exposure. J Appl Toxicol. 2000;20:179–187. doi: 10.1002/(sici)1099-1263(200005/06)20:3<179::aid-jat631>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Dorman DC, Struve MF, Clewell HJ, Andersen ME. Application of pharmacokinetic data to the risk assessment of inhaled manganese. NeuroToxicology. 2006;27(5):752–764. doi: 10.1016/j.neuro.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Dukhande VV, Malthankar-Phatak GH, Hugus JJ, Daniels CK, Lai JCK. Manganese-induced neurotoxicity is differentially enhanced by glutathione depletion in astrocytoma and neuroblastoma cells. Neurochem Res. 2006;31:1349–1357. doi: 10.1007/s11064-006-9179-7. [DOI] [PubMed] [Google Scholar]
- Ellingsen DG, Haug E, Gaarder P, Bast-Pettersen R, Thomassen Y. Endocrine and immunologic markers in manganese alloy production workers. Scand J Work Environ Health. 2003;29(3):230–238. doi: 10.5271/sjweh.726. [DOI] [PubMed] [Google Scholar]
- Ellingsen DG, Chashchin V, Haug E, Chashchin M, Tkachenko V, Lubnina M, Bast-Pettersen R, Thomassen Y. An epidemiological study of reproductive function biomarkers in male welders. Biomarkers. 2007;12(5):497–509. doi: 10.1080/13547500701366496. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Dobson AW, Dorman DC, Aschner M. Manganese exposure and induced oxidative stress in the rat brain. Sci Total Environ. 2004;334–335:409–416. doi: 10.1016/j.scitotenv.2004.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson KM, Dorman DC, Lash LH, Aschner M. Persistent alterations in biomarkers of oxidative stress resulting from combined in utero and neonatal manganese inhalation. Biol Trace Elem Res. 2005;104(2):151–163. doi: 10.1385/BTER:104:2:151. [DOI] [PubMed] [Google Scholar]
- Erikson ME, Dorman DC, Lash LH, Ashner M. Duration of airborne-manganese exposure in rhesus monkeys is associated with brain regional changes inbiomarkers of neurotoxicity. Neurotoxicology. 2008;29:377–385. doi: 10.1016/j.neuro.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Ferroni C, Selis L, Mutti A, Folli D, Bergamaschi E, Franchini I. Neurobehavioral and neuroendocrine effects of occupational exposure to perchloroethylene. Neurotoxicology. 1992;13:243–248. [PubMed] [Google Scholar]
- Finkelstein Y, Milatovic D, Aschner M. Modulation of cholinergic systems by manganese. NeuroToxicology. 2007;28(5):1003–1014. doi: 10.1016/j.neuro.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P, Dinan TG. Prolactin and dopamine: what is the connection? J Psychopharmacol. 2008;9:22, 82–89. doi: 10.1177/0269216307087148. [DOI] [PubMed] [Google Scholar]
- Garcia-Aranda JARA, Wapnir RA, Lifshitz F. In vivo intestinal absorption of manganese in the rat. J Nutr. 1983;113(12):2601–2607. doi: 10.1093/jn/113.12.2601. [DOI] [PubMed] [Google Scholar]
- Greenberg DMWW, Campbell WW. Studies in mineral metabolism with the aid of induced radioactive isotopes IV-manganese. Proc Natl Acad Sci USA. 1940;26(7):448–452. doi: 10.1073/pnas.26.7.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudelsky GA. Tuberoinfundibular dopamine neurons and the regulation of prolactin secretion. Psychoneuroendocrinology. 1981;6:3–16. doi: 10.1016/0306-4530(81)90044-5. [DOI] [PubMed] [Google Scholar]
- Gunter TE, Gavin CE, Aschner M, Gunter KK. Speciation of manganese in cells and mitochondria: A search for the proximal cause of manganese neurotoxicity. Neurotoxicology. 2006;27(5):765–776. doi: 10.1016/j.neuro.2006.05.002. [DOI] [PubMed] [Google Scholar]
- HaMai D, Campbell ASC, Bondy SC. Modulation of oxidative events by multivalent manganese complexes in brain tissue. Free Radic Biol Med. 2001;31(6):763–768. doi: 10.1016/s0891-5849(01)00639-6. [DOI] [PubMed] [Google Scholar]
- Hazell AS, Normandin L. Manganese neurotoxicity: An update of pathophysiologic mechanisms. Metab Brain Dis. 2002;17:375–387. doi: 10.1023/a:1021970120965. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Mo X, Du FQ, Fu X, Zhu XY, Gao HY, Xie JL, Liao FL, Pira E, Zheng W. Effective treatment of manganese-induced occupational Parkinsonism with p-aminosalicylic acid: a case of 17-year follow-up study. J Occup Environ Med. 2006;48(6):644–9. doi: 10.1097/01.jom.0000204114.01893.3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser J. Manganese: a high octane dispute. Science. 2003;9:300, 926–8. doi: 10.1126/science.300.5621.926. [DOI] [PubMed] [Google Scholar]
- Keith M, Erikson KM, Allison W, Dobson AW, Dorman DC, Aschner M. Manganese exposure and induced oxidative stress in the rat brain. Sci Total Environ. 2004;334–335:409–416. doi: 10.1016/j.scitotenv.2004.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EA, Cheong HK, Joo KD, Shin JH, Choi SB, Kim MO, Lee IJ, Kang DM. Effect of occupational manganese exposure on the central nervous system of welders: 1H magnetic resonance spectroscopy and MRI findings. NeuroToxicology. 2007;28(2):276–283. doi: 10.1016/j.neuro.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Kim HY, Lee CK, Lee Jt, Moon CS, Ha SC, Kang SG, Kim DH, Kim HD, Ahn JH, Lee SB, Kang MG. Effects of manganese exposure on dopamine and prolactin production in rat. Neuroreport. 2009;20(1):69–73. doi: 10.1097/WNR.0b013e328315cd35. [DOI] [PubMed] [Google Scholar]
- Ladefoged O, Roswall K, Larsen JJ. Acetone potentiation and influence on the reversibility of 2,5-hexanedione-induced neurotoxicity studied with behavioral and morphometric methods in rats. Pharmacol and Toxicol. 1994;74:294–299. doi: 10.1111/j.1600-0773.1994.tb01114.x. [DOI] [PubMed] [Google Scholar]
- Li GJ, Choi BS, Wang X, Liu J, Waalkes MP, Zheng W. Molecular mechanism of distorted iron regulation in the blood-CSF barrier and regional blood-brain barrier following in vivo subchronic manganese exposure. Neurotoxicology. 2006;27(5):737–744. doi: 10.1016/j.neuro.2006.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Goyer RA, Waalkes MP. In: Toxicology: the basic science of poisons 2008. 7. Klassen CD, Casarett, Doull’s, editors. USA: Mcgraw-Hill; 2008. pp. 955–956. [Google Scholar]
- Lucchini R, Apostoli P, Perrone C, Placidi D, Albini E, Migliorati P. Long-term exposure to “low levels” of manganese oxides and neurofunctional changes in ferroalloy workers. Neurotoxicology. 1999;20:287–297. [PubMed] [Google Scholar]
- Lucchini R, Albini E, Placidi D, Alessio L. Mechanism of neurobehavioral alteration. Toxicol Lett. 2000;112–113:35–39. doi: 10.1016/s0378-4274(99)00251-9. [DOI] [PubMed] [Google Scholar]
- Maeda H, Sato M, Yoshikawa A, Kimura M, Sonomura T, Terada M, Kishi K. Brain MR imaging in patients with hepatic cirrhosis: relationship between high intensity signal in basal ganglia on T1-weighted images and elemental concentrations in brain. Neuroradiology. 1997;39(8):546–550. doi: 10.1007/s002340050464. [DOI] [PubMed] [Google Scholar]
- Malecki EA, Devenyi AG, Beard JL, Connor JR. Existing and emerging mechanisms for transport of iron and manganese to the brain. J Neurosci Res. 1999;56(2):113–122. doi: 10.1002/(SICI)1097-4547(19990415)56:2<113::AID-JNR1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Marreilha dos Santos AP, Santos D, Au C, Milatovic D, Aschner M, Batoreu MCC. Antioxidants prevent the cytotoxicity of manganese in RBE4 cells. Brain Res. 2008;1236:200–205. doi: 10.1016/j.brainres.2008.07.125. [DOI] [PubMed] [Google Scholar]
- Marreilha dos Santos AP, Milatovic D, Au C, Zhaobao Y, Batoreu M, Camila Aschner M. Rat brain endothelial cells are a target of manganese toxicity. Brain Res. 2010;1326:152–161. doi: 10.1016/j.brainres.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard LS, Cotzias GC. The partition of manganese among organs and intracellular organelles of the rat. J Biol Chem. 1955;214(1):489–495. [PubMed] [Google Scholar]
- Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988;263(33):17205–17208. [PubMed] [Google Scholar]
- Meister A. Glutathione deficiency produced by inhibition of its synthesis, and its reversal; Applications in research and therapy. Pharmacol Ther. 1991;51(2):155–194. doi: 10.1016/0163-7258(91)90076-x. [DOI] [PubMed] [Google Scholar]
- Melov S. Modeling mitochondrial function in aging neurons. Trends Neuroscie. 2004;27(10):601–606. doi: 10.1016/j.tins.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Michalke B, Halbach S, Nischwitz V. Speciation and toxicological relevance of manganese in humans. J Environ Monitor. 2007;9:650–656. doi: 10.1039/b704173j. [DOI] [PubMed] [Google Scholar]
- Montes S, Riojas-Rodriguez H, Sabido-Pedraza E, Rios C. Biomarkers of manganese exposure in a population living close to a mine and mineral processing plant in Mexico. Environ Res. 2008;106(1):89–95. doi: 10.1016/j.envres.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Mutti A, Bergamashi E, Alinovi R, Lucchini R, Vettori MV, Franchini I. Serum prolactin in subjects occupationally exposed to manganese. Ann Clin Lab Sci. 1996;26:10–17. [PubMed] [Google Scholar]
- Nicoll CS, Meites J. Estrogen-stimulation of prolactin production by rat adenohypophysis in vitro. Endocrinol. 1962;70:272–277. doi: 10.1210/endo-70-2-272. [DOI] [PubMed] [Google Scholar]
- Popek W, Dietrich G, Glogowski J, Demska-Zakes K, Drag-Kozak E, Sionkowski J, Luszczek-Trojan E, Epler P, Demianowicz W, Sarosiek B, Kowalski R, Jankun M, Zakes Z, Krol J, Czerniak S, Szczepkowski M. Influence of heavy metals and 4-nonylphenol on reproductive function in fish. Reprod Biol. 2006;6(Suppl 1):175–188. [PubMed] [Google Scholar]
- Reaney SH, Bench G, Smith DR. Brain accumulation and toxicity of Mn(II) and Mn(III) exposures. Toxicol Sci. 2006;93(1):114–124. doi: 10.1093/toxsci/kfl028. [DOI] [PubMed] [Google Scholar]
- Roels HA, Ghyselen P, Buchet JP, Ceulemans E, Lauwerys RR. Assessment of the permissible exposure level to manganese in workers exposed to manganese dioxide dust. Brit J Ind Med. 1992;49:25–34. doi: 10.1136/oem.49.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roels H, Meiers G, Delos M, Ortega I, Lauwerys R, Buchet JP, Lison D. Influence of the route of administration and the chemical form (MnCl2, MnO2) on the absorption and cerebral distribution of manganese in rats. Arch Toxicol. 1997;71(4):223–230. doi: 10.1007/s002040050380. [DOI] [PubMed] [Google Scholar]
- Roth JA. Homeostatic and toxic mechanisms regulating manganese uptake, retention and elimination. Biol Res. 2006;39:45–57. doi: 10.4067/s0716-97602006000100006. [DOI] [PubMed] [Google Scholar]
- Shah NJ, Neeb H, Zaitzev M, Steinhoff S, Korcheis G, Amunts K, Haussinger D, Zilles K. Quantitative T1 mapping of hepatic encephalopathy using magnetic Resonance imaging. Hepatology. 2003;38(5):1219–1226. doi: 10.1053/jhep.2003.50477. [DOI] [PubMed] [Google Scholar]
- Sloot WN, Korf J, Koster JF, DeWitt LEA, Gramsbergen JBP. Manganese-induced hydroxyl radical formation in rat striatum is not attenuated by depletion or Iron chelation in vivo. Expt Neurol. 1996;138:236–245. doi: 10.1006/exnr.1996.0062. [DOI] [PubMed] [Google Scholar]
- Smargiassi A, Mutti A. Peripheral biomarkers and exposure to manganese. Neurotoxicology. 1999;20(2–3):401–6. [PubMed] [Google Scholar]
- Smith D, Gwiazda R, Bowler R, Roels H, Park R, Taicher C, Lucchini R. Biomarkers of Mn exposure in humans. Am J Ind Med. 2007;50(11):801–811. doi: 10.1002/ajim.20506. [DOI] [PubMed] [Google Scholar]
- Stredrick DL, Stokes AH, Worst TJ, Freeman WM, Johnson EA, Lash L, Aschner, Vrana KE. Manganese-Induced Cytotoxicity in Dopamine Producing Cells. Neurotoxicology. 2004;25(4):543–553. doi: 10.1016/j.neuro.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Takser L, Mergler D, de Grosbois S, Smargiassi A, Lafond J. Blood manganese content at birth and cord serum prolactin levels. Neurotoxicol Teratol. 2004;26(6):811–815. doi: 10.1016/j.ntt.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Torrente M, Colomina MT, Domingo JL. Behavioral effects of adult rats concurrently exposed to high doses of oral manganese and restraint stress. Toxicology. 2005;211(1–2):59–69. doi: 10.1016/j.tox.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Verity M. Manganese neurotoxicity: a mechanistic hypothesis. Neurotoxicology 1999. 1999;20(2–3):489–97. [PubMed] [Google Scholar]
- Vezér T, Papp A, Hoyk Z, Varga C, Náray M, Nagymatényi L. Behavioral and neurotoxicological effects of subchronic manganese exposure in rats. Environ Toxicol Pharmacol. 2005;19(3):797–810. doi: 10.1016/j.etap.2004.12.046. [DOI] [PubMed] [Google Scholar]
- Weber S, Dorman DC, Lash LH, Erikson K, Vrana KE, Aschner M. Effects of manganese (Mn) on the developing rat brain: Oxidative-stress related endpoints. Neurotoxicology. 2002;23:169–175. doi: 10.1016/s0161-813x(02)00014-1. [DOI] [PubMed] [Google Scholar]
- Yin Z, Aschner JL, dos Santos AP, Aschner M. Mitochondrial-dependent manganese neurotoxicity in rat primary astrocyte cultures. Brain Res. 2008;1203:1–11. doi: 10.1016/j.brainres.2008.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Aschner M, Ghersi-Egea JF. Brain barrier systems: a new frontier in metal neurotoxicological research. Toxicol Appl Pharmacol. 2003;192:1–11. doi: 10.1016/s0041-008x(03)00251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]